Key message
Naringenin exposure altered auxin redistribution via VrPIN1 leading to morphological alterations and significantly reduced the protein precipitable tannins that further enhanced the protein accumulation and bioavailability.
Flavonoid exposure is known to affect the antioxidant profile of legumes. However, a detailed study evaluating the effect of flavonoid naringenin on morphology and biochemical profile of legume is lacking. The present study is a novel report of improved in planta protein bioavailability and antioxidant potential of legume mungbean on naringenin exposure. The quantitative evaluation revealed significant protein accumulation (64–122 μg/g FW) on naringenin exposure. Further, an increase in protein solubility and digestibility compared to control was evident. Naringenin mediated altered α-amylase activity improved the mungbean seed germination rate. Naringenin induced auxin redistribution and altered PIN formed transcript expression reduced lateral root density and increased stem length that was subsequently reverted on exogenous indole acetic acid application. Naringenin enhanced polyphenolic accumulation and improved the antioxidant potential of mungbean. Additionally, the responsiveness of the early gene of the flavonoid biosynthetic pathway, Chalcone isomerase to naringenin concentration was revealed indicating a probable feedback regulation. Further, the presence of alternate liquiritigenin biosynthesis was also evident. The present study, thus reveals the probable potential of phytochemical naringenin towards agricultural sustainability in the changing environmental conditions.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02428-6) contains supplementary material, which is available to authorized users.
Keywords: α-Amylase, Antioxidant, Auxin, Alternate biosynthesis, Chalcone isomerase, Germination, Liquiritigenin, Mungbean, Naringenin, Protein, VrPIN1
Introduction
Naringenin is a plant-derived flavanone abundantly present in citrus fruits and tomatoes (Kawaii et al. 1999). It has considerable antioxidant and anti-inflammatory potential (Esmaeili and Alilou 2014; Li et al. 2015b; Mulvihill et al. 2016; Shakeel et al. 2017; Rehman et al. 2018; Wali et al. 2020). Reports document the medicinal usage of naringenin against diseases including metabolic syndrome, cancer, brain disorders, liver and heart diseases (Wilcox et al. 1999; Yang et al. 2011; Park et al. 2012; Alam et al. 2014; Wu et al. 2016; Hernandez-Aquino and Muriel 2018; Liaquat et al. 2018). Naringenin is synthesized by the phenylpropanoid biosynthetic pathway operating in plants (Jiang et al. 2005). The enzyme encoded by gene Chalcone isomerase (CHI) catalyzes the conversion of phenylpropanoid intermediate naringenin chalcone into naringenin. Naringenin being the central intermediate and key branch point for flavonoid synthesis leads to a diversity of secondary metabolites including flavones, flavonols, anthocyanins, and isoflavonoids (Jiang et al. 2005; Bido et al. 2010). Hence, the enzyme encoded by gene CHI has been considered as the regulatory gene of the flavonoid biosynthetic pathway (Muir et al. 2001; Verhoeyen et al. 2002; Lim and Li 2017).
Flavonoids have also been known to significantly regulate plant growth, morphological development, environmental responses, and cell signaling (Mandal et al. 2010; Ferreyra et al. 2012). However, few studies document the concerted effect of naringenin on germination, morphology, and biochemical composition of plants. Naringenin reportedly enhanced the nodule number of garden pea without affecting their nitrogenase activity (Novak et al. 2002). On the contrary, the inhibitory effect of naringenin on the growth of a few annual gramineous plants has also been reported (Chen et al. 2004; Deng et al. 2004). However, only one report documents the inhibitory role of naringenin on the germination and growth of legume soybean (Bido et al. 2010). Legumes are protein-rich plants known for their enhanced flavonoid mediated antioxidant potential. Hence, revealing the relationship between flavonoids and protein accumulation in legumes becomes important. Earlier, a US Patent, US7268276B2 has documented the enhanced production of oil and protein by suppressing the transcription factor TTG1 from Brassica napus. This disruption was claimed to downregulate the phenylpropanoid pathway of plants (Ruezinsky et al. 2007). However, the underlying mechanism still remained unrevealed. Furthermore, in the present scenario of prevailing protein-energy malnutrition, the focus is on the evaluation of protein enrichment strategies for legumes.
Mungbean (Vigna radiata L.) is the most edible and commonly produced leguminous crop of Asian and South European countries. It is a recommended source of plant protein than several animal proteins. Mungbean seeds and sprouts are a rich source of phenylpropanoids. The sprouts have increased protein bioavailability and significantly higher antioxidant potential than seeds. The polyphenolic derivatives of the phenylpropanoid pathway are responsible for the antioxidant potential and environmental responses of mungbean (Guleria and Kumar 2017; Hou et al. 2019; Lu et al. 2019). Irrespective of the significance of phenylpropanoids towards the growth and development of mungbean, various molecular and biochemical aspects of the phenylpropanoid pathway are still lacking in mungbean (Guleria and Kumar 2017).
Hence keeping in view the previous gaps, findings and need for protein research in legumes, the present article documents the impact of naringenin on protein accumulation and antioxidant potential of legume mungbean. Besides, its effect on mungbean morphology and biochemical accumulation of carbohydrate, flavonoid and polyphenols has also been evidenced. This is a novel report assessing the possible relationship between flavonoid and protein bioavailability in legumes.
Materials and methods
Seed procurement and naringenin treatment
Mungbean seeds, Vigna radiata var. PAU911TK was purchased from Punjab Agricultural University, Ludhiana (Punjab, India), and naringenin was purchased from Sigma Aldrich. The seeds were exposed to five concentrations of naringenin, 0.2, 0.4, 0.6, 0.8, and 1 mM through the soil. The soil without naringenin supplementation was kept as control. The germination data was recorded till 96 h.
In vitro α-amylase assay
The in vitro α-amylase activity of mungbean seeds germinated in the presence and absence of naringenin was estimated (Afiukwa et al. 2009). Equally weighed seeds germinated in control and naringenin supplemented soil collected at different time points were decoated and homogenized in 0.1 M acetate buffer (pH 4.2). The filtered homogenate was centrifuged and the supernatant was used as a crude extract for conducting the in vitro α-amylase assay. The α-amylase activity was estimated by the iodine/KI method described (Afiukwa et al. 2009).
The α-amylase activity was calculated as follows:
Estimation of endogenous indole-3-acetic acid (IAA)
The endogenous IAA accumulation of shoot and root sections of mungbean germinated without and with naringenin supplementation was estimated. The IAA was extracted by following the protocol documented (Kelen et al. 2004). The prepared dried extract was resuspended in phosphate buffer and quantified using a UV–visible spectrophotometer by the method described earlier (Leveau and Lindow 2005).
Exogenous application of IAA
Mungbean seeds were germinated on control and naringenin supplemented soils for IAA treatment to rescue the lateral root density reduction (Zhao et al. 2014). 3 days post-seed germination, seedlings were sprayed with 1 mM IAA once a day for 10 days. The lateral root count was estimated at 0 and 10 days post IAA treatment. Further, the endogenous IAA level of roots and shoots post IAA treatment was also estimated (Kelen et al. 2004; Leveau and Lindow 2005).
Chlorophyll estimation
Chlorophyll content was determined for control as well as naringenin exposed mungbean by using the method described earlier (Guleria et al. 2014). Extract prepared in 80% acetone was used for recording absorbance at 645 (A645) and 663 nm (A663). MacKinney’s specific absorption coefficient was employed to quantify total chlorophyll, chlorophyll a and chlorophyll b contents (Guleria et al. 2014). The following equations were used:
Estimation of total carbohydrates
The carbohydrate accumulation of mungbean grown in the absence and presence of naringenin was performed by the earlier reported method with few modifications (Zhang and Blumwald 2001). The extraction was performed with methanol and supernatant of the extract was used for carbohydrates estimation. The 5% aqueous phenol and conc. H2SO4 was used for colorimetric detection of carbohydrates at 490 nm.
