Abstract
Purpose
Empty follicle syndrome (EFS) refers to the inability to obtain mature oocytes after appropriate ovarian stimulation during the process of in vitro fertilization (IVF). However, the specific cause and mechanism of action underlying EFS remain to be further explored. Herein we aimed to investigate the clinical and genetic characteristics of EFS.
Methods
After data were collected in an infertile family, we performed whole-exome sequencing (WES) on the patient and confirmed the pathogenic mutations through Sanger sequencing. Western immunoblotting, immunofluorescence staining, and minigene assay were further used to investigate the negative effects of these mutations.
Results
Absence of oocytes was observed over 2 cycles of IVF in the patient, and we evaluated the novel compound heterozygous mutations c.2T>A (p. M1K) and c.1112+1G>T of the zona pellucida glycoprotein 1 gene (ZP1, MIM# 195000) by WES. For the family under study, EFS was classified as an autosomal recessive inheritance pattern. The results of western blotting and immunofluorescence staining analyses confirmed that the missense mutation of c.2T>A [p. M1K] resulted in almost missing protein production. Additionally, using a minigene assay, we demonstrated the deleterious effect on the ZP1 gene of the splice site mutation c.1112+1G>T, which caused truncation of ZP1 protein.
Conclusions
The compound heterozygous mutations of ZP1 gene identified in this study by genetic and functional experiments constituted a novel genetic cause of EFS, and further study will expand its use in the clinical and molecular diagnoses of EFS.
Keywords: Empty follicle syndrome, ZP1, Gene mutation, WES
Introduction
Empty follicle syndrome (EFS) is a phenomenon whereby follicles develop well before ovulation in natural or induced-ovulation cycles; even though the size and number of follicles and the concentrations of serum estradiol (E2) are within normal range, oocytes are unable to be obtained after repeated follicle aspiration and washing [1]. Stevenson et al. first proposed in 2008 to divide EFS into genuine EFS (GEFS) and false EFS (FEFS) [2]. FEFS is mainly associated with pharmacological or iatrogenic problems, for example, a patient error in timing, preparation, or administration of the triggering drug, resulting in the low levels of beta-human chorionic gonadotropin (β-hCG) on the day of oocyte retrieval [2]. However, the etiology of GEFS, in which the optimal β-hCG levels are present on the day of oocyte retrieval, remains obscure and limited to date. It has been suggested that GEFS is commonly ascribed to dysfunctional folliculogenesis, ovarian aging, or genetic defects [3–6]. Only a few genetic factors associated with GEFS have thus far been identified, including luteinizing hormone/choriogonadotropin receptor (LHCGR) [7, 8], zona pellucida glycoprotein 1 (ZP1) [9–12], pellucida glycoprotein 3 (ZP3) [13], and the pericentric inversion of chromosome 2 [14]. Therefore, more etiologic studies are certainly needed.
In mammals, the zona pellucida (ZP) is an extracellular matrix that envelops the oocyte [15], and loss-of-function mutations in ZP genes are closely related to female infertility (Table 1). According to the previous studies, patients with ZP1 [9–12, 16] or ZP3 [13] mutations lacked zona pellucida or manifested symptoms of EFS, while the known ZP2 mutations only resulted in thin or absent ZP in patients [18, 19]. Among these ZP genes, ZP1 exhibits the closest relationship with EFS, yet the known ZP1 mutations associated with EFS are still limited to only 11 identified mutations [9–12]. As these mutations can only explain a few EFS cases, any report concerning pathogenic mutations of ZP1 is meaningful.
Table 1.
