Abstract
Monomorphic posttransplant lymphoproliferative disorders (PTLD) have been defined as lymphoid or plasmacytic proliferations that fulfil criteria for one of the B-cell or T/NK-cell neoplasms recognized in immunocompetent hosts in the current WHO Classification. Low grade B-cell neoplasms have historically been excluded from this category, although rare reports of marginal zone lymphoma (MZL) have been described. We report nine cases of posttransplant EBV-negative MZL, all arising in solid organ transplant (SOT) recipients (four renal, three liver, one cardiac, and one liver, pancreas, & small bowel). Seven were extranodal MZL (EMZL) of MALT type, all of which had gastrointestinal (GI) involvement (four colon, one duodenum, one stomach, and one oropharynx/base of tongue). Notably, the preferential involvement of intestine distinguishes posttransplant EMZL from sporadic cases. Immunoglobulin light chain restriction was seen in all cases, with PCR showing a monoclonal pattern in seven of eight cases with successful amplification of PCR products. A clonally unrelated recurrence was seen in one case. Next generation sequencing (NGS) identified recurrent mutations previously reported in MZL in 3/5 cases. MZL was diagnosed at least one year after SOT (median time to presentation, 84 months; range, 13 to 108 months). Median age was 44 (range, 9 to 73 years); male: female ratio 5:4. Mean follow-up was 33.4 months, with an indolent clinical course observed. A subset responded to reduction in immunosuppression and anti-CD20 therapy alone. These data support designation of EBV-negative MZL as an uncommon form of monomorphic PTLD.
INTRODUCTION
Posttransplant lymphoproliferative disorders (PTLDs) are a heterogenous group of lymphoid or plasmacytic proliferations developing secondary to chronic immunosuppression in solid organ transplant (SOT) and hematopoietic stem cell transplant (HSCT) recipients.1 Though rare they are a well-recognized complication of transplant. The incidence of PTLD is variable ranging from 1 to 20% in SOT recipients2 depending on the allograft organ and is much lower, <2% in HSCT recipients.3 Most PTLDs are of B-cell derivation and are associated with Epstein-Barr virus (EBV) infection as seen in 60–80% of cases.4–8 The most significant risk factors for the development of PTLDs include seronegativity for EBV, (especially donor positive/recipient negative), allograft type, type of immune suppression and recipient age.9
The 2017 revision of the fourth Edition of the WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues, classifies PTLD into four major categories: Non-destructive PTLD, Polymorphic PTLD, Monomorphic PTLD and Classic Hodgkin Lymphoma PTLD.10 Monomorphic PTLD is further subclassified into categories of various B-cell or T/NK-cell lymphomas as seen in immunocompetent patients. Indolent B cell lymphomas have been traditionally excluded from monomorphic PTLDs except for EBV-positive marginal zone lymphomas (MZL), recognized only recently.11, 12 Several studies have reported an increased incidence of MZL in the posttransplant setting,4, 13, 14 a finding that distinguishes MZL from other low-grade B-cell lymphomas such as chronic lymphocytic leukemia and follicular lymphoma. Nevertheless, EBV-negative MZL has not been formally accepted as a form of PTLD. Most of the reported cases have been examples of extranodal MZL (EMZL) of mucosa-associated lymphoid tissue (MALT) type involving the gastric mucosa.15–22 These are usually associated with Helicobacter pylori (H. pylori) infection and generally have been shown to resolve with H. pylori eradication therapy.16, 18
Expanding the spectrum of clinical presentations and anatomic localizations of EBV-negative MZL in the posttransplant setting, we present nine SOT recipients with distinct clinical features. Seven cases showed primary involvement of extranodal sites, and two were nodal in origin. Of the seven EMZL cases, only one case had gastric involvement but lacked association with H. pylori. Two cases were remarkably similar, presenting as multiple intestinal polyps following liver transplantation in patients with ulcerative colitis. Overall five of seven EMZL involved either colon (4 cases) or duodenum (1 case).
MATERIALS AND METHODS
Selection of Cases
The pathology archives of National Cancer Institute, National Institutes of Health were searched between January 2005 to March 2019 for cases of EBV-negative MZL with a history of prior transplant. The slides were reviewed by E.S.J. and P.G. The clinical and transplant history, prior diagnosis, treatment following MZL diagnosis and clinical outcome/follow-up were collected from the submitting institution’s medical record whenever possible.
Immunohistochemistry and EBV-encoded Small RNA In Situ Hybridization
Hematoxylin and eosin staining was performed on formalin-fixed, paraffin-embedded (FFPE) tissue sections following routine processing. Immunohistochemical stains were performed on a BenchMark ULTRA automated immunostainer (Roche Diagnostics Corporation, Indianapolis, IN), using the antibodies against CD20 (L26 clone; predilute; Roche), CD3 (2GV6 clone; predilute; Ventana), CD79a (SP18; predilute; Roche), CD138 (B-838; predilute; Roche), kappa (rabbit; predilute; Roche), lambda (rabbit; predilute; Roche), Ki-67 (MIB-1; 1:200 ; Dako), IgG (rabbit; 1:12000 ; Dako), IgM (rabbit; 1:10000 ; Dako), and IgA (rabbit; 1:20000 ; Dako) using procedures per manufacturer’s instructions. EBV-encoded small RNA (EBER) in situ hybridization was performed as previously described.23
Molecular Studies
DNA was extracted from FFPE sections using the QIAamp FFPE Tissue Kit and polymerase chain reaction (PCR) amplified for detection of immunoglobulin gene (IGH and IGκ loci) rearrangements. Rearrangements of the IGH locus were interrogated using the BIOMED-2 primer set described by van Dongen et al.24 and supplied by InVivoScribe Technologies (IGH B-Cell Clonality Assay). Three separate multiplex reactions were performed using 6 VH-FR1 (Tube A), 7 VH-FR2 (Tube B), and 7 VH-FR3 (Tube C) fluorochrome-conjugated forward primers and a consensus JH reverse primer. The products were analyzed by capillary electrophoresis on an ABI 3130xl Genetic Analyzer, and electropherograms were analyzed using GeneMapper software version 4.0 (ABI).
