Abstract
Purpose
Microgravity has severe effects on cellular and molecular structures as well as on metabolic interactions. The aim of this study is to investigate the effects of microgravity (μg) exposure on human frozen sperm samples.
Methods
Sibling samples from 15 normozoospermic healthy donors were frozen using glycerol as cryoprotectant and analyzed under microgravity and ground conditions. Microgravity was obtained by parabolic flights using a CAP10B plane. The plane executed 20 parabolic maneuvers with a mean of 8.5 s of microgravity for each parabola.
Results
Frozen sperm samples preserved in cryostraws and stored in a secure and specific nitrogen vapor cryoshipper do not suffer significant alterations after μg exposure. Comparing the study group (μg) and the control group (1 g), similar results were obtained in the main parameters studied: sperm motility (M/ml) 13.72 ± 12.57 vs 13.03 ± 12.13 (− 0.69 95% CI [− 2.9; 1.52]), progressive a + b sperm motility (%) 21.83 ± 11.69 vs 22.54 ± 12.83 (0.03 95% CI [− 0.08; 0.15]), sperm vitality (%) 46.42 ± 10.81 vs 44.62 ± 9.34 (− 0.04 95% CI [− 0.13; 0.05]), morphologically normal spermatozoa (%) 7.03 ± 2.61 vs 8.09 ± 3.61 (0.12 95% CI [0.01; 0.24]), DNA sperm fragmentation by SCD (%) 13.33 ± 5.12 vs 13.88 ± 6.14 (0.03 95% CI [− 0.09; 0.16]), and apoptotic spermatozoa by MACS (%) 15.47 ± 15.04 vs 23.80 ± 23.63 (− 0.20 95% CI [− 0.66; 1.05]).
Conclusion
The lack of differences obtained between frozen samples exposed to μg and those maintained in ground conditions provides the possibility of considering the safe transport of human male gametes to space. Nevertheless, further research is needed to validate the results and to consider the possibility of creating a human sperm bank outside the Earth.
Trial registration number
ClinicalTrials.gov: NCT03760783
Keywords: Microgravity, Sperm, Motility, Vitality, DNA fragmentation, Apoptosis
Introduction
One of the main effects that bodies, including living ones, undergo as they move away from Earth is weightlessness due to the scant net gravitational force. The term microgravity is used when referring to small gravity levels or low gravity. According to Clément [1], a microgravity environment is one that imparts to an object a net acceleration that is small compared with that produced by the Earth at its surface. Such an environment can be achieved by using different platforms. In practice, these platforms create a near-zero gravity environment in which the residual acceleration ranges from 1% of the Earth’s gravitational acceleration to one part in a million, usually referred to as microgravity. It is known that microgravity alters physiological processes in living organisms and causes a number of adverse effects on cellular and molecular structures and metabolic interactions. It has been reported that the cell membrane, cytoskeleton, cytoplasm, and nucleus are sensitive to gravitational changes and also that microgravity affects enzymatic reactions and leads to conformational changes in lipid structures [2].
The effects of different gravitational environments other than that of the Earth’s gravity on functions of the human body are found in the literature. During a spaceflight, when a human being transitions throughout different gravitational environments, the health of astronauts is affected and different diseases can be induced [3]. The main target of microgravity included musculoskeletal apparatus [4], the nervous system [5], and the endocrine system [6], among others. The effects of microgravity on the cardiovascular system and blood flow have also been widely studied [7].
However, little is known about the effects of microgravity on the human reproductive system. Female astronauts are encouraged to use contraception to avoid menses and to imperatively prevent pregnancy on orbits of long-duration flights, so it is not known what might happen to the natural menstrual cycle in zero gravity and its consequences on oocyte quality, fertilization, and embryo and fetal development processes [8]. Much more information exists about the effects of microgravity on the reproductive system in the animal model, specifically in mammals [9, 10]. In general, alterations in the male and female reproductive systems have been reported, due to the influence of microgravity on their specific functions and their associated endocrine signals [11, 12]. It has also been reported that simulated microgravity affects the regulation of gene expression of some genes involved in mouse inner cell mass formation and blastocyst development [13]. Descriptions also exist regarding the influence of microgravity on the development of mouse embryonic testes [14] and spermatogenesis in rats [15] by affecting cell proliferation, differentiation, germ cell survival, apoptosis, and the secretion of sexual hormones from testicles. Alterations in the physiology of testicular cells are induced, and testicular function is impaired in response to microgravity exposure. However, the ability to maintain a certain capacity of clone-forming and differentiation into round spermatic cell with flagella has also been reported [16]. Using a cell line established from a primary lesion of a testicular seminoma from a human male patient, it has been shown that simulated microgravity activates the oxidative machinery, thereby triggering transient microscopic cell events, such as a reduction in the proliferation rate, changes in the cytoskeleton-driven shape, and autophagy activation [12]. Some studies have been published on the effect of microgravity on sperm motility in different species, and while some authors have identified an increased motility in the bull [17] and the sea urchin [18], others have found a reduced motility in mice [19] and in humans [20].
Different approaches have been adopted in studies on the effects of space gravity variations. Access to a real spaceflight, sounding rockets, and other platforms outside the Earth, such as the International Space Station, is highly limited. Consequently, other platforms operating on the Earth that provide limited periods of microgravity or simulation models for producing microgravity have been designed to enable the gravitational effects in physical and life sciences to be tested. Different approaches have been adopted for conducting experiments in microgravity conditions, for instance, rotating wall vessel bioreactors, which make it possible to perform a rotating cell culture system providing a dynamic simulated microgravity [14, 16, 21, 22]; random positioning machines that allow simulated microgravity exposure [12], clinostats [23, 24], drop towers [25], sounding rockets [26], and finally parabolic flights [2, 7, 27–34].
