Abstract
Recently, a lateral flow rapid diagnostic test (RDT) with good accuracy has been described. This test enables measles specific IgM antibody detection in serum, capillary blood and oral fluid. RDTs have the potential to transform measles surveillance by allowing real-time case confirmation outside of central/regional laboratories and by facilitating a timely public health response. Measles virus genes can also be amplified and sequenced consistently from dried IgM-positive RDTs stored outside of cold chain, which will enable more complete virologic surveillance. Critical questions remain regarding operational use of RDTs as part of global measles surveillance. Projects to evaluate RDT use as part of national surveillance programs and to commercialize the RDT are underway.
Live attenuated measles vaccines have been in widespread use over the last 30 years, either as a single antigen vaccine or as combination vaccines with rubella, mumps, or varicella antigens [1]. Over the last 20 years, the vaccine has been used as part of a global program to control and eliminate measles. All six World Health Organization (WHO) regions have established measles elimination goals, and the Region of the Americas verified elimination in 2016. By the end of 2018, measles elimination was achieved in 82 (42%) countries globally [2•]. During 2000–2018, the annual number of estimated deaths globally dropped by 73% to an estimated 140 000 deaths in 2018 [2•]. However, a global measles resurgence began in 2017–2018, and measles outbreaks in Venezuela and Brazil led to loss of elimination status in the Region of the Americas in 2017, highlighting the ongoing public health challenge [2•,3,4].
Measles infection presents as a rash and fever illness, typically with prodromal symptoms including cough, coryza, and conjunctivitis [5]. Clinical diagnosis is reasonably accurate during outbreaks, but at other times, particularly when measles incidence is low, differential diagnosis based on clinical ground only is unreliable because many other infectious diseases cause rash and fever including dengue, rubella, Zika and parvovirus B19 [5–7]. Laboratory confirmation of cases is an essential component of surveillance and is performed using enzyme immunoassays (EIAs) to detect measles-specific immunoglobulin M (IgM) antibodies in acute serum samples [8]. Global laboratory-based surveillance for measles is provided by the Global Measles Rubella Laboratory Network (GMRLN), a network of over 700 laboratories operating in 191 countries [9,10••,11•]. Over recent years, real-time reverse transcriptase polymerase chain reaction (RT-PCR) has been used increasingly to compliment IgM detection for case confirmation [8]. RT-PCR is particularly useful in settings where the positive predictive value of IgM positive results is low because of low measles incidence [12•].
Measles virus, a morbillivirus with a single-stranded RNA genome, is serologically monotypic, but strains can be grouped into 24 recognized genotypes based on sequence analysis. Genotypes are routinely assigned by analysis of a standard sequencing window of 450 nucleotides in the N gene (N-450), which varies by up to 12% between genotypes [8]. Virologic surveillance of circulating measles viruses is used to monitor progress toward elimination of endemic virus. GMRLN members update the online Measles Nucleotide Surveillance (MeaNS) database regularly, which facilitates analysis of transmission pathways [13,14].
Important recent developments in rapid diagnostic tests (RDTs) for measles IgM testing, in combination with advances in innovation with molecular detection, present new opportunities for the global surveillance program and will be discussed here [15•].
Measles RDT: current status and requirements for use in measles surveillance
High quality measles surveillance is characterized by representative case detection meeting a minimal level of sensitivity and ability to classify cases based on laboratory confirmation (e.g. key indicator target of ≥2 suspected cases per 100 000 population discarded as non-measles and non-rubella) [5]. Currently, laboratory confirmation of cases is based on the detection of measles-specific IgM antibodies in serum samples from a suspected case using EIAs [8]. Much of this testing is performed in laboratories that are part of GMRLN, which take part in an external quality assessment (EQA) scheme and are accredited through WHO [8]. Laboratories work to achieve performance targets for the time required to collect and ship samples, perform testing, and report results [5]. Genotyping strains from a representative proportion of measles outbreaks is another key surveillance target [5,10••].
Despite the many achievements of the GMRLN, the challenges of delivering universal, rapid, and accurate results remain because of limited laboratory capacity, staff capability, and availability of EIA test kits [16•]. In settings with poor infrastructure and hard-to-reach populations (e.g. remote rural, or island), these challenges are often compounded by limited sample transport capacity and a lack of laboratory capacity outside of major cities [17–19]. Approximately 70 network laboratories in resource-limited countries routinely receive EIA kits through WHO. As all WHO Regions now have adopted a measles elimination goal, surveillance has shifted from outbreak confirmation to case-based surveillance with laboratory confirmation, which increases the financial burden on GMRLN.
Different approaches to address the challenges of laboratory-based surveillance have been used. Alternative samples, such as capillary blood that can be tested immediately or collected as dried blood spots and oral fluids, can be used for IgM antibody detection [5]. These samples can be obtained safely and non-invasively without the risks associated with venous blood collection. Use of capillary blood and oral fluid samples improve patient compliance with specimen collection, as the procedures are relatively simple and collection of an oral fluid specimen is painless and non-invasive [20]. Oral fluid contains a transudate of serum with the same composition of antibodies as serum and is widely used for HIV diagnosis and surveillance [21]. Although oral fluids have a slightly lower sensitivity, commercial EIAs have been used with oral fluid samples in the United Kingdom for nearly 20 years to detect measles and rubella IgM and helped document elimination [22–26]. Additionally, oral fluid is the optimum sample for detecting measles virus ribonucleic acid (RNA) by RT-PCR in acute cases [20,27].