Estimation of total polyphenolics
The total flavonoids, phenols, and tannins constitute the plant’s total polyphenolic fractions (Tsao 2010). The accumulation of polyphenols was estimated in mungbean germinated in the absence and presence of naringenin. Total flavonoids were estimated by the method described earlier (Chang et al. 2002). The 10% aluminium chloride and 1 M potassium acetate were added to the methanolic extract and the absorbance was recorded at 415 nm. The total phenols and tannins were estimated by an earlier described method (Makkar et al. 1993). The methanolic extract was spectrophotometrically quantified at 725 nm for total phenolics estimation by using Folin-Ciocalteu reagent. The quantified phenolics were expressed as x. Phenolics were removed from the same extract by polyvinyl polypyrrolidone. Following centrifugation, non-tannin phenolic content was again measured using Folin-Ciocalteu reagent and designated as y. The tannic acid equivalent representing total tannin percentage was calculated by subtracting non-tannin phenols out of total phenolics (x – y).
Estimation of free radical scavenging activity
The free radical scavenging assay was performed using 2,2-diphenyl-1-picrylhydrazyl (DPPH) to evaluate the effect of naringenin on the antioxidant potential of mungbean (Guleria and Yadav 2014). To 50 µl of plant methanolic extract, 0.1 mM methanolic DPPH solution was added and used for UV–Vis spectrophotometric analysis at 517 nm estimated in terms of the percent inhibition of DPPH.
Protein estimation
Protein was extracted from mungbean germinated in the absence and presence of naringenin by using TCA based extraction and quantified as described earlier (Tang et al. 2003). The detailed description is provided in the supplementary information. The digestibility and solubility of isolated protein were evaluated. The protein was digested with 0.2 mg/ml trypsin suspension dissolved in Tris–HCl buffer (pH 7.6) and incubated for 2 h at 37 °C. The reaction was stopped with 50% TCA and incubated for 30 min at 4 °C followed by centrifugation. The obtained pellet was resuspended in sodium hydroxide solution and protein present in TCA precipitate was quantified using Bradford assay (Elkhalil et al. 2001). For solubility measurements, the pH of 1 mg/ml suspension of isolated protein was adjusted separately from pH 2.0–12.0. The prepared suspensions were pellet down and protein was quantified (Tang et al. 2003).
Calculations were performed as follows:
Estimation of protein precipitable tannins
The protein precipitable tannins present in control and naringenin exposed mungbean was quantified (Makkar et al. 1993). The 2 ml BSA was incubated with tannins isolated from mungbean at 4 °C for 16 h. The mixture was pellet down and resuspended in 1% SDS. The SDS-triethanolamine solution was added to suspension followed by a ferric chloride solution. The absorbance of the prepared solution was recorded at 510 nm after incubating it at room temperature for 30 min. The obtained values represented protein-precipitable phenolics, designated as x. From the similar extract, total phenolics were also estimated designated as y to calculate the percentage of total protein precipitable phenolics as (x/y) × 100.
cDNA synthesis and gene expression analysis
Total RNA was isolated from 100 mg of control and naringenin exposed fresh mungbean tissue with SV Total RNA isolation system (Promega, USA). The 1 μg of isolated RNA was used for the synthesis of first-strand cDNA using Superscript III RT (Invitrogen) and oligodT primers. The cDNA equally quantified with 26 s rRNA was used for comparative gene expression analysis (Singh et al. 2004). The relative expression analysis for genes VrPIN1, Superoxide dismutase (VrSOD, GenBank ID: HQ259253.1), Catalase (VrCAT, GenBank ID: HQ260598.1) and Ascorbate peroxidase (VrAPX, GenBank ID: EU652949.1) and Chalcone isomerase (VrCHI, GenBank ID: KP164975.1) was conducted. The gene-specific primer sequences and thermal profile used for transcript analysis are mentioned in Table S1.
Bio-informatic evaluation of V. radiata VrCHI protein binding affinity with substrates
To evaluate the binding affinity of VrCHI protein for substrates naringenin chalcone and isoliquiritigenin, the three-dimensional structure for the protein was predicted using SWISS-MODELLER server and docked with the substrates using molecular docking server (Guleria and Yadav 2012; Waterhouse et al. 2018). The protein molecule was docked individually with both the substrates and repeated twice to obtain the best results.
Statistical analysis
All evaluations were performed in triplicate fashion until and unless stated. Data presented in graphs and tables represent mean ± standard deviation. Least significant differences for the evaluated parameters between control and naringenin exposed mungbean were calculated by Student’s t test corresponding to P < 0.05, P < 0.01 and P < 0.001.
Results
Naringenin enhanced α-amylase activity to increase germination rate
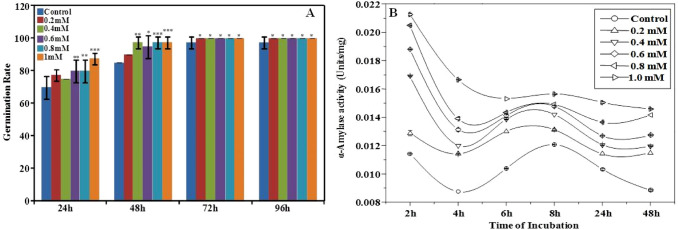
The germination rate of mungbean seeds exposed to naringenin was significantly enhanced than control in a concentration-dependent manner. The germination rate was enhanced by 10–25% at 24 h with an increase in naringenin concentration. Likewise, the increment in germination rate was found to be 6–15% at 48 h and 3% at 72 and 96 h than control (Fig. 1a).
Fig. 1.
Effect of naringenin exposure on mungbean germination rate. a The germination rate of mungbean without and with naringenin exposure was recorded at 24, 48, 72 and 96 h. Naringenin exposure enhanced the germination rate of mungbean. b The line graph shows enhanced α-amylase activity of mungbean seeds on naringenin exposure in a concentration-dependent manner compared to control. Data is represented as mean ± standard deviation of three independent measurements (*P < 0.05, **P < 0.01, ***P < 0.001)
The emergence of root and shoot on seed germination is stimulated by α-amylase mediated hydrolysis of endosperm starch into metabolizable sugars (Kaneko et al. 2002). Hence, the seedlings germinated in the absence and presence of naringenin were evaluated for α-amylase activity. The crude amylase extract isolated from control and naringenin exposed seedlings were added to pre-quantified starch for the quantification of hydrolyzed products. Surprisingly, naringenin exposed seedlings showed enhanced α-amylase activity than control. The activity was consistently increased with an increase in naringenin concentration with maximum activity obtained at 2 h of exposure. The average enhancement in α-amylase activity of seedlings exposed to 0.2, 0.4, 0.6, 0.8, and 1 mM naringenin was found to be 14, 23, 29, 35, and 44% compared to control plants, respectively (Fig. 1b).
Phenotypic characterization of mungbean on naringenin exposure
The seedlings exposed to naringenin were observed for their change in morphology with respect to control (Fig. S1). With the increase in naringenin concentration, exposed seedlings showed 27.2, 34, 34, 31, and 40.7% increase in shoot length (Table 1). However, root length showed non-significant variation than control (Table 1). Whereas the lateral root number was significantly reduced by 28, 32, 32, 34, and 36% on the exposure of 0.2–1 mM naringenin (Table 1). Further, the fresh biomass of naringenin exposed seedlings compared to control was enhanced by 5, 36, 42, 100, and 127%, respectively (Table 1). Likewise, the relative water content was increased by 7, 16, 17, 23, and 25% in the naringenin concentration-dependent manner (Table 1).
Table 1.
