Abstract
Purpose
To investigate if human ovarian grafting with pure virgin human recombinant collagen type-1 from bioengineered plant lines (CollPlant™) or small intestine submucosa (SIS) yields better implantation results for human ovarian tissue and which method benefits more when combined with the host melatonin treatment and graft incubation with biological glue + vitamin E + vascular endothelial growth factor-A.
Methods
Human ovarian tissue wrapped in CollPlant or SIS was transplanted into immunodeficient mice with/without host/graft treatment. The tissue was assessed by follicle counts (including atretic), for apoptosis evaluation by terminal deoxynucleotidyl transferase assay and for immunohistochemical evaluation of neovascularization by platelet endothelial cell adhesion molecule (PECAM) expression, and for identification of proliferating granulosa cells by Ki67 expression.
Results
Human ovarian tissue transplanted with CollPlant or SIS fused with the surrounding tissue and promoted neovascularization. In general, implantation with CollPlant even without additives promoted better results than with SIS: significantly higher number of recovered follicles, significantly fewer atretic follicles, and significantly more granulosa cell proliferation. Moreover, results with CollPlant alone seemed to be at least as good as those after host and graft treatments.
Conclusions
CollPlant is a biomaterial without any potential risks, and grafting ovarian tissue with CollPlant is easy and the procedure may be easily modified, with limited or no foreseeable risks, for auto-transplantation in cancer survivors. Further studies are needed using other novel methods capable of enhancing neovascularization and reducing apoptosis and follicle atresia.
Keywords: Ovarian transplantation, Immunodeficient mice, CollPlant, SIS, Neovascularization, Stroma cell apoptosis, Atretic follicles
Introduction
Anti-cancer therapy, particularly chemotherapy, induces the destruction of ovarian follicles, placing young female survivors at risk of infertility [1, 2]. One of the options for fertility preservation is cryopreservation of human ovarian cortical tissue containing immature primordial follicles [3]. So far, transplantation of frozen-thawed ovarian tissue has resulted in over 130 live births in cancer survivors [4].
However, studies reported extensive follicular loss immediately after grafting, probably as a result of slow graft revascularization resulting in ischemia [1]. Ovarian cellular death occurs mainly by apoptosis, a programmed cell death [5]. Methods to hasten graft vascularization and reduce ovarian cell death are being investigated [6, 7]. Immunodeficient mice engrafted with ovarian tissue serve as follicle development models because they do not reject transplants.
Studies from our laboratory have shown that human ovarian tissue grafting and follicular survival can be increased with the addition of our “improvement protocol” consisting of host treatment with melatonin, a free-radical scavenger with anti-oxidant and anti-apoptotic activities [6, 8], graft treatment with hyaluronic acid (HA)-based biological glue + vitamin E + vascular endothelial growth factor A (VEGF-A). Hyaluronic acid promotes anti-inflammatory and anti-apoptotic signals in ovarian follicles and preimplantation embryos [8]; vitamin E is a free-radical scavenger with anti-oxidant and anti-apoptotic activities [6, 8], and VEGF-A is the most potent neovascularization enhancer. We speculate that it is possible to further improve this method by adding novel substances for tissue fusion and regeneration.
A decellularized human extracellular tissue matrix (ECTM) generated from cadaver skin has been used in cosmetic surgery, breast reconstruction, and other reconstructive procedures to enhance successful tissue implantation and revascularization [9]. When human ovarian cortical pieces were transplanted with ECTM into immunodeficient mice, the tissue integrated into the ECTM and normal primordial follicles was identified after grafting. Two pregnancies, including one live birth, were reported following auto-transplantation of human ovarian tissue with ECTM.
Another study reported embedding ovine ovarian tissue into carrier gels of agar + regular collagen type I combined with VEGF111 [10]. VEGF111 is an isoform that does not bind to the extracellular matrix, diffuses extensively, is resistant to proteolysis, and has a relatively long plasma half-life time (74 min) [10, 11]. The authors found that the addition of VEGF111 led to improved results over agar-collagen gels alone [11].
Others described a novel extracellular (ECM)-like matrix consisting of human recombinant collagen type-1 grown in bioengineered tobacco plant lines from a natural precursor procollagen [12]. Processing yielded a pure, smooth, consistent fibril collagen that formed a three-dimensional scaffold which promoted cell proliferation, differentiation, and tissue culture of primary human cells. The virgin human collagen can be used as a biomaterial/scaffold for tissue repair (orthopedics, wound repair, vascular grafts etc.). It is non-allergenic and non-immunogenic and poses none of the potential viral hazards of animal-derived pathogens (http://www.collplant.com/).
Porcine small intestinal submucosa (SIS) is a growth-factor-rich-extracellular matrix that enhances wound healing and tissue remodeling [13]. SIS is naturally impregnated with glycosaminoglycans and also contains basic fibroblast growth factor (bFGF), an angiogenic agent, and transforming growth factor-β that plays important roles in follicle growth [14]. SIS has been used as a xenograft scaffold in dermal, cardiovascular, orthopedic, and other applications in various animal models [15, 16]. Encouraging results were obtained for muscle tissue regeneration and functional recovery, without an immunologic rejection [17]. In a study from our laboratory, we used both CollPlant and SIS matrices for culturing human ovarian tissue [18]. Moreover, one study described remodeling of rabbit ovaries with SIS after a portion of the ovary was cut [19].
The aims of the present study were to determine if the survival of frozen-thawed human ovarian tissue implanted in immunodeficient mice can be improved by the use of CollPlant or SIS matrices with/without prior host treatment and/or prior graft treatment [8].
Patients and methods/materials
Human ovarian material
Small ovarian samples were derived from nine patients with cancer aged 9–22 years (mean age, 17 ± 4 years) undergoing ovarian retrieval by laparoscopy for fertility preservation before anti-cancer therapy (Table 1). There were no statistical differences between the patient ages in the CollPlant and SIS experiments. Approval for this experiment has been obtained from the Ethics Committee of Rabin Medical Center. Informed consent was obtained from the patients or their parents in the cases of minors [6, 20]. The tissue samples were divided into CollPlant and SIS groups. The distribution of the tissue by source is shown in Table 1. Because of the limited availability of ovarian tissue for research, only samples from patients 1 and 3 (Table 1) were transplanted in parallel in both groups, although tissue from similar aged patients was implanted in both groups. From our experience in previous studies, this number of ovarian samples was sufficient to draw definite conclusions regarding the optimal host and graft treatment for human ovarian tissue [6, 8].
Table 1.
Details of patients and in which part of the study their tissue was used
| Patients’ no. | Age | CollPlant | SIS |
|---|---|---|---|
| 1 | 16 | + | + |
| 2 | 17 | + | |
| 3 | 17 | + | + |
| 4 | 9 | + | |
| 5 | 17 | + | |
| 6 | 20 | + | |
| 7 | 22 | + | |
| 8 | 21 | + | |
| 9 | 13 | + | |
| 9 patients† | 17 ± 4‡ |
†‡SD represents the standard deviation; total patient number; average ± SD; + represents if human ovarian tissue of the specific patient was used in the CollPlant or/and SIS part
One slice measuring 5 × 5 mm from every ovarian sample was fixed in Bouin’s solution (prepared from solutions purchased from Sigma, St. Louis, MO, USA and Biolab, Jerusalem, Israel) before cryopreservation (fresh ungrafted control) to evaluate pre-grafting follicular density (see the “Histological preparation” section). This procedure was conducted to ensure that only follicle-rich samples would be used for grafting.
