Abstract
Background and Objectives
In our previous proof‐of‐principle study, transcranial photobiomodulation (tPBM) with 1,064‐nm laser was reported to significantly increase concentration changes of oxygenated hemoglobin (∆[HbO]) and oxidized‐state cytochrome c oxidase (∆[oxi‐CCO]) in the human brain. This paper further investigated (i) its validity in two different subsets of young human subjects at two study sites over a period of 3 years and (ii) age‐related effects of tPBM by comparing sham‐controlled increases of ∆[HbO] and ∆[oxi‐CCO] between young and older adults.
Study Design/Materials and Methods
We measured sham‐controlled ∆[HbO] and ∆[oxi‐CCO] using broadband near‐infrared spectroscopy (bb‐NIRS) in 15 young (26.7 ± 2.7 years of age) and 5 older (68.2 ± 4.8 years of age) healthy normal subjects before, during, and after right‐forehead tPBM/sham stimulation with 1,064‐nm laser. Student t tests were used to test statistical differences in tPBM‐induced ∆[HbO] and ∆[oxi‐CCO] (i) between the 15 young subjects and those of 11 reported previously and (ii) between the two age groups measured in this study.
Results
Statistical analysis showed that no significant difference existed in ∆[HbO] and ∆[oxi‐CCO] during and post tPBM between the two subsets of young subjects at two study sites over a period of 3 years. Furthermore, the two age groups showed statistically identical net increases in sham‐controlled ∆[HbO] and ∆[oxi‐CCO].
Conclusions
This study provided strong evidence to validate/confirm our previous findings that tPBM with 1,064‐nm laser enables to increase cerebral ∆[HbO] and ∆[oxi‐CCO] in the human brain, as measured by bb‐NIRS. Overall, it demonstrated the robust reproducibility of tPBM being able to improve cerebral hemodynamics and metabolism of the human brain in vivo in both young and older adults. Lasers Surg. Med. © 2020 The Authors. Lasers in Surgery and Medicine published by Wiley Periodicals, Inc.
Keywords: transcranial photobiomodulation, tPBM, cytochrome c oxidase, broadband near‐infrared spectroscopy, reproducibility
INTRODUCTION
Transcranial photobiomodulation (tPBM) is a non‐invasive and non‐thermal approach that uses low‐power light at a near‐infrared (NIR) wavelength between 620 and 1,100 nm to accelerate cellular functions in the human brain/tissue for cognitive benefits of normal aging [1, 2, 3] and/or for clinical treatments of brain disorders and injuries [4, 5, 6, 7]. A recently published book presents a thorough collection of studies on tPBM, covering topics of tPBM on cultured neurons and molecular mechanisms, studies with animal models, and clinical studies of patients with a variety of neurological diseases [8]. Intriguingly, a recent exploratory study with a small sample size reported that tPBM was also able to improve sexual dysfunction by an array of light emitting diodes at 823 nm with an area of 28.7 cm2 on each side of the dorsolateral prefrontal region [9]. It has been well‐accepted by researchers in the field that the mechanism of tPBM with NIR light is based on the photo‐oxidation of cytochrome c oxidase (CCO), the terminal enzyme in the electron transport chain, which catalyzes the oxygen metabolism for cellular adenosine triphosphate (ATP) production [3, 10, 11]. Specifically, the NIR light can convert reduced‐state CCO into its oxidized state, as labeled “oxi‐CCO” hereafter. oxi‐CCO is active in a series of redox reactions, reducing oxygen molecules into water and enhancing the proton gradient at the inner membrane of mitochondria. This photo‐oxidation process of CCO accelerates the utilization of oxygen within mitochondria and the production of intracellular ATP [3, 12]. Accordingly, cells and neurons that have a high concentration of mitochondria will be facilitated to be more active in their mitochondrial oxygen metabolism and thus their functional activities, leading to enhancement of synaptic connections [13, 14, 15].
Recently, this tPBM mechanism of action was supported by objective measures of tPBM‐induced increases in concentrations of cerebral oxygenation and oxi‐CCO in the human brain using non‐invasive broadband near‐infrared spectroscopy (bb‐NIRS) by our group [16]. While the results reported in the study were highly statistically significant, it was the only published report showing effects of tPBM on hemodynamic and metabolic upregulation in the human brain measured by bb‐NIRS in vivo, with a small group of 11 young subjects. It is important to demonstrate the robust reproducibility for the findings to develop tPBM as a non‐invasive neuromodulation tool for cognitive benefits in healthy adult and aging population as well as patients with neurological or psychiatric disorders.