Two additional reactions were performed for the IGκ locus using the BIOMED-2 primer set described by van Dongen et al.,24 and supplied by InVivoScribe Technologies (IGK Gene Clonality Assay - ABI Fluorescence Detection). These reactions interrogate rearrangements involving the Vκ loci and Jκ (Tube A), the Vκ locus and the κDE locus (Tube B), and the κ intron RSS locus and the κDE locus (Tube B). The products were analyzed by capillary electrophoresis on an ABI 3130xl Genetic Analyzer, and electropherograms were analyzed using GeneMapper software version 4.0 (ABI).
In six cases DNA was available for DNA sequencing using the TruSight Oncology 500 panel, which is a targeted clinical sequencing panel for single nuclear variations (SNVs), indels, tumor mutation burden (TMB), and microsatellite instability (MSI) biomarkers. 100 ng of DNA from FFPE tumor tissue was used to prepare libraries using the TruSight Oncology 500 kit (Illumina, USA) according to the manufacturer’s instructions. Amplified pre-enriched libraries were hybridized to probes specific to the 523 genes targeted by the TruSight Oncology 500 panel. Enriched libraries were amplified, quantified and normalized to 2nM then sequenced as paired-end reads on a high-output NextSeq 500/550 flow cell. TruSight Oncology 500 local app was used for alignment, variant calling, and determination of TMB and MSI. The variant annotation, classification, and interpretation were performed on Qiagen Clinical Insight software (Qiagen).
RESULTS
Clinical Features
Nine patients with a diagnosis of EBV-negative MZL in the posttransplant setting were retrieved from the pathology archives (Table 1). These included four females and five males with a median age at presentation of 44 years (range, 9 to 73 years). All patients had received solid organ transplants [kidney (4); liver (3); heart (1) and liver, pancreas and small bowel (1)] and presented at a median of 84 months (range, 13 to 108 months) after SOT. The immunosuppressive regimens included tacrolimus, mycophenolate mofetil and/or prednisone.
Table 1.
Clinical Features of Post-transplant EBV Negative Marginal Zone Lymphoma
| Case | Age/ Sex | Reason for TX | Type of TX | Immunosuppr-ession | Time from TX (mos.) | Site of MZL | Diagnosis | Treatment | Outcome | Follow-up Time (mos.) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1* | 40/F | Auto-immune hepatitis, UC | Liver | Tacrolimus, prednisone | 156, 180, 216 | Colon, multiple polyps | EMZL | RI, pentostatin, rituximab** | Alive, NED for 48 months, recurrence with different clone | 108 |
| 2 | 62/M | Primary sclerosing cholangitis, UC | Liver | Tacrolimus, mycophenolate mofetil | 13 | Colon and small bowel, multiple polyps | EMZL | surgery | Alive, NED | 7 |
| 3 | 44/M | ESRD secondary to FSGS | Kidney | Tacrolimus, mycophenolate mofetil, prednisone | 84 | Mass, Oropharynx/ left base of tongue | EMZL | rituximab | Alive, NED | 6 |
| 4 | 70/F | NI | Liver | NI | 24 | Cecum, mass | EMZL | NI | NI | Lost to follow-up |
| 5 | 73/M | NI | Kidney | Tacrolimus, mycophenolate mofetil, prednisone | 168 | Descending colon polyp | EMZL | NI | NI | Lost to follow-up |
| 6 | 18/F | Idiopathic dilated cardiomyopathy | Heart | Tacrolimus, mycophenolate mofetil | 84 | Duodenum, ulcer; H pylori neg | EMZL | RI, rituximab, cyclophosphamide, prednisone | Alive, NED | 26 |
| 7 | 9/M | Necrotizing enterocolitis | Liver, pancreas, small bowel | Tacrolimus, mycophenolate mofetil, prednisone | 84 | Stomach, H pylori neg | EMZL | RI, rituximab | Alive, NED | 20 |
| 8 | 46/M | NI | Kidney | NI | 180 | Groin, lymph node | Nodal MZL | NI | NI | Lost to follow-up |
| 9 | 52/F | CRF | Kidney | Mycophenolate mofetil | 96 | Mesenteric lymph node | Nodal MZL | NI | NI | Lost to follow-up |
previously published as a case report 22;
currently being treated with rituximab
Abbreviations: UC, ulcerative colitis; CRF, Chronic renal failure; EMZL, Extranodal marginal zone lymphoma; ESRD, End stage renal disease; F, Female; FSGS, Focal segmental glomerulosclerosis; LAD, Lymphadenopathy; M, Male; MZL, Marginal zone lymphoma; NI, no information available; RI, reduced immunosuppression; NED, no evidence of disease.
Two of the patients [case 1 (previously published25) and case 2] had similar past medical histories of ulcerative colitis (UC) and hepatic transplants secondary to autoimmune hepatitis (case 1) and primary sclerosing cholangitis (case 2).
Case 1 is a 40-year-old female with past medical history of UC and autoimmune hepatitis status post liver transplant 18 years prior to her presentation with an episode of abdominal pain, fever, and rectal bleeding. Colonoscopy performed at the time revealed rectal erythema and rectal polyp. The patient underwent multiple colonic biopsies that revealed mucosal infiltration by plasmacytoid cells with lambda light chain restriction. Inguinal lymph node and bone marrow were involved (Stage IV disease). Subsequently, previously obtained colon biopsies were re-reviewed, which revealed the same, clonally related pan-colonic disease. This disease was present 5 years prior (i.e. 13 years after liver transplant). After initial therapy with pentostatin, the patient was disease free for four years. She recently developed recurrent colonic polypoid lesions, which showed a change in the immunoglobulin light chain (now kappa restricted). She is currently receiving rituximab monotherapy with reduction of immune suppression.
Case 2 is a 62-year-old male with past medical history of primary sclerosing cholangitis and UC, who underwent an orthotopic liver transplant 13 months prior to his presentation. In the interim he had multiple colonoscopies for surveillance of active UC and iron deficiency anemia that revealed multiple colonic polyps. The patient subsequently underwent a total colectomy. His pre-operative positron emission tomography/computed tomography (PET/CT) scan revealed multi-focal PET-positive lesions in the colon (intramural, largest 4cm in ascending colon) but with no extra-colonic disease, no lymphadenopathy or bone marrow involvement. He currently has no evidence of disease (NED).
