Abstract
Nine medicinal plants and their stick-type medicinal concentrated beverages (SMCB-I and SMCB-II) with different combination ratio were evaluated on antioxidant, nitric oxide (NO) inhibitory, and antibacterial effects against pathogenic bacteria involved in respiratory system illnesses. Antioxidant activity was high in Syzygium aromaticum, Pueraria lobata, Plantago asiatica, and Kalopanax pictus which have higher contents of total phenolics and total flavonoids. The NO inhibitory activity was high in Syzygium aromaticum, Plantago asiatica, and Glycyrrhiza uralensis. Syzygium aromaticum, Plantago asiatica, Kalopanax pictus and Glycyrrhiza uralensis showed higher antibacterial activity than the other five medicinal plants against Staphylococcus aureus, Corynebacterium diphtheriae, and Mycobacterium sp. SMCB-II exhibited higher antioxidant, NO inhibitory, and antibacterial effects than SMCB-I, since Syzygium aromaticum, Pueraria lobata, and Kalopanax pictus were only used for the production of SMCB-II. The SMCBs would be expected to contribute to an easy-to-carry, easy-to-consume, and high value-added health beverage for the modern people.
Keywords: Antibacterial, Antioxidant, Medicinal plants, Nitric oxide, Stick-type medicinal concentrated beverage
Introduction
The main air pollutants have received growing attention as the key cause of respiratory illnesses in modern society. Respiratory system illnesses can also be caused by various bacteria. According to the Centers for Disease Control and Prevention, the most common cause of nosocomial pneumonia cases was Staphylococcus aureus between 1990 and 1992 in the United States (Emori and Gaynes, 1993). In addition, Corynebacterium diphtheriae is the main cause of diphtheria, a bacterial respiratory infection (Belsey et al., 1969); Streptococcus pyogenes infects the upper airway (nose, pharynx, larynx, etc.) and is the causative pathogen of pharyngitis, pneumonia, and acute respiratory distress syndrome (Stevens, 1992). Mycobacterium sp. is classified into tuberculous and non-tuberculous bacteria, where the latter is predominantly responsible for pulmonary diseases (Stout et al., 2016). A bronchial disease is caused when the bronchial mucosal cells are weakened by the common cold, fine particulate matter, or cigarette smoking, and the secondary infection by pathogenic bacteria results in inflammation that persists and leads to conditions such as asthma, pneumonia, or tuberculosis.
Medicinal plants have traditionally been widely used for beneficial pharmacological effects on human health. Syzygium aromaticum, Plantago asiatica, Glycyrrhiza uralensis, Panax ginseng, and Platycodon grandiflorum have been reported to exhibit antimicrobial effects against the bacteria causing bronchial diseases as well as various microorganisms (Lee et al., 2000; Ravn and Brimer, 1988; Sung and Lee, 2008; Wang et al., 2015). Ongoing studies are also focused on the antimicrobial mechanisms of the extracts or main components of these plants. The anti-inflammatory activity of Syzygium aromaticum, Glycyrrhiza uralensis, Plantago asiatica, Panax ginseng, and Pueraria lobata have been reported to inhibit nitric oxide production and suppress pro-inflammatory mediator expression (Lee et al., 2006; Sung and Lee, 2008; Tanemoto et al., 2015; Türel et al., 2009). Analysis of the DPPH free radical scavenging capacity and ferrous ion (Fe2+) chelating activity shown by the extracts of Plantago asiatica and Kalopanax pictus identified that the antioxidant activity is produced by phenolic acids, polyphenols, and flavonoids (Amakura et al., 2012; Kim et al., 2013).
Presently in 2019, the Ministry of Food and Drug Safety in Korea has 33 categories for the approval and release of functional materials for functional health foods, including intestinal health, cholesterol regulation, body fat reduction, and antioxidant activity; however, a category for approval has not yet been established for the functional ingredients that improve respiratory or bronchial symptoms (MFDS and NFSI). The prevalence and mortality rates of respiratory and chronic lung diseases have increased, not only in Korea but across the globe, due to the deteriorated air quality caused by climate change, air pollutants such as fine particulate matter, and cigarette smoking. This implicates a need for the development of functional ingredients and functional foods with respect to respiratory illnesses. In Korea, research on medicinal plants or their complex extracts for improving respiratory illnesses has been conducted; in particular, toward the development of concentrated beverages as they can be carried around and consumed in a convenient way.
In this study, total phenolics, total flavonoids, and crude saponin contents of the pharmacologically active nine medicinal plants were analyzed; their antioxidant, antibacterial and inhibition of nitric oxide production effects were evaluated with the purpose of investigating the basic efficacy of each medicinal plant for improving respiratory illnesses. By combining Zizyphus jujuba extract with a concentrate of the complex extract produced by mixing different composition ratio of medicinal plants, a stick-type medicinal concentrated beverage (SMCB) was developed, and its antioxidant, antibacterial, and nitric oxide inhibitory activities were assessed to verify the potential application as a functional medicinal beverage.
Materials and methods
Materials
The medicinal plants were provided by the National Institute for Korean Medicine Development (Gyeongsan, Korea): Platycodon grandiflorum (PGA; Yeongju, Korea), Plantago asiatica (PA; China), Panax ginseng (PG; Yeongju, Korea), Citrus unshiu (CU; Jeju, Korea), Zizyphus jujuba (ZJ; Gyeongsan, Korea), Glycyrrhiza uralensis (GU; China), Pueraria lobata (PL; Geochang, Korea), Kalopanax pictus (KP; Hoengseong, Korea), and Syzygium aromaticum (SA; Indonesia). For stick-type medicinal beverages commercially available in Korea: red ginseng concentrated beverage (CGB; JeongKwanJang, Daejeon, Korea) and platycodon ginseng beverage (CPBG; PGA (90%), ZJ (5%), red ginseng (3.4%); Natural Charm Food, Goyang, Gyeonggido, Korea) were purchased.
Dipotassium hydrogen phosphate (K2HPO4), ammonium nitrate (NH4NO3), magnesium sulfate anhydrous (MgSO4), and sodium carbonate (Na2CO3) were purchased from Junsei Chemical Co., Ltd. (Tokyo, Japan). Dextrose, iron(II) sulfate heptahydrate (FeSO4·7H2O), Folin–Ciocalteu phenol reagent, iron(III) sulfate hexahydrate (FeCl3·6H2O), sodium nitrite (NaNO2), quercetin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), aluminum chloride (AlCl3), gallic acid, 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), phosphate buffered saline (PBS), and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). Sodium acetate trihydrate and L(+)-ascorbic acid were purchased from Samchun Pure Chemical Co., Ltd. (Pyeongtaek, Gyeonggi-do, Korea). BBL™ trypticase™ soy broth and Bacto™ yeast extract were purchased from Becton, Dickinson and Company (BD) (Franklin Lakes, NJ, USA). Sulfanilamide and N-1-naphthylethylenediamine dihydrochloride (NED) solutions were purchased from Promega (Madison, WI, USA). Blood agar plates were purchased from Synergy Innovation (Seongnam, Gyeonggi-do, Korea) and agar was purchased from Oriental Chemical Industries (Seoul, Korea). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin–streptomycin, and 0.25% trypsin–EDTA solution were purchased from HyClone (Logen, UT, USA).
