Abstract
Lemon myrtle leaves were extracted with ethanol at different temperatures (25, 50, and 80 °C) and times (2, 4, 6, and 10 h) to examine the effect of extraction conditions on total polyphenol contents (TPC), total flavonoid contents (TFC), their antioxidant, anti-inflammatory activities, and amount of phenolic compounds. Under optimal extraction conditions (80 °C and 6 h), the values were 23.37%, 102.72 mg gallic acid equivalents (GAE/g dry basis), 23.37 mg rutin equivalents (RE/g dry basis), 83.31%, 60.13%, and 1.10% for yield, TPC, TFC, DPPH, ABTS radical scavenging activity, and reducing power, respectively. In addition, total amount of the phenolic compounds of extract was determined as 43.9 μg/g. The anti-inflammatory effect was determined in lipopolysaccharide-stimulated RAW 264.7 cells and inhibited the production of inflammatory mediators such as nitric oxide (NO). These results indicate that extracts of lemon myrtle leaves have potential as a valuable natural product with antioxidant and anti-inflammatory.
Keywords: Lemon myrtle (Backhousia citriodora), Antioxidant activity, Anti-inflammatory activity, Nitric oxide, Antioxidant phenolic compound
Introduction
Oxidative stress, defined as the formation of reactive oxygen species (ROS) or free radicals and the imbalance in antioxidant levels, is known that associated with various diseases, such as degenerative nervous system diseases, aging, and diabetes (Bjelakovic et al., 2014; Reuter et al., 2010). Persistent excessive oxidative stress on cells not only increases gene expression of specific cells that cause degenerative diseases, but also increases cell death, leading to chronic inflammatory responses (Kang, 2011). Oxidative stress increased by ROS induces inflammatory and immune responses and increases proteins such as Cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) (Han et al., 2015). Inflammatory factors are expressed by intracellular signaling pathways and transcription factors and occur due to primary stimuli such as lipopolysaccharides (LPS) (Kim et al., 2008). LPS, which present in the outer membrane of gram negative E. coli, stimulates macrophages to increase inflammatory cytokine production such as interleukin, tumor necrosis factor (TNF)-α, nitric oxide (NO) (Akihisa et al., 1959). NO as an indicator of inflammatory response has a variety of physiological functions such as vasodilation, signal transduction and body defense, but they enhance inflammation when overproduced, causing septic shock, tissue damage, genetic variation, and nerve damage (Bogdan, 2001). Synthetic anti-inflammatory agents are used as inhibitors of inflammatory mediators, but there is increasing interest in natural antioxidants and natural anti-inflammatory agents due to concerns about the side effects caused by synthetic anti-inflammatory agents (Buchaillot et al., 2009; Hyon et al., 2010).
The lemon myrtle oil has been effective in the treatment of skin lesions caused by mollusk disease virus (Burke et al., 2004). Similarly, Wilkinson et al. (2003) reported that the lemon myrtle oil has a potential as a plant fungicide, surface disinfectant or antimicrobial food additive. Studies on lemon myrtle have reported about cytotoxic effects due to presence of essential oils (Hayes and Markovic, 2002), antimicrobial and antifungal activity (Wilkinson et al., 2003), and active components (Konczak et al., 2010). Lemon myrtle leaf has high levels of antioxidant activity which is associated with its phenolic compounds contents including phenolic acids and flavonoids (Kim et al., 2017). However, antioxidant and anti-inflammatory effects of lemon myrtle leaf has been reported rarely.
Therefore, this study was conducted to determine the optimal ethanol extraction conditions for bioactive compounds (polyphenols and flavonoids) in lemon myrtle leaf by evaluating the extraction yield, antioxidant and anti-inflammatory activity at different extraction conditions. In addition, the phenolic compounds extracted from lemon myrtle leaf were identified and quantified using high-performance liquid chromatography with diode array detector (HPLC-DAD).
