The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure upon reasonable request to the corresponding author.
Hospitalized COVID-19 patients frequently have myocardial injury with troponin elevation1–4, but underlying etiologies beyond acute coronary syndromes (ACS) and pulmonary emboli (PE) are ill-defined. We used cardiovascular magnetic resonance (CMR) during early convalescence to assess the presence, type and extent of myocardial injury in troponin-positive COVID-19 patients.
All patients with COVID-19 discharged from the Royal Free London NHS Foundation Trust until 30th April 2020 were reviewed (Figure). Diagnosis was either by (i) positive oro/nasopharyngeal throat swab for SARS-CoV-2 by reverse-transcriptase-polymerase-chain-reaction (RT-PCR), or (ii) negative swabs for SARS-CoV-2 but the triad of: viral illness symptoms (≥1 of cough, fever, myalgia), typical blood biomarkers (≥1 of new lymphopenia, high d-dimer, high ferritin, elevated liver transaminases), and probable likelihood of COVID-19 infection on thoracic imaging. A CMR scan (1.5T, Magnetom Aera, Siemens Healthcare, Erlangen, Germany) was offered to patients discharged with a COVID-19 diagnosis and myocardial injury as indicated by elevated high-sensitivity troponin T (hsTnT, >14ng/L). Exclusions were: hospitalization with ACS or PE, known cardiac pathology likely to cause scar, age ≥80 years, severe renal impairment, pregnancy, medical unsuitability and standard CMR contraindications. CMR included cines, native myocardial T1 and T2 mapping, early and late gadolinium enhancement (LGE) with, if no contraindications, adenosine stress perfusion imaging. The CMR diagnosis of myocarditis followed published expert recommendations5. Ethical approval was obtained (London Hampstead Research Ethics Committee, reference 19/LO/1561) and all patients provided written informed consent.
Figure. CONSORT diagram of patient selection for CMR (left panel) with examples of CMR study diagnoses (right panel).
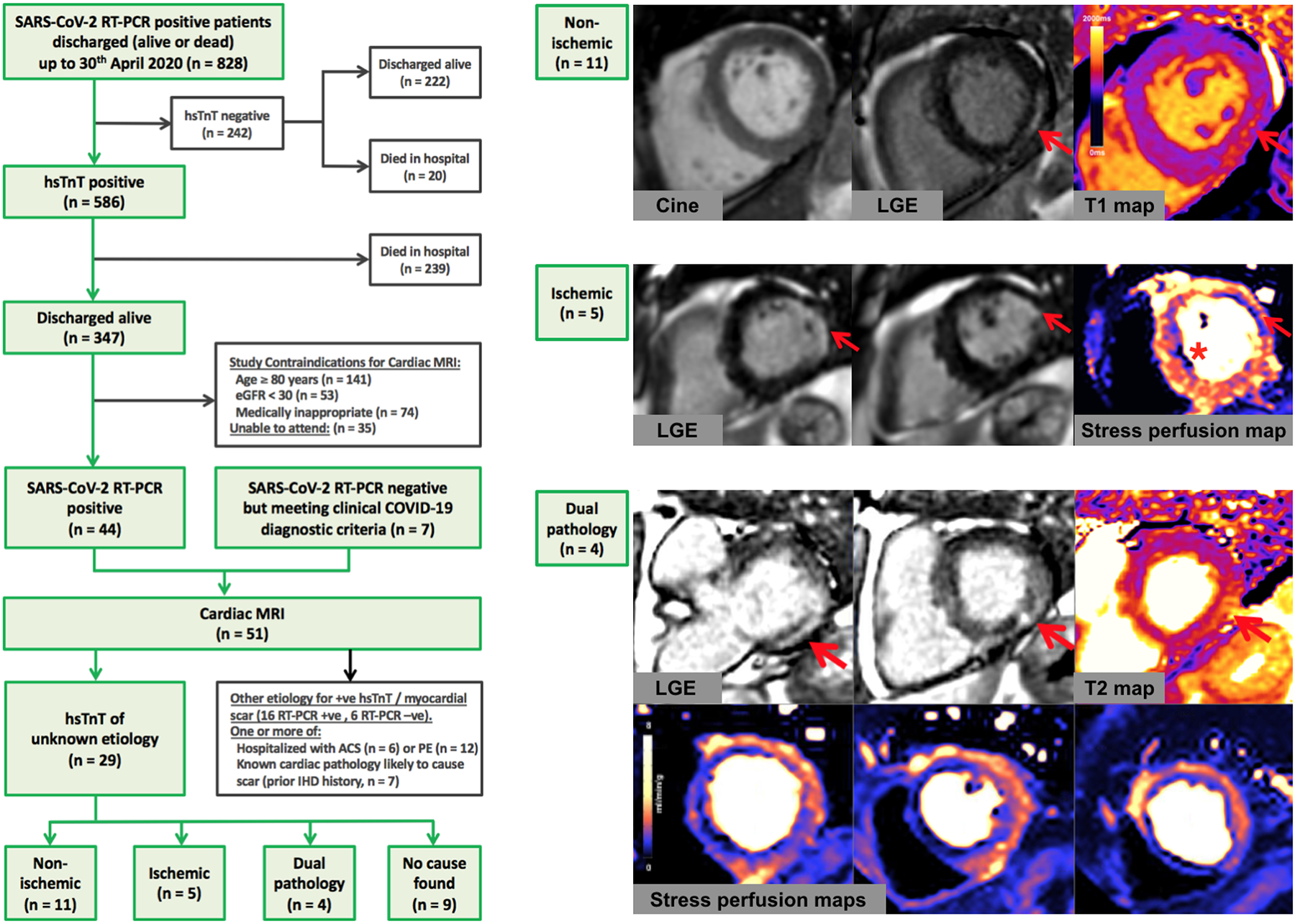
