Abstract
Introduction:
Early onset preeclampsia (EOP) and late onset preeclampsia (LOP) have been differentiated with a cut-point of ≤34 weeks. This classical definition has never been examined with respect to maternal characteristics by different gestational age cut-points. We examined maternal characteristics in a population-based cohort of 1736 preeclamptic deliveries at different gestational age cut-points from 30 to 37 weeks (CO30 to CO37).
Material and methods:
Eighteen-year observational population-based historical cohort study (2001-2018). All consecutive births delivered at the Centre Hospitalier Universitaire Hospitalier Sud Reunion’s maternity. Standardized epidemiological perinatal database.
Results:
The incidence of EOP was lower in adolescents (1.8% vs 3.5%, odds ratio [OR] 0.50, P = .17). Conversely, the odds of LOP was increased for women over 35, beginning at C030 (OR 1.13, P = .02) and this effect (OR = 1.2) was still detectable at C037 (P = .06). Among primigravid women, the incidence of EOP was lower than LOP (OR ranging from 0.71 to 0.82 for different CO). Conversely, the incidence of LOP was higher (adjusted OR about 2.7 [CO30-CO34] with a rise to 3.3 at CO37 (P < .001). Women with EOP had a lower body mass index (BMI) as compared with LOP at CO34 and CO37. The adjusted OR (per 5 kg/m2 increment) declined from 1.06 to 1.03 from CO30 to C037 in EOP women. Conversely, for LOP, the adjusted odds ratio (aOR) increased from 1.04 to 1.06 from CO30 to CO37 (P < .001). Gestational diabetes mellitus was not associated with LOP at any cut-off (aOR 1.07, NS) but was protective against EOP from CO30 to CO34 (aOR 0.42, 0.61 and 0.73, respectively, P < .001). This protective effect disappeared at CO37. Chronic hypertension and history of preeclampsia were both EOP and LOP risks but with a much stronger effect for EOP (chronic hypertension: aOR 6.0-6.5, history of preeclampsia: aOR 12-17).
Conclusions:
The 34th week of gestation appears to provide a reasonable cut-point to differentiate between EOP and LOP. Additional research is needed to better describe the possible differences in the pathophysiology of these different phenotypes.
Keywords: early onset preeclampsia, epidemiology, late onset preeclampsia, preeclampsia
1 ∣. INTRODUCTION
Determining a useful gestational age cut-point to differentiate early onset preeclampsia (EOP) from late onset preeclampsia (LOP) has been a topic of discussion in understanding this persistent complication of pregnancy.1 In 1996, Ness & Roberts2 published a seminal paper highlighting the heterogeneity of preeclampsia. They emphasized that preeclampsia often followed an intrinsically normal placentation and remodeling of the spiral arteries. Further, these cases were frequently associated with maternal systemic inflammation (eg diabetes and obesity) but were not associated with small-for-gestational-age newborns.2 They suggested that ‘maternal preeclampsia’ differed from ‘placental preeclampsia’ based on the extent of trophoblastic invasion in the first and early 2nd trimester of pregnancy as well as high rates of fetal growth restriction. They proposed that ‘placental preeclampsia’ was linked to EOP, whereas ‘maternal preeclampsia’ was linked to LOP.2
This publication led to a general consensus among most researchers that preeclampsia manifested in two distinct phenotypes and a cut-point of 34 weeks’ gestation would differentiate EOP from LOP.3 Other characteristics of pregnancy, however, that may have further supported this conclusion were not explored in a population-based study.4 The late onset form, LOP, is the most prevalent, constituting approximately 90% of cases in developed countries and 70% in developing countries.5,6
The literature has reported that LOP (both preterm and term preeclampsia) was associated with increased maternal body mass index (BMI). This was predominately due to pre-pregnancy maternal weight and different stages of obesity (class I-III). Conversely, EOP had a weaker association with maternal pre-pregnancy BMI (ppBMI).7 These reports may be considered important insights into the epidemiological differences across different parts of the world between the incidences of EOP and LOP.5-7 Notwithstanding, there remains considerable discussion around the paradigm that “EOP is associated with lack of spiral artery remodelling, while LOP may center around interactions between senescence of the placenta and a maternal genetic predisposition to cardiovascular and metabolic disease”.3
In the present investigation, EOP was differentiated from LOP based on the gestational age at which preeclampsia resulted in delivery of the pregnancy. There remains the question, however, of whether the current gestational age cut-point is supported by other clinical observations. The aim of the current study—using a large population cohort—was closely to evaluate different risk profiles at 30, 32, 34 and 37 weeks’ gestation in order to establish whether clinical and demographic data would support an optimal cut-point.
2 ∣. MATERIAL AND METHODS
From 1 January 2001 to 31 December 2018, the hospital records of all women delivered at the maternity department of the University of South Reunion Island (ca. 4300 births per year) were abstracted in a standardized fashion. The study sample was drawn from the hospital perinatal database, which has prospectively recorded data of all mother-infant pairs since 2001; multiple pregnancies were excluded from the study and all normotensive singleton pregnancies included as reference. Information is collected at the time of delivery and at the infant hospital discharge and is regularly audited by appropriately trained staff. This epidemiological perinatal database contained information on obstetrical risk factors, description of deliveries and neonatal outcomes. For the purpose of this study, records were validated and used anonymously. Additionally, during the prenatal follow up during pregnancy, as participants in the French national healthcare system, all pregnant women in Reunion Island have their prenatal visits, biological and ultrasonographic examinations and anthropological characteristics recorded in their maternity booklet before coming to the maternity department for delivery. Preeclampsia, gestational hypertension and eclampsia were diagnosed according to the definition issued by the International Society for the Study of Hypertension in Pregnancy (ISSHP) relative to the guidelines in force in the year of pregnancy.
