Abstract
Purpose
Intracytroplasmatic sperm injection (ICSI) is a common procedure used to improve reproductive results, even among couples without male factor infertility. However, the evidence available is still uncertain on the possible advantages and deficiencies that this procedure may have in patients with no formal indication for ICSI.
Methods
A SWOT (strengths, weaknesses, opportunities, threats) analysis examines the possible advantages and deficiencies of performing ICSI in these patients with no formal indication.
Results
The evidence suggests that ICSI is not justified for non-male factor infertile couples requiring in vitro conception. One of the major strengths associated to the procedure is the virtual elimination of cases further complicated by total fertilization failure and a combination between IVF and ICSI on sibling oocytes has been advised in the literature. Greater technical difficulties, higher costs and performing an unnecessary invasive technique in some cases represent some of the weaknesses of the procedure, and questions regarding safety issues should not be ruled out.
Conclusion
Despite the widespread use of ICSI in patients without a formal diagnosis of male factor infertility, evidence demonstrating its effectiveness in this population is still lacking. Additional large and well-designed randomized controlled trials are needed to clarify definitive indications for ICSI in non-male factor infertility.
Keywords: Intracytoplasmic sperm injection (ICSI), In vitro fertilization (IVF), Severe non-male factor, Pregnancy, Assisted reproduction, Infertility
Introduction
Intracytroplasmatic sperm injection (ICSI) is an assisted fertilization method where a single spermatozoon is injected into a mature oocyte. ICSI was introduced in 1992 as a way to treat couples with severe male infertility [1]. However, over the last 20 years, the use of ICSI has increased considerably, particularly among patients without male factor infertility, where ICSI use has gone from 15% in 1996 to 67% in 2012 [2].
Based on a recent report, in some parts of the world (Egypt, Lebanon), couples went through ICSI in 100% of in vitro fertilization (IVF) cycles and in 65% of IVF cycles in Europe [3]. Despite its increased use in cycles without male factor infertility, evidence is yet unclear on whether or not ICSI improves reproductive outcomes and published well-designed randomized controlled studies analyzing the possible benefits of ICSI in this population are scarce [4].
For this reason, we designed a SWOT (strengths, weaknesses, opportunities, threats) analysis to evaluate the available published evidence on the possible recommendation of ICSI in non-male factor infertility. In addition, an assessment of the scientific Oxford level of evidence for each of the reviewed papers was carried out thereby avoiding subjectivity in their statements.
Methods
In this study, a SWOT analysis was carried out to discern the perceived strengths and drawbacks of the ICSI in non-male factor infertility, to identify the possible opportunities available and the key threats of this technology according to the reviewed bibliography, and to know the experts’ point of view. The SWOT method is only recently applied in fertility medical research to assess the possible applicability of a particular technique when scientific evidence is insufficient, highlight specific issues, and weigh the possible pros and cons [5–8].
Initially, a bibliographic search aimed at “ICSI AND non-male factor infertility” was carried out. Two independent investigators carried out the bibliographic assessment and in case of non-agreement a third one was consulted. The primary review of the literature revealed 361 papers and 93 were classified as relevant articles.
Then, a second manual bibliographic search was carried out to complete those matters of relevance included by the researches in the SWOT clinical outline and not resolved in the first general search. The total number of references was divided among the researchers and an Excel spreadsheet for each of the sections of the SWOT was created, which would be available on the SISGtool.orgplatform along with the corresponding references. In each of the tables, the ideas/phrases identified for each section were noted and each researcher added the studies classified by the degree of evidence to each of them. The quality of the selected articles was assessed using the Oxford Centre for Evidence-Based Medicine (CEBM) Levels of Evidence, which rank the validity of evidence in a hierarchy of levels, with systematic reviews as level 1 (strong evidence) and expert opinions as level 5 (weak evidence) (Oxford CEBM, 2009) (Table 1).
Table 1.
Oxford Centre for Evidence-Based Medicine (CEBM)-Levels of Evidence (March 2009). Available at: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/
| Levels of evidence | Type of study |
|---|---|
| 1a | Systematic reviews (with homogeneity) of randomized controlled trials |
| 1b | Individual randomized controlled trials (with narrow confidence interval) |
| 1c | All or none randomized controlled trials |
| 2a | Systematic reviews (with homogeneity) of cohort studies |
| 2b | Individual cohort study or low-quality randomized controlled trials (e.g., < 80% follow-up) |
| 2c | “Outcomes” Research; ecological studies |
| 3a | Systematic review (with homogeneity) of case-control studies |
| 3b | Individual case-control study |
| 4 | Case-series (and poor-quality cohort and case-control studies) |
| 5 | Expert opinion without explicit critical appraisal, or based on physiology, bench research or “first principles” |
A minus sign “−” may be added to denote evidence that fails to provide a conclusive answer because it is either (a) a single result with a wide confidence Interval; OR (b) a systematic review with troublesome heterogeneity
Results
Strengths
ICSI could increase fertilization rate and decrease fertilization failure in patients with unexplained infertility
In conventional IVF, total fertilization failure occurs in 5–10% and live birth rates of IVF are in the range of 35–45% per embryo transfer [9]. Some data suggest that ICSI could result in increased fertilization rate and decreased total fertilization failure with favorable clinical outcomes in couples with well-defined idiopathic infertility (evidence 2a) [10] or long-term infertility (evidence 2b) [11]. On the other hand, other studies contradict the finding of an increase in fertilization rates with ICSI compared with IVF (see Table 2). The largest meta-analysis published analyzing this matter considered eleven studies including 901 couples with unexplained infertility (female age range 30–35 years) with 11,767 sibling oocytes. In this meta-analysis the pooled relative risk (RR) of a mature oocyte fertilizing was higher with ICSI than with IVF (RR 1.49, 95% confidence interval [CI] 1.35–1.65) and the pooled RR of total fertilization failure (TFF) was significantly greater with conventional IVF than with ICSI (RR 8.22, 95% CI 4.44–15.23) (evidence 2a) [10]. Five subjects treated with ICSI was the amount necessary to prevent one case of TFF. This meta-analysis and other randomized controlled studies using sibling oocytes supports the use of ICSI in decreasing TFF risk in couples with well-defined unexplained infertility (well-defined unexplained infertility was considered if the authors clearly documented that patients included in the analysis had no cause for infertility determined after a complete infertility evaluation) [10]. Discordant results regarding the fertilization rate may be related to the low sample size and different characteristics of the populations analyzed.
Table 2.
Fertilization rate and total fertilization failure (TFF) in prospective RCT of sibling oocytes allocated to IVF or ICSI in non-male factor infertility
| N | Fertilization rate IVF (%) | Fertilization rate ICSI (%) | p | TFF IVF (%) | TFF ICSI (%) | p | Pregnancy rate IVF | Pregnancy rate ICSI | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| Aboulghar et al., 1996 | 22 | 50.7 | 63.0 | n.s. | 22.7 | 0 | < 0.001 | – | – | – |
| Aboulghar et al, 1999 | 131 | 50.2 | 55.0 | – | 17.6 | 1.5 | – | 29.8 | 36.7 | – |
| Ruiz et al, 1997 | 70 | 54.0 | 60.4 | n.s. | 11.4 | 0 | 0.01 | – | – | – |
| Khamsi et al, 2001 | 35 | 57.2 | 71.3 | 0.005 | 11.4 | 2.9 | n.r. | – | – | – |
| Hershlag et al, 2002 | 60 | 48.1 | 65.3 | < 0.001 | 16.7 | 0 | < 0.002 | – | – | – |
| Jaroudi et al, 2003 | 125 | 51.6 | 61.0 | < 0.001 | 19.2 | 0.8 | < 0.001 | – | – | – |
| Wyns et al. 2004 | 60 | 61.6 | 67.9 | n.r. | 13.3 | 0 | n.r. | 36.7 | 50 | – |
| Bungum et al. 2004 | 248 | 46.0 | 68.0 | < 0.005 | 24.2 | 4.4 | < 0.001 | – | 49.2 | – |
| Li et al. 2004 | 11 | 40.1 | 57.6 | n.s. | 36.4 | 0 | < 0.05 | – | – | – |
| Foong et al., 2006 | 60 | 77.2 | 82.4 | n.s. | 2/30 | 0 | – | 50 | 50 | ns |
| Shveiky et al. 2006 | 118 | 31.2 | 60.1 | n.r. | 43.2 | 0 | n.r. | 44 | 49 | nr |
| Chiamchianya et al. 2008 | 36 | 57.0 | 70.3 | n.s. | 13.9 | 0 | 0.04 | 38.5 | 36.4 | ns |
| Kovacs et al. 2011 | 60 | 55.1 | nr | n.r. | 6.6 | 3.3 | n.r. |
n.s. not significant, n.r. not reported, IVF in vitro fertilization, ICSI intracytoplasmic sperm injection
Adapted from Johnson et al., 2013
ICSI decreases fertilization failure in patients with mild male factor infertility or borderline semen
In patients with borderline/subfertile semen (defined by the presence of at least one abnormal semen parameter, that is, concentration < 20 × 106 per milliliter and/or < 40% motility) [12] performing ICSI on at least some of the oocytes has demonstrated to prevent unnecessary fertilization failure and in these cases evidence supports that 25% of cycles could be rescued by ICSI (evidence 1b) [13–15]. The risk ratio for oocyte fertilization in patients with moderate male infertility has been estimated to be 1.9 (95% confidence interval of 1.4 to 2.5) in favor of ICSI, and 3.1 ICSI cycles may be needed to avoid one complete fertilization failure after conventional IVF (95% CI of 1.7 to 12.4) (evidence 1b-) [16]. Until more results are available, some authors suggest the application of combined IVF-ICSI treatment in cases of borderline semen when enough oocytes are available and ICSI when the number of oocytes is too small for a fair chance of fertilization through IVF (evidence 1b-) [13].