Preparation of SMCB
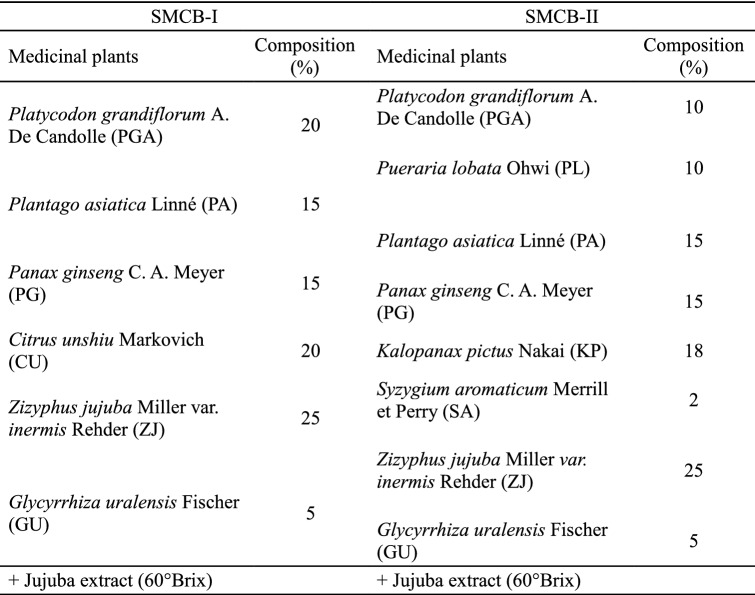
SMCB-I and SMCB-II were prepared based on the composition ratio shown in Table 1. Each medicinal plant was mixed to the weight of 200 g, water (2 L) was added, and then put through two sets of reflux extraction at 100 °C for 2 h. After filtration, the concentrate and ZJ extract (60°Brix, Hanbando, Gyeongsan, Korea) were mixed at a ratio of 1:1 (v/v) by adding water to produce a concentrate of 40°Brix. Each medicinal plant was mixed with hot water (10 times the weight of the plant) and followed by two sets of reflux extraction at 100 °C for 2 h. After filtration, the extract was concentrated, freeze-dried (PVTFD20R, ShinBioBase, Dongducheon, Korea), and stored at − 4 °C for subsequent use.
Table 1.
Composition of medicinal plants for preparation of stick-type medicinal concentrated beverage (SMCB)
Total phenolic content (TPC) analysis
SMCBs and the freeze-dried extracts of medicinal plants were diluted to 0.1 g/5 mL in distilled water for the various analyses. In a test tube, 0.5 mL sample, 4.5 mL distilled water, and 0.5 mL of Folin–Ciocateu reagent were added; 1 mL of Na2CO3 was added to the mixture after 3 min. After leaving the sample mixture in a dark room for 1 h, the absorbance was measured at 725 nm using a microplate reader (Multiskan GO, Thermo Scientific, Waltham, MA, USA). Gallic acid solutions were prepared for the standard curve, and the TPC was calculated and expressed as mg gallic acid equivalent (GAE)/g.
Total flavonoids content (TFC) analysis
In a test tube, 1 mL sample and 4 mL distilled water were added. After leaving it to stand for 5 min, 0.3 mL of 5% NaNO2 solution and 0.3 mL of 10% AlCl3 solution were added. After 6 min, 2 mL of 1 M NaOH solution and 2.4 mL distilled water were added, and the absorbance was measured at 510 nm using a microplate reader. Quercetin solutions were prepared to produce the standard curve, and then the TFC was calculated and expressed as mg quercetin equivalent (QE)/g.
Crude saponin content analysis
In a conical flask, 1–2 g sample and 60 mL distilled water were mixed and transferred to a separatory funnel, to which 60 mL ethyl ether was added for removing lipids. The water layer was taken and transferred to a new separatory funnel; 60 mL water-saturated butanol was added and the mixture was left to stand until the water and butanol layers completely separated. After repeated three times, the upper layer was collected in a separatory funnel, and 50 mL distilled water was added for washing and separation. The upper layer was transferred to a round flask and concentrated with a rotary vacuum evaporator (N-1110, EYELA, Tokyo, Japan) followed by drying in a 105 °C oven for 20 min. The crude saponin content was calculated with the following equation:
A1 = weight of flask (g), A2 = weight of flask after concentrated and dried water-saturated butanol layer (g), S = weight of sample (g).
Antioxidant assays
DPPH free radical scavenging capacity (RSC)
To a test tube, the diluted sample (0.2 mL), ethanol (1.8 mL), and 0.15 mM DPPH solution (2.5 mL) were mixed. The mixture was left in a dark room for 30 min, then the absorbance was measured at 517 nm using a UV/vis spectrophotometer (Optizen 2120UV, Optizen, Daejeon, Korea). The DPPH RSC was calculated using the following equation:
A0 = absorbance of blank; A1 = absorbance of sample.
Ferric reducing antioxidant power (FRAP)
FRAP reagent was prepared by mixing 250 mL of acetate buffer (300 mM, pH 3.6), 50 mL of 10 mM TPTZ solution, and 50 mL of 20 mM FeCl3·6H2O solution. To a test tube, 150 μL of the diluted sample and 4.5 mL of FRAP reagent were added; the mixture was left to react at 37 °C for 4 min and then the absorbance was measured at 593 nm using a microplate reader. Ascorbic acid solutions were prepared for the standard curve, and then the FRAP value was calculated, and expressed as mg ascorbic acid equivalent (AAE)/g.