Overview of the ZP mutations
| Mutated genes | cDNA changes | Genotype | Inheritance | Phenotype | Reference |
|---|---|---|---|---|---|
| ZP1 | c.1169-1176del | Homozygous | AR | Lack of ZP | [16] |
| c.1708G>A | Homozygous | AR | Lack of ZP | [10] | |
| c.1228C>T | Homozygous | AR | Lack of ZP | [10] | |
| c.507del | Homozygous | AR | [10] | ||
| c.1430+1G>T | Compound heterozygous | AR | Lack of ZP | [10] | |
| c.1775-8T>C | Lack of ZP | ||||
| c.181C>T | Compound heterozygous | AR | EFS | [9] | |
| c.1169-1176del | |||||
| c.1100A>G | Compound heterozygous | AR | Lack of ZP | [17] | |
| c.1215del | |||||
| c.170-174del | Compound heterozygous | AR | EFS | [12] | |
| c.1169-1176del | |||||
| c.1510C>T | Homozygous | AR | EFS | [11] | |
| c.1014+1G>A | Homozygous | AR | EFS | [11] | |
| c.123C>A | Compound heterozygous | AR | EFS | [11] | |
| c.1663C>T | |||||
| c.1129-1130del | Homozygous | AR | EFS | [11] | |
| c.508del | Compound heterozygous | AR | EFS | [11] | |
| c.1573-2A>G | |||||
| c.2T>A | Compound heterozygous | AR | EFS | This report | |
| c.1112+1G>T | |||||
| ZP2 | c.1115G>C | Homozygous | AR | Thin or lack of ZP | [10] |
| ZP3 | c.130G>A | Homozygous | AR | Thin ZP | [18] |
| c.1695-2A>G | Homozygous | AR | Thin ZP | [19] | |
| c.1691-1694dup | Homozygous | AR | Thin ZP | [19] | |
| c.400G>A | Heterozygous | AD | EFS | [13] | |
| c.763G>C | Heterozygous | AD | Lack of ZP | [10] |
AR autosomal recessive, AD autosomal dominant, ZP zona pellucida
In the present study, we investigated an infertile female with EFS, carried out whole-exome sequencing (WES) on the woman, and identified the loss-of-function of compound heterozygous mutations in ZP1. Furthermore, we confirmed the negative effects of the mutations on ZP1 expression using in vitro experiments. Our work suggests that the novel compound heterozygous mutations in ZP1 can lead to inadequate oocyte formation, thus causing female infertility.
Materials and methods
Study participants
The infertile woman with EFS was enrolled at the Reproduction Medical Center of West China Second University Hospital of Sichuan University. Family members of the patient were also recruited. A total of 200 unrelated Han Chinese women with normal fertility (giving birth to at least 1 offspring by natural fertilization) were recruited from volunteers to serve as the control group. This study was approved by the Ethical Review Board of West China Second University Hospital, Sichuan University. Informed consent was obtained from each subject in our study.
Genetic studies
We performed WES using patient DNA as follows. Genomic DNA was collected from peripheral blood samples using the FitAmp Plasma/Serum DNA Isolation Kit (Epigentek). Exon capture was then performed using the Agilent SureSelect Human All Exon V6 Kit, and DNA was sequenced on the Illumina HiSeq X system. We used ANNOVAR for functional annotation and the 1000 Genomes Project, HGMD, dbSNP, and ExAC to filter the data. After filtering, the retained non-synonymous SNVs were submitted to PolyPhen-2, SIFT, MutationTaster, and CADD for functional prediction.
We identified candidate pathogenic variants of the patient via Sanger sequencing of the DNA from the patient’s parents as well as from the normal controls. PCR amplification was performed with the ProFlex PCR System (Thermo Fisher), and we conducted DNA sequencing of PCR products on an ABI377A DNA sequencer (Applied Biosystems). Primers used in the PCR were F1, 5′-GCCCTTACAGGCACCCACTT-3′; R1, 5′-CTGCATTCCCTTGATCCCA-3′; F2, 5′-GGAGAGTAAGCGTCCATTC-3′; and R2, 5′-CCTGTACTGAGCTCGCCT-3′.