Risk minimization for cycle cancelation due to total fertilization failure in couples with a prior failed fertilization using conventional insemination
Unfortunately, some couples may not conceive at their first cycle of IVF and will need to consider an additional cycle. An initial cohort study suggested that the use of ICSI following prior total failed fertilization or poor fertilization rates in a prior IVF cycle could reduce the risk of subsequent failed fertilization. This study analyzed 662 sibling MII oocytes from patients with tubal disease and normozoospermic partners, and reported lower total fertilization failure rates for ICSI (3.6%; 95% CI 0.4–12.3) compared with those obtained for IVF (12.5%; 95% CI 5.2–24.1) (evidence 2b) [17].
Other authors suggest that in these couples, if an amount of sibling oocytes are subjected to ICSI, total fertilization failure of an IVF can be prevented and fertilization can be improved maintaining high chances of achieving a pregnancy. Likewise, the optimal fertilization method for subsequent treatment cycles can be determined [18]. In a prospective randomized study where sibling oocytes were allocated to conventional IVF vs. ICSI/IVF combination after total failed fertilization resulted in 12/109 (11%) oocytes fertilized by IVF/conventional insemination and 78/162 (48%) fertilized with ICSI/IVF combination (evidence 2b) [19]. In this study, ICSI/IVF reported higher fertilization rates (60% vs 22%) even in couples which showed a low fertilization rate (< 25%) in a first IVF attempt. However, large well-designed randomized studies should be performed to definitively confirm this finding.
ICSI could improve embryo quality
Some authors suggest a superiority of ICSI embryos because the ICSI procedure avoids oocyte and zygote culture with too many spermatozoa. This thereby reduces exposure to the reactive oxygen species produced by the spermatozoa that might contribute to embryonic damage [20]. To support the hypothesis of a possible improvement of embryo quality with ICSI, a preliminary study that comprised 13 couples found that more grade A embryos were obtained by ICSI than by conventional IVF performed on sibling oocytes (evidence 2b) [21]. Later on, a study using sibling oocytes from 35 couples with non-male factor infertility demonstrated higher formation of good-quality embryos per retrieved oocyte after ICSI than after conventional IVF (64.4 vs. 47.1%, respectively) (evidence 1b) [22] whereas, Ruiz et al. (evidence 2b) [23] failed to note any such difference. Compared fertility outcomes among ICSI and conventional IVF in couples with non-male factor infertility showed similar pronuclear morphology of zygotes (nucleoli, pronuclei, and axis) derived from sibling oocytes, significantly lower rate of abnormal pronuclei formation (3.9% vs. 13.3%, p < 0.01), and better cleavage stage embryo quality in the ICSI group (evidence 2b) [24]. Data gathered and analyzed from patients with non-male factor infertility, aged ≥ 35, undergoing their first IVF/ICSI cycle attempt show higher top quality embryos (TQE) rate (defined as two to four, or six to eight blastomeres on day 2 or 3, respectively, with equally sized blastomeres and < 15% fragmentation) following ICSI compared with conventional insemination (62.8% versus 45.5% respectively; p < 0.001) (evidence 2b) [25]. Nevertheless, these differences in embryo quality need to be confirmed as they could depend on intrinsic factors of the gametes involved rather than on the fertilization process per se.
ICSI could increase pregnancy rates in patients with disordered zona pellucida–induced acrosome reaction
Some authors suggest that ICSI may probably provide some advantages in those cases with sperm–zona pellucida penetration and fertilization disturbances. In fact, up to a third of normozoospermic men have disordered ZP-induced acrosome reaction (AR) and, in patients with normal semen analysis, the sperm–zona pellucida (ZP)–induced acrosome reaction is highly correlated with sperm-ZP penetration and fertilization rate [26]. In patients with unexplained infertility with ZP-induced AR < 16%, average fertilization rates are < 30% with conventional IVF and, interestingly, ICSI has been found to overcome these defects resulting in an improvement of pregnancy rates (evidence 2c) [27–29].
Avoid possible contamination with DNA from spermatozoa in cases where PGT is performed for monogenic conditions
Testing errors may arise from several different problems in a Preimplantation Genetic Testing (PGT) procedure, but the most common and troublesome ones have been those cited to be from the issues related to sample contamination. The use of ICSI as opposed to conventional IVF in PGT cycles is recommended to prevent paternal contamination from the introduction of excess sperm into the zona pellucida, or maternal contamination from granulosa cells especially with PGT performed for single gene defects (evidence 1a) [30].
Weakness
No standardized guidelines to define “severe male factor” infertility
Male factor infertility is generally defined through the use of conventional semen profile (number of spermatozoa present in the ejaculate, the proportion that are motile or progressively motile and the percentage of morphologically normal spermatozoa), but a standardized definition is still needed [31]. Nevertheless, a variety of different markers are used in clinical practice such as total number of motile spermatozoa (TMSC) or WHO criteria. The standard descriptive semen analysis still cannot detect sperm dysfunction and is recognized as a relatively insensitive predictive tool in determining sperm fertilizing potential, except in extreme cases and, interestingly, men can produce dysfunctional sperm even when their semen parameters (sperm concentration and motility) are normal [32–34].
Invasiveness of the technique
ICSI is a complex and invasive technique that requires micromanipulation where a single spermatozoon is injected centrally into the cytoplasm of the oocyte using a fine sharp-tipped glass pipette [35]. The operator selects the spermatozoon for microinjection thereby bypassing the natural selection barrier to fertilization and may contribute to the creation of embryos with molecular disturbances and low implantation potential. One potential problem with ICSI micromanipulation is the oocyte damage (lysis) which is largely unpredictable and unsystematic in nature (evidence 2a) [36, 37] and Al-Inany et al. suggested that the removal of the cumulus cells of the oocyte could affect the reproductive outcomes in ICSI [38].
Operator experience and technical factors are critical
Technical factors are crucial for achieving high fertilization and pregnancy rates with ICSI and include the use of standardized ICSI pipettes, spermatozoon immobilization before injection, aspiration of a minimal amount of ooplasm before reinjection with the sperm, and substantial operator experience [39]. In a prospective trial involving 305 ICSI cycles, oocyte degeneration rate ranged from 5 to 11% depending on laboratory technician performance and the volume of aspirated oocyte cytoplasm during the procedure also affected the percentage of embryos reaching the blastocyst stage [40]. Precision in handling and manipulation, injection procedure and rigorous thermal control is critical for reducing adverse outcomes.
ICSI complications: imprinting defects and DNA methylation, possible sex ratio modification
Imprinting and DNA methylation
DNA methylation changes and imprinting disorders are believed to be influenced by spermatozoa manipulation techniques and embryo culture duration, but reports of the effects of IVF/ICSI on imprinting have been heterogeneous (evidence 1a) [41]. Imprinting disorders and DNA methylation are more prevalent after human IVF or ICSI (evidence 3b) [42], but there is no proof of a causal relationship between imprinted diseases and IVF or ICSI treatments (evidence 2a) [43].