2.1 ∣. Design and study population
The maternity department of Saint-Pierre Hospital is a tertiary care center that performs about 4300 deliveries per year, thus representing about 80% of deliveries of the Southern area of Reunion Island, and is the only level 3 maternity department (the other maternity department is a private level 1 hospital, which is not allowed to follow/deliver preeclamptic pregnancies). Reunion Island is a French overseas region in the Southern Indian Ocean. Virtually the whole population at that time had access to health care, provided free of charge by the French healthcare system, which combines freedom of medical practice with nationwide social security.
2.2 ∣. Definition of exposure and outcomes
We defined early and late onset preeclampsia as the date at which preeclampsia resulted in delivery of the pregnancy. Infants were considered small for gestational age when the age-adjusted birth weight was below the 10th percentile according to normal tables for our specific population.
Renal diseases were defined as known preexisting nephropathies without hypertension (glomerulopathies, tubulopathies, renal failure, diabetic nephropathies). Urological pathologies were excluded. Thyroid diseases were defined as hypo/hyperthyroidism, goiter, thyroiditis and thyroidectomy. Coagulopathies were defined as antiphospholipid syndrome, protein C/protein S deficit, factor 5 Leyden or other coagulation factor deficits at the time they were reported in the records (they were not systematically screened in all women as in a case-control study).
Screening of gestational diabetes mellitus (GDM) is systematically carried out in all pregnant women in the first trimester: until 2016 the O’Sullivan test was used (50 g glucose, blood glucose level after 1 hour), the threshold for hyperglycemia being 1.4 g/L. Since 2016, this test has been replaced in all women by a fasting glycemia in the first trimester, the threshold for positivity being 0.92 g/L. As the incidence of GDM is very high in Reunion, a glucose tolerance test is done (at 24-28 weeks) in all pregnant women (even if they have a normal 1st trimester blood glucose), except those who had a 1st trimester blood glucose >1.26 g/L, these being considered to have Type 2 diabetes.
2.3 ∣. Statistical analyses
Data are presented as numbers and proportions (%) for categorical variables and as mean and standard deviation (SD) for continuous ones. Comparisons between groups were performed using the Chi-square test; odds ratio (OR) with 95% confidence interval was also calculated. Paired t test was used for parametric and the Mann-Whitney U test for non-parametric continuous variables. P-values <.05 were considered statistically significant. Epidemiological data were recorded and analyzed with the software EPI-INFO™ 7.1.5, 2008 (Centers for Disease Control and Prevention, Atlanta, GA, USA), EPIDATA 3.0 (http://www.epidata.dk) and EPIDATA ANALYSIS V2.2.2.183 (http://www.epidata.dk, Denmark).
Further, to validate the independent association of maternal ppBMI or maternal ages and other confounding factors with EOP or LOP we realized a multiple regression logistic model. Variables associated with bivariate analysis, with a P-value <.1 or known to be associated with the outcome in the literature were included in the model. A stepwise backward strategy was then applied to obtain the final model. The goodness of fit was assessed using the Hosmer-Lemeshow test. A P-value <.05 was considered significant. All analyses were performed using MEDCALC software version 12.3.0 (MedCalc Software, Ostend, Belgium).
2.4 ∣. Ethical approval
This study was conducted in accordance with French legislation. As per new French law applicable to trials involving human subjects (Jardé Act), a specific approval of an ethics committee (Comité de Protection des Personnes, COP) is not required for this non-interventional study based on retrospective, anonymized data of authorized collections, and written patient consent is not needed. Nevertheless, the study was registered on UMIN Clinical Trials Registry (identification number UMIN000037012).
3 ∣. RESULTS
During the 18-year period, there were 96 861 births in the South of the Reunion Island with an incidence of preeclampsia of 1842 (1.9%), of which 106 were multiple pregnancies (5.8%). The study population, therefore, consisted of 1736 singleton preeclamptic pregnancies.
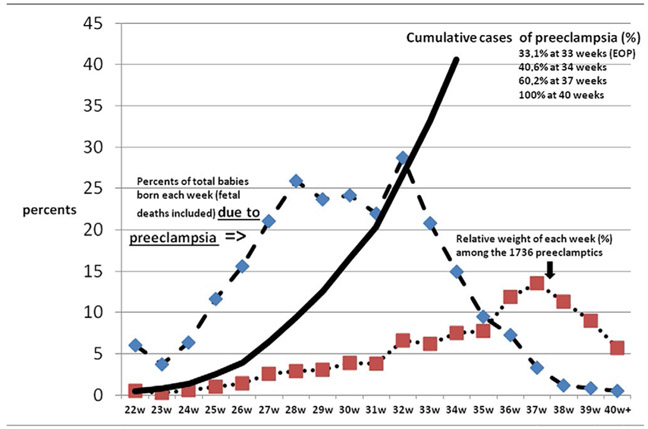
Figure 1 depicts the weight of preeclampsia in the 3rd trimester of pregnancy in this population.
FIGURE 1.