In this study, we aimed to investigate (i) the validity of previous findings in Ref. [16] in two different subsets of young subjects at two study sites over a period of 3 years and (ii) age‐related effects of tPBM by comparing sham‐controlled increases of ∆[HbO] and ∆[oxi‐CCO] between young and older adults. The same bb‐NIRS experimental setup, protocol, and procedures were performed on a group of young (n = 15) and older (n = 5) participants, but with different experimental operators and different participants at a different experimental site with respect to the previous study. Student t tests were used to assess the reproducibility of the findings between the two sets of young adults and between the two age groups. The results indicated high reproducibility of tPBM effects detected by bb‐NIRS in the young‐adult groups. For age effects, the sham‐controlled increases in ∆[HbO] and ∆[oxi‐CCO] were identical between two age groups. These results indicate great potential of neuroplastic effects of tPBM in future applications.
METHODS
Participants
Twenty healthy normal subjects (11 females and 9 males) were recruited from the University of Texas Southwestern Medical Center at Dallas (UTSW), the Southern Methodist University (SMU), and the University of Texas at Arlington (UTA). For this study, the actual measurements took place at the UTSW campus. There were 15 adults (7 females and 8 males; age = 26.7 ± 2.7 years) in the young‐adult group (younger than 60 years) and 5 (4 females and 1 male; age = 68.2 ± 4.8 years) in the older‐adult (older than 60 years) group. The inclusion/exclusion criteria were the same as those in Wang et al. [16]. All subjects had no prior diagnosis or history of neurological conditions and were instructed to keep caffeine and nicotine consumption to a minimum (a cup) prior to the experiment. The study protocol was reviewed and approved by the Institutional Review Board (IRB) of both UTA and UTSW as well as complied with all applicable federal and NIH guidelines. Written informed consent was obtained from each participant prior to the experiments. All the participants were asked to come on two non‐consecutive days for either sham or tPBM in a randomized order. Data from 11 young participants (5 females and 6 males; age = 31.0 ± 13.7 years) recruited in the previous study were also used for examining the reproducibility of tPBM effects on cerebral metabolism and hemodynamics of the human brain in vivo [16]. There was no overlap between the participants in the two studies.
Experiments
1,064‐nm tPBM
Both tPBM and sham experiments were administered using the same continuous‐wave 1064‐nm laser (Model CG‐5000; Cell Gen Therapeutics LLC, Dallas, TX) with respect to the previous study [16]. It is Food and Drug Administration‐cleared for pain relief, such as for muscle, nerve, and joint pain. The laser device was set to a calibrated power of 3.4 W to deliver tPBM on each participant's forehead and was set to a minimum operating power of 0.1 W for the sham experiments. During the sham experiments, the laser aperture was also blocked by a thick black cap to prevent 1,064‐nm light from reaching the participant's forehead. Both tPBM and sham experiments were conducted through a hand‐held aperture at 2 cm away from the forehead scalp/skin without any physical contact, following the common suggested procedure [1]. Light from the aperture was well‐collimated and the illumination area on the human forehead was 13.6 cm2, giving rise to a constant power density of 3.4 W/13.6 cm2 = 0.25 W/cm2.
bb‐NIRS
The same bb‐NIRS system was used as in our previous report [16]. In brief, this system consists of a tungsten halogen lamp (Model 3900; Illumination Technologies Inc., East Syracuse, NY) as the broadband light source (covering 400–1,500 nm light) and a back‐thinned cool‐down CCD spectrometer (QE‐Pro, Ocean Optics Inc.) as the detector. The white light emitted from the light source was delivered on to the human forehead through a fiber optic bundle (see Fig. 1). A portion of the light diffused through the human brain was collected by another fiber bundle 3 cm away from the source bundle, latter of which directed the detected light into the CCD spectrometer. Time‐dependent spectral (740–900 nm) intensities before, during, and after tPBM or sham delivery were recorded and used to quantify concentration changes of oxygenated hemoglobin (Δ[HbO]) and oxidized cytochrome c oxidase Δ[oxi‐CCO] based on the modified Beer‐Lambert law, as described in Ref. [17].