Possible sex ratio modification
In 2009, a retrospective study of the Society for Assisted Reproductive Technology national database that included 15,164 singleton live births by IVF and ICSI indicated that use of ICSI is associated with a decrease in the sex ratio of male infants, particularly with blastocyst-stage embryos (evidence 3b) [44]. The impact of ICSI on sex ratio has also been observed in a recently published study that assessed a total of 59,628 singleton deliveries resulting from different assisted reproductive technologies (IVF, ICSI, intrauterine insemination, ovulation induction) from 101 IVF clinics in Germany. In this study, ICSI was associated with a lower sex ratio of male infants compared with the natural conception (50.0% vs. 51.3%; p < 0.001), whereas IVF was associated with a higher sex ratio (52.2% vs. 51.3%; p = 0.015) and this phenomenon was not influenced by maternal age (evidence 2b) [45].
No benefit of ICSI on women-related factors of poor-prognosis
Oocytes of older women could contain structural damage that could lower fertilization potential and ICSI could be the chosen insemination procedure to avoid fertilization failure. Nevertheless, several retrospective cohort studies and a meta-analysis suggest that, in the absence of male factor infertility diagnosis, ICSI did not improve fertilization rates compared with IVF in advanced maternal age (evidence 2a) [46, 47], poor-quality oocytes/low oocyte yield (evidence 2b) [48], and poor responders (evidence 2a) [46].
No effect on reproductive outcomes
Although ICSI is capable of consistently overcoming unforeseen sperm cell dysfunction and providing increased fertilization rates in some cases, no significant effect on reproductive outcomes have been reported in several randomized studies yielding similar reproductive results in comparison with conventional IVF (Table 3). A retrospective cohort study of 585,065 ART treatment cycles between 2002 and 2013 reports a lower live birth rates using ICSI compared with IVF in non-male factor patients in most years raising the question of its widespread use (evidence 2b) [49]. Moreover, a recently published population-based cohort of 14,693 women (evidence 2b) [50], having undergone their first ever stimulated cycle with fertilization performed for at least one oocyte through either IVF or ICSI demonstrated that among couples with non-male factor infertility, ICSI showed similar cumulative live birth rate results compared with IVF (AHR 0.96, 95% CI 0.85–1.10).
Table 3.
Randomized controlled studies comparing the efficacy of IVF vs. ICSI in couples with non-male infertility
| N | Fertilization rate (%) | p | Pregnancy rate (%) | p | Live born rates | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IVF | ICSI | IVF | ICSI | IVF | ICSI | ||||||
| Aboulghar et al., 1996 | RCT tubal factor | 22 | 64.8 | 70 | ns | 32.0 | 38.0 | ns | – | – | – |
| Aboulghar et al., 1999 | RCT unexplained infertility | 116 | 64.8 | 53.3 | nr | 31.0 | 32.8 | nr | – | – | – |
| Moreno et al., 1998 | RCT poor responders | 96 | 70.2 | 77.7 | ns | 17.3 | 21.1 | ns | – | – | – |
| Bukulmez et al., 2000 | RCT tubal factor | 76 | 67.3 | 69.3 | ns | 21.5 | 21.5 | ns | 15.79 | 18.42 | ns |
| Bhattacharya et al., 2001 | RCT couples with non-male factor infertility | 415 | 58 | 48 | < 0.05 | 33.0 | 26.0 | < 0.05 | – | – | – |
| Poehl et al., 2001 | RCT tubal infertility or hostile cervical mucus | 89 | – | – | – | 33.0 | 23.0 | – | – | – | – |
| Hariprashad et al., 2002 | RCT failed fertilization cycles | 75 | 44.7 | 77 | < 0.05 | 10.7 | 34.1 | < 0.05 | – | – | – |
| Foong et al., 2006 | RCT unexplained infertility | 60 | 77.2 | 82.4 | ns | 50.0 | 50.0 | ns | 46 | 50 | ns |
IVF in vitro fertilization, ICSI intracytoplasmic sperm injection, RCT randomized controlled trial, ns not significant
No evidence of cost-benefit
In the USA, mandates for IVF coverage have been associated with lower ICSI use for non-male factor infertility cycles, probably due to the elevated cost (evidence 2b) [51]. The cost of an average ICSI cycle is 8.5–30% higher than an IVF cycle [52–54]. Ola et al. (2001) found a cost difference of about £600 per fresh cycle between IVF and ICSI and estimated that £60,000 (cost needed to treat, CNT) would be needed to gain one additional live birth when ICSI was used for patients requiring IVF [55]. This includes consumables, personnel, and facility costs. The incremental cost per live birth (for patients unsuited for conventional IVF) has been estimated to be approximately three times the cost of standard IVF [56]. For a patient who would have good fertilization without ICSI, performing ICSI will result in a higher cost. However, it could be cost-saving for a patient who may have very low fertilization or no fertilization. In couples with unexplained infertility, using an adaptive decision model to analyze the cost-effectiveness of split IVF-ICSI showed that, in a single cycle, all IVF is preferred as the ICER of split IVF-ICSI or not all ICSI ($58,766) justified the increased live birth rate (3%). If two cycles were needed, split IVF/ICSI is preferred as the increased cumulative live birth rate (3.3%) was gained at an ICER of $29,666 [57].
Lack of well-designed randomized controlled trials and inconsistencies in the results
The lack of large well-designed randomized controlled trials (RCT) is one important limitation of ICSI and most of them have been criticized due to poor study design. A RCT able to detect a relative increase in live birth rate from IVF to ICSI of 20% (i.e., relative risk = 1.2, e.g., 35 to 42%) would require a minimum of 1200 patients (600 on each group) to achieve acceptable levels of confidence (95%) and statistical power (80%). It seems quite unrealistic that such a trial could be conducted nowadays, given the difficulty to recruit ideal patients and the trend to use ICSI routinely.
Studies comparing IVF and ICSI have had varied methodology, outcome measures, and conclusions and inconsistencies among the results are equivocal at times. For example, although higher fertilization rates are described in most sibling oocytes studies carried out in infertile patients lacking a diagnosis of male factor infertility, no difference in pregnancy rates are observed in the RCT between those undergoing IVF with ICSI and those undergoing IVF without ICSI. Some authors suggest that the limited quality of these studies or the fact that the patients receiving ICSI have more severe infertility conditions that are not easily apparent to the researchers could be a possible explanation for the lack of a positive effect of ICSI on outcomes [58].
Opportunities
Selection of the oocytes
ICSI allows a mechanical selection of the oocytes as patient-specific oocyte quality has been significantly related to the incidence of lysis during the procedure (evidence 2b) [39]. Moreover, the removal of the cumulus cells provides a more direct feedback on the oocyte maturity [59]. Human oocyte grading based on the triple factors (first polar body, size of perivitelline space and cytoplasmic inclusions) has been significantly related to fertilization rate and embryo quality after ICSI (evidence 1c) [60]. In non-male factor infertility, ICSI could be considered when metaphase I (MI) oocytes are matured, since the multi-pronuclei formation rate with ICSI is significantly lower than that with conventional insemination (evidence 2b) [61].
Sperm DNA fragmentation
High sperm DNA fragmentation has adverse effects on Assisted Reproductive Technology (ART) outcomes [62]. Studies report that DNA-damaged sperm cells could still fertilize the oocyte regardless of the degree of DNA damage. Still, embryonic development and full-term pregnancy were closely related to the degree of damage [63, 64], which has been associated with a “late paternal effect” during the activation of male gene expression [65].
Although Esteves et al. suggested that sperm DNA fragmentation could impair the results of both IVF and ICSI cycles [66], the outcomes of a meta-analysis published in 2015 suggest that IVF treatment in men with high sperm DNA fragmentation is associated with lower live birth outcome compared with those with low DNA fragmentation, but this detrimental effect has not been demonstrated when ICSI treatment was used (evidence 1a-) [67]. This finding may suggest a role for ICSI treatment in men with high sperm DNA fragmentation but it is important to emphasize that only two studies addressed this matter but with strict inclusion criteria and adequate sensitivity analysis to minimize the bias. The development of an efficient test that allows choosing the sperm without DNA fragmentation could be an opportunity of improving live birth rates in these cases.
Technical improvements: selection of a good-quality semen with intact sperm genome (IMSI, PICSI, flow cytometry, reotaxis, swim-up)
Prior ICSI procedure, there is a potential to select good-quality sperm (functional cells with intact sperm genome apparatus), other than the three basic sperm parameters [68]. Various techniques for sperm selection have been developed, such as intracytoplasmic morphologically selected sperm injection (IMSI), hyaluronan-selected sperm (physiological ICSI [PICSI]), flow cytometry, rheotaxis, or swim-up.
