Abstract
Purpose
Patients with Klinefelter syndrome (KS) who receive assisted reproductive technology (ART) treatment often experience poor pregnancy rates due to decreased fertilization, cleavage, and implantation rates and even an increased miscarriage rate. Mounting evidence from recent studies has shown that various technological advances and approaches could facilitate the success of ART treatment for KS patients. In this review, we summarize the methods for guiding KS patients during ART and for developing optimal strategies for preserving fertility, improving pregnancy rate and live birth rate, and avoiding the birth of KS infants.
Methods
We searched PubMed and Google Scholar publications related to KS patients on topics of controlled ovarian stimulation protocols, sperm extraction, fertility preservation, gamete artificial activation, round spermatid injection (ROSI), and non-invasive prenatal screening (PGD) methods.
Results
This review outlines the different ovulation-inducing treatments for female partners according to the individual sperm status in the KS patient. We further summarize the methods of retrieving sperm, storing, and freezing rare sperm. We reviewed different methods of gamete artificial activation and discussed the feasibility of ROSI for sterile KS patients who absolutely lack sperm. The activation of eggs in the process of intracytoplasmic sperm injection and non-invasive PGD are urgently needed to prevent the birth of KS infants.
Conclusion
The integrated strategies will pave the way for the establishment of ART treatment approaches and improve the clinical outcome for KS patients.
Keywords: Klinefelter syndrome, Administration strategy, Single sperm cryopreservation, TESE, Fertility preservation
Introduction
Klinefelter syndrome (KS) is the most common male disease characterized by aberrant chromosomes. It severely impairs male health with manifestations including gynaecomastia, small testes, hypoandrogenism, universal azoospermia, infertility, and even the appearance of learning and behavior problems [1, 2]. The majority of KS patients are diagnosed by an infertility clinic. The incidence of KS is estimated to be 0.1–0.2% in the general population and 3.1% in the population of infertile patients [3–6]. This chromosomal disorder shows an aberrant male karyotype with non-mosaic 47, XXY or other mosaic karyotypes, such as 47, XXY/46, XY [6]. The former represents the majority of KS karyotypes. Although KS will result in various health problems, including tall stature, eunuchoid skeleton, small testes, azoospermia, gynaecomastia, and metabolic disorders [7–14], infertility is the most urgent problem that needs to be solved because the sperm retrieval rate (SRR) is closely associated with progression of the pathology.
The resultant infertility causes a series of social issues. The majority of KS patients aspire to have their own children [5]. Infertility related to KS has long been regarded as an untreatable disease. Because the testicular lesion progresses with age, it impairs the production of sperm and finally leads to male infertility. Although the degree of androgenization is associated with the residual function of Leydig cells, it has been demonstrated that most KS adult men have normal testosterone levels [15]. This might be the reason that abundant cases of KS remain undiagnosed until the affected individuals seek medical care for infertility. The damaged testis impairs the male’s fertility function due to asthenospermia or azoospermia. In recent decades, many KS patients have benefited from assisted reproductive technology (ART) by using ICSI, which could guarantee that residual rare sperm can be effectively injected into their partners’ eggs. Once the non-mosaic 47, XXY spermatozoan has been found in the fresh ejaculate of KS patients, it could be easily selected for ICSI, which widely improves the fertilization rate in populations with asthenospermia and azoospermia. However, the probability of obtaining sperm from ejaculates is very low in KS patients, especially when the patient’s age is beyond 35 years. Therefore, the main method to retrieve sperm from KS patients is microsurgical TESE (micro-TESE). Several studies have reported that successful sperm retrieval in KS patients by conventional testicular sperm extraction (c-TESE) and by micro-TESE is defined as a 42–57% retrieval rate [16–18]. Currently, it is widely accepted that younger KS patients have a greater opportunity to retrieve sperm by micro-TESE. Although many new methods have been developed to extract the rare sperm from the testes of KS patients, the pregnancy rate and live birth rate in the in vitro fertilization (IVF) cycle after ICSI have not been significantly improved due to the lack of effective methods and tools to store the rare sperm, especially in cases involving fewer than 5–10 sperm cells in each patient.
Conventional spermatozoa freezing/thawing methods and devices may result in sperm loss during the process of repeated centrifugation and washing [19]. It is hard to extend these techniques to cryopreserve rare spermatozoa, especially the spermatozoa obtained from KS men. On the other hand, repeated surgical procedures for diagnostic or therapeutic extraction will destroy the blood-testicular barrier and impair sexual function. Recently, several cryopreservation methods have been reported, and various containers and novel methodologies have been established to preserve the small number of spermatozoa [20–28], Successful pregnancies have been reported through ICSI, with a few spermatozoa cryopreserved in empty zona pellucida [29]. Most recently, Endo Y et al. reported successful delivery after transfer of a blastocyst derived from ICSI using limited numbers of sperm stored in a cell sleeper [30], despite the fact that they did not obtain motile spermatozoa after warming them from the cell sleeper and cryotop containers. Several studies have also addressed devices called the cryostrip and cryoleaf, which were developed to store spermatozoa obtained from patients with serious oligozoospermia [31]. However, thus far, there are no reported methods to stock sperm extracted from KS patients’ testes.
KS may occur in the general population despite the lower incidence rate. Therefore, men with KS have a greater risk of fathering an infant with KS than normal couples have. A new approach, namely, ICSI combined with preimplantation genetic diagnosis (PGD), was recently used to exclude embryos with 47, XXY karyotypes. This method effectively reduced the KS occurrence rate in male newborns. Nevertheless, it is well known that biopsy during the process of PGD may increase the risk of abortion due to injury of the embryo. Therefore, non-invasive PGD may benefit KS couples; moreover, prenatal diagnosis should be carefully considered.
The aim of this comprehensive review is to summarize methods to manage KS patients when they seek ART therapy and to provide integrated insight into the ART cycle in KS patients, including an optimal controlled ovarian stimulation (COS) protocol, methods of extracting sperm, spermatozoa freezing/thawing, activation of spermatozoa or eggs during ICSI, non-invasive PGD, and prenatal diagnosis. The integrated ART treatment strategy will improve the rates of pregnancy and live birth in KS couples.
Methods
A comprehensive literature review was conducted by searching the PubMed and Google Scholar databases. Our recent unpublished data were also included in this review. The keywords searched included Klinefelter syndrome, infertility, rare sperm, administration strategy, single sperm cryopreservation, non-invasive PGD, micro-TESE, PGD, and prenatal diagnosis. The goal of this review is to provide an overview of the current knowledge regarding ART treatment in KS and to develop a potential, integrated ART strategy for KS patients.
Female therapy strategy
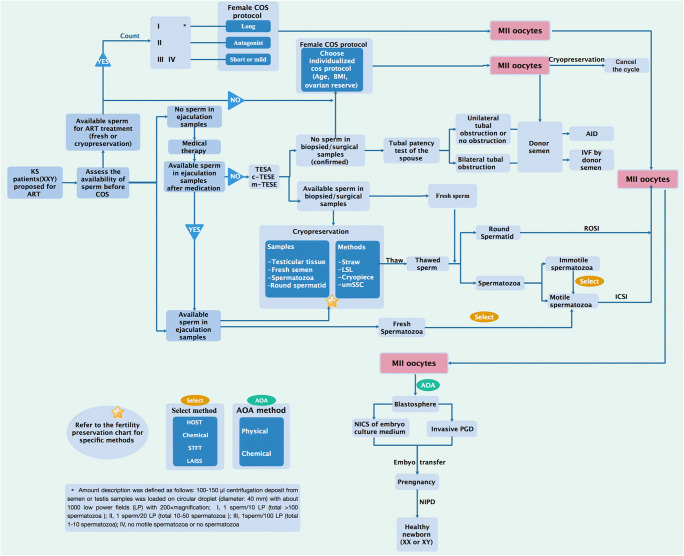
In previous studies (Table 1), ovulation induction of the female partner was performed via different protocols. Ovarian stimulation was carried out by pituitary desensitization with a GnRH agonist (either Decapeptyl Gyn (triptorelin); Ferring, Kiel, Germany or Lucrin (leuprolide acetate); Abbott, Aubonne, Switzerland) or a GnRH antagonist (either Cetrorelix; Serono, Switzerland or Ganirelix; Organon, The Netherlands) combined with recombinant FSH (either Gonal F; Serono, Switzerland or Puregon; Organon, The Netherlands) or hMG (Humegon; Organon, Oss, The Netherlands) and hCG (Pregnyl 10,000 IU, Organon). The most commonly used approach was the routine long protocol of midluteal pituitary suppression with a GnRH agonist followed by recombinant FSH. If sperm is available, undoubtedly, the long protocol is the best choice. However, this treatment cannot be extended to all females with a KS husband because of the widely different sperm statuses among men with KS. Therefore, there has been no consensus on the choice of ovulation induction protocol for female partners of KS patients thus far.
Table 1.
Characteristics and outcomes in KS couples underwent different COS protocols in ART therapy
| Study | No. pts | COS protocol | No. MII ova | No. injected MII ova | Source of sperm | Sperm used for ICSI | No. of ICSI cycles | Clinical pregnancies | Live born children | Men age (years) | Women age (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reubinoff et al. 1998 | 5 | Long | / | 26 | FNA | Fresh | 6 | 1 | 1 | 31.4 | 26.2 |
| Palermo et al. 1998 | 2 | Long | 67 | 46 | Biopsy | Fresh | 3 | 2 | 3 | 33 | 32.5 |
| Ron et al. 2000 | 1 | Long | / | 16 | Biopsy | Fresh | 1 | 1 | 1 | 31 | 26 |
| KITAMURA et al. 2000 | 4 | Long | 10 | 10 |
Biopsy (1) ejaculated (3) |
Fresh | 3 | 2 | 1 | 34.3 | 30.7 |
| Poulakis et al. 2001 | 2 | Long | 54 | 31 | Biopsy | Fresh | 2 | 2 | 2 | 34 | 28.5 |
| Friedler et al. 2001 | 12 | Long | 107 | 107 | Biopsy |
Fresh CP |
5 5 |
5 | 6 | 28.7 | 26.4 |
| Kyono et al. 2001 | 1 | Long | 4 | 4 | Biopsy | Fresh | 1 | 1 | NR | 28 | 31 |
| Bergere et al. 2002 | 3 | Long | 30 | 30 | Biopsy | CP | 4 | 1 | 1 | NR | NR |
| Rosenlund et al. 2002 | 1 | Long | 13 | 13 | Biopsy |
Fresh CP |
1 1 |
1 | 1 | 27 | 26 |
| Staessen et al. 2003 | 20 | Long | 325 | 310 |
Biopsy (31) ejaculated (1) |
Fresh CP |
22 10 |
8 | 4 | NR | 29.5 |
| Ulug et al. 2003 | 12 | Long | 130 | 130 |
Biopsy (11) ejaculated (1) |
Fresh | 12 | 3 | 1 | 33.4 | 30.4 |
| Okada et al. 2005 | 6 | Long | / | 56 | Biopsy | Fresh | 7 | 4 | 3 | 32.2 | 27.3 |
| Schiff et al. 2005 | 42 | Long | / | 285 | Biopsy | Fresh | 39 | 18 | 21 | 32.8 | 33.2 |
| Yarali et al. 2006 | 1 | Long | 18 | 18 | Biopsy | Fresh | 1 | 1 | 1 | 34 | 26 |
| Kyono et al. 2007 | 17 | Long/antagonist | 61 | 55 | Biopsy |
Fresh CP |
6 3 |
7 | 8 | 35.0 | 30.6 |
| Vicdan et al. 2007 | 2 | Long | 35 | 35 | Biopsy | CP | 2 | 2 | 3 | 27 | 25.5 |
| Greco et al. 2008 | 1 |
NR natural |
10 1 |
6 1 |
Biopsy | CP | 1 | 1 | 1 | 33 | 32 |
| Bakircioglu et al. 2011 | 106 | Long | Average 13.4 | / | Biopsy | Fresh | 49 | 26 | 29 | 34.3 | 29.2 |
| Greco et al. 2013 | 15 | Short/long/antagonist |
110/cycle 99/cycle |
83 67 |
Biopsy |
Fresh CP |
10 16 |
7 8 |
8 8 |
34.8 33.7 |
33.6 33.0 |
| Madureira et al. 2014 | 25 | Antagonist |
155 132 |
155 132 |
Biopsy |
Fresh CP |
20 17 |
12 4 |
12 5 |
33.4 | NR |
| Ozer et al. 2018 | 21 | Long/antagonist | 309 | 309 | Biopsy |
Fresh CP |
28 3 |
11 | 9 | 29 | 27.5 |
COS, controlled ovarian stimulation; CP, cryopreserved; NR, not reported
Most KS patients who visit our IVF clinic center have a poor sperm status. Thus, the COS protocols are also various. Thirty-one non-mosaic 47, XXY adults with KS were included in the study, and all patients had testicular tissue recovery. Ovulation induction of the female partners was performed via a GnRH agonist long protocol (1/31, 3.2%), a GnRH antagonist protocol (29/31, 93.5%), or mild stimulation protocol (1/31, 3.2%). Characteristics and outcomes in KS patients at our center are shown in Table 2. Individual detailed information is also listed in Table 2. In our center, the majority of cases involve the GnRH antagonist protocol. It is reasonable to perform a GnRH antagonist protocol in unsure situations to determine whether we can obtain sperm and how many sperm can be retrieved from the KS patients.
Table 2.
