Abstract
Invasive stratified mucinous carcinoma (iSMC) has been suggested to represent an aggressive subtype of endocervical adenocarcinoma. We sought to investigate the outcomes of iSMC and determine which clinical and pathological parameters may influence the prognosis.
Slides from 52 cases of iSMC were collected and classified as follows: pure iSMC (>90% of the entire tumor) and iSMC mixed with other HPVA components (miSMC) (>10% but <90% of the entire tumor). Clinical and pathological parameters were evaluated and compared with overall survival (OS), recurrence free survival (RFS).
One third of patients with iSMC presented with lymph node metastases (LNM) and 25% developed local recurrences while 4 (7.7%) developed distant recurrences. 29 cases (55.8%) were pure iSMC while 23 cases (44.23%) were miSMC. OS was 74.7% in pure iSMC versus 85.2% in miSMC (p: 0.287). RFS was 56.5% in pure iSMC and 72.9% in miSMC (p: 0.185). At 5 years, OS in stage I was 88.9% vs. stage II-IV 30% (p: 0.004) while RFS in stage I was 73.9% vs. stage II-IV 38.1% (p: 0.02). OS was influenced by FIGO stage (p: 0.013), tumor size (p: 0.02), LNM (p: 0.015) and local recurrence (p:0.022), while RFS was influenced by FIGO stage (p: 0.031), tumor size (p: 0.001), local recurrence (p: 0.009), LNM (p: 0.008) and type of surgical treatment (p:0.044).
iSMC is an aggressive cervical tumor biologically different from other human Papillomavirus associated adenocarcinomas (HPVAs) due to propensity for LNM, local/distant recurrence. FIGO stage, tumor size, LNM and presence of local/pelvic recurrences are determinants of outcome in iSMCs.
Keywords: invasive stratified mucin-producing carcinoma, iSMC, recurrence, prognostic factors, outcome
Introduction
Stratified mucin-producing intraepithelial lesion (SMILE), initially described in 2000 by Park et al.1, is a premalignant lesion of the uterine cervix, included in the 2014 World Health Organization (WHO) Classification of Tumors of Female Reproductive Organs as a variant pattern of endocervical adenocarcinoma in situ (AIS), thought to arise from human papillomavirus (HPV) infected reserve cells of the transformation zone.1,2,3 SMILE is characterized by atypical cells containing intracytoplasmic mucin stratified throughout the entire epithelial thickness without classic gland formation, which imparts a hybrid morphology that can mimic high-grade squamous intraepithelial lesion (HSIL). It has been reported in association with conventional HSIL, AIS associated with HPV infection, as well as invasive carcinomas such as adenocarcinoma, adenosquamous carcinoma or squamous cell carcinoma.1,2,4-7
Recently, invasive stratified mucinous carcinoma (iSMC) was described by Lastra et al.8 as a morphologic variant of HPV-related endocervical adenocarcinoma with morphologic similarity to SMILE and proposed to be its invasive counterpart. In that series, there were 8 cases of iSMC; 7 pure and 1 associated with usual type endocervical adenocarcinoma.8 Of 4 iSMC with follow up information up to 36 months, 3 (75%) developed distant tumor recurrence, 2 in the lung and 1 with widespread abdominopelvic disease.8 Onishi et al. subsequently described 9 invasive carcinomas with associated SMILE, of which 2 showed pure iSMC and 1 showed iSMC associated with invasive squamous cell carcinoma, and all 3 of these patients were alive without evidence of disease after a median of 59.3 months.4 More recently, Horn et al. described 5 additional cases of iSMC, all of which developed pelvic recurrences during a mean time of 8 months.9 Two patients also experienced distant metastases (one to lung and one to inguinal lymph nodes, liver and skin) and 4 of the 5 patients died of disease, while one patient is alive with disease.9 The authors also summarize all reported cases in the literature and conclude that clinically, iSMCs are associated with large tumor size, pelvic lymph node involvement at the time of diagnosis and that this may represent an aggressive tumor with early recurrent disease and substantial risk of distant metastatic disease, especially to the lungs.9 The poorer prognosis of iSMC compared to usual type adenocarcinoma has also been demonstrated by Hodgson et. al.10 In that study, adverse events most commonly occurred in patients with iSMC morphology, with 4 of 8 (50%) patients developing recurrence (2 local, 2 distant), and 2 patients (25%) dead of disease. Also in the Hodgson study, iSMCs had significantly worse disease free and disease specific survival when compared to other HPV associated types by univariate analysis (p:0.008 and 0.016, respectively). 10 However, none of these previous publications included a large number of cases of iSMCs.
We sought to investigate the outcomes in iSMC and determine which clinical and pathological parameters may influence the prognosis in a large, multi-institutional international series of cases.
