Abstract
This review discusses evidence suggesting that group III/IV muscle afferents affect locomotor performance by influencing neuromuscular fatigue. These neurons regulate the hemodynamic and ventilatory response to exercise and thus assure appropriate locomotor muscle O2-delivery, which optimizes peripheral fatigue development and facilitates endurance performance. In terms of central fatigue, group III/IV muscle afferents inhibit motoneuronal output and thereby limit exercise performance.
Keywords: Fatigue, cardiovascular, ventilation, corticospinal, central nervous system
SUMMARY for table of contents:
Group III/IV muscle afferents influence whole body endurance capacity by determining the cardiovascular, ventilatory, and neuromuscular fatigue response to exercise.
SUMMARY
Group III/IV afferent feedback from locomotor muscle influences whole body exercise performance through their role in the development of neuromuscular fatigue. These sensory neurons assure appropriate locomotor muscle O2 delivery and ATP cost of muscle contraction and therefore attenuate peripheral fatigue development and facilitate locomotor exercise performance. However, group III/IV muscle afferents also cause central fatigue and this CNS-mediated decline in locomotor muscle activation limits locomotor exercise performance. Future studies should focus on the influence of group III/IV muscle afferents on skeletal muscle energetics and on the circulatory, ventilatory, and neuromuscular fatigue response to long-duration whole body exercise.
INTRODUCTION
This review summarizes our current understanding of the significance of group III and IV afferent feedback from locomotor muscle in determining whole body endurance performance through their role in the development of neuromuscular fatigue during exercise. In keeping with the journal’s guidelines, we mainly focus on our own original research papers as the base for the integrative concept discussed in this review (Figure 1). The limited number of references prevented us from including additional studies supporting, or challenging, the ideas discussed in this writing. For additional, and perhaps complementary, reading on the neuromuscular fatigue response to whole body / locomotor exercise, we refer the reader to two recently published reviews (1, 2).
Figure 1:
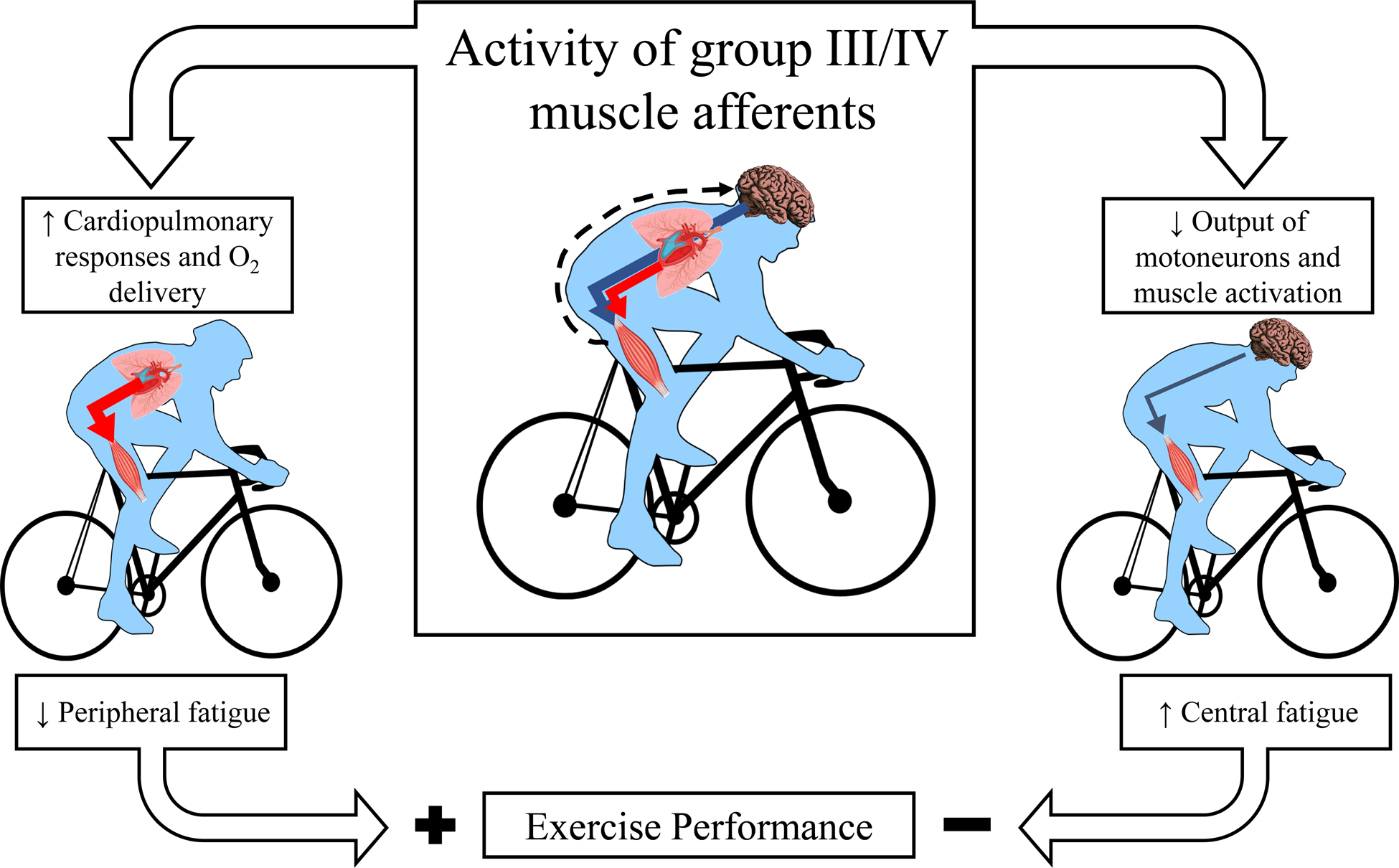
Consequences of group III/IV muscle afferent feedback for the development of neuromuscular fatigue and whole body exercise performance. Muscle contraction-induced increases in group III/IV afferent feedback raise circulation and pulmonary ventilation during exercise and thereby assure adequate oxygen delivery to the working locomotor muscle. This attenuates the development of peripheral fatigue and facilitates exercise performance (left side). On the other side (right side), group III/IV muscle afferent feedback restricts spinal motoneuron output and voluntary muscle activation, i.e. promotes central fatigue and impairs exercise performance. Black dashed arrow represents the central projection of group III/IV muscle afferents during exercise. The red and blue arrows represent locomotor muscle oxygen delivery and descending neural input to the locomotor muscle, respectively.