The spermatozoa selection process using IMSI had a hypothetical potential to improve results in ART cycles through an increase in the number of grade A embryos formed and a decrease in the level of fragmentation in these embryos (evidence 1a) [69] but results from RCTs with this technique do not encourage its clinical use. Studies show no evidence of the effect on live birth or miscarriage and the evidence that IMSI improves clinical pregnancy is of very low quality [70].
Three RCT have analyzed the use of hyaluronic acid binding assays in ICSI cycles to improve clinical outcomes (evidence 1b-) [71–73]. These studies reported that using PICSI for sperm selection decreased miscarriage rates and improved embryo quality and live birth rates compared with ICSI with sperm selected using standard methods. The couples who gained the most from PICSI had low hyaluronan–sperm binding scores. Nevertheless, the largest randomized trial of PICSI recently published provides a robust measure of livebirth and it shows that PICSI did not significantly increase the term livebirth rate compared with standard ICSI, but a significant decrease was observed in miscarriage rates among couples in the PICSI group (evidence 1b) [74]. According to these findings, the application of this technique may provide an improvement in embryo quality and implantation rate, but evidence does not encourage its routine use in all ICSI cycles and the identification of patients that might benefit from this technique needs further study.
Artificial oocyte activation
A failure in fertilization has been mainly attributed to an oocyte activation disruption [75]. The use of ICSI followed by artificial oocyte activation (AOA) has been associated with an increase in the proportion of cleavage stage embryos (RR 5.44, 95% CI 2.98–9.91), top/high-quality cleavage stage embryos (RR 10.02, 95% CI 2.45–40.95) (evidence 1a-) [76], and higher fertilization rates [77] in couples with a history of total fertilization failure in a previous cycle. Therefore, at this time, oocyte activation failure can be overcome clinically by AOA such as calcium ionophores or recombinant PLCζ protein injection. A number of cases have demonstrated that ICSI combined with AOA greatly increases the fertilization rate success and subsequent pregnancy (evidence 3b) [78–80] (even in patients with a history of failed fertilization with ICSI [78, 79, 81, 82]. Several reports on the well-being of children born after ICSI with AOA have provided reassuring insights resulting with neonatal outcome within normal limits concerning birth weight, gestational age, neonatal malformations, occurrence of perinatal mortality motor skills, behavioral profile and early development [79, 80, 83, 84].
Genome editing (CRISP/Cas9)
Innovative genome editing methods have been developed, particularly CRISPR/Cas 9, giving rise to new perspectives on germ line interventions [85]. Germ line genome editing advocates suggest that the procedure could be used as a means of disease prevention.
Threats
Predisposition to use ICSI (indiscriminate use/intolerance to fertilization failure)
The increase of ICSI due to non-male infertility despite the lack of evidence to support it has arisen, at least in part because of the intolerance to fertilization failure and because of the increasing expectation from infertile couples of obtaining a successful pregnancy. Furthermore, the removal of the cumulus cells provides the physician with more direct feedback on the quality of their stimulation, increasing the chance of success of ICSI in patients with poor morphology oocytes. A database analysis conducted using the National Assisted Reproductive Technology Surveillance System (NASS) from the Centers for Disease Control and Prevention (CDC) in the USA reported an overutilization of ICSI with no improved outcomes, particularly when it is used for non-male factor infertility and supports the contention that it is being over applied. Practice patterns for ICSI differ by geographical region and possible explanations may relate to insurance coverage availability, laboratory efficiencies, and/or perceived competition among clinics in specific regions of the country. A major reason why ICSI is performed for non-male factor indications is the pressure on the clinicians in privately funded IVF programs in particular, when results of clinics are up for patients to see in national registries.
Another relevant consideration for further examination is the specific criteria used to define male factor infertility and the indication for ICSI used by clinics in different regions of the country (evidence 2a) [86].
ICSI “overuse” during PGT
The use of PGT by an increasing number of centers has promoted the generation of embryos by ICSI to exclude the risk of interference of contaminating spermatozoa. Recently, doubts on ICSI “overuse” in non-male infertility have arisen, since recent data suggest that it does not offer an advantage over IVF and that IVF should be the preferred insemination methods in PGT cycles while ICSI should be indicated only in cases of male factor infertility (evidence 2b) [87].
Improvement of IVF
A possible improvement in IVF procedures could decrease the use of ICSI. In particular, a recently published retrospective data analysis summarizing the first 15 years of ART activity in Europe for 12 consecutive years (1997–2011) shows a notable increase in the proportion of ICSI versus IVF during this period, but since 2008 is reaching a plateau (evidence 2a) [9] where ICSI and IVF represent around two-thirds and one-third of fresh cycles, respectively. The plateau could be due to the reconsideration of many centers to perform ICSI only when necessary. Thereby, verifying that the results of IVF are comparable to or even better than those of ICSI in those circumstances, probably to avoid problems derived from microinjection or from having achieved laboratory improvements that increase the effectiveness of the process [50].
Possible long-term effects of ICSI on children (infants, adults)
Contradictory results have been published with regard to the possible long-term effects of ICSI on children. From a medical standpoint, an increased risk of adverse neonatal outcome has been reported for ICSI-born children [88–90]. Furthermore, some meta-analysis have shown an increase in congenital malformations in children born after ICSI [91–93], but no clustering of any specific major malformation and no increased risk for major malformations has been found in ICSI children (evidence 1a) [94].
These issues refer mainly to potential changes in genetic material, the possible transmission of foreign genetic material, the use of immature or senescent germ cells, and associations between genetic disorders and some forms of infertility [95–97]. Nevertheless, medical follow-up studies have failed to report adverse outcomes for children born through the ICSI technique [98–100].
With regard to child development, one of the first studies analyzing the possible impact of ICSI on child development showed that 1-year-old ICSI-born children compared with spontaneously conceived and IVF children, revealed an increased risk of mildly to significantly delayed development [95]. However, a concurrent single-center follow-up study found no signs of delayed development in 2-year-old ICSI children (evidence 2b) [95]. Later studies carried out in ICSI children aged up to 5 years show no delayed development [100–104] and although generalization of these findings should be treated with caution, given the many methodological limitations, such as relatively small sample sizes, inclusion of ICSI procedures for male factor deficiency, lack of formal child assessment, and demographic matching of samples.
Comparable or even better cognitive and motor development until the age of 8 years have also been demonstrated in two large-scale multicenter studies that compared developmental outcomes of 151 8-year-old singletons born through ICSI after 32 weeks of gestation with those of 153 singletons of the same age born after spontaneous conception (SC) (evidence 2b) [105]. Authors did not find a higher resting blood pressure or higher stress response after psychological stress in ICSI-conceived pubertal children [106]. Finally, a systematic review of 34 studies comparing ICSI- and IVF-conceived children suggest their physical health, growth, and neuro-development is comparable; although studies are few and limited to childhood. ICSI-conceived children may be at increased risk of autism and intellectual impairment and no difference in risk of childhood cancer was reported in one study. These data are inconclusive and further research into health outcomes in adolescence and adulthood is required before conclusions can be drawn on the long-term safety of ICSI compared with IVF. Until then, ICSI might be better reserved for its original intended use, male factor infertility (evidence 2a) [107].
Conclusion
In summary, despite the widespread use of ICSI in patients without a formal diagnosis of male factor infertility, evidence demonstrating its effectiveness in this population is still lacking. The major advantage of performing ICSI is that cases complicated by total fertilization failure can almost be eliminated. However, reproductive outcomes are similar, or even worse, to those obtained by IVF. These observations have to be taken with caution as most studies comparing IVF and ICSI in non-male factor infertility have low quality and varied methodology, outcome measures, and conclusions.