Case 3 is a 44-year-old male who underwent renal transplant for end stage renal disease, 84 months prior to presenting with a large, multi-lobulated exophytic mass in the midline and left base of tongue. PET/CT scan done at the time of initial presentation revealed involvement of bilateral cervical lymph nodes. Bone marrow was normocellular with a scant infiltrate, but showed 0.17% kappa restricted B-cells, negative for CD5 and CD10; PCR studies confirmed the presence of a clonal population. However, following rituximab therapy, the patient is alive and NED.
Two patients, case 4 (70-year-old female) and case 5 (73-year-old male) developed EMZL involving the colon, 24 and 168 months following liver and kidney transplantation, respectively. Cases 6 and 7 had EMZL involving the upper gastrointestinal tract (duodenum and stomach, respectively); both cases were negative for H. pylori. Neither patient had other sites of involvement, as determined by CT, PET/CT, or magnetic resonance imaging staging studies at diagnosis. Both patients responded well to therapy and are NED.
There were two patients with nodal MZL (cases 8 and 9) both of whom presented with lymphadenopathy following with renal transplant at 180 and 96 months. Details of treatment and follow-up were not available.
Remarkably, all seven of the patients with EMZL had GI tract involvement, with colon being the most common site, seen in 4 of 7 cases. Therapy approaches varied, but included reduced immune suppression in three, and rituximab in four. Four of five patients with available follow-up are NED (Cases 1, 2, 3, 6 and 7; median 20 months; range, 6 months – 108 months). Overall, the prognosis has been excellent, with only one patient with available follow-up developing recurrent disease following treatment.
Morphologic, Immunohistochemical, and Molecular Features
In this case series, 7 patients had EMZL of MALT type and 2 patients presented with nodal MZL (mesenteric lymphadenopathy and inguinal lymphadenopathy). All cases of EMZL had involvement of the GI tract [colon (4), oropharynx/ base of tongue (1), stomach (1) and duodenum (1)] (Table 2).
Table 2.
Morphologic and Immunophenotypic Features of Posttransplant EBV negative Marginal Zone Lymphoma
| Case | Anatomic Site | Microscopic Description | Immunohistochemistry | Molecular | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Architecture | Plasmacytic Differentiation | Monocytoid cells | CD20 | CD79a | CD138 | Light chain | Heavy chain | EBER ISH | Ki67 | IG PCR Clonality | ||
| 1a | Multiple Colon Bx | Atypical lymphoplasmacytic infiltrate expanding lamina propria, extending into the superficial submucosa | Present | Absent | Pos (subset) | Pos | Pos | Lambda | IgG | Neg | ND | Clonal* |
| 1b | Multiple Colon Bx | Atypical lymphoplasmacytic infiltrate expanding lamina propria, extending into the superficial submucosa | Present | Absent | Pos (subset) | Pos | Pos | Lambda | IgG | Neg | ND | ND |
| 1c | Multiple Colon Bx | Atypical lymphoplasmacytic infiltrate expanding lamina propria, extending into the superficial submucosa | Present | Absent | Pos (subset) | Pos | Pos | Lambda | IgG | Neg | ND | Clonal* |
| 1d | BM | Atypical lymphoplasmacytic infiltrate | Present | Absent | ND | Pos | Pos | Lambda | ND | ND | ND | ND |
| 1e | R Inguinal LN | Effaced with diffuse atypical lymphoplasmacytic infiltrate | Present | Absent | Pos | Pos | Pos | Lambda | ND | Neg | Low | Clonal* |
| 1f | Multiple Colon Bx | Atypical lymphoplasmacytic infiltrate | Present | Absent | ND | ND | ND | Lambda | ND | ND | ND | ND |
| 1g | R Colon Bx | Atypical lymphoplasmacytic infiltrate | Present | Absent | Pos | Pos | ND | Kappa | IgG | Neg | ND | Clonal** |
| 2 | Colon and small bowel | Polypoid nodules, marked expansion of the lamina propria by a uniform lymphoplasmacytic infiltrate | Present | Present | Pos | Pos | Pos | Kappa | IgG | Neg | ND | Polyclonal |
| 3a | Oropharynx/ Base of tongue | Diffuse polymorphous infiltrate, no secondary follicles | Present | Absent | Pos (subset) | Pos | Pos (subset) | Kappa^ | IgA | Neg | Low-Mod | Clonal |
| 3b | R Cervical LN | Atypical polymorphous infiltrate | Present | Present | Pos (subset) | Pos | Pos (subset) | Kappa^ | ND | Neg | Mod | Clonal |
| 3c | BM | Scant lymphoplasmacytic infiltrate | Present | Absent | Neg | Pos | Pos | Kappa^A | ND | Neg | Neg | Clonal |
| 4 | Cecum, partial colectomy and paracolic lymph nodes | LELs, atypical infiltrate extending into submucosa and muscularis propria, focal necrosis, 18/18 paracolic lymph nodes involved | Prominent (Dutcher bodies noted) | Present | Pos | Pos | ND | Lambda | IgA | Neg | Low | Clonal |
| 5 | Descending colon polyps | Dense infiltrate expanding lamina propria with replacement of crypts and extending to the submucosa | Present (Dutcher bodies noted) | Absent | Pos | ND | Pos (subset) | Kappa | IgA | Neg | Low-Mod | Clonal |
| 6 | Duodenum | Atypical dense lymphocytic infiltrate with scattered admixed large cells | Present | Present | Pos | Pos | Pos (subset) | Lambda | IgG | Neg | Low-Mod | No Amplification |
| 7 | Stomach | Atypical lymphoplasmacytic infiltrate | Present | Present | Pos (subset) | Pos | Pos | Lambda | IgA | Neg | ND | Clonal |
| 8 | L Inguinal LN | Castleman like features with increased interfollicular B cells | Absent | Present | Pos | Pos (subset) | ND | Lambda^ | Neg | Neg† | ND | Clonal |
| 9 | Mesenteric LN | Diffuse lymphoplasmacytic infiltrate | Present | Present | Pos | Pos | Pos | Lambda | IgG | Neg | Low | Clonal |
Clonally related;
Clonally unrelated;
by flow cytometric analysis;
few positive cells in few germinal centers consistent with reactivation;
, small population (0.17%) of kappa restricted B-cells
Abbreviations: BM, Bone marrow; Bx, Biopsy; L, Left; LEL, Lymphoepithelial lesions; LN, Lymph node; ND, Not done; Mod, Moderate; Neg, Negative; Pos, Positive; R, Right;
The extranodal cases demonstrated a dense, lymphoplasmacytic infiltrate expanding the lamina propria and extending into the superficial submucosa in most cases, with infiltration of muscularis propria in one case (case 4; partial colectomy; Figure 1). All cases revealed prominent plasmacytic differentiation with some cases showing focal aggregates of small to medium sized lymphoid cells with ample amount of pale eosinophilic to clear cytoplasm (monocytoid cells; cases 2, 3, 4 and 7). One case (case 4; partial colectomy) also showed focal areas of necrosis.