Neuromuscular fatigue, determined by a peripheral and a central component, is defined as the exercise-induced decrease in an individual’s torque- and power-generating capacity (3, 4). ‘Peripheral fatigue’, sometimes referred to as contractile fatigue, encompasses biochemical changes within the contracting muscle leading to an attenuated torque / power response to neural excitation. While there are different methods to quantify exercise-induced peripheral fatigue in humans (5), the studies introduced in this review used a supramaximal electrical or magnetic peripheral motor nerve stimulation to evoke a twitch torque generated by the target muscle before and shortly after exercise. The pre- to post-exercise difference in twitch torque, which is highly correlated with exercise-induced intramuscular metabolic perturbations (6), was used to quantify peripheral fatigue. Regardless, the impact of group III/IV muscle afferents on the development of peripheral locomotor muscle fatigue is mediated through their role in optimizing locomotor muscle O2 delivery by regulating the circulatory and the ventilatory response to exercise (7).
‘Central fatigue’, emanating from the central nervous system (CNS) and structurally including the brain and the spinal cord, refers to the decrease in torque / power secondary to a decrease in voluntary muscle activation. Pre- to post-exercise reductions in voluntary muscle activation, which is traditionally quantified via the twitch interpolation technique (4), are generally used to reflect fatigue of the CNS. It is, however, important to recognize that this assessment is based on an isometric, single-joint contraction performed before and after the actual locomotor task and that it may therefore not be a good reflection of central fatigue present during whole body exercise. Despite considerable shortcomings (8), locomotor muscle electromyogram (EMG), when normalized for M waves (i.e. electrically-evoked compound action potentials which reflect peripheral properties of the neuromuscular system), offers an acceptable surrogate for motoneuronal output and muscle activation during locomotor exercise. Important for the context of this review, group III/IV muscle afferent feedback can facilitate CNS fatigue both by restricting descending voluntary motor drive at or ‘upstream’ from the premotor cortex and by compromising the excitability of the central motor pathway. Appropriate responsiveness of this descending motor pathway, which includes the motor cortex and spinal motoneurones and is usually referred to as the corticospinal pathway, is important as it relays motor signals from higher brain areas to the working locomotor muscle (4).
The implications of group III/IV muscle afferent feedback are considerably altered with healthy aging (9) and by both cardiovascular (10–12) and pulmonary (13) disease. Furthermore, afferent feedback from respiratory muscles and other organs can also limit whole body exercise performance (14). Discussions on these issues can be found elsewhere (9–14), this review is limited to studies focusing on the effect of afferent feedback from locomotor muscle in healthy young individuals.
NEURAL FEEDBACK FROM EXERCISING LIMB MUSCLE: GROUP III AND IV MUSCLE AFFERENTS
With the onset of exercise, contraction-induced mechanical and metabolic stimuli begin to activate molecular receptors located on the terminal end of both thinly myelinated (group III, mainly mechanosensitive) and unmyelinated (group IV, mainly metabosensitive) nerve fibers with their receptive fields within skeletal muscle. The exercise-induced activation of these receptors increases the spontaneous discharge of the thin fiber muscle afferents (15, 16) that project, via the dorsal horn of the spinal cord (17), to various spinal and supraspinal sites within the CNS (18, 19). Based on functional differences, metabosensitive group III/IV muscle afferents can be separated into two categories (20–23). One subtype, the so-called metabo- or ergoreceptors, is predominantly activated by the concentrations of intramuscular metabolites (lactate, ATP, protons) existing during ‘normal’ (i.e. freely perfused, aerobic, innocuous) exercise up to strenuous intensities. In contrast, the other subtype, the so-called metabo-nociceptors, only respond to higher (and concurrently noxious) levels of metabolites present in muscle during ischaemic contractions or following hypertonic saline infusions – but not to non-noxious metabolite concentrations associated with conventional exercise (20–23).
Feedback from both mechano- (24, 25) and metabosensitive (26, 27) group III/IV muscle afferents have been documented to evoke cardiovascular and ventilatory changes. However, the functional differences between metaboreceptors and metabo-nociceptors (20–23) caution a careful interpretation of findings from investigations utilizing traditional techniques, such as post exercise circulatory occlusion (PECO; muscle ischaemia maintains afferent firing at rest following exercise) or hypertonic saline infusions to activate and study the role of metabosensitive group III/IV muscle afferents in the cardiovascular, ventilatory, and central fatigue response to exercise. Specifically, these techniques evoke intramuscular metabolic changes in excess of those occurring during normal endurance exercise. Studies utilizing these approaches may therefore mainly focus on the physiological effects of feedback from metabo-nociceptors, the subset of metabosensitive muscle afferents which is largely inactive during normal exercise (20, 22, 23).
GROUP III/IV MUSCLE AFFERENTS INFLUENCE THE DEVELOPMENT OF PERIPHERAL FATIGUE
Direct evidence reflecting the influence of group III/IV muscle afferent feedback on the rate of development of contractile locomotor muscle fatigue during whole body exercise is provided by investigations using lumbar intrathecal fentanyl to attenuate sensory feedback from the legs during bicycle exercise (28–30). Based on the previous use of spinal opioid receptor agonists in animal studies (31–33), we pioneered this approach in exercising humans over a decade ago (34). Briefly, when applied intrathecally via injection into the spinal canal at the lumbar level, fentanyl, a μ-opioid receptor agonist, temporarily attenuates the central projection of group III/IV afferents innervating locomotor muscle (i.e. legs) by approximately 55–60% (35) and, importantly, without affecting the force generating capacity and, thus, central motor drive (34). Human studies based on this method suggest that when constant-load leg cycling is performed with attenuated feedback from group III/IV locomotor muscle afferents, the rate of development of contractile locomotor muscle fatigue is up to 60% faster compared to the same exercise performed with an intact afferent feedback system (28–30) (Figure 2A). Based on these findings, group III/IV muscle afferent feedback can be considered an important determinant for the development of contractile locomotor muscle fatigue during whole body exercise and critical for optimizing fatigue resistance, and therefore performance, during physical activities in healthy humans.