Clinical parameters and outcome of ART treatment in patient with non-mosaic Klinefelter syndrome
| Patient | Cycle | COS protocol | Source of sperm(semen or testis) | Spermatozoa for ICSI (fresh or freeze/stock methods) | Spermatozoa status when performing ICSI | Oocyte activation | Injected MII oocyte (n) | 2PN fertilization rate (%) | Embryo cleaved number/rate (%) | Available embryo number/rate (%) | Embryo transferred (n) |
Clinical outcome (gender, gestational weeks, weight of newborn) |
niPGT-A | Prenatal screening | Birth defects | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amount description | Usable spermatozoa characters after activation (mobility/morphology) | Spermatozoa activating methods | NIPT | Amniocentesis | ||||||||||||||
| 1 | 1 | Long | T | Freeze (cryopiece) | III (22sperms) | M/NM | AACR | N | 12 | 7 (58) | 7 (100) | 5 (71) | Fresh (10 III) | N | ||||
| Freeze |
Single live birth (Female, 39 weeks, 3900 g ) |
/ | Normal | / | Normal | |||||||||||||
| 2 | 1st | Mild | T | Fresh | I | M/AM | AACR | N | 2 | 2 (100) | 2 (100) | 2 (100) | Freeze | N | ||||
| 2nd | Mild | T | Freeze (straw) | I | M/AM | AACR | N | 4 | 4 (100) | 3 (75) | 3 (100) | Freeze |
Single live birth (Female, 38 + 3 weeks, 3250 g ) |
/ | Normal | / | Normal | |
| 3 | 1 | Antagonist | T | Freeze (LSL) | III |
IM/AM (ICSI cancel) |
AACR、HOS | |||||||||||
| 4 | 1 | Antagonist | T | Freeze (straw) | I | M/NM | AACR | N | 18 | 15 (83) | 15 (100) | 3 (20) | Fresh (6IV, 6IV) | N | ||||
| Freeze (4BC) | N | |||||||||||||||||
| 5 | 1 | Antagonist | T | Freeze (straw) | I | M/NM | AACR | N | 17 | 14 (82) | 14 (100) | 9 (64) | Fresh (8I, 10II) |
Twin live birth (Male, 37 weeks, 2900 g/2250 g) |
/ | / | / | Normal |
| 6 | 1 | Antagonist | T | Freeze (straw) | II | M/NM | AACR | N | 12 | 11 (92) | 10 (91) | 10 (91) | Fresh (8I, 8I) |
Single live birth (Male, 40 + 2 weeks, 4100 g ) |
/ | Normal | / | Normal |
| 7 | 1 | Antagonist | T | Freeze (straw) | II | M/NM | AACR | N | 18 | 12 (67) | 12 (100) | 11 (92) | Freeze | N | ||||
| 8 | 1 | Antagonist | T | Freeze (cryopiece) |
III (3 sperms) |
IM/AM (ICSI cancel) |
AACR、HOS | |||||||||||
| 9 | 1 | Antagonist | T | Fresh (overnight) Freeze (LSL) | IV |
IM/AM (ICSI cancel) |
AACR、HOS | |||||||||||
| 10 | 1st | Antagonist | T | Freeze (cryopiece) | III (12 sperms) |
IM/AM (ICSI cancel) |
AACR、HOS | |||||||||||
| 2nd | Fresh | IV |
No spermatozoa (ICSI cancel) |
AACR | ||||||||||||||
| 11 | 1st | Antagonist | T | Freeze (LSL) | IV | IM/NM | AACR、HOS | Y | 8 | 3 (38) | 3 (100) | 2 (67) | Fresh D2 (6II, 4III) | N | ||||
| 2nd | Antagonist | T | Fresh | IV |
IM/AM (ICSI cancel) |
AACR、HOS | ||||||||||||
| 12 | 1 | Antagonist | T | Fresh | IV |
IM/AM (ICSI cancel) |
AACR、HOS | |||||||||||
| 13 | 1 | Antagonist | T | Freeze (LSL) | I | M/NM | AACR | N | 3 | 1 (33) | 1 (100) | 1 (100) | Fresh (8I) | Miscarriage (4 weeks) | ||||
| 14 | 1 | Antagonist | T | Freeze (straw) | II | M/NM | AACR | N | 19 | 11 (58) | 11 (100) | 10 (91) | Freeze | Miscarriage (42 days) | ||||
| Freeze |
Single live birth (Female, 39 + 8 weeks, 3150 g ) |
Normal | Normal | / | Normal | |||||||||||||
| 15 | 1 | Antagonist | T | Fresh | IV | IM/NM | AACR、HOS | Y | 12 | 1 (8) | 0 | 0 | 0 | |||||
| 16 | 1 | Antagonist | T | Fresh | II | M/NM | AACR | N | 10 | 5 (50) | 5 (100) | 2 (40) | Freeze | N | ||||
| 17 | 1 | Antagonist | T | Freeze (straw) | I | M/NM | AACR | N | 3 | 0 | 0 | 0 | 0 | |||||
| 18 | 1 | Antagonist | T | Fresh | IV | IM/NM | AACR | Y | 11 | 5 (45) | 5 (100) | 4 (80) | Fresh (8I, 8I) | N | ||||
| Freeze | N | |||||||||||||||||
| 19 | 1 | Antagonist | T | Fresh | II | M/NM | AACR | Y | 3 | 2 (67) | 2 (100) | 2 (100) | Fresh (12IV, 8III) | N | ||||
| 20 | 1 | Antagonist | T | Freeze (straw) | I | M/NM | AACR | N | 16 | 9 (56) | 9 (100) | 9 (100) | Freeze |
Single live birth (female、33 + 6 weeks, 3350 g ) |
/ | Normal | / | Normal |
| 21 | 1 | Antagonist | T | Fresh | III | M/AM | AACR | N | 16 | 6 (38) | 6 (100) | 3 (50) | Freeze |
Twin live birth (Male, 37 weeks, 2550 g/3100 g) |
/ | / | / | Normal |
| 22 | 1 | Antagonist | T | Fresh | III | M/AM | AACR | N | 21 | 17 (81) | 17(100) | 11 (65) | Freeze |
Single live birth (Male, 39 + 5 weeks, 3400 g ) |
/ | Normal | / | Normal |
| 23 | 1 | Antagonist | T | Fresh | IV | IM/AM | AACR、HOS | Y | 5 | 3 (60) | 0 | 0 | 0 | |||||
| 24 | 1st | Antagonist | T | Freeze (straw) | III |
M/AM (ICSI cancel) |
||||||||||||
| 2nd | Fresh | III | M/AM | AACR | Y | 24 | 18 (75) | 10 (56) | 8 (44) | Freeze (10II, 8III) | N | |||||||
| Freeze | Pregnancy (16 weeks) | / | Normal | |||||||||||||||
| 25 | 1 | Antagonist | T | Fresh | IV | IM/AM | AACR | Y | 10 | 8 (80) | 6 (75) | 2 (11) |
Fresh (6III, 8III) |
Ectopic pregnancy | / | |||
| 26 | 1 | Antagonist | T | Fresh | II | M/AM | AACR | N | 10 | 5 (50) | 5 (100) | 2 (40) |
Fresh (6II, 6III) |
N | ||||
| 27 | 1 | Antagonist | T | Freeze (LSL) | I | M/NM | AACR | N | 13 | 9 (69) | 9 (100) | 9 (100) | Fresh (4I) | Pregnancy (35 + 2 weeks) | / | Normal | ||
| 28 | 1 | Antagonist | T | Fresh | I | M/NM | AACR | N | 11 | 3 (27) | 3 (100) | 3 (100) | Freeze | Pregnancy (19 + 5 weeks) | / | Normal | ||
| 29 | 1 | Antagonist | T | Fresh | II | M/NM | AACR | N | 8 | 7 (88) | 7 (100) | 2 (29) |
Fresh (8I, 8II) |
N | ||||
| 30 | 1 | Antagonist | T | Freeze (LSL) | IV | IM/NM | AACR | Y | 21 | 15 (71) | 11 (73) | 2 (13) | Freeze | N | ||||
| 31 | 1 | Antagonist | T | Fresh | II | M/AM | AACR | Y | 20 | 14 (70) | 12 (86) | 2 (14) | Freeze | N | ||||
IM, immobility; AM, abnormal morphology; NM, normal morphology; AACR, artificial activation with chemical reagents; niPGT-A, noninvasive preimplantation genetic testing for aneuploidy; NIPT, non invasive prenatal DNA testing. Amount description was defined as following: 100–150 μl centrifugation deposit from semen or testis sample was loaded on circular droplet (diameter 40 mm) with about 1000 low power fields (LP) with × 200 magnification; I, 1 sperm/10 LP (total > 100 spermatozoa ); II, 1 sperm/20 LP (total 10–50 spermatozoa ); III, 1 sperm/100 LP (total 1–10 spermatozoa); IV, no motile spermatozoa or no spermatozoa
Since 5–10% of men with non-obstructive azoospermia (NOA) have sperm viable for ICSI in their ejaculates, it is important to perform a semen analysis prior to the planned micro-TESE to assess whether surgical sperm retrieval is needed. In our experience, testicular sperm cryopreservation ensures the flexibility of the ICSI cycle to make the timing most favorable for patients. Given the possibility of failed sperm recovery from the testicles, azoospermic non-mosaic KS patients are ideal candidates for performing a diagnostic testicular biopsy prior to oocyte pick-up. The tissue can be cryopreserved and used for a future ICSI cycle.
Studies have shown that the stimulation period, the concomitant complications, and the oocyte quantity and quality due to different ovulation induction protocols differ [32–36]. As there is only one opportunity to use fresh testicular spermatozoa, the COS protocol should be carefully planned, specifically regarding the optimal method for collecting a sufficient number of MII eggs while avoiding the risk of ovarian hyper-stimulation syndrome (OHSS). Moreover, the COS protocols with a low risk of being cancelled on the day of the micro-TESE as well as the flexibility in starting the ovarian stimulation should be taken into account. All things considered, we suggest that a proper COS protocol for the female partners of KS patients should be personalized based on the number of available spermatozoa as follows:
The GnRH agonist long protocol, which may yield more MII eggs, should be considered for patients with motile sperm in the semen analysis or patients with a sufficient number of cryopreserved sperm before ovulation induction therapy.
The GnRH antagonist protocol should be given for patients with severe oligoasthenozoospermia or azoospermia but a high possibility of obtaining enough sperm through micro-TESE in order to collect a sufficient number of MII eggs and avoid the risk of OHSS.
The GnRH agonist short protocol or mild stimulation protocol should be used for patients with extremely rare sperm (fewer than 5–10 cryopreserved spermatozoa from either ejaculation or micro-TESE) for the sake of obtaining high-quality MII eggs to make the most of the hard-earned spermatozoa and avoid the risk of OHSS.
Male therapy strategy
Approaches to retrieve sperm from KS patients
Generally, KS is always associated with azoospermia. In fact, after the onset of puberty, these patients exhibit progressive and irreversible testicular atrophy and testicular germ cell depletion [37]. Therefore, spontaneous pregnancy in the KS patient’s partner is extremely rare. Approximately 8% of males with KS have the potential to exhibit spermatozoa in their ejaculated seminal fluid [38–41]. The birth of a healthy girl after ICSI treatment with ejaculated spermatozoa from a man with non-mosaic Klinefelter’s syndrome has previously been reported [40]. There are very few cases in which a KS patient fathers a healthy child by ICSI using ejaculated spermatozoa. Although the ejaculated spermatozoa could be harvested, it is still a question whether these sperm could be used for ART treatment because the majority are immotile and show an aberrant morphology. For the KS adolescent, once spermatozoa are present in masturbation semen, the cryopreservation service should be offered. At this stage, the morphology and motility are better than those in older adults due to less lesioning of the testis. In consideration of the very low KS population capable of obtaining sperm from ejaculation, masturbation is not the main method for retrieving sperm in KS males.
As shown in Table 3, surgery, including testicular sperm extraction (TESE), micro-TESE, and testicular sperm aspiration (TESA), is still the main means of obtaining sperm from KS patients. Increasing evidence demonstrates that treatment with hormones and aromatase inhibitors prior to surgery facilitates the success of testicular sperm retrieval [17, 18, 42]. A cohort study has reported the successful extraction of sperm from 70% of young KS adolescents via micro-TESE after the administration of topical testosterone replacement therapy (TRT) and oral aromatase inhibitors for 1–5 years [18]. However, this finding may not be generalizable to the older KS group because age is a negative factor for successful sperm retrieval. In addition, it was not certain whether the sperm retrieval rate (SRR) or the sperm yield would have been higher or lower in KS adolescents without exposure to TRT due to the lack of a control group. A similar observation was addressed in another study: posttreatment testosterone was higher in men in whom sperm were found by microdissection TESE [17]. In this study, KS adults were treated with aromatase inhibitors, clomiphene, or human chorionic gonadotropin before m-TESE, and the SRR was the main evaluation indicator. Patients who underwent medical therapy with a resultant testosterone of 250 ng/dl or higher had a higher SRR than those who received posttreatment testosterone at less than 250 ng/dl. Therefore, they concluded that KS patients with hypogonadism who respond to medical therapy may have a better opportunity for sperm retrieval. Our data, together with other reports, revealed that medical therapy including aromatase inhibitor treatment could induce sperm production in NOA patients. However, this amazing result has never been reported in KS adults, which suggests that surgical sperm retrieval is the main approach, whereas medical therapy will benefit the former in parts of the KS population. In 1998, B.E. Reubinoffet et al. reported a birth after testicular fine needle aspiration combined with ICSI and PGD in a case of non-mosaic KS [43]. Ten punctures were performed in different locations in each testis, and rare sperm were retrieved and used for subsequent ICSI. In our experience, it is difficult to extend the multiple biopsy approach to the majority of KS patients, especially once they reach 35 years of age. In 1996, a successful recovery of sperm by c-TESE in patients with KS and azoospermia was first reported [44]. Later, TESE combined with ICSI was successfully used for retrieving sperm from KS patients and resulted in successful pregnancy [45]. Recently, a review analysed the currently available data from subjects with KS regarding SRRs as the primary outcome [46]. In this review, 37 published studies were enrolled in this investigation, and the reported surgical types included 19 c-TESEs, 14 micro-TESEs, 1 TESA, and 4 mixed procedures. These data suggest that surgery is still the main approach to retrieve sperm from KS patients.
Table 3.
Overview of the reported outcome of different sperm extraction methods in non-mosaic Klinefelter patients
| Reference | Patients (n) |
Age (years) |
Retrieve type | SSR | Sperm count (× 106) | Fertility preservation | ART result |
|---|---|---|---|---|---|---|---|
| Ulug et al. 2003 [90] | 12 | mean 33.4 |
Spermatozoa Spermatozoa round |
1/12 ejaculate 6/11 TESE 4/11 TESE |
/ | / |
The pregnancy rate per ET = 27.2% 2 singleton; 1triplet |
| Chiang et al. 2004 [153] | 14 | 21–39 | Spermatozoa |
2/14 ejaculate 8/13 TESE |
/ | / |
Clinical pregnancy rate = 75%, live delivery rate = 62.5% |
| Wikstrom et al. 2004 [37] | 14 | 10–14 |
Spermatozoa Spermatogonia |
0/14 TESE 7/14 TESE |
/ | / | / |
| Okada et al. 2005 [95] | 51 | 25–43 | Spermatozoa |
0/51 ejaculate 26/51 TESE |
/ | / |
Clinical pregnancy rate = 46%, 8 singleton; 2 twin |
| Schiff et al. 2005 [154] | 42 | Mean 32.8 | Spermatozoa | 29/42 mTESE | / | / |
Clinical pregnancy rate = 46%, 21 live births |
| Koga et al. 2007 [155] | 26 | 28–45 | Spermatozoa |
0/26 ejaculate 13/26 mTESE |
/ | / |
Clinical pregnancy rate = 31%, 2 singleton |
| Ferhi et al. 2008 [156] | 27 | 25–42 | Spermatozoa |
0/27 ejaculate 0/3 TESA 8/24 TESE |
/ | / |
Clinical pregnancy rate = 50% 3 singleton; 2 twin |
| Yarali et al. 2009 [157] | 33 | Mean 32.0 | Spermatozoa | 22/39TESE | / | Testicular tissue |
Clinical pregnancy rate = 39%, Live birth rate/embryo transfer = 28% |
| Bakircioglu et al. 2011 [158] | 106 | Mean 34.3 | spermatozoa |
0/106 ejaculate 50/106 mTESE |
/ | / | Clinical pregnancy rate = 53%, spontaneous abortion rate = 12% |
| Gies et al. 2012 [80] | 7 | 10–16 |
Spermatozoa Spermatogonia |
0/7 ejaculate 0/7 mTESE 4/7 mTESE |
/ | / | / |
| Rives et al. 2013 [159] | 5 | 15–17 |
Spermatozoa elongated |
1/5 TESE 1/5 TESE |
/ | Testicular tissue | / |
| Mehta et al. 2013 [18] | 10 | 14–22 | Spermatozoa | 7/10 mTESE | 0.01–2 | / | / |
| Greco et al. 2013 [160] | 38 | Mean 35.3 | Spermatozoa |
0/38 ejaculate 14/38 TESE 1/38 mTESE |
/ |
Straws-CBS Straws-CBS |
16 babies/26 ICSI cycle |
| Sabbaghian et al. 2014 [161] | 134 | Mean 32.6 | Spermatozoa |
0/134 ejaculate 38/134 mTESE |
Cryotubes |
Live birth/embryo transfer rate = 13% 3 singleton; 1 twin |
|
| Madureira et al. 2014 [74] | 65 | 24–46 | Spermatozoa | 25/65 TESE | / | Straws-L' Aigle |
Clinical pregnancy rate = 44.4%, 11 singleton; 3 twin |
| Plotton et al. 2015 [162] |
41 25 16 |
15–39 15–23 25–39 |
Spermatozoa |
0/41 ejaculate 13/25 TESE 10/16 TESE |
/ |
Straws-CBS Straws-CBS |
/ |
| Rohayem et al. 2015 [50] |
50 85 |
13–19 (adolescent) 20–61 (adult) |
Spermatozoa Spermatozoa elongated |
1/29 ejaculate 19/50 mTESE 0/82 ejaculate 26/85 mTESE 4/85 mTESE |
< 0.1 | / | / |
| Majzoub et al. 2015 [163] | 43 | Mean 32.9 | Spermatozoa |
0/43 ejaculate 6/20 mTESE 0/23 TESE |
/ | / |
Clinical pregnancy rate = 50% 1 singleton; 2 twin |
| Nahata et al. 2016 [61] | 15 | 12–25 | Spermatozoa |
0/15 ejaculate 5/10 mTESE |
/ | / | / |
| Chihara et al. 2017 [164] | 5 | Mean 33.6 | Spermatozoa |
0/5 ejaculate 2/5 mTESE |
/ | / |
Clinical pregnancy rate = 50% 1 singleton |
| Ozer et al. 2018 [165] | 108 | Mean 32.7 | Spermatozoa |
0/108 ejaculate 21/108 mTESE |
/ | Cryotubes |
Clinical pregnancy rate = 35.4% Spontaneous abortion rate = 6.7% |
SSR, successful sperm retrievals; TESE, testicular sperm extraction; mTESE, microsurgical testicular sperm extraction; TESA: testicular sperm aspiration
The critical factor for successful sperm retrieval
Although the successful SRR of TESE in the KS population reaches up to 42–57% [6, 46, 47], critical factors or markers for predicting successful surgical retrieval with TESE are limited. Here, we summarized the candidate factors used to estimate the result of SRR in previous studies.
Since irreversible lesions include hyalinization of seminiferous tubules and progressive degeneration of the testis, surgery for extracting sperm has been suggested to be performed in the early pathological stage, which might result in better clinical outcomes [48, 49]. Therefore, it is well accepted that age is an important factor affecting the SRR. One literature review suggests that spermatozoa could be obtained by TESE in half of the patients with KS aged 16–30 years old and that the retrieval rates of spermatozoa in the adolescent population under 16 years old are lower (0–20%) than those in adolescents and young adults aged from 16 to 30 years (40–70%) [48]. This higher SRR in young adults may be attributed to sexual maturity and fewer lesions in the testicular tissue. Therefore, these findings suggest that a successful SRR is mainly associated with an individual patient’s testicular pathological status and that age may serve as a reference factor but not a criterion to judge the success of sperm retrieval.
In addition, factors including serum FSH, LH, free and total testosterone, E2, inhibin B, SHBG, prolactin, testicular volume, and testicular histology are inconsistent in predicting successful sperm extraction by TESE. A study demonstrated that juvenile and adult KS patients with total serum testosterone levels above 7.5 nmol/l and LH levels below 17.5 U/l have a higher SRR via micro-TESE [50]. We also found that the level of testosterone in the KS population with a successful SRR is much higher than that in the KS population with an unsuccessful SRR (unpublished data). Despite this result, we considered that the samples were too small to support a conclusion that testosterone has significant predictive value in the SRR because the investigation only enrolled 31 cases. Another predictive model demonstrated that male age, a higher level of serum testosterone, and lower levels of FSH and LH were good predictive factors for successful sperm retrieval [51]. However, a recent meta-regression analysis evaluated numerous clinical investigations and concluded that none of the parameters, including age, testis volume and FSH, LH, and testosterone levels, affect the clinical outcome of SRR [46].
Some studies have stated that mosaicism is a critical factor affecting the SRR. Generally, the presence of more than 10% 46, XY cells is considered mosaicism. This boundary was based on clinical practice involving sex chromosome aberrations in Sweden (Wahlstro¨m J., personal communication). Furthermore, peripheral blood karyotypes are not completely consistent with gonadal karyotypes. Accordingly, KS patients do not seem to be a homogeneous group. Some studies have investigated whether cellular chromosome analyses, including peripheral lymphocytes, buccal tissue, testicular somatic cells, and germ cells, have predictive value for successful sperm retrieval in men with non-mosaic KS. However, fluorescence in situ hybridization (FISH) of peripheral lymphocytes and buccal tissue showed no correlation between the distribution of normal 46, XY cells and SRR in non-mosaic KS [52]. Three of four patients with peripheral blood karyotypes indicating non-mosaic KS in fact had testicular mosaicism; testicular spermatozoa were obtained through multiple site testis biopsies, indicating that testicular mosaicism has a high prognostic value for the SRR [53]. Additionally, a study of 6 Klinefelter patients suggested that focal spermatogenesis originates from euploid spermatocytes and that only 46, XY spermatocytes can achieve meiosis [54]. Despite a different hypothesis that 47, XXY spermatogonia undergo meiosis to produce hyperploid spermatozoa, the results also indicated that KS patients (n = 12) who had spermatozoa in their testicular tissue were positive for testicular mosaicism (46, XY/47, XXY). In contrast, those (n = 12) without spermatozoa in their testicular tissue were positive for 47, XXY spermatogonia but negative for 46, XY spermatogonia [55]. Recently, a study of the chromosomal constitution of non-mosaic KS biopsied testicular tissues indicated that the average proportion of the 46 XY and 47 XXY chromosomes was 73.6% (194/265) and 26.4% (71/265), respectively, in the spermatogonium (SG) stage. In one case, all of the SGs exhibited the 46 XY chromosomal constitution (34/34), although the relationship between testicular mosaicism and SRR was not mentioned. The above research results draw attention to rare spermatozoa or spermatids originating from mosaic normal karyotype testicular germ cells in non-mosaic KS patients [56].