Effect of naringenin exposure on the morpho-biochemical parameters of mungbean
| Parameter/sample | Control | 0.2 mM naringenin | 0.4 mM naringenin | 0.6 mM naringenin | 0.8 mM naringenin | 1 mM naringenin |
|---|---|---|---|---|---|---|
| Shoot length (mm) | 103 ± 7 | 131 ± 5c | 138 ± 5c | 138 ± 7c | 135 ± 5c | 145 ± 5c |
| Root length (mm) | 30 ± 8 | 30 ± 5 | 23 ± 2 | 22.5 ± 3.5 | 28 ± 1 | 23 ± 5 |
| Lateral root number | 15.6 ± 2.3 | 11.3 ± 3a | 10.6 ± 1.1a | 10.6 ± 4a | 10.3 ± 3.2a | 10 ± 3.6a |
| Fresh biomass (mg) | 267.7 ± 7 | 280 ± 21 | 363 ± 9c | 378.5 ± 3c | 536 ± 36c | 606 ± 5c |
| Relative water content | 74.75 ± 3.4 | 79.96 ± 0.99 a | 86.92 ± 4.2c | 87.54 ± 4.5 c | 91.59 ± 2.7 c | 93.77 ± 2c |
| Chlorophyll a (mg/g FW) | 22.29 ± 0.42 | 23.72 ± 0.11b | 30.87 ± 1.34c | 33.14 ± 0.47c | 33.7 ± 0.09c | 36.35 ± 0.1c |
| Chlorophyll b (mg/g FW) | 0.435 ± 0.03 | 0.642 ± 0.2 | 4.05 ± 0.44c | 4.36 ± 0.4c | 6.89 ± 0.11c | 13.85 ± 2.05c |
| Total chlorophyll (mg/g FW) | 22.7 ± 0.46 | 24.36 ± 0.09a | 34.92 ± 0.9c | 37.51 ± 0.07c | 40.59 ± 0.01c | 50.21 ± 2.16c |
| Carbohydrates (mg/m FW) | 35.65 ± 3.5 | 39.31 ± 2.0c | 42.45 ± 0.9c | 47.68 ± 0.5c | 51.60 ± 1.6c | 49.64 ± 2.9c |
| TFC (mg/g FW) | 32.86 ± 0.1 | 52.30 ± 6.8c | 56.2 ± 6.8c | 58.63 ± 9.8c | 68.85 ± 2.7c | 73.21 ± 4.1c |
| TPC (μg/g FW) | 262.45 ± 8.6 | 316.3 ± 15.2c | 372.30 ± 0.8c | 408.91 ± 19.5c | 488.61 ± 2.6c | 556.30 ± 45.6c |
| Total Tannins (percent tannic acid equivalent) | 243.07 ± 4.5c | 286.46 ± 10.4c | 338.78 ± 3.6c | 370.61 ± 12.9c | 401.08 ± 1.4c | 438.62 ± 22.9c |
| Free radical scavenging potential (percent inhibition) | 34.09 ± 4.0 | 45.24 ± 3.0b | 52.13 ± 6.0c | 57.38 ± 4c | 58.29 ± 6.0c | 66.49 ± 0.4c |
The measurements are the mean ± SD of four replicates, (aP < 0.05; bP < 0.01; cP < 0.001)
TFC total flavonoid content, TPC total phenolic content
Exogenous IAA rescued lateral root count reduced by naringenin
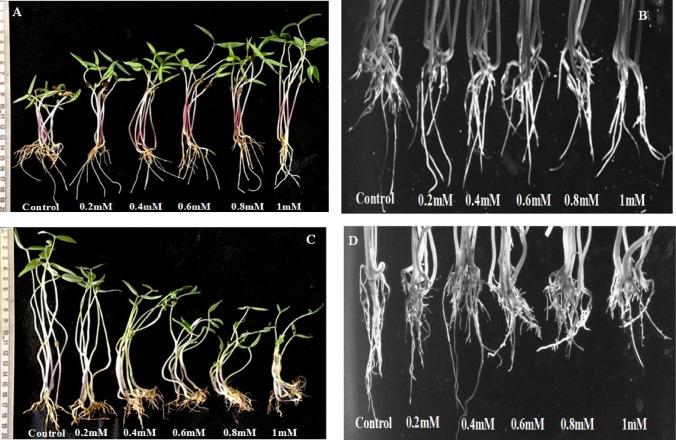
Naringenin significantly reduced the lateral root count of mungbean (Fig. 2a, b). The indole-3 acetic acid (IAA) content is known to regulate lateral root emergence and growth (Overvoorde et al. 2010). Quantitative estimation showed a significant reduction in root IAA content on naringenin exposure than control (Table 2). The IAA content of control roots was estimated to be 29.5 µg/g of fresh weight. Whereas the content was reduced by 9, 24, 37, 44, and 53% in roots exposed to 0.2, 0.4, 0.6, 0.8, and 1 mM naringenin, respectively (Table 2).
Fig. 2.
IAA induced variation in the mungbean shoot length and lateral root count in the presence and absence of naringenin. a Comparison of three days old mungbean stem length and number of lateral roots grown in the absence and presence of naringenin. The naringenin exposed mungbean showed a significant increment in stem length and reduced density of lateral roots compared to control. b Gel documentation system image shows a variation in the lateral root number of V. radiata with and without naringenin. Naringenin exposure reduced the lateral root density compared to control. c Image representing the reversal of shoot elongation and rescue of lateral root density after ten days of IAA spray. d The image is a close view representing the rescue of lateral root count in mungbean post 10 days of IAA spray
Table 2.
Naringenin induced variation in endogenous auxin accumulation leading to a morphological alteration in mungbean shoot and root sections
| Sample/parameter | Shoot length | Lateral root count | Shoot endogenous IAA | Root endogenous IAA |
|---|---|---|---|---|
| Pre-IAA exposure | ||||
| Control | 52.7 ± 2.5 | 10.7 ± 0.57 | 36.5 ± 0.2 | 29.5 ± 0.5 |
| 0.2 mM naringenin | 73.7 ± 7.1c | 8 ± 2b | 38.66 ± 0.4a | 26.96 ± 0.2a |
| 0.4 mM naringenin | 84.3 ± 1.2c | 7.8 ± 0.83b | 42.96 ± 0.4c | 22.43 ± 0.7c |
| 0.6 mM naringenin | 89.0 ± 2.2c | 6.3 ± 1c | 47.81 ± 0.3c | 18.58 ± 0.3c |
| 0.8 mM naringenin | 99.0 ± 3.4c | 6.2 ± 0.44c | 49.81 ± 0.3c | 16.5 ± 0.2c |
| 1 mM naringenin | 112.3 ± 9.3c | 6 ± 1c | 53.27 ± 0.2c | 13.89 ± 0.4c |
| Post-IAA exposure | ||||
| Control | 147.5 ± 19.4 | 18 ± 4 | 35.66 ± 0.1c | 37.27 ± 0.2c |
| 0.2 mM naringenin | 113.8 ± 10.6b | 25 ± 2b | 31.81 ± 0.1c | 49.73 ± 0.4c |
| 0.4 mM naringenin | 92.0 ± 8.2c | 29.2 ± 4c | 29.27 ± 0.2c | 53.04 ± 0.3c |
| 0.6 mM naringenin | 67.3 ± 8.1c | 31.8 ± 2c | 26.27 ± 0.1c | 55.89 ± 0.2c |
| 0.8 mM naringenin | 50.2 ± 13.1c | 33.4 ± 3c | 22.96 ± 0.2c | 59.04 ± 0.3c |
| 1 mM naringenin | 43.8 ± 14.6c | 35.4 ± 1c | 19.66 ± 0.3c | 63.12 ± 0.2c |
The measurements are the mean ± SD of four replicates, (aP < 0.05; bP < 0.01; cP < 0.001)
Exogenous application of 1 mM IAA for 10 days significantly enhanced the lateral root number than control (Fig. 2c, d). At 0 days of IAA spray, the lateral root number was reduced by 25–44% in presence of naringenin. Interestingly, the lateral root count of naringenin exposed plants was recovered and comparatively enhanced than control after the 10th day of IAA spray. Naringenin exposed mungbean showed an increase of 39, 62, 77, 86, and 97% in lateral root number compared to control (Fig. 2d).