Materials and methods
Materials and chemicals
Australian lemon myrtle leaves were purchased from Teazen Co. (Anyang, Korea) Folin-Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonate) (ABTS), gallic acid, ellagic acid, luteoloside, rutin, catechin, and quercetin were purchased from Sigma Chemical Co. (St. Louis, MO, USA). RAW 264.7 cells for anti-inflammatory experiments were obtained from the Korean Cell Line Bank (Seoul, Korea). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), lipopolysaccharide (LPS), and other supplements for cell culture were purchased from Gibco (Thermo Fisher Scientific, Waltham, USA). Other reagents and solvents in this study used analytical reagent grade.
Extraction of lemon myrtle leaves
Dried lemon myrtle leaves were pulverized with a coffee grinder (Hamilton Beach Inc., Glenalan, VA, USA), and distilled water and 80% ethanol were added 10 times compared to solid and extracted using a reflux condenser according to the extraction conditions of temperature (25, 50, and 80 °C) and times (2, 4, 6, and 10 h). Then the extracts were filtered (Whatman No. 1), concentrated at 55 °C with rotary evaporator (UT-1000, EYELA, Tokyo, Japan), freeze-dried and stored at − 20 °C for future experiments.
Yields, total polyphenol and flavonoid content
Each extract was freeze-dried to obtain a dry weight and then extracted as a percentage of the dry weight of the raw materials used to prepare the extract to obtain an extraction yield. Total polyphenol content (TPC) was measured by modified the AOCS method (AOCS, 1990). Folin-Ciocalteu reagent (10% (v/v), 0.75 mL) and the same volume of 6% sodium carbonate solution (w/v) were added to 0.2 mL of diluted crude extract (1 mg/mL). The mixture was kept in the dark for 90 min at room temperature; afterwards, the absorbance was measured at 725 nm. TPC was expressed as mg of gallic acid equivalents per g of dry sample (mg GAE/g).
Total flavonoid content (TFC) was measured by the method of Zhishen et al. (1999). TFC was expressed as milligrams of rutin equivalents per g of dry sample (mg RE/g).
Antioxidant activity
DPPH and ABTS radical scavenging activity
DPPH radical scavenging activity was measured by modifying the method of Blosis (1958). Briefly, 100 μL of diluted sample (1 mg/mL) was added to 1 mL of 0.2 mM DPPH radical solution and incubated for 30 min at room temperature. Absorbance was measured at 525 nm with ascorbic acid as a control. DPPH radical scavenging activity of sample was calculated using the following equation,
ABTS radical scavenging activity was measured by modifying the method of Re et al. (1999). ABTS stock solution was diluted with 5.0 mM phosphate-buffered saline (pH 7.4) to obtain an absorbance of approximately 1.80 at 735 nm. Fifty microliters of diluted crude extract (1 mg/mL) was added to 1 mL of ABTS stock solution then reacted at room temperature for 6 min. Absorbance was read at 735 nm with ascorbic acid as a control. ABTS radical scavenging activity of samples was calculated using the following equation,
Reducing power
Reducing power of the crude extract was measured by modifying the method of Oyaizu (1986). Absorbance was measured at 700 nm with ascorbic acid as a control and reducing power was expressed as the absorbance value measured.
Quantification of phenolic compounds by HPLC with diode-array detection (DAD)
Phenolic compounds in the crude extract were analyzed by HPLC-DAD (Agilent 1200 system) using the conditions described in Table 1. Phenolic compounds in crude extract were identified by comparing their retention time with those of internal standards.
Table 1.