Preeclampsia and the third trimester of pregnancy in Reunion island
Using the internationally accepted cut-off of 34 weeks’ gestation (CO34), EOP represented 33% of the preeclampsia deliveries (dark line). For each week of gestation, preeclampsia newborns (intrauterine fetal demise and alive) represented 25%-30% of deliveries between week 27 and week 33 (mostly iatrogenic preterm birth) (blue line). For the preeclamptic cohort (red line: 1736 singleton pregnancies), the peak of deliveries was at 37 weeks, with a rise beginning at week 26.
Figure 2 depicts the association of maternal ppBMI with the incidence of EOP and LOP, beginning with very lean women (15-19 kg/m2) to the morbidly obese (BMI 45 kg/m2 onward). LOP was predominately associated with rising maternal ppBMI, with a much weaker association with EOP when considering different cut-points: CO30 to CO34. This association disappeared at CO37 (in Reunion, LOP women after 37 weeks represent 40% of preeclampsia cases; Table 4). At CO37, EOP and LOP were equivalent, with a parallel trends, but there was still an ongoing rise with increases of maternal ppBMI.
FIGURE 2.
Association between maternal pre-pregnancy body mass index (BMI) (from lean women to obesity class I-III) with early onset preeclampsia (EOP) and late onset preeclampsia (LOP): overweight and rising obesities are always linearly linked to LOP and not to EOP until the cut-off 34 weeks. PE, preeclampsia
TABLE 4.
Cut-off at 37 weeks. Crude results. Differences between early onset preeclampsia (EOP) and late onset preeclampsia (LOP): EOP: n = 1047 (60.3%); LOP, n = 689 (39.7%). Multiparae with preeclampsia: EOP, n = 578 (64.3%); LOP, n = 321 (35.7%)
|
Nonsignificant results Left numbers EOP n = 466 Right numbers LOP n = 1270 |
P value |
Significant results EOP vs LOP OR [95% CI] Underlined: difference with cut-off at 34 weeks |
P value | ||||
|---|---|---|---|---|---|---|---|
| Adolescents (<18 y) | 3.2% vs 3.8% | OR 0.89 | .48 | Mother age (y, SD) | 29.3 vs 28.3 | .002 | |
| Grand multiparae (5+) | 10.4% vs 9.1% | OR 1.15 | .38 | Primigravidity | 32.0% vs 39.9% | 0.71 [0.58-0.87] | .001 |
| BMI ≥25 kg/m2 | 53.4% vs 53.9% | OR 0.98 | .46 | Chronic hypertension | 11.8% vs 7.8% | 1.58 [1.13-2.2] | .007 |
| Preexisting diabetes | 4.2% vs 4.6% | OR 0.91 | .69 | Pre-pregnancy/booking BMI | 26.6 vs 27.3 Kg/m2 | .03 | |
| Coagulopathyb | 0.8% vs 0.4% | OR 1.98 | .29 | ||||
| Smoking | 9.4% vs 8.3% | OR 1.14 | .44 | ||||
| Antecedent | 31.5% vs 29.7% | OR 1.09 | .53 | ||||
| miscarriage | |||||||
| Antecedent abortion | 25.4% vs 22.5% | OR 1.18 | .26 | ||||
| Antecedent thyroid diseasea | 2.4% vs 1.3% | OR 1.8 | .11 | ||||
| Antecedent renal Disease | 1.4% vs 0.7% | OR 1.8 | .23 | ||||
| Allergy/asthma | 7.3% vs 6.7% | OR 1.11 | .32 | ||||
| 35 y+ | 26.2% vs 22.2% | OR 1.24 | .06 | ||||
| Medically induced | 2.1% vs 1.0% | OR 2.1 | .08 | ||||
| BMI ≥30 kg/m2 | 27.4% vs 31.5% | OR 0.82 | .07 | ||||
| Differences from cut-off at 34 week’s gestation | |||||||
| Items becoming NON significant | Items becoming SIGNIFICANT | ||||||
| Antecedent | 12.6% vs 9.9% | 1.31 | .16 | Gestation (mean, | 2.9 vs 2.6 | .006 | |
| preeclampsia | SD) | ||||||
| Antecedent perinatal | 11.8% vs 9.4% | 0.77 | .21 | Parity (mean, SD | 1.28 vs 1.10 | .02 | |
| deaths | |||||||
| Gestational diabetes | 15.5% vs 13.9% | 1.13 | .38 | Primiparity | 44.8% vs | OR 0.71 | .001 |
| 53.4% | |||||||
| Single mother | 35.3% vs 40.1% | OR 0.82 | .04 | ||||
| Years of schooling ≥10 | 53.5% vs 58.34% | OR 0.82 | .05 | ||||
Thyroid diseases: goiter, hypo/hyperthyroidy, thyroidectomy, thyroid node, thyroiditis.
“Coagulopathies”: antiphospholipid syndrome, protein C/protein S deficit, factor 5, Leyden or other coagulation factors.
EOP women had a higher mean age than LOP women with a gap of 1.7 years (30.4 vs 28.7) at CO30, 1.1 years at CO32 and CO34, and 1.0 year at CO37. In Table 5, in the logistic regression for both EOP and LOP, the adjusted odds ratio (aOR) was ≈1.04 (increase of 4% per each increment of 5 years of age). The effect was the same from CO30 to CO37, but always with an “older advantage” for EOP women. Younger women had a higher LOP, particularly adolescents at CO30 and CO32 and women <25 years at CO34. Conversely, older women had a higher EOP: age ≥30 years for CO30 and CO32 and age ≥35 years at CO37.
