Abstract
Dispersion of rice protein (RP) at a neutral pH is highly important for its application in the food industry. We analyzed the solubility of RP at different pH conditions and found higher solubility at pH < 3 and pH > 8 than at a neutral pH. Furthermore, at pH 2, the RP solubility improved from 30 to 63% with sonication; however, the samples precipitated when the pH was increased from 2 to 7. To circumvent this, anionic pectin and sodium alginate were added to the RP solution at pH 2. Pectin formed a complex with RP at pH 2, showing a shift in the zeta-potential from 17.3 mV (RP only) to − 1.0 mV (RP plus 1% pectin). Interestingly, the formation of this RP-pectin complex allowed RP to remain dispersed when the pH was increased to 7. Moreover, a stable emulsion could be prepared using the RP-pectin complex as an emulsifier.
Keywords: Rice protein, Water solubility, Emulsion, Pectin
Introduction
Recently there have been efforts to replace milk proteins with plant proteins due to allergenicity, economic, sustainability and dietary issues (Ozturk and McClements, 2016; Xu et al., 2016). In particular, rice protein (RP) has garnered increasing interest in the food industry because it is hypoallergenic, rich in essential amino acids, and reportedly has higher digestibility and nutritive value than other major cereals, such as wheat, corn, and barley (Amagliani et al., 2017a; Han et al., 2015).
RP is also considered one of the vegetable protein sources that can replace milk and soy in infant formulas and add variety to gluten-free diets. However, the applications of RP in the food industry are limited because of its relatively low water-solubility. RP consists of four fractions with different solvent solubilities: albumin (water-soluble, 1–5%), globulin (salt-soluble, 4–15%), glutelin (alkali/acid-soluble, 80%), and prolamin (alcohol-soluble, 2–8%). The predominant RP, glutelin, is hydrophobic and highly aggregated by disulfide bonds; thus, the extracted RPs are highly insoluble (Amagliani et al., 2017a).
Many attempts have been made to improve the solubility of RP and increase its functional properties, including physical modification, enzymatic hydrolysis, and chemical modification. Several studies indicated that limited proteolysis was able to increase RP solubility, expose buried hydrophobic groups, increase surface hydrophobicity, reduce molecular weight, and increase the emulsion-forming ability of rice protein (Amagliani et al., 2017a; 2017b; Paraman et al., 2007). There are also many studies showing the effects of ultrasound on the properties of vegetable proteins (Hu et al., 2013; O'Sullivan et al., 2016; Yildiz et al., 2017) finding that solubility, gelation, and emulsification increased.
In a preliminary test, we confirmed that sonication of RP solutions increased the solubility of RP suspensions at pH 2, where RP was relatively more soluble, so that protein precipitation was not observed by eye after sonication. From these results, we hypothesized that if RP is completely dispersed under acidic pH 2 conditions using sonication and then anionic polysaccharides are added, interactions between RP and the polysaccharides may allow RP to remain in solution across a range of pH values, including neutral pH. This hypothesis was based on the fact that the protein molecules present positive charges at pH values below their pI. If RP could be dispersed without precipitation over a wide pH range, the possibility that RP could be used as a functional food ingredient would be increased.
Therefore, the purpose of this study was to investigate whether RP and polysaccharides can form a complex in acidic conditions (pH 2) after improving the solubility of RP by sonication and then examine whether the formation of RP-polysaccharide complexes can be used to disperse RP under various pH conditions. We also examined the suitability of RP-polysaccharide complex as an emulsifier.
Materials and methods
Materials
The rice used to isolate RP was waxy rice (rice variety: Sinseonchal) provided by the National Institute in Crop Science, Rural Development Administration (Iksan, South Korea). Pectin (from apple, DE 50–75%) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Sodium alginate and all of the other chemicals were of analytic grade and purchased from Showa Chemical Industry Co., Ltd. (Tokyo, Japan). Soybean oil was purchased from a local supermarket and used without further purification.
Isolation of RP
100 g of rice grains was soaked in 1000 mL of 0.2% NaOH for 1 h. The mixture was then ground with a food mixer (Type HR2096, Main Power Industrial Co. Ltd., Nanjing, China) and passed through a series of differently sized sieves. The starch slurry was centrifuged (1646 g, 10 min) and the supernatant was collected. The pH of the supernatant was adjusted to pH 4.5 to precipitate the RP. The precipitate was recovered by centrifugation (2569 g, 10 min), washed with deionized water, adjusted to pH 7.0, and freeze-dried.