Figure 2:
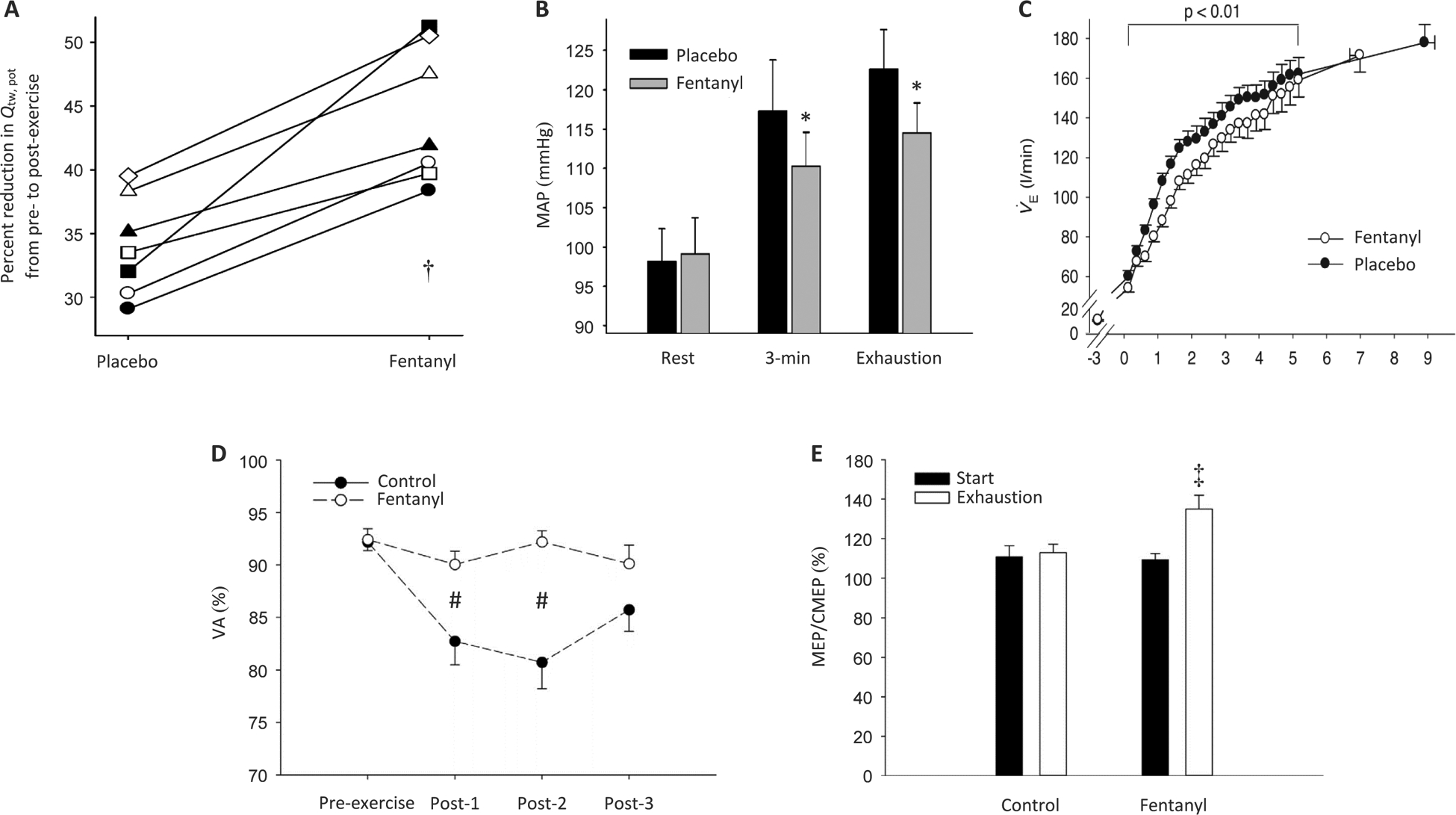
Blood pressure, ventilatory, and neuromuscular responses to exhaustive whole body exercise with intact (Placebo or CTRL) and blocked (Fentanyl or FENT) group III/IV muscle afferent feedback. Panel A, peripheral locomotor muscle fatigue following constant-load cycling (80% of peak power) to exhaustion (Placebo ~9 min; Fentanyl ~7 min); exercise-induced quadriceps fatigue is illustrated as the pre- to post-exercise decrease in potentiated twitch torque of the quadriceps (Qtw,pot). †P < 0.05 vs Placebo. Panel B, mean arterial pressure (MAP) at rest, 3 minutes into strenuous constant-load cycling (80% of peak power), and at exhaustion (Placebo ~9 min; Fentanyl ~7 min). * P < 0.05 vs. Placebo. Panel C, pulmonary ventilation (V̇E) during constant-load cycling exercise to exhaustion. Panel D, exercise-induced central fatigue, illustrated as the percent change in voluntary quadriceps activation (VA) from before to after constant-load cycling exercise to exhaustion (80% of peak power; ~8.5 min in both conditions) (post-1, post-2, and post-3 measure were taken ~1 min, ~2.5 min, and ~4 min, respectively, after exercise). #P < 0.05 vs. Control. Panel E, changes in motor cortical excitability during strenuous cycling exercise to task failure (80% of peak power; ~8.5 min in both conditions); increases in the ratio of motor-evoked potential (MEP) and cervicomedullary motor-evoked potential (CMEP) reflect an increase in motor cortical excitability; the illustrated ratios were obtained at the start of exercise and again at task failure (i.e. exhaustion). ‡P < 0.05 vs. Start. (Panels A–C. Reprinted from (30). Copyright © 2011 John Wiley and Sons. Used with permission.) (Panels D-E. Reprinted from (28). Copyright © 2017 Elsevier. Used with permission.)
How Do Muscle Afferents Mediate Peripheral Fatigue?
Group III/IV muscle afferents contribute to the development of peripheral fatigue during exercise by influencing the rate of accumulation of metabolites (e.g., H+, phosphates) known to cause failure of the excitation-contraction coupling within muscle fiber. To appreciate this relationship, it needs to be recognized that decreases in muscle blood flow/O2 delivery accelerate, whereas increases attenuate, the intramuscular accumulation of metabolites known to cause contractile fatigue [33]. Furthermore, group III/IV muscle afferents influence muscle O2 delivery, and thus the development of contractile fatigue, by determining the circulatory and the ventilatory response to exercise. Recent studies using lumbar intrathecal fentanyl and 31P-MRS suggested that group III/IV muscle afferents might also influence the development of contractile fatigue presumably independent of their role in regulating muscle O2 delivery. Indeed, it was proposed that the increased rate of accumulation of intramuscular fatigue metabolites during isometric knee-extensor exercise executed with blocked muscle afferents was, at least in part, due to an increased ATP cost of muscle contraction (36, 37). The reasons for this increased ATP cost during exercise with blocked muscle afferents remain elusive. It was, however, suggested that the influence of group III/IV muscle afferents on the ATP cost of muscle contraction is likely through an indirect mechanism, such as motor unit recruitment strategies, rather than a direct role of these afferents in regulating intracellular metabolic processes.
The impact of the muscle O2 delivery-mediated effect of group III/IV muscle afferents on the development of peripheral fatigue during whole body exercise was, until recently, not fully appreciated. Specifically, despite important evidence from animal (38, 39) and human (40, 41) investigations supporting significant cardiovascular (called the ‘exercise pressor reflex’ (41)) and ventilatory effects of group III and IV muscle afferent feedback when studied in isolation [e.g. PECO (26, 27), or passive (25, 42) or electrically-evoked exercise (35, 43)], their relative contribution and actual significance during whole body exercise remained controversial. This uncertainty resulted from the fact that the cardioventilatory response to voluntary whole body exercise is also influenced by strong feedforward influences (i.e. central command) (44), reflex interactions (35, 45), and potentially neural occlusions (i.e. central motor drive inhibits group III/IV muscle afferent feedback) (46).