Cryopreservation and thawing of ovarian tissue
The ovarian samples were sliced and placed in cryogenic vials (Nalge Nunc International, Delta, Roskilde, Denmark) filled with 1.5 M dimethylsulfoxide (DMSO, Sigma) [6]. Samples were kept on ice for 30 min for equilibration. They were slowly frozen in a programmable freezer (Kryo 10; series 10/20, Planer Biomed, Sunbury on Thames, UK), and then immediately placed in liquid nitrogen. Thawing was conducted by washouts with decreasing concentration gradients of DMSO (1 M, 0.5 M, and phosphate buffered saline with 20% synthetic serum) at room temperature, with a final incubation at 37 °C. One slice similar in size to the fresh ungrafted control was fixed immediately in Bouin’s solution (thawed ungrafted control).
Preparation of matrices
Matrices of CollPlant (a generous gift from CollPlant Ltd., Weizmann Science Park, Ness Ziona, Israel) and SIS (a generous gift from Cook Biotech, West Lafayette, Indiana, USA, produced under the United States Food and Drug Administration guidance) were sterilized at our laboratory by exposure to ultraviolet light for 1 h (Fig. 1A and B), as reported by us previously [18]. The discs were placed in wells of 48-well plates (CELLSTAR, Greiner Bio-One GmbH, Frickenhausen, Germany), which were then filled with 1 ml alpha minimal essential medium (αMEM, Biological Industries, Beit Ha’emek, Israel) and human serum albumin (HSA, Irvine Scientific, Santa Ana, CA, USA), and incubated overnight at 37 °C.
Fig. 1.
Representative photographs of matrices. A—CollPlant matrices. B—SIS matrix
Transplantation into immunodeficient mice
For tissue transplantation, we used 146 immunodeficient nu/nu female Balb/C mice aged 10–12 weeks (Harlan, Jerusalem, Israel) [6]. From our experience, in past studies, this was a sufficient number of hosts to draw clear statistical conclusions [6, 8].
The operations were carried out in sterile conditions under anesthesia in special animal facilities [8]. We chose the back muscle as a transplantation site because it is not richly vascularized, and can mimic ovarian conditions. After dissecting the back muscle, one ovarian slice per host (measuring 5 × 5 mm, similar to the ungrafted controls) was pushed inside and the opening was sutured. The same sizes of ovarian samples were used for transplantation with and without the matrices (CollPlant or SIS). This part of the experiment was approved by the Animal Experimentation Supervision Committee of Rabin Medical Center.
Experimental groups (Table 1, Fig. 2)
Fig. 2.
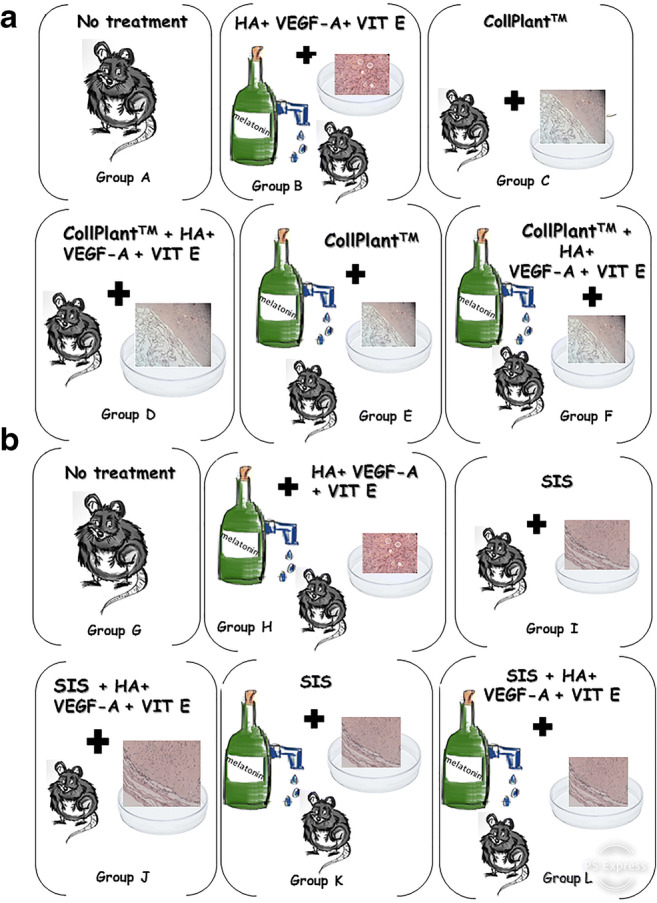
Illustration of the experimental groups. A thawed ungrafted control specimen was fixed immediately for histological evaluation in both SIS and CollPlant groups. The remaining thawed samples were divided for implantation into 12 treatment subgroups, as follows: (a) CollPlant subgroups. A—Engrafted without further treatment, B—engrafted with the improvement protocol only: both host and graft treatment (host treatment with melatonin and graft treatment with HA + VEGF-A + vitamin E), C—engrafted with ovarian tissue encapsulated with CollPlant, D—engrafted after graft incubation with HA + VEGF-A + vitamin E and encapsulated with CollPlant, E—engrafted with host treatment with melatonin and graft encapsulated with CollPlant, F—engrafted with host treatment with melatonin and graft treatment with HA + VEGF-A + vitamin E and encapsulated with CollPlant. (b) SIS subgroups. G—Engrafted without further treatment, H—engrafted and treated as in group B, I—engrafted with ovarian tissue encapsulated with SIS, J—engrafted with graft treatment with HA + VEGF-A + vitamin E and encapsulated with SIS, K—engrafted with host treatment with melatonin and graft encapsulated with SIS, L—engrafted with host treatment with melatonin and graft treatment with HA + VEGF-A+ vitamin E and graft encapsulated with SIS
Each of the CollPlant and SIS groups was further divided into six subgroups for implantation of the ovarian tissue with/without host and/or graft treatment and with/without the respective matrix. Figure 2 was created in order to clarify the different subgroups with SIS and CollPlant:
CollPlant groups
Thawed/ungrafted controls
-
Group A
—untreated hosts and grafts
-
Group B
—treated host and treated graft (improvement protocol) without matrix. Hosts were treated with melatonin (240 mg/l) (Sigma) in the drinking water and grafts were incubated for 2 h with hyaluronic acid enriched-biological glue (Uterine Transfer Medium, MediCult, Jyllinge, Denmark) + VEGF-A (200 ng/ml) (Biological Industries, Beit Ha’emek, Israel) + vitamin E (E-400 natural, 400 IU/ml-oily form SOFT GEL Technologies Inc., Los Angeles, CA, USA) and implanted [6–8]. Explanations of the doses of melatonin, VEGF-A, and vitamin E were given in detail elsewhere [6–8].
-
Group C
—untreated hosts and untreated grafts, wrapped and encapsulated within the matrix without stitching. The grafts were encapsulated with CollPlant and implanted.
-
Group D
—untreated host and treated graft, with matrix. The grafts were incubated for 2 h as in group B and then encapsulated with CollPlant and implanted.
-
Group E
—treated host and untreated graft, with matrix. The hosts were treated as in group B and the grafts were encapsulated with CollPlant and implanted.
-
Group F
—treated host and treated graft (improvement protocol), with matrix. The hosts and the grafts were treated as in group B and the grafts were encapsulated with CollPlant and implanted.
SIS groups
Thawed/ungrafted controls
-
Group G
—untreated host and graft
-
Group H
—treated host and graft (improvement protocol), without matrix. The hosts and the grafts were treated as in group B and the grafts were implanted.
-
Group I
—untreated host and untreated graft, wrapped and encapsulated within the matrix without stitching. The grafts were encapsulated with SIS and implanted.
-
Group J
—untreated hosts and treated grafts, with matrix. The grafts were incubated for 2 h as in group B and encapsulated with SIS and implanted.
-
Group K
—treated hosts and untreated grafts, with matrix. The hosts were treated as in group B and the grafts were encapsulated with SIS and implanted.