Subsequently, post-IAA exposure, the endogenous IAA content of naringenin exposed roots was also enhanced than control (Table 2). The IAA level of control and 1 mM naringenin exposed root was estimated to be 37.27 and 63.12 μg/g fresh weight, respectively. The percent increase in root IAA content compared to control was 33, 42, 50, 58, 69% in 0.2–1 mM naringenin exposed mungbean, respectively (Table 2). Hence, naringenin mediated reduction in root IAA accumulation led to inhibition of lateral root emergence and density.
Exogenous IAA reversed naringenin mediated shoot elongation
Mungbean grown in presence of naringenin showed significant enhancement in the stem length (Fig. 2a). The shoot length was increased by 40–113% on 0.2–1 mM naringenin exposure than control (Table 2). Auxins are well documented to regulate plant morphogenesis including alterations in shoot and root architecture (Lavenus et al. 2013). Likewise, the present study reports increased IAA accumulation in mungbean shoots on naringenin exposure. The IAA content was quantified to be 36.5 ± 0.2 and 53.27 ± 0.2 μg/g fresh weight in control and 1 mM naringenin exposed shoots, respectively (Table 2). Naringenin exposure enhanced the shoot IAA accumulation by 6, 18, 31, 36, and 46% compared to control (Table 2).
However, naringenin shoots were significantly shortened post-IAA spray (Fig. 2c). The percent reduction in naringenin exposed stem length than control was found to be 23, 38, 54, 66, and 70% post-IAA spray (Table 2). Moreover, IAA accumulation in naringenin shoots showed a significant reduction of 11–45% post-IAA spray (Table 2). Hence, naringenin mediated alteration in shoot–root IAA accumulation was responsible for the observed morphological variations of mungbean.
VrPIN1 expression altered on naringenin exposure
The present study documents naringenin mediated variation in the shoot-root endogenous auxin levels with a probable role in regulating auxin transport from shoot to roots. PIN FORMED (PIN) proteins are auxin efflux regulators (Ganguly et al. 2010). PIN1 has been specifically identified to regulate the movement of auxins from stem to roots (Galweiler et al. 1998). To date, the transcriptomic analysis of mungbean has validated the presence of only one auxin efflux transporter gene Vr21159 corresponding to PIN1 protein (Li et al. 2015a). Since 1 mM naringenin exposure showed the most significant alteration in IAA accumulation compared to control, hence the expression of VrPIN1 designated for transcript Vr21159 was evaluated in the identified samples.
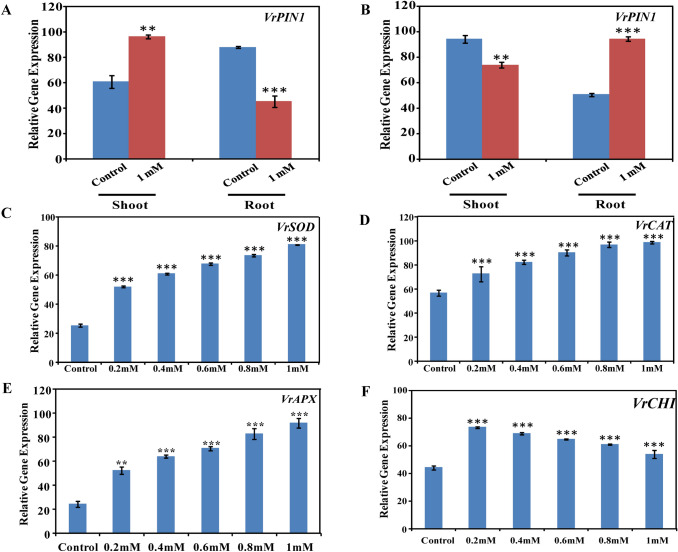
Naringenin significantly altered the VrPIN1 expression in shoot and root sections (Fig. S2A). The transcript expression was enhanced by 58% in naringenin exposed shoots than control. However compared to the control, the naringenin exposed roots showed a 48% reduction in the VrPIN1 mRNA accumulation (Fig. 3a). Likewise post IAA spray, VrPIN1 transcript expression of naringenin exposed mungbean tissues was significantly reversed to attain the control expression (Fig. S2B). The 22% reduction and 87% enhancement in the VrPIN1 mRNA accumulation in naringenin exposed shoot and root was noticed (Fig. 3b). Thus, a probable potential of naringenin to alter auxin source-sink distribution by the plausible regulation of PIN1 protein was indicated.
Fig. 3.
Comparative gene expression analysis of control and naringenin exposed mungbean. a The bar diagram represents the integrated density values (IDV) of VrPIN1 amplicon as measured with ImageJ software in shoot and root of control and 1 mM naringenin exposed mungbean before (a) and after (b) IAA spray. Each bar in the bar diagram presents the IDV of the amplicons representing genes, (c) VrSOD, (d) VrCAT and (e) VrAPX in control and naringenin exposed mungbean. f The bar graph shows the IDV of VrCHI transcript expression in control and naringenin exposed mungbean. The gene encoding 26 s rRNA was used as an internal control. Data is represented as mean ± standard deviation of three independent measurements (**P < 0.01, ***P < 0.001)
Enhanced chlorophyll and carbohydrate accumulation on naringenin exposure
Mungbean showed an enhanced biomass accumulation on naringenin exposure. The plant biomass accumulation is directly related to their chlorophyll content (Langton et al. 2003). Hence, naringenin exposed mungbean showed enhanced chlorophyll content compared to the control. The chlorophyll a and b content was increased by 6–63 and 47–3083% in presence of naringenin (Table 1). An overall increase of 7, 54, 65, 79, and 121% in total chlorophyll content of mungbean in presence of 0.2–1 mM naringenin was noticed (Table 1).
The chlorophyll content of plants is responsible for their productivity in terms of carbohydrate accumulation (Benett et al. 2008). The carbohydrate accumulation in the presence of 0.2–1 mM naringenin was increased by 10, 19, 34, 45, and 39% than control, respectively (Table 1). Hence, naringenin mediated increment in chlorophyll accumulation of mungbean positively affected their fresh biomass and carbohydrate accumulation.
Naringenin enhanced polyphenolics and antioxidant potential of mungbean
The total polyphenolic accumulation of mungbean was considerably enhanced in presence of naringenin. The total flavonoids, phenolics, and tannin fraction of mungbean were increased by 59–123, 21–112, and 18–80% on 0.2–1 mM naringenin exposure than control, respectively (Table 1).
Since polyphenolics, in majority flavonoids contribute to the antioxidant potential of legumes, mungbean exposed to naringenin showed enhanced free radical scavenging potential than control (Mierziak et al. 2014). An increment of 33, 53, 68, 71 and 95% compared to control in the presence of 0.2–1 mM naringenin was evident (Table 1). Further, the transcript expression of VrSOD, VrCAT, and VrAPX was comparatively enhanced on naringenin exposure (Fig. 3c–e). Compared to control, the mRNA accumulation of VrSOD, VrCAT and VrAPX was enhanced by 105–218, 28–74 and 67–278% on 0.2–1 mM naringenin exposure (Fig. 3c–e).