Immunofluorescence staining
The cumulus-oocyte complexes (COCs) were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, blocked with 5% BSA, and then incubated with primary antibodies (1:50, sc-365,435; Santa Cruz, CA, USA) overnight at 4 °C. On the following day, samples were washed three times with 1 × PBS, incubated with DyLight 488-labeled secondary antibodies (1:800, 1,927,937, Thermo Fisher) for 1 h at 25 °C, and then counterstained with 4,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich) to label the nuclei. Images were obtained using a laser scanning confocal microscope (Olympus).
Western immunoblotting
Proteins in the cultured cells were extracted using a universal protein extraction lysis buffer (Bioteke) containing a protease inhibitor cocktail (Roche). Denatured proteins were separated on 10% SDS-polyacrylamide gels and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore) for immunoblot analysis.
Minigene assay
We used functional splicing reporter minigene assays to assess the effect of sequence variants on splicing. A genomic fragment containing exon 5, intron 5, exon 6, intron 6, and exon 7 of the ZP1 gene was amplified by PCR from the genomic DNA of the patient and normal control and cloned into a minigene Vector pSPL3. After transient transfected into COS7 cells, the splicing patterns of transcripts produced by the wild-type and mutant were compared by RT-PCR analysis and sequencing. The primers for genomic amplification were F: 5′-ACCAGAATTCTGGAGCTCGAGCGGCCGC-3′ and R: 5′-CCAAACATTATGTACCTCTGTATCATATG-3′. Primers for RT-PCR were SD6 (F): 5′-TCTGAGTCACCTGGACAACC-3′ and SA2 (R): 5′-ATCTCAGTGGTATTTGTGAGC-3′.
Results
Identification of EFS in an infertile patient
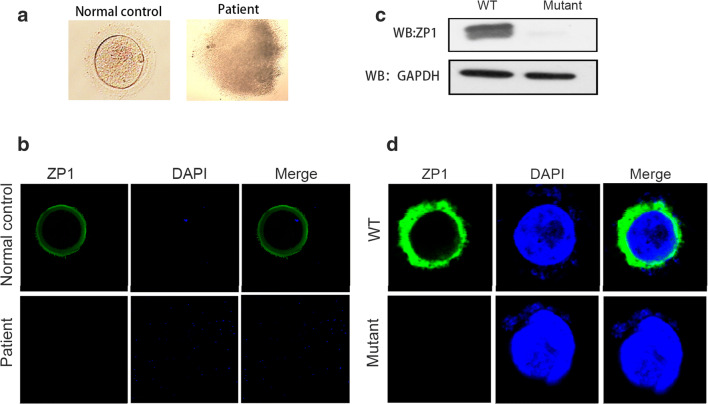
The patient (II-1) was female, 27 years of age, married for 3 years, maintained a normal sex life, not pregnant without contraception, and then diagnosed with primary infertility. Her chromosomal karyotyping was 46, XX. She exhibited regular menses since menarche at the age of 14, with normal sex hormone concentrations (Table 2); hysterosalpingography (HSG) showed that the fallopian tubes were unobstructed bilaterally and that vaginal B-ultrasonography showed no abnormalities in her uterus or appendages. The husband’s semen was normal upon routine examination. The patient underwent two cycles of IVF treatment, but both failed. The granulosa cells of the patient’s COCs were loose and irregular and presented as empty mucous masses containing no oocytes (Fig. 2a). Therefore, we classified this patient suffered from EFS.
Table 2.
Clinical characteristics of the patient
| Patient | ||
|---|---|---|
| Age (year) | 26 | |
| Length of primary infertility history(y) | 3 | |
| Basal sexual hormone | FSH (IU/L) | 7.8 |
| LH (IU/L) | 2.58 | |
| AMH (ng/ml) | 4.58 | |
| E2 (pg/ml) | 46.8 | |
| Cycle 1 | Protocol | short |
| E2 (pg/ml) | 2366.9 | |
| No. of follicles ≥ 14 mm | 7 | |
| No. of oocytes retrieved | 6 degenerated oocytes | |
| Cycle 2 | Protocol | mild |
| E2 (pg/ml) | 2064.3 | |
| No. of follicles ≥ 14 mm | 9 | |
| No. of oocytes retrieved | 1 degenerated oocytes | |
FSH follicle-stimulating hormone, LH luteinizing hormone, AMH anti-Mullerian hormone, E2 estradiol
Fig. 2.