Parabolic flights have been conducted for a long time. Among other aircraft, the European Space Agency has used the Airbus A300 ZERO-G and the Airbus 310 ZERO-G for research experiments in microgravity. These aircraft provide up to 25 s of microgravity and have been widely used for research purposes [2, 27–30]. Since 2011, a smaller single-engine aerobatic aircraft has also been used with successful results for different experimental studies under microgravity conditions [7, 31–34]. This method has been employed in different investigations because a high level of repetitiveness is achieved, thus obtaining a mean of 8.5 s of microgravity for each parabola when parabolas are executed by trained and specialized pilots. As compared with an orbit of the International Space Station around the Earth (approximately 90 min), this exposure time is but a small fraction of a possible exposure time in such an orbit. Nonetheless, parabolic flights have been used since the beginnings of space exploration as a reliable way to obtain meaningful data from a variety of experiments in the field of space medicine [1, 30, 34]. They are particularly appropriate for this experiment to test our hypothesis.
Studies investigating the effects of altered gravity on animals, and specifically on human beings, are required if life and even reproduction outside the Earth arise as a challenge to be addressed in the future. Should assisted reproductive techniques (ART) be used as an alternative method for natural conception in microgravity conditions, one of the first aspects to be studied is how microgravity may affect frozen human sperm samples and also whether they can be safely sent into space for possible future use under different gravitational conditions outside the Earth. The effects of microgravity on fresh or cryostored human gametes and embryos remain unknown. The objective of this study was to analyze the effect of microgravity obtained by parabolic flights on frozen human sperm under in vitro conditions.
Material and methods
Parabolic flights
This is a prospective study carried out in a university-affiliated ART center in collaboration with a Polytechnic University and an Aeroclub specialized in aerobatic parabolic flights for scientific research, located at 25 km from the ART laboratory. A CAP10B plane that provides short-duration microgravity exposure was chosen to obtain microgravity conditions. It is a twin-seater aircraft, thus enabling a space in the cockpit to be used for the experiment. A container suitable for air transport and holding the frozen sperm samples was placed in the cockpit space and securely attached to prevent any movement during the flight. This container is a shipping container of lightweight aluminum located inside a bigger protective dry shipper that keeps materials at liquid nitrogen temperatures with no free-flowing liquid (Arctic express cryogenic dry shipper; Thermo Fisher).
A total of three parabolic flights carrying five samples in each experimental flight were performed during 2018–2019, always in appropriate weather conditions in order to ensure that visual flight operations were not jeopardized. In every flight, the plane executed 20 parabolic maneuvers of up to 8.5 s of microgravity (~ 0.05 g) for each parabola. The maneuvers started at an altitude of 1000 m above ground level (AGL) and rose up to 1200 m AGL at the peak of the parabola before descending to the previous flight level.
Sperm samples
Sixteen healthy male volunteers (not fertility patients) were asked to participate in the study and informed of all the details of the procedure. After signing the specific informed consent for the study, they donated a sperm sample in accordance with the standard WHO recommendations [35]. After liquefaction, samples were checked to evaluate concentration and motility, and only 15 normozoospermic males (sperm concentration of more than 15 million/ml and a progressive motility greater than 32%) were included in the study. The volunteers were aged between 26 and 40 years old, 14 of whom were without offspring and one was a father of two children. Their mean BMI was 23.9 (18.6–27.2). Five of these volunteers were moderately overweight (BMI > 25), but none was classified as obese (BMI > 30).
Sperm freezing protocol
Each sperm sample was split in two before freezing in order to obtain sibling samples for exposure to different gravitational conditions: μg and 1 g (control). Both fractions were frozen according to the slow freezing method [36]. A stepwise cooling using glycerol as cryoprotectant (CryoSperm™, Origio) was performed, after which the samples were aliquoted in 0.5 ml CBS™ high security straws (Cryo Bio System). Semen was mixed 1:1 with the cryoprotectant and left for 10 min at room temperature. Next, straws were sealed by heat and left in nitrogen vapor for 30 min before being kept in liquid nitrogen for storage until the day of the experiment.
On the day of the flight, cryopreserved sperm samples from microgravity group (μg) were transported to the Aeroclub in liquid nitrogen vapor using the specified container. The control samples (1 g) were kept in nitrogen vapor at ground conditions.
Semen analysis
After the parabolic flight, frozen samples exposed to microgravity were quickly transported to the ART laboratory where both μg samples and the control group maintained at 1 g were thawed just prior to the start of each test. Straws were warmed by being placed on a heated plate at 37 °C for 5 min, after which the content was recovered in a clean tube and immediately used for the different assays: sperm concentration, motility, vitality, morphology, DNA fragmentation, and apoptosis.
Sperm concentration and motility were evaluated using a Makler chamber and SCA® CASA System (Microptic), which allows an automatic assessment of the samples by analyzing 150–200 spermatozoa from a drop of 4 μl of the thawed sperm sample. The following different motile parameters were analyzed: total motility (M/ml and %), percentage of progressive motility a + b (%), mean curvilinear velocity (μm/s) (VCL) as the time-averaged velocity of a sperm head along its curvilinear path, rectilinear velocity or straight-line velocity (μm/s) (VSL) as the average velocity of a sperm head along the straight line between its first position and its last position detected, and the linearity index (%) (LIN) as a path linearity indicator (LIN = VSL/VCL).