Initial attempts to address the relative importance of group III/IV muscle afferents for the cardioventilatory responses to human whole body exercise included the injection of local anesthetics (e.g. lidocaine or bupivacaine) into the lumbar epidural space to block sensory feedback from locomotor muscle (47, 48). However, local anesthetics also reduce efferent nerve traffic to the limbs, thereby “weakening” the locomotor musculature (49). As a consequence, subjects were required to increase central motor drive to overcome the weakened muscles and to maintain a given work rate. Since augmented central command exerts additional feedforward influences on the cardioventilatory response to exercise (49, 50), epidural anesthesia created a condition of reduced feedback in the face of increased feedforward influences and was therefore unsatisfactory to assess the relative importance of group III/IV muscle afferents in regulating the cardioventilatory response to whole body exercise. More recent investigations circumvented this issue by utilizing lumbar intrathecal fentanyl to study the significance of lower limb muscle afferent feedback in determining O2 delivery by regulating the cardiovascular and ventilatory response to exercise (51, 52). Since intrathecal fentanyl has no effect on neuromuscular function (34), this approach allowed for a scenario characterized by reduced group III/IV-mediated afferent feedback combined with unaltered feedforward influences.
Studies taking advantage of this method found that bicycle exercise performed with blocked group III/IV locomotor muscle afferent feedback features significantly reduced heart rate and blood pressure responses (Figure 2B) and is characterized by hypoventilation (Figure 2C) causing considerable CO2 retention and, at higher workloads, arterial desaturation (28, 30, 51, 53). Additional investigations, based on dynamic single leg knee-extension exercise, documented that afferent blockade attenuates cardiac output via chronotropic and inotropic effects, and decreases leg perfusion pressure, leg blood flow, and leg O2 delivery during exercise (9, 35, 45, 52). These studies clearly highlighted the necessity of group III/IV muscle afferent feedback in regulating the normal circulatory and ventilatory responses in rhythmically exercising humans and were the first to emphasize the implications of this feedback mechanism for locomotor muscle O2 delivery, and thus the development of contractile fatigue, during whole body exercise. Importantly, since fentanyl blockade reduces only ~60% of group III/IV-mediated feedback (35), the observed relative contribution of group III/IV muscle afferents to the cardioventilatory response to exercise was likely an underestimation, the actual influence of these afferents is presumably much larger. Furthermore, the faster accumulation of contractile fatigue during constant-load cycling exercise with fentanyl-blocked muscle afferents will, eventually, require an increase in central motor drive to compensate for fatigued motor units. Since increases in central motor drive enhance the cardiovascular and ventilatory response to exercise (44), this increase causes a further underestimation of the relative contribution of group III/IV muscle afferents to the cardioventilatory and contractile fatigue response to exercise. However, a significantly greater central motor drive during constant-load exercise with blocked muscle afferents does not occur, even during high intensity cycling, until after 3–4 minutes into the exercise (30, 51). This allows for an important window to study the effects of group III/IV muscle afferents largely independent of the confounding influence of elevated central motor drive.
Reflex Interactions Modulate Cardiovascular Impact of Group III/IV Muscle Afferents
The impact of group III/IV muscle afferent feedback on the cardioventilatory response to exercise is, at least in part, modulated by considerable influences resulting from the interaction of the reflexes mediated by these muscle afferents (e.g. exercise pressor reflex, EPR) with other cardiovascular and ventilatory reflexes (26, 54, 55). For example, Hureau et al. recently investigated the significance of afferent feedback in carotid baroreflex responsiveness and resetting to operate at the higher blood pressures associated with electrically-evoked (no central command) and voluntary (requiring central command) knee extension exercise (35). Based on the findings from experiments utilizing afferent blockade (intrathecal fentanyl) during exercise, it was concluded that group III/IV muscle afferent feedback is, independent of central command, critical for the resetting of the carotid baroreflex blood pressure and heart rate operating points, but not for spontaneous baroreflex responsiveness. Although this study did not investigate the peripheral hemodynamic consequence of the interaction between the EPR and the baroreflex, earlier animal studies found no direct consequence on muscle blood flow during exercise (56). It could therefore be argued that the EPR-baroreflex interaction may not affect the development of peripheral fatigue during exercise.
Furthermore, Wan et al. utilized spinal anesthesia and gas breathing-induced changes in arterial blood gases (hypoxemia or hypercapnia) to manipulate the EPR and the chemoreflex (CR), respectively, with the goal of determining the interactive effects of these reflexes on the hemodynamic response to voluntary knee extension exercise in humans (45). It was documented that the mode of interaction resulting from the co-activation of the EPR and the hypercapnia-induced chemoreflex is characterized by a simple addition of the hemodynamic responses evoked by each reflex in alone (i.e., additive interaction). This suggests that the interaction does not result in additional consequences on muscle O2 delivery and thus the development of contractile fatigue during exercise. However, during the co-activation of the EPR and the CR triggered by hypoxemia, both heart rate and blood pressure rose higher than the sum of the responses to the activation of each reflex alone (i.e. hyper-additive interaction), while leg blood flow and leg vascular conductance were lower than the summated responses (i.e. hypo-additive interaction). In other words, when the CR was triggered by hypoxemia, the EPR:CR interaction potentiated the tachycardic and pressor responses to exercise whereas exercise-induced hyperemia and vasodilation were further restricted in the working muscle (45). The cardiovascular consequences of the EPR:CR interaction are therefore of particular relevance for humans at altitude, an environmental condition where this reflex interaction, per se, imposes an additional impairment on O2 delivery to the working limb muscles and thus the development of peripheral fatigue during exercise.
GROUP III/IV MUSCLE AFFERENTS FACILITATE CENTRAL FATIGUE
The effects of group III/IV muscle afferents on CNS fatigue during whole body exercise were first documented by studies using lumbar intrathecal fentanyl to attenuate sensory feedback from the legs during high-intensity time trial bicycling (i.e. subject can change power output ad libitum). These investigations revealed that motoneuronal output and locomotor muscle activation, estimated by quadriceps EMG, were significantly higher during the time trial performed with blocked, compared to intact, feedback from group III/IV locomotor muscle afferents (6, 34, 57). Although these observations suggested that afferent feedback facilitates CNS fatigue during locomotor exercise, this effect was not always reflected in the changes in voluntary muscle activation from before to after the exercise. Specifically, some investigations confirmed the EMG-based evidence of less central fatigue during cycling and found a smaller decrease in voluntary muscle activation (i.e., less CNS fatigue) after exercise with blocked compared to unblocked muscle afferents (28, 29) (Figure 2D). Other studies, however, reported no difference (57), or found an even greater reduction (6, 34) in voluntary muscle activation following bicycle exercise with blocked compared to unblocked afferents. The discrepancy between EMG-based evidence of CNS fatigue during bicycling and changes in voluntary muscle activation from before to after exercise is, perhaps, not surprising. In fact, the difference has been attributed to a variety of reasons including the sometimes delayed assessment of post-exercise voluntary muscle activation (34), temporary motor control issues impairing the subjects’ ability to perform adequate MVCs (6), and the inability and inappropriateness of pre/post-exercise changes in voluntary muscle activation to capture and reflect CNS fatigue during whole body exercise (58). Finally, it should be mentioned that feedback from group III/IV locomotor muscle afferents during exhaustive cycling exercise can also compromise voluntary activation of a remote muscle group (i.e. elbow flexors) not directly involved in the task (53). Taken together, these recent findings directly emphasize the significant involvement of group III/IV muscle afferents in the development of central fatigue during locomotor exercise.