FIGURE 1.
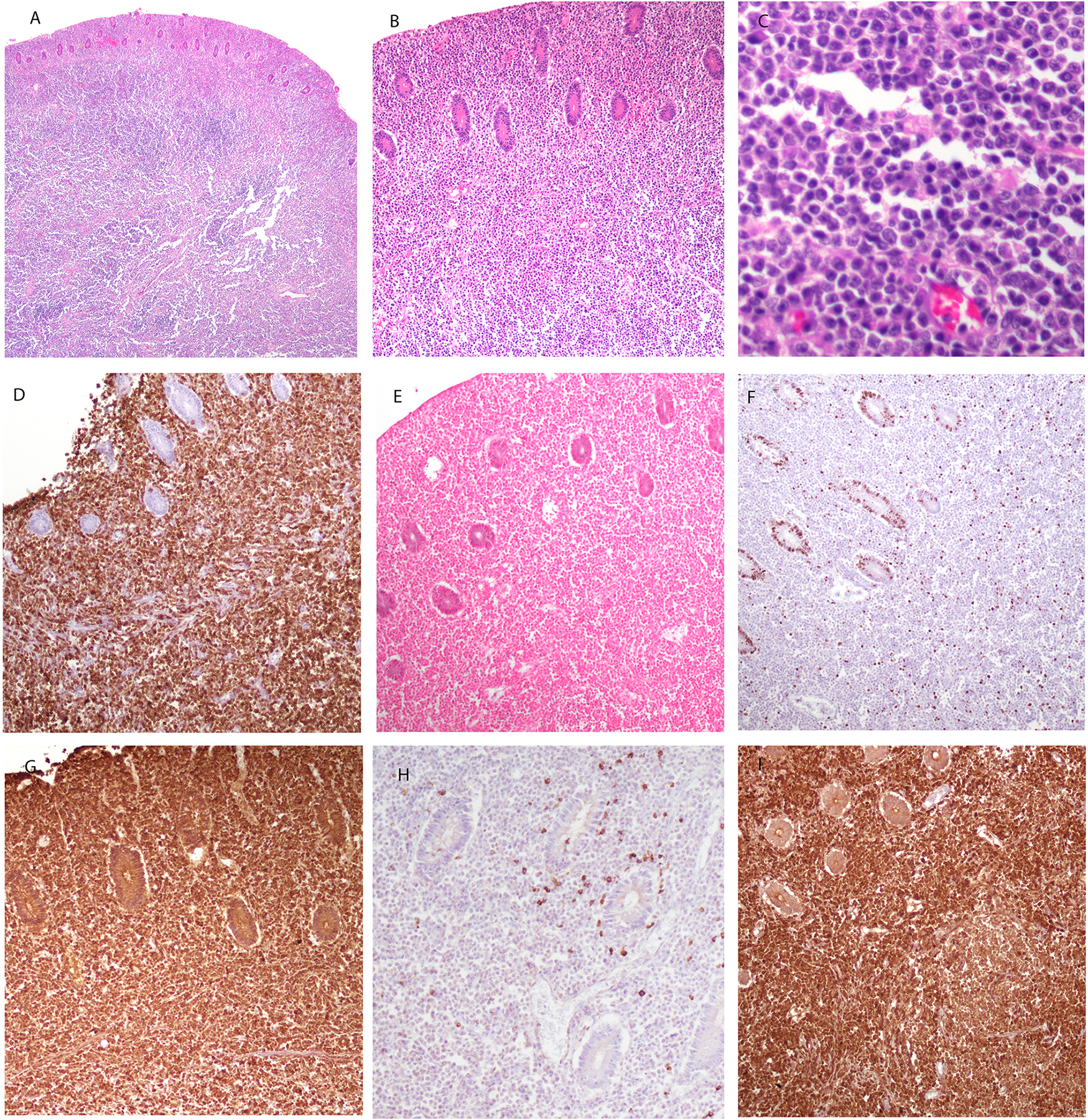
EBV-negative EMZL, MALT type in a 70-year-old female involving the colon (case 4). A, B and C, atypical lymphoplasmacytic proliferation infiltrating the muscularis propria. Cells are small to medium in size, with plasmacytoid features. D. Atypical proliferation is positive for CD79a. E. EBER is negative. F. Ki-67 demonstrates a very low proliferation index in the neoplastic cells, while being positive in the residual colonic crypts. G. Lymphoid cells are positive for IgA, are negative for kappa light chain (H), and positive for lambda (I).
Two of the cases (case 1, colon involvement and case 3, oropharynx/ base of tongue involvement) had either histologic (case 1) or flow cytometric (case 3) evidence of bone marrow involvement. These two cases also showed additional nodal involvement (case 1, inguinal lymph node and case 3, cervical lymph node) with effacement of nodal architecture by an atypical proliferation composed of small lymphoid cells, plasma cells and medium sized cells with ovoid nuclei, condensed chromatin and pale eosinophilic cytoplasm (Figures 2 and 3).