TABLE 5.
Adjusted odds ratios. Logistic model. Outcomes: (a) early onset preeclampsia (EOP), (b) late onset preeclampsia (LOP). Preeclamptic women (n = 1736), vs controls (normotensive women, n = 71 090)
| Cut-off 30 weeks aOR | P value | Cut-off 32 weeks aOR | P value | Cut-off 34 weeks aOR | P value | Cut-off 37 weeks aOR | P value | |
|---|---|---|---|---|---|---|---|---|
| EOP | ||||||||
| Maternal age (increment/5 y of age) | 1.04 [1.01-1.07] | .005 | 1.05 [1.02-1.07] | <.0001 | 1.04 [1.02-1.06] | <.0001 | 1.04 [1.03-1.06] | <.0001 |
| BMI (increment/5 kg/m2) | 1.06 [1.03-1.09] | <.0001 | 1.05 [1.03-1.07] | <.0001 | 1.04 [1.02-1.05] | <.0001 | 1.035 [1.02-1.04] | <.0001 |
| Antecedent preeclampsia | 17.04 [10.5. 27.5] | <.0001 | 12.6 [8.3.19.3] | <.0001 | 12.8 [9.2-17.9] | <.0001 | 9.4 [7.2-12.4] | <.0001 |
| Coagulopathies | 3.85 [0.91-16.2] | .06 | 3.7 [1.16-12.2] | .02 | 2.95 [1.06-8.2] | .04 | 3.1 [1.5-6.12] | .001 |
| GDM | 0.42 [0.23-0.77] | .005 | 0.61 [0.4-0.93] | .02 | 0.73 [0.52-1.0] | .05 | 1.05 | .61 |
| IVF | 2.8 | .16 | 1.39 | .64 | 2.06 | .12 | – | – |
| Chronic HBP | 6.4 [3.8-10.82] | <.0001 | 6.0 [3.9-9.26] | <.0001 | 6.51 [4.6-9.2] | <.0001 | 6.2 [4.9-7.8] | <.0001 |
| Renal diseases | 0.0 | .94 | 1.25 | .77 | 1.43 | .53 | 1.43 | .53 |
| Primiparity | 2.16[1.2-3.7] | .005 | 2.6 [1.7-3.9] | <.0001 | 2.53 [1.9-3.4] | <.0001 | 2.38 [2.0-2.8] | <.0001 |
| ART pregnancy | 3.7 | .19 | 4.2 [1.01-17.7] | .05 | 3.9 [1.2-12.7] | .02 | – | – |
| Smoking | 0.66 | .19 | 0.76 | .27 | 0.89 | .52 | 0.80 [0.63-1.0] | .06 |
| Thyroid diseases | 1.18 | .17 | 1.26 | .61 | 1.39 | .33 | 1.32 | .20 |
| LOP | ||||||||
| Maternal age (increment/5 y of age) | 1.04 [1.03-1.06] | <.0001 | 1.04 [1.02-1.057] | <.0001 | 1.04 [1.02-1.04] | <.0001 | 1.038 [1.02-1.05] | <.0001 |
| BMI (increment/5 kg/m 2) | 1.044 [1.03-1.05] | <.0001 | 1.047 [1.04-1.06] | <.0001 | 1.05 [1.04-1.06] | <.0001 | 1.063 [1.05-1.07] | <.0001 |
| Antecedent preeclampsia | 8.27 [6.4-10.6] | <.0001 | 8.2 [6.3.10.6] | <.0001 | 7.11 [5.4-9.4] | <.0001 | 8.3 [5.8-11.9] | <.0001 |
| Coagulopathies | 2.35 [1.12-4.9] | .02 | 1.7 | .17 | 1.66 | .22 | 1.4 | .54 |
| GDM | 1.07 | .41 | 1.08 | .36 | 1.12 | .19 | 0.90 | .40 |
| IVF | 0.98 | .97 | 1.1 | .70 | 1.03 | .91 | 0.74 | .51 |
| Chronic HBP | 4.8 [3.8-6.1] | <.0001 | 4.7 [3.8-5.9] | <.0001 | 4.45 [3.5-5.6] | <.0001 | 3.4 [2.5-4.8] | <.0001 |
| Renal diseases | 2.17 [1.06-4.4] | .03 | 1.99 [1.04-3.8] | .04 | 2.02 [1.01-4.0] | .05 | 1.9 | .17 |
| Primiparity | 2.68 [2.2-3.3] | <.0001 | 2.7 [2.4-3.1] | <.0001 | 2.75 [2.4-3.2] | <.0001 | 3.3 [2.8-4.0] | <.0001 |
| ART pregnancy | 1.31 | .64 | 0.89 | .80 | 0.58 | .36 | 0.65 | .54 |
| Smoking | 0.89 | .30 | 0.76 [0.6-0.9] | .006 | 0.75 [0.6-0.9] | .008 | 0.72 [0.54-0.95] | .02 |
| Thyroid diseases | 1.02 | .91 | 1.07 | .74 | 0.94 | .81 | 0.79 | .50 |
ART, assisted reproductive technology; BMI, body mass index; GDM, gestational diabetes mellitus; HBP, high blood pressure; IVF, in vitro fertilization.