Protein solubility
RPs were dispersed in citrate–phosphate buffers with a range of pH values (pH 2 to pH 11). Prior to analysis, the mixtures were stirred at room temperature for 1 h, and then centrifuged at 3061 g for 20 min. The protein solubility was calculated as the protein content of the supernatant as a percentage of the total protein content of the isolate. The soluble protein content in the supernatant was analyzed using the Kjeldahl method.
Sonication of RP solution
RP was dispersed in citrate–phosphate buffers with a range of pH values (pH 2 to pH 11). The mixtures were sonicated for 30 s at a frequency of 20 kHz, amplitude of 60% and duty cycle of 0.5 s (VCX 750, Sonics & Materials, Inc., Newtown, CT, USA) and centrifuged 2569 g for 10 min, and the supernatants were collected to analyze protein solubility.
Preparation and characterization of emulsions
Coarse emulsions (0.5 and 1.0 wt% oil) were prepared by homogenizing soybean oil with the RP-pectin dispersion (ration oil to RP, 1:1, pH 7.0) using a high-speed blender (ULTRA-TURRAX model T25 digital, IKA, Staufen im Breisgau Germany) for 2 min, and passing the mixture through a microfludizer (Picomax MN 250A, Micronox, Seongnam, Korea) three times at 6.89 MPa.
The particle diameter and ζ-potential of the droplets were analyzed by dynamic light scattering using a Zetasizer (ZS90, Malvern Instruments Ltd., Worcestershire, UK). Prior to measurement, emulsions (100 μL) were diluted with 10 mM phosphate buffer (4900 μL) to avoid multiple scattering. Mean particle diameter (Z-average) was obtained from the graph of signal intensity.
Statistical analysis
All data presented were the mean ± standard deviation. Statistical analysis was performed using SPSS for windows (ver. 21.0, IBM Corp., Armonk, N.Y., USA). A one-way ANOVA test followed by a Duncan’s multiple range test was conducted to identify statistical significances (p < 0.05).
Results and discussion
Effect of sonication treatment on the solubility of RP
In this set of experiments, RP dispersions at different pH values were sonicated to see whether sonication increases the solubility of RP. The influence of pH on the solubility of isolated RP in the absence of sonication and the change in solubility after sonication are shown in Fig. 1. Similar to that in previous studies (Mun et al., 2016; Pinciroli et al., 2009), solubility of RP was higher under acidic (pH < 3) and alkali (pH > 8) conditions than at other pH values. Because alkaline extracted RP is mainly composed of glutelin which contains disulfide bonds and shows extensive aggregation, it is not soluble in moderate pH ranges (Amagliani et al., 2017b; Hamada, 2000; Xu et al., 2016).
Fig. 1.
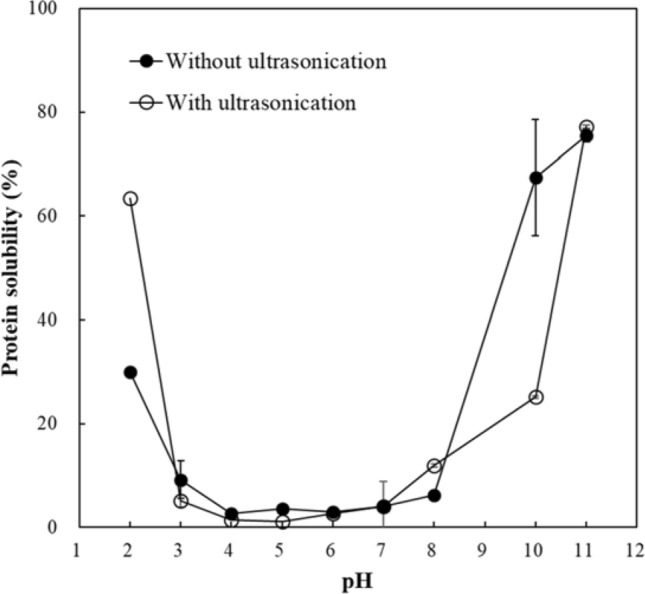
Rice protein solubility at different pH values with and without sonication
After sonication, the solubility of RP improved only at pH 2. This result might be due to structural changes such as unfolding of glutelin protein that occurs at extreme pH values. Under pH 2 and over pH 11, the binding force of glutelin seems to be weakened and therefore, it seems that RP dispersed in pH 2 and pH 11 are more easily affected by the physical force caused by sonication. It has been reported that pH-shifts to extremely acidic or basic pH values allow the protein molecules to unfold (Jiang et al., 2017; Lee et al., 2016; Yildiz et al., 2017). Yildiz et al. (2017) reported that SPI unfolded by high pH (pH 12) became more susceptible to breakdown by the physical forces produced by acoustic cavitation. At pH 11, solubility already exceeded 75% before sonication. Hence there was no significant difference in solubility in this study. Jiang et al. (2017) reported that pea protein solubility was highest at pH 12 in combination with ultrasound treatment, suggesting that pea protein molecules became more flexible and partially unfolded after the shift to pH12, which allowed the physical forces produced by acoustic cavitation to alter its microstructures resulting in an improvement in solubility. Therefore, the increase in solubility of RP at pH 2 obtained after sonication might be attributable to the partial unfolding of RP structure.