The empty follicle syndrome phenotype in the patient. a The patient’s follicle contained no oocyte, but some granulosa cells in cumulus-oocyte complexes were observed compared with the normal control. b No ZP1 expression was detected in the patient compared with the normal control using immunofluorescence staining (green, ZP1; blue, DAPI). c The western immunoblotting results showed that the ZP1 protein was barely detectable in CHO cells transfected with mutant-ZP1 plasmid compared with the cells with WT-ZP1 plasmid. d The absence of ZP1 protein was observed in CHO cells transfected with mutant-ZP1 plasmid compared with the cells with WT-ZP1 plasmid using immunofluorescence staining (green, ZP1; blue, DAPI)
Compound heterozygous mutations of ZP1 identified in an infertile patient with EFS
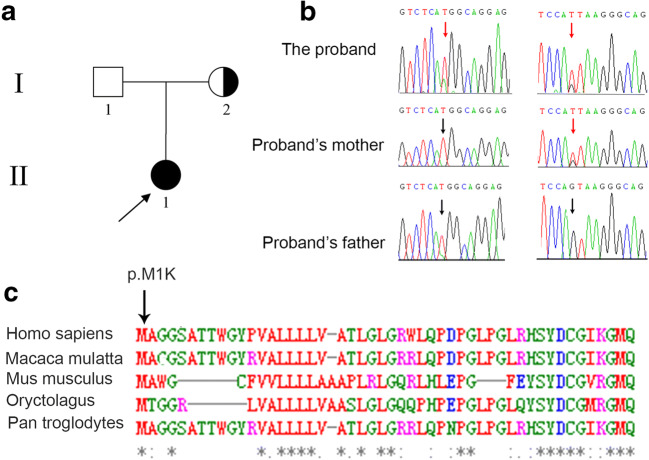
To illuminate the genetic cause of EFS in the present study, we performed WES analysis on the proband. Variants were removed if the following conditions were met: (a) the minor allele frequency was greater than or equal to 1% in ExAC Browser, gnomAD, or the 1000 Genome Projects, considering that pathogenic variants that cause EFS are rare in humans; (b) the variant was not predicted to be deleterious by SIFT, PolyPhen-2, and MutationTaster tools; (c) the variant was in noncoding exons, 3′- or 5′-untranslated regions, or intronic sequences, except for canonical splice sites. After filtering, the two heterozygous variants (c.2T>A [p. M1K] and c.1112+1G>T) in ZP1 were identified, and we confirmed the missense mutation c.2T>A [p. M1K] to be 100% conserved across several species (Fig. 1c). In order to understand the distribution of these two variations in the family (Fig. 1a), we carried out Sanger sequencing on the unaffected parents as well as the patient. The mother carried a heterozygous variant of c.1112+1G>T, and no variation in ZP1 was found in the father suggesting that c.2T>A (p. M1K) was a de novo variant in this family (Fig. 1b). EFS is associated to autosomal dominant inheritance and autosomal recessive inheritance [5–13]; this will have different ramifications for our research, thus clarifying which of these our patient falls under is crucial. In our study, the healthy mother harbored the heterozygous variant; this indicated that the genetic pattern of the variations of ZP1 in the patient should be classified as autosomal recessive inheritance. To prove this, we performed SNP linkage analysis on the patient, and the results showed that the two variants were located on two homologous chromosomes, suggesting that the compound heterozygous variants were the genetic cause of EFS in this family, which is consistent with the previous studies. Strikingly, we were unable to detect the two variants among our sample of 200 Chinese Han controls, further confirming the pathogenicity of the mutations in the affected female.