Figure 1.
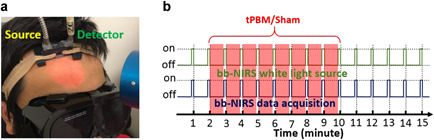
(a) A picture of experimental setup showing (1) two fiber bundles for tPBM/sham light delivery (source) and detection (detector) on a human forehead and (2) circular area on the forehead under 4‐cm‐diameter laser illumination. Note that the laser aperture shown is more than 2 cm away from the forehead to show a clear illumination spot/area. (b) Experimental protocol of interleaved measurements. tPBM, transcranial photobiomodulation.
Experimental protocol
Both tPBM and sham experiments were conducted in a dark locked room without any reflective surfaces. When the laser system was in use, a warning sign was placed on the door to further dissuade individuals from entering. Protective goggles were worn by all the individuals in the lab room to ensure eye safety for both the participants and operators. Participants were also asked to keep their eyes closed during the entire experiment procedures to further reduce the possibility of eye injury and maintained blind between the sham versus tPBM conditions. Participants had no information on which condition they were given until they completed both of their visits to the lab. As soon as the participants entered the lab, they were asked to sit on an inclined chair comfortably. The horizontally I‐shaped bb‐NIRS probe was then placed on the subject's right forehead above the eyebrow and secured using an elastic bandage (see Fig. 1a). After the probe was attached comfortably on the scalp, the bb‐NIRS system was switched on to test the stability of the spectral intensities and to confirm artifact‐free/motion‐free time‐dependent recordings under resting state.
Time‐dependent Δ[HbO] and Δ[oxi‐CCO]
The same interleaved experimental protocol was followed with respect to the previous study [16]. In brief, each experiment consisted of a 2‐minute baseline, an 8‐minute tPBM/sham light delivery, and a 5‐minute recovery period, as shown in Figure 1b. Near the end of each minute, tPBM/sham was paused for 5 seconds for bb‐NIRS recording of the spectral intensities that diffused back from the human brain. In this way, 15 spectral data points were obtained at the end of each experiment and further processed/quantified as time‐dependent Δ[HbO] and Δ[oxi‐CCO]. Among the 15 data points recorded from each participant in each experiment, the first two points recorded before the onset of tPBM/sham were regarded as baselines. They were used as references to quantify Δ[HbO] and Δ[oxi‐CCO] for the following 13 data points during and after tPBM/sham, leading to 13 time‐dependent Δ[HbO] or Δ[oxi‐CCO] readings in μM for each experimental run. This time‐dependent data process was repeated for each participant in both tPBM and sham experiments.
Statistical Analysis for Comparisons of Two Studies
Data used for comparisons in this study included the data sets collected from 11 young adults at UTA in 2016 and the data sets collected from 15 young and five older adults at UTSW in 2019. We conducted three steps of statistical evaluations/comparisons over these data sets for assessing tPBM‐induced effects on cerebral hemodynamic and metabolic activities: (i) comparison between two young‐adult groups measured under different operating conditions (i.e., at different experimental sites with different operators from different participating subjects with a 3‐year time interval); (ii) comparison between two age groups measured under the same operating conditions; (iii) comparison of tPBM‐induced sham‐controlled changes between two age groups measured under the same operating conditions. Specifically, in Step I, two‐sample t tests were performed on time‐dependent Δ[HbO] and Δ[oxi‐CCO] values at each time point (over a total of 13 temporal points excluding the two baseline readings) taken from young adults between the current (n = 15) and previous (n = 11) studies. The comparisons between the two young‐adult groups were analyzed per each time point under tPBM and sham conditions separately. Likewise, in Step II, two‐sample t tests were performed on time‐dependent Δ[HbO] and Δ[oxi‐CCO] values of the participants in two different age groups (n = 15 for young and n = 5 for older) measured under the identical operating conditions in this study. In Step III, sham‐controlled changes of Δ[HbO] and Δ[oxi‐CCO] were quantified by subtracting sham‐derived (Δ[HbO] or Δ[oxi‐CCO]) values from tPBM‐derived (Δ[HbO] or Δ[oxi‐CCO]) values at each of the 15 time points for both age groups separately, followed by two‐sample t tests to test age‐related effects of tPBM. A two‐tailed level of 0.01 < P < 0.05 and P < 0.05 was chosen to be statistically significant in these tests.