Reduced protein precipitable tannins enhanced protein accumulation and bioavailability on naringenin exposure
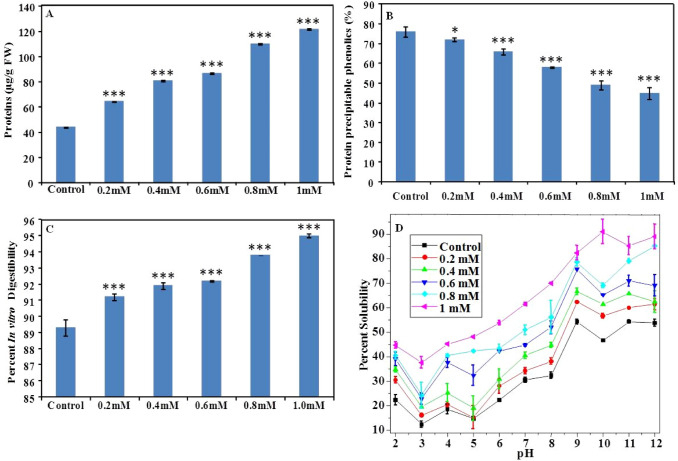
Naringenin is a metabolite of phenylpropanoid pathway that emerges as a diversion of shikimate pathway synthesizing phenylalanine, tryptophan, and tyrosine (Guleria and Kumar 2017). These amino acids play an important in plant protein biosynthesis, hence it becomes important to evaluate the impact of naringenin exposure on mungbean protein accumulation (Maeda and Dudareva 2012). Mungbean protein content was significantly enhanced by 46, 84, 97, 151, and 177% on naringenin exposure in a concentration-dependent manner (Fig. 4a).
Fig. 4.
Effect of naringenin exposure on the accumulation and bioavailability of protein in mungbean. a Bar graph shows an increase in the protein content of naringenin exposed mungbean plants compared to the control. Each bar represents the mean of three independent measurements (***P < 0.001). b The bar diagram shows the protein in vitro digestibility of mungbean in control and naringenin supplemented soil. c The graph depicts the protein solubility in response to naringenin concentrations with respect to control. d The bar diagram shows the percent of protein precipitable phenolics estimated from mungbean grown in the absence and presence of naringenin. Naringenin supplementation increased the protein content with a significant increment in protein in vitro digestibility and solubility. The considerable reduction in the fraction of protein precipitable phenolics was also evident
The protein precipitable tannins are anti-nutritional factors of legumes that make proteins less soluble and less digestible on binding to reduce the overall protein bioavailability (Mohamed et al. 2009; Gilani et al. 2012; Sarwar et al. 2012). Hence in the present study, naringenin mediated protein enhancement could be significant only if the proteins are considerably bioavailable. Surprisingly, naringenin exposure reduced the protein precipitable tannins compared to control (Fig. 4b). The percent reduction in tannin fraction than control was 5, 13, 24, 36, and 41% in a naringenin concentration-dependent manner (Fig. 4b). Moreover, protein digestibility and solubility were enhanced in the presence of naringenin (Fig. 4c, d). An increment of 2–6% in protein digestibility than control on exposure of naringenin was evident (Fig. 4c). Likewise, naringenin enhanced protein solubility of mungbean compared to control. The protein showed reduced solubility at acidic pH and enhanced solubility at alkaline pH (Fig. 4d). The protein solubility was evaluated to be 25% and 30–45% in the absence and presence of naringenin at pH 2. At pH 9 and beyond, the protein solubility was more than 50%, with increased solubility in the presence of naringenin (Fig. 4d). Hence, naringenin mediated reduction in protein precipitable tannins evidently enhanced the protein bioavailability of mungbean.
Naringenin decreased VrCHI mRNA accumulation
Chalcone isomerase (CHI) diverts the phenylpropanoid pathway towards flavonoid biosynthesis by catalyzing the conversion of chalconoid naringenin chalcone into flavonoid naringenin. Interestingly, enhancement in naringenin concentration showed consistent downregulation of VrCHI expression. With an increase in naringenin concentration beyond 0.2 mM, the VrCHI mRNA accumulation was downregulated by 6, 12, 17, and 26%, respectively. However in comparison to control, the transcript expression was comparatively higher in presence of naringenin (Fig. 3f). Hence, naringenin mediated probable feedback regulation of VrCHI along with its involvement in an alternate metabolic step was indicated.
VrCHI showed highest affinity for isoliquiritigenin
Presence of CHI isozymes with specificity for substrates naringenin chalcone and isoliquiritigenin (Fig. S3A) has been reported from legumes (Kimura et al. 2001). To validate the hypothesis in mungbean, a three-dimensional structure of VrCHI protein was predicted and validated (Fig. S3B, C, D). On molecular docking of VrCHI model with naringenin chalcone and isoliquiritigenin, the least binding energy for isoliquiritigenin, − 6.37 kcal/mol than for naringenin chalcone, − 4.15 kcal/mol was estimated (Fig. S3E, F). The least binding energy of protein with a ligand indicates the highest affinity of their interaction (Guleria and Yadav 2012; Guleria and Yadav 2013). VrCHI further showed an enhanced number of polar, hydrogen, and hydrophobic interactions for isoliquiritigenin than naringenin chalcone (Fig. S3G, H). Hence, VrCHI protein/enzyme might be involved in alternate biosynthesis of liquiritigenin in addition to its catalytic activity towards naringenin biosynthesis (Patil et al. 2010; Guleria and Yadav 2013).
Discussion
Naringenin is a plant-derived flavonoid synthesized by the phenylpropanoid biosynthetic pathway. It is synthesized from naringenin chalcone diverting the phenylpropanoid pathway towards flavonoids by the enzyme encoded by gene CHI (Mierziak et al. 2014). Naringenin like other flavonoids is important because of its antioxidant potential that imparts environmental responsiveness/ resistance to plants (Mierziak et al. 2014). Enhanced accumulation of flavonols has detoxified the free radicals accumulated in tomato on abiotic stress exposure (Mierziak et al. 2014). A possible improvement in plant stress tolerance by engineering phenylpropanoid metabolism has also been hypothesized (Martinez et al. 2016). However, the lack of molecular evaluation of phenylpropanoid metabolism in legumes has delayed the respective process (Guleria and Kumar 2017). Keeping the facts in view, the present study evaluated and for the first time reports the naringenin mediated promotion of mungbean vegetative growth and biochemical accumulation. The early gene of the flavonoid pathway, VrCHI showed responsiveness towards naringenin. Most importantly, the naringenin exposed mungbean showed enhanced protein content with increased protein bioavailability.
α-Amylase is the predominant hydrolytic enzyme in the seed aleurone layer that provides energy for radicle and plumule emergence by hydrolyzing starch into metabolizable sugars (Kaneko et al. 2002). The significant role of α-amylase activity towards seed germination has already been reported (Helland et al. 2002; Kaneko et al. 2002; Singh et al. 2007; Liu et al. 2018). Recently, enhancement in the α-amylase activity of rice seeds on silver nanoparticles exposure was attributed to their improved germination (Mahakham et al. 2017). Likewise, naringenin induced enhancement in α-amylase activity was responsible for mungbean germination rate.
Naringenin exposed mungbean showed increased shoot length but reduced lateral root density. However, the parameters were significantly reversed on IAA exogenous spray. Auxins are well documented to regulate plant morphogenesis including shoot and root parameters (Lavenus et al. 2013). In the present study, naringenin mediated enhancement and reduction in IAA accumulation of shoot and root was noticed, respectively. Earlier, naringenin exposure has been reported to reduce the root growth of model plant Arabidopsis thaliana (Brown et al. 2001). Enhanced IAA accumulation in maize roots than shoots was likewise reported to increase root growth with a simultaneous reduction in stem length (Li et al. 2018). Inhibited transport of polar auxins at the root- shoot junction on the application of auxin transport inhibitor reduced the free IAA accumulation of A. thaliana roots, thus decreasing the lateral root density (Casimiro et al. 2001). PIN FORMED (PIN) proteins are the essential regulators of auxin efflux. The PIN mRNA accumulation has been documented to show a positive relationship with the IAA content (Benkova et al. 2003; Oochi et al. 2019). Likewise, in the present study naringenin mediated enhanced VrPIN1 expression in mungbean shoots than roots indicated increased auxin accumulation in shoot sections. Earlier, increased movement of auxin from shoot to root reduced the shoot length but enhanced the lateral root number (Li et al. 2018). Hence, naringenin mediated altered VrPIN expression probably reduced the auxin transport from shoot to root with enhanced shoot auxin leading to increased shoot length and reduced lateral root number. Collectively, naringenin was identified as a negative regulator of IAA/auxin accumulation in mungbean responsible for the observed morphological variations.