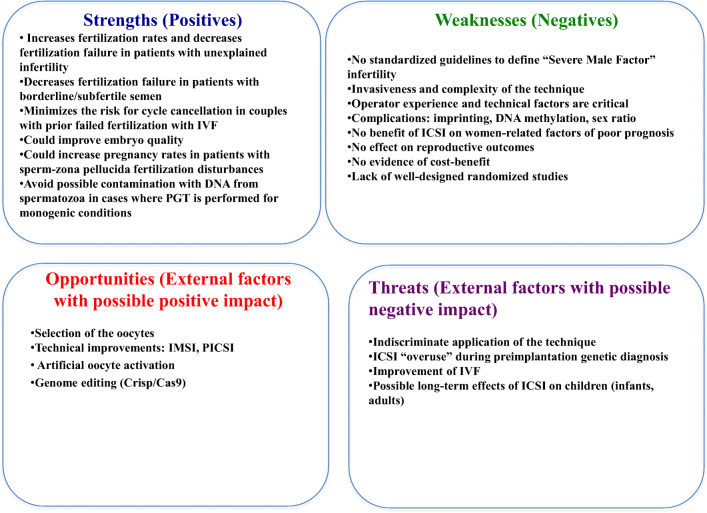
This procedure has some disadvantages. It involves more technical difficulties, higher costs, and performing an unnecessary invasive technique in some cases. Moreover, questions regarding safety issues cannot be ruled out. Furthermore, in the long-term, outcomes that may arise from the development and transfer of genetic disorders in cases where the ICSI procedure was used are still unknown. It may be concluded that ICSI is not justified in all couples requiring in vitro conception. In idiopathic infertility and relative male factor infertility, a possible strategy might be to perform a diagnostic comparison between conventional IVF and ICSI in all first treatment cycles. This would be especially beneficial in preventing total fertilization failure. Nevertheless, additional large and well-designed RCT are needed to clarify definitive indications for ICSI in non-male factor infertility (Fig. 1).
Fig. 1.
A SWOT analysis of ICSI versus IVF for non-male factor infertility in assisted reproduction
Acknowledgments
The author would like to thank Ana Isabel Ortega who provided medical writing assistance.
Authors’contribution
All authors have contributed to the conception and design, analysis and interpretation of data, drafting the article, and revising it critically for important intellectual content, and to the final approval of the version to be published.
Funding information
No funding or sponsorship was received for this study. Support for editorial assistance was funded by Angelini.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Palermo GD, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–18. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 2.Boulet SL, Mehta A, Kissin DM, Warner L, Kawwass JF, Jamieson DJ. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA. 2015;313:255–263. doi: 10.1001/jama.2014.17985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamson GD, de Mouzon J, Chambers GM, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Dyer S. International Committee for Monitoring Assisted Reproductive Technology: world report on assisted reproductive technology, 2011. Fertil Steril. 2018;110:1067–1080. doi: 10.1016/j.fertnstert.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 4.van Rumste MM, Evers JL, Farquhar CM. ICSI versus conventional techniques for oocyte insemination during IVF in patients with non-male factor subfertility: a Cochrane review. Hum Reprod. 2004;19(2):223–227. doi: 10.1093/humrep/deh061. [DOI] [PubMed] [Google Scholar]
- 5.Barrow P. Revision of the ICH guideline on detection of toxicity to reproduction for medicinal products: SWOT analysis. Reprod Toxicol. 2016;64:57–63. doi: 10.1016/j.reprotox.2016.03.048. [DOI] [PubMed] [Google Scholar]
- 6.Blockeel C, Drakopoulos P, Santos-Ribeiro S, Polyzos NP, Tournaye HA. A fresh look at the freeze-all protocol: a SWOT analysis. Hum Reprod. 2016;31(3):491–497. doi: 10.1093/humrep/dev339. [DOI] [PubMed] [Google Scholar]
- 7.Engmann L, Benadiva C, Humaidan P. GnRH agonist trigger for the induction of oocyte maturation in GnRH antagonist IVF cycles: a SWOT analysis. Reprod BioMed Online. 2016;32(3):274–285. doi: 10.1016/j.rbmo.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Esteves SC, Agarwal A, Cho CL, Majzoub A. A strengths-weaknesses-opportunities-threats (SWOT) analysis on the clinical utility of sperm DNA fragmentation testing in specific male infertility scenarios. Transl Androl Urol. 2017;6(Suppl 4):S734–S760. doi: 10.21037/tau.2017.08.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferraretti A, Nygren K, Nyboe A, de Mouzon A, Kupka J, Calhaz-Jorge M, et al. Trends over 15 years in ART in Europe: an analysis of 6 million cycles. Human Reproduction Open. 2017;2017. 10.1093/hropen/hox012. [DOI] [PMC free article] [PubMed]
- 10.Johnson LN, Sasson IE, Sammel MD, Dokras A. Does intracytoplasmic sperm injection improve the fertilization rate and decrease the total fertilization failure rate in couples with well-defined unexplained infertility? A systematic review and meta-analysis. Fertil Steril. 2013;100(3):704–711. doi: 10.1016/j.fertnstert.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 11.Shi XY, Wu FR, Chen SL, Wang QL, Luo C, Ni YP, Zheng HY, Qiu ZL, Zhang WQ, Yang J, Chen X. In vitro fertilization versus intracytoplasmic sperm injection for primary and secondary infertility using sibling oocytes: clinical analysis of the outcomes. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30(10):2263–2266. [PubMed] [Google Scholar]
- 12.World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge: Cambridge University Press; 1999
- 13.van der Westerlaken L, Naaktgeboren N, Verburg H. Conventional in-vitro fertilization versus intracytoplasmic sperm injection in patients with borderline semen: a randomized study using sibling oocytes. Fertil Steril. 2006;85:395–400. doi: 10.1016/j.fertnstert.2005.05.077. [DOI] [PubMed] [Google Scholar]
- 14.Plachot M, Belaisch-Allart J, Mayenga JM, Chouraqui A, Tesquier L, Serkine AM. Outcome of conventional IVF and ICSI on sibling oocytes in mild male factor infertility. Hum Reprod. 2002;17(2):362–369. doi: 10.1093/humrep/17.2.362. [DOI] [PubMed] [Google Scholar]
- 15.van Rumste MM, Evers JL, Farquhar CM, Blake DA. Intra-cytoplasmic sperm injection versus partial zona dissection, subzonal insemination and conventional techniques for oocyte insemination during in vitro fertilization. Cochrane Database Syst Rev. 2000;2:CD001301. doi: 10.1002/14651858.CD001301. [DOI] [PubMed] [Google Scholar]
- 16.Tournaye H, Verheyen G, Albano C. Intracytoplasmic sperm injection versus in vitro fertilization: a randomized controlled trial and a meta-analysis of the literature. Fertil Steril. 2002;78(5):1030–1037. doi: 10.1016/s0015-0282(02)03377-0. [DOI] [PubMed] [Google Scholar]
- 17.Staessen C, Camus M, Clasen K. Conventional in-vitro fertilization versus intracytoplasmic sperm injection in sibling oocytes from couples with tubal infertility and normozoospermic semen. Hum Reprod. 1999;14:2474–2479. doi: 10.1093/humrep/14.10.2474. [DOI] [PubMed] [Google Scholar]
- 18.Bungum L, Bungum M, Humaidan P, Andersen CY. A strategy for treatment of couples with unexplained infertility who failed to conceive after intrauterine insemination. Reprod BioMed Online. 2004;8:584–589. doi: 10.1016/s1472-6483(10)61107-8. [DOI] [PubMed] [Google Scholar]
- 19.van der Westerlaken L, Helmerhorst F, Dieben S, Naaktgeboren N. Intracytoplasmic sperm injection as a treatment for unexplained total fertilization failure or low fertilization after conventional in vitro fertilization. Fertil Steril. 2005;83(3):612–617. doi: 10.1016/j.fertnstert.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 20.Quinn P, Lydic ML, Ho M. Confirmation of the beneficial effects of brief coincubation of gametes in human in vitro fertilization. Fertil Steril. 1998;69:399–402. doi: 10.1016/s0015-0282(97)00576-1. [DOI] [PubMed] [Google Scholar]
- 21.Yang D, Shahata MA, Al-Bader M, Al-Natsha SD, Al-Flamerzia M, Al-Shawaf T. Intracytoplasmic sperm injection improving embryo quality: comparison of the sibling oocyte of non-male-factor couples. J Assist Reprod Genet. 1996;13:351–355. doi: 10.1007/BF02070151. [DOI] [PubMed] [Google Scholar]
- 22.Khamsi F, Yavas Y, Roberge S, Wong JC, Lacanna IC, Endman M. Intracytoplasmic sperm injection increased fertilization and good-quality embryo formation in patients with non-male factor indications for in vitro fertilization: a prospective randomized study. Fertil Steril. 2001;75(2):342–347. doi: 10.1016/s0015-0282(00)01674-5. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz A, Remohi J, Minguez Y, Guanes PP, Simon C, Pellicer A. The role of in vitro fertilization and intracytoplasmic sperm injection in couples with unexplained infertility after failed intrauterine insemination. Fertil Steril. 1997;68:171–173. doi: 10.1016/s0015-0282(97)81497-5. [DOI] [PubMed] [Google Scholar]
- 24.Kim JY, Kim JH, Jee BC, Lee JR, Suh CS, Kim SH. Can intracytoplasmic sperm injection prevent total fertilization failure and enhance embryo quality in patients with non-male factor infertility? Eur J Obstet Gynecol Reprod Biol. 2014;178:188–191. doi: 10.1016/j.ejogrb.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 25.Farhi J, Cohen K, Mizrachi Y, Weissman A, Raziel A, Orvieto R. Should ICSI be implemented during IVF to all advanced-age patients with non-male factor subfertility? Reprod Biol Endocrinol. 2019;17(1):30. doi: 10.1186/s12958-019-0474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bastiaan HS, Windt ML, Menkveld R, Kruger TF, Oehninger S, Franken DR. Relationship between zona pellucida-induced acrosome reaction, sperm morphology, sperm-zona pellucida binding, and in vitro fertilization. Fertil Steril. 2003;79(1):49–55. doi: 10.1016/s0015-0282(02)04548-x. [DOI] [PubMed] [Google Scholar]
- 27.Liu DY, Bourne H, Baker HWG. High fertilization and pregnancy rates after intracytoplasmic sperm injection in patients with disordered zona pellucida-induced acrosome reaction. Fertil Steril. 1997;67:955–958. doi: 10.1016/s0015-0282(97)81415-x. [DOI] [PubMed] [Google Scholar]
- 28.Liu DY, Baker HWG. Defective sperm–zona pellucida interaction: a major cause of failure of fertilization in clinical in-vitro fertilization. Hum Reprod. 2000;15:702–708. doi: 10.1093/humrep/15.3.702. [DOI] [PubMed] [Google Scholar]
- 29.Liu DY, Baker G. Disordered zona pellucida–induced acrosome reaction and failure of in vitro fertilization in patients with unexplained infertility. Fertil Steril. 2003;79(1):74–80. doi: 10.1016/s0015-0282(02)04555-7. [DOI] [PubMed] [Google Scholar]
- 30.Choi HY, Kim SK, Kim SH, Choi YM, Jee BC. Impact of sperm DNA fragmentation on clinical in vitro fertilization outcomes. Clin Exp Reprod Med. 2017;44(4):224–231. doi: 10.5653/cerm.2017.44.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Cambridge: Cambridge University Press; 2010.