RESULTS
Comparisons of Δ[HbO] and Δ[oxi‐CCO] Between Two Young Adult Groups
The time‐dependent cross‐subject means as well as standard errors of Δ[HbO] and Δ[oxi‐CCO] from the current and previous study [16] are plotted in Figure 2a and b, respectively. On the basis of two‐tailed two‐sample t tests, there was no significant difference (P > 0.05) observed between these two groups under either tPBM or sham condition for both (i) Δ[HbO] and (ii) Δ[oxi‐CCO], indicating a high reproducibility of using bb‐NIRS to measure tPBM effects on young human adults.
Figure 2.
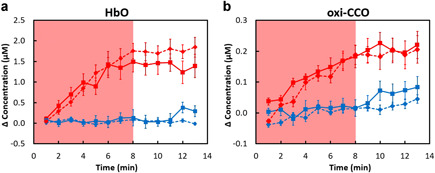
Comparison of concentration changes of (a) Δ[HbO] and (b) Δ[oxi‐CCO] measured from two groups of young adults in the current (solid lines; n = 15) and previous (dashed lines; n = 11) study. The red symbols and lines indicate the results from the tPBM experiment, while the blue symbols and lines indicate those from the sham experiment. The error bars are standard errors of mean. The shades indicate the time durations of tPBM/sham illumination. No significant differences between the two young‐adult groups (solid lines: current study; dashed lines: previous study) are marked with * P < 0.05 for tPBM and sham condition respectively. [HbO], oxygenated hemoglobin; [oxi‐CCO], oxidized‐state cytochrome c oxidase.
Comparisons of Δ[HbO] and Δ[oxi‐CCO] Between Two Age Groups
Time‐dependent Δ[HbO] and Δ[oxi‐CCO] values measured from 15 young adults and five older adults in this study are plotted in Figure 3a and b, respectively. While the time‐dependent changes in Δ[HbO] between the two age groups showed very similar trends under either tPBM or sham condition, significant differences were observed in Δ[oxi‐CCO] between the two age groups. Specifically, for both the tPBM and sham experiments, the older group presents a significantly lower level of Δ[oxi‐CCO] than the younger group.
Figure 3.
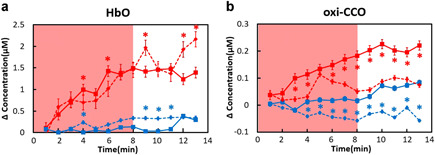
Comparison of concentration changes of (a) Δ[HbO] and (b) Δ[oxi‐CCO] measured in this study from a young adult group (solid lines; n = 15) and an older adult group (dashed lines, n = 5). The red color represents the tPBM experiment, while the blue color denotes the sham experiment. The error bars are standard errors of mean. The shades indicate the time durations of tPBM/sham illumination. Significant differences between two age groups (solid: young adults; dashed lines: older adults) are marked with *P < 0.05 for tPBM and sham conditions repectively.
Comparisons of Net Changes in Δ[HbO] and Δ[oxi‐CCO] Between Two Age Groups
To determine relative effects of tPBM from two age groups, sham‐controlled changes of Δ[HbO] and Δ[oxi‐CCO] were quantified for each subject by obtaining net changes with respect to the results under the sham condition. The time‐dependent, group‐averaged net changes of Δ[HbO] and Δ[oxi‐CCO] from 15 young adults (red symbols and lines) and five older adults (blue symbols and lines) were plotted in Figure 4a and b, respectively. The two‐tailed, two‐sample t tests revealed that there was no statistically significant difference between the two age groups for tPBM‐induced Δ[HbO] and Δ[oxi‐CCO] effects with respect to the sham condition.
Figure 4.
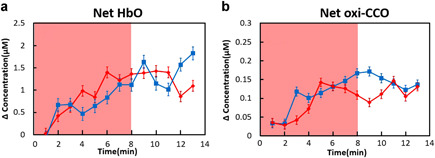
Net changes of (a) Δ[HbO] and (b) Δ[oxi‐CCO] measured in this study from a young adult group (red symbols and lines; n = 15) and an older adult group (blue symbols and lines; n = 5). The error bars are standard errors of mean. The shades indicate the time durations of tPBM/sham illumination. No significant differences between the two age groups are marked with *P < 0.05 for both tPBM and sham conditions.