Sperm vitality was evaluated using eosin-nigrosin staining (VitalScreen, FertiPro), which makes it possible to identify membrane-intact spermatozoa. The lower reference limit used was 58% of live spermatozoa according to the WHO guidelines for semen analysis [35].
Morphology was assessed by staining with eosin and blue for fast staining in accordance with the manufacturer’s protocol (PanReac AppliChem) and classified according to the WHO criteria. A mean of 200 spermatozoa per sample were evaluated. The lower reference limit for normal morphology was 4% [35].
DNA fragmentation was evaluated by sperm chromatin dispersion (SCD) test (Halosperm®, Halotech). A mean of 500 spermatozoa per sample were evaluated. Spermatozoa with a small halo (halo width similar to or smaller than 1/3 of the diameter of the core) or without halo were classified as sperm with fragmented DNA. The reference limit for normal sperm DNA fragmentation was < 30% [37].
For the evaluation of sperm apoptosis, magnetic-activated cell sorting (MACS) using Annexin-V microbeads (MACS® Annexin V ART System, Miltenyi Biotec) was used in accordance with the manufacturer’s instructions. Samples were submitted to density gradients (PureSperm®, Nidacon), and the pellet was washed with Annexin-binding buffer and finally incubated with Annexin-V reagent. Apoptotic spermatozoa were retained in the column containing magnetic microbeads and placed in a magnet, while non-apoptotic spermatozoa with intact membranes went through it. Both fractions were collected, the sperm concentration was measured using SCA® CASA System, and the percentage of apoptotic spermatozoa was calculated.
Statistics
All sperm parameters analyzed were evaluated as continuous variables. Concentration, progressive motility, vitality, morphology, and DNA fragmentation were additionally evaluated as categorical variables according to the defined standards of normality. Continuous variables were expressed as mean and standard deviations and categorical variables as frequencies.
McNemar’s test was applied to compare categorical variables between the study group and the control group. For continuous variables, we performed 95% confidence intervals for differences of mean. Paired data were considered for all analyses. Mean differences were statistically significant when confidence intervals did not contain 0.
Statistical analyses were performed with IBM© SPSS© Statistics v 22. This study was registered at the Clinical Trials Database (ref: ClinicalTrials.gov) with the registration number: NCT03760783.
Results
Comparison of mean values between frozen samples exposed to microgravity (μg) and samples maintained on earth conditions (1 g) with automatic assessment after thawing showed similar results in terms of total sperm concentration (39.01 ± 32.02 vs 39.29 ± 36.53 M/ml) and total motile sperm concentration (13.72 ± 12.57 vs 13.03 ± 12.13 M/ml). Moreover, we observed no statistically significant differences in the percentage of sperm progressive motility (21.83 ± 11.69 vs 22.54 ± 12.83) nor in the sperm concentration of grade a (5.80 ± 6.40 vs 5.22 ± 5.58 M/ml), b (3.46 ± 3.15 vs 4.03 ± 4.16 M/ml), or c (4.47 ± 4.18 vs 3.78 ± 3.22 M/ml) (Table 1).
Table 1.
Comparison between normozoospermic frozen samples exposed to microgravity (μg) and the control group maintained on earth conditions (1 g): sperm concentration and motility after thawing
| μg | 1 g | ||||
|---|---|---|---|---|---|
| Mean ± SD | [Range] | Mean ± SD | [Range] | DIF [95% CI] | |
| Total sperm concentration (M/ml) | 39.01 ± 32.02 | [11.04–115.90] | 39.29 ± 36.53 | [8.90–127.74] | 0.28 [−3.40; 3.96] |
| Motile sperm concentration (M/ml) | 13.72 ± 12.57 | [1.20–43.58] | 13.03 ± 12.13 | [0.86–37.86] | − 0.69 [− 2.9; 1.52] |
| % Motility | 32.82 ± 14.19 | [10.85–50.18] | 32.27 ± 15.84 | [9.77–62.28] | 0.09 [− 0.12; –0.30] |
| Grade a sperm concentration (M/ml) | 5.80 ± 6.40 | [0.25–23.74] | 5.22 ± 5.58 | [0.36–21.03] | − 0.58 [− 1.28; 0.12] |
| Grade b sperm concentration (M/ml) | 3.46 ± 3.15 | [0.20–9.70] | 4.03 ± 4.16 | [0.19–14.67] | 0.58 [− 0.96; 2.11] |
| Grade c sperm concentration (M/ml) | 4.47 ± 4.18 | [0.36–13.93] | 3.78 ± 3.22 | [0.32–9.52] | − 0.69 [− 1.93; 0.55] |
| Progressive motility (a + b) (%) | 21.83 ± 11.69 | [5.35–37.37] | 22.54 ± 12.83 | [6.21–45.10] | 0.03 [− 0.08; 0.15] |
Mean values for the additional parameters automatically assessed to further characterize possible alterations in motility patterns also failed to show any differences: VCL (39.88 ± 8.70 vs 43.50 ± 9.82 μm/s), VSL (22.23 ± 8.00 vs 24.57 ± 8.12 μm/s), and LIN (62.60 ± 10.39 vs 65.88 ± 11.30%) Table 2.
Table 2.