Materials and methods
Slides from 52 cases of iSMCs were collected from 10 institutions (USA: Memorial Sloan Kettering Cancer Center [MSKCC], New York, and Massachusetts General Hospital, Boston; Romania: University of Medicine, Pharmacy, Sciences and Technology of Targu Mures and Regional Institute of Oncology, Iasi; Japan: Jikei University School of Medicine, Tokyo; Mexico: Hospital de Oncología Mexico City, Mexico City; Israel: Sheba Medical Center, Tel-Hashomer, Ramat Gan; Italy: Ospedale Sacro Cuore Don Calabria, Negrar; Canada: Sunnybrook Health Sciences Center, Toronto; Germany University Hospital Leipzig, Leipzig). The initial set of cases were reviewed by three of the authors (SS, RAS, KJP).The cases from Toronto and Leipzig were initially reviewed by the submitting authors and subsequently reviewed and confirmed by RAS and/or KJP. In all cases, hematoxylin and eosin slides containing tumor (average of 12 slides per case) were examined at a multiheaded microscope. A consensus diagnosis was reached in every case, with at least 2, and as many as 4, study pathologists reviewing slides. The 52 study cases were classified according to the International Endocervical Adenocarcinoma Criteria and Classification (IECC)11 a new classification proposal for invasive endocervical adenocarcinomas (ECAs) which recognizes iSMC as a variant of HPV-related mucinous ECA. Invasive stratified mucinous carcinoma was diagnosed in the presence of a stromal-invasive carcinoma composed of nests filled with stratified tumor cells containing intracytoplasmic mucin, as seen in the in-situ counterpart (SMILE)4. For the purposes of this study, pure iSMC was classified separately from iSMC mixed with other HPV-associated components (miSMC) such as usual type adenocarcinoma (UEA), mucinous (MUC) not otherwise specified (NOS), adenosquamous carcinoma (ASC) and neuroendocrine carcinoma (NEC). Tumors were classified as mixed if the iSMC portion made up >10% but <90% of the entire tumor. Cases where iSMC represented less than 10% of the entire tumor were excluded. The following parameters were evaluated: age, International Federation of Gynecology and Obstetrics (FIGO) stage, treatment, HPV status, tumor size, histological grade, lympho-vascular invasion (LVI), Silva pattern of invasion12, lymph node metastases (LNM), local/pelvic recurrence, overall survival (OS) and recurrence free survival (RFS). There is no universally accepted grading system for ECAs, but in a recent survey conducted by the International Society of Gynecological Pathologists, the majority of responders reported using the same grading criteria as for FIGO grading of endometrial carcinoma. Microscopic grading was therefore performed according to the FIGO grading system used for endometrioid endometrial carcinomas (grade 1: ≤5% solid growth; grade 2: 6-50% solid growth; grade 3: >50% solid growth).
HPV testing was done using in situ hybridization for all except 7 cases (Germany University Hospital Leipzig) in which polymerase chain reaction (PCR) was performed. Both techniques were described in detail in previous papers9, 11.
Data were tabulated using Microsoft Excel (Microsoft) software and analyzed using SPSS for Microsoft Windows, version 20.0 (Chicago, IL, USA). Continuous and qualitative variables were, respectively, described by mean and percent. Overall survival was defined as the time from surgery until death by any cause. Recurrence free survival was defined as the time from randomization to the first of either local or regional recurrence, or death. Statistical analysis was performed using cross-tabulation test for comparison of pure iSMC to miSMC; group t-student test to compare patient age median and range; Kaplan-Meier for survival curve estimates; and the log rank Mantel Cox test to compare groups. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using the Cox proportional hazards regression model and p<0.05 was considered statistically significant.
Institutional approval for this study was obtained from each of the participating centers.
Results
The mean follow-up period was 36.5 months (1-108 months) with patient age ranging from 22-78 years (mean 40 years). In most cases (80%) iSMC occurred in patients <50 years old. Most tumors were stage FIGO I (70%), with 5 FIGO II (9.6%), 6 FIGO III (11.5%) and 1 FIGO IV (1.9%). Staging information was unavailable in 4 cases.
There were 29 (55.8%) pure iSMC and 23 (44.2%) miSMC: 13 with usual type adenocarcinoma, 6 with adenosquamous carcinoma, 3 with mucinous adenocarcinoma NOS and 1 with neuroendocrine carcinoma (Figures 1,2,3).