FIGURE 2.
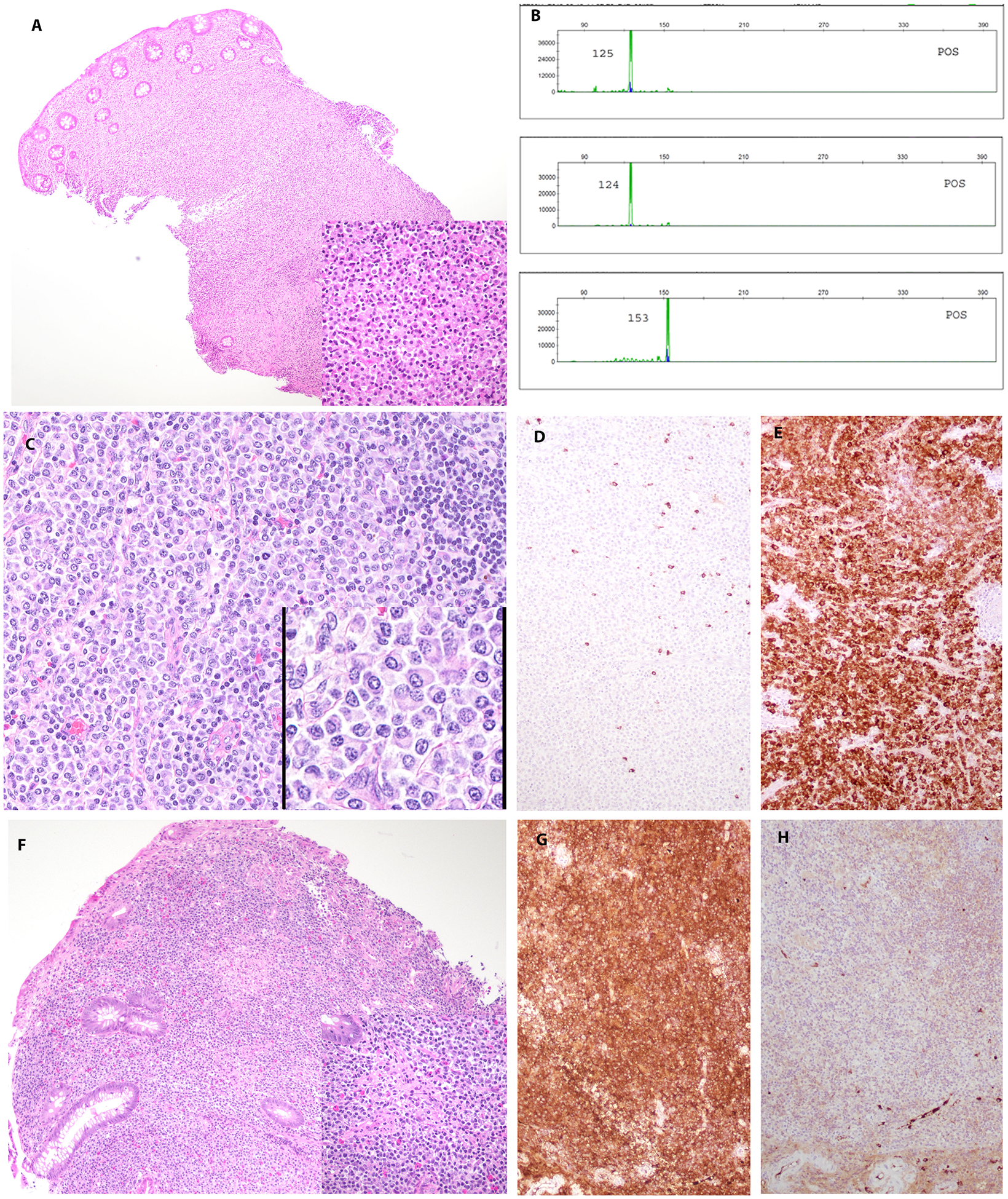
EBV-negative MALT lymphoma in a 40-year-old female with past medical history of autoimmune hepatitis and ulcerative colitis (case 1). Patient had a long-protracted course with multiple recurrences in the colon. A. The atypical infiltrate involves the mucosa and submucosa of the right colon. B. Comparison of PCR for immunoglobulin gene rearrangements from colon specimens at 156 months, 216 months and 264 months’ time point. The study reveals common IG rearrangement pattern in first 2 specimens (156 months, 216 months; top 2 plots) and different clonal peaks in IGH Fr III in the third specimen (264 months; bottom plot). C. The lymph node (216 months posttransplant) with an atypical infiltrate composed of plasmacytoid cells. The infiltrate is positive for lambda (E) and negative for kappa (D) light chain. F. Colonic biopsy (264 months posttransplant) with atypical infiltrate positive for kappa (G) and negative for lambda (H) light chain.
FIGURE 3.