Therefore, because the lesion status of the testis varies in individual KS patients, factors that could function as a marker to predict the SRR are still elusive.
Fertility preservation for KS patients and single sperm cryopreservation
Ejaculated semen preservation
To our knowledge, no definite biomarkers or clinical parameters have been developed to predict the SRR in males with KS. The challenge for KS patients is to preserve their fertility because the degenerative process of the testis may start during mid-fetal life [57, 58], and progressive hyalinization of seminiferous tubules is observed after puberty [48, 49]. Thus, preservation of rare spermatogenesis is necessary for KS patients who wish to bear a child. However, the lack of advanced equipment for stocking rare sperm has limited the fertility preservation in KS patients.
The best fertility status for the KS patients is that the sperm is present in their semen. However, several cohort studies have reported successful ejaculation of spermatozoa in 7.4–8.4% of young adult KS patients [6, 12, 41, 59, 60] and in 0–5% of KS adolescents [12, 60–62]. These patients typically exhibit cryptozoospermia or severe oligospermia with sperm concentrations lower than 1 × 106/mL and impairments in sperm motility and morphology [12]. To date, a growing number of live-born children resulting from ICSI with ejaculated semen spermatozoa have been reported [40, 63–66]. However, the ejaculated spermatozoa preservation procedure cannot be applied pre-puberty due to the patient’s lack of ability to produce spermatozoa. Recently, a study of 50 adolescent KS patients aged 13–19 years revealed that only one 17-year-old adolescent patient had severe oligoasthenoteratozoospermia (< 0.1 mill/mL), among 29 psycho-sexually mature adolescent KS patients who could provide a semen sample for analysis. The remaining participants had no spermatozoa identified in their semen samples [50]. Such sperm are very precious to KS patients; therefore, it is necessary to cryopreserve the spermatozoa for as long as possible once sperm is present in the patients’ ejaculated semen. Currently, straw tubes have been widely used to preserve rare sperm. Nevertheless, it is difficult to stock extremely rare sperm (fewer than 10 cells in the whole ejaculated semen). Thus, we established a cryopreservation system specifically designed for extremely rare sperm [67]. Because the ejaculated sperm number is sharply fluctuant in different KS patients, the selection of sperm preservation methods should be based on their sperm status. According to our experience and that of others, we created a flow chart, shown in Fig. 1. To date, various novel fertility stock techniques have been established and successfully applied in the clinic [67, 68] and are considered promising applications for KS patients. To summarize, the cryopreservation of available spermatozoa from the semen of KS patients is necessary, even for sperm containing very low numbers or morphological abnormalities of spermatozoa, because the degenerative process of the testis is strictly associated with age and is irreversible.
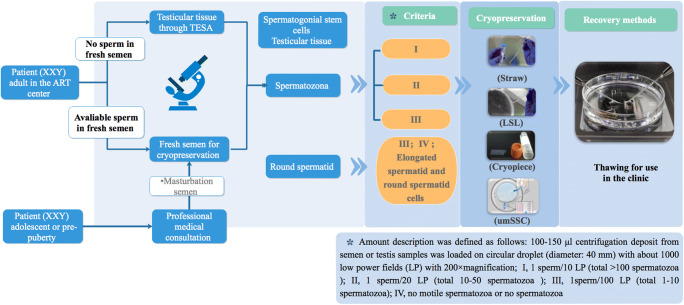
Fig. 1.
Flow chart for cryopreserving the sperm from KS patients. Adolescent or pre-pubertal KS patients need a professional medical consultation. If sperm is found in their masturbation semen, cryopreservation should be performed as soon as possible. For adult KS patients, regardless of the use of fresh semen or surgically obtained spermatozoa, if the concentration is beyond 0.1 × 106/ml, a straw tube is suitable for stocking this kind of spermatozoan; for 10/ml to 0.1 × 106/ml, an LSL tube; for less than 10/ml, the cryopiece system; and for less than 10/ml or less than 5-10 sperm cells, elongated spermatids, or round spermatid cells, the umSSC system is the optimal choice for cryopreservation
The cryopreservation for operative spermatozoa
Although some non-mosaic KS patients could father a child through obtaining motile sperm in the ejaculate and even via spontaneous pregnancies ([60, 69–71]), the majority of non-mosaic KS patients need to use donor semen due to severe oligozoospermia or azoospermia. Over the past two decades, technical advances in TESE combined with ICSI have benefited infertile males, especially KS patients with NOA. Numerous successful live births with the help of this technical advance have been reported [45, 72]. Most importantly, the SRR of TESE in KS patients is not lower than that of patients with NOA resulting from other causes [73], and the offspring appear to have normal karyotypes [74].
If fresh ejaculation spermatozoa are not found in KS males, TESE is the optimal method to obtain available spermatozoa in the ICSI cycle. However, patients may be diagnosed with KS at different life stages, ranging from the prenatal period via amniocentesis to adulthood. The most prominent feature of KS patients is progressive hyalinization of seminiferous tubules after puberty with worsening in adulthood [48]. Therefore, the identification of KS as early as possible is a critical step for fertility preservation. Affected individuals could choose to carry out TESE procedures in their early life stage or at the optimal time for spermatozoa retrieval. In addition to the sperm, the rest of the tissues should also be carefully preserved because they may contain a few sperm that were not found at that time. Furthermore, a recent study has reported that cryopreserved testis tissues may be used for culturing mature sperm, resulting in the successful delivery of healthy offspring [75].
Fertility preservation possibility in pre-puberty
It is hard to identify KS in prenatal and pre-pubertal populations because they have no obvious clinical features and fertility requirements at that time. Given that the degenerative process of the testis and the progressive hyalinization of seminiferous tubules are irreversible, increasing studies have begun to pay attention to fertility preservation in pre-puberty. Some studies have suggested estimating the possibility of fertility preservation by histological assessment of the testicular tissue in KS patients ranging from prenatal to pre-puberty ages, with the main reason being the lack of mature sperm in this population. It was reported that the degeneration of germ cells happens as early as during the fetal life of KS patients, despite the appearance of normal testicular tubules and mesenchymal structures [57]. Numerous evidence in previous studies has shown a reduced number of spermatogonia to different degrees compared with that in normal pre-pubertal children, although these KS patients in childhood present normal levels of hormones including testosterone, FSH, LH, inhibin B, AMH, and E2 [37, 76–81]. Therefore, whether TESE should be carried out before the onset of puberty to cryopreserve spermatogonial stem cells (SSCs) or testicular tissue is an urgent issue to be considered. The following data prompt us to estimate the possibility of performing TESE and carrying out fertility preservation in pre-puberty. First, a recent systematic review revealed that the SRR per TESE cycle was high (up to 44%) in 1248 KS patients with a mean age of 30.9 ± 5.6 years [46]. Second, germ cells were detectable in testicular biopsies from 21% of adult men for whom no spermatozoa could be retrieved by TESE and in 31.5% of peripubertal KS boys. Rare spermatogonia (0.03–0.06 spermatogonia/tubule) were detected in three out of four (75%) pre-pubertal patients [82]. Therefore, according to the numerous studies mentioned above, several issues such as the following should be considered: (1) Is it necessary for boys with KS to undergo fertility preservation, including that of germ cells and testicular tissues, during their pre-puberty stage? As is well known, some KS patients have the opportunity to extract sperm when they are sexually mature. Thus, fertility preservation by TESE at this stage of their lives may impair their testis and further destroy their chance to retrieve sperm when they grow up. In contrast, for another group of KS boys who may have absolutely no sperm even in their adult period, we are concerned that they will lose the final chance to preserve fertility if we do not stock their germ cells and testicular tissues during pre-puberty. Thus, all of these possible factors should be taken into account prior to fertility preservation in pre-puberty. (2) If a KS patient undergoes fertility preservation in his pre-puberty stage, the TESE should be performed again in the adult period when he decides to accept ART treatment. Once spermatozoa or spermatids have been found at sexual maturity, these gametes have a better developmental potentiality than those cryopreserved in pre-puberty. (3) If the preservation of pre-pubertal testicular tissue from KS patients with a normal architecture and a higher number of spermatogonia is performed, the degenerative process of germ cells and seminiferous tubules still cannot be completely prevented even under the condition of in vitro maturation, germ cell transplantation, or tissue grafting.
Fertility preservation in adolescents and adults
The comparison study of the SRR between adolescent and adult KS patients showed that the SRR per TESE cycle was as high as 44%, with a mean age of 30.9 ± 5.6 years. Overall, a total of 218 biochemical pregnancies after 410 ICSI cycles were observed, with 43% pregnancy rates (PRs) [46]. The majority of retrospective studies focused on the relationship between SRR and clinical parameters such as age, testicular volume, serum levels of FSH, LH, and testosterone, and the presence of mosaicism, while there are no reliable clinical and biological predictors for successful sperm retrieval in NOA patients with KS [46, 74, 83]. Unfortunately, the residual potential fertility, including SSCs, round spermatids, and remaining testicular tissue after TESE, is commonly ignored if the surgery fails to extract spermatozoa. In fact, these approaches should be considered as methods of fertility preservation in KS patients. Recently, the retrieval of testicular spermatozoa by TESE in four age groups (fetal, pre-pubertal, peripubertal, and adult) revealed that testicular spermatozoa were collected by TESE in 48.1% of the adult KS patients, while spermatozoa were recovered after TESE in only one peripubertal patient (5.0%). Germ cells were detectable in testicular biopsies from 21% of adult men for whom no spermatozoa could be retrieved by TESE and in 31.5% of peripubertal KS boys [82]. Therefore, it is necessary to pay more attention to potential fertility preservation in individuals in whom no spermatozoa have been found, although the potential fertility presents some controversial issues before clinical treatment. Vitrification cryopreservation of either testicular tissue or SSCs is the optimal choice for these patients whose germ cell depletion is caused by some toxicity or malignancy [84–87]. These procedures for fertility preservation offer the potential possibilities for SSC transplantation (SSCT), testicular tissue grafting, and in vitro spermatogenesis. In sum, fertility ability preservation with SSCs in KS children is experimental; so far, it has not been applied in clinical practice. Unfortunately, it is unlikely that techniques such as tissue grafting, SSCT, xenografts, or in vitro maturation of SSCs will produce viable spermatozoa in men with KS within the coming few years. In IVF centers, these NOA patients have commonly been considered for the use of donor spermatozoa or for cancelling the ART cycle by the vitrification and cryopreservation of oocytes, especially for KS couples for whom no sperm is found by TESE on egg retrieval day. However, some studies have reported that a certain proportion of non-obstructive azoospermic men with neither spermatozoa nor late-stage spermatids possess round spermatids [88, 89]. For KS patients with NOA, the presumptive round spermatids were cryopreserved using our different methods of vitrification and following manipulation in round spermatid injection (ROSI) as a last resort for even the spermatozoa found in the enzymatically dissociated testicular tissue because the spermatozoa obtained from KS patients by TESE are always characterized by rare numbers, an abnormal morphology and non-motile activity. ROSI only was considered as an experimental technology rather than a recommended clinical practice because of the potential risk to safety despite the fact that healthy infants were obtained after ROSI, and on-going pregnancies have been reported [90]. Recently, the publication of two reports seemed to strengthen the confidence regarding ROSI application in humans. In these two reports, researchers revealed the details of the ROSI procedure and achieved fourteen infants [91]. They compared the physical and cognitive development of babies born after ROSI with those born after natural conception, including body weight increase, response to parents, and understanding and speaking simple language. No significant difference was found during the first 2 years after birth [92]. Therefore, the ROSI procedure is also a promising technical advance for fertility preservation in adult KS patients in the future.
Single sperm or spermatid cell cryopreservation
During the past several decades, important achievements have been made regarding fertility preservation. Effective sperm preservation for KS patients is a crucial step for fertility preservation according to the patient’s age and testicular status [93]. Rare literature addresses the ICSI results with cryopreserved sperm in patients with non-mosaic KS. Two studies reported that one out of five KS patients with NOA and two out of six patients had to be injected with immotile cryopreserved-thawed spermatozoa with a low fertilization rate [94, 95]. Another study pointed out that it is urgent to determine how to cryopreserve and to effectively retrieve the thawed spermatozoa by TESE in KS patients. Currently, there is a lack of effective methods to stock residual rare fresh spermatozoa after ICSI and to increase the recovery efficiency when the cryopreserved spermatozoa have been thawed [96]. In KS patients, especially those with fewer than 5–10 sperm, establishing a system with a high recovery rate to cryopreserve a single human sperm will contribute to fertility preservation. Although it is critical to successfully obtain spermatozoa or spermatid cells from KS patients with NOA by TESE, how to well preserve the precious spermatozoa, even a single sperm, is the main obstacle for final successful pregnancy and delivery [67]. The method using the zona pellucida for cryopreserving a single spermatozoa is intriguing and reliable [29, 97]. However, an ethical problem exists, and the source of this material is not easily available. In previous studies [30, 31], the cryostrip and cryoleaf devices have been developed to store rare spermatozoa, but this method is not practical and is rarely available. Recently, we established a novel cryopiece system to effectively preserve individual spermatozoa from patients with severe oligozoospermia and NOA [67]. The spermatozoa with normal morphology and higher motility were captured under a microscope using the ICSI pipettes equipped on the micromanipulator and then loaded onto the indicated medium drops (1–2 ul) on the cryopiece system. We compared the fertilization rate, embryo cleavage rate, ET rate, and implantation rate between ICSI series using fresh testicular spermatozoa and thawed testicular spermatozoa cryopreserved with the cryopiece system. No remarkable difference was observed between the groups. The cryopreserved system has been authorized for patents in China (Numbers: ZL 2016 2 0544372.5 and ZL 2016 1 0396509.1) and well applied in clinical practice. The data analysis has demonstrated that the spermatozoa recovery rate is 83% and that the mobile spermatozoa rate is 36% after thawing.
Although the mobility rate is slightly low, this method could effectively avoid ART cycle cancellations resulting from no sperm or no retrieval of living sperm cryopreserved by straw tubes or cell stock tubes. Furthermore, the pregnancies were obtained by ICSI using thawed immotile spermatozoa because the spermatozoa were living prior to being selected for cryopreservation. Some researchers have also found that these immotile spermatozoa have the same developmental potentiality in single spermatozoa preservation methods [30, 67]. Therefore, the cryopiece system works well in stocking rare spermatozoa and is one of the rare alternative methods for spermatozoa cryopreservation successfully applied in clinical practice in populations with severe male factor infertility.