Figure 1:
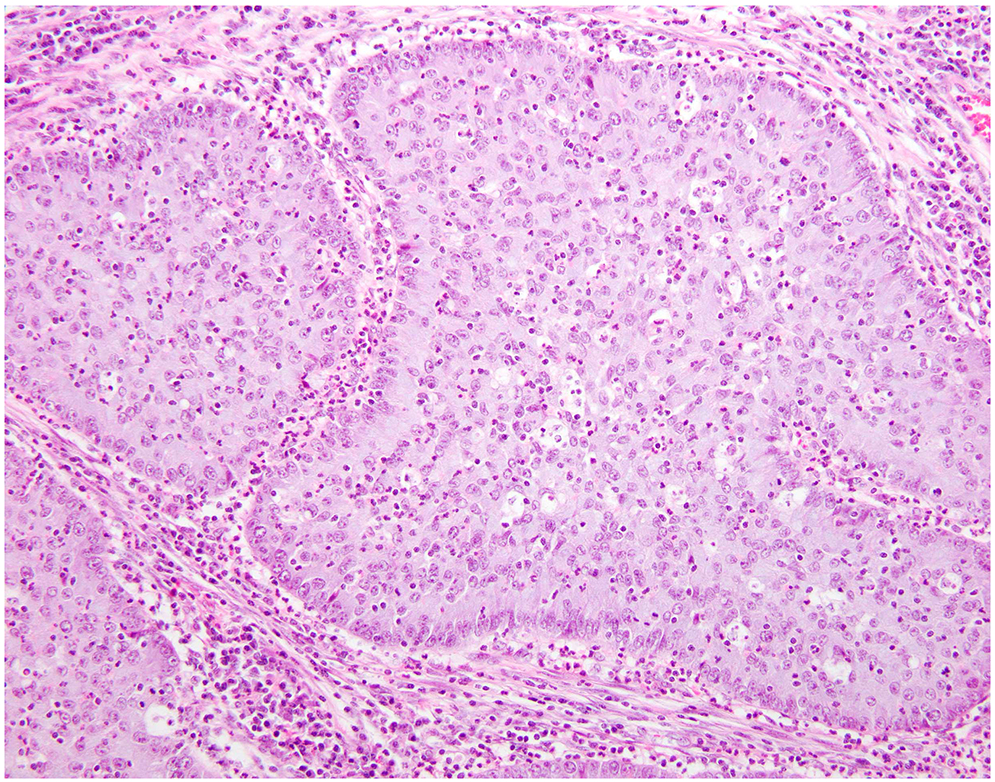
Pure iSMC represented by nests filled with stratified tumor cells containing intracytoplasmic mucin invading the cervical stroma
Figure 2:
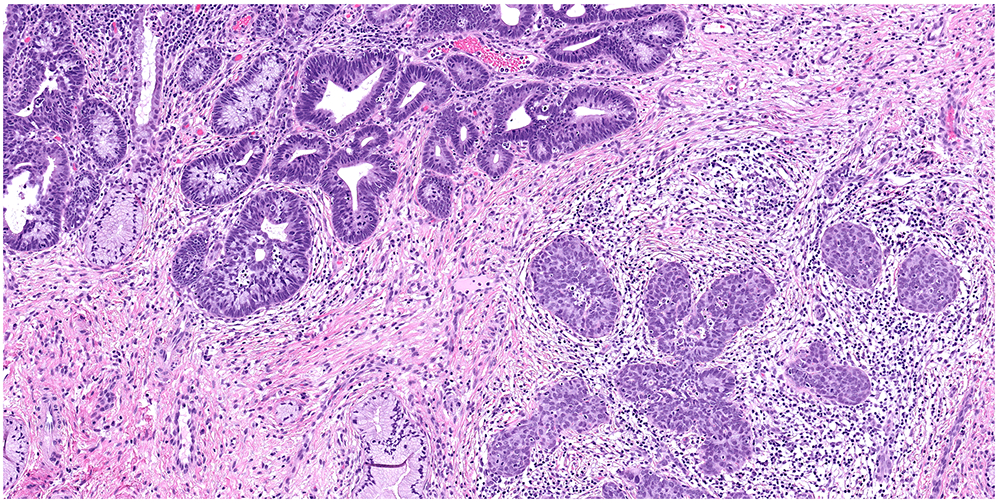
miSMC with usual type adenocarcinoma
Figure 3:
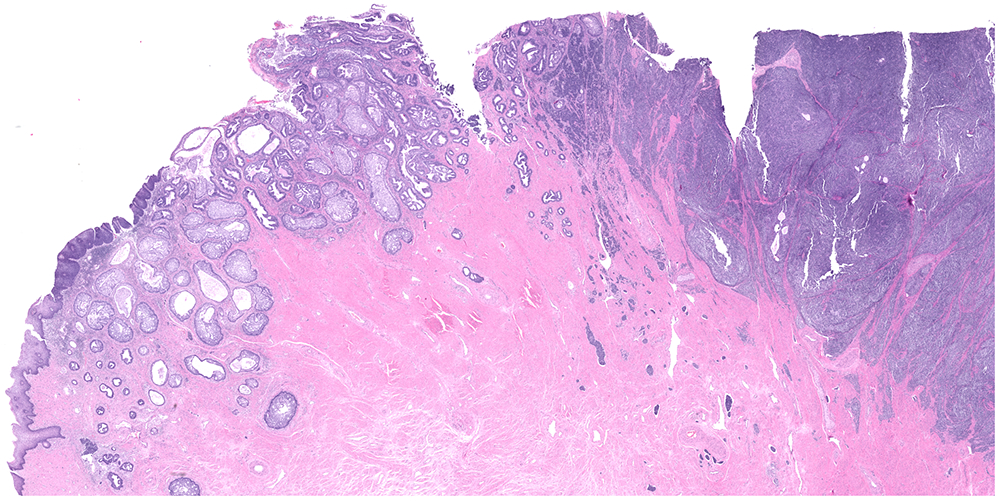
miSMC with neuroendocrine carcinoma
Regarding HPV status, 44 out of 45 cases tested by in situ hybridization and 6 out of 7 cases tested by PCR were positive for HPV. In our study, most prevalent high-risk HPV subtype was 18.