Lateral-flow RDTs are used increasingly for diagnosis of many diseases including malaria, dengue, HIV, and other sexually transmitted infections [28,29]. RDTs are performed in a single incubation step at ambient temperature, and results can be interpreted visually, often within 30 min. This allows rapid diagnosis not only to be made by the designated laboratory but also in the field or at minimally equipped peripheral health facilities, increasing the diagnostic capacity in low-resource settings, with the potential to inform clinical management and enable a more immediate public health response [28,29].
A lateral flow RDT has been developed that enables the rapid detection (<30 min) of measles specific IgM antibodies in both serum and oral fluid specimens [30••,31]. With serum, the RDT had a sensitivity and a specificity of 91% (69/76) and 94% (88/94), respectively; with oral fluid, the sensitivity and the specificity were 90% (63/70) and 96% (200/208), respectively [30••]. Measles virus RNA could be recovered from used IgM positive RDTs after dry storage for five weeks at 20–25°C, and the RNA could be sequenced to obtain a genotype [30••,31].
This prototype RDT has been redesigned for use with capillary blood, using the ASSURED criteria1 [32] and manufactured commercially. A clinical trial batch of measles IgM RDTs was evaluated against a reference assay, the Enzygnost® anti-measles virus IgM EIA (Siemens Healthcare GmbH, Marburg, Germany), using a representative panel of 125 sera from the Brazilian measles surveillance program and sera collected from acute dengue cases. The RDT showed high sensitivity (95%) and specificity (98%) [33]. In the same study, the accuracy of visual reading was compared to the result with quantitative signal measurement by scanning densitometry using the ESEQuant reader (Qiagen Lake Constance GmbH, Stockach, Germany). Excellent agreement (Kappa statistic >0.9) was observed between the three independent visual readings, supporting the interpretation of the measles RDT results based on visual reading alone [33].
In recent unpublished field trials in India and Uganda, local surveillance teams used RDTs with both capillary blood and oral fluid samples following a two-day training program. The oral fluid collection, RDT, and visual reading were well-accepted as shown by the high compliance in sample collection (98%) and the agreement between readers (98%) (L. Warrener, J. Bwogi, L. Sangal and D.W. Brown, personal communication). The sensitivity of measles IgM detection in capillary blood using the RDT was comparable to published performance with RDT and to current laboratory-based EIA testing; sensitivity was slightly lower using oral fluid. The used IgM positive RDTs were air-dried, shipped together with other samples in cold chain and the specimen filter pads from IgM-positive RDTs were tested by RT-PCR, with similar performance to earlier laboratory-based studies (L. Warrener and D.W. Brown, personal communication).
These results demonstrate that using RDTs with capillary blood samples produces comparable performance to the current IgM EIA test. Further, performance of RDTs used with oral fluid is good, but with slightly lower sensitivity and specificity. Both approaches are sufficiently accurate for use in many epidemiological settings, allowing expansion of testing outside central/regional laboratories, and can provide faster case confirmation than EIAs (30 min versus ~3–14 days including sample transport time and return of results). Using the RDT enables real time case confirmation and once dried, used RDTs can be shipped to national laboratories outside the cold chain, where confirmation by RT-PCR and genotyping can be performed. Currently, collection of a separate specimen (throat or nasopharyngeal swab, or urine) for virus detection is recommended [5], but is often omitted because of logistical constraints as specimens need a reverse cold chain and dedicated specimen collection devices need to be on site [10••,11•]. Thus, RDTs have the potential to strengthen molecular epidemiological studies by improving the representativeness of strains characterized.
The ASSURED scorecard for current measles IgM RDT includes the following characteristics: affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free and deliverable to those who need it [32]. Manufactured in small batches, the RDT device costs about USD 4.5. Commercial production may decrease the costs significantly. The target product profile (TPP) includes minimal performance criteria of >90% sensitivity and specificity for capillary blood and oral fluid, stable up to 40°C and with a shelf life >1 year. With capillary blood, the RDT is simple to perform and requires minimal training. A dilution buffer is the only accompanied equipment needed when using capillary blood; an oral fluid collector is needed when using oral fluid. The final commercial product will be delivered in small, individual packages containing desiccant accompanied by all required reagents and supplies. Field studies to support regulatory approval are planned and these will establish the accuracy of the RDTs for measles and rubella. Different levels of RDT performance may be needed for outbreak investigations and for case confirmation in countries close to disease elimination (e.g. in low prevalence settings).
Efforts to introduce RDTs into measles surveillance programs
In low-resource settings, measles and rubella testing primarily occurs for surveillance, rather than for clinical diagnostic purposes. Critical questions remain regarding the operational use of the measles RDT to support its introduction into surveillance programs (Table 1) [32,34]. For example, what is the relative benefit of using measles RDTs in countries close to elimination versus in endemic settings? In countries close to elimination with strong health systems, improved timeliness of RDT results has the potential to accelerate the measles public health response and reduce the burden of investigating and responding to suspected cases before laboratory confirmation. In endemic settings, the primary benefit of RDT use could be expanded laboratory capacity beyond central labs, but it is unclear whether providing faster results would improve the timeliness of public health response, without further strengthening of the health system [28,35].