Fig. 1.
A compound heterozygous variant of ZP1 in an infertility female. a Family pedigree. The square represents male pedigree members, and circle represents female pedigree members; the arrow points to the proband. b Sanger sequencing of the compound heterozygous variant was confirmed in this family. The red arrow points to the mutant position, and the black arrow points to the wide-type position. c Multiple sequence alignment of the ZP1 protein among different species
Mutations of ZP1 impair its function
To further understand the damaged effects of ZP1 mutations on its expression, we used an immunofluorescence assay on the COCs of the patient as well as the controls. In the control group, ZP1 was primarily distributed in the ZP; however, no ZP1 expression was detected in the patient (Fig. 2b). To confirm the potential deleterious influence of the missense mutation in ZP1, we constructed expression vectors for WT-ZP1 (His-tagged wild-type human ZP1) and mutated ZP1 (His-tagged human mutant ZP1 with c.2T>A), and then transiently transfected into CHO-K1 cells. Compared with WT-ZP1 expression, the mutant-ZP1 protein was not detected using western immunoblotting analysis (Fig. 2c). Moreover, subsequent analysis of immunofluorescence staining showed that ZP1 was visibly expressed in the cytoplasm of cells transfected with WT-ZP1 plasmid, while the ZP1 staining was marginally detectable in these cells overexpressed with mutant-ZP1 plasmid (Fig. 2d), which was consistent with results of western blotting. We speculated that the missense mutation damaged the expression of ZP1, thus affecting the ZP formation.
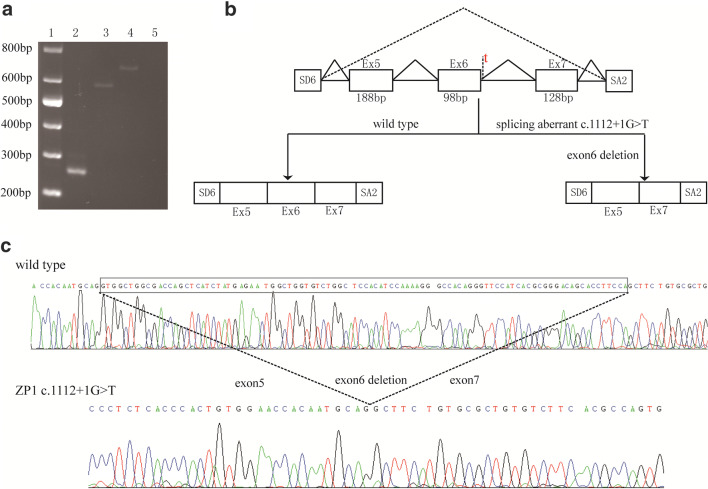
Bioinformatics analysis and predicted results showed that the original splicing site was disrupted by the c.1112+1G>T variant. In order to validate the aberrant splicing transcript caused by this mutation, we carried out a minigene splicing assay, and the results showed that the RT-PCR product of the wild-type was longer than that of the variant (Fig. 3a). Sequence analysis revealed that this variation caused a complete deletion of exon 6 and resulted in premature termination (p. Val339Aspfs*11)—generating a truncated protein that easily degenerated (Fig. 3b and c). Overall, EFS was thus manifested in the patient.
Fig. 3.
Functional effects of the splice-site mutation on the ZP1 gene transcript. a Agarose gel electrophoresis of RT-PCR products obtained from wild-type and mutant plasmids. The first channel is a marker, and the second is the empty vector. A significant decrease in molecular weight was observed in the mutant plasmid (channel 3) compared with the wild-type plasmid (channel 4). The negative control was shown in channel 5. b Proposed model of missing exon 6 caused by the splice site mutation. The primers SD6 and SA2 were used to amplify the exon 5, 6, and 7 of ZP1 gene. c Sequence analysis of the RT-PCR product obtained from the mutant plasmid exhibited a deletion of 98 nucleotides in exon 6
Discussion
In the present study, we identified the novel compound heterozygous mutations c.2T>A [p. M1K] and c.1112+1G>T in ZP1 gene sourced from an infertile woman with EFS. These two mutations disrupted ZP1 expression and led to the absence of the ZP, causing a failure in oocyte formation. ZP1 is a critical glycoprotein that contributes to the formation of zona pellucida, which is vital in the normal development of oocyte [20, 21]. The functional analysis in our study indicated that the compound heterozygous of ZP1 mutations resulted in a defect in the ZP1 function during oocyte development. These findings thus provide a unique insight into the pathogenic roles of such novel variants.