Mungbean showed enhancement in fresh biomass, chlorophyll and carbohydrate accumulation on naringenin exposure. Earlier, chlorophyll content was positively correlated to biomass accumulation in A. thaliana transgenics overexpressing SrUGT85C2 from Stevia rebaudiana (Guleria et al. 2014). Hence, increased chlorophyll on naringenin exposure enhanced the fresh biomass of mungbean in terms of increased carbohydrate accumulation. Moreover, naringenin exposure enhanced total polyphenolics accumulation in mungbean. Earlier, exogenously applied unnatural tryptamine was readily taken up by the Catharanthus roseus hairy root cultures to induce in vivo synthesis of desired artificial unnatural novel alkaloids (Runguphan et al. 2009). Likewise, in the present study exogenously applied naringenin might be taken up by mungbean via roots to enhance the in vivo naringenin levels leading to the enhanced biosynthesis and accumulation of downstream polyphenolic metabolites, i.e. flavonoids, tannins, and phenols.
Polyphenolics essentially flavonoids enhance the free radical scavenging potential of plants (Nakabayashi et al. 2014; Soengas et al. 2018). Enhanced polyphenolics of legumes than non-leguminous plants are responsible for their significant antioxidant potential (Diaz-Batalla et al. 2006). MYB transcription factor overexpressing A. thaliana transgenics hyperaccumulate flavonoids to enhance their free radical scavenging and high water retention potential (Nakabayashi et al. 2014). The present study likewise documents naringenin mediated enhanced polyphenolic accumulation leading to increased antioxidant potential and relative water content of mungbean. Flavonoids further interact and enhance the enzymatic regulators of free radicals, i.e., superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) in plant cytosol (Hernandez et al. 2009). In the present study, enhanced expression of antioxidant genes VrSOD, VrCAT and VrAPX along with polyphenolics increased the antioxidant potential of mungbean on naringenin exposure.
A negative correlation between isoflavonoid and protein accumulation of soybean has already been reported (Charron et al. 2005). Plants with altered phenylpropanoid pathway have also been documented to enhance seed oil and protein content without any understanding of the underlying mechanism (Charron et al. 2005). The phenylpropanoid naringenin exposure in the present study, likewise reports enhanced protein accumulation. Protein solubility and in vitro digestibility are the critical factors determining protein nutritional bioavailability (Butts et al. 2012). Hence, naringenin significantly enhanced protein bioavailability with a consistent reduction in the protein precipitable tannins. However, a detailed study to understand the underlying basis of protein alteration on phenylpropanoid variation is still required.
Further, possible feedback regulation of the phenylpropanoid pathway on naringenin exposure was indicated. Earlier, exogenous application of catechin was reported to downregulate the expression of the flavanone-3 hydroxylase gene of the flavonoid pathway in tea indicating a feedback inhibition mechanism (Singh et al. 2008). The mungbean VrCHI protein moreover showed the highest affinity for isoliquiritigenin than naringenin chalcone on bio-informatic analysis. Earlier, considerable bifurcation of the steviol glycoside biosynthesis pathway in Stevia has also been hypothesized using bioinformatic prediction and docking studies (Guleria and Yadav 2013). Although the existence and activity of CHI isozyme are subjected to vary with the type of leguminous plant (Kimura et al. 2001). These observations thus collectively necessitate a detailed in vivo study in mungbean to reveal the existence of CHI isoforms, alternate liquiritigenin biosynthesis, and the existence of feedback inhibition mechanism.
Conclusion
In conclusion, the present study documents a considerable enhancement in protein bioavailability on naringenin exposure reflecting a possible positive relationship between flavonoids and protein. Naringenin mediated alterations in VrPIN1 mRNA confirmed auxin redistribution leading to variation in mungbean morphology. Further, the responsiveness of the VrCHI transcript towards naringenin was indicated. Additionally, the probable presence of alternate liquiritigenin biosynthesis in mungbean with a requirement of a detailed study was indicated. Hence, the present study for the first time documents growth and nutritional enrichment property of naringenin towards mungbean with an observed significant impact on protein bioavailability. These findings further recommend the possible potential role of naringenin towards stress tolerance and agricultural sustainability of plants including legumes. In the current scenario of global climate change, the use of phytochemicals for sustained and improved crop growth and survival will offer a novel approach.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 Three-dimensional VrCHI protein and interaction with naringenin chlacone and isoliquiritigenin. (DOCX 1828 kb)
Acknowledgements
The authors are thankful to the Vice Chancellor, DAV University, Jalandhar for his continuous encouragement and support. Financial support from DST under StartUP Project YSS/2015/000719 is duly acknowledged. PS is thankful to DST SERB, Government of India for giving a fellowship in the form of Project Assistantship.
Author contributions
PS: experiment designing, performed experiments, data collection and analysis, manuscript draft preparation. VK: designing experiments, data analysis, manuscript draft preparation. RK: manuscript draft preparation and experiment designing. PG: experiment designing, data analysis, final manuscript preparation and communication.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest between any of the authors.
Contributor Information
Priya Sharma, Email: psdavuniversity@gmail.com.
Vineet Kumar, Email: vineetkumar22@gmail.com.
Rajiv Khosla, Email: khosla30@yahoo.com.
Praveen Guleria, Email: pvihbt@gmail.com.
References
- Afiukwa CA, Ibiam UA, Edeogu CO, Nweke FN, Chukwu UE. Determination of amylase activity of crude extract from partially germinated mango seeds (Mangifera oraphila) Afr J Biotechnol. 2009;8:3294–3296. [Google Scholar]
- Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr. 2014;5:404–417. doi: 10.3945/an.113.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benett CGS, Buzetti S, Silva KS, Bergamaschine AF, Fabricio JA. Productivity and bromatological composition of marandu grass at sources and doses of nitrogen1. Cienc Agrotec. 2008;32:1629–1636. [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- Bido GS, Ferrarese MLL, Marchiosi R, Ferrarese-Filho O. Naringenin inhibits the growth and stimulates the lignification of soybean root. Braz Arch Biol Technol. 2010;53:533–542. [Google Scholar]
- Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 2001;126:524–535. doi: 10.1104/pp.126.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts CA, Monro JA, Moughan PJ. In vitro determination of dietary protein and amino acid digestibility for humans. Br J Nutr. 2012;108:S282–S287. doi: 10.1017/S0007114512002310. [DOI] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inze D, Sandberg G, Casero PJ, Bennett M. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell. 2001;13:843–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Charron CS, Allen FL, Johnson RD, Pantalone VR, Sams CE. Correlations of oil and protein with isoflavone concentration in soybean [Glycine max (L.) Merr. J Agri Food Chem. 2005;53:7128–7135. doi: 10.1021/jf050610o. [DOI] [PubMed] [Google Scholar]