Table 1.
Questions related to the operational use of measles IgM rapid diagnostic tests (RDTs) to support introduction into surveillance programs
| Topic area | Question |
|---|---|
| Test use |
|
| Process and implementation |
|
| Test results |
|
| Cost and shelf-life |
|
The related framework in Figure 1 presents four possible settings for measles RDT use: 1) hard-to-reach communities, 2) health clinics, 3) regional and local laboratories, and 4) central or national public health laboratories in the GMRLN. The relative benefit of measles RDT use in these different settings is likely to vary by country and within a country. One difference between the scenarios is that capillary blood and oral fluid samples could be used at community and clinic levels, improving patient compliance with testing, whereas serum samples would likely be used in laboratories having capacity for centrifugation, per current procedures. RDT implementation at lower levels of the health system would create higher resource demands in terms of training, supply management systems, quality assurance, and processes/systems for reporting of results [36,37,38•].
Figure 1.
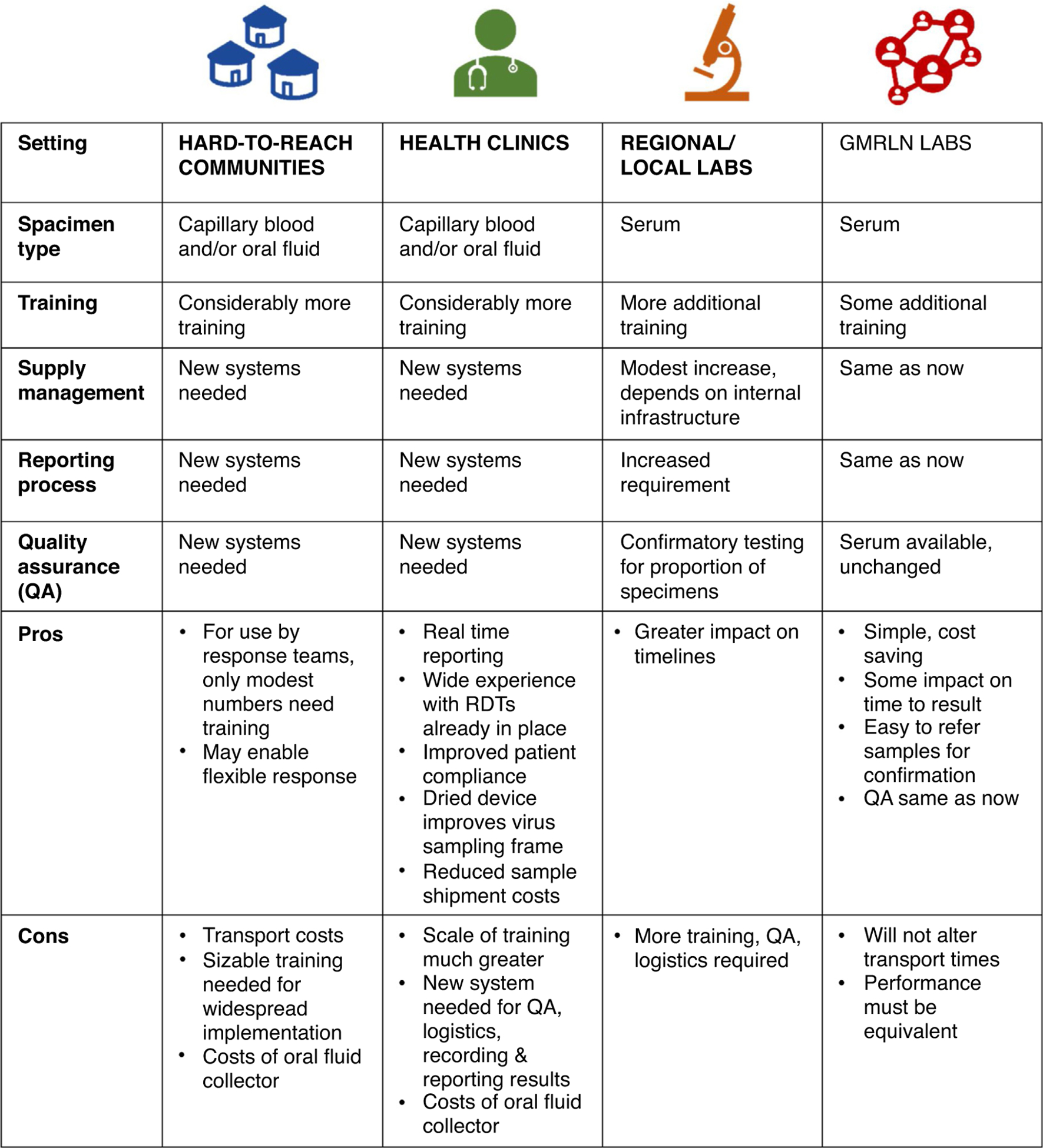
Diversity of potential settings and implications for measles rapid diagnostic test (RDT) use.