RP dispersions produced at pH 2 with sonication were centrifuged and the clear suspension was readjusted to different pH values (pH 3–7) to determine whether the RP would remain in solution at other pH values. However, precipitation was observed after adjusting the pH. Therefore, we examined whether the addition of polysaccharides would maintain RP in solution. Previous studies have reported that at pH values close to a protein’s isoelectric point (pI), protein-polysaccharide complexes improved the protein’s colloidal stability (Benichou et al., 2007; Li et al., 2019). If the pH of the protein solution is below the pI, the protein solution will have a net positive charge, which will interact with anionic polysaccharides to form a complex. Some studies have also reported that the stability of protein-stabilized emulsions at low pH could be increased by creating polysaccharide multilayers around oil droplets (Xu et al., 2017; Yildiz et al., 2018).
Effect of polysaccharide addition on water dispersibility of RP
Negatively charged polysaccharides (pectin and sodium alginate) were added to the RP dispersions, which were prepared by sonication in pH 2, and the ability of the RP-polysaccharide complex to maintain the dispersibility of RP under various pH conditions was examined.
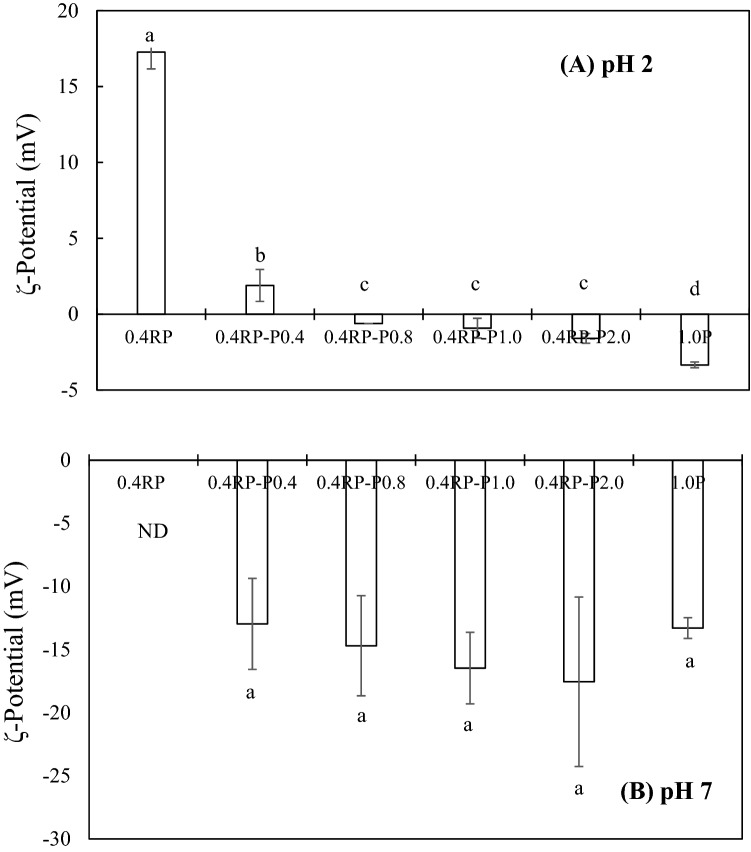
In order to determine the most suitable ratio of RP to polysaccharide, 0–2.0 wt% pectin and sodium alginate were added to 0.4% RP dispersions (pH 2). The zeta-potential of the 0.4% RP suspension in the absence of polysaccharides was around 17.3 mV, since pH 2 was below the pI of RP, (pI ≈ 4.5) net charge of protein was positive. As the concentration of pectin increased, the zeta-potential shifted towards a negative charge (Fig. 2A). With the addition of 0.4 wt% the charge was still positive, however, the zeta-potential became negative when the pectin level exceeded 0.8 wt%, suggesting that pectin was present on the surface of the RP molecules. The change in zeta-potential from positive to negative further indicated the formation of RP-pectin complexes. However, In the case of sodium alginate, precipitation occurred at all concentrations. Hence, it was difficult accurately measure the zeta-potential of the mixture of RP and sodium alginate.