Analytical condition of HPLC with diode-array detection (DAD) for the determination of quantification of phenolic compounds from lemon myrtle leaves
| Instrument | Agilent 1200 series HPLC system |
| Detector | Diode array at 257, 280, 325 and 365 nm |
| Column | YMC-Triart C18 column (4.6 mm × 250 mm) |
| Column temp. | 35 °C |
| Flow rate | 0.8 mL/min |
| Mobile phase | 2% formic acid in water (A) and 2% formic acid in methanol (B) was used as follows: water containing 2% acetic acid (water / acetic acid 98:2, (A)) and methanol (B), the composition gradient was: 95% (A), 5% (B) for 2 min; 25% (B) until 10 min; after, 40, 50, 60, 70, and 80% (B) every 10 min, respectively. |
| Injection vol. | 10 μL |
Immunomodulatory activities
Cell culture
RAW 264.7 cells, a mouse macrophage line, were subcultured in 100 mm dishes (Falcon, Bedford, MA, USA) in DMEM supplemented with 10% FBS and 1% antibiotic (100 U/mL penicillin G, 100 μg/mL streptomycin), and were incubated at 37 °C with 5% CO2 supplemented.
Cell viability
Cell viability was measured using the cell counting kit-8 (CCK-8, Dojindo, Japan). RAW 264.7 cell (1 × 105 cells/well) were seeded into 96 well culture plates with diluted crude extracts at a series of concentration (5–100 μg/mL) for 2 h and stimulated with 200 ng/mL of LPS for 24 h. Then, CCK-8 solution (10%, 100 μL) was added and incubated at 37 °C for 30 min. Absorbance was measured at 450 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). The results were expressed as percent (%) relative to LPS alone control cells.
Nitric oxide (NO) assay
RAW 264.7 cells (1 × 105 cells/well) were plated onto 96 well culture plates for 2 h. Subsequently, non-adherent cells were removed by washing with PBS, and incubated for 2 h by adding diluted crude extracts (5–100 μg/mL) to adherent macrophages; afterward, 200 ng/mL of LPS was stimulated for 24 h and NO levels in culture media were measured using a Promega Griess Reagent System Kit (WI, USA).
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Science (SPSS) version 12.0 (SPSS Inc., Chicago, IL, USA). Differences (p < 0.05) among groups were evaluated by a one-way Analysis of variance (ANOVA) and Duncan’s multiple range tests. Statistical significance was determined using the two tailed Student’s t-test. All data are presented as the mean ± standard deviation (SD, n = 3).
Results and Discussion
Analysis of yield, total polyphenol and flavonoid content
Extraction yield, total polyphenol and total flavonoid content of lemon myrtle according to the extraction conditions are shown in Table 2. As the temperature and time increase, the yield was increased. The highest values for extraction yield in lemon myrtle extracts were obtained following extraction at 80 °C for 10 h; however, there was no significant difference in extraction for more than 6 h. In general, insoluble cell walls are dissolve by heat treatment, thereby increasing water-soluble fiber. During the water solubilization process, the dietary fiber component dissolves from the insoluble cell wall due to structural changes in the plant tissue (Hwang et al., 1994). In reflux extraction method of this experiment, as temperature and time increase, insoluble components were dissolved by heat and pressure to promote dissolution. When extracting more than 6 h lemon myrtle leaves, there is no significant difference in yield, so it is thought that extraction at 80 °C for 6 h will be economical for industrial applications.
Table 2.