DISCUSSION
Reproducible Hemodynamic and Metabolic Effects of tPBM in Young Adults
The photo‐oxidation effects of PBM on CCO have been discussed for decades. More recently tPBM‐induced behavioral improvements on human cognition [1, 2, 3] and mental illness [4, 5, 6, 7] have been reported. Without objective measures of changes in the redox state of CCO in the human brain in vivo, it would be difficult to design successful tPBM protocols and intervention for safe and optimal photobiomodulation effects in future applications. To fulfill this goal, we recently applied bb‐NIRS and non‐invasively quantified the changes of tPBM‐induced Δ[HbO] and Δ[oxi‐CCO] in the brain of young human adults [16]. Its conclusions helped to understand the mechanism of action of tPBM. However, any new development of methodology and scientific findings require reliable reproducibility with a reasonable sample size. Since the previous study was the first published report of tPBM effects on cerebral Δ[HbO] and Δ[oxi‐CCO] measured by bb‐NIRS and based on a small sample of 11 young controls, the reproducibility of the findings is important to be examined and statistically tested.
By applying statistical two‐sample t tests, we compared the young healthy subjects, 11 sets of data collected in year 2016 at UTA versus the 15 sets of data collected from a new group of young subjects in year 2019 using the same experimental setup at UTSW. As shown in Figure 2, the cross‐subject means of Δ[HbO] and Δ[oxi‐CCO] from the two groups of young adults have no statistical difference under both tPBM and sham conditions, demonstrating the high reproducibility for the measured respective parameters. Specifically, at each of temporal points, all the two‐sample t test comparisons reveal no significant difference between the measurements of the two sets of data.
Comparison of Hemodynamic and Metabolic Effects of tPBM in Two Age Groups
While Figure 2b shows statistically reproducible photon‐oxidation effects of tPBM on the cerebral redox state of CCO in young adults, we also compared the effects of tPBM on cerebral Δ[HbO] and Δ[oxi‐CCO] between the 15 young and five older adults measured under the same operating conditions at UTSW. The statistical analysis revealed that non‐significant difference exists in tPBM‐induced Δ[HbO], but tPBM‐induced Δ[oxi‐CCO] values were significantly lower in older adults than in young adults. Interestingly, such an age‐related difference disappeared when we compared the sham‐controlled or sham‐subtracted changes in Δ[HbO] and Δ[oxi‐CCO] between the two age groups (see Fig. 4). This set of results indicate that tPBM can induce the same amount of hemodynamic and metabolic effects with respect to the sham experiment regardless of age differences. This finding may imply that use of tPBM can boost cerebral metabolism in an older brain with the amount of effect equal or equivalent to that in a young brain, serving as a non‐invasive and promising means of neuromodulation.
Potential Benefits of tPBM on Older Adults
Aging is a risk factor for many health conditions, such as Alzheimer's disease, hypertension, and cardiovascular disease [18, 19]. One of the leading reason of aging is thought to be the accumulation of oxidative stress over time [20]. Oxidative stress can be caused by the natural generation of reactive oxygen species (ROS) during ATP production. The ROS hold an extra electron in their molecular structure, making them chemically reactive to damage cellular structures and thus causing cell death [21]. While a small amount of ROS plays a vital role in boosting cellular functions [22], oxidative stress occurs when the generation of ROS becomes faster than the speed that they can be removed by the natural cellular mechanisms [23]. The healthy functioning of oxidized CCO is believed to efficiently convert ROS into a chemically steady state without the extra electron. Therefore, having a large concentration of [oxi‐CCO] can inhibit the fast accumulation of cellular ROS, which protects the cellular environment [24]. In the case of aging, when CCO gradually decreases its function, less concentration of [oxi‐CCO] exists within cells and/or neurons. Therefore, ROS will accumulate increasingly to induce oxidative stress [25]. Given the observation that tPBM was able to boost Δ[oxi‐CCO] in an equal amount between young and older adults (Fig. 4), we speculate or expect that tPBM may be able to induce cognition‐enhancing benefits on older brains. This speculation needs to be further investigated/tested in future research while several recent publications reported supporting evidence based on behavioral measures [26].