- Chen W, Yun MS, Deng F, Yogo Y. Effects of root-applied naringenin and chalcone on the growth of annual plants. Weed Biol Manag. 2004;4:118–122. [Google Scholar]
- Deng F, Aoki M, Yogo Y. Effect of naringenin on the growth and lignin biosynthesis of gramineous plants. Weed Biol Manag. 2004;4:49–55. [Google Scholar]
- Diaz-Batalla L, Widholm JM, Fahey GC, Jr, Castano-Tostado E, Paredes-Lopez O. Chemical components with health implications in wild and cultivated Mexican common bean seeds (Phaseolus vulgaris L.) J Agric Food Chem. 2006;54:2045–2052. doi: 10.1021/jf051706l. [DOI] [PubMed] [Google Scholar]
- Elkhalil EAJ, El Tinay AH, Mohamed BE, Elshseikh EAE. Effect of malt pretreatment on phytic acid and in vitro protein digestibility of sorghum flour. Food Chem. 2001;72:29–32. [Google Scholar]
- Esmaeili MA, Alilou M. Naringenin attenuates CCl4-induced hepatic inflammation by the activation of an Nrf2-mediated pathway in rats. Clin Exp Pharmacol Physiol. 2014;41:416–422. doi: 10.1111/1440-1681.12230. [DOI] [PubMed] [Google Scholar]
- Ferreyra MLF, Rius SP, Casati P. Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci. 2012;3:222. doi: 10.3389/fpls.2012.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- Ganguly A, Lee SH, Cho M, Lee OR, Yoo H, Cho HT. Differential auxin-transporting activities of PIN-FORMED proteins in Arabidopsis root hair cells. Plant Physiol. 2010;153:1046–1061. doi: 10.1104/pp.110.156505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani GS, Xiao CW, Cockell KA. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br J Nutr. 2012;108:S315–S332. doi: 10.1017/S0007114512002371. [DOI] [PubMed] [Google Scholar]
- Guleria P, Kumar V. Understanding the phenylpropanoid pathway for agronomical and nutritional improvement of mungbean. J Hortic Sci Biotech. 2017;92:335–348. [Google Scholar]
- Guleria P, Yadav SK. Insights into steviol glycoside biosynthesis pathway enzymes through structural homology modeling. Am J Biochem Mol Biol. 2012;3:1–19. [Google Scholar]
- Guleria P, Yadav SK. Agrobacterium mediated transient gene silencing (AMTS) in Stevia rebaudiana: insights into steviol glycoside biosynthesis pathway. PLoS ONE. 2013;8:e74731. doi: 10.1371/journal.pone.0074731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria P, Yadav SK. Overexpression of a glycosyltransferase gene SrUGT74G1 from Stevia improved growth and yield of transgenic Arabidopsis by catechin accumulation. Mol Biol Rep. 2014;41:1741–1752. doi: 10.1007/s11033-014-3023-y. [DOI] [PubMed] [Google Scholar]
- Guleria P, Masand S, Yadav SK. Overexpression of SrUGT85C2 from Stevia reduced growth and yield of transgenic Arabidopsis by influencing plastidial MEP pathway. Gene. 2014;539:250–257. doi: 10.1016/j.gene.2014.01.071. [DOI] [PubMed] [Google Scholar]
- Helland MH, Wicklund T, Narvhus JA. Effect of germination time on alpha-amylase production and viscosity of maize porridge. Food Res Int. 2002;35:315–321. [Google Scholar]
- Hernandez I, Alegre L, Van Breusegem F, Munne-Bosch S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci. 2009;14:125–132. doi: 10.1016/j.tplants.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Hernandez-Aquino E, Muriel P. Beneficial effects of naringenin in liver diseases: molecular mechanisms. World J Gastroenterol. 2018;24:1679–1707. doi: 10.3748/wjg.v24.i16.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D, Yousaf L, Xue Y, Hu J, Wu J, Hu X, Feng N, Shen Q. Mung bean (Vigna radiata L.): bioactive polyphenols, polysaccharides, peptides, and health benefits. Nutrients. 2019;11:1238. doi: 10.3390/nu11061238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wood KV, Morgan JA. Metabolic engineering of the phenylpropanoid pathway in Saccharomyces cerevisiae. Appl Environ Microbiol. 2005;71:2962–2969. doi: 10.1128/AEM.71.6.2962-2969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Itoh H, Ueguchi-Tanaka M, Ashikari M, Matsuoka M. The α-amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiol. 2002;128:1264–1270. doi: 10.1104/pp.010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M. Quantitation of flavonoid constituents in citrus fruits. J Agric Food Chem. 1999;47:3565–3571. doi: 10.1021/jf990153+. [DOI] [PubMed] [Google Scholar]
- Kelen M, Demiralay EC, Sen S, Ozkan G. Separation of abscisic acid, indole-3-acetic acid, gibberellic acid in 99 R (Vitis berlandieri x Vitis rupestris) and rose oil (Rosa damascena Mill.) by reversed phase liquid chromatography. Turk J Chem. 2004;28:603–610. [Google Scholar]
- Kimura Y, Aoki T, Ayabe S. Chalcone isomerase isozymes with different substrate specificities towards 6’-hydroxy and 6’-deoxychalcones in cultured cells of Glycyrrhiza echinata, a leguminous plant producing 5-deoxyflavonoids. Plant Cell Physiol. 2001;42:1169–1173. doi: 10.1093/pcp/pce130. [DOI] [PubMed] [Google Scholar]
- Langton FA, Adams SR, Cockshull KE. Effects of photoperiod on leaf greenness of four bedding plant species. J Hortic Sci Biotech. 2003;78:400–404. [Google Scholar]
- Lavenus J, Goh T, Roberts I, Guyomarch S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L. Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci. 2013;18:450–458. doi: 10.1016/j.tplants.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Leveau JHJ, Lindow SE. Utilization of plant hormone IAA for growth by Pseudomonas putida stain 1290. Appl Environ Microbiol. 2005;71:2365–2371. doi: 10.1128/AEM.71.5.2365-2371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SW, Shi RF, Leng Y. De novo characterization of the mung bean transcriptome and transcriptomic analysis of adventitious rooting in seedlings using RNA-Seq. PLoS ONE. 2015;15:e0132969. doi: 10.1371/journal.pone.0132969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YR, Chen DY, Chu CL, Li S, Chen YK, Wu CL, Lin CC. Naringenin inhibits dendritic cell maturation and has therapeutic effects in a murine model of collagen-induced arthritis. J Nutr Biochem. 2015;26:1467–1478. doi: 10.1016/j.jnutbio.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang X, Zhao Y, Li Y, Zhang G, Peng Z, Zhang J. Enhancing auxin accumulation in maize root tips improves root growth and dwarfs plant height. Plant Biotechnol J. 2018;16:86–99. doi: 10.1111/pbi.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaquat L, Batool Z, Sadir S, Rafiq S, Shahzad S, Perveen T, Haider S. Naringenin-induced enhanced antioxidant defence system meliorates cholinergic neurotransmission and consolidates memory in male rats. Life Sci. 2018;194:213–223. doi: 10.1016/j.lfs.2017.12.034. [DOI] [PubMed] [Google Scholar]
- Lim W, Li J. Synergetic effect of the Onion CHI gene on the PAP1 regulatory gene for enhancing the flavonoid profile of tomato skin. Sci Rep. 2017;7:12377. doi: 10.1038/s41598-017-12355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Xia W, Li H, Zeng H, Wei B, Han S, Yin C. Salinity inhibits rice seed germination by reducing α-amylase activity via decreased bioactive gibberellin content. Front Plant Sci. 2018;9:275. doi: 10.3389/fpls.2018.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Chang X, Guo X. Dynamic changes of ascorbic acid, phenolics biosynthesis and antioxidant activities in mung beans (Vigna radiata) until maturation. Plants. 2019;8:75. doi: 10.3390/plants8030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Dudareva N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu Rev Plant Biol. 2012;63:73–105. doi: 10.1146/annurev-arplant-042811-105439. [DOI] [PubMed] [Google Scholar]
- Mahakham W, Sarmah AK, Maensiri S, Theerakulpisut P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci Rep. 2017;7:8263. doi: 10.1038/s41598-017-08669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar HPS, Bluemmel M, Borowy NK, Becker K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J Sci Food Agric. 1993;61:161–165. [Google Scholar]