Yields, total polyphenol contents (TPC) and total flavonoid contents (TFC) of the various extraction conditions from lemon myrtle leaves
| Extraction conditions | Yields (%) | TPC (mg GAE/g) | TFC (mg RE/g) |
|---|---|---|---|
| 25 °C | |||
| 4 h | 14.90 ± 0.46e1 | 53.63 ± 0.50d | 15.36 ± 1.49e |
| 50 °C | |||
| 4 h | 17.02 ± 0.31c | 79.52 ± 0.38c | 17.34 ± 1.44e |
| 80 °C | |||
| 2 h | 16.77 ± 0.24d | 79.55 ± 0.74c | 19.89 ± 0.67d |
| 4 h | 16.55 ± 0.30d | 81.04 ± 0.81b | 25.75 ± 1.09c |
| 6 h | 23.37 ± 0.31b | 102.72 ± 0.58a | 33.09 ± 1.22a |
| 10 h | 24.48 ± 1.01a | 103.56 ± 0.59a | 30.18 ± 0.74b |
1Lower case letters (a–e) indicate significant differences between groups (p < 0.05) using Duncan’s multiple range tests
TPC in the lemon myrtle extract ranges from 53.63–103.56 mg GAE/g, the highest TPC among the total extracts was 103.56 ± 0.59 mg GAE/g which was extracted for 10 h at 80 °C, and there was no difference in extraction for more than 6 h. The polyphenol content was significantly increased at 80 °C rather than 25 °C. According to study Jeong et al. (2004) the total polyphenol content of citrus peel extract was 71.8 μM without treatment, but increased to 165.4 μM with heat treatment at 150 °C for 1 h. In addition, peanut shells roasted at high temperatures have been reported to total polyphenol content was increased according to the increased extraction temperature and time (Lee et al., 2006). As the heat temperature and time increase, the polyphenolic compound tends to increase. One reason for this is the bound polyphenol compounds which forms a covalent bond with insoluble polymers in the plant cell walls are converted to the free polyphenol compounds by heat treatment and thus it is easy to elute. The other is the high molecular weight phenolic compound is broken down into low molecular weight phenolic compounds (Turkmen et al., 2005). In order to clarify the change of TPC of the extracts according to the heat treatment conditions, it is thought that the composition ratio of each phenolic acid should be compared by separating the bound and free phenolic compounds from heat treated extracts.
TFC in the lemon myrtle extract ranges from 15.36–33.09 mg GAE/g and the highest TFC among the total extracts was 33.09 ± 1.22 mg RE/g which was extracted at 80 °C for 6 h. This is the same trend as the previous TPC and as the temperature and time increase, the TFC was increase. Kang and Lee (2013) showed that the cell walls of plants in the mugwort (Artemisia) were destroyed by reflux and high-pressure extraction to release the bound polyphenols, thereby increasing the content of TPC and TFC. Jiratanan and Liu (2004) reported that the useful components and antioxidant activity by heat treatment depend on the type of plant and the bonding structure.
DPPH, ABTS radical scavenging activities and reducing power
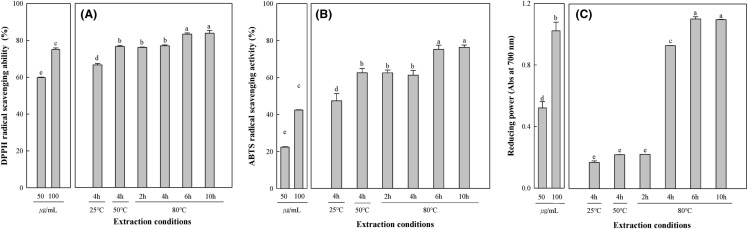
Antioxidant properties of various extraction conditions were compared using DPPH, ABTS radical scavenging activity and reducing power assays. DPPH radical scavenging activity of lemon myrtle extract according to the extraction conditions was shown in Fig. 1A. As the temperature and time increase, DPPH radical scavenging activity gradually increased from 66.59% to 83.80% (p < 0.05). Kwon et al. (2006) reported that antioxidant activity, polyphenols, flavonoids, and browning indicator HMF (5-hydroxymethylfurfural) were significantly increased during extraction at high temperature and pressure. Therefore, we determined that as the temperature and time increase, the reason why DPPH radical scavenging activity is increase, that polyphenol content and browning substance contained.
Fig. 1.
Antioxidant activities of various extraction conditions determined with DPPH and ABTS free radical scavenging activity (%), and reducing power. The tested concentration was 100 µg/mL, respectively, any means in the same column followed by the same letter are not significantly (p < 0.05) different by Duncan’s multiple range test
ABTS radical scavenging activity of lemon myrtle extract according to the extraction conditions was shown in Fig. 1B. ABTS radical scavenging activity gradually increased from 35.21% to 60.32% (p < 0.05) with increasing of the temperature and time. Jang et al. (2012) reported that the bound polyphenols were converted into free form by heat treatment or reflux extraction, thereby increasing the antioxidant activity. Thus, the components of the plant extract are decomposed or polymerized to produce antioxidants during heat extraction, increasing TPC, TFC, DPPH and ABTS radical scavenging activity (%).