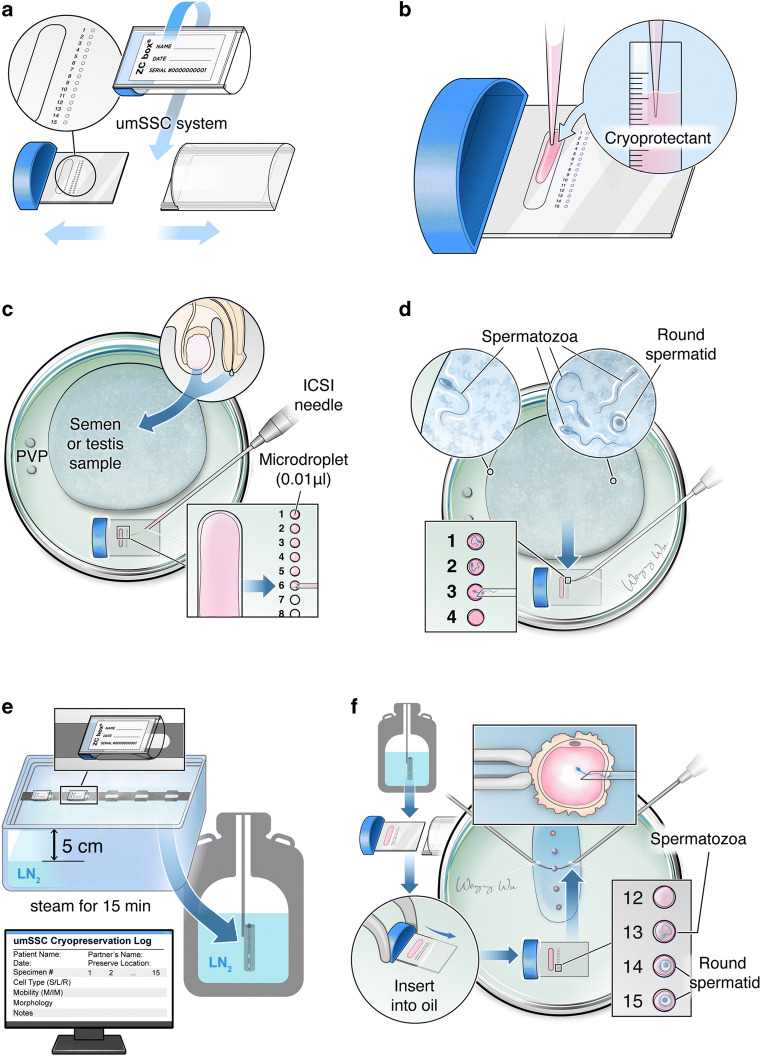
Most importantly, the preheating cover oil was tactically used to thaw the micro-drop containing cryopreserved sperm. Additionally, the system has several advantages in preserving fresh testicular spermatozoa or testicular tissue. First, the cryopiece-based system overcomes the limitation of preserving fewer than 5–10 sperm. Traditionally, these sperm have been stocked in commercial straw tubes together with other debris and sediment, which causes the limited sperm not to be found easily after thawing. Thus, the traditional method usually fails to store extremely rare sperm. Second, the available spermatozoa were easily identified and separated from the mixtures with testicular tissue, various sediments, and dead sperm cells. Last but not the least, the timing of TESE is flexible. The KS patient can make an appointment with the doctor for TESE in his free time. Although the recovery rate reaches up to 80% in general NOA patients when their sperm is stocked with a cryopiece system, the approximately 20% loss of sperm in the thawing procedure is not acceptable in KS patients because some severe NOA KS patients could exhibit fewer than 5 sperm in total. For this kind of patient, each sperm is invaluable. According to the clinical request, we established a new single sperm cryopreservation system to store the KS sperm one by one and rank them on the cryopiece on the basis of their quality, mainly including morphology and mobility. In fact, there is no real, single spermatozoa cryopreservation method (including the above technique in which we introduced the cryopiece system) so far because small numbers of spermatozoa are cryopreserved in minuscule (1–5 ul) droplets rather than as individually isolated spermatozoa. It is difficult to compare the characteristic change in thawed spermatozoa with that before cryopreservation and to trace the developmental potentiality of spermatozoa with different morphologies or spermatids. Currently, we named the system ultramicro-single spermatozoa cryopreservation (umSSC, Fig. 2) because this system could be loaded and could cryopreserve single spermatozoa or spermatid cells. This system includes the semi-cylinder cryopreservation container and information registration form for spermatozoa or spermatids. The components of the device and cryopreservation procedure are shown in detail in Fig. 2. The semi-cylinder container presented a faster cooling rate than with the cylinder freezing tube in the cryopiece system we previously established. The most significant modification is that a single spermatozoa or spermatid is arrested in ultramicro-droplets produced by the ICSI injection needle (Fig. 2a–d), whose volume is only 0.05 μl and the diameter is only 2–3 times that of spermatozoa and gives the serial number with detailed characteristics in the registration form (Fig. 2e). Thus, the stocked sperm in ultramicro-droplets could be easily found and increase the recovery rate. In the current system, our data revealed that the spermatozoa recovery rate of the umSSC system after thawing reaches up to 100% (two cases listed in Table 4). The spermatozoa cannot swim away and can be observed clearly by inverted microscopy in ultramicro-droplets because the diameter of the ultramicro droplet is only two- to threefold that of spermatozoa. These modifications, including the smaller volume, using homemade cryoprotectant and semi-cylinder containers, make the umSSC system closer to vitrification. As a result, the warmed spermatozoa motility rate (60–80%) in the umSSC system is comparable to that of vitrified sperms in the human or mouse zona pellucida [21, 98] and Volvox globator algae [99]. This method greatly improves the embryo quality because excellent sperm is selected to be injected into eggs according to the rank recorded in the registration table (Fig. 2e–f).
Fig. 2.
Schematic illustration of the single sperm cryopreservative system. (A) Droplets, polypropylene carrier and ZC (Z.Z and C.W designed the device, named it ZC box) box of a single sperm cryopreservative system. (B) The cryoprotectant was loaded onto the polypropylene carrier. (C) The polypropylene carrier with cryoprotectant was placed into the bottom of the dish, and the cryoprotectant was then divided into a 0.01 μl micro-drop. The prepared extremely rare spermatozoa sample from ejaculated sperm, TESE or thawing sperm was loaded on the dish and then covered with mineral oil. (D) The motile spermatozoa were captured and loaded on the microdrops by ICSI pipettes, following storage in the liquid nitrogen tank and recorded for each sperm’s information. (E) The polypropylene carrier with spermatozoa was inserted into the bottom of a dish and covered with warm mineral oil for thawing, and the recovered spermatozoa were prepared for ICSI
Table 4.
Clinical parameters and outcome after intracytoplasmic sperm injection with vitrified spermatozoa cryopreservation in cryopiece and umssc system
| Patient | Diagnosis | Cycle | Spermatozoa origin | Single spermatozoa cryopreservation type | No. motile spermatoza/no.cryopiece | No. retrieved/no. vitrified spermaozoa (%) |
No. motile spermatozoa/no. retrieved spermatozoa (%) |
Clinic procedure | Oocyte activation | Injected MII oocyte | 2PN fertilization [n (%)] |
Embryo cleaved [n (%)] | Available embryo | Outcome (pregnancy date) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NOA | 1 | m-TESE | Cryopiece | 20/2 | 18/20 (90) | 10/18 (56) | ICSI | No | 6 | 5 (83) | 5 (100) | 4 | Single live birth (2015–08–31) |
| 2 | Oligo | 1 | Ejaculate | Cryopiece | 40/2 | 30/40 (75) | 12/30 (40) | ICSI | No | 11 | 8 (73) | 7 (88) | 3 | Twin live birth (2015–09–24) |
| 3 | NOA | 1 | m-TESE | Cryopiece | 26/1 | 22/26 (85) | 10/22 (45) | ICSI | No | 8 | 5 (63) | 4 (80) | 1 | No pregnancy |
| 4 | NOA | 1 | m-TESE | Cryopiece | 20/1 | 18/20 (90) | 6/18 (33) | ICSI | No | 5 | 4 (80) | 3 (75) | 2 | Single live birth (2016–09–12) |
| 5 | NOA (KS) | 1 | m-TESE | Cryopiece | 22/1 | 18/22 (82) | 3/18 (17) | ICSI | No | 12 | 7 (58) | 7 (100) | 5 | Single live birth (2016–05–8) |
| 6 | NOA | 1 | m-TESE | Cryopiece | 25/1 | 18/25 (72) | 3/18 (17) | ICSI | No | 2 | 1 (50) | 1 (100) | 1 | No pregnancy |
| 7 | Oligo | 1 | Ejaculate | Cryopiece | 45/2 | 35/45 (78) | 10/35 (29) | ICSI | No | 18 | 3 (17) | 2 (67) | 2 | No pregnancy |
| 2 | Ejaculate | Cryopiece | 39/2 | 30/39 (77) | 9/30 (30) | ICSI | No | 9 | 3 (33) | 2 (67) | 2 | No pregnancy | ||
| 8 | Oligo | 1 | Ejaculate | Cryopiece | 21/1 | 16/21 (76) | 10/16 (63) | ICSI | No | 10 | 9 (90) | 9 (100) | 6 | Single live birth (2016–08–13) |
| 9 | Oligo | 1 | Ejaculate | Cryopiece | 33/2 | 25/33 (76) | 5/25 (20) | ICSI | No | 5 | 3 (60) | 3 (100) | 2 | No pregnancy |
| 10 | NOA (KS) | 1 | m-TESE | Cryopiece | 3/1 | 3/3 (100) | 0/3 | Donor semen | ||||||
| 11 | Oligo | 1 | Ejaculate | Cryopiece | 8/1 | 8/8 (100) | 6/8 (75) | ICSI | No | 6 | 3 (50) | 2 (67) | 0 | No pregnancy |
| 12 | NOA (KS) | 1 | m-TESE | Cryopiece | 20/1 | 12/20 (60) | 0/20 | Oocyte cryopreservation | ||||||
| 13 | NOA | 1 | m-TESE | Cryopiece | 11/1 | 7/11 (64) | 4/7 (57) | ICSI | No | 4 | 3 (75) | 3 (100) | 3 | No pregnancy |
| 14 | Oligo | 1 | Ejaculate | Cryopiece | 27/2 | 21/27 (78) | 8/21 (38) | ICSI | No | 8 | 6 (75) | 3 (50) | 3 | Single live birth (2017–11–10) |
| 15 | NOA | 1 | m-TESE | Cryopiece | 6/1 | 5/6 (83) | 0/6 (0) | ICSI | No | 5 | 2 (40) | 0 (0) | 0 | No pregnancy |
| 16 | Oligo | 1 | Ejaculate | Cryopiece | 21/1 | 21/21 (100) | 14/21 (67) | ICSI | No | 14 | 5 (36) | 5 (100) | 2 | No pregnancy |
| 17 | Oligo | 1 | Ejaculate | Cryopiece | 22/3 | 17/22 (77) | 7/17 (41) | ICSI | No | 7 | 3 (43) | 2 (67) | 1 | No pregnancy |
| 18 | Oligo | 1 | Ejaculate | Cryopiece | 14/1 | 13/14 (93) | 10/13 (77) | ICSI | No | 10 | 3 (30) | 3 (100) | 2 | No pregnancy |
| 19 | NOA | 1 | m-TESE | Cryopiece | 11/1 | 9/11 (82) | 3/9 (33) | ICSI | Yes | 5 | 4 (80) | 4 (100) | 3 | Single live birth (2018–03–26) |
| 20 | Oligo | 1 | Ejaculate | umSSC | 20/2 | 20/20 (100) | 14/20 (70) | ICSI | No | 8 | 5 (63) | 5 (100) | 2 | Single live birth (2018–07–05) |
| 21 | Oligo | 1 | m-TESE | Cryopiece | 7/1 | 5/7 (71) | 1/5 (20) | ICSI | No | 1 | 0 (0) | 0 (0) | 0 | No pregnancy |
| 22 | Oligo | 1 | Ejaculate | Cryopiece | 15/1 | 11/15 (73) | 7/11 (64) | ICSI | No | 14 | 9 (64) | 9 (100) | 7 | Single live birth (2018–11–01) |
| 23 | Oligo | 1 | Ejaculate | Cryopiece | 11/1 | 7/11 (64) | 3/7 (43) | ICSI | Yes | 5 | 5 (100) | 5 (100) | 2 | No pregnancy |
| 24 | Oligo | 1 | Ejaculate | Cryopiece | 12/1 | 9/12 (75) | 1/9 (11) | ICSI | Yes | 9 | 2 (22) | 2 (100) | 2 | Singleton ongoing pregnancy (2018–11–08) |
| 25 | Oligo | 1 | Ejaculate | umSSC | 22/2 | 20/22 (100) | 10/20 (50) | ICSI | No | 10 | 7 (70) | 7 (100) | 3 | Twins ongoing pregnancy (2019–01–8) |
| 26 | Oligo | 1 | Ejaculate | Cryopiece | 47/2 | 38/47 (81) | 7/38 (18) | ICSI | Yes | 8 | 6 (75) | 6 (100) | 4 | No pregnancy |
| 27 | Oligo | 1 | Ejaculate | Cryopiece | 12/1 | 9/12 (75) | 4/9 (44) | ICSI | Yes | 6 | 5 (83) | 4 (80) | 2 | No pregnancy |
umSSC, ultramicro single spermatozoa cryopreservation system
The method could be extended to stock round spermatids or elongated spermatids if no sperm has been found after TESE. Using this round spermatid vitrification strategy, we can prepare the identified round spermatids prior to the ICSI procedure and trace the developmental potentiality of different types of round spermatids because the key to ROSI success is the ability to identify round spermatids accurately before oocyte injection [91]. To date, two pregnancies have been obtained using spermatozoa cryopreserved in the umSSC system in severe NOA patients, and identified round spermatids special for ROSI have been cryopreserved with the umSSC system. Cryopreservation of single spermatozoa or elongated spermatids is a novel and promising method for fertility preservation. Moreover, single spermatozoa cryopreservation of the umSSC system combined with ROSI is a promising technique in fertility preservation for severe NOA patients, especially KS patients with severe NOA.
Selection and assisted activation of the available spermatozoa from KS patients
Fathering is a severe issue in the KS population. A cohort study enrolling almost 200 Dutch men with KS documented that these patients and their partners strongly wished to have their own children and had a positive attitude towards TESE–ICSI treatment [13]. The meta-analysis for ICSI outcomes in KS showed that live children could be obtained in approximately 16% of subjects who undergo TESE [46], which is slightly lower than the 25% in non-KS subjects with NOA [51]. A critical cause of the lower live birth rate might be the poor quality of the spermatozoa because of the genetic defect or abnormal structure, which impairs the motility of the sperm in the KS population. The other cause might be based on very heterogeneous events, and immotility is a consequence of oxidative stress, infection, or numerous other influences [100, 101]. Furthermore, the injuries to the sperm resulting from the cryopreserving-thawing process also contribute to the weak immotility and poor clinical outcome. Thus, the selection of immotile but viable spermatozoa on the day of ICSI is a major challenge for embryologists if no moving sperm has been found.
In the routine work of ART laboratories, the selection of valid spermatozoa from poor quality KS sperm mixtures mostly depends on their morphology and vitality. Of course, “handsome” and active sperm have more opportunity to be selected for ICSI. Nevertheless, how to choose immotile but living sperm is a challenge. Currently, several methods have been widely used for selecting this kind of sperm, including the hypo-osmotic swelling test (HOS), artificial activation with chemical reagents, the sperm tail flexibility test (STFT), and laser-assisted immotile sperm selection (LAISS) [102]. Considering possible injury due to the dyes’ (such as eosin) toxicity to sperm, the HOS test is the only recommended approach for immotile sperm selection according to the latest version of the WHO’s (2010) manual as an alternative to dye exclusion tests. To improve the feasibility and effect of the HOS test combined with the ICSI procedure, various modifications according to the basic protocol have been developed [103–105]. Certainly, this protocol greatly improves the clinical outcome when sperm obtained by TESE was selected for ICSI. Given this advantage of the HOS test, it has been extended to select the sperm of Kartagener’s syndrome characterized [105] with primary ciliary dyskinesia [106–108]. Although the HOS test could benefit choosing living sperm from immotile sperm, spontaneously developing tail swelling (SDTS) would limit the skill used in selecting valid sperm in cryopreserved-thawed TESE spermatozoa [109]. This phenomenon is commonly observed in the KS patients’ cryopreserved sperm and TESE tissue mixtures, which will impair the embryologist’s estimation of the HOS test results.
The TESE sperm from KS patients generally exhibits frail mobility, which is more so the case for cryopreserved-thawed KS sperm. Thus, some of the chemical reagents could facilitate this kind of sperm’s movement. Although these reagents sometimes only stimulate the sperm’s tail to wiggle, they will facilitate valid sperm selection. These reagents mainly comprise pentoxifylline and theophylline, both of which belong to the same family of methylxanthine derivatives. They function by inhibiting phosphodiesterase activity and thus increasing intracellular cAMP levels and then inducing sperm motility [102]. Therefore, the incubation of the mixture from TESE of ejaculated spermatozoa with a chemical substance will result in activation of the whole spermatozoa pool rather than single immotile spermatozoa activation or selection by HOS, which greatly saves time used for selecting motile spermatozoa [110]. One previous study comparing pentoxifylline treated against the HOS test identified spermatozoa and revealed that pentoxifylline treatment was more effective in both fertilization (62.05% vs. 41.07%) and clinical PR (32% vs. 16%) [111]. Increasing evidence certified that stimulation with pentoxifylline could potently improve the embryonic quality, related laboratorial factors, and clinical outcomes, except for the pregnancy rate [110, 112, 113]. Although no birth defect has been reported after artificial activation of sperm with pentoxifylline or theophylline, a remarkable increase in spermatozoa DNA damage was observed, and negative effects on blastocyst development were found with high concentrations of pentoxifylline in the animal experiment [114, 115]. Therefore, the dose should be considered in ART clinical practice.
The STFT is based upon the observation that immotile spermatozoa have a flexible tail, and this analysis provides great advantages, such as the lack of unclear negative effects and the absence of additives, over employing activating substances and HOS tests. Nonetheless, the STFT requires skillful experience and sharp observation. The STFT mainly depends on the embryologist’s evaluation according to the sperm tail observation. Therefore, subjectivity may affect living sperm selection, especially for thawed KS TESE sperm. Its advantage is that physical stirring causes no injury to the sperm.
Viable but immotile spermatozoa were first identified by the appearance of curling or coiling at the tip of the flagellum after treatment with a single laser shot [116]. The main advantage of laser usage is that it carries no risk of genetic mutation or embryotic toxicity and only requires a laser instrument used for embryo-assisted hatching or biopsy. The fertilization rate injected with immotile but vital spermatozoa by ICSI significantly improved when applying laser-based selection compared with the control protocol (45.4% vs. 20.4%, p = 0.006), and the take-home infant rate increased from 5.9 to 19.0%. Furthermore, no negative effect of the laser on birth rates was seen [117].
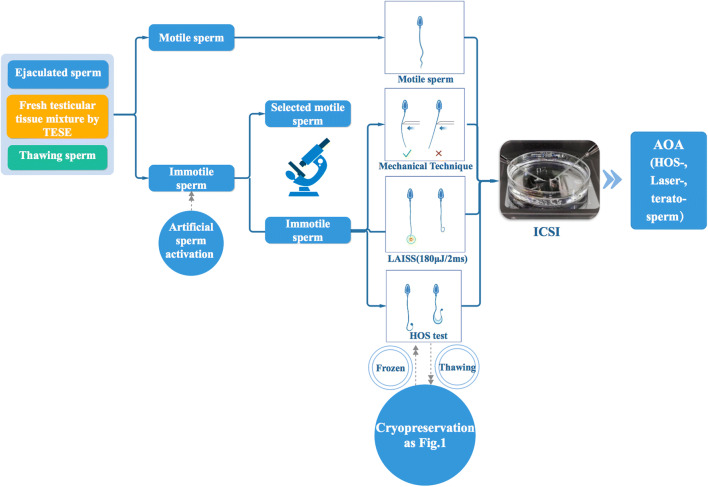
Considering the characteristics of these methods of selecting immotile but viable spermatozoa of KS patients, we established a flow chart to direct the treatment of KS patients’ spermatozoa samples in our ART laboratory (Fig. 3). It is crucial that embryologists estimate whether the sample contains available spermatozoa as soon as possible, especially when oocyte retrieval and TESE are carried out simultaneously. Therefore, according to our flow chart (Fig. 3), if no motile spermatozoa are available, chemical substances for the induction of tail movements (e.g., pentoxifylline) would be immediately used for incubation with the spermatozoa sample. The motile and slightly swimming spermatozoa can be identified under an inverted microscope (× 10–20 magnification), rather than engaging in a time-consuming search for spermatozoa in tissue fragments one by one to identify the vitality through other methods. The HOS test and laser methods are employed in the following situations: no viable spermatozoa were found after treatment with chemical substances; not enough viable spermatozoa were injected into oocytes. In our own trial using chemical substance activation combined with laser testing (tending to cryopreserved TESE spermatozoa) and the HOS test (tending to fresh TESE spermatozoa), we found that these methods are quick, easy, and feasible for KS spermatozoa viability testing (Table 2 for KS spermatozoa situation). One of the weaknesses of the current method is that the determination of viable spermatozoa in KS patients was based on a small sample size and should be further validated by a larger sample size. Representative sperm activation methods and clinical outcomes are summarized in Table 5.