NO assay
Mouse-derived macrophage RAW 264.7 cells were purchased from Korea Cell Line Bank (Seoul, Korea). The cells were cultured in DMEM containing 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin in a 5% CO2 incubator at 37 °C and 95% humidity. RAW 264.7 cells were seeded and cultured in a 24 well plate at 1 × 105 cell/mL/well. After removing the medium, DMEM containing 1% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, and 100 ng/mL PBS (for the negative control) or 100 ng/mL LPS (for LPS-treated group or sample-treated groups) were added, and cultured for 96 h. For the sample-treated groups, the extract of medicinal plants (0.2 mg/mL) or SMCB (20 mg/mL) were also added. Afterwards, 50 μL of the supernatant taken from each well and 50 μL of sulfanilamide solution were mixed in a 96 well plate and left in a dark room for 5–10 min. Then 50 μL NED solution was added for another 5–10 min and the absorbance was measured at 535 nm. Sodium nitrite solutions were prepared for a standard curve and the level of NO production was calculated. The NO production of each sample-treated group was converted to % based on the NO production of the LPS-treated group.
Determination of minimum inhibitory concentration (MIC)
Staphylococcus aureus (KCCM 12256), C. diphtheriae (KCCM 40413), and S. pyogenes (KCCM 11873) were purchased from the Korean Culture Center of Microorganisms (KCCM, Seoul, Korea). Mycobacterium sp. (KCTC 1829) and Escherichia coli (KCTC 2593) were purchased from the Korean Collection for Type Cultures (KCTC, Jeongeup, Korea). S. aureus and E. coli were cultured using trypticase soy agar and trypticase soy broth, and C. diphtheriae and S. pyogenes were cultured using blood agar plates and tryptic soy broth, at 37 °C for 24 h. Mycobacterium sp. was cultured using the medium for KCTC 1829 at 29 °C. One colony was taken from each microbial strain culture using a platinum loop and inoculated in the liquid broth. S. aureus, C. diphtheriae, S. pyogenes, and E. coli were cultured at 37 °C for 24 h, whereas Mycobacterium sp. was cultured at 29 °C for 66 h. The cultured bacterial medium was diluted until the absorbance at 660 nm was 0.03 (1–2 × 107 CFU/mL). A SMCB mixed medium was prepared at a concentration of 1280 mg/mL and diluted by twofold serial dilutions. In a sterilized 96 well plate (Simport® Scientific, QC, Canada), 1 mL of SMCB mixed medium was added and 10 μL of the bacterial culture medium was inoculated; the plate was incubated for 24 h in a shaking incubator (150 rpm). The concentration of the SMCB mixed medium where bacterial growth could not be visually detected was selected as the MIC. In addition, when microbial survival could not be ascertained due to a strong sample color, the absorbance was measured at 600 nm for comparison against the control and the MIC was determined. The MIC of the medicinal plant extracts was also measured by inoculating the microbial strain of mixed medium.
Statistical analysis
TPC, TFC, crude saponin content, and antioxidant activity results were presented as mean ± standard deviation (SD). Analysis of variance (ANOVA) was performed with Statistical Analysis System 9.2 (SAS Institute Inc., Cary, NC, USA) and Duncan’s multiple range test was used to determine the statistical significance of the means at α = 0.05. The result of NO production was expressed as the mean ± standard error of the mean (SEM); GraphPad Prism 5 (San Diego, CA, USA) was used for performing one-way ANOVA and Newman–Keuls Multiple Comparison Test for the statistical significance of the means at α = 0.05. The IBM SPSS statistics 25 (Armonk, NY, USA) was used for the correlation analysis, and the Pearson correlation coefficient (r) and p value were calculated and used to determine the statistical significance at α = 0.05 or α = 0.01.
Results and discussion
TPC
The medicinal plant extracts showed a TPC of 5.08–304.92 mg GAE/g, which indicated a broad range with substantial differences (Table 2). The order of TPC was SA > PL > PA > GU > CU > KP > ZJ > PG > PGA, and in particular, SA exhibited 3.8 times and 60.0 times higher content of phenolic compounds than PL and PGA, respectively. The TPC of SMCBs developed in this study was significantly higher than the commercially available CGB or CPGB (p < 0.05), with the TPC of SMCB-II being 1.43 times higher than SMCB-I, 3.14 times higher than CGB, and 1.95 times higher than CPGB (p < 0.05) (Table 2). Looking into the type and the composition ratio of the medicinal plants used in SMCB preparation, PA (15%), PG (15%), ZJ (25%), and GU (5%) were used across all SMCBs with an identical composition ratio. CU (20%) was used in SMCB-I only, and SA (2%), PL (10%), and KP (18%) were used in SMCB-II only. PGA was used in SMCB-I and SMCB-II with the composition ratio of 20% and 10%, respectively (Table 1). In SMCB-II, despite the low content of SA and PL, the high TPC of each contributed to the higher TPC in SMCB-II than in SMCB-I. In addition, the lower TPC of CGB and CPGB may be attributed to composition with medicinal plants with low TPC in red ginseng (100%) and platycodon (90%), respectively.
Table 2.
Total phenolics, total flavonoids and crude saponin contents of medicinal plant extracts and stick-type medicinal concentrated beverages (SMCB)
| Sample | Total phenolics (mg GAE1/g) | Total flavonoids (mg QE2/g) | Crude saponin (g%) |
|---|---|---|---|
| Stick-type medicinal concentrated beverages | |||
| SMCB-I | 10.09 ± 0.29b | 3.88 ± 0.44b | 23.85 ± 1.03b |
| SMCB-II | 14.43 ± 0.49a | 10.29 ± 0.33a | 35.66 ± 0.35a |
| CGB3 | 4.59 ± 0.20c | 0.02 ± 0.03d | 16.40 ± 0.41c |
| CPGB4 | 1.95 ± 0.12d | 0.54 ± 0.13c | 7.71 ± 0.02d |
| Medicinal plants5 | |||
| SA6 | 304.92 ± 5.74a | 98.76 ± 2.27b | NA7 |
| PL | 79.82 ± 7.51b | 71.70 ± 0.84c | 37.55 ± 2.09a |
| PA | 65.71 ± 5.33c | 132.39 ± 3.35a | NA |
| KP | 32.87 ± 3.69d | 54.53 ± 2.80d | 29.91 ± 0.35b |
| CU | 36.92 ± 6.42d | 10.25 ± 0.55f | NA |
| PGA | 5.08 ± 0.12f | 3.66 ± 0.29g | 4.74 ± 0.19e |
| GU | 45.13 ± 8.74d | 24.99 ± 2.65e | 24.53 ± 0.63c |
| PG | 7.48 ± 0.38ef | 1.56 ± 0.19g | 19.66 ± 2.09d |
| ZJ | 19.05 ± 2.73e | 3.33 ± 0.40g | NA |
1GAE, gallic acid equivalent
2QE, quercetin equivalent
3Commercial red ginseng concentrated beverage
4Commercial platycodon red ginseng concentrated beverage
5Freeze-dried water extract of medicinal plants
6The medial plant of the abbreviation is the same as Table 1
7Not available
a–gMeans within the same column with different superscript letters are significant different by Duncan’s multiple range test at p < 0.05 (n = 3)
The main phenolic compounds in medicinal plants are phenolic acids (hydroxybenzoic acid and hydroxycinnamic acid), flavonoids (flavonols, flavones, flavanones, isoflavone, anthocyanidins, and tannins), and lignans. The phenolic acids are present in the forms of ester, glycosides, or amides (Tanase et al., 2019). The main phenolic compounds in SA are eugenol (4-allyl-2-methoxyphenol), eugenyl acetate (2-methoxy-4-(2-prophenyl)-phenol), and pyrogallol; these compounds play a vital role with antioxidant and antimicrobial activities (Hemalatha et al., 2016). PL contains protocatechuic acid, glucosyringic acid, and chlorogenic acid (Sano et al., 1991); PA contains caffeic acid and the derivatives (chlorogenic acid and plantamajoside) (Ferrazzano et al., 2015); and KP contains syringin, liriodendrin, chlorogenic acid, protocatechuic acid, glucosyringic acid, and vanillic acid (Kim et al., 2013; Sano et al., 1991). CU contains caffeic acid, ferulic acid, p-coumaric acid, and sinapinic acid (Bocco et al., 1998).