Several assessments are ongoing to evaluate the feasibility and potential impact of introducing the measles RDT into ongoing case-based measles surveillance with laboratory confirmation. One evaluation is in Malaysia, which is close to measles elimination with a robust public health response (e.g. contact tracing, response immunization to both suspected and confirmed measles cases), and another is in Uganda, which is a control setting where the public health response to cases is often inadequate. In evaluations in both countries, we are (i) assessing the agreement of direct visual readings of RDT results by health facility staff versus RDT photos and machine (scanning densitometry) readings by laboratory staff; (ii) evaluating RDT acceptability, knowledge, attitudes, and practices of staff involved in the public health management of measles; and (iii) assessing the impact of RDTs on measles surveillance program performance. For this purpose, capillary blood and oral fluid samples are being used with RDTs at health facilities in parallel with standard surveillance testing of serum by EIA. Of interest, we observed widespread (>90%) reported previous experience using RDTs among trainees in Uganda and Malaysia, especially for HIV, malaria, and dengue, which may benefit the measles surveillance program in terms of RDT introduction (H.M. Scobie, M. Donadel, L. Warrener, D. Featherstone, P. Rota, M. Mulders, J. Bwogi, A. Senin and D.W. Brown, personal communication).
In assessments in Ghana and Cameroon, serum samples are currently being tested with RDTs in district laboratories. This is to test a different scenario where RDTs may be used to decentralize and expand the extent of serum testing beyond the central laboratory. An evaluation will be conducted in each country to assess: (i) the accuracy of test results between district and reference laboratory readers, (ii) the timeliness of reporting of RDT results, (iii) the acceptability of the measles RDT by users, and (iv) operational requirements of measles RDT use. Preliminary results from all studies are expected at the end of 2020 (D. Waku, P. Rota, A. Balajee, M. Mulders and D.W. Brown, personal communication).
Currently, limitations exist regarding measles RDT use for surveillance. At this time, the measles RDT is manufactured in batches according to product specifications. The commercialization phase of the RDT has started, lasting until early to mid-2021; the regulatory review and approval process will follow. Because the standard protocol for most GMRLN laboratories is to test measles IgM negative serum samples for rubella IgM, a current limitation to the use of the measles RDT for surveillance is that there is not yet a rubella RDT. The rubella RDT is under development as part of commercialization of the measles RDT, including development of a combination measles and rubella IgM test.
Implications of RDTs and other advances on virologic surveillance for measles
In the measles elimination phase, genetic characterization of circulating wild-type measles viruses is particularly important. After countries and regions achieve elimination, measles continues to be imported, but importations usually have limited spread because of high vaccination coverage [5]. The combination of molecular analysis and standard epidemiological investigation in elimination settings provides a sensitive means to document the interruption of endemic transmission [13,39]. Absence of endemic genotype(s) is one of the criteria for verifying measles elimination in a country [40].
High quality virologic surveillance has been made possible by establishing a global sequence database, MeaNS [41]. Unfortunately, global virologic surveillance is incomplete in many regions [2•,41]. Resource limitations in several regions have restricted the number of virus detections and sequences reported to MeaNS (Table 2) [10••,41]. The introduction of the RDT will help to address this challenge since, as described above, viral RNA can be extracted from RDTs with IgM positive results and used for genotyping, eliminating the need for reverse cold chain transport of separate specimens (e.g. throat swab) for viral detection [30••]. Thus, RDTs have the potential to facilitate increased representativeness of virologic surveillance, especially from resource-limited settings.
Table 2.
Number of confirmed measles cases and measles sequences, 2018–2019
| Region | Total number of confirmed measles cases | Number of sequences (percent of total) |
|---|---|---|
| AFR | 344 315 | 152 (0.04) |
| AMR | 36 233 | 1589 (4.39) |
| EMR | 78 589 | 416 (0.53) |
| EUR | 193 113 | 9911 (5.13) |
| SEAR | 118 987 | 2023 (1.70) |
| WPR | 92 619 | 3121 (3.37) |
| Total | 863 856 | 17 212 (1.99) |
WHO regions as follows, AFR: African, AMR: Americas, EMR: Eastern Mediterranean, SEAR: Southeast Asian, WPR: Western Pacific). Monthly measles case-based data reported to WHO [URL: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/measles_monthlydata/en/] and number of measles sequences reported to MeaNS [10••,41].
The diversity of circulating measles genotypes has decreased with only 4 genotypes detected as of the end of 2019, with >99% of sequences being genotypes B3 and D8 (Figure 2) [41]. The decrease in the number of circulating genotypes has presented a challenge to virologic surveillance for measles because the genotype designation alone no longer provides enough resolution for tracking transmission. To address this issue, GMRLN developed a convention for nominating specific N-450 sequences as “named strains” [10••,14]. Tracking named strains allows linkage of measles strains that are circulating around the world. For 2019, the most frequently reported named strain for genotype D8 was MVs/Gir Somnath.IND/42.16/, with 4239 reports in 64 countries [41]. An additional challenge for virologic surveillance in countries that have achieved measles elimination is that analysis of the N-450 region is not able to distinguish between multiple introductions of the same named strain and continued endemic circulation of that strain [42••]. Recent studies from Canada, UK, and USA have shown that extending the sequence window or performing whole genome sequencing (WGS) can greatly improve the discrimination of molecular epidemiological studies [43–45]. Although extended sequencing and WGS are still considered research methods and need to be evaluated for use with RDT derived samples, these molecular techniques are predicted to become more important components of virologic surveillance for measles.