Post-thaw progressive rate and linear movement from frozen samples exposed to both gravitational conditions (μg vs 1 g)
| μg | 1 g | ||||
|---|---|---|---|---|---|
| Mean ± SD | [Range] | Mean ± SD | [Range] | DIF [95% CI] | |
| Curvilinear velocity, VCL (μm/s) | 39.88 ± 8.70 | [25.71–53.91] | 43.50 ± 9.82 | [23.75–57.57] | 3.62 [− 0.03; 7.27] |
| Straight-line (rectilinear velocity, VSL (μm/s) | 22.23 ± 8.00 | [9.87–34.44] | 24.57 ± 8.12 | [10.24–38.95] | 2.34 [− 1.11; 5.78] |
| Linearity index, LIN (%) | 62.60 ± 10.39 | [46.29–81.01] | 65.88 ± 11.30 | [42.82–82.17] | 0.05 [− 0.03; 0.13] |
The mean percentage of live spermatozoa assessed by eosin-nigrosin staining (46.42 ± 10.81 vs 44.62 ± 9.34) observed in both thawed samples (μg vs 1 g) indicates that the vitality of frozen sperm samples does not undergo any change after microgravity exposure under the conditions of this experimental study. The results of morphological sperm assessment showed a similar proportion of normal morphological sperm (7.03 ± 2.61 vs 8.09 ± 3.61%), and in both cases, they were above the lower reference limit for normal morphology (Table 3).
Table 3.
Percentage of live spermatozoa, morphological normal forms, DNA fragmented sperm, and apoptotic sperm in the group exposed to microgravity (μg) versus control group without microgravity exposure (1 g)
| μg | 1 g | ||||
|---|---|---|---|---|---|
| Mean ± SD | [Range] | Mean ± SD | [Range] | DIF [95% CI] | |
| Vitality (%) | 46.42 ± 10.81 | [29.00–66.75] | 44.62 ± 9.34 | [24.75–59.50] | − 0.04 [− 0.13; 0.05] |
| Normal sperm morphology (%) | 7.03 ± 2.61 | [3.13–13.00] | 8.09 ± 3.61 | [3.50–18.00] | 0.12 [0.01; 0.24] |
| Sperm DNA fragmentation (%) | 13.33 ± 5.12 | [7.60–25.20] | 13.88 ± 6.14 | [7.00–26.20] | 0.03 [− 0.09; 0.16] |
| % Apoptotic sperm | 15.47 ± 15.04 | [2.55–56.72] | 23.80 ± 23.63 | [0.16–93.96] | 0.20 [− 0.66; 1.05] |
Similar results were also observed in relation to the mean percentage of sperm with DNA fragmentation measured by SCD. Samples exposed to microgravity conditions had a mean of 13.33 ± 5.12% sperm fragmentation, while control samples had a mean of 13.88 ± 6.14%. The mean percentage of DNA fragmentation was similar in both groups and lower than the reference limit (Table 3).
Neither the proportion of apoptotic sperm with externalized phosphatidylserine, attached to the Annexin-V column (15.47 ± 15.04 vs 23.80 ± 23.63%), nor the non-apoptotic spermatozoa with intact membranes that went throughout the column (84.53 ± 15.04 vs 76.20 ± 23.63%) showed any statistical differences between μg and 1 g groups (Table 3).
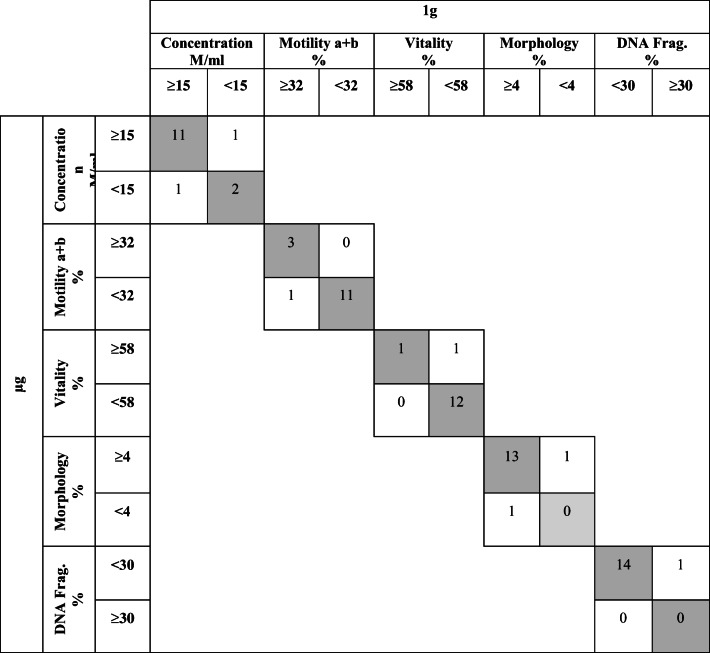
Regarding the diagnosis of each parameter analyzed according to the defined standards of normality, a high degree of diagnostic concordance (> 85%) between both groups of thawed samples exposed to different gravity conditions was observed. With respect to progressive motility and DNA fragmentation, the degree of concordance between the study and the control group at ground conditions was 93.3% (14/15). With regard to sperm concentration, vitality diagnosis, and morphological assessment, concordance was observed in 86.7% (13/15) of the samples. Overall, the vast majority of samples (90%) have been classified in the same diagnostic category after being exposed to microgravity conditions (Table 4).
Table 4.