-
Group L
—treated hosts and treated grafts, with matrix. The hosts and the grafts were treated as in group B and the grafts were encapsulated in SIS and implanted.
The mice were sacrificed 3 weeks post-operatively, as described by us previously [6–8], and the grafts were removed and fixed in Bouin’s solution (Sigma and Biological Industries).
Histological preparation
The transplanted ovarian tissue specimens and the thawed ungrafted controls were fixed in Bouin’s solution and dehydrated, embedded in paraffin, sectioned, and stained with hematoxylin (Pioneer Research Chemicals Ltd., Essex, UK) and eosin (Sigma), as reported by us previously [6–8]. The follicles were counted with a computerized image analyzer (analySIS, Soft Imaging System, Digital Solutions for Imaging and Microscopy, System GmbH, Munster, Germany) from the stained sections [6, 8]. The number of follicles in the ungrafted and grafted samples was counted throughout the field (magnification × 100) in two levels per specimen (with at least 50 μm between levels to avoid counting the same follicle twice); this method was used by us previously and is reliable for evaluation of follicle number distribution [6–8]. Atretic follicles were identified by the characteristic pyknotic cells, eosinophilia of the ooplasm, and clumping of the chromatin material [3]. These results of all grafted groups were compared with the thawed ungrafted control specimens.
Unstained sections were placed on OptiPlus positive charged microscope slides (BioGenex Laboratories, San Ramon, CA, USA) for apoptosis evaluation by the terminal deoxynucleotidyl transferase (TdT) assay (TUNEL) and for immunohistochemical evaluation of neovascularization by platelet endothelial cell adhesion molecule (PECAM) expression [7, 21], and for identification of proliferating granulosa cells by Ki67 expression [22].
TdT assay
Ovarian cell death occurs mainly via apoptosis [5], whereby an endonuclease is activated to specifically cleave DNA at regularly spaced nucleosomal units. Therefore, sensitive in situ DNA end–labeling assays have been developed for its identification. We used a TdT assay (ApopTag In Situ Detection Kit; Intergen Company, Purchase, NY, USA) [6] for two sections per case. The slides were deparaffinized, dehydrated, and rinsed. Positive controls were prepared by pretreating samples with DNase I (specific activity 10,000–1000 U/mL; Sigma). A second positive control was a section from a rat mammary gland 4 days after weaning, which was included with the kit. Thereafter, all slides were treated with proteinase K (Sigma). The samples were quenched with 3% hydrogen peroxide (Gadot Biochemical Industries LTD, Haifa Bay, Israel) in the dark to block endogenous peroxidase activity, and then incubated with working-strength TdT at 37 °C. The negative controls were incubated with distilled water. Rinsing the sections in stop/wash buffer terminated the reaction.
Samples were then transferred to a humidified chamber for incubation with conjugate anti-digoxigenin peroxidase, and exposed to a diaminobenzidine (DAB) urea H2O2 solution (brown staining = apoptosis) (Sigma Fast tablets, Sigma). The sections were counterstained with Mayer hematoxylin (Pioneer Research Chemicals Ltd., Colchester, UK). Finally, the slides were rinsed with running tap water and rehydrated.
Stroma cell staining level was graded by intensity on a 4-point scale: 0 = no TUNEL staining, 1 = low intesity TUNEL staining, 2 = TUNEL medium staining intensity, and 3 = TUNEL high staining intensity. This grading system has been previously used by our laboratory [6–8]. Follicles that were unstained for DAB and appeared with only blue counterstaining were considered non apoptotic. The slides were viewed independently and blindly by two of the authors (for CollPlant groups: D.S. and R.A.; for SIS groups: Y.T. and R.A.).
Immunohistochemistry for Ki67 and PECAM
Sections were first dehydrated. To enhance antigen retrieval, all slides were microwaved with citrate buffer at pH 6.0 (CheMate buffer, DAKOCytomation, Glostrup, Denmark), and to block endogenous peroxidase activity, they were quenched in 3% hydrogen peroxide (Gadot Biochemical Industries Ltd., Haifa, Israel) [22, 23]. The sections were then incubated for 1 h with either a rabbit polyclonal anti-Ki67 antibody at a concentration of 1: 10 and 1:30 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or an anti-PECAM antibody at a concentration of 1: 250 (Santa Cruz Biotechnology). For the negative control, we used a normal rabbit IgG antibody (sc-2027, Santa Cruz Biotechnology) diluted to the same concentrations as the primary antibody. For a positive control for Ki67, we used a section of a human tonsil (Cell Marque, Rocklin, CA, USA) and for PECAM, a section of a human kidney (described as a positive control by IHCWORLD, Life Science Products & Services, Woodstock, MD, USA). The use of these controls was according to the Histochemical Society’s standards of practice [24]. Solutions from a Dako EnVision System, horseradish peroxidase–3-amino-9-ethylcarbazole (AEC) kit (Dako Corporation, Glostrup, Denmark), were used for immunostaining: (red-brown AEC staining indicating Ki67 or PECAM expression). Follicles were considered to be positively stained for Ki67 if at least one of their granulosa cells expressed Ki67 [22, 23]. The slides were viewed independently and blindly by two of the authors (for CollPlant groups: D.S. and R.A.; for SIS groups: Y.T. and R.A.).
Statistical analysis
Data were statistically analyzed by analysis of variance, chi-square test, and Fisher’s exact test, as required. All analyses were compared within the matrix subgroups (CollPlant and SIS subgroups) and also between the matrices (CollPlant subgroups vs. SIS subgroups). P values less than 0.05 were considered statistically significant.
Results
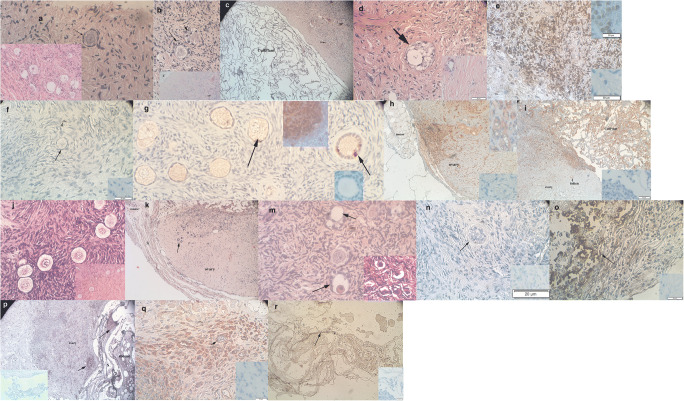
Grafts in all groups except the untreated controls (groups A and G) retained their size and did not shrink after implantation. It is, however, noteworthy that although all grafts were retrieved, in most cases, the matrices attached the tissue to the murine muscle, and we recovered most of the grafts in parts and not as whole slices. Their existence was clear only after histological preparation. Figure 3 shows representative ovarian sections at various stages of the study. Good fusion to host tissue was found when the grafts were transplanted either with CollPlant (Fig. 3c, h, and i) or with SIS (Fig. 3k and p). In most cases, the recovered grafts showed only a limited number of follicles regardless of matrix.
Fig. 3.