Figure 2.
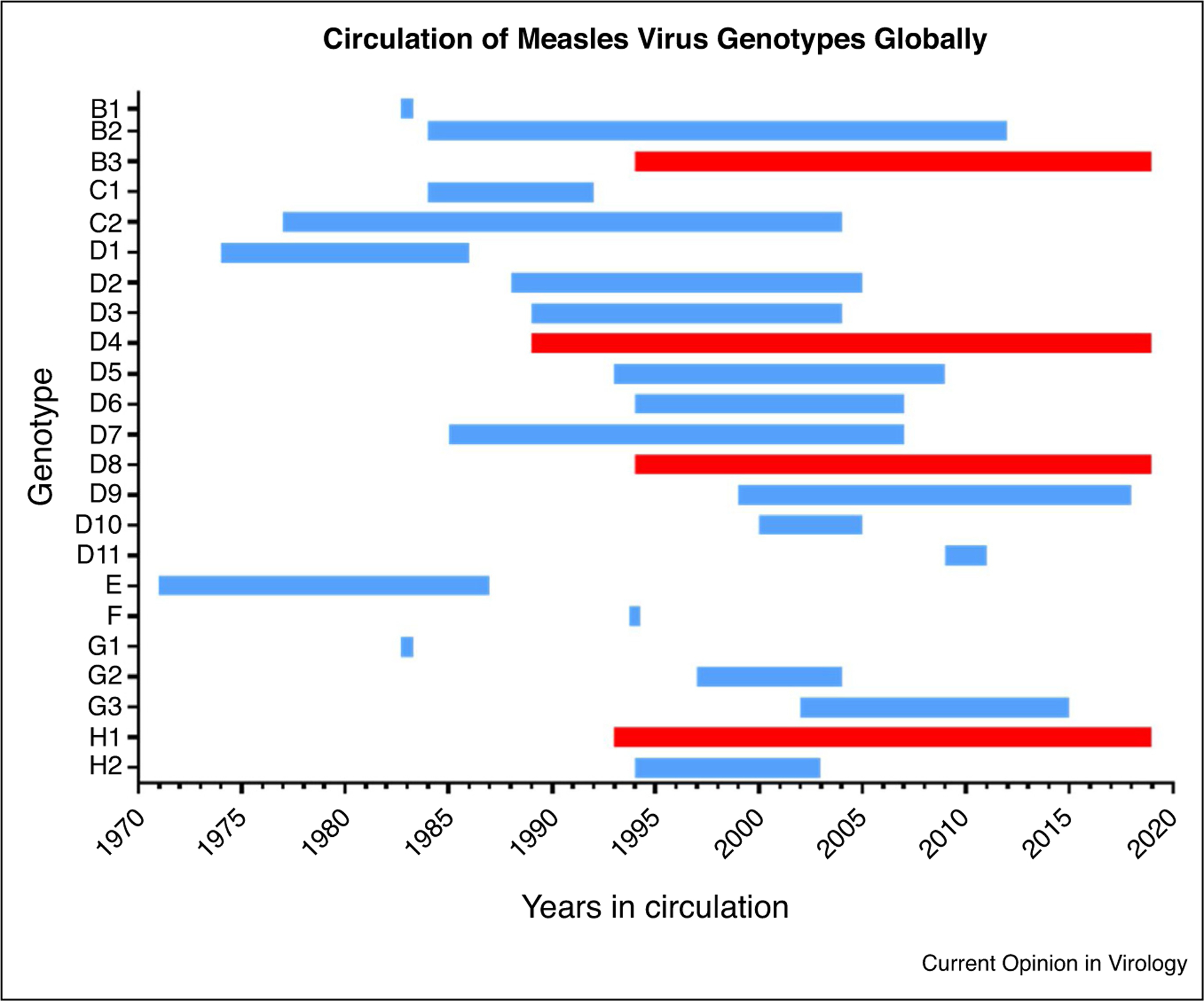
Circulation of measles virus genotypes globally reported through virologic surveillance, 1970–2019.
Figure shows year of first and last detection of measles wild-type virus genotypes. The first and last years of documented circulation reported to the MeaNS database (or GenBank before 2005) for all 24 measles virus genotypes are shown. First year detection determined from earliest reported reference strain and does not necessarily indicate first year of circulation. The four currently circulating genotypes (detected in 2019) are indicated in red (B3, D4, D8, H1). Measles vaccines were derived from genotype A which is not shown.
Conclusions
Although the global program to control and eliminate measles has made great progress over the last 20 years, significant challenges remain. The introduction of a measles RDT using capillary blood or oral fluid samples can transform current measles surveillance by improving the representativeness and timeliness of case-based surveillance and enabling a timely public health response. Measles RDTs use also has the potential to enhance the representativeness of viral strains genotyped. Standardisation and systematic use of RDTs, in combination with new molecular approaches, will help inform the picture of measles transmission in the elimination phase.