80% (pure iSMC and miSMC) were grade 3 (G3), 42.3% had LVI and 57.7% had concurrent precursor lesions (such as HSIL, AIS and/or SMILE). According to the Silva pattern of invasion, most cases (92%) were pattern C (both iSMC and iSMCmixed) with only 2 cases each for pattern A (iSMC) and B (1 iSMC and 1 miSMC).
One third of all patients presented with LNM and 25% developed local recurrences, while 4 (7.6%) developed distant recurrences and 7 (13.4%) died of disease.
The cross tabulation test comparing clinico-pathologic parameters in pure versus mixed tumors showed no statistically significant differences in age (p=0.268), FIGO stage (p=1), tumor size (p=0.698), adjuvant treatment (p=1), HPV status (p=1) FIGO histological grade (p=0.08), Silva pattern of invasion (p=0.310), LVI (p=0.08), LNM, (p=0.298) local recurrence (p=0.324) or outcome (p=0.437) (Table 1). We did find a statistical difference, however, in surgical treatment (radical resection with or without lymph node dissection) between pure versus mixed tumors (p=0.015). Lymph node dissection was performed less frequently for miSMC tumors (39.1%, 9/23 cases,) than for pure iSMC tumors (77.3%, 17/22 cases).
Table 1.
Analysis of clinic-pathological parameters and outcome in pure versus mixed SMCs, cross tabulation and t student test; NA: no data; DOD: died of disease; n: number of cases; LN: lymph node
| Pure SMC, n, % |
miSMC, n, % | p, OR, 95%CI | |
|---|---|---|---|
| Cases | 29 (55.76%) | 23 (44.23%) | |
| Age, median | 41.82 | 38.39 | p=0.316 (t-student test) |
| range | 22-78 | 26-60 | |
| Age <50 years | 22 | 21 | p=0.268; OR=3.341; (0.621-17.95) |
| >=50 years | 7 | 2 | |
| FIGO stage I | 20 | 16 | p=1; OR=1.042 (0.268-4.047) |
| II-IV | 6 | 5 | |
| Tumor size >=4cm | 5 | 3 | p=0.698; OR=1.765 (0.364-8.549) |
| <4cm | 17 | 18 | |
| NA | 7 | 2 | |
| FIGO histological grade G3 | 26 | 16 | p=0.08; OR=3.792; (0.855-16.813) |
| G1, G2 | 3 | 7 | |
| LVI Yes | 8 | 14 | p=0.08 OR=0.321; (0.09-1.059) |
| No | 16 | 9 | |
| NA | 5 | none | |
| Silva pattern C | 28 | 20 | p=0.310 OR=4.2; (0.406-43.399) |
| A, B | 1 | 3 | |
| LNM Yes | 5 | 7 | P=0.298 OR=0.298 (0.104-1.763) |
| No | 15 | 9 | |
| NA | 9 | 7 | |
| Local/pelvic recurrences Yes | 9 | 4 | p=0.324 OR=2.25 (0.566-8.93) |
| No | 15 | 15 | |
| NA | 5 | 4 | |
| Surgical treatment Radical with LN dissection | 17 | 9 | p=0.015 OR=5.289 (1.438-19.454) |
| Radical without LN | 5 | 14 | |
| NA | 7 | none | |
| Adjuvant treatment Yes | 11 | 7 | p=1 OR=0.982 (0.226-4.252) |
| No | 8 | 5 | |
| NA | 10 | 11 | |
| HPV status (HPV-testing) Positive | 15 | 7 | p=1 OR=0.41 (0.017-9.741) |
| Negative | 2 | 0 | |
| NA | 12 | 16 | |
| Outcome DOD | 5 | 2 | p=0.437 OR=2.237 (0.382-13.080) |
| Alive | 19 | 17 | |
| NA | 5 | 4 |
Kaplan Meier survival analysis revealed 5-year OS and RFS of 78.8% and 63.6%, respectively. Comparing pure to mixed iSMC, there was no difference in OS (74.7% vs. 85.2%, p=0.287) or RFS (56.5% vs. 72.9%, p=0.185). Five-year OS was 88.9% for stage I vs. 30% for stage II-IV (p=0.004) (Figure 4), while 5-year RFS was 73.9% for stage I vs. 38.1% for stage II-IV (p=0.02) (Figure 5). Using Log rank Mantel Cox analysis, OS was influenced by FIGO stage (p=0.013) (95% CI=1.490-30.500) (HR=6.76), tumor size (p=0.02) (95% CI=3.583-287.821) (HR= 32.11), LNM (p=0.015) (95% CI=1.694-131.912) (HR= 14.95) and local recurrence (p=0.022) (95% CI= 1.436-105.770) (HR= 12.32), while RFS was influenced by FIGO stage (p=0.031) (95% CI=1.12-10.746) (HR=3.46), tumor size (p= 0.001) (95% CI=2.619-41.794) (HR=10.26), local recurrence (p=0.009) (95% CI=2.04-160.361) (HR=18.089), LNM (p=0.008) (95%CI=1.569-19.424) (HR=5.52) and type of surgical treatment, without versus with lymph node dissection (p=0.044) (95% CI=1.041-21.974) (HR=4.78) (Table 2).