Concordance in the diagnosis of normality in sibling thawed samples (μg and 1 g) according to the standards of normality defined
Discussion
If the number of space missions increases in the coming years, and the establishment of human colonies outside the Earth becomes an option, it is important to study the effects of human exposure to space conditions and to seriously consider the possibility of reproduction beyond the Earth. This study was conceived as an initial step in the investigation of the effects of microgravity conditions on human reproductive cells. In this case, the study was performed with human frozen sperm samples in order to predict if they can be safely sent and stored in space.
One of the main challenges involved in conducting microgravity research on the ground is that such a condition is difficult to obtain, because the Earth’s gravity is an inevitable force that cannot be avoided. We have chosen aerobatic parabolic flights because they are recognized as a powerful method for experimental studies, despite the limited microgravity time obtained and the short periods of hypergravity before and after microgravity periods. Both limitations are common for small and large aircraft. We decided to use the CAP10B aircraft because of its accessibility and its user-friendliness. The parabolic flights were conducted by an experienced pilot, and the proximity between the airport and the ART center facilitated the procedures. Other platforms have been described in different gravity studies [38, 39]. For intance, the effects of microgravity on marrow mesenchymal cells have been successfully studied using a clinostat [24]. This method has proven to be suitable for simulating microgravity effects on cultured cells, but in our case, it would have been impractical to hold frozen samples stored in liquid nitrogen inside the clinostat for a long period of time. Furthermore, although the effect of continuously rotating the sample in a clinostat or a rotating position machine may have no effect on frozen sperm samples, it will certainly affect the motility features of fresh sperm samples [20], which could later be compared with the behavior of the frozen samples studied herein. Drop towers might also be considered, but the size of the cylinder containing the sample makes it unpractical to hold and maintain frozen sperm samples, so parabolic flights seemed to be the best option regardless of other facilities operating beyond the Earth. Sounding rockets, suborbital flights, or the in-flight research facilities of the International Space Station provide longer exposure time to microgravity, and despite their highly limited access, they should be taken into account in the future for more in-depth studies. In this context, NASA announced an experiment on the International Space Station with bull and human sperm samples, but at the time of this publication, their results remain unpublished.
The results of a previous parabolic flight experiment for studying microgravity effects on the motility of human sperm samples were also published [20]. In that case, the authors found a lower motility rate and progressive velocity after microgravity exposure by parabolic flights using fresh semen. They suggested that the decline in sperm motility might be due to chemical changes in the intracellular environment during microgravity exposure. In our study, the undetectable differences observed in the parameters analyzed (% motile sperm, % a + b; VCL, VSL, LIN) could suggest that the effects of microgravity on sperm motility was minimized, because the samples were frozen and sperm integrity was shielded by cryoprotectants.
The vitality results showed similar values in microgravity and ground conditions, but in both circumstances, they were below the reference limit. In no case are the lower results related to gravity conditions; they may be attributable to the fact that sperm membrane integrity can be affected during freezing and thawing processes and the established reference limit is generally applied to fresh samples. With respect to the other parameters analyzed (morphology, DNA fragmentation, and apoptosis), we also obtained similar results between both groups (μg vs 1 g), which reinforces the case for better protection against microgravity in the frozen samples when compared with the fresh ones.
Regarding the diagnosis of the different tests performed, it is important to highlight that the results obtained showed a concordance of more than 85% in all parameters. The minor variations observed were more likely to be due to heterogeneity of the sperm samples rather than to the effect of exposure to different gravity conditions. In conclusion, frozen sperm samples preserved in cryostraws and stored in a specific nitrogen vapor cryoshipper undergo no significant alterations after exposure to microgravity obtained by parabolic flights and under the specific conditions of this study.
The lack of differences observed in all the sperm parameters studied suggests that frozen sperm samples exposed to microgravity do not suffer significant alterations in comparison with the control samples maintained in ground conditions. This finding has an important clinical significance, since it represents a starting point for assessing the possibility of transporting human gametes into space. However, the limitations due to the reduced periods of microgravity obtained by parabolic flights suggest that it would be desirable to corroborate this finding by further studies with different platforms that provide longer microgravity exposure and a larger sample. We are aware of the limitation of the reduced sample size, but due to the large battery of tests carried out in this study and the need to perform all the analyses immediately after the flight, no more than 5 samples could be included in each experiment.
Concerning the method of human sperm cryopreservation, we have used the slow freezing method with liquid nitrogen vapor, which despite its limitations is the method that has been successfully employed for decades in ART and fertility preservation. It is known that in spite of the great variability between samples and donors, a reduction in sperm motility is usually observed after freezing/thawing with this method. Although in our study only normozoospermic donors were included, the number of motile spermatozoa also decreased after freezing/thawing, but with no differences between the two groups.
We know that liquid nitrogen will be difficult to manipulate, supply, and store without risks in space. In order to secure the transport and maintenance of frozen sperm samples, other cryopreservation methods will need to be considered. Evaporation and freeze-drying have been suggested as new methods of mouse sperm cryopreservation [40, 41]. In the case of mice, freeze-dried spermatozoa have succeeded in fertilizing through ICSI, and full-term development and normal offspring have been achieved [41]. Freeze-dry methods have also been experimentally tested in human sperm samples [42], but to date, they have yet to be used in human-assisted reproduction. Slow freezing, or more recently vitrification [43, 44], is currently regarded as being the methods of choice. More research is needed to assess the feasibility of these or other alternative methods in human sperm cryopreservation in order to optimize transport outside the Earth.