Although the exact mechanisms mediating the influence of group III/IV muscle afferents on the reduction in spinal motoneuron output and muscle activation are not fully understood, it is thought that these sensory neurons exert inhibitory influences on voluntary descending drive ‘upstream’ of the motor cortex (59) and compromise corticospinal excitability during fatiguing cycling exercise (1, 28, 29, 53). To address the latter, Sidhu et al. (28) recently compared alterations in motor cortical and motoneuronal excitability during the identical cycling exercise (80% of peak power output for ~8 min) performed with and without muscle afferent blockade.
[Briefly, transcranial magnetic stimulations of the motor cortex and electric transmastoid stimulations were used during exercise to track changes in motor-evoked potentials (MEP) and cervicomedullary motor-evoked potentials (CMEP), respectively. Increases in MEP size reflect an increase in the net excitability of the corticospinal pathway, whereas decreases reflect a reduction. Increases in CMEP size represent an increase in the excitability of the motoneuron pool, whereas decreases reflect a reduction. Finally, changes in motor cortical excitability can be acquired by normalizing MEPs to CMEPs; increases in this ratio reflect increases in motor cortical excitability, decreases reflect a reduction (1).]
During the control trial performed with intact afferent feedback, the net excitability of the corticospinal pathway remained unaltered from the start to the final minute of exercise. However, when feedback from group III/IV muscle afferents was attenuated (lumbar intrathecal fentanyl) during the identical exercise, corticospinal excitability increased (28). Since motoneuronal excitability remained unchanged throughout exercise, both with intact and blocked afferent feedback, it was suggested that group III/IV muscle afferent feedback compromises the excitability of the motor cortex during fatiguing locomotor exercise (28) (Figure 2E). This postulate was later confirmed by a study during which the corticospinal pathway was assessed during brief cycling bouts, which were matched for EMG [which influences corticospinal excitability (60) and can mask a reflex inhibition (61)], performed before and after the fatiguing constant-load trial (29). Furthermore, exercise-induced increases in the length of the TMS-evoked silent period allowed the authors to speculate that the afferent-mediated motor cortical depression during exercise could be related to intracortical inhibitory mechanisms (28).
To further investigate the role of intracortical inhibition as a potential mechanism of the group III/IV-mediated motor cortical depression, a specific paired pulse paradigm was utilized in a later study (62). This paradigm, which is believed to primarily evaluate GABAB-mediated inhibitory networks (63), was employed during low intensity cycling (matched for EMG) performed before and again immediately after fatiguing constant-load cycling exercise (80% of peak power output, ~8 min). During exercise performed with intact afferent feedback, the authors discovered an increased activity of inhibitory pathways within the motor cortex, presumably related to the GABAB mediated inhibitory networks. Importantly, when afferent feedback was pharmacologically attenuated during the same exercise, this effect was abolished suggesting that the group III/IV-mediated inhibition of the motor cortex results, at least in part, from the facilitating effect of these sensory neurons on intracortical inhibitory networks, potentially mediated by GABAB receptors (29).
Little is known about the mechanisms mediating the impact of group III/IV muscle afferents on voluntary descending drive upstream of the motor cortex, i.e. the effect of afferent feedback on mechanisms driving the motor cortex. Studies focusing on experimental muscle pain, which engages sensory neurons with little activity during conventional whole body exercise, document significant effects of nociceptive muscle afferents on various brain structures including the primary sensory cortex and motor regions (64). Although direct evidence is currently missing, findings from fMRI studies have led to the hypothesis that fatigue-related group III/IV muscle afferent feedback limits motor cortical activation via their inhibitory influence on the prefrontal cortex, which plays an important role in goal directed movements and thus maximizing exercise performance (65), and the cingulate and insular cortices (66–68). However, indirect evidence might be offered by studies documenting the effects of muscle afferent blockade during exercise on the conscious sensation of exertion, a perception mediated by complex processes upstream of the motor cortex (69). Specifically, the rating of perceived exertion, which has been suggested to limit endurance performance (69), is considerably lower during whole body (51, 53) and single joint (36, 37) exercise performed with blocked, compared to unblocked, group III/IV muscle afferents. This suggests that afferent feedback might, via its contribution to the performance limiting sensation of exertion, contribute to the decrease in motoneuron output and muscle activation, i.e. central fatigue, by limiting voluntary descending drive upstream of the motor cortex.
Side Note:
The group III/IV muscle afferent feedback-mediated restriction on motoneuron output and muscle activation has been proposed as an integral part of a regulatory mechanism aiming to prevent an excessive exercise-induced homeostatic challenge in working limb muscle and ‘unpleasant’ sensations during exercise. Details on the idea of a ‘critical threshold of fatigue’ and a ‘sensory tolerance limit’ have recently been summarized (70).
EFFECT OF GROUP III/IV MUSCLE AFFERENTS ON CENTRAL FATIGUE LIMITS LOCOMOTOR PERFORMANCE
Investigating the effect of group III/IV muscle afferents on whole body exercise performance is challenging. The difficulty arises from the twofold role (Figure 1) these neurons play in an exercising human. Specifically, although group III/IV muscle afferent feedback facilitates CNS fatigue (i.e. limiting effect on performance), it simultaneously attenuates the rate of development of contractile fatigue (i.e. facilitating effect on performance). Therefore, manipulating muscle afferents during exercise affects both sides and the net effect depends on how, or whether, one effect outweighs the other.
With the exception of a study on patients with COPD (13) and findings from an investigation utilizing single-joint exercise in healthy individuals (71), direct experimental evidence for a group III/IV-mediated limitation of locomotor exercise performance was, until recently, missing. While studies based on time trial cycling found no effect (6, 34), others, based on constant-load cycling, either confirmed this lack of an effect (28, 29, 53) or observed a performance enhancing effect (30) of locomotor muscle afferent feedback. For example, during a 5-km cycling time trial performed with attenuated group III/IV muscle afferent feedback (lumbar intrathecal fentanyl), power output was, compared with the control trial performed with intact afferent feedback, ~10% greater during the first half and ~10% lower during the second half (6, 34). Interestingly, although the pacing strategy adopted by the participants was drastically different during the time trial with attenuated feedback, overall exercise performance was nearly identical compared with the control and the placebo trial (6, 34). Regardless, the overall inconclusive findings from these studies were attributed to the opposing effects of muscle afferent feedback, i.e. facilitating endurance performance by optimizing locomotor muscle O2 delivery vs limiting performance by restricting locomotor muscle activation.