First pregnancies had a lower EOP: OR 0.71 at CO37 (P = .01), 0.78 at CO34 (P = .02), 0.82 at CO32 (P = .09) and .76 at CO30 (P = .06). In the logistic regression model, the odds for LOP for first pregnancy were approximately ≈2.7 (CO30 to CO34) with a rise at 3.3 at CO37, P < .001.
EOP women had a lower BMI compared with women with LOP. This association was observed beginning at CO34 and at CO37 (26.6 vs 27.3 kg/m2, P = .03). When considering Table 5, the logistic regression model, the aOR declined from 1.06 (6% increase for each increment of 5 kg/m2) to 1.03 from CO30 to CO37 in EOP women. The reverse was observed for LOP: aOR 1.04-1.06 from CO30 to CO37.
In the logistic regression, controlling for maternal ages and maternal ppBMI, GDM was not associated with LOP (aOR = 1.07) (Table 5). In contrast, it was protective against EOP from CO30 to CO34 (aOR 0.42, 0.61, 0.73, respectively). This apparent “protective effect” disappeared at CO37.
Chronic hypertension: Chronic hypertension was associated with both EOP and LOP risks (Table 5) (aOR 4.5 for LOP vs aOR 6.0-6.5 for EOP) predominantly from CO30 to CO37 (aOR 1.45-1.6; Tables 1-4).
TABLE 1.
Cut-off at 34 weeks (“Reference”). Crude results. Differences between early onset preeclampsia (EOP) and late onset preeclampsia (LOP): EOP, n = 574 (33.0%); LOP, n = 1162 (67%). Multiparae with preeclampsia: EOP, n = 314 (34.9%); LOP, n = 585 (65.0%)
|
Nonsignificant results Left numbers EOP n =574 Right numbers LOP n=1162 |
P value |
Significant results EOP vs LOP ODDS ratios [95% CI] |
P value | ||||
|---|---|---|---|---|---|---|---|
| Gestation (mean, SD) | 2.91 vs 2.73 | .10 | Mother’s age (y, SD) | 29.7 vs 28.6 | .002 | ||
| Parity (mean, SD | 1.29 vs 1.17 | .25 | Primigravidity | 31.4% vs 37.0% | 0.78 [0.63-0.96] | .02 | |
| Primiparity | 45.3% vs 49.7% | OR 0.84 | .11 | Chronic hypertension | 12.7% vs 9.2% | 1.47 [1.05-1.9] | .02 |
| Adolescents (<18 y) | 3.1% vs 3.5% | OR 0.89 | .29 | Gestational diabetes | 11.9% vs 17.9% | 0.68 [0.50-0.92] | .009 |
| Grand multiparae (5+) | 11.2% vs 9.5% | OR 1.20 | .29 | Antecedent perinatal deaths | 12% vs 7.4% | 1.78 [1.2-2.7] | .05 |
| Mother living Single | 36.1% vs 37.72% | OR 0.93 | .53 | Antecedent preeclampsia | 18.1% vs 11.4% | 1.73 [1.2-2.5] | .001 |
| Years of schooling ≥10 | 55.3% vs 55.4% | OR 1.0 | .98 | Pre-pregnancy/booking BMI | 26.4 vs 27.1 kg/m2 | .06 | |
| BMI ≥25 kg/m2 | 55.2% vs 53.1% | OR 1.09 | .47 | ||||
| BMI ≥30 kg/m2 | 28.6% vs 29.3% | OR 0.97 | .79 | ||||
| Preexisting diabetes | 4.3% vs 4.4% | OR 0.99 | .96 | ||||
| Smoking | 9.9% vs 8.6% | OR 1.17 | .40 | ||||
| Antecedent miscarriage | 31.2% vs 30.7% | OR 1.03 | .85 | ||||
| Antecedent abortion | 27.4% vs 23.1% | OR 1.26 | .12 | ||||
| Antecedent thyroid diseasea | 1.5% vs 1.3% | OR 1.12 | .79 | ||||
| Antecedent renal disease | 1.7% vs 0.9% | OR 1.99 | .20 | ||||
| Medically induced | 2.1% vs 1.5% | OR 1.4 | .34 | ||||
| Allergy/asthma | 6.8% vs 7.2% | OR 0.93 | .40 | ||||
| Coagulopathyb | 1.3% vs 0.5% | OR 2.7 | .07 | ||||
| 35 y+ | 27.5% vs 23.5% | OR 1.23 | .09 | ||||
Thyroid diseases: goiter, hypo/hyperthyroidsm, thyroidectomy, thyroid node, thyroiditis.
“Coagulopathies”: antiphospholipid syndrome, protein C/protein S deficit, factor 5, Leyden or other coagulation factors.
In the same way, antecedent preeclampsia was involved in both EOP and LOP risks (Table 5; AOR 8 for LOP), but with a much stronger effect (aOR 12-17) for the EOP risk: occurring predominantly from CO30 to CO37 (aOR 1.45-1.6; Tables 1-4). It is of note that antecedent preeclampsia began this “stronger effect” (aOR 17 and crude OR 2.0) at week 30.
There was a strong association with EOP (Table 5) of “coagulopathies” (aOR 3-3.8) , the highest seen at CO30 (P = .001). In stimulated pregnancies, the adjusted OR was 4 (P = .02) until CO34 (it is of note that in vitro fertilization [crude OR 2] did not reach the level of significance; Table 5). The association with LOP of renal diseases (glomerulo-tubulopathies) (aOR 2; P = .05), began at CO30.