Figure 4:
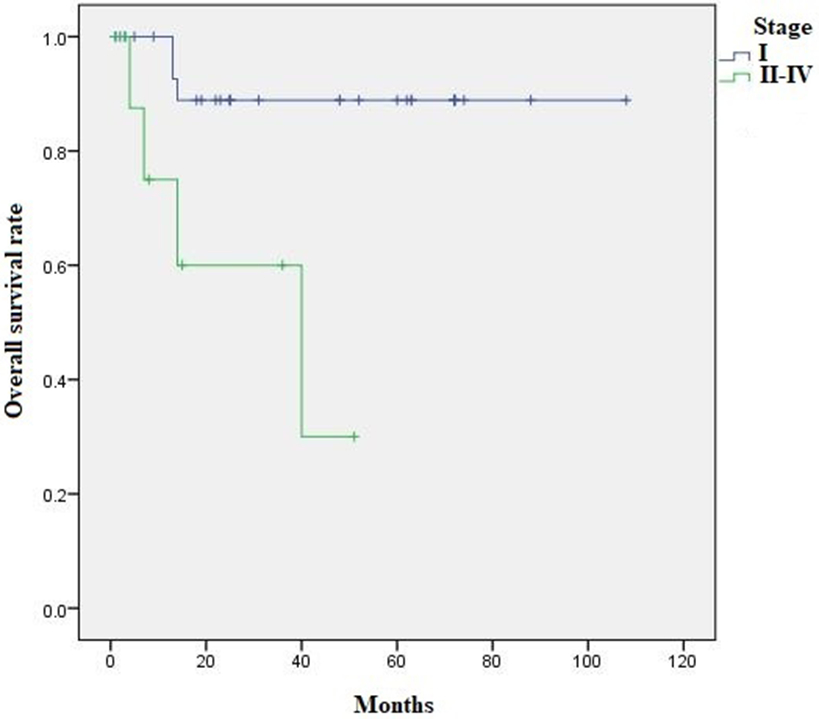
Kaplan Meier survival analysis: 5-year OS for stage I was 88.9% vs. 30% for stage II-IV (p=0.004)
Figure 5:
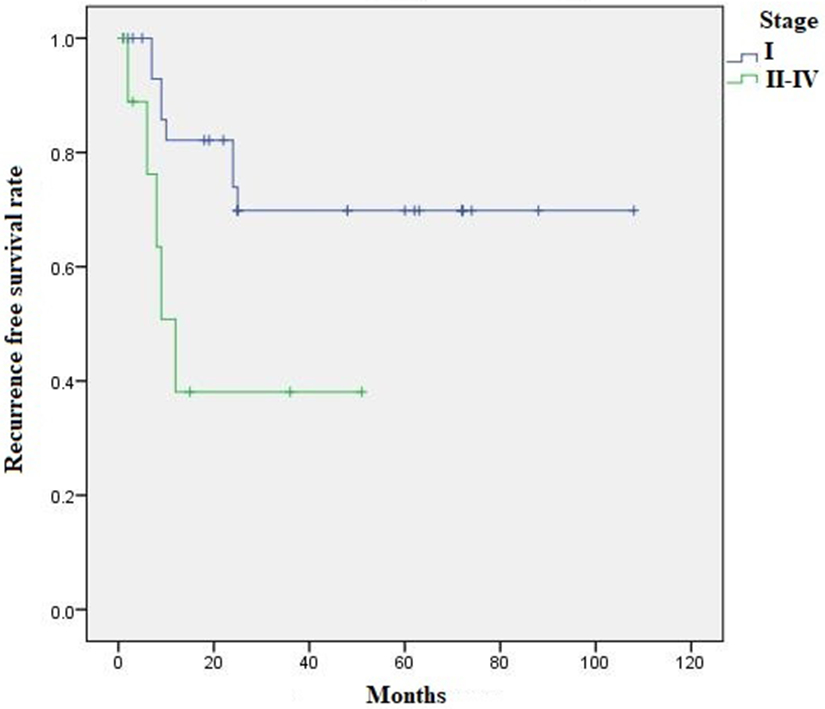
Kaplan Meier survival analysis: 5-year RFS for stage I was 73.9% vs. 38.1% for stage II-IV (p=0.02)
Table 2.