Zona pellucida is the glycoprotein layer that surrounds the oocyte and plays an important role in oocyte development, fertilization, and early embryonic development [21–23]. Zona pellucida plays vital roles throughout various reproductive processes: e.g., in oogenesis, the zona pellucida promotes the growth of oocytes and the development of follicles [24]; and in fertilization, the zona pellucida ensures specific recognition of sperm-egg binding, induces sperm exocytosis and the acrosome reaction, and prevents polyspermic fertilization [20, 25]. Before embryonic implantation, zona pellucida maintains the integrity of embryo, prevents blastomeres from dispersing, and enhances the communication between the blastomeres [26]. Studies of gene-knockout mice further confirmed the important reproductive functions of the zona pellucida proteins. The zona pellucida structure in Zp1−/− female mice was loose, and although the oocytes could be fertilized, the ensuing pregnancy rate was significantly lower than in normal controls [27]. Zp2−/− mice produced few oocytes, with the oocytes exhibiting an extremely thin zona pellucida, and they were not fertilized [28]. The oocytes produced by Zp3−/− mice were not even enveloped by a zona pellucida and also were not fertilized [29]. Therefore, the ZP proteins play an irreplaceable role in female reproduction.
With the development of assisted reproduction technology (ART), it helps more and more patients such as tubal obstruction (TO) to get pregnancy. During the process of assisted reproduction, abnormalities in the zona pellucida of oocytes may result in poor pregnancy outcomes in vitro [30]. For patients with EFS, ART has failed since mature and integrate oocyte is a prerequisite for fertilization. In our study, we observed no oocytes in the patient’s assisted pregnancy cycles due to ZP1 mutations that disturbed ZP1 expression, resulting in EFS. Having identified the causative factor for this patient, we suggested to the patient to consider accepting donated oocytes avoiding the repeated failure of IVF. Overall, we were able to uncover the cause of EFS in this patient and elucidate its pathogenesis, which should help guide future clinical treatments.
In conclusion, we identified a novel, pathogenic, compound heterozygous mutations of ZP1 in a Chinese Han family, and this finding enriched the variant spectrums of ZP1 gene and reminded that genetic analysis can play a crucial role in EFS diagnosis and prognosis, which provides additional beneficial knowledge related to genetic counseling.
Funding information
This work was supported by General Program of China Post-doctoral Science Foundation (2018 M640920).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohan Liu and Ying Shen contributed equally to this work.