TFC
Medicinal plant extracts showed TFC of 1.56–132.39 mg QE/g, indicating a broad range of flavonoid content with substantial differences (Table 2). The order of TFC was PA > SA > PL > KP > GU > CU > PGA > ZJ > PG with statistical significance (p < 0.05), and PA exhibited 1.3 times and 88.9 times higher TFC than SA and PG, respectively. The TFC of SMCBs was significantly higher than CGB or CPGB (p < 0.05), with the TFC of SMCB-II being 2.7 times higher than SMCB-I, and 514.5 times and 19.1 times higher than CGB and CPGB, respectively (p < 0.05) (Table 2). CGB and CPGB showed considerably lower TFC, which may be attributed to the low flavonoid content of red ginseng or platycodon used in the manufacture. The particularly high TFC of SMCB-II may be the result of the exclusive use of SA, PL, and KP among medicinal plants with high flavonoid content, whereas the TFC was lower in SMCB-I as it did not contain those plants.
The main flavonoids in PA include apigenin, luteolin, luteolin-7-O-glucoside, baicalein, and plantaginin (Ferrazzano et al., 2015), while PL contains the isoflavonoids (genistein and daidzein) and their glycosides (puerain and daidzin) (Reppert et al., 2008). CU contains the glycosides of hesperidn and naringin, and polymethoxylated flavones of nobiletin and tangerein (Li et al., 2006; Rafiq et al., 2018); GU contains chanlones (isoliquiritigenin and isoliquiritin) and flavanones (liquiritigenin and liquiritin) (Tanemoto et al., 2015). SA contains the flavonoids including kaempferol, rhamnetin, eugenitin, and myricetin, which are known to exhibit antioxidant, antimicrobial, and anti-inflammatory effects (Mittal et al., 2014).
Crude saponin content
The crude saponin content in medicinal plant extract showed the order of PL > KP > GU > PG > PGA, with a significance (p < 0.05) (Table 2). The saponins are structurally steroid and triterpenoid glycosides produced by plants and have diverse biological activities. KP contains kalopanax saponins (A, B, G), pericap saponin PJ3, and hederasaponin B (Sano et al., 1991); GU contains the triterpene glycoside (glycyrrhizin) (Na et al., 2008); PGA contains the triterpenoid type saponin: platycodigenin and platycogenic acid A, B, and C (Kubota et al., 1969). PL contains oleanane-type glycosides such as kudzusaponins and soyasaponins (Arao et al., 1997), while PG contains over 30 types of saponins (ginsenosides) that are divided into 20(S)-protopanaxdiol type saponin (PPD; Rb1, Rb2, Rc and Rd) and 20(S)-protopanaxatriol type saponin (PPT, Re, Rf, Rg1 and Rg2) (Lee et al., 2006).
SMCB-II and SMCB-I had crude saponin contents of 35.66 g% and 23.85 g%, respectively, which were significantly higher than CGB (16.40 g%) or CPGB (7.71 g%) (p < 0.05) (Table 2). CGB (100% red ginseng) had 2.1 times higher saponin content than CPGB (90% platycodon and 3.4% red ginseng), which is thought to be due to the higher saponin content of the main ingredient red ginseng than platycodon.
Antioxidant activity
The antioxidant activity of SMCB was assessed based on the FRAP value and DPPH RSC; these values were higher in SMCB-II than in SMCB-I by 1.6 times and 1.7 times, respectively, while the antioxidant activity of SMCB was significantly higher than CGB or CPGB (p < 0.05) (Table 3). The DPPH RSC of medicinal plant extract showed the order of PA > SA > KP > GU > PL > CU > ZJ > PGA > PG (p < 0.05), while the FRAP value showed the order of SA > PA > PL > KP > CU > GU > PGA > PG > ZJ (p < 0.05). SA exhibited a markedly higher FRAP value than the other medicinal plants: 7.8 times higher than PA, and 192.3 times higher than ZJ (Table 3). The higher antioxidant activity of SMCB-II than SMCB-I appears to be due to the use of SA, PA, and KP, the top three plants exhibiting the highest DPPH RSC and FRAP values.
Table 3.