Although in this study we have not analyzed the effect of space radiation, we are nevertheless aware that not only microgravity has to be considered before sending human sperm samples into space. Ionizing radiation is another important phenomenon to be taken into consideration and requires in-depth study because it has been proven to be a threat to human health and therefore is expected to impair the quality and viability of human reproductive cells as well. Cell apoptosis and sperm DNA damage have been described as being induced by simulated microgravity and carbon ion irradiation in mice [45]. These authors venture that sperm DNA damage may be one of the underlying mechanisms behind male fertility decline under space environment. On the other hand, an interactive effect of microgravity and radiation on the cellular responses has been reported, suggesting that altered gene expression caused by microgravity may be further modified by ionizing radiation [41, 46]. It is necessary to conduct further research into the effect of both factors acting together on human reproductive cells. It is also important to take into consideration the period of time that frozen sperm samples should be kept in the space, because the detrimental effects of radiation are accumulative. In order to ensure such a safe storage, the addition of antioxidants or other specific protective measures needs to be explored.
In conclusion, frozen sperm samples preserved in cryostraws and stored in a specific nitrogen vapor cryoshipper undergo no significant alterations after exposure to microgravity obtained by parabolic flights and under the specific conditions of this study. The lack of differences observed in the sperm parameters analyzed, between frozen samples exposed to microgravity and those maintained in ground conditions, opens up the prospect of achieving the safe transport of human male gametes into space. Nevertheless, further research is required in order to validate the results obtained before the possibility of creating a human sperm bank outside the Earth can be realized. It is necessary to validate these results with a larger sample as well as to progress to further assays in other platforms in order to obtain longer periods of microgravity exposure for a better evaluation of the effects of microgravity on male gametes. The possible effects of microgravity on human oocytes and embryos also need to be considered.
Acknowledgments
The authors thank Ignacio Rodriguez for his support in the statistical analysis.
Code availability
Not applicable.
Authors’ contributions
M. Boada and A. Perez-Poch conceived the study; D.V. González conducted the parabolic flights; M. Ballester and S. García-Monclús performed the seminal tests; S. García performed statistical analysis, M. Boada, A. Perez-Poch, M. Ballester, S. García-Monclús, and A.Veiga analyzed the data and wrote the paper. All authors read, reviewed, and approved the final manuscript.
Funding information
This work was performed under the auspices of Càtedra d’Investigació en Obstetrícia y Ginecologia of the Department of Obstetrics, Gynaecology and Reproduction, Dexeus Women’s Health and the Universitat Autònoma de Barcelona. The study was supported by a research grant from “Fundación Dexeus Mujer 2019” in the area of Basic Science (Reproductive Medicine).
Data availability
All relevant data are within the paper.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This work was approved by the Ethics Committee and Review Board of the Center.
Consent to participate
Study participants were informed of the procedure and gave their consent to participate by signing the informed consent.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Clément G. Fundamentals of space medicine. 1st ed. Kluwer; 2003. p. 4.
- 2.Vaquer S, Cuyàs E, Rabadan A, González A, Fenollosa F, de la Torre R. Active transmembrane drug transport in microgravity: a validation study using an ABC transporter model. F1000Research. 2014;3:201. doi: 10.12688/f1000research.4909.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pietsch J, Bauer J, Egli M, Infanger M, Wise P, Ulbrich C, Grimm D. The effects of weightlessness on the human organism and mammalian cells. Curr Mol Med. 2011;11:350–364. doi: 10.2174/156652411795976600. [DOI] [PubMed] [Google Scholar]
- 4.Narici M, de Boer MD. Disuse of musculo-skeletal system in space and on earth. Eur J Appl Physiol. 2011;111:403–420. doi: 10.1007/s00421-010-1556-x. [DOI] [PubMed] [Google Scholar]
- 5.Mandsager KT, Robertson D, Diedrich A. The function of the autonomic nervous system during space flight. Clin Auton Res. 2015;25:141–151. doi: 10.1007/s10286-015-0285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macho L, Kvetnansky R, Fickova M, Popova IA, Grigoriev A. Effects of exposure to space flight on endocrine regulations in experimental animals. Endocr Regul. 2001;35:101–114. [PubMed] [Google Scholar]
- 7.Osborne J, Alonsopérez MV, Ferrer D, Goswami N, González DV, Moser M, Grote V, Garcia-Cuadrado G, Perez-Poch A. Effect of mental arithmetic on heart rate responses during parabolic flights: the Barcelona zero-G challenge. Microgravity Sci Technol. 2014;26:11–16. doi: 10.1007/s12217-014-9365-1. [DOI] [Google Scholar]
- 8.Jennings R, Baker E. Gynecological and reproductive issues for women in space: a review. Obstet Gynecol Surv. 2000;55:109–116. doi: 10.1097/00006254-200002000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Serova LV, Denisova LA, Lavrova EA, Makeyeva VF, Natochin YV, Pustynnikova AM, Shakhmatova EI. Parameters of the reproductive function of the mammals: Fetal and placental characteristics. In: OG Gazenko editors. Ontogenesis of mammals in microgravity. NASA TM-103978, Washington DC. 1993. pp. 35–6.