Smoking had a specific protective effect on LOP (aOR 0.7) which appeared strongest at CO32. There is no protective effect for EOP.
Thyroid diseases never reached a level of significance as an effect on either EOP or LOP.
4 ∣. DISCUSSION
To our knowledge, this is the first study to explore different cut-points to define EOP and LOP with the additional inclusion of demographics/complications of pregnancy. We tested whether the chosen cut-off at 34 weeks proposed in the late 1990s and now widely accepted,4 would identify different risk profiles if the cut-off cursor was assigned to another week gestation. Considering EOP, which is the more serious form of preeclampsia, these results identified strong associations with women with a higher mean age, history of previous preeclampsia or perinatal deaths, chronic hypertension and stimulated pregnancies. For LOP, associations were observed for primigravidity or primiparity, younger ages and equivalent patterns of linear risk with increased maternal ppBMI (from overweight to obesity class I-III). Regardless of gestational age cut-point, young women were more likely to manifest LOP and older women ≥30 years more likely to manifest EOP. Women wiith primigravidity and nulliparity were more likely to develop LOP at every cut-off tested. For ppBMI, rising BMI (overweight, obesity class I-III) was associated only with LOP, having only a weak association with EOP from CP30 to CP34, thus confirming a recent report.7 Controlling for maternal age and BMI, GDM was not associated with risk for LOP irrespective of the cut-point (aOR 1.07, NS) and was protective against EOP from CP30 to CP34 (aOR 0.42, 0.61, 0.73, respectively, P < .001). It should be noted, however, that testing for GDM typically occurs at 26-28 weeks, ie, preeclampsia diagnosed prior to the regular time for the glucose tolerance test is by default not associated with GDM in the case of EOP. However, our results seem valid concerning the absence of association with LOP. As a matter of fact, several studies previously proposed GDM as a risk factor (risk X by 2 or 48). Reports associate diabetes and preeclampsia,9-11 especially with LOP.11 However, a few studies 12,13 did not find such an association when controlling for maternal weight and age.
Previous history of preeclampsia, stimulated pregnancies and “coagulopathies” are risk factors for EOP beginning at week 30. This association was reversed with respect to preexisting renal diseases. It is of note that in vitro fertilization pregnancies had a tendency to be associated with EOP beginning at week 30 (aOR 2.8), although not statistically significant. Rising maternal age had a homogeneous effect (aOR 1.04) (ie, a 4% increase for each increment of 5 years of age) for both EOP and LOP. There were no significant differences between EOP and LOP in allergy/asthma risk (Tables 1-4).14 Smoking was protective (30% decrease) only for LOP.
We report a high proportion of EOP in Reunion island, representing 33% of preeclampsia cases using the standard 34-week definition) as contrasted with 10% typically reported in the literature.12-19 This observation was identified by Iacobelli et al in 2016.19 Differences in EOP appear to vary across geographic areas and have the highest incidence in developing counties (88% of world births).6 However, as LOP is more specifically associated with pre-pregnancy maternal overweight or obesity, the effect clearly seen after 30 weeks’ gestation may explain the wide differences we witness around the world concerning the specific weights of LOP in preeclamptic pregnancies among different populations.5-7
The Centre Hospitalier Universitaire Sud-Reunion’s maternity department (Level 3, European standards of care) is the only public hospital in the southern part of Reunion Island (Indian Ocean, French overseas department). It serves the whole population of the area (ca. 360 000 inhabitants and 5100 births per year in the south of the island), but with 4300 births per year the university maternity handles also 82% of the total births of the area. Concerning preeclamptic cases, the university maternity, being level 3, is sole to be allowed to handle these women (the other maternity is a private clinic, level 1). Therefore we are sure all the preeclampsia cases were referred to our hospital during the 18.5-year period. This is then a real population-based study. As a limitation of the study, we have to consider the retrospective nature of the study, which may have meant that although the information recorded is comprehensive, some characteristics were missed, such as length of sexual relationship and/or primipaternity. “Coagulopathies” were not systematically screened in all women (cases and references). However, when a woman was known to have one of these characteristics, she was scrupulously included in the database. Another limitation is we had no information on change of male partner, and we did not test the interval between pregnancies,15, 16 which would have allowed us to test whether there was a “new couple” effect in multiparae, and whether delayed pregnancies could be associated with one form or the other of preeclampsia.17 The strengths of this study are mostly related to the homogeneity of data in such a large cohort, as they were collected in a single center (no intercenter variability) and not based on national birth registers but directly from medical records (avoiding inadequate codes).
5 ∣. CONCLUSION
Based on this detailed dataset in a large population cohort, we have to conclude that there is no “magical” cut-off for differentiating between early and late preeclampsia. Therefore, the current consensual 34-week cut-off can be considered an acceptable compromise until there is a better understanding of underlying pathophysiological pathways of preeclampsia.
TABLE 2.