Representative photographs of ungrafted controls and grafted samples. A—Thawed ungrafted ovarian section from a 9-year-old girl (patient 5). Note the primordial follicle (arrow). Hematoxylin and Eosin, Original magnification × 400. Note also at the lower left hand side the same section with several primordial follicles but at original magnification of × 200. B—Ovarian section from a 13-year-old girl (patient 1) after grafting with CollPlant alone (group C). Note the two secondary follicles (arrows). Hematoxylin and Eosin, Original magnification × 400. Note also at the bottom the same section with a visible part of the CollPlant matrix (C) and follicles (arrow) at lower magnification, original magnification × 200. C—Ovarian section from the same patient as in panel B, after grafting with CollPlant alone (group C). Note the primordial follicles (arrow head) and the CollPlant matrix that attached the human ovarian tissue to the murine muscle (not shown in this photo). Hematoxylin and Eosoin, original magnification × 100. D—Ovarian section from the same patient as in panel B, after grafting with CollPlant combined with the improvement protocol (group F). Note the atretic follicle (arrow). Hematoxylin and Eosoin, original magnification × 400. Note also at the bottom left hand size the same section at a lower magnification, original magnification × 200. E—Ovarian section from the same patient as in panel B, transplanted without treatment (group A). Note the brown staining indicating apoptosis in the stroma cells. Original magnification × 400. Note also at the upper right hand corner the red-brown staining in the positive control from a rat mammary gland four days after weaning and in the lower right corner the totally blue negative control. F—Thawed ungrafted ovarian section from the same patient as in panel A. Note the light brown staining indicating apoptosis in the stroma cells (arrow head) and lack of apoptosis in a primordial follicle (arrow). Original magnification × 400. Note also at the lower right corner the totally blue negative control. The positive control is the same as in Fig. 3e. G—Ovarian section from the same patient as in panel B from group C. Note the primordial and primary follicles with positively stained red-brown Ki67 staining (arrow), indicating proliferation. Original magnification × 400. Note also at the upper right side the positive control with its blue and red-brown staining and at the lower right side the negative control with a primary follicle and totally blue staining. H—Ovarian section from the same patient as in panel B from group F. Note the red-brown PECAM staining mostly in the borders of the graft but also in its center. The human ovarian tissue is fully fused with the mouse tissue. Original magnification × 400. Note also at the upper right corner the positive control with its blue and red-brown staining and at the lower right corner the totally blue negative control. I—Ovarian section from the same patient as in panel A, after host treatment with melatonin and graft transplantation with CollPlant (group E). Note the PECAM staining mainly in the border of the graft and in the CollPlant matrix but also in the graft’s center (blood vessels). The human ovarian tissue is fully fused with the CollPlant and the mouse tissue. Original magnification × 100. Note also at the lower right corner the totally blue negative. The positive control is shown in Fig. 3h. J—Ungraffted-thawed ovarian section from patient 7. Note the normal primordial follicles. Hematoxylin and Eosin. Original magnification × 400. Note also at the lower right corner the same section at lower magnification, original magnification, × 200. K—Ovarian sample from patient 9 grafted with SIS (S) after improvement protocol (group L). Note that SIS envelops the ovarian slice and the follicles in the graft (arrow) and graft is attached to the mouse tissue. Hematoxylin and eosin. Original magnification × 100. L—Ovarian sample as in K. Note the normal primary follicles with the red-brown. Ki67 staining in the granulosa cells and oocytes (arrows). Original magnification × 400. Note also at the upper right corner the negative control with totally blue follicles. The positive control with Ki67 staining is shown in Fig. 3g. M—Ovarian sample from patient 1 from group L. Note the atretic follicles (arrow). Hematoxylin and eosin. Original magnification × 400. Note also at the lower right corner the same section at a lower magnification, original magnification × 200. N—Ovarian sample from patient 1 grafted with SIS after pre-incubation with HA + VEGF-A + vitamin E (group J). Note the follicle (not midsection, arrow) and the overall blue staining and lack of brown apoptosis staining. Original magnification × 400. Note also at the lower right corner the totally blue negative control. The positive control is shown in Fig. 3e. O—Ovarian sample from patient 6 grafted with SIS (group I). Note the strong brown staining in the stroma cells (arrow), indicating apoptosis. Original magnification × 400. Note also at the lower right corner the totally blue negative control. The positive control is shown in Fig. 3e. P—Ovarian sample as in K. Note the red-brown PECAM staining in the SIS matrix (S) and the ovarian graft (arrow). The SIS matrix is between the graft and the mouse tissue. Original magnification × 100. Note also at the left lower corner the totally blue negative control. The positive control is shown in Fig. 3h. Q—Ovarian sample from patient 8 grafted in group J. Note the red-brown PECAM staining in the ovarian graft and in its blood vessels (arrow). Original magnification × 400. Note also at the right lower corner the totally blue negative control. The positive control is shown in Fig. 3h. R—Partially loose SIS matrix (S) after implantation of ovarian tissue from patient 7 in group I. Note the darker PECAM staining in the SIS matrix (arrow). Original magnification × 400. Note also the totally blue negative control at the right lower corner. The positive control is shown in Fig. 3h
In all groups except the thawed/ungrafted controls, positive PECAM staining was identified in the region between the human ovarian graft, CollPlant or SIS, and the murine muscle. There was also diffuse PECAM staining within the tissue itself in all CollPlant and SIS groups and also in blood vessels (Fig. 3h, i, p–r).
CollPlant groups (Figs. 4–7)
Fig. 4.
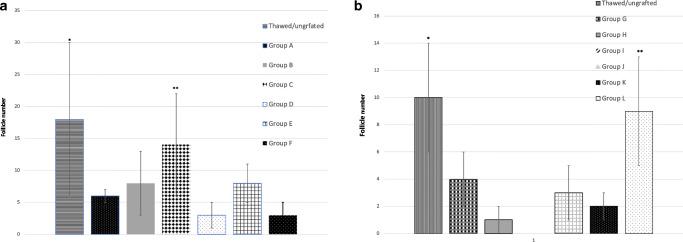
Follicle numbers. Results are presented as mean ± standard deviation (SD). The x axis represents the six experimental groups and the thawed ungrafted control. The y axis represents the mean follicular numbers. (a) CollPlant groups: the bars represent the different subgroups, as follows: Single asterisk indicates significantly higher than all groups (P > 0.0001 than all groups but group C, P = 0.006). Double asterisks indicate significantly higher than group BC (P = 0.04), group D (P = 0.005), group E (P = 0.04), and group F (P = 0.04). (b) SIS groups: single asterisk indicates significantly higher than in group H (treated host and treated graft without SIS, P = 0.02 and P = 0.0061, respectively), group I (untreated host and graft with SIS, P = 0.01 and P = 0.0033, respectively) and group K (treated host and untreated graft with SIS, P = 0.04 and P = 0.0058, respectively). Double asterisks indicate significantly higher than in groups G (untreated grafts without SIS, P = 0.013), I (untreated host and treated graft with SIS, P = 0.033) and J (untreated hosts and treated grafts with SIS, P = 0.0072)
Fig. 7.
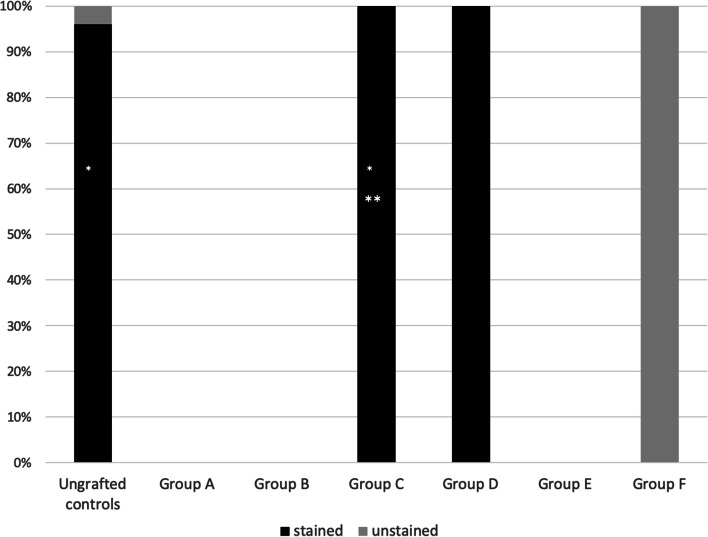
Percent of Ki67 staining in the CollPlant subgroups. Single asterisk indicates significantly more Ki67 positively stained follicles in ungrafted controls and group C (P < 0.0001 for both), and group D (P = 0.02) for group D than in group A. Double asterisks indicate significantly more Ki67 positively stained follicles (P < 0.0001) than group F
CollPlant groups
Thawed/ungrafted controls
-
Group A
—untreated hosts and grafts
-
Group B
—treated host and treated graft (improvement protocol) without matrix. Hosts were treated with melatonin (240 mg/l) (Sigma) in the drinking water and grafts were incubated for 2 h with hyaluronic acid enriched-biological glue (Uterine Transfer Medium, MediCult, Jyllinge, Denmark) + VEGF-A (200 ng/ml) (Biological Industries, Beit Ha’emek, Israel) + vitamin E (E-400 natural, 400 IU/ml-oily form SOFT GEL Technologies Inc., Los Angeles, CA, USA) and implanted (Abir et al., 2017; Friedman et al., 2012).