Determinants of outcome: Overall Survival (OS) and Recurrence Free Survival (RFS) in 52 iSMC cases −Cox regression analyses
| OS | RFS | |||||
|---|---|---|---|---|---|---|
| HR | CI 95% | p | HR | CI 95% | p | |
| Tumor type (pure iSMC vs miSMC) | 2.444 | 0.471-12.675 | 0.287 | 2.158 | 0.663-7.020 | 0.201 |
| Age 50<;>50 years | 1.834 | 0.347-9.702 | 0.476 | 1.379 | 0.160-3.273 | 0.676 |
| FIGO stage (I vs II-IV) | 6.765 | 1.490-30.500 | 0.013 | 3.469 | 1.120-10.746 | 0.031 |
| Surgical treatment (without vs with lymph node dissection) | 57.526 | 0.077-42858,438 | 0.23 | 4.782 | 1.041-21.974 | 0.044 |
| Adjuvant treatment (yes vs no) | 3.382 | 0.378-28.493 | 0.281 | 3.324 | 0.700-15.792 | 0.131 |
| Tumor size (<4cm vs >=4cm) | 32.114 | 3.583-287.821 | 0.02 | 10.261 | 2.619-41.794 | 0.001 |
| HPV status (HPV testing) (pos vs neg) | 3.69 | 0.055-1.347 | 0.111 | 2.197 | 0.112-1.856 | 0.273 |
| Tumor grade (1,2 vs 3) | 2.032 | 0.244-16.923 | 0.512 | 3.841 | 0.495-29.831 | 0.198 |
| Silva pattern (A, B vs C) | 22.565 | 0.000-8545346.7 | 0.634 | 1.187 | 0.109-6.494 | 0.869 |
| Lympho-vascular invasion (yes vs no) | 3.289 | 0.633-17.093 | 0.157 | 2.998 | 0.893-10.071 | 0.076 |
| Lymph node metastasis (yes vs no) | 14.95 | 1.694-131.912 | 0.015 | 5.52 | 1.569-19.424 | 0.008 |
| Local/pelvic recurrences (yes vs no) | 12.323 | 1.436-105.770 | 0.022 | 18.089 | 2.040-160.361 | 0.009 |
Discussion
iSMC is a morphologic variant of HPV-related endocervical adenocarcinoma comprised of tumor nests containing cells with round/ovoid nuclei and variable amounts of intracytoplasmic mucin vacuoles stratified throughout its thickness, often with nuclear palisading along the periphery of the nests.8 It is recognized as a mucinous subtype of HPV-associated adenocarcinoma by the International Endocervical Adenocarcinoma Criteria and Classification (IECC),11 which classifies endocervical adenocarcinomas into HPV-associated (HPVA) and HPV-independent (HPVI) based on morphology alone. In the IECC study, all tested iSMCs were positive for high-risk HPV by mRNA in-situ hybridization (7/7 cases), and diffusely p16 positive by immunohistochemistry (8/8 cases).11
iSMC can present architectural diversity and various cytologic features, posing diagnostic challenges since it can simulate other tumors13. Tumors can be composed almost entirely of the stratified mucinous component (pure iSMC) or be mixed with other well established components (miSMC), as in the present series in which of the 52 cases of iSMC, 13 were associate with usual type adenocarcinoma, 6 with adenosquamous carcinoma, 3 with mucinous adenocarcinoma NOS and 1 with neuroendocrine carcinoma (Figure 3). Mixed cases have also been reported by Onishi, Lastra and Horn4, 8, 9.
iSMCs, in general, have a slightly different immunohistochemical profile compared to usual type HPVA endocervical adenocarcinoma; p40 and p63 are often expressed in the nuclei of cells along the periphery of the nests, which often impart a distinct peripheral palisade appearance; in addition, a significant subset of cases show mutation-type p53 staining and less frequent PAX8 labeling14,15. Onishi et al.4 described cases of iSMC that were all positive for high-risk HPV by in situ hybridization, most of which were positive for HPV 18 (5 cases) or HPV 16 (1 case) by polymerase chain reaction when tested. p16 and CAM 5.2 were positive, while p63, and CK 5/6 expression was only focally detected.
The mean age of patients in this series was 41.8 years, similar to that reported by Horn et.al (47.1 years), as well as to patients with invasive squamous cell carcinoma and UEA9. Typically, iSMC presents with larger size at diagnosis comparing to usual type HPVA and can show a polypoid and exophytic appearance9; 15% of the present cases were larger than 4 cm in diameter at the time of diagnosis (Table 3).