- 10.Ronca A. Mammalian development in space. In: Marty H-J, editor. Developmental Biology Research in Space. Elsevier Science; 2003. p. 217–51. [DOI] [PubMed]
- 11.Pellegrini M, Di Siena S, Claps G, Di Cesare S, Dolci S, Rossi P, Geremia R, Grimaldi P. Microgravity promotes differentiation and meiotic entry of postnatal mouse male germ cells. PLoS One. 2010;5(2):e9064. doi: 10.1371/journal.pone.0009064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morabito C, Guarnieri S, Catizone A, Schiraldi C, Ricci G, Mariggio MA. Transient increases in intracellular calcium and reactive oxygen species levels in TCam-2 cells exposed to microgravity. Sci Rep. 2017;7:15648. doi: 10.1038/s41598-017-15935-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinde V, Brungs S, Henry M, Wegener L, Nemade H, Rotshteyn T, Acharya A, Baumstark-Khan C, Hellweg C, Hescheler J, Hemmersbach R, Sachinidis A. Simulated microgravity modulates differentiation processes of embryonic stem cells. Cell Physiol Biochem. 2016;38:1483–1499. doi: 10.1159/000443090. [DOI] [PubMed] [Google Scholar]
- 14.Nowacki D, Klinger F, Mazur G, De Felici M. Effects of culture in simulated microgravity on the development of mouse embryonic testes. Adv Clin Exp Med. 2015;24:769–774. doi: 10.17219/acem/27920. [DOI] [PubMed] [Google Scholar]
- 15.Tash JS, Johnson DC, Enders GC. Long term (6 wk) hind limb suspension inhibits spermatogenesis in adult male rats. J Appl Physiol. 2002;92:1191–1198. doi: 10.1152/japplphysiol.00931.2001. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Li L, Bai Y, Shi R, Wei H, Zhang S. Mouse undifferentiated spermatogonial stem cells cultured as aggregates under simulated microgravity. Andrologia. 2014;46:1013–1021. doi: 10.1111/and.12189. [DOI] [PubMed] [Google Scholar]
- 17.Engelmann U, Krassnigg F, Schill WB. Sperm motility under conditions of weightlessness. J Androl. 1992;13:433–436. [PubMed] [Google Scholar]
- 18.Tash JS, Bracho GE. Microgravity alters protein phosphorylation changes during initiation of sea urchin sperm motility. FASEB J. 1999;13:S43–S54. doi: 10.1096/fasebj.13.9001.s43. [DOI] [PubMed] [Google Scholar]
- 19.Kamiya H, Sasaki S, Ikeuchi T, Umemoto Y, Tatsura H, Hayashi Y, Kaneko S, Kohri K. Effect of simulated microgravity on testosterone and sperm motility in mice. J Androl. 2003;24:885–890. doi: 10.1002/j.1939-4640.2003.tb03140.x. [DOI] [PubMed] [Google Scholar]
- 20.Ikeuchi T, Sasaki S, Umemoto Y, Kubota Y, Kubota H, Kaneko T, Kohri K. Human sperm motility in a microgravity environment. Reprod Med Biol. 2005;4:161–167. doi: 10.1111/j.1447-0578.2005.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C, Guo X, Wang F, Li X, X Cindy T, Li L, Wu Z. Simulated microgravity compromises mouse oocyte maturation by disrupting meiotic spindle organization and inducing cytoplasmic blebbing. PLoS One. 2011;6(7):e22214. doi: 10.1371/journal.pone.0022214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin SC, Gou GH, Hsia CW, Ho CW, Huang KL, Wu YF, Lee SY, Chen YH. Simulated microgravity disrupts cytoskeleton organization and increases apoptosis of rat neural crest stem cells via upregulating CXCR4 expression and RhoA-ROCK1-p38MAPK-p53 signaling. Stem Cells Dev. 2016;25:1172–1193. doi: 10.1089/scd.2016.0040. [DOI] [PubMed] [Google Scholar]
- 23.Barjaktarović Z, Nordheim A, Lamkemeyer T, Fladere C, Madlung J, Hampp R. Time-course of changes in amounts of specific proteins upon exposure to hyper-g, 2-D clinorotation, and 3-D random positioning of Arabidopsis cell cultures. J Exp Bot. 2007;58:4357–4363. doi: 10.1093/jxb/erm302. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa M, Ohgushi H, Tamai N, Osuga K, Uemura M, Yoshikawa H, Myoui A. The effect of simulated microgravity by three-dimensional clinostat on bone tissue engineering. Cell Transplant. 2005;14:829–835. doi: 10.3727/000000005783982477. [DOI] [PubMed] [Google Scholar]
- 25.Kufner E, Blum J, Callens N, Eigenbrod C, Koudelka O, Orr A, Vedernikov A, Will S, Reimann J, Wurm G. ESA’s drop tower utilization activities 2000 to 2011. Microgravity Sci Technol. 2011;23:409–425. doi: 10.1007/s12217-011-9261-x. [DOI] [Google Scholar]
- 26.Dannenberg K. Swedish space activities - an overview with focus on balloons and rockets. In: Proceedings of the 200th ESA Symposium on European rocket and balloon programmes and related research. ESA Special publications. 2011. pp 33–5.