Fig. 3.
The flow chart for selection and assisted activation of the available spermatozoa from KS patients. The motile sperm from ejaculated sperm, TESE or thawing sperm was directly used for ICSI. If no motility was found, the immotile sperm was loaded onto the lanes as indicated in the figure and then selected by a mechanical technique, LAISS or HOS test. If the terato-sperm was chosen for ICSI or the immotile sperm underwent HOS or laser treatment, artificial ovarian activation is recommended
Table 5.
Overview of the reported outcome of different representative sperm activation methods
| Study ID | Activation method | Retrieval methoed | No. of immotile sperm | No. of live sperm | Fertilization rate | ETs | ART result |
|---|---|---|---|---|---|---|---|
| Terriou et al. 2000 | Pentoxifylline |
TESE or MESAor PESA |
/ / / |
/ / / |
45.2% | 20 |
Clinical pregnancy rate= 30%, Two deliveries of healthy children and four ongoing pregnancies |
| Soares et al. 2003 | STFT | Ejaculated | / | / | 40/132(30.3%) | 9 | Clinical pregnancy rate = 11.12% |
| Ma´tya´s et al. 2004 |
STFT HOST |
TESE TESE |
/ / |
/ / |
66.9% 52.3% |
30 6 |
Clinical pregnancy rate= 17.5% |
| Oliveira et al. 2004 | STFT |
TESE (frozen-thawed) TESE(fresh) |
/ / / |
/ / |
65.7% 73.4% |
Clinical pregnancy rate/cycle = 33.3%, 5 singleton |
|
| Montjean et al. 2004 |
Pentoxifylline HOST |
Ejaculated Ejaculated |
/ / |
/ / |
3/15 (20%) 5/17 (29.4%) |
2 2 |
Clinical pregnancy rate= 25%, 1 singleton |
| Aktan et al. 2004 |
LAISS HOST |
TESE Ejaculated TESE Ejaculated |
/ / / / |
/ / / / |
45.4% 64.2% 20.4% 49.5% |
/ / / / |
Take-home baby rate/cycle =19.0 %, Take-home baby rate/cycle =5.9%, Take-home baby rate/cycle =28.0%, Take-home baby rate/cycle =16.7% |
| Tal et al. 2005 | STFT | Ejaculated | / | / | 27/49 (55.1%) | 5 |
Clinical pregnancy rate= 20%, 1 singleton |
| Sallam et al. (2005) | HOST | TESE | / | / | 129/296 (43.6%) | 44 |
Clinical pregnancy rate =27.3%, Ongoing pregnancy rates=20.5% |
| KOVACˇ IC et al. 2006 | Pentoxifylline |
TESA TESE |
/ / |
/ / |
219/332(66%) | 47 | Clinical pregnancy rate= 38.3% |
| Gerber et al. 2008 |
LAISS HOST |
Ejaculated Ejaculated |
/ / |
/ / |
4/7 (57%) 2/4 (50%) |
1 1 |
1 singleton, no pregnancy was achieved |
| Mangoli et al. 2010 |
Pentoxifylline HOST |
TESE TESE |
/ / |
/ / |
193/311 (62.05%) 138/336 (41.07%) |
25 25 |
Clinical pregnancy rate =32%, Clinical pregnancy rate = 16% |
| Nordhoff et al. 2012 | LAISS | TESE | / | / | 292/554 (52.7%) | 57 | / |
| Terriou et al. 2015 |
Papaverine Pentoxifylline |
TESE or MESA |
/ / |
/ / |
/ / |
/ / |
Motility after Pentoxifylline=23%, Motility after Papaverine=27% |
| Navas et al. 2017 | Pentoxifylline |
Testicular Epididymal Ejaculated |
/ / / |
/ / / |
/ / / |
/ / / |
82 singletons; 20 twins |
| Huanhua Chen et al. 2017 | LAISS |
TESE Ejaculated |
5811 2991 |
2888 (49.7%) 663 (22.2%) |
139/173 (80.3%) 37/47 (78.7%) |
/ / |
/ / |
| Yi-Fan Gu et al. 2018 | SperMagic medium |
TESE Ejaculated |
/ / |
/ / |
415/506 (82%) 291/355 (81.7%) |
45 32 |
Clinical pregnancy rate= 68%, 45 live births |
| Ozkavukcu et al. 2018 | LAISS | Ejaculated | / | / | 45.5% | 1 | Live births of healthy triplets |
| Huanhua Chen et al. 2019 |
Eosin-nigrosin test LAISS |
TESE Ejaculated TESE Ejaculated |
421 946 436 920 |
88 (20.9%) 660 (69.8%) 0 (0%) 212 (23%) |
TESE (87.0%) ejaculate (81.8%) |
2 |
Clinical pregnancy rate=50%, 1 singleton |
HOST: hypo-osmotic swelling test; STFT: sperm tail flexibility test; LAISS: laser assisted immotile sperm selection; ETs: Embryo transfer cycles; TESA: Testicular sperm extraction; MESA: Microsurgical epididymal sperm aspiration; PESA: percutaneous epididymal sperm aspiration
ROSI
For KS patients, the average SRR reaches 50%. Therefore, most KS patients fail to achieve sperm by micro-TESE. If neither spermatozoa nor late-stage spermatids are found in the testis, the men will be considered sterile. In 1995, Atsuo Ogura et al. pointed out that mouse oocytes injected with cryopreserved round spermatids can develop into normal offspring [118]. In the next year, they reported the birth of a normal young mouse after the electrofusion of oocytes with spermatids [119]. Subsequently, another research group demonstrated that round spermatids of cynomolgus monkeys can be used as substitute gametes to support embryonic development at least to mid-gestation [120]. These results imply that round spermatids have potential clinical value in infertility therapy as substitute gametes. Atsushi Tanaka et al. first injected round spermatids into human oocytes and achieved fourteen infants [91]. Apparently, the safety of ROSI is the critical issue we pay attention to. In our opinion, we should not conduct ROSI in ART treatments until the safety and efficiency have been approved. Most recently, the same research group has provided data to address this question. They compared the physical and cognitive development of babies born after ROSI with those born after natural conception, including body weight increase, response to parents, and understanding and speaking simple language. No significant differences were found during the first 2 years after birth [92]. Despite the birth of healthy infants, human ROSI is limited due to the difficulty in identifying round spermatids and the lower fertility rate. Nevertheless, in KS patients without spermatozoa or elongated spermatids, the injection of round spermatids into oocytes has become the last resort to use their own genetic material to produce offspring. However, the contrary conclusion has been made in other groups. The authors considered ROSI not efficacious for men with KS in terms of achieving pregnancy [90]. In this study, the oocytes of four patients with KS were subjected to ROSI; 41.6% of them were fertilized and developed embryos to the stage of transfer, while no pregnancy was detected. Regardless of the presence or absence of a successful pregnancy, at least ROSI provides another possibility for the sterile KS male to father a child. Thus, ROSI needs additional careful consideration and further study.
Artificial oocyte activation
In clinical practice, the harvested sperm from KS patients commonly shows an aberrant morphology, which is the major factor of non-fertilization. As described above, we could only find less than 10 sperm in some of the KS cases. These sperm might be immobilized by a gentle touch of the injection needle when we catch them for single sperm cryopreservation. This results in difficulty in identifying whether the thawed sperm is living or dead when we perform ICSI. Moreover, the cryopreservation procedure may impair sperm activity. These factors contribute to the poor fertilization rate in the ICSI cycle. Similarly, as a premature sperm, the ROSI also leads to a low fertilization rate [91, 92]. Nevertheless, the issue has been well overcome by artificial oocyte activation with ROSI combined with electric stimulation [91, 92]. In our clinical practice, artificial oocyte activation potently improved the fertilization rate in KS patients. Mechanistically, various stimulations induce repetitive intracellular Ca2+ oscillation, which is an essential component of oocyte activation and a prerequisite of normal fertilization and embryo development [121]. Currently, the popular approaches for artificial oocyte activation include chemical activation, electric stimulation, and mechanical stimulations. In 2010, Sugaya S reported a successful pregnancy outcome after calcium ionophore A23187 oocyte activation in an infertile couple with a repeated failure of achieving fertilization after ICSI [122]. In this case, the patient underwent two pregnancy failures because none of the oocytes was fertilized. In the third IVF cycle, the oocytes after ICSI were incubated with calcium ionophore for 5 min, which resulted in the fertilization of two of three oocytes and a subsequent successful pregnancy and delivery. The different calcium ionophores have various effects on oocyte activation and fertilization. It was demonstrated that ionomycin, one of the calcium ionophores, was more potent than A23187 (another calcium ionophore) in provoking Ca2+ oscillation and more efficient in improving the fertilization rate [123]. Moreover, SrCl2 was also used in assisted oocyte activation (AOA) and has been certified as an efficient treatment strategy to overcome poor fertilization and improve embryonic quality [124, 125]. In addition, exposure to 7% anhydrous alcohol function as AOA has been documented to improve the fertilization rate, high-quality embryo formation rate, and blastocyst formation rate [126]. This is contrary to the ordinary role of prohibition in exposing the embryo or oocyte in alcoholic conditions. Although these chemicals could facilitate oocyte fertilization and produce high-quality embryos, their safety should not be ignored. Compared with chemical activation, electric stimulation may have fewer side effects on a healthy birth, attributed to no cytotoxicity and less injury to oocytes when performing AOA with this kind of stimulation [127, 128]. To date, ninety healthy babies have been born after combining electric stimulation function with AOA after ROSI [92]. The contrary view has also been shown that electric stimulation increases reactive oxide species (ROS) levels in embryos and impairs embryo development [129]. Therefore, the long-term effect of electric stimulation on infant health should be evaluated. Undoubtedly, these approaches could overcome fertilization failure, which is a critical obstacle for pregnancy in KS couples, and more importantly could facilitate embryo development. Therefore, according to these successful reports and our experience, artificial oocyte activation is recommended as a routine procedure after ICSI in the KS patients’ IVF cycle.
Non-invasive prenatal diagnosis and PGD
The prevalence of KS is estimated at 0.1–0.2% in male newborns even if the parents are normal individuals, and the rate might be higher in infants with a KS father. Therefore, PGD and prenatal diagnosis should be performed to guarantee healthy newborn infants. In clinical practice, a general or 3D ultrasound assessment is commonly performed to monitor the status of the fetus. To some extent, ultrasonic examination could prevent birth defects. However, this approach fails to identify the KS fetus because there is no sharp difference between normal and KS fetuses in phenotype. The critical diversity lies in their chromosomes. In 1997, Y M Dennis Lo et al. pioneered a non-invasive prenatal diagnosis by detecting fetal DNA in maternal plasma and serum [130]. Subsequently, this method was extended to detect a single-copy fetal DNA sequence, RhD status, beta-thalassemia gene, and so on [131–133]. Currently, it has been widely used to screen aneuploid diseases, including Down’s syndrome, Turner syndrome, XYY syndrome, and KS [134–136]. Perez-Carbajo, E. et al. pointed out that an invasive test, such as amniocentesis, must be further carried out to assess the karyotype normality in cases of the prenatal diagnosis of abnormalities [137]. We agree with their opinion because there are false positive results in the non-invasive prenatal diagnosis.
Although these approaches could be effectively used to screen and identify KS fetuses, identification is never the goal of the non-invasive diagnosis, as KS couples are then faced with the dilemma of whether to terminate the pregnancy or to carry it to term [137–139]. On the basis of this condition, we developed a new concept, namely, non-invasive PGD during the KS patients’ ICSI cycle. In fact, the traditional PGD depending on segmentation-sphere biopsy could identify the extra chromosome in the KS fetus. In 1990, Handyside AH et al. blocked an X-linked genetic disease from passing onto the next generation for the first time through sex identification of the fetus by amplifying the Y chromosome-specific repeat sequence of a single cell at the cleavage stage [140]. PGD can be routinely carried out via direct genetic assessment by removing a single or multiple cells from preimplantation embryos in either the cleavage stage or blastocyst stage [141–144]. Although blastomere biopsy does directly assess the genomic DNA of in vitro fertilized embryos, this invasive procedure is more likely to result in reducing the developmental potential of embryos and may increase the risk of abortion [145]. In addition, genetic diagnosis in this stage is not as reliable, especially in embryos with mosaicism [146]. Therefore, further study revealed that genomic DNA was present in approximately 90% of blastocyst fluid samples harvested during the vitrification procedure [147]. Based on this finding, researchers reported a medium-based PGD test for α-thalassemia-SEA by comparing a single embryo cell biopsy and the spent culture medium [148]. The diagnostic efficiency of medium-based α-thalassemia-SEA detection significantly increased compared with that of embryo cell biopsy by fluorescent gap PCR analysis. Similarly, our work also provided solid evidence that the embryonic culture medium contains the embryonic DNA genetic information by amplification of the SRY gene [149]. According to this evidence, a new non-invasive chromosome screening (NICS) method based on sequencing the genomic DNA secreted into the culture medium from the human blastocyst was developed [150]. Since then, it has been widely used in the identification of concurrent mutations and aneuploidy screening to diagnose beta-thalassemia disorders at the single- and multiple-cell levels and to detect PKD1 mutations [151, 152]. The researchers used multiple annealing and looping-based amplification cycles (MALBACs) for whole-genome amplification (WGA) combined with next-generation sequencing (NGS) [150]. Therefore, a positive genetic link between the embryo and the spent culture medium was established. This method has been successfully used in balanced translocation, azoospermia, or recurrent pregnancy loss [150]. We also detected the extra chromosome in the KS patients’ spent culture medium in the blastocyst stage by using the above approach (unpublished data). Therefore, non-invasive PGD by determining the spent culture medium provides an optimal approach to avoiding the birth of KS infants to the KS couples who are seeking ART medical therapy.
Conclusions and future directions
It has long been commonly accepted that men with KS are infertile. In recent years, there have been many clinical reports about KS patients successfully fathering healthy offspring through ART. However, clinical management guidelines for ART treatment of KS patients have not yet been developed. It is well known that KS patients face many challenges in the process of ART treatment, including sperm retrieval, cryopreservation of rare sperm, activation of thawed immotile sperm, ROSI, pre-transplantation chromosome screening, and fertility preservation. Therefore, in this review, we systematically summarized the key points of the clinical and laboratory management of KS patients receiving ART treatment. Based on a large number of research results and our own experience, we proposed a clinical management guideline for assisted reproduction therapy in patients with KS (Fig. 4). On the one hand, we recommend that patients should undergo fertility preservation as soon as possible when they are diagnosed with KS. Once KS patients need ART treatment, except for those with ejaculated sperm, the remaining azoospermia KS patients should be re-evaluated after a short period of medication treatment. Patients who still present with azoospermia after medication should undergo microsurgery for sperm extraction. Then, different methods are selected for sperm cryopreservation according to the number and morphology of the retrieved sperm. For extremely rare sperm, a single sperm cryopreservation system is preferred. Subsequently, ICSI is performed after activating immotile sperm in combination with a variety of methods, and ROSI is conducted in patients with only round spermatids. Oocyte activation should be considered for all KS patients to increase the fertility rate. In addition, non-invasive PGD and strict prenatal diagnosis are helpful to avoid the transfer of 47, XXY genotype embryos and the birth of KS children. On the other hand, the selection of the COS protocol for the spouses of KS patients should be determined according to the status of sperm retrieved in the KS patients. The goal of COS is to obtain an appropriate number of mature eggs and minimize the risk of OHSS. Overall, we hope that this strategy will provide us with a clear understanding of how to manage KS patients during ART treatment. However, special attention should be paid to the fact that due to the lack of a large sample analysis of birth defects in ROSI offspring, several follow-up studies are needed to clarify the safety of ROSI in the future. With progress in ART-related technology, more advanced methods are expected to increase the future success rate of ART treatment for KS patients.
Fig. 4.
Schematic illustration of the integrated administration strategies in KS patients’ ART treatment. For spouses of KS patients, the selection of the COS protocol should be determined according to the status of sperm retrieved from KS patients in order to obtain an appropriate number of mature eggs and minimize the risk of OHSS. We recommend choosing a GnRH agonist long protocol if sperm concentration > 0.1 × 106/ml, a GnRH antagonist protocol if sperm concentration < 0.1 × 106/ml but >10 cells, and a GnRH agonist short protocol or mild stimulation protocol if sperm<10 cells. For KS patients who are confirmed to have no sperm after microsurgery, the COS protocol of their spouses should be determined individually on the basis of age, BMI and ovarian reserve. The obtained MII eggs are subsequently used for ICSI or ROSI. If no spermatozoa are found, the cycle should be cancelled and the eggs frozen until sperm is available. Artificial insemination by donor (AID) or IVF by donor semen will be selected according to the tubal patency test of the spouses of KS patients who eventually have no sperm. Regarding KS patients, except for those with ejaculated sperm, the remainder of azoospermia KS patients should be re-evaluated after a short period of medication treatment. Patients who still present azoospermia after medication should undergo surgery, such as TESA, c-TESE or micro-TESE, for sperm extraction. Then, different methods are selected for sperm cryopreservation according to the number and morphology of the retrieved sperm. For extremely rare sperm, a single sperm cryopreservation system is preferred. Subsequently, ICSI is performed after activating immotile sperm in combination with a variety of methods, and ROSI is performed in patients with only round spermatids. Oocyte activation should be considered for all KS patients to increase the fertility rate. In addition, non-invasive PGD and strict prenatal diagnosis are helpful to avoid the transfer of 47, XXY genotype embryos and the birth of children with KS.