Acknowledgements
Diane Waku-Kouomou is contracted to the Division of Viral Disease, CDC. The authors express their gratitude to David Williams at PHE, and Melissa Coughlin at CDC, for their assistance with figure preparation. The authors would like to thank David Featherstone, WHO technical consultant; Arun Balajee, CDC; Varja Grabovac, WHO Regional Office for the Western Pacific Region; as well as key study collaborators at the WHO Malaysia Country Office and Malaysia Ministry of Health: A’aisah Senin, Hajah Noorliza Mohamad Noordin, Jamiatul Aida Md. Sani, Yu Kie Chem; key study collaborators at the Uganda Ministry of Health: Josephine Bwogi, Theopista Kabaliisa, Henry Bukenya; key study collaborators at the Ghana Health Services and the WHO Ghana Country Office: David Opare, Franklin Asiedu-Bekoe, Fred Osei-Sarpong; key study collaborators at the Cameroon Ministry of Health, the Centre Pasteur du Cameroun, the National Public Health Laboratory and the WHO Cameroon Country Office: Georges Etoundi, Richard Njouom, Marie Claire Okomoand, Marlise Dontsop; and finally key study collaborators at the WHO India Country Office, the WHO South-East Asia Regional Office, the WHO National Polio Surveillance Project, and the Indian Council of Medical Research-National Institute of Virology: Lucky Sangal, Sunil Bahl, Ashutosh Agarwal, Kunwar Abhishek, Ashok Munivenkatatappa and Diamond Prakash Sinha. The authors would also like to thank all the many other key study contributors and enrolled patients for their time and support.
Funding
This work was supported by Public Health England; the U.S. Centers for Disease Control and Prevention; and the Bill and Melinda Gates Foundation, Seattle, WA [grant number OPP1066619].
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention nor that of Public Health England.
Conflict of interest statement
Nothing declared.
Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free, Deliverable to end-users
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.World Health Organization: Measles vaccines: WHO position paper - April 2017. Wkly Epidemiol Rec 2017, 92:205–227.28459148 [Google Scholar]
- 2.•.Patel MK, Dumolard L, Nedelec Y, Sodha SV, Steulet C, Gacic-Dobo M, Kretsinger K, McFarland J, Rota PA, Goodson JL: Progress toward regional measles elimination - worldwide, 2000–2018. MMWR Morb Mortal Wkly Rep 2019, 68:1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]; In this most recent of an annual series, the authors summarize progress towards global measles elimination by WHO region in terms of routine immunization coverage, campaign coverage, reported incidence and estimated measles mortality. The article highlights the remaining gaps in immunization programs and surveillance in order to achieve regional measles elimination goals.
- 3.Durrheim DN, Crowcroft NS, Blumberg LH: Is the global measles resurgence a “public health emergency of international concern”? Int J Infect Dis 2019, 83:95–97. [DOI] [PubMed] [Google Scholar]
- 4.Thornton J: Measles cases in Europe tripled from 2017 to 2018. BMJ 2019, 364:l634. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization: Vaccine Preventable Diseases Surveillance Standards: Measles chapter. 2018. [https://www.who.int/immunization/monitoring_surveillance/burden/vpd/standards/en/].
- 6.Hubschen JM, Bork SM, Brown KE, Mankertz A, Santibanez S, Ben Mamou M, Mulders MN, Muller CP: Challenges of measles and rubella laboratory diagnostic in the era of elimination. Clin Microbiol Infect 2017, 23:511–515. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira SA, Siqueira MM, Camacho LA, Nogueira RM, Spinetti CC, Cubel Garcia RC, Knowles W, Brown DW: The aetiology of maculopapular rash diseases in Niteroi, State of Rio de Janeiro, Brazil: implications for measles surveillance. Epidemiol Infect 2001, 127:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization: Manual for the Laboratory-based Surveillance of Measles, Rubella, and Congenital Rubella Syndrome. 2018. [https://www.who.int/immunization/monitoring_surveillance/burden/laboratory/manual/en/].
- 9.World Health Organization: Expanded Programme on Immunization (EPI). Standardization of the nomenclature for describing the genetic characteristics of wild-type measles viruses. Wkly Epidemiol Rec; 1998, 73:265–269. [PubMed] [Google Scholar]
- 10.••.Brown KE, Rota PA, Goodson JL, Williams D, Abernathy E, Takeda M, Mulders MN: Genetic characterization of measles and rubella viruses detected through global measles and rubella elimination surveillance, 2016–2018. MMWR Morb Mortal Wkly Rep 2019, 68:587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors describe recent measles molecular epidemiology and the WHO-agreed upon nomenclature for describing genetic diversity used by surveillance programs. The article highlights the impact of the global vaccination program on reducing viral diversity, the use of named strains to improve discrimination within genotypes, and the need for extended sequencing to support future surveillance.
- 11.•.Mulders MN, Rota PA, Icenogle JP, Brown KE, Takeda M, Rey GJ, Ben Mamou MC, Dosseh AR, Byabamazima CR, Ahmed HJ et al. : Global measles and rubella laboratory network support for elimination goals, 2010–2015. MMWR Morb Mortal Wkly Rep 2016, 65:438–442 [DOI] [PubMed] [Google Scholar]; The authors provide an overview of the GMRLN structure and activities, including serology, molecular testing and external quality performance, as well summarizing GMRLN laboratory performance towards achieving measles and rubella elimination.