Reducing power of lemon myrtle extract according to the extraction conditions was shown in Fig. 1C. The temperature and time increase, reducing power increased gradually increased from 0.17 to 1.10 (Abs at 700 nm) (p < 0.05), which is similar to DPPH and ABTS radical scavenging activity. Osawa (1994) reported that phenolic substances have a variety of physiological effects, including antioxidant activity, mainly because of reducing power. Reducing power assays are used to evaluate the ability of an antioxidant to provide hydrogen or an electron, the antioxidants converts or reduces the ferricyanide (Fe3+) complexes to the ferrous form (Fe2+) (Agrawal et al., 2016). Further studies on the transformed ferrocyanide into ferrous form from lemon myrtle extracts would be necessary to confirm their potential antioxidative activity.
Quantification of phenolic compounds by HPLC-DAD
The phenolic compound, an active ingredient of lemon myrtle extract, was quantitatively analyzed using HPLC-DAD (Table 3). Chromatogram analysis of lemon myrtle extract showed that the retention times of luteoloside, rutin, ellagic acid, gallic acid, catechin and quercetin were 29.654, 30.569, 32.303, 10.355, 16.573, and 40.092 min, respectively. Total amounts of the six phenolic compounds in the lemon myrtle extract ranged from 35.8–43.9 μg/g. Although there was no significant difference in total phenolic compound content of lemon myrtle according to extraction temperature and time, the highest total phenolic compound among the extracts was 43.9 μg/g which was extracted at 80 °C for 6 h. TPC of lacquer tree (Rhus vemiciflua) extract increased significantly according to extraction temperature and time, but decreased when extracted above a certain temperature and time (Park et al., 2013). The reasons why the total phenol content decreases over time is that polyphenols, such as tannin, are insoluble in prolonged heat treatment, thereby reducing migration to the extract, and the longer the extraction time, the lower the antioxidant activity due to heat denaturation. It is though that the total phenol content decreased during extraction at 80 °C for 10 h due to carbonization, and this issue requires additional research. Phenolic compounds widely distributed in plant systems have various structures and molecular weights, and are well known to have physiological functions such as antioxidant, anticancer, and antibacterial properties through phenolic hydroxyl groups (Duval and Shetty, 2001). In particular, polyphenolic compounds are effective for the prevention of chronic cardiovascular disease, flavonoid compounds containing hydroxyl groups have been reported to be responsible for radical scavenging effects in plants (Spencer, 2010). Further research is needed to study the antioxidant activity mechanisms of effective compound such as gallic acid, ellagic acid, luteoloside, rutin, catechin, and quercetin, isolated from the lemon myrtle at the molecular level.
Table 3.