Acknowledgments
The authors thank all members of the IVF center in Shanghai General hospital for helpful discussions when preparing this manuscript.
Authors’roles
C.W, B. M, and Y. Y collected the information, analyzed the data, and wrote the manuscript. S. D, Y. Y, and S. J designed the pictures. Z. Z supervised the conception, design, and development of all aspects of the article and was involved in the data analysis and manuscript preparation. W. S, W. Y, F.Y, W.Y, and Z. Z review and editing of the final article. All the authors have seen and approved the final version.
Funding information
This work was supported by grants from the National Natural Science Foundation of China (grants 81672562, 81872111, 81370074, 81902630), National Key Technology R&D Program of China (2019YFC1005200 and 2019YFC1005201), Shanghai Municipal Science and Technology Committee of Shanghai outstanding academic leaders plan (19XD1423100), the project of Outstanding Medical Doctor for ZZ, the cross project of Medical and Engineering (YG2016MS27), Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20181714), the projects sponsored by the development fund for Shanghai talents, the Shanghai Municipal Public Health Bureau (grant XYQ2013119), and the “Chenxing Project” from Shanghai Jiao Tong University to Z.Z.
Compliance with ethical standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei Chen, Ming Zhu Bai and Yixia Yang contributed equally to this work.
References
- 1.Bonomi M, Rochira V, Pasquali D, Balercia G, Jannini EA, Ferlin A, et al. Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. J Endocrinol Investig. 2017;40(2):123–134. doi: 10.1007/s40618-016-0541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capalbo A, Hoffmann ER, Cimadomo D, Ubaldi FM, Rienzi L. Human female meiosis revised: new insights into the mechanisms of chromosome segregation and aneuploidies from advanced genomics and time-lapse imaging. Hum Reprod Update. 2017;23(6):706–722. doi: 10.1093/humupd/dmx026. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen J, Wohlert M. Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Hum Genet. 1991;87(1):81–83. doi: 10.1007/BF01213097. [DOI] [PubMed] [Google Scholar]
- 4.Abramsky L, Chapple J. 47,XXY (Klinefelter syndrome) and 47,XYY: estimated rates of and indication for postnatal diagnosis with implications for prenatal counselling. Prenat Diagn. 1997;17(4):363–368. doi: 10.1002/(sici)1097-0223(199704)17:4<363::aid-pd79>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Grace RJ. Klinefelter’s syndrome: a late diagnosis. Lancet. 2004;364(9430):284. doi: 10.1016/S0140-6736(04)16679-8. [DOI] [PubMed] [Google Scholar]
- 6.Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter’s syndrome. Lancet. 2004;364(9430):273–283. doi: 10.1016/S0140-6736(04)16678-6. [DOI] [PubMed] [Google Scholar]
- 7.Ferlin A, Schipilliti M, Di Mambro A, Vinanzi C, Foresta C. Osteoporosis in Klinefelter’s syndrome. Mol Hum Reprod. 2010;16(6):402–410. doi: 10.1093/molehr/gaq026. [DOI] [PubMed] [Google Scholar]
- 8.Hieronimus S, Lussiez V, Le Duff F, Ferrari P, Bstandig B, Fenichel P. Klinefelter's syndrome and bone mineral density: is osteoporosis a constant feature? Ann Endocrinol. 2011;72(1):14–18. doi: 10.1016/j.ando.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Greco E, Rienzi L, Ubaldi F, Tesarik J. Klinefelter’s syndrome and assisted reproduction. Fertil Steril. 2001;76(5):1068–1069. doi: 10.1016/s0015-0282(01)02734-0. [DOI] [PubMed] [Google Scholar]
- 10.Radicioni AF, Ferlin A, Balercia G, Pasquali D, Vignozzi L, Maggi M, Foresta C, Lenzi A. Consensus statement on diagnosis and clinical management of Klinefelter syndrome. J Endocrinol Investig. 2010;33(11):839–850. doi: 10.1007/BF03350351. [DOI] [PubMed] [Google Scholar]
- 11.Nieschlag E, Ferlin A, Gravholt CH, Gromoll J, Kohler B, Lejeune H, et al. The Klinefelter syndrome: current management and research challenges. Andrology. 2016;4(3):545–549. doi: 10.1111/andr.12208. [DOI] [PubMed] [Google Scholar]
- 12.Selice R, Di Mambro A, Garolla A, Ficarra V, Iafrate M, Ferlin A, et al. Spermatogenesis in Klinefelter syndrome. J Endocrinol Investig. 2010;33(11):789–793. doi: 10.3275/6935. [DOI] [PubMed] [Google Scholar]
- 13.Maiburg M, Repping S, Giltay J. The genetic origin of Klinefelter syndrome and its effect on spermatogenesis. Fertil Steril. 2012;98(2):253–260. doi: 10.1016/j.fertnstert.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Franik S, Smeets D, van de Zande G, Gomes I, D'Hauwers K, Braat DDM, Fleischer K, Ramos L. Klinefelter syndrome and fertility-impact of X-chromosomal inheritance on spermatogenesis. Andrologia. 2018;50(5):e13004. doi: 10.1111/and.13004. [DOI] [PubMed] [Google Scholar]
- 15.Aksglaede L, Andersson AM, Jorgensen N, Jensen TK, Carlsen E, McLachlan RI, et al. Primary testicular failure in Klinefelter’s syndrome: the use of bivariate luteinizing hormone-testosterone reference charts. Clin Endocrinol. 2007;66(2):276–281. doi: 10.1111/j.1365-2265.2006.02722.x. [DOI] [PubMed] [Google Scholar]
- 16.Aksglaede L, Juul A. Testicular function and fertility in men with Klinefelter syndrome: a review. Eur J Endocrinol. 2013;168(4):R67–R76. doi: 10.1530/EJE-12-0934. [DOI] [PubMed] [Google Scholar]
- 17.Ramasamy R, Ricci JA, Palermo GD, Gosden LV, Rosenwaks Z, Schlegel PN. Successful fertility treatment for Klinefelter’s syndrome. J Urol. 2009;182(3):1108–1113. doi: 10.1016/j.juro.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Mehta A, Bolyakov A, Roosma J, Schlegel PN, Paduch DA. Successful testicular sperm retrieval in adolescents with Klinefelter syndrome treated with at least 1 year of topical testosterone and aromatase inhibitor. Fertil Steril. 2013;100(4):970–974. doi: 10.1016/j.fertnstert.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Nawroth F, Isachenko V, Dessole S, Rahimi G, Farina M, Vargiu N, Mallmann P, Dattena M, Capobianco G, Peters D, Orth I, Isachenko E. Vitrification of human spermatozoa without cryoprotectants. Cryo-Letters. 2002;23(2):93–102. [PubMed] [Google Scholar]
- 20.Hallak J, Sharma RK, Wellstead C, Agarwal A. Cryopreservation of human spermatozoa: comparison of TEST-yolk buffer and glycerol. Int J Fertil Women’s Med. 2000;45(1):38–42. [PubMed] [Google Scholar]
- 21.Hsieh Y, Tsai H, Chang C, Lo H. Cryopreservation of human spermatozoa within human or mouse empty zona pellucidae. Fertil Steril. 2000;73(4):694–698. doi: 10.1016/s0015-0282(99)00612-3. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Zheng XZ, Baramki TA, Compton G, Yazigi RA, Katz E. Cryopreservation of a small number of fresh human testicular spermatozoa and testicular spermatozoa cultured in vitro for 3 days in an empty zona pellucida. J Androl. 2000;21(3):409–413. [PubMed] [Google Scholar]
- 23.Schuster TG, Keller LM, Dunn RL, Ohl DA, Smith GD. Ultra-rapid freezing of very low numbers of sperm using cryoloops. Hum Reprod. 2003;18(4):788–795. doi: 10.1093/humrep/deg162. [DOI] [PubMed] [Google Scholar]
- 24.Herrler A, Eisner S, Bach V, Weissenborn U, Beier HM. Cryopreservation of spermatozoa in alginic acid capsules. Fertil Steril. 2006;85(1):208–213. doi: 10.1016/j.fertnstert.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J, Garrisi GJ, Congedo-Ferrara TA, Kieck KA, Schimmel TW, Scott RT. Cryopreservation of single human spermatozoa. Hum Reprod. 1997;12(5):994–1001. doi: 10.1093/humrep/12.5.994. [DOI] [PubMed] [Google Scholar]
- 26.Montag M, Rink K, Dieckmann U, Delacretaz G, van der Ven H. Laser-assisted cryopreservation of single human spermatozoa in cell-free zona pellucida. Andrologia. 1999;31(1):49–53. doi: 10.1046/j.1439-0272.1999.00236.x. [DOI] [PubMed] [Google Scholar]
- 27.Isachenko V, Isachenko E, Katkov II, Montag M, Dessole S, Nawroth F, van der Ven H. Cryoprotectant-free cryopreservation of human spermatozoa by vitrification and freezing in vapor: effect on motility, DNA integrity, and fertilization ability. Biol Reprod. 2004;71(4):1167–1173. doi: 10.1095/biolreprod.104.028811. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Li L, Qian Y, Xu C, Zhu Y, Huang H, Jin F, Ye Y. Small-volume vitrification for human spermatozoa in the absence of cryoprotectants by using Cryotop. Andrologia. 2015;47(6):694–699. doi: 10.1111/and.12320. [DOI] [PubMed] [Google Scholar]
- 29.Walmsley R, Cohen J, Ferrara-Congedo T, Reing A, Garrisi J. The first births and ongoing pregnancies associated with sperm cryopreservation within evacuated egg zonae. Hum Reprod. 1998;13(Suppl 4):61–70. doi: 10.1093/humrep/13.suppl_4.61. [DOI] [PubMed] [Google Scholar]
- 30.Endo Y, Fujii Y, Kurotsuchi S, Motoyama H, Funahashi H. Successful delivery derived from vitrified-warmed spermatozoa from a patient with nonobstructive azoospermia. Fertil Steril. 2012;98(6):1423–1427. doi: 10.1016/j.fertnstert.2012.07.1128. [DOI] [PubMed] [Google Scholar]
- 31.Peng QP, Cao SF, Lyu QF, Xue SG, Jin W, Liu XY, Zhang WJ, Nielsen HI, Kuang YP. A novel method for cryopreservation of individual human spermatozoa. In Vitro Cellular Develop Biol Animal. 2011;47(8):565–572. doi: 10.1007/s11626-011-9428-1. [DOI] [PubMed] [Google Scholar]
- 32.Greco E, Litwicka K, Ferrero S, Baroni E, Sapienza F, Rienzi L, Romano S, Minasi MG, Tesarik J. GnRH antagonists in ovarian stimulation for ICSI with oocyte restriction: a matched, controlled study. Reprod BioMed Online. 2007;14(5):572–578. doi: 10.1016/s1472-6483(10)61048-6. [DOI] [PubMed] [Google Scholar]
- 33.Rashid MRZ, Ong FB, Omar MH, Ng SP, Nurshaireen A, Sharifah-Teh NSMN, et al. GnRH agonist and GnRH antagonist in intracytoplasmic injection cycles. Med J Malays. 2008;63(2):113–117. [PubMed] [Google Scholar]
- 34.Moez K, Meriem E, Fethi Z, Amel Z. Short vs long agonist protocols in poor responders undergoing IVF. La Tunisie Medicale. 2014;92(10):604. [PubMed] [Google Scholar]
- 35.Ochin H, Ma X, Wang L, Li X, Song J, Meng Y, Shen J, Cui YG, Liu J. Low dose clomiphene citrate as a mild stimulation protocol in women with unsuspected poor in vitro fertilization result can generate more oocytes with optimal cumulative pregnancy rate. J Ovarian Res. 2018;11(1):37. doi: 10.1186/s13048-018-0408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reyftmann L, Déchaud H, Loup V, Anahory T, Brunet-Joyeux C, Lacroix N, Hamamah S, Hédon B. Natural cycle in vitro fertilization cycle in poor responders. Gynecol Obstet Fertil. 2007;35(4):352–358. doi: 10.1016/j.gyobfe.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 37.Wikstrom AM, Raivio T, Hadziselimovic F, Wikstrom S, Tuuri T, Dunkel L. Klinefelter syndrome in adolescence: onset of puberty is associated with accelerated germ cell depletion. J Clin Endocrinol Metab. 2004;89(5):2263–2270. doi: 10.1210/jc.2003-031725. [DOI] [PubMed] [Google Scholar]
- 38.Gurbuz AS, Salvarci A, Ozcimen N, Zamani AG. Early morphokinetic monitoring of embryos after intracytoplasmic sperm injection with fresh ejaculate sperm in nonmosaic Klinefelter syndrome: a different presentation. Case Reports Genet. 2015;2015:827656–827654. doi: 10.1155/2015/827656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu FC, Bancroft J, Davidson DW, Nicol K. The behavioural effects of testosterone undecanoate in adult men with Klinefelter’s syndrome: a controlled study. Clin Endocrinol. 1982;16(5):489–497. doi: 10.1111/j.1365-2265.1982.tb02763.x. [DOI] [PubMed] [Google Scholar]
- 40.Cruger D, Toft B, Agerholm I, Fedder J, Hald F, Bruun-Petersen G. Birth of a healthy girl after ICSI with ejaculated spermatozoa from a man with non-mosaic Klinefelter’s syndrome. Hum Reprod. 2001;16(9):1909–1911. doi: 10.1093/humrep/16.9.1909. [DOI] [PubMed] [Google Scholar]
- 41.Kitamura M, Matsumiya K, Koga M, Nishimura K, Miura H, Tsuji T, et al. Ejaculated spermatozoa in patients with non-mosaic Klinefelter’s syndrome. Int J Urol. 2000;7(3):88–92. [PubMed] [Google Scholar]
- 42.Gies I, Unuane D, Velkeniers B, De Schepper J. Management of Klinefelter syndrome during transition. Eur J Endocrinol. 2014;171(2):R67–R77. doi: 10.1530/EJE-14-0213. [DOI] [PubMed] [Google Scholar]
- 43.Reubinoff BE, Abeliovich D, Werner M, Schenker JG, Safran A, Lewin A. A birth in non-mosaic Klinefelter’s syndrome after testicular fine needle aspiration, intracytoplasmic sperm injection and preimplantation genetic diagnosis. Hum Reprod. 1998;13(7):1887–1892. doi: 10.1093/humrep/13.7.1887. [DOI] [PubMed] [Google Scholar]
- 44.Tournaye H, Staessen C, Liebaers I, Van Assche E, Devroey P, Bonduelle M, et al. Testicular sperm recovery in nine 47. XXY Klinefelter patients. Hum Reprod. 1996;11(8):1644–1649. doi: 10.1093/oxfordjournals.humrep.a019462. [DOI] [PubMed] [Google Scholar]
- 45.Palermo GD, Schlegel PN, Sills ES, Veeck LL, Zaninovic N, Menendez S, Rosenwaks Z. Births after intracytoplasmic injection of sperm obtained by testicular extraction from men with nonmosaic Klinefelter’s syndrome. N Engl J Med. 1998;338(9):588–590. doi: 10.1056/NEJM199802263380905. [DOI] [PubMed] [Google Scholar]
- 46.Corona G, Pizzocaro A, Lanfranco F, Garolla A, Pelliccione F, Vignozzi L, Ferlin A, Foresta C, Jannini EA, Maggi M, Lenzi A, Pasquali D, Francavilla S, On behalf of the Klinefelter ItaliaN Group (KING) Sperm recovery and ICSI outcomes in Klinefelter syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2017;23(3):265–275. doi: 10.1093/humupd/dmx008. [DOI] [PubMed] [Google Scholar]
- 47.Paduch DA, Fine RG, Bolyakov A, Kiper J. New concepts in Klinefelter syndrome. Curr Opin Urol. 2008;18(6):621–627. doi: 10.1097/MOU.0b013e32831367c7. [DOI] [PubMed] [Google Scholar]
- 48.Franik S, Hoeijmakers Y, D’Hauwers K, Braat DD, Nelen WL, Smeets D, et al. Klinefelter syndrome and fertility: sperm preservation should not be offered to children with Klinefelter syndrome. Hum Reprod. 2016;31(9):dew179. doi: 10.1093/humrep/dew179. [DOI] [PubMed] [Google Scholar]