Robustness of the Quantification Method and Post‐Stimulation Effects
While CCO is undoubtedly the most important chromophore in PBM, there is emerging evidence that other primary chromophores such as opsins, flavins, and cryptochromes, may mediate the biological absorption of light, particularly at shorter wavelengths (blue and green) [27]. Also, nitric oxide (NO) is another key neurotransmitter or character that is hypothesized [28] and demonstrated to closely associate with the outcome of tPBM [29] or PBM [30]. Specifically, Eshaghi et al. [29] showed that changes of NO levels in the prefrontal cortex and hippocampus of tPBM‐treated mice with depression were related to the anti‐depressive effect of tPBM. Mitchell and Mack [30] demonstrated that PBM on the human forearm gave rise to increases of NO levels in venous blood draining near the treatment site in healthy subjects. However, neither of these studies could be performed non‐invasively for quantitative measures of changes in NO induced by PBM.
Detailed confirmation of the CCO‐driven mechanism of tPBM needs further biochemistry‐based investigations but is beyond the topic of this paper. What we presented in this current study is to report objective and reproducible observation that oxidized CCO concentration, [oxi‐CCO], is increased after few minutes of tPBM initiation. The method used for determination of [oxi‐CCO] is well‐developed and tested through laboratory, animal, and human studies in the last several years [31, 32, 33, 34, 35, 36, 37, 38]. Therefore, this study has provided solid evidence on tPBM‐induced increases in both Δ[HbO] and Δ[oxi‐CCO], which appeared to maintain elevation during the 5‐minute recovery time. Furthermore, while water does absorb light, the total amount of light absorption of water does not change during tPBM. Thus, water absorption does not influence the quantification of Δ[HbO] and Δ[oxi‐CCO], rather affecting the penetration depth of light propagation within the brain.
Although our current results do not enable us to predict how long the after‐effects will last, a few recent studies have reported post‐treatment benefits. Yang et al. [39] used a hypoxia‐ischemia model in neonatal rats to investigate tPBM preconditioning and reported that ATP content in the hippocampus collected 6 hours after tPBM was highest compared with ATP levels collected 3, 5, and 8 hours after tPBM. Song et al. [40] demonstrated that tPBM given on the primary motor cortex significantly increased motor‐evoked potentials, which reached its maximum 15 minutes post the stimulation.
Limitations and Future Work
While this study has provided high reproducibility of tPBM being able to upregulate hemodynamic and metabolic activities measured in two different groups of young adults, there are several limitations of the study that need to be further investigated. First, the bb‐NIRS system is very sensitive to motion artifacts during the 15‐minute measurements. The current setup requires the subjects to keep minimal motion and an experienced operator to collect stable and meaningful data. In future studies, an effective methodology for motion artifact detection/removal in the bb‐NIRS data should be developed to warrant the quality and accuracy of tPBM‐induced effects. Second, we need to increase the sample size for the older adults in order to confirm the findings that tPBM induces the same amount of hemodynamic and metabolic increases with respect to the sham condition regardless of age differences. Last, it is unclear how much the measured Δ[oxi‐CCO] signal is affected by the human scalp where a certain concentration of mitochondria exists and their redox state of CCO should contribute to the detected signal. There is no clear answer to solve this problem yet. A potential solution could be a time‐resolved bb‐NIRS approach, allowing for layer‐resolved quantification of Δ[oxi‐CCO] to separate the signals coming from the superficial scalp layer and the cortical regions.
CONCLUSIONS
In this study, we were able to show high reproducibility of sham‐controlled, tPBM‐induced increases in cerebral Δ[HbO] and Δ[oxi‐CCO] measured by bb‐NIRS in a young adult group versus a different group reported in a previous study [16]. The statistical analysis demonstrated no significant differences in tPBM‐induced cerebral effects between the two groups of young participants at two different sites (UTA, UTSW). We also demonstrated the agreement of net tPBM effects with respect to the sham conditions on both young and older adults. These findings indicated the robust reproducibility or reliability of bb‐NIRS in quantifying tPBM effects in healthy humans.