Identification and quantification of the phenolic compounds of the various extraction conditions from lemon myrtle leaves using HPLC with diode-array detection (DAD)
| Extraction conditions | Gallic acida | Ellagic acidb | Luteolosideb | Rutinb | Catechina | Quercetinc | Total amount (μg/g) | |
|---|---|---|---|---|---|---|---|---|
| 25 °C | 4 h | 1.2 | 3.6 | 10.7 | 17.0 | 6.6 | 1.1 | 40.3 |
| 50 °C | 4 h | 1.6 | 3.1 | 10.2 | 16.5 | 7.3 | 0.8 | 39.5 |
| 80 °C | 2 h | 1.5 | 3.2 | 9.2 | 14.8 | 8.0 | 0.9 | 37.5 |
| 4 h | 1.7 | 3.9 | 8.9 | 14.4 | 9.2 | 0.7 | 38.8 | |
| 6 h | 1.8 | 4.4 | 10.4 | 16.6 | 9.4 | 1.2 | 43.9 | |
| 10 h | 1.6 | 3.5 | 10.6 | 15.4 | 4.4 | 0.2 | 35.8 | |
All chromatography operations were carried out at ambient temperature
Chromatography peaks were detected at a280 nm for gallic acid, and catechin; b257 nm for luteoloside, rutin, and ellagic acid; c365 nm for quercetin using HPLC-DAD
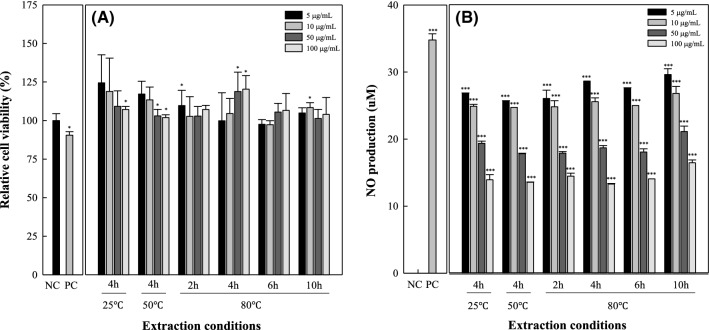
In vitro cell viability and nitric oxide (NO) producution
The present study was undertaken to examine the potential in vitro anti-inflammatory activity of lemon myrtle extract using the LPS-induced on macrophage-derived cell line RAW 264.7. Cells were treated with various concentration of lemon myrtle extract (5–100 μg/mL) for 24 h followed by LPS stimulation (Fig. 2A). Lemon myrtle extracts treatment did not exhibit any cytotoxic effects on RAW 264.7 cells at concentration up to 100 μg/mL after treatment for 24 h. To investigated the effect of lemon myrtle extract treatment on NO production, LPS (20 μg/mL) treatment was performed on RAW 264.7 cells to induce intracellular NO production, followed by lemon myrtle extracts of 5–100 μg/mL (Fig. 2B). The NO production of LPS-induced macrophages was found to be effective as an inflammatory modulator by decreasing concentration-dependently (p < 0.001) in each extract.
Fig. 2.
Effect of lemon myrtle of various extraction conditions on cell viability (A) and nitric oxide (NO) production (B) in LPS-stimulated RAW 264.7 cells. A total of 20 ng/mL lipopolysaccharide (LPS) was used as the positive control (PC) and media used as negative control (NC). One-way ANOVA was used for comparisons of multiple group means followed by t-test (significant as compared to control; **p < 0.01, ***p < 0.001)
As the extraction temperature increased, the levels of LPS-induced NO production activity significantly decreased, however, there was no significant difference in NO production inhibition activity with extraction time increased. Koh et al. (2009) showed that the extraction temperature decrease, the NO production of hot water extracts of dandelion leaves with different extraction temperature decreased. On the other hand, Kang et al. (2017) reported that the higher extraction temperature, the NO production of ethanol extract of red garlic with different extraction temperature decreased. These results were showed that the conditions for extracting the active component differ depending on the characteristics of the raw material.
Under optimal extraction conditions (80 °C, and 6 h extraction time), extracts showed the strongest antioxidant and anti-inflammatory activities and the largest amount of phenolic compound. Crud ethanol extracts from lemon myrtle leaves could be used as a natural antioxidant in the food industry and a dietary supplement, resistant to oxidative disease. Further research is needed to investigate the antioxidant and anti-inflammatory mechanisms of active compounds isolated at the molecular level, to analyze the safety of animals and to establish effective dose levels.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eun-Jung Kang, Email: gogowud@naver.com.
Jae-Kwon Lee, Email: jglee@kyonggi.ac.kr.
Hye-Ryung Park, Email: pooh-lup@hanmail.net.
Hoon Kim, Email: hkim81@khu.ac.kr.
Hyun-Seok Kim, Email: khstone@kyonggi.ac.kr.
Jiyong Park, Email: foodpro@yonsei.ac.kr.