Cut-off at 30 weeks. Crude results. Differences between early onset preeclampsia (EOP) and late onset preeclampsia (LOP): EOP, n = 218 (12.5%); LOP, n = 1518 (87.5%). Multiparae with preeclampsia: EOP, n = 126 (14.0%); LOP, n = 773 (86%)
|
Nonsignificant results Left numbers EOP nN= 218 Right numbers LOP n =1518 |
P value |
Significant results EOP vs LOP OR [95% CI] Underlined: difference from cut-off at 34 weeks |
P value | ||||
|---|---|---|---|---|---|---|---|
| Gestation (mean, SD) | 2.9 vs 2.8 | .42 | Mother’s age (y, SD) | 30.4 vs 28.7 | .0006 | ||
| Parity (mean, SD | 1.37 vs 1.18 | .21 | |||||
| Adolescents (<18 y) | 1.8% vs 3.6% | OR 0.50 | .17 | Chronic hypertension | 14.7% vs 9.6% | 1.62 [1.07-2.4] | .02 |
| Grand multiparae (5+) | 12.4% vs 9.6% | OR 1.34 | .19 | Gestational diabetes | 9.4% vs 15.6% | 0.56 [0.35-0.91] | .02 |
| Single mother | 33.5% vs 37.8% | OR 0.83 | .22 | Antecedent perinatal deaths | 15.8% vs 8.1% | 2.12 [1.2-3.4 | .001 |
| Years of schooling ≥10 | 56.5% vs 55.3% | OR 1.05 | .74 | Antecedent preeclampsia | 19.1% vs 10.5% | 2.0 [1.26-3.15] | .002 |
| BMI ≥25 kg/m2 | 57.8% vs 53.1% | OR 1.21 | .22 | ||||
| BMI ≥30 kg/m2 | 29.2% vs 29.1% | OR 1.0 | .98 | ||||
| Preexisting diabetes | 4.5% vs 4.3% | OR 1.03 | .92 | ||||
| Smoking | 9.2% vs 8.9% | OR 1.03 | .89 | ||||
| Coagulopathyb | 1.8% vs 0.5% | OR 3.5 | .08 | ||||
| Antecedent miscarriage | 27.6% vs 31.3% | OR 0.84 | .85 | ||||
| Antecedent abortion | 25.0% vs 24.2% | OR 1.04 | .83 | ||||
| Antecedent thyroid diseasea | 2.3% vs 1.9% | OR 1.2 | .70 | ||||
| Antecedent renal disease | 1.4% vs 1.1% | OR 1.3 | .40 | ||||
| Medically induced | 2.3% vs 1.6% | OR 1.46 | .44 | ||||
| Allergy/asthma | 6.9% vs 7.1% | OR 0.96 | .49 | ||||
| Differences with cut-off at 34 weeks’ gestation | |||||||
| Items becoming NON significant | Items becoming SIGNIFICANT | ||||||
| Pre-pregnancy/booking BMI | 27.2 vs 26.8 Kg/M2 | .43 | Primiparity | 42.2% vs 49.1% | 0.76 [0.57-1.01] | .06 | |
| Primigravidity | 30.3% vs 35.80% | OR 0.78 | .10 | 35 y+ | 31.2% vs 23.6% | 1.13 [1.07-2.0] | .02 |
Thyroid diseases: goiter, hypo/hyperthyroidism, thyroidectomy, thyroid node, thyroiditis.
“Coagulopathies”: antiphospholipid syndrome, protein C/protein S deficit, factor 5, Leyden or other coagulation factors.
TABLE 3.
Cut-off at 32 weeks. Crude results. Differences between early onset preeclampsia (EOP) and late onset preeclampsia (LOP): EOP, n = 466 (26.8%); LOP: n = 1270 (73.2%). Multiparae with preeclampsia: EOP, n = 255 (28.4%); LOP, ∣n = 644 (71.6%)
|
Nonsignificant results Left numbers EOP, n = 466 Right numbers LOP, n = 1270 |
P value |
Significant results EOP vs LOP ODDS ratios [95% CI] Underlined: difference with cut-off 34 weeks |
P value | ||||
|---|---|---|---|---|---|---|---|
| Gestation (mean,SD) | 2.9 vs 2.75 | .18 | Mother’s age (y,SD) | 29.7 vs 28.6 | .002 | ||
| Parity (mean, SD | 1.30 vs 1.17 | .14 | Chronic hypertension | 12.9% vs 9.3% | 1.44 [1.04-2.0] | .02 | |
| Primiparity | 45.3% vs 49.3% | OR 0.85 | .14 | Gestational diabetes | 12.1% vs 15.9% | 0.73 [0.53-1.01] | .06 |
| Adolescents (<18 y) | 2.6% vs 3.7% | OR 0.69 | .25 | Antecedent perinatal deaths | 13.9% vs 9.7% | 1.49 [1.0-2.2] | .05 |
| Grand multiparae (5+) | 11.2% vs 9.5% | OR 1.2 | .29 | Antecedent preeclampsia | 14.5% vs 7.5% | 2.07 [1.4-3.0] | .0001 |
| Single mother | 36.1% vs 37.7% | OR 0.93 | .53 | ||||
| Years of schooling ≥10 | 55.3% vs 55.4% | OR 1.0 | .97 | ||||
| BMI ≥25 kg/m2 | 55.2% vs 53.1% | OR 1.09 | .47 | ||||
| BMI ≥30 kg/m2 | 28.6% vs 29.3% | OR 0.97 | .79 | ||||
| Preexisting diabetes | 4.3% vs 4.4% | OR 0.99 | .96 | ||||
| Smoking | 9.9% vs 8.6% | OR 1.12 | .40 | ||||
| Antecedent miscarriage | 312% vs 30.7% | OR 1.03 | .85 | ||||
| Antecedent abortion | 27.4% vs 23.1% | OR 1.26 | .12 | ||||
| Antecedent thyroid diseasea | 1.5% vs 1.3% | OR 1.12 | .79 | ||||
| Antecedent renal disease | 1.7% vs 0.9% | OR 1.99 | .20 | ||||
| Medically induced | 2.1% vs 1.5% | OR 1.4 | .34 | ||||
| Allergy/asthma | 7.1% vs 7.1% | OR 0.99 | .49 | ||||
| Differences from cut-off at 34 weeks’ gestation | |||||||
| Items becoming NON significant | Items becoming SIGNIFICANT | ||||||
| Primigravidity | 32.0% vs 36.3% | OR 0.82 | .09 | Coagulopathyb | 1.3% vs 0.5% | OR 2.7 | .07 |
| Pre-pregnancy/booking BMI | 26.7 vs 26.9 kg/M2 | .60 | 35 y+ | 27.5% vs 23.5% | OR 1.23 | .09 | |
Thyroid diseases: goiter, hypo/hyperthyroidism, thyroidectomy, thyroid node, thyroiditis.