- 49.Gies I, Oates R, De Schepper J, Tournaye H. Testicular biopsy and cryopreservation for fertility preservation of prepubertal boys with Klinefelter syndrome: a pro/con debate. Fertil Steril. 2016;105(2):249–255. doi: 10.1016/j.fertnstert.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Rohayem J, Fricke R, Czeloth K, Mallidis C, Wistuba J, Krallmann C, Zitzmann M, Kliesch S. Age and markers of Leydig cell function, but not of Sertoli cell function predict the success of sperm retrieval in adolescents and adults with Klinefelter’s syndrome. Andrology. 2015;3(5):868–875. doi: 10.1111/andr.12067. [DOI] [PubMed] [Google Scholar]
- 51.Cissen M, Meijerink AM, D’Hauwers KW, Meissner A, Van DWN, Mochtar MH, et al. Prediction model for obtaining spermatozoa with testicular sperm extraction in men with non-obstructive azoospermia. Hum Reprod. 2016;31(9):1934. doi: 10.1093/humrep/dew147. [DOI] [PubMed] [Google Scholar]
- 52.Westlander G, Ekerhovd E, Granberg S, Hanson L, Hanson C, Bergh C. Testicular ultrasonography and extended chromosome analysis in men with nonmosaic Klinefelter syndrome: a prospective study of possible predictive factors for successful sperm recovery. Fertil Steril. 2001;75(6):1102–1105. doi: 10.1016/s0015-0282(01)01793-9. [DOI] [PubMed] [Google Scholar]
- 53.Bergère M, Wainer R, Nataf V, Bailly M, Gombault M, Ville Y, et al. Biopsied testis cells of four 47,XXY patients: fluorescence in-situ hybridization and ICSI results. Hum Reprod. 2002;17(1):32–37. doi: 10.1093/humrep/17.1.32. [DOI] [PubMed] [Google Scholar]
- 54.Sciurano RB, Hisano CV, Luna RMI, Olmedo S, Brugo VG, Rey CR, et al. Focal spermatogenesis originates in euploid germ cells in classical Klinefelter patients. Hum Reprod. 2009;24(9):2353–2360. doi: 10.1093/humrep/dep180. [DOI] [PubMed] [Google Scholar]
- 55.Yasuhisa Y, Nikolaos S, Yasuyuki M, Dimitrios L, Apostolos K, Ikuo M. Morphometric and cytogenetic characteristics of testicular germ cells and Sertoli cell secretory function in men with non-mosaic Klinefelter’s syndrome. Hum Reprod. 2002;17(4):886–896. doi: 10.1093/humrep/17.4.886. [DOI] [PubMed] [Google Scholar]
- 56.Miki T, Nagayoshi M, Takemoto Y. Genetic risk of Klinefelter’s syndrome in assisted reproductive technology. Reproduct Med Biol. 2017;16(2):188–195. doi: 10.1002/rmb2.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coerdt W, Rehder H, Gausmann I, Johannisson R, Gropp A. Quantitative Histology of Human Fetal Testes in Chromosomal Disease. Pediatr Pathol. 1985;3(2-4):245–259. doi: 10.3109/15513818509078785. [DOI] [PubMed] [Google Scholar]
- 58.Zondek LH, Zondek T. KLINEFELTER’S SYNDROME IN A FETUS. Lancet. 1974;304:347. doi: 10.1016/s0140-6736(74)91720-6. [DOI] [PubMed] [Google Scholar]
- 59.Kamischke A, Baumgardt A, Horst J, Nieschlag E. Clinical and diagnostic features of patients with suspected Klinefelter syndrome. J Androl. 2003;24(1):41–48. [PubMed] [Google Scholar]
- 60.Aksglaede L, Jorgensen N, Skakkebaek NE, Juul A. Low semen volume in 47 adolescents and adults with 47,XXY Klinefelter or 46,XX male syndrome. Int J Androl. 2009;32(4):376–384. doi: 10.1111/j.1365-2605.2008.00921.x. [DOI] [PubMed] [Google Scholar]
- 61.Nahata L, Yu RN, Paltiel HJ, Chow JS, Logvinenko T, Rosoklija I, et al. Sperm retrieval in adolescents and young adults with Klinefelter syndrome: a prospective, pilot study. J Pediatr. 2016;170:260–5.e2. doi: 10.1016/j.jpeds.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 62.Gies I, Schepper JD, Goossens E, Saen DV, Pennings G, Tournaye H. Spermatogonial stem cell preservation in boys with Klinefelter syndrome: to bank or not to bank, that’s the question. Fertil Steril. 2012;98(2):284–289. doi: 10.1016/j.fertnstert.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 63.Shinji K, Isao H, Yukari H, Akiko H, Hiroyuki K, Nobuyuki K, et al. Birth of healthy neonates after intracytoplasmic injection of ejaculated or testicular spermatozoa from men with nonmosaic Klinefelter’s syndrome: a report of 2 cases. J Reprod Med. 2004;49(2):126–130. [PubMed] [Google Scholar]
- 64.Bourne H, Stern K, Clarke G, Pertile M, Speirs A, Baker HW. Delivery of normal twins following the intracytoplasmic injection of spermatozoa from a patient with 47, XXY Klinefelter’s syndrome. Hum Reprod. 1997;12(11):2447–2450. doi: 10.1093/humrep/12.11.2447. [DOI] [PubMed] [Google Scholar]
- 65.Gérard T, Nelly F, Nicole MD, Anne Le D, Renato F, Michel V, et al. Reproductive genetic counselling in non-mosaic 47,XXY patients: implications for preimplantation or prenatal diagnosis: Case report and review. Hum Reprod. 2003;18(2):271–275. doi: 10.1093/humrep/deg070. [DOI] [PubMed] [Google Scholar]
- 66.Staessen C, Tournaye H, Assche EV, Michiels A, Landuyt LV, Devroey P, et al. PGD in 47,XXY Klinefelter’s syndrome patient. Hum Reprod Update. 2003;9(4):319–330. doi: 10.1093/humupd/dmg029. [DOI] [PubMed] [Google Scholar]
- 67.Sun J, Chen W, Zhou L, Hu J, Li Z, Zhang Z, Wu Y. Successful delivery derived from cryopreserved rare human spermatozoa with novel cryopiece. Andrology. 2017;5(4):832–837. doi: 10.1111/andr.12380. [DOI] [PubMed] [Google Scholar]
- 68.Liu F, Zou SS, Zhu Y, Sun C, Liu YF, Wang SS, Shi WB, Zhu JJ, Huang YH, Li Z. A novel micro-straw for cryopreservation of small number of human spermatozoon. Asian J Androl. 2017;19(3):326–329. doi: 10.4103/1008-682X.173452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laron Z, Dickerman Z, Zamir R, Galatzer A. Paternity in Klinefelter’s syndrome—a case report. Arch Androl. 1982;8(2):149–151. doi: 10.3109/01485018208987032. [DOI] [PubMed] [Google Scholar]
- 70.Terzoli G, Lalatta F, Lobbiani A, Simoni G, Colucci G. Fertility in a 47,XXY patient: assessment of biological paternity by deoxyribonucleic acid fingerprinting. Fertil Steril. 1992;58(4):821–822. doi: 10.1016/s0015-0282(16)55334-5. [DOI] [PubMed] [Google Scholar]
- 71.Lin YM, Huang WJ, Lin JSN, Kuo PL. Progressive depletion of germ cells in a man with nonmosaic Klinefelter’s syndrome: optimal time for sperm recovery. Urology. 2004;63(2):380–381. doi: 10.1016/j.urology.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 72.Fullerton G, Hamilton M, Maheshwari A. Should non-mosaic Klinefelter syndrome men be labelled as infertile in 2009? Hum Reprod. 2010;25(3):588–597. doi: 10.1093/humrep/dep431. [DOI] [PubMed] [Google Scholar]
- 73.Schlegel PN. Nonobstructive azoospermia: a revolutionary surgical approach and results. Semin Reprod Med. 2009;27(02):165–170. doi: 10.1055/s-0029-1202305. [DOI] [PubMed] [Google Scholar]
- 74.Madureira C, Cunha M, Sousa M, Neto AP, Pinho MJ, Viana P, Gonçalves A, Silva J, Teixeira da Silva J, Oliveira C, Ferraz L, Dória S, Carvalho F, Barros A. Treatment by testicular sperm extraction and intracytoplasmic sperm injection of 65 azoospermic patients with non-mosaic Klinefelter syndrome with birth of 17 healthy children. Andrology. 2014;2(4):623–631. doi: 10.1111/j.2047-2927.2014.00231.x. [DOI] [PubMed] [Google Scholar]
- 75.Fayomi AP, Peters K, Sukhwani M, Valli-Pulaski H. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science. 2019;363(6433):1314–1319. doi: 10.1126/science.aav2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Christiansen P, Andersson AM, Skakkebaek NE. Longitudinal studies of inhibin B levels in boys and young adults with Klinefelter syndrome. J Clin Endocrinol Metab. 2003;88(2):888–891. doi: 10.1210/jc.2002-021379. [DOI] [PubMed] [Google Scholar]
- 77.Wikstrom AM, Painter JN, Raivio T, Aittomaki K, Dunkel L. Genetic features of the X chromosome affect pubertal development and testicular degeneration in adolescent boys with Klinefelter syndrome. Clin Endocrinol. 2010;65(1):92–97. doi: 10.1111/j.1365-2265.2006.02554.x. [DOI] [PubMed] [Google Scholar]
- 78.María Gabriela B, Rey RA, Ignacio B, Patricia B, Luz A, Graciela DR, et al. Establishment of testicular endocrine function impairment during childhood and puberty in boys with Klinefelter syndrome. Clin Endocrinol. 2010;67(6):863–870. doi: 10.1111/j.1365-2265.2007.02977.x. [DOI] [PubMed] [Google Scholar]
- 79.Zeger MPD, Zinn AR, Lahlou N, Ramos P, Kowal K, Samango-Sprouse C, Ross JL. Effect of ascertainment and genetic features on the phenotype of Klinefelter syndrome. J Pediatr. 2008;152(5):716–722. doi: 10.1016/j.jpeds.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gies I, De Schepper J, Van Saen D, Anckaert E, Goossens E, Tournaye H. Failure of a combined clinical- and hormonal-based strategy to detect early spermatogenesis and retrieve spermatogonial stem cells in 47,XXY boys by single testicular biopsy. Hum Reprod. 2012;27(4):998–1004. doi: 10.1093/humrep/des002. [DOI] [PubMed] [Google Scholar]
- 81.Saen D, Van Gies I, Schepper J, De Tournaye H, Goossens E. Can pubertal boys with Klinefelter syndrome benefit from spermatogonial stem cell banking? Hum Reprod. 2012;27(2):323–330. doi: 10.1093/humrep/der425. [DOI] [PubMed] [Google Scholar]
- 82.Van SD, Vloeberghs V, Gies I, Mateizel I, Sermon K, De SJ, et al. When does germ cell loss and fibrosis occur in patients with Klinefelter syndrome? Hum Reprod. 2018;33(6):1009–1022. doi: 10.1093/humrep/dey094. [DOI] [PubMed] [Google Scholar]
- 83.Vicdan K, Akarsu C, Sozen E, Buluc B, Vicdan A, Yilmaz Y, et al. Outcome of intracytoplasmic sperm injection using fresh and cryopreserved-thawed testicular spermatozoa in 83 azoospermic men with Klinefelter syndrome. J Obstet Gynaecol Res. 2016;42(11):1558–1566. doi: 10.1111/jog.13090. [DOI] [PubMed] [Google Scholar]
- 84.Kvist K, Thorup J, Byskov AG, Hoyer PE, Mollgard K, Yding Andersen C. Cryopreservation of intact testicular tissue from boys with cryptorchidism. Hum Reprod. 2006;21(2):484–491. doi: 10.1093/humrep/dei331. [DOI] [PubMed] [Google Scholar]
- 85.Picton HM, Wyns C, Anderson RA, Goossens E, Jahnukainen K, Kliesch S, Mitchell RT, Pennings G, Rives N, Tournaye H, van Pelt AMM, Eichenlaub-Ritter U, Schlatt S. A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod. 2015;30(11):2463–2475. doi: 10.1093/humrep/dev190. [DOI] [PubMed] [Google Scholar]
- 86.Baert Y, Braye A, Struijk RB, Pelt AMMV, Goossens E. Cryopreservation of testicular tissue before long-term testicular cell culture does not alter invitro cell dynamics. Fertil Steril. 2015;104(5):1244–52.e4. doi: 10.1016/j.fertnstert.2015.07.1134. [DOI] [PubMed] [Google Scholar]
- 87.Sá R, Cremades N, Malheiro I, Sousa M. Cryopreservation of human testicular diploid germ cell suspensions. Andrologia. 2012;44(6):366–372. doi: 10.1111/j.1439-0272.2012.01290.x. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka A, Tanaka I, Nagayoshi M, Awata S, Himeno N, Kusunoki H. V-10: In vitro culture of the immature spermatogenic cells. Fertil Steril. 2006;86(3):S518–S5S9. [Google Scholar]
- 89.Gianaroli L, Selman HA, Magli MC, Colpi G, Fortini D, Ferraretti AP. Birth of a healthy infant after conception with round spermatids isolated from cryopreserved testicular tissue. Fertil Steril. 1999;72(3):539–541. doi: 10.1016/s0015-0282(99)00285-x. [DOI] [PubMed] [Google Scholar]
- 90.Ulug U, Bener F, Akman MA, Bahceci M. Partners of men with Klinefelter syndrome can benefit from assisted reproductive technologies. Fertil Steril. 2003;80(4):903–906. doi: 10.1016/s0015-0282(03)01157-9. [DOI] [PubMed] [Google Scholar]
- 91.Tanaka A, Nagayoshi M, Takemoto Y, Tanaka I, Kusunoki H, Watanabe S, Kuroda K, Takeda S, Ito M, Yanagimachi R. Fourteen babies born after round spermatid injection into human oocytes. Proc Natl Acad Sci U S A. 2015;112(47):14629–14634. doi: 10.1073/pnas.1517466112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tanaka A, Suzuki K, Nagayoshi M, Tanaka A, Takemoto Y, Watanabe S, Takeda S, Irahara M, Kuji N, Yamagata Z, Yanagimachi R. Ninety babies born after round spermatid injection into oocytes: survey of their development from fertilization to 2 years of age. Fertil Steril. 2018;110(3):443–451. doi: 10.1016/j.fertnstert.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 93.Onofre J, Baert Y, Faes K, Goossens E. Cryopreservation of testicular tissue or testicular cell suspensions: a pivotal step in fertility preservation. Hum Reprod Update. 2016;22(6):744–761. doi: 10.1093/humupd/dmw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Friedler S, Raziel A, Strassburger D, Schachter M, Bern O, Ronel R. Outcome of ICSI using fresh and cryopreserved–thawed testicular spermatozoa in patients with non-mosaic Klinefelter’s syndrome. Hum Reprod. 2001;16(12):2616–2620. doi: 10.1093/humrep/16.12.2616. [DOI] [PubMed] [Google Scholar]
- 95.Okada H, Goda K, Muto S, Maruyama O, Koshida M, Horie S. Four pregnancies in nonmosaic Klinefelter’s syndrome using cryopreserved-thawed testicular spermatozoa. Fertil Steril. 2005;84(5):1508.e13–1508.e16. doi: 10.1016/j.fertnstert.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 96.Kyono K, Uto H, Nakajo Y, Kumagai S, Araki Y, Kanto S. Seven pregnancies and deliveries from non-mosaic Klinefelter syndrome patients using fresh and frozen testicular sperm. J Assist Reprod Genet. 2007;24(1):47–51. doi: 10.1007/s10815-006-9079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fusi F, Calzi F, Rabellotti E, Papaleo E, Gonfiantini C, Bonzi V, et al. Fertilizing capability of frozen-thawed spermatozoa recovered from microsurgical epidydimal sperm aspiration and cryopreserved in oocyte-free human zona pellucida. Hum Reprod. 2001;16:117. [Google Scholar]
- 98.Levi-Setti PE, Albani E, Negri L, Cesana A, Novara P, Bianchi S. Cryopreservation of a small number of spermatozoa in yolk-filled human zonae pellucidae. Arch Ital Urol Androl. 2003;75(4):195–198. [PubMed] [Google Scholar]
- 99.Alexander J, Irmhild G, Martina WB, Johann L, Andreas O, Heinz S. Novel method for the cryopreservation of testicular sperm and ejaculated spermatozoa from patients with severe oligospermia: A pilot study. Fertil Steril. 2004;82(2):445–447. doi: 10.1016/j.fertnstert.2003.12.050. [DOI] [PubMed] [Google Scholar]
- 100.Lecomte PJ, Barthelemy C, Nduwayo L, Hamamah S. Necrospermia: etiology and management. 1999.