- 32.Hamada A, Esteves S, Agarwal A. Unexplained male infertility—looking beyond routine semen analysis. Eur Urol Rev. 2012;7:90–96. [Google Scholar]
- 33.Alasmari W, Barratt C, Publicover S, Whalley K, Foster E, Kay V, Da Silva SM, Oxenham S. The clinical significance of calcium signalling pathways mediating human sperm hyperactivation. Hum Reprod. 2013;28:866–876. doi: 10.1093/humrep/des467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper TG, Noonan E, Von Eckardstein S, Auger J, Baker HG, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 35.Rahman A. Intracytoplasmic sperm injection-revolution in human and animal assisted reproduction: a review. Biotechnology. 2010;9:392–410. [Google Scholar]
- 36.Rosen MP, Shen S, Dobson AT, Fujimoto VY, McCulloch CE, Cedars MI. Oocyte degeneration after intracytoplasmic sperm injection: a multivariate analysis to assess its importance as a laboratory or clinical marker. Fertil Steril. 2006;85(6):1736–1743. doi: 10.1016/j.fertnstert.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Sfontouris IA, Kolibianakis EM, Lainas GT, Navaratnarajah R, Tarlatzis BC, Lainas TG. Live birth rates using conventional in vitro fertilization compared to intracytoplasmic sperm injection in Bologna poor responders with a single oocyte retrieved. J Assist Reprod Genet. 2015;32(5):691–697. doi: 10.1007/s10815-015-0459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Inany HG, Abou-Setta AM, Aboulghar MA, Mansour RT, Serour GI. Highly purified hMG achieves better pregnancy rates in IVF cycles but not ICSI cycles compared with recombinant FSH: a meta-analysis. Gynecol Endocrinol. 2009;25:372–378. doi: 10.1080/09513590802630120. [DOI] [PubMed] [Google Scholar]
- 39.McCulloh D, Goorbarry BA, Shah MS, Ahmad K. Oocyte lysis following intracytoplasmic sperm injection: association with measures of oocyte quality and technician performance. J Reprod Stem Cell Biotechnol. 2011;2(1):46–54. [Google Scholar]
- 40.Dumoulin JM, Coonen E, Bras M, Bergers-Janssen JM, Ignoul-Vanvuchelen RC, van Wissen LC, Geraedts JP, Evers JL. Embryo development and chromosomal anomalies after ICSI: effect of the injection procedure. Hum Reprod. 2001;16(2):306–312. doi: 10.1093/humrep/16.2.306. [DOI] [PubMed] [Google Scholar]
- 41.Lazaraviciute G, Kauser M, Bhattacharya S, Haggarty P, Bhattacharya S. A systematic review and meta-analysis of DNA methylation levels and imprinting disorders in children conceived by IVF/ICSI compared with children conceived spontaneously. Hum Reprod Update. 2015;21(4):555–557. doi: 10.1093/humupd/dmv017. [DOI] [PubMed] [Google Scholar]
- 42.Whitelaw N, Bhattacharya S, Hoad G, Horgan GW, Hamilton M, Haggarty P. Epigenetic status in the offspring of spontaneous and assisted conception. Hum Reprod. 2014;29(7):1452–1458. doi: 10.1093/humrep/deu094. [DOI] [PubMed] [Google Scholar]
- 43.Vermeiden JP, Bernardus RE. Are imprinting disorders more prevalent after human in vitro fertilization or intracytoplasmic sperm injection? Fertil Steril. 2013;99(3):642–651. doi: 10.1016/j.fertnstert.2013.01.125. [DOI] [PubMed] [Google Scholar]
- 44.Luke MB, Brown DA, Grainger VL, Baker E, Ginsburg JE, Stern and S. f. A. R. T. W. Group The sex ratio of singleton offspring in assisted-conception pregnancies. Fertil Steril. 2009;92(5):1579–1585. doi: 10.1016/j.fertnstert.2008.08.107. [DOI] [PubMed] [Google Scholar]
- 45.Cirkel C, König IR, Schultze-Mosgau A, Beck E, Neumann K, Griesinger G. The use of intracytoplasmic sperm injection is associated with a shift in the secondary sex ratio. Reprod BioMed Online. 2018;37(6):703–708. doi: 10.1016/j.rbmo.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Sunderan S, Boulet SL, Kawwass JF, Kissin DM. Comparing fertilization rates from intracytoplasmic sperm injection (ICSI) to in vitro fertilization (IVF) in women older than 38 years with no male factor infertility: a meta-analysis. Fertil Steril. 2018;110:e221. doi: 10.1016/j.fertnstert.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tannus S, Son WY, Gilman A, Younes G, Shavit T, Dahan MH. The role of intracytoplasmic sperm injection in non-male factor infertility in advanced maternal age. Hum Reprod. 2017;32(1):119–124. doi: 10.1093/humrep/dew298. [DOI] [PubMed] [Google Scholar]
- 48.Luna M, Bigelow C, Duke M, Ruman J, Sandler B, Grunfeld L, Copperman AB. Should ICSI be recommended routinely in patients with four or fewer oocytes retrieved? J Assist Reprod Genet. 2011;28(10):911–915. doi: 10.1007/s10815-011-9614-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chambers GM, Wand H, Macaldowie A, Chapman MG, Farquhar CM, Bowman M, Molloy D, Ledger W. Population trends and live birth rates associated with common ART treatment strategies. Hum Reprod. 2016;31(11):2632–2641. doi: 10.1093/humrep/dew232. [DOI] [PubMed] [Google Scholar]
- 50.Li Z, Wang AY, Bowman M, Hammarberg K, Farquhar C, Johnson L, Safi N, Sullivan EA. ICSI does not increase the cumulative live birth rate in non-male factor infertility. Hum Reprod. 2018;33(7):1322–1330. doi: 10.1093/humrep/dey118. [DOI] [PubMed] [Google Scholar]
- 51.Dieke AC, Mehta A, Kissin DM, Nangia AK, Warner L, Boulet SL. Intracytoplasmic sperm injection use in states with and without insurance coverage mandates for infertility treatment, United States, 2000-2015. Fertil Steril. 2018;109(4):691–697. doi: 10.1016/j.fertnstert.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 52.Kjellberg AT, Carlsson P, Bergh C. Randomized single versus double embryo transfer: obstetric and paediatric outcome and a cost-effectiveness analysis. Hum Reprod. 2006;21(1):210–216. doi: 10.1093/humrep/dei298. [DOI] [PubMed] [Google Scholar]
- 53.Kovacs P, Kovats T, Sajgo A, Szollosi J, Matyas S, Kaali SG. The role of hyaluronic acid binding assay in choosing the fertilization method for patients undergoing IVF for unexplained infertility. J Assist Reprod Genet. 2011;28:49–54. doi: 10.1007/s10815-010-9479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouwmans CA, Lintsen BM, Eijkemans MJ, Habbema JD, Braat DD, Hakkaart L. A detailed cost analysis of in vitro fertilization and intracytoplasmic sperm injection treatment. Fertil Steril. 2008;89:331–341. doi: 10.1016/j.fertnstert.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Ola B, Afnan M, Sharif K, Papaioannou S, Hammadieh N, Barratt C. Should ICSI be the treatment of choice for all cases of in-vitro conception. Considerations of fertilisation and embryo development, cost effectiveness and safety? Hum Reprod. 2001;12:2485–2490. doi: 10.1093/humrep/16.12.2485. [DOI] [PubMed] [Google Scholar]