The top case shows an example CMR images of a patient with myocarditis. The basal short-axis slice with LGE showed subepicardial scar of the basal inferolateral wall (red arrow) and the corresponding native T1 map demonstrated elevated myocardial T1 in the corresponding area consistent with myocardial scar and/or edema. The middle case shows an example of a potential ischemic etiology for troponin leak. There is anterolateral subendocardial LGE (red arrow) signifying myocardial infarction along with septal inducible myocardial ischemia (asterisk) on quantitative stress perfusion maps performed during adenosine-induced hyperemia. The bottom case shows example CMR images of a patient with a dual diagnosis of extensive inducible myocardial ischemia along with myocarditis. The LGE showed diffuse patchy subepicardial enhancement of the myocardium predominantly in the basal inferolateral wall (red arrows) with the T2 map showing associated myocardial edema. Quantitative stress perfusion maps showed extensive inducible ischemia predominantly in the left anterior descending and right coronary artery territories. Abbreviations: eGFR, estimated glomerular filtration rate; hsTnT, high-sensitivity troponin T; LGE, late gadolinium enhancement; MRI, magnetic resonance imaging; RT-PCR, reverse-transcriptase-polymerase-chain-reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The majority (71%) of the 828 RT-PCR-positive patients had elevated hsTnT, which was associated with higher in-patient mortality (elevated vs normal hsTnT: 239/586 (41%) died vs 20/242 (8%), respectively, p<0.001). Fifty-one patients were referred for CMR, twenty-two of whom had one or more identifiable causes of troponin elevation (six with ACS, twelve with PE) and/or known cardiac pathology (seven had a history of ischemic heart disease). The remaining twenty-nine patients that were included in the final analysis had unexplained myocardial injury and no cause for prior myocardial scarring, nineteen of whom (66%) underwent additional adenosine stress perfusion. The intervals between CMR and symptom onset, diagnosis or discharge were 46±15 days, 37±10 days and 27±11 days, respectively.