Table 3.
Comparison of clinico-pathologic features in iSMC versus usual type HPVA
| iSMC (52 cases) | Usual type HPVA (262 cases)9 |
|
|---|---|---|
| Mean age (years) | 41.8 | 42 |
| Tumor size (median) (mm) | 21 | 23 |
| LVI (% of cases) | 42.3 | 49.8 |
| LNM (% of cases) | 33 | 12.2 |
| Local recurrence (% of cases) | 25 | 8.8 |
| Distant recurrence (% of cases) | 7.6 | 5.4 |
| FIGO stage II or higher (% of cases) | 30 | 14 |
| Silva C pattern (%of cases) | 92 | 76 |
| Died of disease (% of cases) | 13.4 | 7.2 |
A previous study from our group demonstrated for the first time that there are no statistically significant differences in OS and DFS when comparing pure iSMC and miSMCs on a small series of cases15. Here, we extended the study by including a larger series comprising 52 cases. We report no statistically significant differences between the groups, demonstrating that having a component of iSMC is clinically significant, regardless of its extent. That being said, pure iSMC cases were more likely than miSMC cases to have large tumors, present at high stage, have lymphovascular invasion and lymph node metastases with subsequent pelvic recurrences.
It is well established that FIGO stage, presence of LVI, pelvic/para-aortic lymph node metastases, as well as distant metastases have impact on prognosis in cervical adenocarcinoma16. In the present study, 42.3% of cases (both pure and mixed iSMC) had LVI, 33.3% had LNM and 25% developed local recurrences, while 4 (7.6%) developed distant recurrences and 7 (13.4%) died of disease. This is in concordance with previously published results by Lastra8, Horn9 and Hodgson10 where iSMCs were also frequently associated with LNM at time of diagnosis and developed local and distant recurrences. In contrast to iSMC, a study from our group showed that usual type ECAs presented with LVI in 49.8% of cases but LNM was found in only 12.2%, while local recurrence occurred in only 8.8% and distant recurrence in 5.4% 17. Of interest, mucinous NOS ECAs also presented with local recurrences in 33.3% of cases in that study but no distant recurrences were noted17.
In the present study, 30% of iSMCs were FIGO stage II or higher, which contrasts with UEA in which 14% were similarly staged17. Also, iSMCs more frequently exhibited diffusely destructive stromal invasion (Silva Pattern C) compared to UEA (92% versus 76%)18. This is significant since Silva Pattern C is frequently associated with disease recurrence (22%) and death from disease (8%),12 all of which suggests that iSMC may be an aggressive variant of HPV-associated endocervical adenocarcinoma. The outcome of iSMC has been previously studied by other groups, but the cohort sizes were relatively limited8-10.
We previously demonstrated that by using multivariate survival analysis, significant differences (p=0.04) in progression-free survival were found when comparing usual type to mucinous type HPVA adenocarcinomas (including iSMC)17. In the present study, by analyzing 52 cases of iSMC using Kaplan Meier analysis we have demonstrated that OS and RFS at 5 years is statistically different in advanced stages versus FIGO stage I (p=0.004 and p=0.02 respectively). In addition, using Log rank Mantel Cox analysis, we demonstrated that OS was influenced by FIGO stage (p= 0.013), tumor size (p=0.02), LNM (p=0.015) and local recurrence (p=0.022), while RFS was influenced by FIGO stage (p=0.031), tumor size (p= 0.001), local recurrence (p=0.009), LNM (p=0.008) and type of surgical treatment (without versus with lymph node dissection) (p=0.044). These data suggest that iSMC should be treated with radical surgery and lymph node dissection regardless of the size of the tumor and percentage of the tumor represented by an iSMC component.
Conclusions
Invasive stratified mucinous carcinoma of the cervix is an aggressive tumor that is morphologically, immunohistochemically and potentially biologically distinct from other HPVA adenocarcinomas due to its propensity for advanced FIGO stage, LVI, LNM, destructive stromal invasion (Silva Pattern C) and local/distant recurrence, factors which determine outcomes in most cervical carcinomas. In addition, it appears that the type of surgical treatment is an important determinant of outcome in iSMC. Whether these tumors are inherently and biologically different from other HPVA adenocarcinomas due to underlying molecular mechanisms is yet to be determined, though there are some initial data to suggest this may be the case (higher rate of p53 abnormality). Regardless, iSMC should be recognized and diagnosed (pure or mixed) as a distinct entity so that further analysis can be conducted going forward.