ACKOWLEDGMENTS
This work was supported in part by the National Institute of Mental Health/National Institutes of Health under the BRAIN Initiative (RF1MH114285). We also acknowledge the support in part from the STARS program by the University of Texas System. The authors would like to thank Ms. Devarshi Desai for her assistance with human data collection.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
REFERENCES
- 1. Barrett DW, Gonzalez‐Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 2013;230:13–23. [DOI] [PubMed] [Google Scholar]
- 2. Blanco NJ, Maddox WT, Gonzalez‐Lima F. Improving executive function using transcranial infrared laser stimulation. J Neuropsychol 2017;11(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rojas JC, Gonzalez‐Lima F. Low‐level light therapy of the eye and brain. Eye Brain 2011;3:49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamblin MR. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin 2016;6:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cassano P, Petrie SR, Hamblin MR, Henderson TA, Iosifescu DV. Review of transcranial photobiomodulation for major depressive disorder: Targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics 2016;3(3):031404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naeser MA, Martin PI, Ho MD, et al. Transcranial, red/near‐infrared light‐emitting diode therapy to improve cognition in chronic traumatic brain injury. Photomed Laser Surg 2016;34(12):610–626. [DOI] [PubMed] [Google Scholar]
- 7. Naeser MA, Ho MD, Martin PI, Hamblin MR, Koo BB. Increased functional connectivity within intrinsic neural networks in chronic stroke following treatment with red/near‐infrared transcranial photobiomodulation: Case series with improved naming in Aphasia. Photobiomodul Photomed Laser Surg 2019;38(2):115–131. [DOI] [PubMed] [Google Scholar]
- 8. Hamblin MR, Huang YY. Photobiomodulation in the Brain. San Diago, CA: Acamemic Press; 2019. [Google Scholar]
- 9. Cassano P, Dording C, Thomas G, et al. Effects of transcranial photobiomodulation with near‐infrared light on sexual dysfunction. Lasers Surg Med 2019;51(2):127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez‐Lima F, Valla J, Cada A. Brain cytochrome oxidase activity and how it relates to the pathophysiology of memory and Alzheimer's disease In: Ozben T, editor. Free Radicals, Oxidative Stress and Antioxidants: Pathological and Physiological Significance. New York, NY: Plenum Press; 1998. 296:pp 205–227. [Google Scholar]
- 11. De Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low‐level light therapy. IEEE J Sel Top Quantum Electron 2016;22:7000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gonzalez‐Lima F, Barksdale BR, Rojas JC. Mitochondrial respiration as a target for neuroprotection and cognitive enhancement. Biochem Pharmacol 2014;88(4):584–593. [DOI] [PubMed] [Google Scholar]
- 13. Khakh BS, Gittermann D, Cockayne DA, Jones A. ATP modulation of excitatory synapses onto interneurons. J Neurosci 2003;23(19):7426–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang YY, Gupta A, Vecchio D, et al. Transcranial low level laser (light) therapy for traumatic brain injury. J Biophotonics 2012;5(11–12):827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawauchi S, Sato S, Ooigawa H, Nawashiro H, Ishihara M, Kikuchi M. Simultaneous measurement of changes in light absorption due to the reduction of cytochrome c oxidase and light scattering in rat brains during loss of tissue viability. Appl Opt 2008;47(22):4164–4176. [DOI] [PubMed] [Google Scholar]
- 16. Wang X, Tian F, Reddy DD, et al. Up‐regulation of cerebral cytochrome‐c‐oxidase and hemodynamics by transcranial infrared laser stimulation: A broadband near‐infrared spectroscopy study. J Cereb Blood Flow Metab 2017;37(12):3789–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang X, Tian F, Soni SS, Gonzalez‐Lima F, Liu H. Interplay between up‐regulation of cytochrome‐c‐oxidase and hemoglobin oxygenation induced by near‐infrared laser. Sci Rep 2016;6:30540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol 2001;64(6):575–611. [DOI] [PubMed] [Google Scholar]
- 19. Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: Links to heart disease. Circulation 2003;107(2):346–354. [DOI] [PubMed] [Google Scholar]
- 20. Kadenbach B, Ramzan R, Vogt S. Degenerative diseases, oxidative stress and cytochrome c oxidase function. Trends Mol Med 2009;15(4):139–147. [DOI] [PubMed] [Google Scholar]
- 21. Simon HU, Haj‐Yehia A, Levi‐Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000;5(5):415–418. [DOI] [PubMed] [Google Scholar]