“Coagulopathies”: antiphospholipid syndrome, protein C/protein S deficit, factor 5, Leyden or other coagulation factors.
Key message.
In an analysis of 1700 preeclamptic cases, different cut-offs (from 30 to 37 weeks’ gestation) were tested to define early vs late onset preeclampsia. The standard 34th week of gestation appears to provide a reasonable cutoff point.
Abbreviations:
- aOR
adjusted odds ratio
- BMI
body mass index
- CH
chronic hypertension
- CO30
cutoff gestational age week 30
- CO34
cutoff gestational age week 34
- CO37
cutoff gestational age week 37
- EOP
early onset preeclampsia
- GDM
gestational diabetes mellitus
- LOP
late onset preeclampsia
- OR
odds ratio
- ppBMI
pre-pregnancy BMI
Footnotes
CONFLICT OF INTEREST
None.
REFERENCES
- 1.Robillard PY, Dekker G, Chaouat G, Scioscia M, Iacobelli S, Hulsey TC. Historical evolution of ideas on eclampsia/preeclampsia: a proposed optimistic view of preeclampsia. J Reprod Immunol. 2017;123:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol. 1996;175:1365–1370. [DOI] [PubMed] [Google Scholar]
- 3.Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. [DOI] [PubMed] [Google Scholar]
- 4.Tranquilli AL, Brown MA, Zeeman GG, Dekker G, Sibai BM. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens. 2013;3:44–47. [DOI] [PubMed] [Google Scholar]
- 5.Robillard PY, Dekker G, Iacobelli S, Chaouat G. An essay of reflection: why does preeclampsia exist in humans, and why are there such huge geographical differences in epidemiology? J Reprod Immunol. 2016;114:44–47. [DOI] [PubMed] [Google Scholar]
- 6.Robillard PY, Dekker G, Chaouat G, Elliot MG, Scioscia M. High incidence of early onset preeclampsia is probably the rule and not the exception worldwide. 20th anniversary of the reunion workshop. A summary. J Reprod Immunol. 2019;133:30–36. [DOI] [PubMed] [Google Scholar]
- 7.Robillard P-Y, Dekker G, Scioscia M, et al. Increased BMI has a linear association with late-onset preeclampsia: a population-based study. PLoS ONE. 2019;14:e0223888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostlund I, Haglund B, Hanson U. Gestational diabetes and preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2004;113: 12–16. [DOI] [PubMed] [Google Scholar]
- 10.Bryson CL, loannou GN, Rulyak SJ, Critchlow C. Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol. 2003;15(158):1148–1153. [DOI] [PubMed] [Google Scholar]
- 11.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early-versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1–544.e12. [DOI] [PubMed] [Google Scholar]
- 12.Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Risk factors and clinical manifestations of pre-eclampsia. BJOG. 2000;107:1410–1416. [DOI] [PubMed] [Google Scholar]
- 13.Conde-Agudelo A, Belizan JM. Risk factors for pre-eclampsia in a large cohort of Latin American and Caribbean women. BJOG. 2000;107:75–83. [DOI] [PubMed] [Google Scholar]
- 14.Sande AK, Torkildsen EA, Sande RK, Morken NH. Maternal allergy as an isolated risk factor for early-onset preeclampsia: an epidemic-ological study. J Reprod Immunol. 2018;127:43–47. [DOI] [PubMed] [Google Scholar]
- 15.Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and the risk of preeclampsia. N Engl J Med. 2002;3(346): 33–38. [DOI] [PubMed] [Google Scholar]
- 16.Saftlas AF, Levine RJ. The interval between pregnancies and preeclampsia. N Engl J Med. 2002;6(346):1831–1832. [DOI] [PubMed] [Google Scholar]
- 17.Dekker G, Robillard PY. The birth interval hypothesis—does it really indicate the end of the primipaternity hypothesis. J Reprod Immunol. 2003;59:245–251. [DOI] [PubMed] [Google Scholar]
- 18.Raymond D, Peterson E. A critical review of early-onset and late-on-set preeclampsia. Obstet Gynecol Surv. 2011;66:497–506. [DOI] [PubMed] [Google Scholar]
- 19.Iacobelli S, Bonsante F, Robillard PY. Pre-eclampsia and preterm birth in Reunion Island: a 13 years cohort-based study. Comparison with international data. J Matern Fetal Neonatal Med. 2016;29:3035–3040. [DOI] [PubMed] [Google Scholar]