References
- 1.Coulam CB, Bustillo M, Schulman JD. Empty follicle syndrome. Fertil Steril. 1986;46:1153–1155. doi: 10.1016/S0015-0282(16)49898-5. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson TL, Lashen H. Empty follicle syndrome: the reality of a controversial syndrome, a systematic review. Fertil Steril. 2008;90:691–698. doi: 10.1016/j.fertnstert.2007.07.1312. [DOI] [PubMed] [Google Scholar]
- 3.Younis JS. The genuine empty follicle syndrome: is the king naked? Fertil Steril. 2012;98:e20–e21. doi: 10.1016/j.fertnstert.2012.07.1098. [DOI] [PubMed] [Google Scholar]
- 4.Inan MS, Al-Hassan S, Ozand P, Coskun S. Transcriptional profiling of granulosa cells from a patient with recurrent empty follicle syndrome. Reprod BioMed Online. 2006;13:481–491. doi: 10.1016/S1472-6483(10)60634-7. [DOI] [PubMed] [Google Scholar]
- 5.Yariz KO, Walsh T, Uzak A, Spiliopoulos M, Duman D, Onalan G. Inherited mutation of the luteinizing hormone/choriogonadotropin receptor (LHCGR) in empty follicle syndrome. Fertil Steril. 2011;96:e125–e130. doi: 10.1016/j.fertnstert.2011.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zreik TG, Garcia-Velasco JA, Vergara TM, Arici A, Olive D, Jones EE. Empty follicle syndrome: evidence for recurrence. Hum Reprod. 2000;15:999–1002. doi: 10.1093/humrep/15.5.999. [DOI] [PubMed] [Google Scholar]
- 7.Yuan P, He Z, Zheng L, Wang W, Li Y, Zhao H, Zhang VW, Zhang Q, Yang D. Genetic evidence of ‘genuine’ empty follicle syndrome: a novel effective mutation in the LHCGR gene and review of the literature. Hum Reprod. 2017;32:944–953. doi: 10.1093/humrep/dex015. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Xu X, Kong L, Li P, Zhou F, Zhao S. Novel homozygous nonsense mutations in LHCGR lead to empty follicle syndrome and 46, XY disorder of sex development. Hum Reprod. 2018;33:1364–1369. doi: 10.1093/humrep/dey215. [DOI] [PubMed] [Google Scholar]
- 9.Yuan P, Li R, Li D, Zheng L, Ou S, Zhao H, Zhang Q, Wang W. Novel mutation in the ZP1 gene and clinical implications. J Assist Reprod Genet. 2019;36:741–747. doi: 10.1007/s10815-019-01404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Z, Ni C, Wu L, Chen B, Xu Y, Zhang Z, Mu J, Li B, Yan Z, Fu J, Wang W, Zhao L, Dong J, Sun X, Kuang Y, Sang Q, Wang L. Novel mutations in ZP1, ZP2, and ZP3 cause female infertility due to abnormal zona pellucida formation. Hum Genet. 2019;138:327–337. doi: 10.1007/s00439-019-01990-1. [DOI] [PubMed] [Google Scholar]
- 11.Dai C, Chen Y, Hu L, Du J, Gong F, Dai J, Zhang S, et al. ZP1 mutations are associated with empty follicle syndrome: evidence for the existence of an intact oocyte and a zona pellucida in follicles up to the early antral stage. A case report. Hum Reprod. 2019;34:2201–2207. doi: 10.1093/humrep/dez174. [DOI] [PubMed] [Google Scholar]
- 12.Sun L, Fang X, Chen Z, Zhang H, Zhang Z, Zhou P, Xue T, Peng X, Zhu Q, Yin M, Liu C, Deng Y, Hu H, Li N. Compound heterozygous ZP1 mutations cause empty follicle syndrome in infertile sisters. Hum Mutat. 2019;40:2001–2006. doi: 10.1002/humu.23864. [DOI] [PubMed] [Google Scholar]
- 13.Chen T, Bian Y, Liu X, Zhao S, Wu K, Yan L, Li M, Yang Z, Liu H, Zhao H, Chen ZJ. A recurrent missense mutation in ZP3 causes empty follicle syndrome and female infertility. Am J Hum Genet. 2017;101:459–465. doi: 10.1016/j.ajhg.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vujisic S, Stipoljev F, Bauman R, Dmitrovic R, Jezek D. Pericentric inversion of chromosome 2 in a patient with the empty follicle syndrome: case report. Hum Reprod. 2005;20:2552–2555. doi: 10.1093/humrep/dei083. [DOI] [PubMed] [Google Scholar]