Fig. 2.
Effect of pectin on the zeta-potential value of rice protein dispersions at pH 2 (A) and 7 (B). ND, not detected. Superscripted letters indicate a significant difference at p < 0.05. (0.4RP, 0.4% RP solution; 0.4RP-P0.4, 0.4% RP and 0.4% pectin mixture; 0.4RP-P0.8, 0.4% RP and 0.8% pectin mixture; 0.4RP-P1.0, 0.4% RP and 1.0% pectin mixture; 0.4RP-P2.0, 0.4% RP and 2.0% pectin mixture; 1.0P, 1.0% pectin solution.)
Generally, the interactions between proteins and polysaccharides can be divided into attractive and repulsive interactions depending upon the nature of the biopolymers, their concentrations, and the solution conditions. When a protein and polysaccharide carry opposite charges, they agglomerate to form either soluble complexes or insoluble precipitates. On the contrary, when two non-interacting biopolymers (i.e. carry the same charge) are mixed, they form either a single phase where two biopolymers distribute uniformly throughout the medium, or two distinct phases where each phase is concentrated within one of the two biopolymers. In the current study, RP and pectin had opposite charges, so it was likely that two biopolymer mixtures would exist either as a soluble complex or as an insoluble precipitate (Wusigale et al., 2020). The extent of complexation between two biopolymers is known to be affected not only by the solution conditions (pH, ionic strength, temperature, etc.) but also by the type of biopolymers and their molecular weight, charge density, and structure (Doublier et al., 2000).
It is well known that the negative charge on the alginate and pectin molecules is lower at pH 2 since the carboxyl group (pka ≈ 3.5) on pectin and alginate become partially protonated at pH 2. Nevertheless, there were differences between the two polysaccharides that can be attributed to their different molecular characteristics (Xu et al., 2017).
A previous study reported that secondary emulsions containing pectin were more stable to droplet aggregation and creaming at pH 2 than emulsions containing xanthan gum, gum Arabic, or alginate, although the zeta-potential values of all the emulsions were around zero. They explained that this result might be due to steric repulsion by pectin, which have long side chains, suggesting that the overall emulsion stability can be affected by the electrical characteristics of the interfacial layer and by other factors that influence the thickness and porosity of the polysaccharide layer (Xu et al., 2017).
At pH 2, the mean particle diameter of the 0.4% RP dispersion was 300 nm in the absence of polysaccharides. When pectin was added to the RP dispersion (0.4 wt% RP, 0–2.0 wt% polysaccharides, pH 2), the particle diameter increased. At 0.8% and 1.0% pectin, the mean particle diameters were 634 and 893 nm, respectively. With the addition of pectin, the particle diameter gradually increased (Fig. 3A), however, precipitation was not observed.
Fig. 3.
Effect of pectin on the mean particle diameter of rice protein dispersions at pH 2 (A) and 7 (B). ND, not detected. Superscripted letters indicate a significant difference at p < 0.05
The pH of RP dispersions containing pectin (0.4 wt% RP, 0.8 and 1.0 wt% pectin, pH 2) was changed to pH 7 and particle diameter and zeta-potential were measured (Figs. 2B and 3B). In the absence of pectin, the RP dispersion rapidly precipitated as soon as the pH value shifted to pH 7. However, the RP suspensions containing 0.8 and 1.0 wt% pectin remained clear suspensions, while precipitation occurred slowly with 0.4% pectin. A suspension of 0.4 wt% RP and 0.8 wt% pectin (0.4RP-0.8P) was chosen for the next experiments, since the results indicated that this was the lowest concentration of pectin that surrounded all existing RP molecules.