References
- Agrawal H, Joshi R, Gupta M. Isolation, purification and characterization of antioxidative peptide of pearl millet (pennisetum glaucum) protein hydrolysate. Food Chem. 2016;204:365–372. doi: 10.1016/j.foodchem.2016.02.127. [DOI] [PubMed] [Google Scholar]
- Akihisa T, Kokke W, Kimura Y, Tamura T. Isokarounidiol (d: C-friedooleana-6, 8-diene-3. Alpha., 29-diol]: The first naturally occurring triterpene with a. Delta. 6, 8-conjugated diene system. Iodine-mediated dehydrogenation and isomerization of its diacetate. J. Org. 58: 1959-1962 (1993)
- AOCS. Offical Tentative Methods of the American Oil Chemists’ Society. 4th ed. Method Ce 8. American Oil Chemist’ Society, Champaign, IL (1990)
- Bjelakovic G, Nikolova D, Gluud C. Antioxidant supplements and mortality. Curr. Opin. Clin. Nutr. Metab. Care. 2014;17:40–44. doi: 10.1097/MCO.0000000000000009. [DOI] [PubMed] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Bogdan C. 2001. Nitric oxide and the immune response. Nat. Immunol. 2: 907-916 (2001) [DOI] [PubMed]
- Buchaillot A, Caffin N, Bhandari B. Drying of lemon myrtle (Backhousia citriodora) leaves: Retention of volatiles and color. Drying Technol. 2009;27:445–450. doi: 10.1080/07373930802683740. [DOI] [Google Scholar]
- Burke BE, Baillie J-E, Olson RD. Essential oil of australian lemon myrtle (Backhousia citriodora) in the treatment of molluscum contagiosum in children. Biomed. Pharmacother. 2004;58:245–247. doi: 10.1016/j.biopha.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Duval B, Shetty K. The stimulation of phenolics and antioxidant activity in pea (pisum sativum) elicited by genetically transformed anise root extract. J. Food Biochem. 2001;25:361–377. doi: 10.1111/j.1745-4514.2001.tb00746.x. [DOI] [Google Scholar]
- Han JH, Moon HK, Chung SK, Kang WW. Comparison of physiological activities of radish bud (raphanus sativus l.) according to extraction solvent and sprouting period. J. Korean Soc. Food Sci. Nutr. 44: 549-556 (2015)
- Hayes A, Markovic B. Toxicity of australian essential oil Backhousia citriodora (lemon myrtle). Part 1. Antimicrobial activity and in vitro cytotoxicity. Food Chem. Toxicol. 40: 535-543 (2002) [DOI] [PubMed]
- Hwang JK, Kim CT, Hong SI, Kim CJ. Solubilization of plant cell walls by extrusion. J. Korean Soc. Food Sci. Nutr. 1994;23:358–370. [Google Scholar]
- Hyon JS, Kang SM, Senevirathne M, Koh WJ, Yang TS, Oh MC, Oh CK, Jeon YJ, Kim SH. Antioxidative activities of extracts from dried citrus sunki and c. Unshiu peels. J. Korean Soc. Food Sci. Nutr. 2010;39:1–7. doi: 10.3746/jkfn.2010.39.1.001. [DOI] [Google Scholar]
- Jang GY, Kim HY, Lee SH, Kang YR, Hwang IG, Woo KS, Kang TS, Lee JS, Jeong HS. Effects of heat treatment and extraction method on antioxidant activity of several medicinal plants. J. Korean Soc. Food Sci. Nutr. 2012;41:914–920. doi: 10.3746/jkfn.2012.41.7.914. [DOI] [Google Scholar]
- Jeong SM, Kim SY, Kim DR, Jo SC, Nam K, Ahn D, Lee SC. Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J. Agric. Food Chem. 2004;52:3389–3393. doi: 10.1021/jf049899k. [DOI] [PubMed] [Google Scholar]
- Jiratanan T, Liu RH. Antioxidant activity of processed table beets (beta vulgaris var, conditiva) and green beans (phaseolus vulgaris l.). J. Agric. Food Chem. 52: 2659-2670 (2004) [DOI] [PubMed]