In order to isolate the central fatigue-mediated impact of group III/IV muscle afferents on endurance performance, the consequences of manipulating afferent feedback on arterial oxygenation and limb O2 delivery had to be controlled for. To address this issue, Hureau et al. asked healthy participants to perform 5 km cycling time trials with intact and with blocked group III/IV muscle afferent feedback while assuring (and verifying) similar and adequate locomotor muscle O2 delivery during both trials by using a hyperoxic inspirate (57). This approach eliminated the effect of afferent blockade on the development of contractile fatigue (i.e. exacerbation) and allowed for a suitable scenario to study the CNS fatigue-mediated impact of muscle afferents on endurance performance. The temporary blockade of group III/IV muscle afferents attenuated the centrally-mediated restriction in motoneuronal output (i.e. less CNS fatigue) and resulted in significantly greater muscle activation and a substantial improvement in exercise performance (Figure 3). This study provided the first solid evidence indicating that group III/IV muscle afferents limit cycling performance by restricting the neural activation of locomotor muscle in healthy humans. The authors, however, cautioned that the experimental exposure of the performance limiting aspect of these sensory neurons requires careful control of their impact on locomotor muscle O2 delivery.
Figure 3:
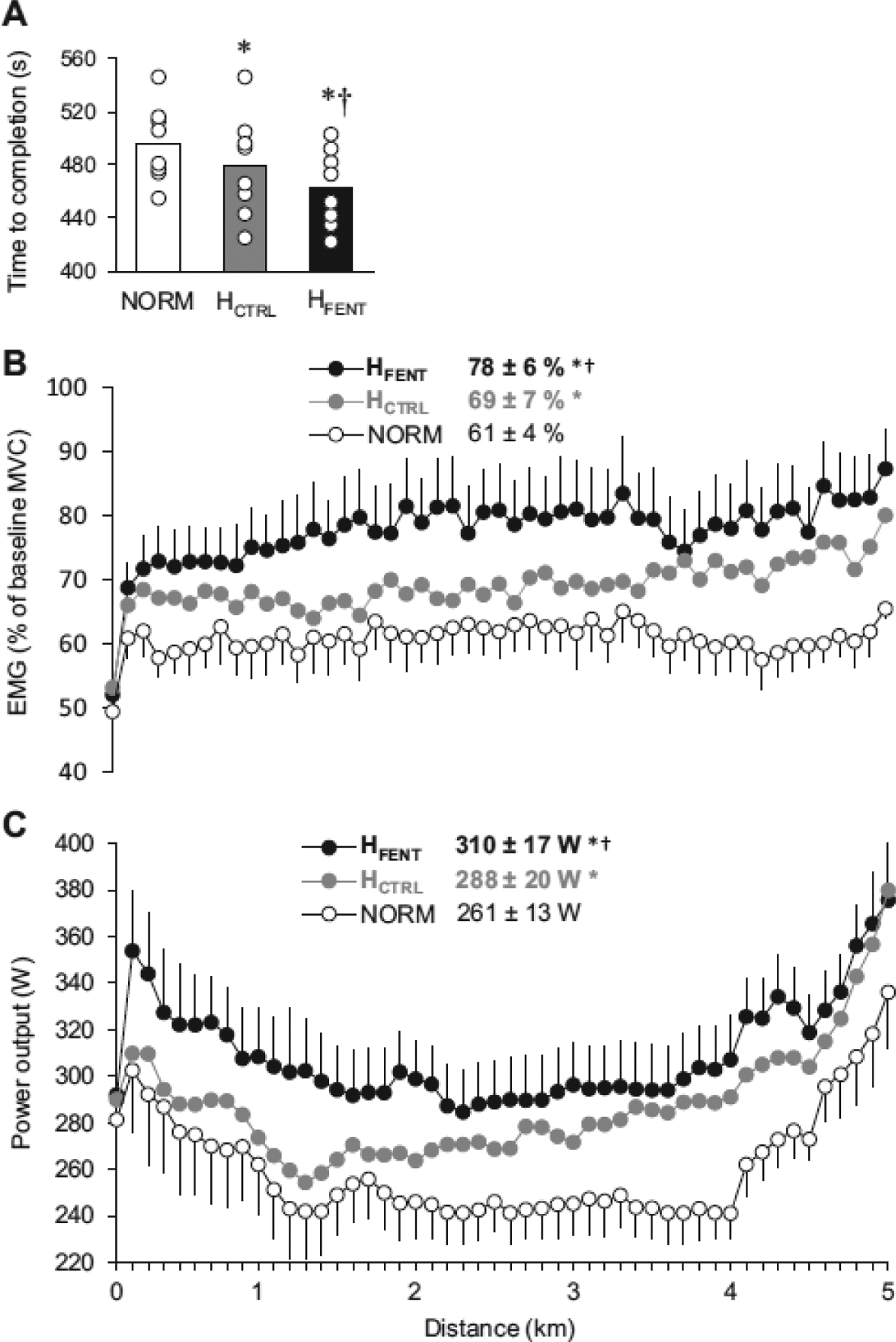
Time to completion, muscle activation, and power output during the 5 km cycling time trials performed under normoxic conditions (NORM) and with a hyperoxic inspirate (H). This strategy compensated for the impact of afferent blockade on convective O2 transport and permitted adequate locomotor muscle O2 delivery during the time trial performed with intact (HCTRL) and blocked (HFENT) group III/IV muscle afferents. Panel A, time to complete the 5 km time trials. Panel B, vastus lateralis EMG normalized to the EMG response recorded during pre-exercise maximal voluntary contraction. Panel C, power output during the time trials. * P < 0.05 vs NORM, † P < 0.05 vs HCTRL. (Reprinted from (57). Copyright © 2019 The American Physiological Society. Used with permission.)
KEY POINTS.
Feedback from group III/IV locomotor muscle afferents increases with the onset of locomotor exercise and influences key determinants of performance, namely, the development of peripheral and central fatigue.
The impact of group III/IV muscle afferents on peripheral fatigue is mediated through their role in facilitating locomotor muscle O2 delivery by regulating the hemodynamic and ventilatory response to exercise. Appropriate muscle O2 delivery slows peripheral fatigue development during exercise. Therefore, group III/IV muscle afferent feedback promotes endurance performance.
Group III/IV muscle afferents restrict motoneuronal output and locomotor muscle activation and therefore limit exercise performance by facilitating central fatigue. This impact results from the group III/IV-mediated inhibition of voluntary descending drive ‘upstream’ of the motor cortex and the group III/IV-mediated depression of motor cortical excitability.