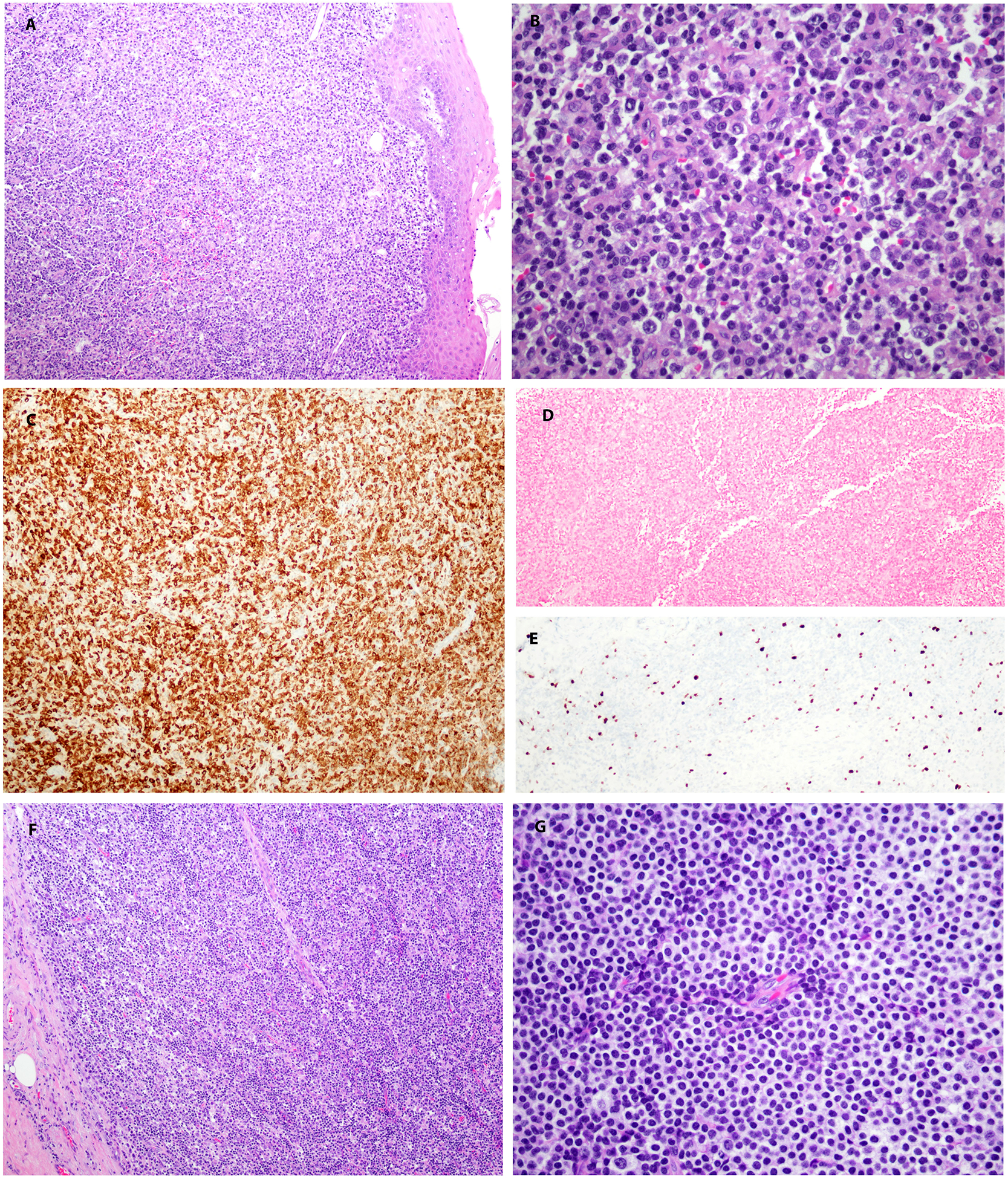
EBV-negative EMZL, MALT type in a 44-year-old male involving the midline and left base of tongue (case 3). A, and B, atypical lymphoplasmacytic proliferation, positive for CD79a (C), and negative for EBER (D). E, Ki-67 demonstrates a low proliferation index in the neoplastic cells. F. Diffuse involvement of lymph node. G. Cells have a rim of pale cytoplasm with a monocytoid appearance.
One case of nodal MZL (case 8) revealed altered lymph node architecture by atypical follicles with Castleman-like features (scattered regressed lymphoid follicles with atretic germinal centers, some containing penetrating hyalinized blood vessels). Immunostain for HHV8 was negative. The interfollicular areas showed increased number of B cells, many of which had monocytoid features. Sections of case 9 showed a largely diffuse infiltrate composed of lymphocytes, plasmacytoid cells and monocytoid cells (Figure 4). Scattered Russell bodies and extracellular immunoglobulin deposits were identified. Focal residual areas of normal paracortex were seen.
FIGURE 4.
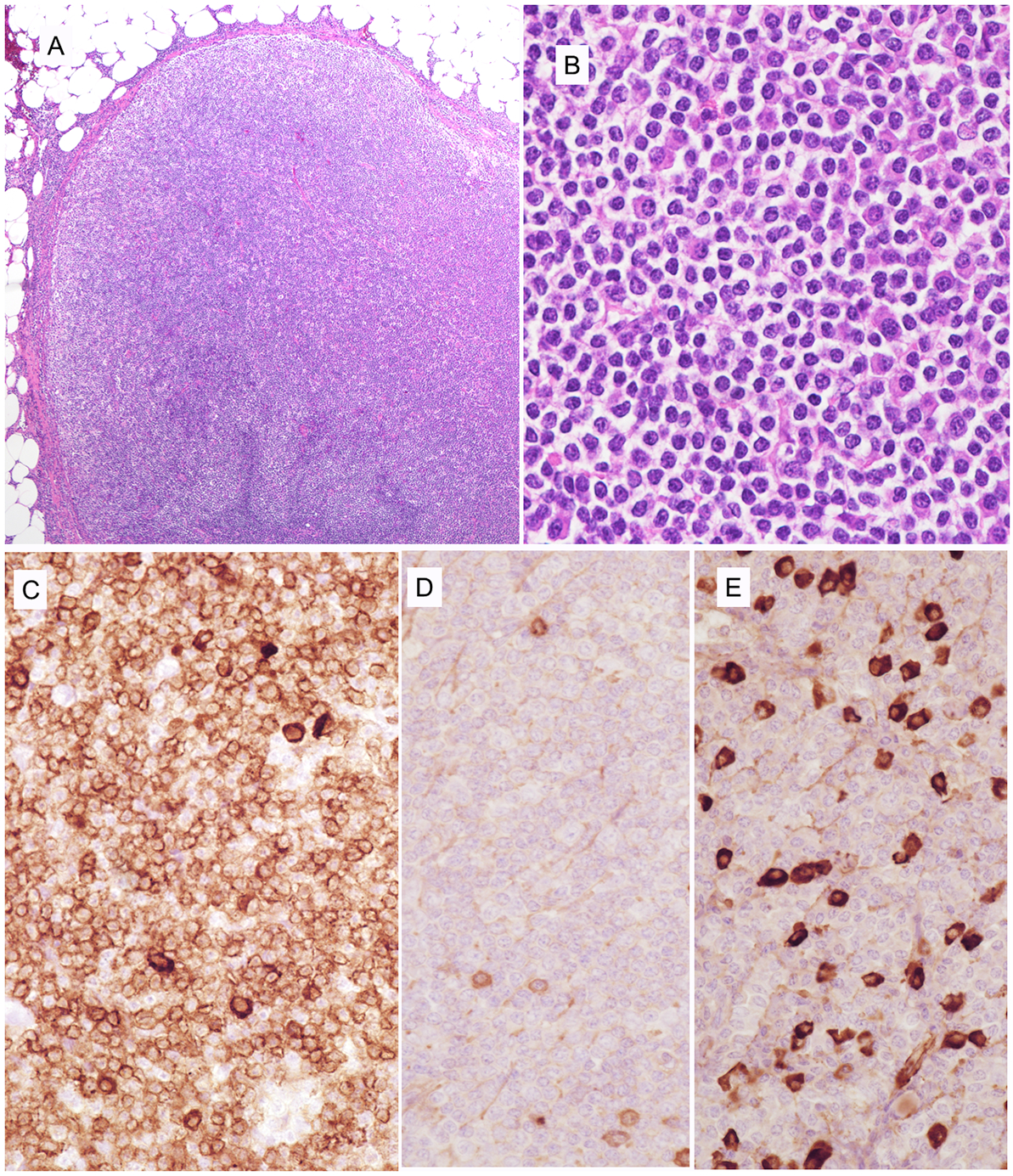
EBV-negative MZL, in a 52-year-old female post renal transplant involving mesenteric lymph node (case 9). A. A diffuse infiltrate effaces nodal architecture. B. Atypical cells are small, many with pale cytoplasm resembling monocytoid cells. Many cells show plasmacytoid features. C. Cells are diffusely positive for CD79a, with strongly staining in plasmacytoid cells. D. Few normal plasma cells are positive for kappa. E. Cells with plasmacytoid differentiation are strongly positive for lambda light chain.
EBER by in situ hybridization was negative in the neoplastic cells in all the cases with one nodal case (case 8) showing few positive cells in few germinal centers consistent with reactivation. All cases demonstrated light chain restriction either by immunohistochemical staining or flow cytometric analysis (data not shown). Six cases were lambda light chain restricted and three cases were kappa light chain restricted. One patient (case 1), presented with a colonic recurrence 4 years later, revealing a different light chain restriction (lambda light chain restriction in earlier samples and kappa light chain restriction in the recurrence; Figure 2). Four cases expressed IgG heavy chain and four cases expressed IgA heavy chain. The prevalence of IgA is consistent with the presentation in the GI tract. Ki-67 revealed overall low proliferation rate (<25%).
Immunoglobulin gene rearrangement studies showed clonal peaks in 7 of the 8 cases with successful amplification; one case showed a polyclonal rearrangement pattern and one case failed PCR amplification due to suboptimal DNA quality. In case 1, a comparison of the specimens from multiple time points (156 months, 216 months and 264 months) indicated that the most recent colon biopsy, differed in its IG gene rearrangement pattern from the earlier specimens. The earlier specimens share a common IG rearrangement pattern. Thus, it was concluded that the most recent biopsy was clonally unrelated to the earlier biopsies (Figure 2).