Emulsifying properties
When emulsions were manufactured with 0.5 and 1.0% oil concentrations, RP alone could not form stable emulsions at pH 7. The dispersion with a 1:2 ratio of RP and pectin (0.4RP-0.8pectin) was tested for its suitability as an emulsifier. The emulsion prepared with 0.5% oil separated, whereas emulsions with 1.0% oil, were stable to creaming and maintained stability to separation after storage for one week. The mean particle diameter and zeta-potential of droplets within the RP-pectin complex-stabilized emulsion prepared with 1.0 wt% oil were 731.8 nm and − 18.9 mV, respectively, and these values were not changed after storage for 1 week. The difference between 0.5 wt% oil and 1.0 wt% oil emulsions might be ascribed to a difference in viscosity. The amount of RP and pectin added to stabilize 1.0 wt% oil is highly effective at increasing the viscosity of the emulsion, which may have prevented the oil droplets from creaming. Further studies related to the rheology of RP dispersions containing pectin are needed.
Dispersion of RP at a neutral pH is highly important for its application in the food industry. However, unmodified RP shows poor solubility at a neutral pH. To address this, physical, chemical, and enzymatic treatments of RP have been tested in an attempt to improve the functional properties (e.g., emulsifying capacity) of RP. For example, hydrolysates of rice or a combination of RP hydrolysates with a non-ionic surfactant have been used to form stable emulsions (Chen et al., 2016; Paraman et al., 2007; Xu et al., 2016). In the present study, we showed that the formation of a RP-pectin complex at pH 2 allowed the RP to remain dispersed when the pH was increased to 7. Additionally, a stable emulsion could be prepared using this RP-pectin complex as an emulsifier. These findings suggest that the formation of a complex of RP with anionic polymers could allow the preparation of highly dispersible RP at a neutral pH and thus expand its scope of application in the food industry.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2018R1A2B6005003).
Compliance with ethical standards
Conflict of interest
The authors declared that they have no conflict of interst.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Saehun Mun, Email: saehun@snu.ac.kr.
Jeonghee Surh, Email: jsurh@kangwon.ac.kr.
Malshick Shin, Email: msshin@chonnam.ac.kr.
References
- Amagliani L, O’Regan J, Kelly AL, O'Mahony JA. The composition, extraction, functionality and application of rice proteins: a review. Trends Food Sci. Technol. 2017;64:1–12. doi: 10.1016/j.tifs.2017.01.008. [DOI] [Google Scholar]
- Amagliani L, O’Regan J, Kelly AL, O'Mahony JA. Composition and protein profile analysis of rice protein ingredients. J. Food Compos. Anal. 2017;59:18–26. doi: 10.1016/j.jfca.2016.12.026. [DOI] [Google Scholar]
- Benichou A, Aserin A, Lutz R, Garti N. Formation and characterization of amphiphilic conjugates of whey protein isolate (WPI)/xanthan to improve surface activity. Food Hydrocoll. 2007;21:379–391. doi: 10.1016/j.foodhyd.2006.04.013. [DOI] [Google Scholar]
- Chen X, Li W, Zhao Q, Selomulya C, Zhu X, Xiong H. Physical and oxidative stabilities of OW emulsions formed with rice dreg protein hydrolysate: Effect of xanthan gum rheology. Food Bioprocess Technol. 2016;9:1380–1390. doi: 10.1007/s11947-016-1727-9. [DOI] [Google Scholar]
- Doublier JL, Garnier C, Renard D, Sanchez C. Protein-polysaccharide interactions. Curr. Opin. Colloid Interface Sci. 2000;5:202–214. doi: 10.1016/S1359-0294(00)00054-6. [DOI] [Google Scholar]