- 12.•.Bolotin S, Lim G, Dang V, Crowcroft N, Gubbay J, Mazzulli T, Schabas R: The utility of measles and rubella IgM serology in an elimination setting, Ontario, Canada, 2009–2014. PLoS One 2017, 12:e0181172. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show a low positive predictive value of measles and rubella IgM serology alone in the context of an elimination setting using retrospective analysis laboratory and epidemiological data from Ontario. The article highlights that Ontario’s measles case definition was modified in 2014, so that a positive IgM result does not confirm a case unless also accompanied by an epidemiological link, an appropriate travel history, or virus detection by culture or PCR.
- 13.Rota JS, Heath JL, Rota PA, King GE, Celma ML, Carabana J, Fernandez-Munoz R, Brown D, Jin L, Bellini WJ: Molecular epidemiology of measles virus: identification of pathways of transmission and implications for measles elimination. J Infect Dis 1996, 173:32–37. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization: Genetic diversity of wild-type measles viruses and the global measles nucleotide surveillance database (MeaNS). Wkly Epidemiol Rec; 2015, 90:373–380. [PubMed] [Google Scholar]
- 15.•.Grant GB, Masresha BG, Moss WJ, Mulders MN, Rota PA, Omer SB, Shefer A, Kriss JL, Hanson M, Durrheim DN et al. : Accelerating measles and rubella elimination through research and innovation - findings from the measles & rubella initiative research prioritization t. Vaccine 2019, 37:5754–5761 [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors provide of summary of potential research topics and innovations identified by the Measles and Rubella Initiative as priorities to achieve measles and rubella elimination. The article points out that research is needed to develop a rubella IgM RDT and to optimize measles IgM RDT use in the field as part of existing case-based surveillance systems.
- 16.•.Patel MK, Gibson R, Cohen A, Dumolard L, Gacic-Dobo M: Global landscape of measles and rubella surveillance. Vaccine 2018, 36:7385–7392 [DOI] [PubMed] [Google Scholar]; The authors report on the global status of national measles and rubella surveillance systems in 2016, using data reported by 164 (85%) countries to the WHO. This article assesses whether essential components are in place and of sufficient quality to conduct elimination-standard surveillance for measles and rubella.
- 17.Scobie HM, Ilunga BK, Mulumba A, Shidi C, Coulibaly T, Obama R, Tamfum JJ, Simbu EP, Smit SB, Masresha B et al. : Antecedent causes of a measles resurgence in the Democratic Republic of the Congo. Pan Afr Med J 2015, 21:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breakwell L, Moturi E, Helgenberger L, Gopalani SV, Hales C, Lam E, Sharapov U, Larzelere M, Johnson E, Masao C et al. : Measles outbreak associated with vaccine failure in adults—federated states of Micronesia, February-August 2014. MMWR Morb Mortal Wkly Rep 2015, 64:1088–1092. [DOI] [PubMed] [Google Scholar]
- 19.Mulders MN, Serhan F, Goodson JL, Icenogle J, Johnson BW, Rota PA: Expansion of surveillance for vaccine-preventable diseases: building on the global polio laboratory network and the global measles and rubella laboratory network platforms. J Infect Dis 2017, 216:S324–S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization: Recommendations from an ad hoc Meeting of the WHO Measles and Rubella Laboratory Network (LabNet) on use of alternative diagnostic samples for measles and rubella surveillance. MMWR Morb Mortal Wkly Rep; 2008, 57:657–660. [PubMed] [Google Scholar]
- 21.Pascoe SJ, Langhaug LF, Mudzori J, Burke E, Hayes R, Cowan FM: Field evaluation of diagnostic accuracy of an oral fluid rapid test for HIV, tested at point-of-service sites in rural Zimbabwe. AIDS Patient Care STDS 2009, 23:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Public Health England: Measles notifications and confirmed cases by oral fluid testing 2013 to 2019. [https://www.gov.uk/government/publications/measles-confirmed-cases/measles-notifications-and-confirmed-cases-by-quarter-in-england-2013-to-2015]. [Google Scholar]
- 23.Ramsay Mary E, Jin L, White J, Litton P, Cohen B, Brown D: The elimination of indigenous measles transmission in England and Wales. J Infect Dis 2003, 187:S198–S207. [DOI] [PubMed] [Google Scholar]
- 24.Manikkavasagan G, Bukasa A, Brown KE, Cohen BJ, Ramsay ME: Oral fluid testing during 10 years of rubella elimination, England and Wales. Emerg Infect Dis 2010, 16:1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown DW, Ramsay ME, Richards AF, Miller E: Salivary diagnosis of measles: a study of notified cases in the United Kingdom, 1991–3. BMJ 1994, 308:1015–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown D, Miller E: Facing the measles epidemic. Practitioner 1994, 238:778–781. [PubMed] [Google Scholar]
- 27.Jin L, Vyse A, Brown DW: The role of RT-PCR assay of oral fluid for diagnosis and surveillance of measles, mumps and rubella. Bull World Health Organ 2002, 80:76–77. [PMC free article] [PubMed] [Google Scholar]
- 28.Pai NP, Vadnais C, Denkinger C, Engel, Pai: Point-of-caretesting for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Med 2012, 9:e1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osorio L, Garcia JA, Parra LG, Garcia V, Torres L, Degroote S, Ridde V: A scoping review on the field validation and implementation of rapid diagnostic tests for vector-borne and other infectious diseases of poverty in urban areas. Infect Dis Poverty 2018, 7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.••.Warrener L, Slibinskas R, Chua KB, Nigatu W, Brown KE, Sasnauskas K, Samuel D, Brown D: Apoint-of-caretest formeasles diagnosis: detection of measles-specific IgM antibodies and viral nucleic acid. Bull World Health Organ 2011, 89:675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show good accuracy of a prototype measles IgM RDT in a laboratory evaluation with serum and oral fluid specimens and sensitive recovery of viral RNA from RDTs performed with oral fluid samples. The article highlights the potential of the RDT for real time diagnosis and for improving the representativeness of virologic surveillance.