- 15.Wassarman PM. Zona pellucida glycoproteins. Annu Rev Biochem. 1988;57:415–442. doi: 10.1146/annurev.bi.57.070188.002215. [DOI] [PubMed] [Google Scholar]
- 16.Huang HL, Lv C, Zhao YC, LiW HXM, Li P, et al. Mutant ZP1 in familial infertility. N Engl J Med. 2014;370:1220–1226. doi: 10.1056/NEJMoa1308851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu W, Li K, Bai D, Yin J, Tang Y, Chi F, Zhang L, Wang Y, Pan J, Liang S, Guo Y, Ruan J, Kou X, Zhao Y, Wang H, Chen J, Teng X, Gao S. Dosage effects of ZP2 and ZP3 heterozygous mutations cause human infertility. Hum Genet. 2017;136:975–985. doi: 10.1007/s00439-017-1822-7. [DOI] [PubMed] [Google Scholar]
- 18.Barbaux S, El Khattabi L, Ziyyat A. ZP2 heterozygous mutation in an infertile woman. Hum Genet. 2017;136:1489–1491. doi: 10.1007/s00439-017-1844-1. [DOI] [PubMed] [Google Scholar]
- 19.Dai C, Hu L, Gong F, Tan Y, Cai S, Zhang S, Dai J, Lu C, Chen J, Chen Y, Lu G, du J, Lin G. ZP2 pathogenic variants cause in vitro fertilization failure and female infertility. Genet Med. 2019;21:431–440. doi: 10.1038/s41436-018-0064-y. [DOI] [PubMed] [Google Scholar]
- 20.Bleil JD, Greve JM, Wassarman PM. Identification of a secondary sperm receptor in the mouse egg zona pellucida: role in maintenance of binding of acrosome-reacted sperm to eggs. Dev Biol. 1988;128:376–385. doi: 10.1016/0012-1606(88)90299-0. [DOI] [PubMed] [Google Scholar]
- 21.Wassarman PM, Jovine L, Litscher ES. A profile of fertilization in mammals. Nat Cell Biol. 2001;3:E59–E64. doi: 10.1038/35055178. [DOI] [PubMed] [Google Scholar]
- 22.Wassarman PM, Litscher ES. Mammalian fertilization: the egg's multifunctional zona pellucida. Int J Dev Biol. 2008;52:665–676. doi: 10.1387/ijdb.072524pw. [DOI] [PubMed] [Google Scholar]
- 23.Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 24.Familiari G, Relucenti M, Heyn R, Micara G, Correr S. Three-dimensional structure of the zona pellucida at ovulation. Microsc Res Tech. 2006;69:415–426. doi: 10.1002/jemt.20301. [DOI] [PubMed] [Google Scholar]
- 25.Pang PC, Chiu PC, Lee CL, Chang LY, Panico M, Morris HR, et al. Human sperm binding is mediated by the sialyl-Lewis(x) oligosaccharide on the zona pellucida. Science. 2011;333:1761–1764. doi: 10.1126/science.1207438. [DOI] [PubMed] [Google Scholar]
- 26.Wassarman PM. Zona pellucida glycoproteins. J Biol Chem. 2008;283:24285–24289. doi: 10.1074/jbc.R800027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rankin T, Talbot P, Lee E, Dean J. Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development. 1999;126:3847–3855. doi: 10.1242/dev.126.17.3847. [DOI] [PubMed] [Google Scholar]
- 28.Rankin TL, O'Brien M, Lee E, Wigglesworth K, Eppig J, Dean J. Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development. 2001;128:1119–1126. doi: 10.1242/dev.128.7.1119. [DOI] [PubMed] [Google Scholar]
- 29.Liu C, Litscher ES, Mortillo S, Sakai Y, Kinloch RA, Stewart CL, Wassarman PM. Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc Natl Acad Sci U S A. 1996;93:5431–5436. doi: 10.1073/pnas.93.11.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sousa M, Teixeira da Silva J, Silva J, et al. Embryological, clinical and ultrastructural study of human oocytes presenting indented zona pellucida. Zygote. 2015;23:145–157. doi: 10.1017/S0967199413000403. [DOI] [PubMed] [Google Scholar]