- 27.Pletser V. Short duration microgravity experiments in physical and life sciences during parabolic flights: the first 30 ESA campaigns. Acta Astronaut. 2004;55:829–854. doi: 10.1016/j.actaastro.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Callens N, Ventura-Traveset J, De Lophem TL, Lopez de Echazarreta C, Pletser V, Van Loon J. ESA Parabolic flights, drop tower and centrifuge opportunities for university students. Microgravity Sci Technol. 2011;23:181–189. doi: 10.1007/s12217-010-9181-1. [DOI] [Google Scholar]
- 29.Pletser V, Winter J, Bret-Dibat T, Friedrich U, Clervoy JF, Gharib T, Gai F, Minster O, Sundblad P. The first joint European partial-G parabolic flight campaign at Moon and Mars gravity levels for science and exploration. Microgravity Sci Technol. 2012;24:383–395. doi: 10.1007/s12217-012-9304-y. [DOI] [Google Scholar]
- 30.Pletser V, Rouquette S, Friedrich U, Clervoy J, Gharib T, Gai F, Mora C. European parabolic flight campaigns with Airbus zero-g: looking back at the A300 and looking forward to the A310. Adv Space Res. 2015;56:1003–1013. doi: 10.1016/j.asr.2015.05.022. [DOI] [Google Scholar]
- 31.Brigos M, Perez-Poch A, Alpiste F, Torner J. Parabolic flights with single-engine aerobatic aircraft: flight profile and a computer simulator for its optimization. Microgravity Sci Technol. 2014;26:229–239. doi: 10.1007/s12217-014-9382-0. [DOI] [Google Scholar]
- 32.Clément G, Allawey H, Demel M, Golemis A, Kindrat A, Melinyshyn A, Merali T, Thirsk R. Long duration spaceflight increases depth ambiguity of reversible perspective figures. PLoS One. 2015;10(7):e0132317. doi: 10.1371/journal.pone.0132317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuster A, Boccia V, Perez-Poch A, Gonzalez DV. Estimation of relative distance between two objects in microgravity conditions during parabolic flight. Proceedings of the Elgra Symposium and general assembly. Elgra news. 2015;31:120.
- 34.Perez-Poch A, Ventura D, Lopez D. Hypogravity research and educational parabolic flight activities conducted in Barcelona: a new hub of innovation in Europe. Microgravity Sci Technol. 2016;28:603–609. doi: 10.1007/s12217-016-9516-7. [DOI] [Google Scholar]
- 35.World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5. Switzerland: World Health Organization; 2010. [Google Scholar]
- 36.Polge C. Low-temperature storage of mammalian spermatozoa. Proc R Soc Lond B Biol Sci. 1957;147:498–508. doi: 10.1098/rspb.1957.0068. [DOI] [PubMed] [Google Scholar]
- 37.Evenson D, Wixon R. Meta-analysis of sperm DNA fragmentation using the sperm chromatin structure assay. Reprod BioMed Online. 2006;12:466–472. doi: 10.1016/S1472-6483(10)62000-7. [DOI] [PubMed] [Google Scholar]
- 38.Kamal K, Herranz R, van Loon JJWA, Medina FJ. Simulated microgravity, Mars gravity, and 2g hypergravity affect cell cycle regulation, ribosome biogenesis, and epigenetics in Arabidopsis cell cultures. Sci Rep. 2018;8:6424. doi: 10.1038/s41598-018-24942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimm D, Egli M, Krüger M, Riwaldt S, Corydon TJ, Kopp S, Wehland M, Wise P, Infanger M, Mann V, Sundaresan A. Tissue engineering under microgravity conditions -use of stem cells and specialized cells. Stem Cells Dev. 2018;27:787–804. doi: 10.1089/scd.2017.0242. [DOI] [PubMed] [Google Scholar]
- 40.Wakayama T, Yanagimachi R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat Biotechnol. 1998;16:639–641. doi: 10.1038/nbt0798-639. [DOI] [PubMed] [Google Scholar]
- 41.Wakayama S, Kamada Y, Kohda T, Suzuki H, Shimazu T, Tada M, Osada I, Nagamatsu A, Kamimura S, Nagatomo H, Mizutani E, Ishino F, Yano S, Wakayama T. Healthy offspring from freeze-dried mouse spermatozoa held on the International Space Station for 9 months. PNAS. 2017;23:5988–5993. doi: 10.1073/pnas.1701425114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gianaroli L, Magli MC, Stanghellini I, Crippa A, Crivello AM, Pescatori ES, Ferraretti AP. DNA integrity is maintained after freeze-drying of human spermatozoa. Fertil Steril. 2012;5:1067–1073. doi: 10.1016/j.fertnstert.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 43.Isachenko E, Isachenko V, Katkov II, Dessole S, Nawroth F. Vitrification of mammalian spermatozoa in the absence of cryoprotectants: from past practical difficulties to present success. Reprod BioMed Online. 2003;10:191–200. doi: 10.1016/S1472-6483(10)61710-5. [DOI] [PubMed] [Google Scholar]
- 44.Isachenko V, Isachenko E, Montag M, Zaeva V, Krivokharchenko I, Nawroth F, Dessole S, Katkov II, van der Ven H. Clean technique for cryoprotectant-free vitrification of human spermatozoa. Reprod BioMed Online. 2005;10:350–354. doi: 10.1016/S1472-6483(10)61795-6. [DOI] [PubMed] [Google Scholar]
- 45.Li HY, Zhang H, Miao GY, Xie Y, Sun C, Di CX, Liu Y, Zhang X, Ma XF, Xu S, Gan L, Zhou X. Simulated microgravity conditions and carbon ion irradiation induce spermatogenic cell apoptosis and sperm damage. Biomed Environ Sci. 2013;26:726–734. doi: 10.3967/0895-3988.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Yatagai F, Ishioka N. Are biological effects of space radiation really altered under the microgravity environment? Life Sci Space Res. 2014;3:76–89. doi: 10.1016/j.lssr.2014.09.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.