-
Group C
—untreated hosts and untreated grafts, wrapped and encapsulated within the matrix without stitching. The grafts were encapsulated with CollPlant and implanted.
-
Group D
—untreated host and treated graft, with matrix. The grafts were incubated for 2 h as in group B and then encapsulated with CollPlant and implanted.
-
Group E
—treated host and untreated graft, with matrix. The hosts were treated as in group B and the grafts were encapsulated with CollPlant and implanted.
-
Group F
—treated host and treated graft (improvement protocol), with matrix. The hosts and the grafts were treated as in group B and the grafts were encapsulated with CollPlant and implanted.
Figure 4a shows the average follicle number in all CollPlant groups. There was a significantly higher number of follicles in the thawed/ungrafted controls than in the other CollPlant subgroups (with/without host and/or graft treatment with/without CollPlant; P < 0.0001 compared with groups A, D–F; P = 0.006 compared with group C). Follicle number was significantly higher in group C (untreated host and graft with CollPlant) than under the other host/graft treatment/non treatment conditions, with/without implantation with the matrix (group B P = 0.04, group D P = 0.005, group E P = 0.04, and group F P = 0.04). Regarding follicle classification in the thawed group, 65.7 were primordial and 34.3 were primary; group A = 66.7% were primordial and 33.3% were primary; group B = 77.3% were primordial and 22.7% were primary; group C = 14.8% were primordial, 81.8% were primary, and 18.5% were secondary; group D = 100% were primordial (only five follicles in this group); group E = 23.1% were primordial, 76.9% were primary, and 7.7% were secondary; group E = 23.1% were primordial, 76.9% were primary, and 7.7% were secondary; group F = 100% were primordial (only five follicles in this group). There were no significant differences between the groups. We chose to represent the percent of follicles and not their numbers as follicle growth occurs when the primordial follicle rate increases in a parallel with increase in primary and secondary follicles [3].
Figure 5a shows the percent of atretic follicles in all CollPlant groups. Significantly, higher atretic follicles were found in the thawed/ungrafted controls in groups A and F (treated host and graft with CollPlant) than those in groups B (P = 0.0018, P = 0.03, P = 0.008, respectively) and E (P = 0.001, P = 0.03, P = 0.008, respectively); in the thawed/ungrafted controls and in group F than in group C (P = 0.0002, P = 0.03, respectively).
Fig. 5.
Atretic follicle percent. The x axis represents the six experimental groups and the thawed ungrafted control (for both CollPlant and SIS groups).The y axis represents the percent of atretic follicles. The bars represent the different subgroups as in Fig. 4. (a) CollPlant groups: single asterisk indicates significantly higher than group B (P = 0.008, P = 0.03 and P = 0.0018, respectively). Double asterisks indicate significantly higher than group E (P = 0.008, P = 0.03, P = 0.001, respectively). Triple asterisks indicate significantly higher than group C (P = 0.03, P = 0.0002, respectively). (b) SIS groups: single asterisk indicates significantly higher than the thawed ungrafted group, groups K and L (all P < 0.0001) and group IH (P = 0.04). Double asterisks indicate significantly higher than the thawed ungrafted group (P = 0.002) and group IH (P = 0.003). Triple asterisks indicate significantly higher than the thawed ungrafted group and group L (both P < 0.0001), groups IH (P = 0.03) and K (P = 0.049). Quadruple asterisks indicate significantly higher than the thawed ungrafted group (P = 0.002) and group H (P < 0.0001)
Figure 6a shows the stroma cell apoptosis level in all CollPlant groups. Apoptosis levels were significantly higher in groups A and F than in thawed ungrafted controls (P = 0.001, P = 0.015, respectively), group B (P < 0.0001, P = 0.03, respectively), and group C (P = 0.0015, P = 0.03, respectively); in group E than in group B (P = 0.0015), and in group A than in group E (P = 0.02). None of the follicles had any brown apoptosis staining (Fig. 3f).
Fig. 6.
Apoptosis expression in thawed controls and grafts. Results are presented as mean ± standard deviation (SD). The x axis represents the six experimental groups and the thawed ungrafted control. The y axis represents the level of TUNEL staining. The apoptosis level grading scores are described in the “Methods” section. (a) CollPlant groups: single asterisk indicates significantly higher than group B (P = 0.03, P = 0.0015, P < 0.0001, respectively). Double asterisks indicate significantly higher than group C (P = 0.03, P = 0.0015, respectively). Triple asterisks indicate significantly higher than the thawed ungrafted group (P = 0.015, P = 0.001, respectively). Quadruple asterisks indicate significantly higher than group E (P = 0.02). (b) SIS groups: single asterisk indicates significantly higher than group H (P = 0.001 for the ungrafted controls and groups K and L, P = 0.002 for group B and P = 0.02 for group I).
There were significantly more Ki67 positively stained follicles in the ungrafted controls (P < 0.0001), in groups C (graft implanted only with CollPlant, P < 0.0001) and D (P = 0.02) than in group A; significantly more Ki67 positively stained follicles in group C than in group F (P < 0.0001) (Fig. 7). There were also more Ki67 positively stained follicles in the thawed/ungrafted controls than in group F (close to significance, P > 0.07).
SIS groups (Figs. 4–6)
Figure 4b shows follicle number in all SIS groups. Significantly, higher number of follicles was detected in group L (treated host and treated graft with SIS) and in the ungrafted controls than in group H (treated host and treated graft without SIS, P = 0.02 and P = 0.0061, respectively), group I (untreated host and graft with SIS, P = 0.01 and P = 0.0033, respectively), and group K (treated host and untreated graft with SIS, P = 0.04 and P = 0.0058, respectively). Significantly, higher number of follicles was also identified in the ungrafted controls than in groups I (untreated host and treated graft with SIS, P = 0.033) and J (untreated hosts and treated grafts with SIS, P = 0.0072) and group G (untreated grafts without SIS, P = 0.013). Regarding follicle classification in the thawed group: 70% were primordial, 26% were primary, and 4% were secondary; group G = 77.3% were primordial and 22.7% were primary; group H = 70.6% were primordial and 29.4% primary; group I = 100% were primordial (only two follicles were identified); group J = 67.4% were primordial and 32.6% were primary; group K = 100% were primordial; group L = 76.4% were primordial and 23.6% primary. There were no significant differences between the groups.
Figure 5b shows percent of atretic follicles in all SIS groups. Significantly, higher number of atretic follicles was found in group L than in thawed/ungrafted controls (P = 0.002) and group H (P < 0.0001) (Fig. 3m); in group G (untreated grafted controls) than in thawed/ungrafted controls (P < 0.0001) and groups H (P = 0.04), K (P < 0.0001), and L (P < 0.0001); in group I than in thawed/ungrafted controls (P = 0.002) and group H (P = 0.003); and group J than in thawed/ungrafted controls (P < 0.0001) and groups H (P = 0.03), K (P = 0.049) and L (P < 0.0001). Positive Ki67 staining was noted in granulosa cells of all morphologically normal grafted follicles (Fig. 3l) with no significant differences among the groups.