Ferric reducing antioxidant power (FRAP) and DPPH free radical scavenging capacities (RSC) of medicinal plant extracts and stick-type medicinal concentrated beverages (SMCB)
| Sample | FRAP value (AAE1 mg/g) | DPPH RSC (%) |
|---|---|---|
| Stick-type medicinal concentrated beverages | ||
| SMCB-I | 4.20 ± 0.17b | 37.38 ± 0.50b |
| SMCB-II | 6.54 ± 0.04a | 62.85 ± 1.83a |
| CGB2 | 1.85 ± 0.06c | 18.87 ± 1.00c |
| CPGB3 | 0.81 ± 0.02d | 9.08 ± 1.17d |
| Medicinal plants4 | ||
| SA5 | 415.47 ± 7.55a | 93.43 ± 3.72ab |
| PL | 33.98 ± 6.29c | 80.50 ± 1.20d |
| PA | 52.07 ± 4.19b | 95.58 ± 0.90a |
| KP | 32.20 ± 2.10c | 91.41 ± 0.40b |
| CU | 11.45 ± 2.10d | 75.28 ± 2.23e |
| PGA | 2.97 ± 0.23de | 23.82 ± 0.16g |
| GU | 9.96 ± 1.68de | 85.76 ± 1.00c |
| PG | 2.59 ± 0.02de | 21.29 ± 0.72g |
| ZJ | 2.16 ± 0.63e | 48.34 ± 0.67f |
1AAE, ascorbic acid equivalent
2Commercial red ginseng concentrated beverage
3Commercial platycodon red ginseng concentrated beverage
4Freeze-dried water extract of medicinal plants
5The medial plant of the abbreviation is the same as Table 1
a–gMeans within the same column with different superscript letters are significant different by Duncan’s multiple range test at p < 0.05 (n = 3)
The correlation between the antioxidant activity of medicinal plants and the TPC, TFC, and crude saponin content was analyzed; the resulting Pearson’s correlation coefficient (r) and p value are presented in Table 4. The r values for TPC, TFC, and crude saponin content in correlation to DPPH RSC were 0.511, 0.566, and 0.658, respectively, and all showed a strong positive correlation with statistical significance (p < 0.05). Hence, the higher the TPC, TFC, and crude saponin content of the medicinal plant, the higher the DPPH RSC. For the FRAP value, a strong positive correlation was shown for TPC and crude saponin content. The correlation was particularly strong with TPC, with r = 0.981 and a coefficient of determination (r2) = 0.43, which indicated approximately 96% correlation while with TFC, a non-significant positive correlation (p = 0.139) was found. The crude saponin content was analyzed for PL, KP, PGA, GU, and PG: PL and KP with their high saponin content exhibited high DPPH RSC and FRAP value since these plants contain high TPC and TFC.
Table 4.
Pearson’s correlation matrix between the contents of total phenolic, total flavonoids and crude saponin, and the activities of antioxidant and NO production inhibitory in medicinal plant extracts and stick-type medicinal concentrated beverages (SMCB)
| Variables | TPC | TFC | Crude Saponin content | Antioxidant activity | Anti-inflammatory activity | |
|---|---|---|---|---|---|---|
| DPPH RSC (%) | FRAP value | NO production (%) | ||||
| Medicinal plant extracts | ||||||
| TPC | r = 1 | |||||
| TFC |
r = 0.381 (P = 0.119) |
r = 1 | ||||
| Saponin |
r = 0.539 (P = 0.108) |
r = 0.366 (P = 0.298) |
r = 1 | |||
| DPPH RSC |
r = 0.511* (P = 0.030) |
r = 0.566* (P = 0.014) |
r = 0.658* (P = 0.039) |
r = 1 | ||
| FRAP value |
r = 0.981** (P = 0.000) |
r = 0.363 (P = 0.139) |
r = 0.653* (P = 0.041) |
r = 0.409 (P = 0.092) |
r = 1 | |
| NO production (%) |
r = − 0.540* (P = 0.021) |
r = − 0.477* (P = 0.012) |
r = 0.017 (P = 0.962) |
r = − 0.523* (P = 0.026) |
r = − 0.461 (P = 0.054) |
r = 1 |
| Stick-type medicinal concentrated beverages (SMCB) | ||||||
| TPC | r = 1 | |||||
| TFC |
r = 0.851** (P = 0.007) |
r = 1 | ||||
| Saponin |
r = 0.987** (P = 0.000) |
r = 0.867** (P = 0.005) |
r = 1 | |||
| DPPH RSC |
r = 0.911** (P = 0.000) |
r = 0.909** (P = 0.002) |
r = 0.989** (P = 0.000) |
r = 1 | ||
| FRAP value |
r = 0.996** (P = 0.000) |
r = 0.880** (P = 0.004) |
r = 0.987** (P = 0.000) |
r = 0.996** (P = 0.000) |
r = 1 | |
| NO production (%) |
r = − 0.274 (P = 0.511) |
r = 0.683* (P = 0.014) |
r = − 0.196 (P = 0.642) |
r = − 0.194 (P = 0.645) |
r = − 0.257 (P = 0.539) |
r = 1 |
TPC total phenolic content, TFC total flavonoids content, FRAP ferric reducing antioxidant power
*Correlation is significant at p < 0.05; **correlation is significant at p < 0.01
The DPPH RSC and FRAP values of the medicinal concentrated beverages (SMCBs, CGB and CPGB) showed a strong positive correlation (r > 0.88) with TPC, TFC, and crude saponin content, indicating that the increase in their contents led to an increase in the antioxidant activity (p < 0.01) (Table 4). The concentrated beverage with high TPC also had high TFC and high crude saponin content, which resulted in outstanding DPPH RSC and FRAP values. The r value was 0.989 between DPPH RSC and crude saponin content, and 0.996 between the FRAP value and TPC, showing approximately 98% and 99% correlation, respectively. This was in line with the correlation analysis results for medicinal plants. Thus, antioxidant activity was higher for the medicinal plants with high levels of TPC, TFC, and crude saponin content, and the use of such medicinal plants in producing the complex extract for concentrated beverages was shown to lead to the consequently strong antioxidant activity.
Phenolic compounds are known to have anti-inflammatory, antibacterial, and antiviral biological activities (Tanase et al., 2019). Polyphenols are molecules with one or more hydroxyl groups attached to at least one aromatic ring, and are potent antioxidants by preventing damage from reactive oxygen species through radical scavenging or by preventing generation of the species; the antioxidant action is largely dependent on the number of hydroxyl groups (Watson et al., 2014). The antioxidant activity of SA comes from its content of phenolic compounds (eugenol, eugenyl acetate, and pyrogallol) and of flavonoids (kaempferol, rhamnetin, eugenitin, and myricetin) (Mittal et al., 2014; Hemalatha et al., 2016). The antioxidant activity of PA comes from the following phenolic compounds: vanillic acid, p-hydroxybenzoic acid, plantamajoside, desrhamnosyl acteoside, calceorioside B, and phenylethyl glycoside (Amakura et al., 2012). Plantamajoside, in particular, as a bioactive caffeic acid derivative and a dihydroxyphenethyl glucoside in polyphenolic compounds, has been reported to exhibit antibacterial, anti-inflammatory, and antioxidant activities (Ravn et al., 2015).