- 31.Shonhai A, Warrener L, Mangwanya D, Slibinskas R, Brown K, Brown D, Featherstone D, Samuel D: Investigation of a measles outbreak in Zimbabwe, 2010: potential of a point of care test to replace laboratory confirmation of suspected cases. Epidemiol Infect 2015, 143:3442–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peeling RW, Holmes KK, Mabey D, Ronald A: Rapid tests for sexually transmitted infections (STIs): the way forward. Sex Transm Infect 2006, 82(Suppl. 5):v1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warrener L, Andrews N, Koroma H, Alessandrini I, Haque M, Garcia CC, et al. Evaluation of a rapid diagnostic test for measles IgM detection; accuracy and the reliability of visual reading using sera from the Measles surveillance programme in Brazil, 2013 to 2015. PLoS, submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosack CS, Page AL, Klatser PR: A guide to aid the selection of diagnostic tests. Bull World Health Organ 2017, 95:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Speed and convenience aren’t everything with diagnostics. PLoS Med 2011, 8:e1001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asiimwe C, Kyabayinze DJ, Kyalisiima Z, Nabakooza J, Bajabaite M, Counihan H, Tibenderana JK: Early experiences on the feasibility, acceptability, and use of malaria rapid diagnostic tests at peripheral health centres in Uganda-insights into some barriers and facilitators. Implement Sci 2012, 7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuupiel D, Bawontuo V, Drain PK, Gwala N, Mashamba-Thompson TP: Supply chain management and accessibility to point-of-care testing in resource-limited settings: a systematic scoping review. BMC Health Serv Res 2019, 19:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.•.Pai NP, Wilkinson S, Deli-Houssein R, Vijh R, Vadnais C, Behlim, Steben M, Engel N, Wong T: Barriers to implementation of rapid and point-of-care tests for human immunodeficiency virus infection: findings from a systematic review (1996–2014). Point Care 2015, 14:81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors summarize numerous existing barriers to HIV RDT implementation at the patient, provider and health system levels, and also with the test devices themselves (e.g. performance, storage). The review highlights the need to strengthen integration of RDTs within local contexts, including testing protocols, training, quality control processes, and linkage to response for improved health outcomes before global scale-up of implementation.
- 39.Gay NJ, De Serres G, Farrington CP, Redd SB, Papania MJ: Assessment of the status of measles elimination from reported outbreaks: United States, 1997–1999. J Infect Dis 2004, 189(Suppl. 1):S36–42. [DOI] [PubMed] [Google Scholar]
- 40.Castillo-Solorzano C, Reef SE, Morice A, Andrus JK, Ruiz Matus C, Tambini G, Gross-Galiano S: Guidelines for the documentation and verification of measles, rubella, and congenital rubella syndrome elimination in the region of the Americas. J Infect Dis 2011, 204(Suppl. 2):S683–689. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization: Measles Nucleotide Surveillance database (MeaNS). [http://www.who-measles.org].
- 42.••.World Health Organization: The role of extended and whole genome sequencing for tracking transmission of measles and rubella viruses: report from the Global Measles and Rubella Laboratory Network meeting, 2017. Wkly Epidemiol Rec 2018, 93:55–59 [PubMed] [Google Scholar]; The authors report on the applications and limitations of WGS for measles and rubella surveillance. The article highlights that WGS will become a more important component of virologic surveillance in support of goals to eliminate measles and rubella in five of the six WHO Regions by 2020, based on the Global Vaccine Action Plan.
- 43.Thomas S, Hiebert J, Gubbay JB, Gournis E, Sharron J, Severini A, Jiaravuthisan M, Shane A, Jaeger V, Crowcroft NS et al. : Measles outbreak with unique virus genotyping, Ontario, Canada, 2015. Emerg Infect Dis 2017, 23:1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardy JL, Naus M, Amlani A, Chung W, Kim H, Tan M, Severini A, Krajden M, Puddicombe D, Sahni V et al. : Whole-genome sequencing of measles virus genotypes H1 and D8 during outbreaks of infection following the 2010 Olympic winter games reveals viral transmission routes. J Infect Dis 2015, 212:1574–1578. [DOI] [PubMed] [Google Scholar]
- 45.Penedos AR, Myers R, Hadef B, Aladin F, Brown KE: Assessment of the utility of whole genome sequencing of measles virus in the characterisation of outbreaks. PLoS One 2015, 10: e0143081. [DOI] [PMC free article] [PubMed] [Google Scholar]