Figure 6b shows apoptosis levels in all SIS groups. Compared with group H (treated host and graft without SIS), stroma cell apoptosis levels were significantly higher in the thawed/ungrafted controls (P = 0.001), groups G (P = 0.002), I (P = 0.02), J (P = 0.001), K (P = 0.001), and L (P = 0.001) (Figs. 3n and o and 4b). It is noteworthy that occasionally, when retrieving the grafts, the SIS matrix got released from the tissue, although positive PECAM staining was identified within the matrix (Fig. 3r).
CollPlant vs. SIS
There was a significantly higher number of atretic follicles in group G than in groups D and E; in groups H–J than in group C; in group H than group E; and in groups H than groups C and E (P < 0.0001 for all comparisons). At the same time, there was a significantly higher rate of stroma cell apoptosis staining in group F than in groups I (P = 0.003), J (P = 0.001), and K (P = 0.03) (Fig. 4a and b).
Discussion
The present study showed that human ovarian tissue transplantation with CollPlant and SIS resulted in fusion with the surrounding tissue and vascularization. The CollPlant matrix even without additives (group C) promoted the best results for human ovarian grafting. It was associated with significantly higher numbers of recovered follicles, fewer atretic follicles (all CollPlant subgroups compared with most SIS subgroups), significantly more Ki67 granulosa cell staining (two CollPlant subgroups), and significantly less stroma cell apoptosis (CollPlant alone). However, follicle atresia levels were significantly higher in CollPlant ungrafted controls than in most groups transplanted with CollPlant. Both peripheral and diffuse positive PECAM stainings were demonstrated in samples grafted either with CollPlant or SIS. In the SIS experiment, although combining SIS with host and graft treatment (group L) led to significantly higher number of recovered follicles, this group also had significantly more atretic follicles than SIS ungrafted controls and group H (improvement protocol without SIS). Positive granulosa-cell Ki67 staining was noted in all morphologically normal follicles, regardless of group. However, there was a significantly higher rate of stroma-cell apoptosis in group F (improvement protocol with CollPlant) than in groups I–K (untreated or treated host and graft or host treatment only, with SIS), and the apoptosis rate was significantly higher in the ungrafted controls than in most SIS groups.
Young age is strongly correlated with high follicular density [3]. We, therefore, chose ovarian tissue from young patients. The low numbers of follicles identified in the recovered grafts relative to the thawed ungrafted controls of patients 1 and 3 in the SIS experiment could indicate accelerated follicular loss after transplantation. However, follicles are distributed unevenly in the human ovary [1, 20, 25], and as such, we might have inadvertently transplanted slices with no or only few follicles. This possibility seems likely considering the difference in follicular counts in ungrafted controls from patients 1 and 3 in the SIS and CollPlant experiments. Both the SIS-and CollPlant-treated samples retained their original size after implantation, and almost all the recovered samples in the SIS experiment contained a higher follicle number, although many of the follicles were atretic. As the number of recovered follicles was low, we could not find any statistical differences between the groups regarding follicle classification (primordial, primary, and secondary). This result was quite expected as development of primordial follicles to more advanced-stage follicles occurs within 6 to 9 months [2, 3], and we removed the grafts after 3 weeks.
The significantly high follicular atresia in the thawed ungrafted controls of the CollPlant experiments is in line with a report of high follicular abnormalities in pre-pubertal human ovaries [26]. Indeed, the ovarian tissue used in the CollPlant experiment was from younger patients (although only patient 4 used only in the CollPlant experiments was pre-pubertal) than the tissue used in the SIS experiment, but the difference was not significant. Otherwise, these samples were frozen with our usual slow freezing protocol that is used also by others [2, 6–8], and no unexpected problems were noted, nor did we report on an increase in follicle atresia in our previous grafting or culture studies [6–8, 18, 22, 23]. The significantly high stroma cell apoptosis level in the thawed ungrafted ovarian samples of the SIS experiments may have been due to the cryopreservation-thawing procedure affecting stroma cells rather than follicles, although we applied our standard freezing/thawing protocol [18]. It is possible that grafting reversed stroma cell apoptosis at least in the SIS groups just like it did follicle atresia in the CollPlant group. We have no explanation for the significantly high apoptosis in group F (improvement protocol, with CollPlant) as neovascularization was demonstrated by PECAM staining. In general, in the CollPlant experiment, apoptosis was higher after grafting in all groups (except for group B-improvement protocol, without matrix) than in their ungrafted controls, but none did the apoptosis levels rose relative to the ungrafted controls, suggesting that CollPlant reduced post-implantation apoptosis. We also postulate that the additives (hyaluronic acid-biological glue + vitamin E + VEGF-A) might have partially blocked the pores mostly in the SIS matrix but also in the CollPlant group (which explains less atretic follicles with CollPlant alone).
The positive peripheral and diffuse PECAM staining in grafts transplanted with CollPlant or SIS indicated that both matrices promoted successful fusion of the ovarian graft with the host tissue and neovascularization. However, the better results with the CollPlant matrices compared with the SIS matrices and the high follicle atretic rate in the SIS only group can possibly be explained by SIS attaching to the tissue at a lesser strength as we identified upon recovery only loose SIS fragments and not CollPlant. In future studies, we plan to investigate blood vessel origin (from hosts or grafts), as described by Van Eyck et al. [27]. The positive Ki67 staining in granulosa cells indicated follicular viability and proliferation. Moreover, grafting tissue in group C (untreated host and graft, with CollPlant) increased granulosa cell proliferation compared with group A (untreated host and graft, without CollPlant).
One study investigated the transplantation into immunodeficient mice of ovine ovarian tissue embedded in carrier gels of agar combined with collagen type I that included VEGF111 [10, 11]. Improved results were found attributable with VEGF111 rather than the collagen itself: accelerated blood vessel recruitment and improved ovarian cortex viability due to reduction in ischemia and tissue damage [11]. It is noteworthy that the authors used regular rat tail collagen and not novel pure CollPlant collagen. The VEGF-A used in our study has a shorter half-life in plasma (~ 30 min) [28] than VEGF111, and we did not test the effect of VEGF-A alone in our previous studies [6, 7].
SIS has been shown to serve as an efficient scaffold for remodeling rabbit ovaries after resection [19]. The rabbit ovaries were not frozen-thawed as in the present investigation. As in our study, the ovarian tissue transplanted with SIS retained its original size and fused well with the SIS matrix. However, in the rabbits, the SIS material induced an inflammatory response after 4 weeks but did not increase vascularization [19], whereas we clearly demonstrated neovascularization through the SIS matrix 3 weeks after human ovarian implantation in mice. Furthermore, in the rabbits, 24 weeks after surgery, the granulosa cells stained positively for proliferating cell nuclear antigen [19], a similar marker to Ki67 used in our study. Twenty-eight weeks after surgery, the primary and primordial follicles were well organized around the SIS ovary boundary and in the SIS matrix, a finding not observed in the present study. The differences between our results and those obtained in rabbits [19] can be explained by our use of immunodeficient mice, whereas Celik et al. [19] used rabbits with a normal immune system, which might have played a role in tissue remodeling, in addition to the longer duration of the rabbit study (up to 7 months), species-dependent differences, our use of frozen-thawed ovarian material, and differences in surgical techniques. Celik et al. [19] sutured the SIS matrix to the ovary, whereas we wrapped the implanted ovarian samples with the SIS matrix without stitching. As we found loose SIS fragments, it is possible that stitching would have improved our results.