Funding:
The authors are supported by the NIH (National Heart, Lung, and Blood Institute HL-116579 and HL-139451) and the Salt Lake City Veterans Affairs Medical Center Geriatrics Research, Education, and Clinical Center
Footnotes
Conflict of Interest: None.
REFERENCES
- 1.Weavil JC, Amann M. Corticospinal excitability during fatiguing whole body exercise. Prog. Brain Res 2018;240:219–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weavil JC, Amann M. Neuromuscular fatigue during whole body exercise. Current Opinion in Physiology. 2019;10:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol. Rev 2008;88(1):287–332. [DOI] [PubMed] [Google Scholar]
- 4.Taylor JL, Amann M, Duchateau J, Meeusen R, Rice CL. Neural Contributions to Muscle Fatigue: From the Brain to the Muscle and Back Again. Med. Sci. Sports Exerc 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairns SP, Knicker AJ, Thompson MW, Sjogaard G. Evaluation of models used to study neuromuscular fatigue. Exerc. Sport Sci. Rev 2005;33(1):9–16. [PubMed] [Google Scholar]
- 6.Blain GM, Mangum TS, Sidhu SK et al. Group III/IV muscle afferents limit the intramuscular metabolic perturbation during whole body exercise in humans. The Journal of physiology. 2016;594(18):5303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amann M, Sidhu SK, Weavil JC, Mangum TS, Venturelli M. Autonomic responses to exercise: Group III/IV muscle afferents and fatigue. Autonomic neuroscience : basic & clinical. 2015;188:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J. Appl. Physiol 2004;96(4):1486–95. [DOI] [PubMed] [Google Scholar]
- 9.Sidhu SK, Weavil JC, Venturelli M et al. Aging alters muscle reflex control of autonomic cardiovascular responses to rhythmic contractions in humans. American journal of physiology. Heart and circulatory physiology 2015;309:H1479–H89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amann M, Venturelli M, Ives SJ et al. Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int. J. Cardiol 2014;174:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sidhu SK, Weavil JC, Rossman MJ et al. Exercise Pressor Reflex Contributes to the Cardiovascular Abnormalities Characterizing Hypertensive Humans During Exercise. Hypertension. 2019;74(6):1468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson TP, Joyner MJ, Eisenach JH, Curry TB, Johnson BD. Influence of locomotor muscle afferent inhibition on the ventilatory response to exercise in heart failure. Exp. Physiol 2014;99(2):414–26. [DOI] [PubMed] [Google Scholar]
- 13.Gagnon P, Bussieres JS, Ribeiro F et al. Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 2012;186(7):606–15. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey JA, Miller JD, Romer L, Amann M, Smith CA. Exercise-induced respiratory muscle work: effects on blood flow, fatigue and performance. Adv. Exp. Med. Biol 2008;605:209–12. [DOI] [PubMed] [Google Scholar]
- 15.Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J. Appl. Physiol 1997;82(6):1811–7. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J. Appl. Physiol 1983;55(1 Pt 1):105–12. [DOI] [PubMed] [Google Scholar]
- 17.Wilson LB, Andrew D, Craig AD. Activation of spinobulbar lamina I neurons by static muscle contraction. J. Neurophysiol 2002;87(3):1641–5. [DOI] [PubMed] [Google Scholar]
- 18.Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27(1):201–9. [DOI] [PubMed] [Google Scholar]
- 19.Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J. Comp. Neurol 1995;361(2):225–48. [DOI] [PubMed] [Google Scholar]
- 20.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J. Neurophysiol 2008;100(3):1184–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birdsong WT, Fierro L, Williams FG et al. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68(4):739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jankowski MP, Rau KK, Ekmann KM, Anderson CE, Koerber HR. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J. Neurophysiol 2013;109(9):2374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollak KA, Swenson JD, Vanhaitsma TA et al. Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp. Physiol 2014;992:368–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trinity JD, Amann M, McDaniel J et al. Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. American journal of physiology. Heart and circulatory physiology 2010;299(5):H1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva TM, Aranda LC, Paula-Ribeiro M et al. Hyperadditive ventilatory response arising from interaction between the carotid chemoreflex and the muscle mechanoreflex in healthy humans. J Appl Physiol (1985). 2018;125(1):215–25. [DOI] [PubMed] [Google Scholar]
- 26.Bruce RM, White MJ. Muscle afferent activation causes ventilatory and cardiovascular responses during concurrent hypercapnia in humans. Exp. Physiol 2012;97(2):208–18. [DOI] [PubMed] [Google Scholar]
- 27.Rowell LB, Hermansen L, Blackmon JR. Human cardiovascular and respiratory responses to graded muscle ischemia. J. Appl. Physiol 1976;41(5 Pt. 1):693–701. [DOI] [PubMed] [Google Scholar]
- 28.Sidhu SK, Weavil JC, Mangum TS et al. Group III/IV locomotor muscle afferents alter motor cortical and corticospinal excitability and promote central fatigue during cycling exercise. Clin. Neurophysiol 2017;128(1):44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidhu SK, Weavil JC, Thurston TS et al. Fatigue-related group III/IV muscle afferent feedback facilitates intracortical inhibition during locomotor exercise. The Journal of physiology. 2018;596(19):4789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high intensity endurance exercise performance in humans. The Journal of physiology. 2011;589:5299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill JM, Kaufman MP. Attenuation of reflex pressor and ventilatory responses to static muscular contraction by intrathecal opioids. J. Appl. Physiol 1990;68(6):2466–72. [DOI] [PubMed] [Google Scholar]
- 32.Meintjes AF, Nobrega AC, Fuchs IE, Ally A, Wilson LB. Attenuation of the exercise pressor reflex. Effect of opioid agonist on substance P release in L-7 dorsal horn of cats. Circ. Res 1995;77(2):326–34. [DOI] [PubMed] [Google Scholar]
- 33.Pomeroy G, Ardell JL, Wurster RD. Spinal opiate modulation of cardiovascular reflexes in the exercising dog. Brain Res. 1986;381(2):385–9. [DOI] [PubMed] [Google Scholar]
- 34.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. The Journal of physiology. 2009;587(Pt 1):271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hureau TJ, Weavil JC, Thurston TS et al. Identifying the role of group III/IV muscle afferents in the carotid baroreflex control of mean arterial pressure and heart rate during exercise. The Journal of physiology. 2018;596(8):1373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broxterman RM, Hureau TJ, Layec G et al. Influence of group III/IV muscle afferents on small muscle mass exercise performance: a bioenergetics perspective. The Journal of physiology. 2018;596(12):2301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broxterman RM, Layec G, Hureau TJ et al. Bioenergetics and ATP Synthesis during Exercise: Role of Group III/IV Muscle Afferents. Med. Sci. Sports Exerc 2017;49(12):2404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. The Journal of physiology. 1972;224(1):173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. The Journal of physiology. 1971;215(3):789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strange S, Secher NH, Pawelczyk JA et al. Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. The Journal of physiology. 1993;470:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Secher NH, Amann M. Human investigations into the exercise pressor reflex. Exp. Physiol 2012;97(1):59–69. [DOI] [PubMed] [Google Scholar]