- 101.Ortega C, Verheyen G, Raick D, Camus M, Devroey P, Tournaye H. Absolute asthenozoospermia and ICSI: what are the options? Hum Reprod Update. 2011;17(5):684–692. doi: 10.1093/humupd/dmr018. [DOI] [PubMed] [Google Scholar]
- 102.Nordhoff V. How to select immotile but viable spermatozoa on the day of intracytoplasmic sperm injection? An embryologist's view. Andrology. 2015;3(2):156–162. doi: 10.1111/andr.286. [DOI] [PubMed] [Google Scholar]
- 103.Esteves SC, Varghese AC. Laboratory handling of epididymal and testicular spermatozoa: what can be done to improve sperm injections outcome. J Human Reproduct Sci. 2012;5(3):233–243. doi: 10.4103/0974-1208.106333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sallam H, Farrag A, Agameya A, Ezzeldin F, Eid A, Sallam A. The use of a modified hypo-osmotic swelling test for the selection of viable ejaculated and testicular immotile spermatozoa in ICSI. Hum Reprod. 2001;16(2):272–276. doi: 10.1093/humrep/16.2.272. [DOI] [PubMed] [Google Scholar]
- 105.Sallam HN, Ashraf F, Abdel-Fattah A, Yehia EG, Fathy E. The use of the modified hypo-osmotic swelling test for the selection of immotile testicular spermatozoa in patients treated with ICSI: a randomized controlled study. Hum Reprod. 2005;20(12):3435–3440. doi: 10.1093/humrep/dei249. [DOI] [PubMed] [Google Scholar]
- 106.Westlander G, Barry M, Petrucco O, Norman R. Different fertilization rates between immotile testicular spermatozoa and immotile ejaculated spermatozoa for ICSI in men with Kartagener’s syndrome: case reports. Hum Reprod. 2003;18(6):1286–1288. doi: 10.1093/humrep/deg240. [DOI] [PubMed] [Google Scholar]
- 107.Kordus RJ, Price RL, Davis JM, Whitman-Elia GF. Successful twin birth following blastocyst culture of embryos derived from the immotile ejaculated spermatozoa from a patient with primary ciliary dyskinesia: a case report. J Assist Reprod Genet. 2008;25(9-10):437–443. doi: 10.1007/s10815-008-9254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Geber S, Lemgruber M, Taitson PF, Valle M, Sampaio M. Birth of healthy twins after intracytoplasmic sperm injection using ejaculated immotile spermatozoa from a patient with Kartagener’s syndrome. Andrologia. 2012;44(s1):842–844. doi: 10.1111/j.1439-0272.2011.01224.x. [DOI] [PubMed] [Google Scholar]
- 109.Hossain A, Osuamkpe C, Hossain S, Phelps JY. Spontaneously developed tail swellings (SDTS) influence the accuracy of the hypo-osmotic swelling test (HOS-test) in determining membrane integrity and viability of human spermatozoa. J Assist Reprod Genet. 2010;27(2-3):83–86. doi: 10.1007/s10815-009-9375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Griveau JF, Lobel B, Laurent MC, Michardière L, Lannou DL. Interest of pentoxifylline in ICSI with frozen–thawed testicular spermatozoa from patients with non-obstructive azoospermia. Reprod BioMed Online. 2006;12(1):14–18. doi: 10.1016/s1472-6483(10)60974-1. [DOI] [PubMed] [Google Scholar]
- 111.Vijay M, Ranjana M, Sucheta D, Kavita S, Sadhana D. Selection of viable spermatozoa from testicular biopsies: a comparative study between pentoxifylline and hypoosmotic swelling test. Fertil Steril. 2011;95(2):631–634. doi: 10.1016/j.fertnstert.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 112.Kovacic B, Vlaisavljevic V, Reljic M. Clinical use of pentoxifylline for activation of immotile testicular sperm before ICSI in patients with azoospermia. J Androl. 2006;27(1):45–52. doi: 10.2164/jandrol.05079. [DOI] [PubMed] [Google Scholar]
- 113.Terriou P, Hans E, Giorgetti C, Spach JL, Salzmann J, Urrutia V, Roulier R. Pentoxifylline initiates motility in spontaneously immotile epididymal and testicular spermatozoa and allows normal fertilization, pregnancy, and birth after intracytoplasmic sperm injection. J Assist Reprod Genet. 2000;17(4):194–199. doi: 10.1023/A:1009435732258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Unsal E, Turan V, Aktuna S, Hurdag C, Bereketoglu G, Canillioglu Y, Baltacı A, Ozcan S, Karayalcin R, Batırbaygil H, Baltacı V. Effects of pentoxifylline and platelet activating factor on sperm DNA damage. Eur J Obstet Gynecol Reprod Biol. 2016;197:125–129. doi: 10.1016/j.ejogrb.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 115.Ain R, Seshagiri PB. Micromolar concentration of pentoxifylline improves development in vitro of hamster 8-cell embryos: confirmation of biological viability by embryo transfer. Reprod Fertil Dev. 1997;9(7):697. doi: 10.1071/r97052. [DOI] [PubMed] [Google Scholar]
- 116.Aktan TM, Montag M, Duman S, Gorkemli H, Rink K, Yurdakul T. Use of a laser to detect viable but immotile spermatozoa. Andrologia. 2004;36(6):366–369. doi: 10.1111/j.1439-0272.2004.00636.x. [DOI] [PubMed] [Google Scholar]
- 117.Nordhoff V, Schuring AN, Krallmann C, Zitzmann M, Schlatt S, Kiesel L, et al. Optimizing TESE-ICSI by laser-assisted selection of immotile spermatozoa and polarization microscopy for selection of oocytes. Andrology. 2013;1(1):67–74. doi: 10.1111/j.2047-2927.2012.00020.x. [DOI] [PubMed] [Google Scholar]
- 118.Ogura A, Matsuda J, Yanagimachi R. Birth of normal young after electrofusion of mouse oocytes with round spermatids. Proc Natl Acad Sci U S A. 1994;91(16):7460–7462. doi: 10.1073/pnas.91.16.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ogura A, Matsuda J, Asano T, Suzuki O, Yanagimachi R. Mouse oocytes injected with cryopreserved round spermatids can develop into normal offspring. J Assist Reprod Genet. 1996;13(5):431–434. doi: 10.1007/BF02066177. [DOI] [PubMed] [Google Scholar]
- 120.Ogonuki N, Tsuchiya H, Hirose Y, Okada H, Ogura A, Sankai T. Pregnancy by the tubal transfer of embryos developed after injection of round spermatids into oocyte cytoplasm of the cynomolgus monkey (Macaca fascicularis) Hum Reprod. 2003;18(6):1273–1280. doi: 10.1093/humrep/deg212. [DOI] [PubMed] [Google Scholar]
- 121.Ozil JP, Banrezes B, Toth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol. 2006;300(2):534–544. doi: 10.1016/j.ydbio.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 122.Sugaya S. Pregnancy following calcium ionophore oocyte activation in an oligozoospermia patient with repeated failure of fertilization after ICSI. Clin Exp Obstet Gynecol. 2010;37(4):261–262. [PubMed] [Google Scholar]
- 123.Nikiforaki D, Vanden Meerschaut F, de Roo C, Lu Y, Ferrer-Buitrago M, de Sutter P, et al. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil Steril. 2016;105(3):798–806. doi: 10.1016/j.fertnstert.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 124.Chen J, Qian Y, Tan Y, Mima H. Successful pregnancy following oocyte activation by strontium in normozoospermic patients of unexplained infertility with fertilisation failures during previous intracytoplasmic sperm injection treatment. Reprod Fertil Dev. 2010;22(5):852–855. doi: 10.1071/RD09268. [DOI] [PubMed] [Google Scholar]
- 125.Kim JW, Kim SD, Yang SH, Yoon SH, Jung JH, Lim JH. Successful pregnancy after SrCl2 oocyte activation in couples with repeated low fertilization rates following calcium ionophore treatment. Syst Biol Reprod Med. 2014;60(3):177–182. doi: 10.3109/19396368.2014.900832. [DOI] [PubMed] [Google Scholar]
- 126.Liu Y, Cao YX, Zhang ZG, Xing Q. Artificial oocyte activation and human failed-matured oocyte vitrification followed by in vitro maturation. Zygote. 2013;21(1):71–76. doi: 10.1017/S0967199411000530. [DOI] [PubMed] [Google Scholar]
- 127.Baltaci V, Ayvaz OU, Unsal E, Aktas Y, Baltaci A, Turhan F, et al. The effectiveness of intracytoplasmic sperm injection combined with piezoelectric stimulation in infertile couples with total fertilization failure. Fertil Steril. 2010;94(3):900–904. doi: 10.1016/j.fertnstert.2009.03.107. [DOI] [PubMed] [Google Scholar]
- 128.Mansour R, Fahmy I, Tawab NA, Kamal A, El-Demery Y, Aboulghar M, et al. Electrical activation of oocytes after intracytoplasmic sperm injection: a controlled randomized study. Fertil Steril. 2009;91(1):133–139. doi: 10.1016/j.fertnstert.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 129.Koo OJ, Jang G, Kwon DK, Kang JT, Kwon OS, Park HJ, Kang SK, Lee BC. Electrical activation induces reactive oxygen species in porcine embryos. Theriogenology. 2008;70(7):1111–1118. doi: 10.1016/j.theriogenology.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 130.Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350(9076):485–487. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- 131.Bischoff FZ, Hahn S, Johnson KL, Simpson JL, Bianchi DW, Lewis DE, Weber WD, Klinger K, Elias S, Jackson LG, Evans MI, Holzgreve W, de la Cruz F. Intact fetal cells in maternal plasma: are they really there? Lancet. 2003;361(9352):139–140. doi: 10.1016/S0140-6736(03)12191-5. [DOI] [PubMed] [Google Scholar]
- 132.Lo YM, Hjelm NM, Fidler C, Sargent IL, Murphy MF, Chamberlain PF, et al. Prenatal diagnosis of fetal RhD status by molecular analysis of maternal plasma. N Engl J Med. 1998;339(24):1734–1738. doi: 10.1056/NEJM199812103392402. [DOI] [PubMed] [Google Scholar]
- 133.Lo YM, Patel P, Sampietro M, Gillmer MD, Fleming KA, Wainscoat JS. Detection of single-copy fetal DNA sequence from maternal blood. Lancet. 1990;335(8703):1463–1464. doi: 10.1016/0140-6736(90)91491-r. [DOI] [PubMed] [Google Scholar]
- 134.Lo YD, Chiu RW. Non-invasive prenatal diagnosis of Down’s syndrome. Lancet. 2007;369(9578):1997. doi: 10.1016/S0140-6736(07)60935-0. [DOI] [PubMed] [Google Scholar]
- 135.Hulten M. Non-invasive prenatal diagnosis of Down’s syndrome. Lancet. 2001;357(9260):963–964. doi: 10.1016/S0140-6736(05)71670-6. [DOI] [PubMed] [Google Scholar]
- 136.Kou KO, Poon CF, Kwok SL, Chan KY, Tang MH, Kan AS, et al. Effect of non-invasive prenatal testing as a contingent approach on the indications for invasive prenatal diagnosis and prenatal detection rate of Down’s syndrome. Hong Kong Med J. 2016;22(3):223–230. doi: 10.12809/hkmj154730. [DOI] [PubMed] [Google Scholar]
- 137.Perez-Carbajo E, Zapardiel I, Sanfrutos-Llorente L, Cruz-Melguizo S, Martinez-Payo C, Iglesias-Goy E. Prenatal diagnosis of concurrent achondroplasia and klinefelter syndrome. Case Rep Obstet Gynecol. 2015;2015:980749–980742. doi: 10.1155/2015/980749. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 138.Mansfield C, Hopfer S, Marteau TM. Termination rates after prenatal diagnosis of Down syndrome, spina bifida, anencephaly, and Turner and Klinefelter syndromes: a systematic literature review. European Concerted Action: DADA (Decision-making After the Diagnosis of a fetal Abnormality) Prenat Diagn. 1999;19(9):808–812. [PubMed] [Google Scholar]
- 139.Pimpolari L, Liberati N, Martini M, Colloridi F, Radicioni A, Duse M, Tarani L. Prenatal genetic counseling in Klinefelter syndrome: comments on the article by Lalatta et al. [2013] and a proposal of a new approach. Am J Med Genet A. 2015;167A(2):450–454. doi: 10.1002/ajmg.a.36875. [DOI] [PubMed] [Google Scholar]
- 140.Handyside AH, Kontogianni EH, Hardy K, Winston RM. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature. 1990;344(6268):768–770. doi: 10.1038/344768a0. [DOI] [PubMed] [Google Scholar]
- 141.el-Hashemite N, Wells D, Delhanty JD. Preimplantation genetic diagnosis of beta-thalassaemia. Lancet. 1996;348(9027):620–621. doi: 10.1016/s0140-6736(05)64841-6. [DOI] [PubMed] [Google Scholar]
- 142.Sermon K, Van Steirteghem A, Liebaers I. Preimplantation genetic diagnosis. Lancet. 2004;363(9421):1633–1641. doi: 10.1016/S0140-6736(04)16209-0. [DOI] [PubMed] [Google Scholar]
- 143.Handyside AH, Pattinson JK, Penketh RJ, Delhanty JD, Winston RM, Tuddenham EG. Biopsy of human preimplantation embryos and sexing by DNA amplification. Lancet. 1989;1(8634):347–349. doi: 10.1016/s0140-6736(89)91723-6. [DOI] [PubMed] [Google Scholar]
- 144.Schoolcraft WB, Fragouli E, Stevens J, Munne S, Katz-Jaffe MG, Wells D. Clinical application of comprehensive chromosomal screening at the blastocyst stage. Fertil Steril. 2010;94(5):1700–1706. doi: 10.1016/j.fertnstert.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 145.De Vos A, Staessen C, De Rycke M, Verpoest W, Haentjens P, Devroey P, et al. Impact of cleavage-stage embryo biopsy in view of PGD on human blastocyst implantation: a prospective cohort of single embryo transfers. Hum Reprod. 2009;24(12):2988–2996. doi: 10.1093/humrep/dep251. [DOI] [PubMed] [Google Scholar]
- 146.Gleicher N, Vidali A, Braverman J, Kushnir VA, Barad DH, Hudson C, et al. Accuracy of preimplantation genetic screening (PGS) is compromised by degree of mosaicism of human embryos. Reprod Biol Endocrinol. 2016;14(1):54. doi: 10.1186/s12958-016-0193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Palini S, Galluzzi L, De Stefani S, Bianchi M, Wells D, Magnani M, et al. Genomic DNA in human blastocoele fluid. Reprod BioMed Online. 2013;26(6):603–610. doi: 10.1016/j.rbmo.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 148.Wu H, Ding C, Shen X, Wang J, Li R, Cai B, Xu Y, Zhong Y, Zhou C. Medium-based noninvasive preimplantation genetic diagnosis for human alpha-thalassemias-SEA. Medicine. 2015;94(12):e669. doi: 10.1097/MD.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang L, Lv Q, Chen W, Sun J, Wu Y, Wang Y, et al. resence of embryonic DNA in culture medium. Oncotarget. 2017;8(40):67805–67809. doi: 10.18632/oncotarget.18852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu J, Fang R, Chen L, Chen D, Xiao JP, Yang W, Wang H, Song X, Ma T, Bo S, Shi C, Ren J, Huang L, Cai LY, Yao B, Xie XS, Lu S. Noninvasive chromosome screening of human embryos by genome sequencing of embryo culture medium for in vitro fertilization. Proc Natl Acad Sci U S A. 2016;113(42):11907–11912. doi: 10.1073/pnas.1613294113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu W, Zhang H, Hu D, Lu S, Sun X. The performance of MALBAC and MDA methods in the identification of concurrent mutations and aneuploidy screening to diagnose beta-thalassaemia disorders at the single- and multiple-cell levels. J Clin Lab Anal. 2018;32(2). 10.1002/jcla.22267. [DOI] [PMC free article] [PubMed]
- 152.Li W, Ma Y, Yu S, Sun N, Wang L, Chen D, Yang G, Lu S, Li Y, Yang B, Mei C. The mutation-free embryo for in vitro fertilization selected by MALBAC-PGD resulted in a healthy live birth from a family carrying PKD 1 mutation. J Assist Reprod Genet. 2017;34(12):1653–1658. doi: 10.1007/s10815-017-1018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chiang CM, Lin CJ, Lee LM, Chen SM. Outcome of Intracytoplasmic Injection of Sperm Obtained by Testicular Sperm Extraction from 14 Azoospermic Men Suffering from 47,XXY Non-mosaic Klinefelter's Syndrome. Taiwanese J Obstet Gynecol. 2004;43:88–96. 10.1016/S1028-4559(09)60062-0.
- 154.Schiff JD, Palermo GD, Veeck LL, Marc G, Zev R, Schlegel PN. Success of testicular sperm extraction [corrected] and intracytoplasmic sperm injection in men with Klinefelter syndrome. J Clin Endocrinol Metab. 2005;90(11):6263–7. [DOI] [PubMed]
- 155.Koga M, Tsujimura A, Takeyama M, Kiuchi H, Takao T, Miyagawa Y, et al. Clinical Comparison of Successful and Failed Microdissection Testicular Sperm Extraction in Patients with Nonmosaic Klinefelter Syndrome. Urology. 2007;70(2):341–5. [DOI] [PubMed]
- 156.Ferhi K, Avakian R, Griveau JF, Guille F. Age as only predictive factor for successful sperm recovery in patients with Klinefelter's syndrome. Andrologia. 2009;41(2):84–7. 10.1111/j.1439-0272.2008.00875.x. [DOI] [PubMed]
- 157.Yarali H, Polat M, Bozdag G, Gunel M, Alpas I, Esinler I, et al. TESE-ICSI in patients with non-mosaic Klinefelter syndrome: a comparative study. Reprod BioMed Online. 2009;18(6):756–60. [DOI] [PubMed]
- 158.Bakircioglu ME, Ulug U, Erden HF, Tosun S, Bayram A, Ciray N, et al. Klinefelter syndrome: does it confer a bad prognosis in treatment of nonobstructive azoospermia? Fertil Steril. 2011;95(5):1696–9. 10.1016/j.fertnstert.2011.01.005. [DOI] [PubMed]
- 159.Rives N, Milazzo JP, Perdrix A, Castanet M, Joly-Helas G, Sibert L, et al. The feasibility of fertility preservation in adolescents with Klinefelter syndrome. Hum Reprod. 2013;28(6):1468–79. 10.1093/humrep/det084. [DOI] [PubMed]
- 160.Greco E, Scarselli F, Minasi MG, Casciani V, Zavaglia D, Dente D, et al. Birth of 16 healthy children after ICSI in cases of nonmosaic Klinefelter syndrome. Hum Reprod 2013;28(5):1155–60. [DOI] [PubMed]
- 161.Sabbaghian M, Modarresi T, Hosseinifar H, Hosseini J, Farrahi F, Dadkhah F, et al. Comparison of Sperm Retrieval and Intracytoplasmic Sperm Injection Outcome in Patients With and Without Klinefelter Syndrome. Urology. 2014;83(1):107–10. 10.1016/j.urology.2013.09.021. [DOI] [PubMed]
- 162.Plotton I, d'Estaing SG, Cuzin B, Brosse A, Benchaib M, Lornage J, et al. Preliminary Results of a Prospective Study of Testicular Sperm Extraction in Young Versus Adult Patients With Nonmosaic 47, XXY Klinefelter Syndrome. J Clin Endocrin Metab. 2015;100(3):961–7. 10.1210/jc.2014-3083. [DOI] [PubMed]
- 163.Majzoub A, Arafa M, Al Said S, Agarwal A, Seif A, Al Naimi A, et al. Outcome of testicular sperm extraction in nonmosaic Klinefelter syndrome patients: what is the best approach? Andrologia. 2016;48(2):171–6. 10.1111/and.12428. [DOI] [PubMed]
- 164.Chihara M, Ogi K, Ishiguro T, Yoshida K, Godo C, Takakuwa K, et al. Microdissection testicular sperm extraction in five Japanese patients with nonmosaic Klinefelter's syndrome. Reproduc Med Biol. 2018;17(2):209–16. [DOI] [PMC free article] [PubMed]
- 165.Ozer C, Caglar AP, Goren MR, Toksoz S, Gul U, Turunc T. Sperm retrieval by microdissection testicular sperm extraction and intracytoplasmic sperm injection outcomes in nonobstructive azoospermic patients with Klinefelter syndrome. Andrologia. 2018;50(3):e12983. [DOI] [PubMed]