- 22. Hill S, Remmen HVan. Mitochondrial stress signaling in longevity: A new role for mitochondrial function in aging. Redox Biol 2014;2:936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leutner S, Eckert A, Muller WE. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. J Neural Transm (Vienna) 2001;108(8–9):955–967. [DOI] [PubMed] [Google Scholar]
- 24. Collman JP, Devaraj NK, Decreau RA, et al. A cytochrome C oxidase model catalyzes oxygen to water reduction under rate‐limiting electron flux. Science 2007;315(5818):1565–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Musatov A, Robinson NC. Susceptibility of mitochondrial electron‐transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radic Res 2012;46(11):1313–1326. [DOI] [PubMed] [Google Scholar]
- 26. Vargas E, Barrett DW, Saucedo CL, et al. Beneficial neurocognitive effects of transcranial laser in older adults. Lasers Med Sci 2017;32(5):1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamblin MR. Mechanisms of photobiomodulation in the brain In: Hamblin MR, Huang YY, editors. Photobiomodulation in the Brain. San Diago, CA: Academic Press; 2019. [Google Scholar]
- 28. Hamblin MR. The role of nitric oxide in low level light therapy In: Hamblin MR, Waynant RW, Anders J, editors. Mechanisms for Low‐Light Therapy III. SPIE ‐ International Society for Optics and Photonics; 2008;6846:pp 684602–1. [Google Scholar]
- 29. Eshaghi E, Sadigh‐Eteghad S, Mohaddes G, Rasta SH. Transcranial photobiomodulation prevents anxiety and depression via changing serotonin and nitric oxide levels in brain of depression model mice: A study of three different doses of 810 nm laser. Lasers Surg Med. 2019;51(7):634–642. [DOI] [PubMed] [Google Scholar]
- 30. Mitchell UH, Mack GL. Low‐level laser treatment with near‐infrared light increases venous nitric oxide levels acutely: A single‐blind, randomized clinical trial of efficacy. Am J Phys Med Rehabil 2013;92(2):151–156. [DOI] [PubMed] [Google Scholar]
- 31. Tachtsidis I, Koh PH, Stubbs C, Elwell CE. Functional optical topography analysis using statistical parametric mapping (SPM) methodology with and without physiological confounds. Adv Exp Med Biol 2010;662:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kolyva C, Tachtsidis I, Ghosh A, et al. Systematic investigation of changes in oxidized cerebral cytochrome c oxidase concentration during frontal lobe activation in healthy adults. Biomed Opt Express 2012;3(10):2550–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kolyva C, Ghosh A, Tachtsidis I, et al. Cytochrome c oxidase response to changes in cerebral oxygen delivery in the adult brain shows higher brain‐specificity than haemoglobin. NeuroImage 2014;85(Pt 1):234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferrari M, Quaresima V. A brief review on the history of human functional near‐infrared spectroscopy (fNIRS) development and fields of application. NeuroImage 2012;63(2):921–935. [DOI] [PubMed] [Google Scholar]
- 35. Bale G, Mitra S, Meek J, Robertson N, Tachtsidis I. A new broadband near‐infrared spectroscopy system for in‐vivo measurements of cerebral cytochrome‐c‐oxidase changes in neonatal brain injury. Biomed Opt Express 2014;5(10):3450–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bainbridge A, Tachtsidis I, Faulkner SD, et al. Brain mitochondrial oxidative metabolism during and after cerebral hypoxia‐ischemia studied by simultaneous phosphorus magnetic‐resonance and broadband near‐infrared spectroscopy. NeuroImage 2014;102(Pt 1):173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bale G, Elwell CE, Tachtsidis I. From Jobsis to the present day: A review of clinical near‐infrared spectroscopy measurements of cerebral cytochrome‐c‐oxidase. J Biomed Opt 2016;21(9):091307. [DOI] [PubMed] [Google Scholar]
- 38. Rajaram A, Bale G, Kewin M, et al. Simultaneous monitoring of cerebral perfusion and cytochrome c oxidase by combining broadband near‐infrared spectroscopy and diffuse correlation spectroscopy. Biomed Opt Express 2018;9(6):2588–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang L, Dong Y, Wu C, et al. Photobiomodulation preconditioning prevents cognitive impairment in a neonatal rat model of hypoxia‐ischemia. J Biophotonics 2019;12(6):e201800359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Song P, Han T, Lin H, et al. Transcranial near‐infrared stimulation may increase cortical excitability recorded in humans. Brain Res Bull 2020;155:155–158. [DOI] [PubMed] [Google Scholar]