- Hamada JS. Characterization and functional properties of rice bran proteins modified by commercial exoproteases and endoproteases. J. Food Sci. 2000;2:305–309. doi: 10.1111/j.1365-2621.2000.tb15998.x. [DOI] [Google Scholar]
- Han S-W, Chee K-M, Cho S-J. Nutritional quality of rice bran protein in comparison to animal and vegetable protein. Food Chem. 2015;172:766–769. doi: 10.1016/j.foodchem.2014.09.127. [DOI] [PubMed] [Google Scholar]
- Hu H, Wu J, Li-Chan ECY, Zhu L, Zhang F, Xu X, Fan G, Wang L, Huang X, Pan S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013;30:647–655. doi: 10.1016/j.foodhyd.2012.08.001. [DOI] [Google Scholar]
- Jiang S, Ding J, Andrade J, Rababah TM, Almajwal A, Abulmeaty MM, Feng H. Modifying the physicochemical properties of pea protein by pH-shifting and ultrasound combined treatments. Ultrason. Sonochem. 2017;38:835–842. doi: 10.1016/j.ultsonch.2017.03.046. [DOI] [PubMed] [Google Scholar]
- Lee H, Yildiz G, dos Santos LC, Jiang S, Andrade JE, Engeseth NJ, Feng H. Soy protein nano-aggregates with improved functional properties prepared by sequential pH treatment and ultrasonication. Food Hydrocoll. 2016;55:200–209. doi: 10.1016/j.foodhyd.2015.11.022. [DOI] [Google Scholar]
- Li Q, Lan H, Zhao Z. Protection effect of sodium alginate against heat-induced structural changes of lactoferrin molecules at neutral pH. LWT Food Sci. Technol. 2019;99:513–518. doi: 10.1016/j.lwt.2018.10.019. [DOI] [Google Scholar]
- Mun S, Shin M, Kim YR. Emulsifying properties of proteins isolated from various rice cultivars. Food Bioporcess Technol. 2016;9:813–821. doi: 10.1007/s11947-015-1667-9. [DOI] [Google Scholar]
- O'Sullivan J, Murray B, Flynn C, Norton I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016;53:141–154. doi: 10.1016/j.foodhyd.2015.02.009. [DOI] [Google Scholar]
- Ozturk B, McClements DJ. Progress in natural emulsifiers for utilization in food emulsions. Curr. Opin. Food Sci. 2016;7:1–6. doi: 10.1016/j.cofs.2015.07.008. [DOI] [Google Scholar]
- Paraman I, Hettiarachchy NS, Schaefer C, Beck MI. Hydrophobic, solubility, and emulsifying properties of enzymatic-modified rice endosperm protein. Cereal Chem. 2007;84:343–349. doi: 10.1094/CCHEM-84-4-0343. [DOI] [Google Scholar]
- Pinciroli M, Vidal AA, Añón M, Martínez EN. Comparison between protein functional properties of two rice cultivars. LWT Food Sci. Technol. 2009;42:1605–1610. doi: 10.1016/j.lwt.2009.06.003. [DOI] [Google Scholar]
- Wusigale Liang L, Luo Y. Casein and pectin: structures, interactions and applications. Trends Food Sci. Technol. 2020;97:391–403. doi: 10.1016/j.tifs.2020.01.027. [DOI] [Google Scholar]
- Xu X, Liu W, Liu C, Luo L, Chen J, Luo S, McClements DJ, Wu L. Effect of limited enzymatic hydrolysis on structure and emulsifying properties of rice glutelin. Food Hydrocoll. 2016;61:251–260. doi: 10.1016/j.foodhyd.2016.05.023. [DOI] [Google Scholar]
- Xu X, Luo L, Liu C, McClements DJ. Utilization of anionic polysaccharides to improve the stability of rice glutelin emulsions: Impact of polysaccharide type, pH, salt, and temperature. Food Hydrocoll. 2017;64:112–122. doi: 10.1016/j.foodhyd.2016.11.005. [DOI] [Google Scholar]
- Yildiz G, Andrade J, Engeseth NE, Feng H. Functionalizing soy protein nano-aggregates with pH-shifting and mano-thermo-sonication. J. Colloid Interface Sci. 2017;505:836–846. doi: 10.1016/j.jcis.2017.06.088. [DOI] [PubMed] [Google Scholar]
- Yildiz G, Ding J, Andrade J, Engeseth NJ, Feng H. Effect of plant protein-polysaccharide complexes produced by mano-thermo-sonication and pH-shifting on the structure and stability of oil-in-water emulsions. Innov. Food Sci. Emerg. Technol. 2018;47:317–325. doi: 10.1016/j.ifset.2018.03.005. [DOI] [Google Scholar]