Inhibitory activity of NO production
The anti-inflammatory effects of medicinal plant extracts and medicinal concentrated beverages were evaluated based on NO production by induction of inflammation in RAW 264.7 cells using LPS. NO production in the LPS-treated group (100%) showed a substantial increase compared to the negative control (7.3%), while that in the groups treated with medicinal plant extracts (0.2 mg/mL) was 7.3%-82.3%, which showed a significant decrease compared to the LPS-treated group (p < 0.001, p < 0.01, or p < 0.05) (Fig. 1). The inhibitory activity of medicinal plant extracts on NO production showed the following order: SA > PA > GU > PL > PG > KP > CU > ZJ > PGA. The inhibition of NO production by SA was 5.0 times higher than PA, and 11.3 times higher than PGA. Of note, the level of NO inhibition was similar to the negative control without LPS treatment (Fig. 1). The increase in TPC and TFC of each medicinal plant led to a significant decrease in NO production, indicating a negative correlation (r = − 0.540 and r = − 0.477), although no correlation was shown for crude saponin content (r = 0.017). Between NO production and antioxidant activity based on DPPH RSC, a strong negative correlation was found with statistical significance (p < 0.05); hence, the higher the antioxidant activity, the higher the inhibitory effect on NO production (Table 4).
Fig. 1.
The effect of stick-type medicinal concentrated beverages and medicinal plant extracts on LPS-induced NO production in RAW 264.7 cells; (A) stick-type medicinal concentrated beverages; (B) medicinal plant extracts. *Significant difference between LPS and each medicinal plants (***p < 0.001; **p < 0.01; *p < 0.05). a–eMeans with different letters on the bars are significant different among the medicinal plants or among the medicinal concentrated extract (p < 0.05)
The anti-inflammatory activity of SA has been reported by the phenolic compounds, eugenol and its derivatives, that exert an inhibitory effect on the activity of the pro-inflammatory enzymes cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) (Leem et al., 2011). Plantamajoside in PA is a well-known polyphenolic compound that exhibits anti-inflammatory activity (Ravn et al., 2015), and ethanolic extracts of PA exhibited inhibitory effect of NO production by suppressing the mRNA expression of pro-inflammatory mediators (IL-1β, IL-6, iNOS, COX-2 and NF-κB) in macrophages (Ay et al., 2017). For GU, the major component glycyrrihizin and the minor constituents, isoliquiritigenin, isoliquiritin and liquiritigene, were shown to suppress NO production in IL-1β-treated rat hepatocytes (Tanemoto et al., 2015). The PG-specific saponins are ginsenosides, among which the PPD-based saponins (ginsenoside-Rb1, Rb2, Rc, Rd, etc.) can inhibit NO production and suppress the expression of inflammatory cytokines (Lee et al., 2006).
The inhibitory effect of medicinal concentrated beverage on NO production showed the following order: SMCB-II > CGB > CPGB > SMCB-I (Fig. 1). The significantly higher inhibitory effect on NO production from SMCB-II than SMCB-I appears to be due to the considerably higher anti-inflammatory activity of SA used as a material in SMCB-II preparation. In addition, CGB (100% red ginseng) showed a higher level of inhibition on NO production than SMCB-I (using platycodon, PA, PG, ZJ, and GU) and CPGB (90% platycodon 90% and 3.4% red ginseng), which could be due to the use of 100% red ginseng produced from PG processing as the source material that effectively inhibits NO production. Ginseng is divided into white ginseng after a process of drying and red ginseng after a process of steaming and drying. The PG used in the preparation of SMCBs was white ginseng. Both white ginseng and red ginseng have anti-inflammatory effects, although red ginseng has been reported to more effectively inhibit NO and IFN-γ production, compared to white ginseng (Hyun et al., 2009).
Antibacterial activity
The antibacterial effects of medicinal plant extracts and medicinal concentrated beverages were evaluated based on the MIC for S. aureus, C. diphtheriae, S. pyogenes, Mycobacterium sp., and E. coli, the bacteria commonly isolated from the phlegm of patients with a bronchial disease (Table 5).
Table 5.
Minimum inhibitory concentration (MIC) of medicinal plant extracts and stick-type medicinal concentrated beverages (SMCB)
| Sample | S. aureus1 | C. diphtheriae | S. pyogenes | Mycobacterium sp. | E. coli |
|---|---|---|---|---|---|
| Stick-type medicinal concentrated beverages | |||||
| SMCB-I | 160 | 80 | 320 | 320 | 640 |
| SMCB-II | 320 | 40 | 160 | 320 | 640 |
| CGB2 | 1280 | 80 | 1280 | 80 | 1280 |
| CPGB3 | 640 | 320 | 320 | 320 | 1280 |
| Medicinal plants4 | |||||
| SA5 | 1 | 1 | 4 | 2 | 8 |
| PL | > 320 | > 320 | > 320 | 160 | > 320 |
| PA | 1 | 2 | 640 | 6 | 320 |
| KP | 10 | 10 | 40 | 40 | 20 |
| CU | 160 | 160 | 160 | 6 | 160 |
| PGA | 160 | 160 | 320 | 320 | 320 |
| GU | 0.5 | 1 | 320 | 2 | 320 |
| PG | 80 | 160 | 160 | 40 | 160 |
| ZJ | 80 | 160 | 320 | 160 | 320 |
1S. aureus, Staphylococcus aureus; C. diphtheriae, Corynebacterium diphtheriae; S. pyogens, Streptococcus pyogenes; E. coli, Escherichia coli (n = 2)
2Commercial red ginseng concentrated beverage
3Commercial platycodon red ginseng concentrated beverage
4Freeze-dried water extract of medicinal plants
5The medial plant of the abbreviation is the same as Table 1
SA extract showed markedly low MIC (1–8 mg/mL) for all the bacteria, with higher antibacterial activity compared to other medicinal plants. The antibacterial effect of SA extract showed the following order: S. aureus, C. diphtheriae > Mycobacterium sp. > S. pyogenes > E. coli, with an inhibitory effect on the growth of bacteria that induce bronchial diseases at ≤ 4 mg/mL. According to Xu et al. (2016), strong antibacterial activity against S. aureus was exhibited by the essential oil of SA (MIC = 0.625 mg/mL). It was suggested that SA essential oil destroys bacterial cell wall and membrane, which causes the loss of vital intracellular materials and ultimately leads to bacterial death; alternatively, the essential oil may penetrate through the cytomembrane to cause cell structure destruction and inhibit the normal synthesis of DNA and proteins required for bacterial growth. The main component responsible for the antimicrobial activity of SA is eugenol, and the possible antibacterial mechanism against S. aureus may be by the simultaneous disruption of the cellular membrane and ROS generation, which induce membrane collapse, resulting in cellular decomposition and eventually bacterial death (Das et al., 2016).