Next generation sequencing was performed on six cases with available material. Five cases (Cases 1, 2, 3, 4 and 5; Table 3) had pathogenic or likely pathogenic genetic alterations. FAS mutations were seen in two cases, and two cases contained mutations in TNFAIP3. NOTCH2, LRP1B and TP53 mutations were seen in a single case. None of the other mutations were recurrent. Molecular studies on one case (Case 9) did not reveal any pathogenic or likely pathogenic genetic alterations.
Table 3.
Genetic Aberrations in Posttransplant EBV negative Marginal Zone Lymphoma
| Case | Gene | Nucleotide change | Amino Acid change | VAF (%) | Mutation Type |
|---|---|---|---|---|---|
| 1 | FAS | c.886A>T | p.Lys296* | 27 | Nonsense |
| TNFAIP3 | c.421delC | p.Arg141fs*75 | 21 | Frameshift deletion | |
| 2 | TET2 | c.4131_4132delCT | p.Phe1377fs*23 | 58 | Frameshift deletion |
| ARID1B | c.1483C>T | p.Gln495* | 29 | Nonsense | |
| B2M | c.2T>C | p.Met1Thr | 26 | Missense | |
| NFKBIA | c.331C>T | p.Gln111* | 24 | Nonsense | |
| TNFAIP3 | c.821delC | p.Pro274fs*13 | 22 | Frameshift deletion | |
| 3 | TSHR | c.1349G>A | p.Arg450His | 52 | Missense |
| 4 | CUX1 | c.622C>T | p.Arg208* | 30 | Nonsense |
| 5 | TP53 | c.641A>G | p.His214Arg | 14 | Missense |
| BTG1 | c.136_138delGAGinsTAA | p.Glu46* | 5.56 | InDel (Nonsense) | |
| FAS | c.616_617dupAA | p.Asn206fs*11 | 12 | Frameshift insertion | |
| LRP1B | c.2863C>T | p.Gln955* | 8.32 | Nonsense | |
| NOTCH2 | c.6418C>T | p.Gln2140* | 11 | Nonsense |
Abbreviations: VAF, variant allele frequency
DISCUSSION
PTLDs exhibit a spectrum of clinical and pathological features, ranging from benign polymorphous proliferations to aggressive B-cell and T/NK-cell lymphomas. PTLDs are responsible for significant morbidity and mortality in transplant recipients. A recent study utilizing linked transplantation and cancer registry data reported that approximately 13% of deaths in SOT recipients were attributable to cancer, with non-Hodgkin lymphoma (NHL) being the largest contributor of mortality among children and second largest contributor overall.26
EBV has been shown to play an important role in the pathogenesis of majority of PTLDs.4, 27, 28 This association is pronounced in early PTLDs occurring within the first year of transplantation. In contrast PTLDs occurring later, after the first year of transplantation are more frequently EBV-negative.2, 4–6, 29 The late PTLDs are also more commonly monomorphic.29
The current WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues does not include low-grade B cell lymphoma in the category of monomorphic PTLD, with the exception of EBV positive MZL.1 Nevertheless, indolent B cell lymphomas, especially EMZL have been described in the posttransplant setting.11, 12, 15–19, 30–38 The rationale for exclusion as a form of PTLD has been that most cases have been gastric (Table 4) with a strong association with H. pylori infection.15–18, 20, 21 Moreover, some cases responded to H. pylori eradication therapy alone.16, 18 These findings suggested that H. pylori was a primary factor in their pathogenesis, similar to that seen in immunocompetent host. In absence of any convincing evidence of a causal relationship between a chronic immunosuppressed state secondary to transplant and EMZL, a coincidental association was thought to be more likely.