Of the twenty-nine patients with elevated hsTnT of unknown etiology (average age 64±9 years), most were male (twenty-four, 83%) and RT-PCR positive (twenty-eight, 97%). Median in-patient stay was nine days (6–16) and ten (34%) required intensive care unit (ICU) ventilatory support. Admission hsTnT levels were 23.0ng/L (19.0–32.8). Patients had lymphopenia (lymphocyte count 0.76±0.37×109/L) and elevated C-reactive protein (CRP, 227.8±126.5mg/L) during admission. On CMR, twenty (69%) patients had residual lung parenchymal changes. Four (14%) had pleural effusions and two (7%) had pericardial effusions. Mean biventricular systolic function for the overall cohort was normal (LVEF 67.7±11.4%, RVEF 63.7%±9.5%); one patient had mild left ventricular (LV) dysfunction, one had severe biventricular dysfunction. Using the LGE technique and (where possible) stress perfusion imaging, twenty patients (69%) had an identifiable mechanism of myocardial injury (Figure), classified as non-ischemic heart disease-related (eleven patients, 38%), ischemic heart disease-related (five, 17%) or dual ischemic and non-ischemic pathology (four, 14%).
A non-ischemic etiology of elevated hsTnT was conferred by the presence of non-infarct pattern LGE (not corresponding to a coronary territory and sparing the endocardium). The LGE patterns were myocarditis-like in thirteen patients (45%) by international criteria5, with two other patients having non-specific mid-wall LGE only. These patients all had normal LV function (LVEF 70.4±6.9%) with no regional wall motion abnormalities. The median extent of the myocarditis-pattern LGE was 2 (1–2.5) segments with no significant residual myocardial edema in the overall cohort (peak myocardial T2 51.0ms (49–54) in myocarditis-like LGE patients vs 51.0ms (48–53) non-myocarditis, p=0.68). Peak CRP (211±103 vs 255±150mg/L, p=0.35) and hsTnT (25.0 (18.5–37.5) vs 27.0ng/L (20.3–47.0), p=0.48) were not significantly elevated compared to non-myocarditis patients. Four of the patients admitted to ICU had evidence of myocarditis (40% of the ICU subgroup).
Nine of the 29 patients were considered to have an ischemic etiology of their elevated hsTnT. Of these, 7 had inducible ischemia, 1 had a prior unknown myocardial infarction by LGE, and 1 had both inducible ischemia and a prior infarction by LGE. When present, inducible ischemia was multi-territorial in half of these cases (ischemic burden 7.3±5.8 segments). Four patients had dual ischemic and non-ischemic pathology: two had myocarditis-pattern LGE and inducible ischemia, and two had non-specific mid-wall LGE along with either inducible ischemia or a MI.
In summary, myocardial injury is common in hospitalized COVID-19 patients and not exclusive to those with ACS or PE. In this single-centre, single-timepoint convalescent study, myocardial injury was associated with cardiac abnormalities detected by CMR where troponin elevation is unexplained even when cardiac function is normal. The main limitation of this study is its cross-sectional design which prompts caution regarding causality of myocardial injury and its relationship to previous COVID-19 infection. Nevertheless, CMR frequently revealed occult coronary artery disease, high rates of myocarditis-like LGE and sometimes dual pathology. The lack of edema in these patients suggests the myocarditis-like scar may be permanent. Further serial study would clarify this and assess the long-term clinical consequences of these findings.
Acknowledgments:
We would like to thank Sarah Anderson, lead radiographer, for her invaluable contribution to this work.
Sources of funding: Dr Daniel Knight is directly supported by the National Institute for Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre. Professor James Moon is directly supported by the (UCLH) and Barts NIHR Biomedical Research Centres and through a BHF Accelerator Award. Dr Marianna Fontana is funded by a British Heart Foundation (BHF) Intermediate Fellowship.
Footnotes
Disclosures: All authors declare no competing interests directly related to the submitted work.
References
- 1.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X and Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-30185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei JF, Huang FY, Xiong TY, Liu Q, Chen H, Wang H, Huang H, Luo YC, Zhou X, Liu ZY, et al. Acute myocardial injury is common in patients with covid-19 and impairs their prognosis. Heart. 2020. doi: 10.1136/heartjnl-2020-317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]