Acknowledgments
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 (Dr. Soslow, Dr. Park).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Park JJ, Sun D, Quade BJ, et al. Stratified mucin-producing intraepithelial lesions of the cervix: adenosquamous or columnar cell neoplasia? Am J Surg Pathol. 2000; 24:1414–1419. [DOI] [PubMed] [Google Scholar]
- 2.Boyle DP, McCluggage WG. Stratified mucin-producing intraepithelial lesion: report of a case series with associated pathological findings. Histopathology. 2015; 66:658–663. [DOI] [PubMed] [Google Scholar]
- 3.Stoler M, Bergeron C, Colgan TJ, et al. Tumours of the Uterine Cervix In: Kurman RJ, Carcangiu ML, Herrington CS, et al. , eds. WHO Classification of Tumours of Female Reproductive Organs, 4th ed. Lyon, France: IARC Press, 2014:184. [Google Scholar]
- 4.Onishi J, Sato Y, Sawaguchi A, et al. Stratified mucin-producing intraepithelial lesion with invasive carcinoma: 12 cases with immunohistochemical and ultrastructural findings. Hum Pathol. 2016; 55:174–81. [DOI] [PubMed] [Google Scholar]
- 5.Schwock J, Ko HM, Dubé V, et al. Stratified Mucin-Producing Intraepithelial Lesion of the Cervix: Subtle Features Not to Be Missed. Acta Cytol. 2016; 60(3):225–31 [DOI] [PubMed] [Google Scholar]
- 6.Park KJ, Soslow RA. Current concepts in cervical pathology. Arch Pathol Lab Med. 2009; 133:729–738 [DOI] [PubMed] [Google Scholar]
- 7.Backhouse A, Stewart CJ, Koay MH, et al. Cytologic findings in stratified mucin-producing intraepithelial lesion of the cervix: A report of 34 cases. Diagn Cytopathol. 2016; 44(1):20–5. [DOI] [PubMed] [Google Scholar]
- 8.Lastra RR, Park KJ, Schoolmeester JK. Invasive stratified mucin producing carcinoma and stratified mucin-producing intraepithelial lesion (SMILE): 15 cases presenting a spectrum of cervical neoplasia with description of a distinctive variant of invasive adenocarcinoma. Am J Surg Pathol. 2016; 40:262–269. [DOI] [PubMed] [Google Scholar]
- 9.Horn LC, Handzel R., Borte G., et al. : Invasive stratified mucin-producing carcinoma (i-SMILE) of the uterine cervix: report of a case series and review of the literature indicating poor prognostic subtype of cervical adenocarcinoma. J Cancer Res Clin Oncol. 2019, August 5. doi: 10.1007/s00432-019-02991-3. [DOI] [PubMed] [Google Scholar]
- 10.Hodgson A, Olkhov-Mitsel E, Howitt BE, et al. : International endocervical adenocarcinoma criteria and classification (IECC): correlation with adverse clinicopathological features and patient outcome. J Clin Pathol. 2019, 72: 347–353 [DOI] [PubMed] [Google Scholar]
- 11.Stolnicu S, Barsan I, Hoang L, et al. International endocervical adenocarcinoma criteria and classification (IECC): a new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am J Surg Pathol. 2018; 42:214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz De Vivar A, Roma AA, Park KJ, et al. Invasive endocervical adenocarcinoma: proposal for a new pattern-based classification system with significant clinical implications: a multi-institutional study. Int J Gynecol Pathol. 2013; 32(6):592–601. [DOI] [PubMed] [Google Scholar]
- 13.Park KJ, Barsan I, Fix D et al. : Invasive Stratified Mucin-producing Carcinoma (SMPC)– A Study in Morphology, Immunohistochemistry and Human Papillomavirus Status. Virchows Arch. 2017; 471(suppl 1):1–352 [Google Scholar]
- 14.Stolnicu S, Barsan I, Hoang L, et al. Diagnostic algorithmic proposal based on comprehensive immunohistochemical evaluation of 297 invasive endocervical adenocarcinoma. Am J Surg Pathol. 2018; 42(8): 989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stolnicu S, Hoang L, Hanko-Bauer O, et al. Cervical adenosquamous carcinoma: detailed analysis of morphology, immunohistochemical profile, and clinical outcomes in 59 cases. Mod Pathol. 2019; 32(2):269–279 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Baalbergen A, Ewing-Graham PC, Hop WC, et al. Prognostic factors in adenocarcinoma of the uterine cervix. Gynecol Oncol. 2004; 92(1):262–267 [DOI] [PubMed] [Google Scholar]
- 17.Stolnicu S, Hoang L, Chiu D, et al. Clinical Outcomes of HPV-associated and Unassociated Endocervical Adenocarcinomas Categorized by the International Endocervical Adenocarcinoma Criteria and Classification (IECC). Am J Surg Pathol. 2019; April;43(4):466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stolnicu S, Barsan I, Hoang L, et al. Stromal invasion pattern identifies patients at lowest risk of lymph node metastasis in HPV-associated endocervical adenocarcinomas, but is irrelevant in adenocarcinomas unassociated with HPV. Gynecol Oncol. 2018;150(1):56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