- Kang KM, Lee SH. Effects of extraction methods on the antioxidative activity of artemisia sp. J. Korean Soc. Food Sci. Nutr. 2013;42:1249–1254. doi: 10.3746/jkfn.2013.42.8.1249. [DOI] [Google Scholar]
- Kang KO. Physiological and antioxidant activities of green, oolong and black tea extracts. J. East Asian Soc. Diet Life. 2011;21:243–249. [Google Scholar]
- Kang MJ, Kim DG, Shin JH. Antioxidant and anti-inflammatory effects of red garlic compositions. J. Korean Food Preserv. 2017;24:446–454. doi: 10.11002/kjfp.2017.24.3.446. [DOI] [Google Scholar]
- Kim PK, Jung KI, Choi YJ, Gal SW. Anti-inflammatory effects of lemon myrtle (Backhousia citriodora) leaf extracts in lps-induced raw 264.7 cells. J. Life Sci. 27: 986-993 (2017)
- Kim YW, Zhao RJ, Park SJ, Lee JR, Cho IJ, Yang CH, Kim SG, Kim SC. Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of nf-κb-dependent inos and proinflammatory cytokines production. Br. J. Pharmacol. 2008;154:165–173. doi: 10.1038/bjp.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh YJ, Park YK, Kim YS, Cha DS, Choi HD. Preparation of hot water extracts of dandelion leaves to increase anti-inflammatory activity. J. Korean Soc. Food Sci. Nutr. 2009;38:391–395. doi: 10.3746/jkfn.2009.38.3.391. [DOI] [Google Scholar]
- Konczak I, Zabaras D, Dunstan M, Aguas P. Antioxidant capacity and phenolic compounds in commercially grown native australian herbs and spices. Food Chem. 2010;122:260–266. doi: 10.1016/j.foodchem.2010.03.004. [DOI] [Google Scholar]
- Kwon OC, Woo KS, Kim TM, Kim DJ, Hong JT, Jeoung HS. Physicochemical characteristics of garlic on the high temperature and pressure by different assays. J. Korean Soc. Food Cult. 2006;22:353–358. [Google Scholar]
- Lee SC, Jeong SM, Kim SY, Park HR, Nam K, Ahn D. Effect of far-infrared radiation and heat treatment on the antioxidant activity of water extracts from peanut hulls. Food Chem. 2006;94:489–493. doi: 10.1016/j.foodchem.2004.12.001. [DOI] [Google Scholar]
- Osawa T. Novel natural antioxidants for utilization in food and biological systems. Tokyo, Japan: Japan Scienctific Societies Press; 1994. pp. 241–251. [Google Scholar]
- Oyaizu M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction. Jpn. J. Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Park HJ, Yoon GM, Lee SH, Jang GY, Kim MY, Meishan L, Lee JS, Jeong HS. Effects of extraction temperature and time on antioxidant activities of rhus verniciflua extract. J. Korean Soc. Food Sci. Nutr. 2013;42:1776–1782. doi: 10.3746/jkfn.2013.42.11.1776. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radical Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radical Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JP. Beyond antioxidants: The cellular and molecular interactions of flavonoids and how these underpin their actions on the brain. Proc. Nutr. Soc. 2010;69:244–260. doi: 10.1017/S0029665110000054. [DOI] [PubMed] [Google Scholar]
- Turkmen N, Sari F, Velioglu YS. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005;93:713–718. doi: 10.1016/j.foodchem.2004.12.038. [DOI] [Google Scholar]
- Wilkinson JM, Hipwell M, Ryan T, Cavanagh HM. Bioactivity of Backhousia citriodora: Antibacterial and antifungal activity. J. Agric. Food Chem. 2003;51:76–81. doi: 10.1021/jf0258003. [DOI] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]