The antibacterial activity of PA and GU extracts against S. aureus, S. diphtheriae, and Mycobacterium sp. was outstanding (MIC, 0.5–6 mg/mL), but it was low against S. pyogenes and E. coli (MIC, 320–640 mg/mL). For GU, triterpene (glycyrrhizin) and flavones (18β-glycyrrhetinic acid, liquiritigenin, licochalcone A and E, and glabridin) decrease the expression of microbe genes, inhibit microbe growth and reduce the production of microbe toxins (Wang et al., 2015). Ravn and Brimer (1988) reported that the antibacterial activity of PA comes from plantamajoside (a phenylethanoid), and the MIC for S. aureus and E. coli was 2.0 mg/mL and > 2.5 mg/mL, respectively, showing an outstanding antibacterial effect against S. aureus, which was in line with the result in this study. KP extract showed an overall high level of antibacterial activity based on a MIC of 10–40 mg/mL, leading to the effective growth inhibition of all bacteria. CU extracts showed substantially higher antibacterial activity against Mycobacterium sp. (MIC, 6 mg/mL) than against other strains (MIC, 160 mg/mL). PG extracts led to growth inhibition with MIC of 40–160 mg/mL where the antibacterial activity was higher against S. aureus and Mycobacterium sp. The antibacterial activity of PL was the lowest among the medicinal plants.
Many saponins are known to be antimicrobial and protect plants from insect attack. Saponins consist of a sugar moiety (glucose, galactose, glucuronic acid, xylose, rhamnose etc.) linked to a hydrophobic aglycone (sapogenin) which are a steroid and triterpenoid (Desai et al., 2009). The antimicrobial effects of saponins has been associated with microbial cell membrane permeability, and this action is the result of the affinity of the aglycone moiety for the phospholipids in the cell membrane (Desai et al., 2009).
The SMCBs showed higher antibacterial activity than CGB or CPGB against S. aureus, C. diphtheriae, S. pyogens, and E. coli, but the highest activity against Mycobacterium sp. was shown by CGB (Table 5). SMCB-I had the strongest effect against S. aureus, while SMCB-II had the strongest effect against C. diphtheriae and S. pyogens. SMCBs were prepared with medicinal plants such as SA, PA, KP, and GU that exhibit outstanding antibacterial activity; this may account for the higher antimicrobial effects, since CGB is solely composed of red ginseng and CPGB is mostly composed of platycodon.
Medicinal plants were evaluated on antioxidant, anti-inflammatory and antibacterial effects, and then SMCBs were prepared with the different composition ratio of medicinal plants, and their efficacies were determined. The medicinal plants with higher TPC, TFC and saponin content showed a higher level of antioxidant activity and the stronger the inhibitory effect on NO production, which indicated higher anti-inflammatory activity. The antibacterial effect was outstanding for SA against all bacteria strains, while PA, KP, and GU showed great effects against the bronchial disease-causing bacteria (S. aureus, C. diphtheriae, S. pyogenes, and Mycobacterium sp.). For preparation of SMCBs, PGA, PA, PG, GU, and ZJ were used as the basic ingredients. SMCB-II was prepared by addition of PL, KP and SA with high levels of TPC, TFC and saponin content while SMCB-I was prepared by adding CU. SMCB-II showed higher antioxidant, NO production inhibitory, and antimicrobial effects than SMCB-I, however SMBC-II has unique bitterness and flavor of herbal medicine stronger than SMCB-I. SMCBs, compared to commercial medicinal beverages, showed higher TPC, TFC and saponin content, as well as antioxidant activity, NO production inhibition, and antibacterial activity. This study could contribute to the need for medicinal products to combat the rise in respiratory illnesses due to particulate matter air pollution and smoking. The medicinal compounds in SCMB-I and SCMB-II have highly effective antioxidant and antibacterial properties, and are superior to the current commercial products. The SMCBs are anticipated to be an easy-to-carry, easy-to-consume and high value-added health beverage for the health of the modern people, with outstanding antioxidant, anti-inflammatory, and antimicrobial effects.
Acknowledgements
This work (Grants No. S2601370) was supported by project for Cooperative R&D between Industry, Academy, and Research Institute funded Korea Ministry of SMEs and Startups in 2018.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hyeon-Jun Chang, Email: chj931116@naver.com.
Yoon-Hee Kim, Email: kimyh0128@daegu.ac.kr.
Yun-Hwan Kang, Email: k_yunhwan@nikom.or.kr.
Myung-Hwan Choi, Email: maesro@naturegarden.kr.
Jeung-Hee Lee, Email: jeunghlee@daegu.ac.kr.
References
- Amakura Y, Yoshimura A, Yoshimura M, Yoshida T. Isolation and characterization of phenolic antioxidants from plantago Herb. Molecules. 2012;17:5459–5466. doi: 10.3390/molecules17055459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arao T, Kinjo J, Nohara T, Isobe R. Oleanene-type triterpene glycosidesfrom puerariae radix. IV. Six new saponins from Pueraria lobate. Chem. Pharm. Bull. 1997;45:362–366. doi: 10.1248/cpb.45.362. [DOI] [PubMed] [Google Scholar]
- Ay NV, Kh A, Enkhchimeg V, Baatartsogt O. Anti-inflammatory effect of Plantago sp. ethan-olc extract in murine RAW 264.7 macrophage cells. Mong J. Agric. Sci. 2017;21:35–42. doi: 10.5564/mjas.v21i02.903. [DOI] [Google Scholar]
- Belsey MA, Sinclair M, Roder MR, LeBlanc DR. Corynebacterium diphtheriae skin infec-tions in alabama and louisiana: a factor in the epidemiology of diphtheria. N. Engl. J. Med. 1969;280:135–141. doi: 10.1056/NEJM196901162800304. [DOI] [PubMed] [Google Scholar]
- Bocco A, Cuvelier ME, Richard H, Berset C. Antioxidant activity and phenolic composition of citrus peel and seed extracts. J. Agric. Food Chem. 1998;46:2123–2129. doi: 10.1021/jf9709562. [DOI] [Google Scholar]
- Das B, Mandal D, Dash SK, Chattopadhyay S, Tripathy S, Dolai DP, Dey SK, Roy S. Eugenol provokes ROS-mediated membrane damage-associated antibacterial activity against clinically isolated multidrug-resistant Staphylococcus aureus strains. Infect. Dis. (Auckl.) 2016;9:11–19. doi: 10.4137/IDRT.S31741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai SD, Desai DG, Kaur H. Saponins and their biological activities. Pharm. Times. 2009;41:13–16. [Google Scholar]
- Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin. Microbiol. Rev. 1993;6:428–442. doi: 10.1128/CMR.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrazzano GF, Cantile T, Roberto L, Ingenito A, Catania MR, Roscetto E, Palumbo G, Zarrelli A, Pollio A. Determination of the in vitro and in vivo antimicrobial activity on salivary streptococci and lactobacilli and chemical characterisation of the phenolic content of a Plantago lanceolate infusion. Biomed. Res. Int. 2015;2015:1–8. doi: 10.1155/2015/286817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemalatha R, Nivetha P, Mohanaproya C, Sharmila G, Muthukumaran C, Gopinath M. Phyrochemical composition, GC-MS analysis, in vitro antioxidant and antibacterial potential of clove flower bud (Eugenia caryophyllus) methanolic extract. J. Food Sci. Technol. 2016;53:1189–1198. doi: 10.1007/s13197-015-2108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun MS, Hur JM, Shin YS, Song BJ, Mun YJ, Woo WH. Comparison study of white ginseng, red ginseng, and fermented red ginseng on the protective effect of LPS-induced inflammation in RAW 264.7 cells. J. Appl. Biol. Chem. 2009;52:21–27. doi: 10.3839/jabc.2009.004. [DOI] [Google Scholar]
- Kim MH, Kim JM, Yoon KY. Effects of blanching on antioxidant activity and total phenolic content according to type of medicinal plants. Food Sci. Biotechnol. 2013;22:817–823. doi: 10.1007/s10068-013-0150-5. [DOI] [Google Scholar]
- Kubota T, Kitatani H, Hinoh H. The structure of platycogenic acids A, B, and C, further trter-penoid constituents of Platycodon grandiflorum A. De Candolle. J. Chem. Soc. D. 1969;19:1313–1314. doi: 10.1039/c29690001313. [DOI] [Google Scholar]
- Lee IS, Choi MC, Moon HY. Effect of Platycodon grandiflorum A. DC extract on the bronchus diseases bacteria. Korean J. Biotechnol. Bioeng. 2000;15:162–166. [Google Scholar]
- Lee WM, Kim SD, Kim KS, Song YB, Kwak YS, Cho JY, Park HJ, Oh JW, Rhee MH. Protopanaxadiol modulates LPS-induced inflammatory activity in murine macrophage RAW 264.7 cells. J. Ginseng. Res. 2006;30:181–187. doi: 10.5142/JGR.2006.30.4.181. [DOI] [Google Scholar]
- Leem HH, Kim EO, Seo MJ, Choi SW. Antioxidant and anti-inflammatory activities of eugenol and its derivatives from clove (Eugenia caryophyllata Thunb.) J. Korean Soc. Food Sci. Nutr. 2011;40:1361–1370. doi: 10.3746/jkfn.2011.40.10.1361. [DOI] [Google Scholar]
- Li RW, Theriault AG, Au K, Douglas TD, Casaschi A, Kurowska EM, Mukherjee R. Citrus polymethoxylated flavones improve lipid and glucose homeostasis and modulate adipocytokines in fructose-induced insulin resistant hamsters. Life Sci. 2006;79:365–373. doi: 10.1016/j.lfs.2006.01.023. [DOI] [PubMed] [Google Scholar]
- MFDS (Ministry of Food and Drug Safety) and NFSI (National Food Safety Information Service). Information by function of health function food. Available from: https://www.foodsafetykorea.go.kr/
- Mittal M, Gupta N, Parashar P, Mehra V, Khatri M. Phytochemical evaluation and pharmacological activity of Syzygium aromaticum: a comprehensive review. Int. J. Pharm. Pharm. Sci. 2014;6:67–72. [Google Scholar]
- Na IS, Park MJ, Noh CH, Min JW, Bang MH, Yang DC. Production of flavonoid aglycone from Korean Glycyrrhizae radix by biofermentation process. J. Physiol. Pathol. Korean Med. 2008;22:569–574. [Google Scholar]
- Rafiq S, Kaul R, Sofi SA, Bashir N, Nazir F, Nayik GA. Citrus peel as a source of functional ingredient: a review. J. Saudi Soc. Agric. Sci. 2018;17:351–358. [Google Scholar]
- Ravn H, Brimer L. Structure and antibacterial activity of plantamajoside, A caffeic acid sugar ester from Plantago major subsp. major. Phytochemistry. 1988;27:3433–3437. doi: 10.1016/0031-9422(88)80744-1. [DOI] [Google Scholar]
- Ravn HW, Mondolot L, Kelly MT, Lykke AM. Plantamajoside—a current review. Phytochem. Lett. 2015;12:42–53. doi: 10.1016/j.phytol.2015.02.002. [DOI] [Google Scholar]
- Reppert A, Yousef GG, Rogers RB, Lila MA. Isolation of radiolabeled isoflavones from kudzu (Pueraria lobate) root culture. J. Agric. Food Chem. 2008;59:7860–7865. doi: 10.1021/jf801413z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano K, Sanada S, Ida Y, Shoji J. Studies on the constituents of the bark of Kalopanax pictus Nakai. Chem. Pharm. Bull. 1991;39:865–870. doi: 10.1248/cpb.39.865. [DOI] [Google Scholar]
- Stevens DL. Invasive group A streptococcus infections. Clin. Infect. Dis. 1992;14:2–13. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- Stout JE, Koh WJ, Yew WW. Update on pulmonary disease due to non-tuberculous mycobacteria. Int. J. Infect. Dis. 2016;45:123–134. doi: 10.1016/j.ijid.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Sung WS, Lee DG. The combination effect of Korean Red ginseng saponins with kanamycin and cefotaxime against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2008;31:1614–1617. doi: 10.1248/bpb.31.1614. [DOI] [PubMed] [Google Scholar]
- Tanase C, Cosarca S, Muntean DL. A critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules. 2019;24:1182–1199. doi: 10.3390/molecules24061182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanemoto R, Okuyama T, Matsuo H, Okumura T, Ikeya Y, Nishizawa M. The constituesnts of licorice (Glycyrrhiza uralensis) differentially suppress nitric oxide production in inerleukin-1β-treated hepatocytes. Biochem. Biophys. Rep. 2015;2:153–159. doi: 10.1016/j.bbrep.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türel I, Özbek H, Erten R, Öner AC, Cengiz N, Yilmaz O. Hepatoprotective and anti-inflammatory activities of Plantago major L. Indian J. Pharmacol. 2009;41:120–124. doi: 10.4103/0253-7613.55211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yang R, Yuan B, Liu Y, Liu C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta. Pharm. Sin. B. 2015;5:310–315. doi: 10.1016/j.apsb.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RR, Preedy VR, Zibadi S. Polyphenols in Human Health and Disease. Cambridge, MA, USA: Academic Press; 2014. pp. 253–265. [Google Scholar]
- Xu JG, Liu T, Hu QP, Cao XM. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules. 2016;21:1194–1206. doi: 10.3390/molecules21091194. [DOI] [PMC free article] [PubMed] [Google Scholar]