Oktay et al. [9] transplanted human ovarian tissue from cadavers with ECTM to immunodeficient mice for 10 days. Similar to our results with CollPlant and SIS, ECTM fused well with the ovarian tissue without any pathological changes, and normal primordial follicles were detected in the grafted tissue. However, the ECTM-transplanted tissue was grafted subcutaneously, and as we had previously found that this implantation site was inefficient [29], we used the back muscle [6, 8] in the present study. Moreover, the earlier study [9] did not use any methods apart from standard histology to assess the quality of the transplanted ovarian tissue. Thereafter, the authors transplanted ovarian tissue after slow freezing-thawing with ECTM into two women, while we have not yet reached a clinical trial level.
Further studies are needed using other novel methods [2, 30–32] or matrices, blood-derived products [2, 33, 34], or drugs/growth factors [2, 35–41] capable of enhancing neovascularization and reducing apoptosis. In the present study, grafting with CollPlant promoted better results after implantation than SIS, and results with CollPlant alone (without host or graft treatment) seemed to be at least as good as those with our improvement protocol alone [7, 8]. Moreover, pure human bioengineered recombinant collagen produced in plant lines is a biomaterial without any potential risks of animal-derived pathogens or issues related to cadaver tissue [9]. Grafting ovarian tissue with CollPlant is easy and the procedure may be easily modified, with limited or no foreseeable risks, for auto-transplantation in cancer survivors. New and efficient methods to evaluate ovarian tissue quality after implantation are still required.
Acknowledgments
We are grateful to Ms. Gloria Ganzach from the Editorial Board of Rabin Medical Center for the English editing and to Ms. Carmela Felz for the histological sections.
Authors’ contributions
R. Abir designed the study and collected the data, assisted in IMH studies and viewed the sections, conducted most of the statistical analysis, and wrote most of the manuscript. D. Stav conducted the CollPlant part of the study, wrote parts of the manuscript, and edited all the figures. Y. Taib conducted the SIS part of the study and wrote parts of the manuscript. R. Gabbay-Benziv conducted the SIS transplantations, assisted in the various drafts of the manuscript and in the analysis of the results, and approved its final version and also suggested the use of CollPlant. M. Kirshner conducted the CollPlant transplantations, assisted in the various drafts of the manuscript and in the analysis of the results, and approved its final version. A. Ben-Haroush provided some of the ovarian samples and reviewed the final draft of the manuscript. E. Freud performed the surgery on the patients, wrote certain parts of the manuscript, and critically proofed the final manuscript. S. Ash recruited most of the patients, helped in writing the manuscript, and critically proofed the final manuscript. I. Yaniv recruited most of the patients, helped in writing the manuscript, and critically proofed the final manuscript. M. Herman-Edelstein contributed the positive PECAM control, helped in writing the manuscript, and critically proofed the revised manuscript. B. Fisch assisted in designing the study, assisted in the various drafts of the manuscript, the analysis of the results, and approved its final version. Y. Shufaro assisted in designing the study, assisted in the various drafts of the manuscript, the analysis of the results, and approved its final version.
Funding information
The study was partially funded by a research grant from the Israel Ministry of Health (for R.A. and B.F. no 3-6098-00000).
Footnotes
This work was part of the requirements for a "Medical Doctor" degree at the Sackler Faculty of medicine, Tel-Aviv University, Israel for Dana Stav and Yossi Taieb.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ronit Abir, Dana Stav and Yossi Taieb contributed equally to this work, and, therefore, should be considered joint first authors.
References
- 1.Feigin E, Freud E, Fisch B, Orvieto R, Kravarusic D, Avrahami G, Ben-Haroush A, Abir R. Cancer in female adolescents. Hauppauge, New York: Nova Science Publishers Inc; 2008. Fertility preservation in female adolescents with malignancies; pp. 103–138. [Google Scholar]
- 2.Fisch B, Abir R. Female fertility preservation: past, present and future. Reproduction. 2018;156:F11–F27. doi: 10.1530/REP-17-0483. [DOI] [PubMed] [Google Scholar]
- 3.Gougeon A. Regulations of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 4.Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377:1657–1665. doi: 10.1056/NEJMc1715731. [DOI] [PubMed] [Google Scholar]
- 5.Tilly JL. Apoptosis and ovarian function. Rev Reprod. 1996;1:162–172. doi: 10.1530/ror.0.0010162. [DOI] [PubMed] [Google Scholar]
- 6.Abir R, Fisch B, Jessel S, Felz C, Ben-Haroush A, Orvieto R. Improving posttransplantation survival of human ovarian tissue by treating the host and graft. Fertil Steril. 2011;95:1205–1210. doi: 10.1016/j.fertnstert.2010.07.1082. [DOI] [PubMed] [Google Scholar]
- 7.Abir R, Fisch B, Fisher N, Samara N, Lerer-Serfaty G, Magen R, Herman-Edelstein M, Ben-Haroush A, Stein A, Orvieto R. Attempts to improve human ovarian transplantation outcomes of needle-immersed vitrification and slow-freezing by host and graft treatments. J Assist Reprod Genet. 2017;34:633–644. doi: 10.1007/s10815-017-0884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman O, Orvieto R, Fisch B, Felz C, Freud E, Ben-Haroush A, Abir R. Possible improvements in human ovarian grafting by various host and graft treatments. Hum Reprod. 2012;27:474–482. doi: 10.1093/humrep/der385. [DOI] [PubMed] [Google Scholar]
- 9.Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol. 2016;214(1):94.e1–94.e9. doi: 10.1016/j.ajog.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mineur P, Colige AC, Deroanne CF, Dubail J, Kesteloot F, Habraken Y, Noël A, Vöö S, Waltenberger J, Lapière CM, Nusgens BV, Lambert CA. Newly identified biologically active and proteolysis-resistant VEGF-A isoform VEGF111 is induced by genotoxic agents. J Cell Biol. 2007;179:1261–1273. doi: 10.1083/jcb.200703052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labied S, Delforge Y, Munaut C, Blacher S, Colige A, Delcombel R, Henry L, Fransolet M, Jouan C, Perrier d’Hauterive S, Noël A, Nisolle M, Foidart JM. Isoform 111 of vascular endothelial growth factor (VEGF111) improves angiogenesis of ovarian tissue xenotransplantation. Transplantation. 2013;95:426–433. doi: 10.1097/TP.0b013e318279965c. [DOI] [PubMed] [Google Scholar]
- 12.Stein H, Wilensky M, Tsafrir Y, Rosenthal M, Amir R, Avraham T, Ofir K, Dgany O, Yayon A, Shoseyov O. Production of bioactive, post-translationally modified, heterotrimeric, human recombinant type-I collagen in transgenic tobacco. Biomacromolecules. 2009;10:2640–2645. doi: 10.1021/bm900571b. [DOI] [PubMed] [Google Scholar]
- 13.McPherson TB, Liang H, Record RD, Badylak SF. Galalpha (1,3) Gal epitope in porcine small intestinal submucosa. Tissue Eng. 2000;6:233–239. doi: 10.1089/10763270050044416. [DOI] [PubMed] [Google Scholar]