- Mandal SM, Chakraborty D, Dey S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav. 2010;5:359–368. doi: 10.4161/psb.5.4.10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V, Mestre TC, Rubio F, Girones-Vilaplana A, Moreno DA, Mittler R, Rivero RM. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front Plant Sci. 2016;7:838. doi: 10.3389/fpls.2016.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierziak J, Kostyn K, Kulma A. Flavonoids as important molecules of plant interactions with the environment. Molecule. 2014;19:16240–16265. doi: 10.3390/molecules191016240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed TK, Zhu K, Issoufou A, Fatmata T, Zhou H. Functionality, in vitro digestibility and physicochemical properties of two varieties of defatted foxtail millet protein concentrates. Int J Mol Sci. 2009;10:5224–5238. [Google Scholar]
- Muir SR, Collins GJ, Robinson S, Hughes S, Bovy A, Ric De Vos CH, van Tunen AJ, Verhoeyen ME. Overexpression of petunia chalcone isomerase in tomato results in fruit containing increased levels of flavonols. Nat Biotechnol. 2001;19:470–474. doi: 10.1038/88150. [DOI] [PubMed] [Google Scholar]
- Mulvihill EE, Burke AC, Huff MW. Citrus flavonoids as regulators of lipoprotein metabolism and atherosclerosis. Annu Rev Nutr. 2016;36:275–299. doi: 10.1146/annurev-nutr-071715-050718. [DOI] [PubMed] [Google Scholar]
- Nakabayashi R, Yonekura-Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T, Matsuda F, Kojima M, Sakakibara H, Shinozaki K, Michael AJ, Tohge T, Yamazaki M, Saito K. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014;77:367–379. doi: 10.1111/tpj.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak K, Chovanec P, Skrdleta V, Kropacova M, Lisa L, Nemcova M. Effect of exogenous flavonoids on nodulation of pea (Pisum sativum L.) J Exp Bot. 2002;53:1735–1745. doi: 10.1093/jxb/erf016. [DOI] [PubMed] [Google Scholar]
- Oochi A, Hajny J, Fukui K, Nakao Y, Gallei M, Quareshy M, Takahashi K, Kinoshita T, Harborough SR, Kepinski S, Kasahara H. Pinstatic acid promotes auxin transport by inhibiting PIN internalization. Plant Physiol. 2019;180:1152–1165. doi: 10.1104/pp.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overvoorde P, Fukaki H, Beeckman T. Auxin control of root development. CSH Perspect Biol. 2010;2:a001537. doi: 10.1101/cshperspect.a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Kim GY, Choi YH. Naringenin attenuates the release of pro-inflammatory mediators from lipopolysaccharide-stimulated BV2 microglia by inactivating nuclear factor-kappaB and inhibiting mitogen-activated protein kinases. Int J Mol Med. 2012;30:204–210. doi: 10.3892/ijmm.2012.979. [DOI] [PubMed] [Google Scholar]
- Patil R, Das S, Stanley A, Yadav L, Sudhakar A, Varma AK. Optimized hydrophobic interactions and hydrogen bonding at the target–ligand interface leads the pathways of drug-designing. PLoS ONE. 2010;5:e12029. doi: 10.1371/journal.pone.0012029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman MU, Rahman Mir MU, Farooq A, Rashid SM, Ahmad B, Bilal Ahmad S, Ali R, Hussain I, Masoodi M, Muzamil S, Madkhali H, Ahmad Ganaie M. Naringenin (4,5,7-trihydroxyflavanone) suppresses the development of precancerous lesions via controlling hyperproliferation and inflammation in the colon of Wistar rats. Environ Toxicol. 2018;33:422–435. doi: 10.1002/tox.22528. [DOI] [PubMed] [Google Scholar]
- Ruezinsky DM, Bennett KA, Jander G (2007) Production of increased oil and protein in plants by the disruption of the phenylpropanoid pathway. US patent, US7268276B2
- Runguphan W, Maresh JJ, O'Connor SE. Silencing of tryptamine biosynthesis for production of nonnatural alkaloids in plant culture. Proc Natl Acad Sci USA. 2009;106:13673–13678. doi: 10.1073/pnas.0903393106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar GG, Wu XC, Cockell KA. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br J Nutr. 2012;108(Suppl 2):S315–S332. doi: 10.1017/S0007114512002371. [DOI] [PubMed] [Google Scholar]
- Shakeel S, Rehman MU, Tabassum N, Amin U, Mir MUR. Effect of naringenin (a naturally occurring flavanone) against pilocarpine-induced status epilepticus and oxidative stress in mice. Pharmacogn Mag. 2017;13(Suppl 1):S154–S160. doi: 10.4103/0973-1296.203977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Raizada J, Bhardwaj P, Ghawana S, Rani A, Singh H, Kaul K, Kumar S. 26S rRNA-based internal control gene primer pair for reverse transcription-polymerase chain reactionbased quantitative expression studies in diverse plant species. Anal Biochem. 2004;335:330–333. doi: 10.1016/j.ab.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Singh D, Nath K, Sharma YK. Response of wheat seed germination and seedling growth under copper stress. J Environ Biol. 2007;28:409. [PubMed] [Google Scholar]
- Singh K, Rani A, Kumar S, Sood P, Mahajan M, Yadav SK, Singh B, Ahuja PS. An early gene of the flavonoid pathway, flavanone 3-hydroxylase, exhibits a positive relationship with the concentration of catechins in tea (Camellia sinensis) Tree Physiol. 2008;28:1349–1356. doi: 10.1093/treephys/28.9.1349. [DOI] [PubMed] [Google Scholar]
- Soengas P, Cartea ME, Velasco P, Francisco M. Endogenous circadian rhythms in polyphenolic composition induce changes in antioxidant properties in Brassica cultivars. J Agric Food Chem. 2018;66:5984–5991. doi: 10.1021/acs.jafc.8b01732. [DOI] [PubMed] [Google Scholar]
- Tang S, Hettiarachchy NS, Horax R, Eswaranandam S. Physicochemical properties and functionality of rice bran protein hydrolyzate prepared from heat-stabilized defatted rice bran with the aid of enzymes. J Food Sci. 2003;68:152–157. [Google Scholar]
- Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeyen ME, Bovy A, Collins G, Muir S, Robinson S, de Vos CH, Colliver S. Increasing antioxidant levels in tomatoes through modification of the flavonoid biosynthetic pathway. J Exp Bot. 2002;53:2099–2106. doi: 10.1093/jxb/erf044. [DOI] [PubMed] [Google Scholar]
- Wali AF, Rashid S, Rashid SM, Ansari MA, Khan MR, Haq N, Alhareth DY, Ahmad A, Rehman MU. Naringenin regulates doxorubicin-induced liver dysfunction: impact on oxidative stress and inflammation. Plants (Basel) 2020;9:550. doi: 10.3390/plants9040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox LJ, Borradaile NM, Huff MW. Antiatherogenic properties of naringenin, a citrus flavonoid. Cardiovasc Drug Rev. 1999;17:160–178. [Google Scholar]
- Wu LH, Lin C, Lin HY, Liu YS, Wu CY, Tsai CF, Chang PC, Yeh WL, Lu DY. Naringenin suppresses neuroinflammatory responses through inducing suppressor of cytokine signaling 3 expression. Mol Neurobiol. 2016;53:1080–1091. doi: 10.1007/s12035-014-9042-9. [DOI] [PubMed] [Google Scholar]
- Yang J, Li Q, Zhou XD, Kolosov VP, Perelman JM. Naringenin attenuates mucous hypersecretion by modulating reactive oxygen species production and inhibiting NF-kappaB activity via EGFR-PI3K-Akt/ERK MAPKinase signalling in human airway epithelial cells. Mol Cell Biochem. 2011;351:29–40. doi: 10.1007/s11010-010-0708-y. [DOI] [PubMed] [Google Scholar]
- Zhang HX, Blumwald E. Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol. 2001;19:765–768. doi: 10.1038/90824. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Xing L, Wang X, Hou YJ, Gao J, Wang P, Duan CG, Zhu X, Zhu JK. The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77-dependent transcription of auxin- responsive genes. Sci Signal. 2014;7:ra53. doi: 10.1126/scisignal.2005051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Three-dimensional VrCHI protein and interaction with naringenin chlacone and isoliquiritigenin. (DOCX 1828 kb)