- 42.Venturelli M, Ce E, Limonta E et al. Central and peripheral responses to static and dynamic stretch of skeletal muscle: mechano- and metaboreflex implications. J Appl Physiol (1985). 2017;122(1):112–20. [DOI] [PubMed] [Google Scholar]
- 43.Asmussen E, Nielsen M, Wieth-Pedersen G. Cortical or reflex control of respiration during muscular work. Acta Physiol. Scand 1943;6(2–3):168–75. [Google Scholar]
- 44.Waldrop TG, Eldridge FL, Iwamoto GA, Mitchell JH. Central neural control of respiration and circulation during exercise In: Rowell LB, Shepherd JT editors. Handbook of Physiology Section 12: Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996, pp. 333–80. [Google Scholar]
- 45.Wan HY, Weavil JC, Thurston TS et al. The exercise pressor reflex and chemoreflex interaction: cardiovascular implications for the exercising human. The Journal of physiology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Degtyarenko AM, Kaufman MP. Fictive locomotion and scratching inhibit dorsal horn neurons receiving thin fiber afferent input. American journal of physiology. Regulatory, integrative and comparative physiology 2000;279(2):R394–403. [DOI] [PubMed] [Google Scholar]
- 47.Fernandes A, Galbo H, Kjaer M, Mitchell JH, Secher NH, Thomas SN. Cardiovascular and ventilatory responses to dynamic exercise during epidural anaesthesia in man. The Journal of physiology. 1990;420:281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friedman DB, Brennum J, Sztuk F et al. The effect of epidural anaesthesia with 1% lidocaine on the pressor response to dynamic exercise in man. The Journal of physiology. 1993;470:681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amann M, Proctor LT, Sebranek JJ, Eldridge MW, Pegelow DF, Dempsey JA. Somatosensory feedback from the limbs exerts inhibitory influences on central neural drive during whole body endurance exercise. J. Appl. Physiol 2008;105(6):1714–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asmussen E, Johansen SH, Jorgensen M, Nielsen M. On the Nervous Factors Controlling Respiration and Circulation During Exercise. Experiments with Curarization. Acta Physiol. Scand 1965;63:343–50. [DOI] [PubMed] [Google Scholar]
- 51.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J. Appl. Physiol 2010;109(4):966–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amann M, Runnels S, Morgan DE et al. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. The Journal of physiology. 2011;589(Pt 15):3855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sidhu SK, Weavil JC, Venturelli M et al. Spinal mu-opioid receptor-sensitive lower limb muscle afferents determine corticospinal responsiveness and promote central fatigue in upper limb muscle. The Journal of physiology. 2014;592(Pt 22):5011–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith SA, Querry RG, Fadel PJ et al. Partial blockade of skeletal muscle somatosensory afferents attenuates baroreflex resetting during exercise in humans. The Journal of physiology. 2003;551(Pt 3):1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JK, Sala-Mercado JA, Rodriguez J, Scislo TJ, O’Leary DS. Arterial baroreflex alters strength and mechanisms of muscle metaboreflex during dynamic exercise. American journal of physiology. Heart and circulatory physiology 2005;288(3):H1374–80. [DOI] [PubMed] [Google Scholar]
- 56.Waldrop TG, Mitchell JH. Effects of barodenervation on cardiovascular responses to static muscular contraction. Am. J. Physiol 1985;249(4 Pt 2):H710–4. [DOI] [PubMed] [Google Scholar]
- 57.Hureau TJ, Weavil JC, Thurston TS et al. Pharmacological attenuation of group III/IV muscle afferents improves endurance performance when oxygen delivery to locomotor muscles is preserved. J Appl Physiol (1985). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Place N, Millet GY. Quantification of Neuromuscular Fatigue: What Do We Do Wrong and Why? Sports Med. 2020;50(3):439–47. [DOI] [PubMed] [Google Scholar]
- 59.Taylor JL, Allen GM, Butler JE, Gandevia SC. Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J Appl Physiol (1985). 2000;89(1):305–13. [DOI] [PubMed] [Google Scholar]
- 60.Weavil JC, Sidhu SK, Mangum TS, Richardson RS, Amann M. Intensity-dependent alterations in the excitability of cortical and spinal projections to the knee extensors during isometric and locomotor exercise. American journal of physiology. Regulatory, integrative and comparative physiology 2015;308(12):R998–R1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weavil JC, Sidhu SK, Mangum TS, Richardson RS, Amann M. Fatigue diminishes motoneuronal excitability during cycling exercise. J. Neurophysiol 2016;116(4):1743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sidhu SK, Weavil J, Thurston T et al. Fatigue modulates the effect of group III/IV muscle afferents on GABAB-mediated inhibtion and corticospinal excitability. Journal of Physiology. 2018. [Google Scholar]
- 63.Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr. Clin. Neurophysiol 1992;85(6):355–64. [DOI] [PubMed] [Google Scholar]
- 64.Macefield VG, Henderson LA. Autonomic responses to exercise: cortical and subcortical responses during post-exercise ischaemia and muscle pain. Autonomic neuroscience : basic & clinical. 2015;188:10–8. [DOI] [PubMed] [Google Scholar]
- 65.Rauch HG, Schonbachler G, Noakes TD. Neural correlates of motor vigour and motor urgency during exercise. Sports Med. 2013;43(4):227–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu JZ, Dai TH, Sahgal V, Brown RW, Yue GH. Nonlinear cortical modulation of muscle fatigue: a functional MRI study. Brain Res. 2002;957(2):320–9. [DOI] [PubMed] [Google Scholar]
- 67.Liu JZ, Shan ZY, Zhang LD, Sahgal V, Brown RW, Yue GH. Human brain activation during sustained and intermittent submaximal fatigue muscle contractions: an FMRI study. J. Neurophysiol 2003;90(1):300–12. [DOI] [PubMed] [Google Scholar]
- 68.Tanaka M, Watanabe Y. Supraspinal regulation of physical fatigue. Neurosci. Biobehav. Rev 2012;36(1):727–34. [DOI] [PubMed] [Google Scholar]
- 69.Okano AH, Fontes EB, Montenegro RA et al. Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise. Br. J. Sports Med 2015;49(18):1213–8. [DOI] [PubMed] [Google Scholar]
- 70.Hureau TJ, Romer LM, Amann M. The ‘sensory tolerance limit’: A hypothetical construct determining exercise performance? European journal of sport science. 2018;18(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amann M, Venturelli M, Ives SJ et al. Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J Appl Physiol (1985). 2013;115(3):355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