- 14.Voytik-Harbin S, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67l:478–491. doi: 10.1002/(SICI)1097-4644(19971215)67:4<478::AID-JCB6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 15.Cobb MA, Badylak SF, Janas W, Boop FA. Histology after dural grafting with small intestinal submucosa. Surg Neurol. 1996;46:389–393. doi: 10.1016/S0090-3019(96)00202-9. [DOI] [PubMed] [Google Scholar]
- 16.Hodde J, Badylak SF, Shelbourne KD. The effect of range of motion on remodeling of small intestinal submucosa (SIS) when used as an Achilles tendon repair material in the rabbit. Tissue Eng. 1997;3:27–37. doi: 10.1089/ten.1997.3.27. [DOI] [Google Scholar]
- 17.Allman AJ, McPherson TB, Badylak SF, Merrill LC, Kallakur B, Sheehan C, Raeder RH, Metzger DW. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation. 2001;71:1631–1640. doi: 10.1097/00007890-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 18.Younis AJ, Lerer-Serfaty G, Stav D, Sabbah B, Shochat T, Kessler-Icekson G, Zahalka M, Shachar-Goldenberg M, Ben-Haroush A, Fisch B, Abir R. Extracellular-like matrices and leukemia inhibitory factor for in vitro culture of human primordial follicles. Reprod Fertil Dev. 2017;29:1982–1994. doi: 10.1071/RD16233. [DOI] [PubMed] [Google Scholar]
- 19.Celik O, Esrefoglu M, Hascali S, Gul M, Tagluk ME, Elter K, Aydin E. Use of porcine small intestinal submucosa to reconstruct an ovarian defect. Int J Gynecol Obstet. 2009;106:218–222. doi: 10.1016/j.ijgo.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 20.Abir R, Ben-Haroush A, Felz C, Okon E, Raanani H, Orvieto R, Nitke S, Fisch B. Selection of patients before and after anticancer treatment for ovarian cryopreservation. Hum Reprod. 2008;23:869–877. doi: 10.1093/humrep/dem41. [DOI] [PubMed] [Google Scholar]
- 21.Rahimi G, Isachenko V, Kreienber R, Sauer H, Todorov P, Tawadros S, Mallmann P, Nawroth F, Isachenko E. Re-vascularisation in human ovarian tissue after conventional freezing or vitrification and xenotransplantation. Eur J Obstet Gynecol Reprod Biol. 2010;149:63–67. doi: 10.1016/j.ejogrb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Lerer-Serfaty G, Samara N, Fisch B, Shachar M, Kossover O, Seliktar D, Ben-Haroush A, Abir R. Attempted application of bioengineered/biosynthetic supporting matrices with phosphatidylinositol-trisphosphate-enhancing substances to organ culture of human primordial follicles. J Assist Reprod Genet. 2013;30:1279–1288. doi: 10.1007/s10815-013-0052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lande Y, Fisch B, Tsur A, Farhi J, Prag-Rosenberg R, Ben-Haroush, Kessler-Icekson G, Zahalka M, Ludeman SM, Abir R. Short term exposure of human ovarian follicles to cyclophosphamide metabolites seems to promote follicular activation in vitro. Reprod BioMed Online. 2017;34:104–114. doi: 10.1016/j.rbmo.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Hewitt SM, Baskin DG, Frevert CW, Stahl WL, Rosa-Molinar E. Controls for immunohistochemistry: the Histochemical Society’s standards of practice for validation of immunohistochemical assays. J Histochem Cytochem. 2014;62:693–697. doi: 10.1369/0022155414545224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt KLT, Byskov AG, Nyobe Andersen A, Muller J, Andersen CY. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18:1158–1164. doi: 10.1093/humrep/deg246. [DOI] [PubMed] [Google Scholar]
- 26.Anderson RA, McLaughlin M, Wallace WHB, Albertini DF, Telfer EE. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod. 2014;29:97–106. doi: 10.1093/humrep/det388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Eyck AS, Bouzin C, Feron O, Rome L, Van Langendonckt A, Donnez J, Dolmans MM. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93:1676–1685. doi: 10.1016/j.fertnstert.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 28.Tang J, Hua Y, Su J, Zhang P, Zhu X, Wu L, Niu Q, Xiao H, Ding X. Expression of VEGF and neural repair after Alprostadil treatment in a rat model of sciatic nerve crush injury. Neurol India. 2009;57:387–394. doi: 10.4103/0028-3886.55583. [DOI] [PubMed] [Google Scholar]
- 29.Abir R, Orvieto R, Raanani H, Feldberg D, Nitke S, Fisch B. Parameters affecting successful transplantation of frozen-thawed human fetal ovaries into immunodeficient mice. Fertil Steril. 2003;80:421–428. doi: 10.1016/S0015-0282(03)00658-7. [DOI] [PubMed] [Google Scholar]
- 30.Demeestere I, Simon P, Buxant F, Robin V, Aguilar Fernandez S, Centner J, Delbaere A, Englert Y. Ovarian function and spontaneous pregnancy after combined heterotopic and orthotopic cryopreserved ovarian tissue transplantation in a patient previously treated with bone marrow transplantation: case report. Hum Reprod. 2006;21l:2010–2014. doi: 10.1093/humrep/del092. [DOI] [PubMed] [Google Scholar]
- 31.Demeestere I, Simon P, Dedeken L, Moffa F, Tsepelidis S, Brachet C, Delbaere A, Devreker F, Ferster A. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod. 2015;3:2107–2109. doi: 10.1093/humrep/dev128. [DOI] [PubMed] [Google Scholar]
- 32.Israeli T, Nevo N, Harmelin A, Neeman M, Tsafriri A. Reducing ischemic damage in rodent ovarian xenografts transplanted into granulation tissue. Hum Reprod. 2006;21:1368–1379. doi: 10.1093/humrep/del010. [DOI] [PubMed] [Google Scholar]
- 33.Callejo J, Salvador C, Gonzalez-Nunez S, Almeida L, Rodriguez L, Marques L, Valls A, Lailla JM. Live birth in a woman without ovaries after autograft of frozen-thawed ovarian tissue combined with growth factors. J Ovarian Res. 2013;6:33. doi: 10.1186/1757-2215-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahmoodi M, Soleimani Mehranjani M, Shariatzadeh SM, Eimani H, Shahverdi A. Effects of erythropoietin on ischemia, follicular survival, and ovarian function in ovarian grafts. Reproduction. 2014;147:733–741. doi: 10.1530/REP-13-0379. [DOI] [PubMed] [Google Scholar]
- 35.Cohen Y, Dafni H, Avni R, Fellus L, Bochner F, Rotkopf R, Raz T, Walsh K, Neeman M. Genetic and pharmacological modulation of Akt1 for improving ovarian graft revascularization in a mouse model. Biol Reprod. 2016;94(14):1–11. doi: 10.1095/biolreprod.115.131987. [DOI] [PubMed] [Google Scholar]
- 36.Kang BJ, Wang Y, Zhang L, Xiao Z, Li SW. bFGF and VEGF improve the quality of vitrified-thawed human ovarian tissues after xenotransplantation to SCID mice. J Assist Reprod Genet. 2016;33:281–289. doi: 10.1007/s10815-015-0628-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, Lee JR, Youm HW, Suh CS, Kim SH. Effect of preoperative simvastatin treatment on transplantation of cryopreserved-warmed mouse ovarian tissue quality. Theriogenology. 2015;83:285–293. doi: 10.1016/j.theriogenology.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Kim EJ, Kong HS, Youm HW, Lee JR, Suh CS, Kim SH. A combination of simvastatin and methylprednisolone improves the quality of vitrified-warmed ovarian tissue after auto-transplantation. Hum Reprod. 2015;30:2627–2638. doi: 10.1093/humrep/dev222. [DOI] [PubMed] [Google Scholar]
- 39.Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6:1109–1114. doi: 10.1038/80442. [DOI] [PubMed] [Google Scholar]
- 40.Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS One. 2000;6:e19475. doi: 10.1371/journal.pone.0019475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu D, Lei Y, Tong Y, Tang F, Qian Y, Zhou Y. Angiogenesis of the frozen-thawed human fetal ovarian tissue at the early stage after xenotransplantation and the positive effect of Salviae miltiorrhizae. Anat Rec (Hoboken) 2010;293:2154–2162. doi: 10.1002/ar.21228. [DOI] [PubMed] [Google Scholar]