- 56.Hollingsworth BH, Duncan AM. The cost effectiveness of intracyctoplasmic sperm injection (ICSI) J Assist Reprod Genet. 2008;24:571–577. doi: 10.1007/s10815-007-9175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vitek WS, Galárraga O, Klatsky PC, Robins JC, Carson SA, Blazar AS. Management of the first in vitro fertilization cycle for unexplained infertility: a cost-effectiveness analysis of split in vitro fertilization-intracytoplasmic sperm injection. Fertil Steril. 2013;100(5):1381–1388. doi: 10.1016/j.fertnstert.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HH, Bundorf MK, Behr B, McCallum SW. Use and outcomes of intracytoplasmic sperm injection for non-male factor infertility. Fertil Steril. 2007;88(3):622–628. doi: 10.1016/j.fertnstert.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 59.Orief Y, Dafopoulos KA. Should ICSI be used in non-male factor infertility? Reprod BioMed Online. 2004;9:348–356. doi: 10.1016/s1472-6483(10)62152-9. [DOI] [PubMed] [Google Scholar]
- 60.Balaban B, Barut T, Urman B. Assessment of oocyte quality. In: Nagy ZP, Varghese AC, Agarwal A, editors. Practical Manual of In Vitro Fertilization: advanced methods and novel devices. London: Springer Science & Bussiness Media; 2012. p. 105–19.
- 61.Harton GL, De Rycke M, Fiorentino F, European Society for Human Reproduction and Embryology (ESHRE) PGD Consortium ESHRE PGD consortium best practice guidelines for amplification-based PGD. Hum Reprod. 2011;26(1):33–40. doi: 10.1093/humrep/deq231. [DOI] [PubMed] [Google Scholar]
- 62.Ahmadi A, Ng SC. Fertilizing ability of DNA-damaged spermatozoa. J Exp Zool. 1999;284:696–704. doi: 10.1002/(sici)1097-010x(19991101)284:6<696::aid-jez11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 63.Seli E, Gardner DK, Schoolcraft WB, Moffatt O, Sakkas D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil Steril. 2004;82:378–383. doi: 10.1016/j.fertnstert.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 64.Tesarik J, Greco E, Mendoza C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum Reprod. 2004;19:611–615. doi: 10.1093/humrep/deh127. [DOI] [PubMed] [Google Scholar]
- 65.Esteves SC, Sánchez-Martín F, Sánchez-Martín P, Schneider DT, Gosálvez J. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril. 2015;104(6):1398–1405. doi: 10.1016/j.fertnstert.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 66.Osman A, Alsomait H, Seshadri S, El-Toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod BioMed Online. 2015;30(2):120–127. doi: 10.1016/j.rbmo.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 67.Park JH, Jee BC, Kim SH. Comparison of normal and abnormal fertilization of in vitro-matured human oocyte according to insemination method. J Obstet Gynaecol Res. 2016;42(4):417–421. doi: 10.1111/jog.12916. [DOI] [PubMed] [Google Scholar]
- 68.Alasmari A. Importance of the assessment of intracellular Ca 2+ level as diagnostic tool of dysfunctional sperm. Middle East Fertil Soc J. 2017;22:170–173. [Google Scholar]
- 69.Wilding M, Coppola G, di Matteo L, Palagiano A, Fusco E, Dale B. Intracytoplasmic injection of morphologically selected spermatozoa (IMSI) improves outcome after assisted reproduction by deselecting physiologically poor quality spermatozoa. J Assist Reprod Genet. 2010;28(3):253–262. doi: 10.1007/s10815-010-9505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McDowell S, Kroon B, Ford E, Hook Y, Glujovsky D, Yazdani A. Advanced sperm selection techniques for assisted reproduction. Cochrane Database Syst Rev. 2014;10:CD010461. doi: 10.1002/14651858.CD010461.pub2. [DOI] [PubMed] [Google Scholar]
- 71.Majumdar G, Majumdar A. A prospective randomized study to evaluate the effect of hyaluronic acid sperm selection on the intracytoplasmic sperm injection outcome of patients with unexplained infertility having normal semen parameters. J Assist Reprod Genet. 2013;30:1471–1475. doi: 10.1007/s10815-013-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parmegiani L, Cognigni GE, Bernardi S. Comparison of two ready-to-use systems designed for sperm–hyaluronic acid binding selection before intracytoplasmic sperm injection: PICSI vs sperm slow: a prospective, randomized trial. Fertil Steril. 2012;98:632–637. doi: 10.1016/j.fertnstert.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 73.Worrilow KC, Eid S, Woodhouse D. Use of hyaluronan in the selection of sperm for intracytoplasmic sperm injection (ICSI): significant improvement in clinical outcomes—multicenter, double-blinded and randomized controlled trial. Hum Reprod. 2013;28:306–314. doi: 10.1093/humrep/des417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller D, Pavitt S, Sharma V, Forbes G, Hooper R, Bhattacharya S. Physiological, hyaluronan-selected intracytoplasmic sperm injection for infertility treatment (HABSelect): a parallel, two-group, randomised trial. Lancet. 2019;393(10170):416–422. doi: 10.1016/S0140-6736(18)32989-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sousa M, Tesarik J. Ultrastructural analysis of fertilization failure after intracytoplasmic sperm injection. Hum Reprod. 1994;9:2374–2380. doi: 10.1093/oxfordjournals.humrep.a138455. [DOI] [PubMed] [Google Scholar]
- 76.Sfontouris IA, Nastri CO, Lima ML, Tahmasbpourmarzouni E, Raine-Fenning N, Martins WP. Artificial oocyte activation to improve reproductive outcomes in women with previous fertilization failure: a systematic review and meta-analysis of RCTs. Hum Reprod. 2015;30(8):1831–1841. doi: 10.1093/humrep/dev136. [DOI] [PubMed] [Google Scholar]
- 77.Takihara H. The treatment of obstructive azoospermia in male infertility--past, present, and future. Urology. 1998;51:150–155. doi: 10.1016/s0090-4295(98)00089-2. [DOI] [PubMed] [Google Scholar]
- 78.Heindryckx B, De Gheselle S, Gerris J, Dhont M, De Sutter P. Efficiency of assisted oocyte activation as a solution for failed intracytoplasmic sperm injection. Reprod BioMed Online. 2008;17:662–668. doi: 10.1016/s1472-6483(10)60313-6. [DOI] [PubMed] [Google Scholar]
- 79.Heindryckx B, Van der Elst J, De Sutter P, Dhont M. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum Reprod. 2005;20:2237–2241. doi: 10.1093/humrep/dei029. [DOI] [PubMed] [Google Scholar]
- 80.Kyono K, Takisawa T, Nakajo Y, Doshida M, Toya M. Birth and follow-up of babies born following ICSI with oocyte activation using strontium chloride or calcium ionophore A23187. Journal of Mammalian Ova Research. 2012;29:35–40. [Google Scholar]
- 81.Eldar-Geva T, Brooks B, Margalioth EJ, Zylber-Haran E, Gal M, Silber SJ. Successful pregnancy and delivery after calcium ionophore oocyte activation in a normozoospermic patient with previous repeated failed fertilization after intracytoplasmic sperm injection. Fertil Steril. 2003;79(3):1656–1658. doi: 10.1016/s0015-0282(03)00369-8. [DOI] [PubMed] [Google Scholar]
- 82.Chi HJ, Koo JJ, Song SJ, Lee JY, Chang SS. Successful fertilization and pregnancy after intracytoplasmic sperm injection and oocyte activation with calcium ionophore in a normozoospermic patient with extremely low fertilization rates in intracytoplasmic sperm injection cycles. Fertil Steril. 2004;82(2):475–477. doi: 10.1016/j.fertnstert.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 83.Yanagida K, Katayose H, Yazawa H, Kimura Y, Sato A, Yanagimachi H, Yanagimachi R. Successful fertilization and pregnancy following ICSI and electrical oocyte activation. Hum Reprod. 1999;14:1307–1311. doi: 10.1093/humrep/14.5.1307. [DOI] [PubMed] [Google Scholar]
- 84.Vanden F, D’Haeseleer E, Roeyers H, Oostra A, Van Lierde K, De Sutter P. Neonatal and developmental outcome of children born following assisted oocyte activation (AOA) Fertil Steril. 2012;98:S16. [Google Scholar]
- 85.Vassena R, Heindryckx B, Peco R, Pennings G, Raya A, Sermon K, Veiga A. Genome engineering through CRISPR/Cas9 technology in the human germline and pluripotent stem cells. Hum Reprod Update. 2016;22(4):411–419. doi: 10.1093/humupd/dmw005. [DOI] [PubMed] [Google Scholar]
- 86.Zagadailov P, Hsu A, Seifer DB, Stern JE. Differences in utilization of Intracytoplasmic sperm injection (ICSI) within human services (HHS) regions and metropolitan megaregions in the U.S. Reprod Biol Endocrinol. 2017;15:45. doi: 10.1186/s12958-017-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feldman B, Aizer A, Brengauz M, Dotan K, Levron J, Schiff E, Orvieto R. Pre-implantation genetic diagnosis-should we use ICSI for all? J Assist Reprod Genet. 2017;34(9):1179–1183. doi: 10.1007/s10815-017-0966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim JW, Lee WS, Yoon TK, Seok HH, Cho JH, Kim YS, Lyu SW, Shim SH. Chromosomal abnormalities in spontaneous abortion after assisted reproductive treatment. BMC Med Genet. 2010;11:153. doi: 10.1186/1471-2350-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Knoester M, Helmerhorst FM, van der Westerlaken LA, Walther FJ, Veen S, Leiden Artificial Reproductive Techniques Follow-up Project (L-art-FUP) Matched follow-up study of 5 8-year-old ICSI singletons: child behaviour, parenting stress and child (health-related) quality of life. Hum Reprod. 2007;22(12):3098–3107. doi: 10.1093/humrep/dem261. [DOI] [PubMed] [Google Scholar]
- 90.Lie RT, Lyngstadaas A, Ørstavik KH, Bakketeig LS, Jacobsen G, Tanbo T. Birth defects in children conceived by ICSI compared with children conceived by other IVF-methods; a meta-analysis. Int J Epidemiol. 2005;34(3):696–701. doi: 10.1093/ije/dyh363. [DOI] [PubMed] [Google Scholar]
- 91.Wen J, Jiang J, Ding C, Dai J, Liu Y, Xia Y, Liu J, Hu Z. Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: a meta-analysis. Fertil Steril. 2012;97(6):1331–7.e1–4. doi: 10.1016/j.fertnstert.2012.02.053. [DOI] [PubMed] [Google Scholar]
- 92.Bowen JR, Gibson FL, Leslie GI, Saunders DM. Medical and developmental outcome at 1 year for children conceived by intracytoplasmic sperm injection. Lancet. 1998;351:1529–1534. doi: 10.1016/S0140-6736(98)10168-X. [DOI] [PubMed] [Google Scholar]
- 93.Tournaye H. ICSI: a technique too far? Int J Androl. 2003;26(2):63–69. doi: 10.1046/j.1365-2605.2003.00380.x. [DOI] [PubMed] [Google Scholar]
- 94.Van de Velde H, De Vos A, Joris H, Nagy ZP, Van Steirteghem AC. Effect of timing of oocyte denudation and micro-injection on survival, fertilization and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1998;13(11):3160–3164. doi: 10.1093/humrep/13.11.3160. [DOI] [PubMed] [Google Scholar]
- 95.Kurinczuk JJ. Safety issues in assisted reproduction technology. From theory to reality--just what are the data telling us about ICSI offspring health and future fertility and should we be concerned? Hum Reprod. 2003;18(5):925–931. doi: 10.1093/humrep/deg217. [DOI] [PubMed] [Google Scholar]
- 96.Devroey P, Van Steirteghem A. A review of ten year experience of ICSI. Hum Reprod Update. 2004;10(1):19–28. doi: 10.1093/humupd/dmh004. [DOI] [PubMed] [Google Scholar]
- 97.Bonduelle M, Wennerholm UB, Loft A, Tarlatzis BC, Peters C, Henriet S, Mau C, Victorin-Cederquist A, Van Steirteghem A, Balaska A, Emberson JR, Sutcliffe AG. A multi-Centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Hum Reprod. 2005;20(2):413–419. doi: 10.1093/humrep/deh592. [DOI] [PubMed] [Google Scholar]
- 98.Sutcliffe AG, Barnes J, Wennerholm UB, Loft A, Tarlatzis BC, Ponjaert-Kristoferson I, Bonduelle M. Laterality in five-year-olds conceived by intracytoplasmic sperm injection, standard in vitro fertilisation and natural conception: a European study. BJOG. 2005;112(10):1397–1401. doi: 10.1111/j.1471-0528.2005.00677.x. [DOI] [PubMed] [Google Scholar]
- 99.Leslie GI, Gibson FL, McMahon C, Cohen J, Saunders DM, Tennant C. Children conceived using ICSI do not have an increased risk of delayed mental development at 5 years of age. Hum Reprod. 2003;18(10):2067–2072. doi: 10.1093/humrep/deg408. [DOI] [PubMed] [Google Scholar]
- 100.Papaligoura Z, Panopoulou-Maratou O, Solman M, Arvaniti K, Sarafidou J. Cognitive development of 12 month old Greek infants conceived after ICSI and the effects of the method on their parents. Hum Reprod. 2004;19(6):1488–1493. doi: 10.1093/humrep/deh270. [DOI] [PubMed] [Google Scholar]
- 101.Squires J, Carter A, Kaplan P. Developmental monitoring of children conceived by intracytoplasmic sperm injection and in vitro fertilization. Fertil Steril. 2003;79(2):453–454. doi: 10.1016/s0015-0282(02)04685-x. [DOI] [PubMed] [Google Scholar]
- 102.Leunens L, Celestin-Westreich S, Bonduelle M, Liebaers I, Ponjaert-Kristoffersen I. Cognitive and motor development of 8-year-old children born after ICSI compared to spontaneously conceived children. Hum Reprod. 2006;21(1):2922–2929. doi: 10.1093/humrep/del266. [DOI] [PubMed] [Google Scholar]
- 103.Belva F, Roelants M, De Schepper J, Roseboom TJ, Bonduelle M, Devroey P, Painter RC. Blood pressure in ICSI-conceived adolescents. Hum Reprod. 2012;27(10):3100–3108. doi: 10.1093/humrep/des259. [DOI] [PubMed] [Google Scholar]
- 104.Catford SR, McLachlan RI, O'Bryan MK, Halliday JL. Long-term follow-up of intra-cytoplasmic sperm injection-conceived offspring compared with in vitro fertilization-conceived offspring: a systematic review of health outcomes beyond the neonatal period. Andrology. 2017;5(4):610–621. doi: 10.1111/andr.12369. [DOI] [PubMed] [Google Scholar]
- 105.Rimm AA, Katayama AC, Diaz M, Katayama KP. A meta-analysis of controlled studies comparing major malformation rates in IVF and ICSI infants with naturally conceived children. J Assist Reprod Genet. 2004;21(12):437–443. doi: 10.1007/s10815-004-8760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Källén B, Finnström O, Nygren KG, Olausson PO. In vitro fertilization (IVF) in Sweden: risk for congenital malformations after different IVF methods. Birth Defects Res A Clin Mol Teratol. 2005;73(3):162–169. doi: 10.1002/bdra.20107. [DOI] [PubMed] [Google Scholar]
- 107.Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346(10):725–730. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]