Table 4.
Previously Reported Cases of EBV negative Marginal Zone Lymphoma in Transplant Patients
| Age/Sex | Type of Transplant | Time from Transplant (months) | Anatomic Site | H.pylori | EBV status | Treatment | Follow-up | Reference |
|---|---|---|---|---|---|---|---|---|
| 53/F | Kidney | 60 | Gastric | Pos | NI | Antibiotics | Alive, no active disease at 24 months | Le Meur Y et al. |
| 46/F | Kidney & pancreas | 110 | Gastric | Pos | Neg | Antibiotics, RI | Alive, no active disease at 12 months | Aull MJ et al. |
| 58/M | Heart | 42 | Gastric | Pos | NI | Antibiotics, RI | Alive, no active disease at 12 months | Aull MJ et al. |
| 52/M | Kidney | 51 | Gastric | Pos | NI | Antibiotics, RI, RT | Alive, no active disease at 34 months | Aull MJ et al. |
| 51/F | Kidney | 67 | Gastric | Neg | NI | Antibiotics, RI | Alive, no active disease at 7 months | Aull MJ et al. |
| 51/M | Heart | 52 | Gastric | Pos | NI | Antibiotics, RI, Surgery & Chemotherapy | Dead at 15 months (Infection) | Aull MJ et al. |
| 61/F | Kidney | 51 | Gastric | Pos | NI | Antibiotics, RI | Alive, no active disease at 38 months | Aull MJ et al. |
| 57/M | Liver | 104 | Gastric | Pos | Neg | Antibiotics | Alive, no active disease at 48 months | Shehab TM et al. |
| 61/F | Liver | 94 | Gastric | Pos | Neg | Antibiotics | Alive, no active disease at 8 months | Shehab TM et al. |
| 54/F | Liver | 33 | Gastric | Pos | Neg | Antibiotics | Alive, no active disease at 10 months | Shehab TM et al. |
| 48/M | Liver | 60 | Gastric | Pos | Neg | Antibiotics, RT | Died at 36 months secondary to metastatic gastric adenocarcinoma | Hsi ED et al. and Shehab TM et al. |
| 47/F | Kidney | 132 | Parotid | NA | Neg | RT | Alive, no active disease at 14 months | Hsi ED et al. |
| 63/F | Liver | 131 | Parotid | NA | Neg | RI | Alive, no active disease at 24 months | Hsi ED et al. |
| 50/M | Heart | 14 | Gastric | Pos | Neg | Antibiotics, RT, Surgery | Alive, no active disease at 12 months | Hsi ED et al. |
| 48/M | Heart | 84 | Gastric | Pos | Neg | RI, Surgery | Alive, no active disease at 11 months | Hsi ED et al. |
| 52/M | Liver | 52 | Colon | NA | Neg | RI, Surgery | Alive | Kim MJ et al. |
| 43/M | Liver | 27.5 | Liver, spleen, BM, LN | NA | Neg | Rituximab-Chemotherapy | Alive | Oertel SH et al. |
| 58/F | Liver | 24 | R Lower Lobe, Lung | NA | Neg | Surgery | Alive, no active disease at 12 months | Shoji F et al. |
| 60/M | Heart | 72 | Gastric | Pos | Neg | Antibiotics, Chemotherapy | Alive, no active disease at 48 months | Boissonnat P et al. |
| 28/F | Kidney | 72 | Gastric | Pos | Neg | Antibiotics, RI, Chemotherapy | Alive, no active disease at 60 months | Bakanay SM et al. |
| 52/M | Heart | 60 | Gastric | Pos | Neg | Chemotherapy | Alive, no active disease at 24 months | Wotherspoon AC et al. |
| 70/M | Kidney | 60 | Gastric with involvement of oral cavity, lung, LN | Pos | Neg | Chemotherapy | Alive, no active disease at 10 months | Wotherspoon AC et al. |
| 50/M | Kidney | 136 | Gastric | NI | NI | Rituximab-Chemotherapy | Alive, no active disease | Ashrafi F et al. |
| 60/F | HSCT | 132 | Cecum | NA | Neg | RT | Alive, no active disease at 6 months | Sundararajan S et al. |
BM, Bone marrow; LN, Lymph node; NA, Not applicable; Neg, Negative; NI, No information available; Pos, Positive; R, Right; RI, reduced immunosuppression; RT, Radiotherapy
In the current study, we describe EBV-negative MZL in nine SOT recipients, with seven cases having features of EMZL. Next generation sequencing studies in four of the six cases, all of which were EMZL, revealed genetic alterations that have been previously reported in hematological malignancies. Three cases harbored mutations well-described in EMZL, namely TNFAIP3, TNFRSF14, LRP1B, FAS and NOTCH2.39–41 These results support the categorization of these cases as monomorphic PTLD and indicate a common pathogenesis with EMZL in the non-transplant setting. Chronic antigenic stimulation has been postulated to play a role in the pathogenesis of EMZL,42 and in this setting we postulate that the transplanted organ fulfills this role. The common mutational profile between sporadic MZL and posttransplant MZL is like that observed for other forms of EBV-negative PTLD. Ferreiro et al. reported that cases of EBV-negative monomorphic PTLD classified as diffuse large B-cell lymphoma and Burkitt lymphoma shared a common mutational profile with cases arising without a history of transplant, and differed from their EBV-positive counterparts.43
Our study provides evidence that EBV-negative MZL, in particular EMZL, should be accepted as a form of monomorphic PTLD. Significantly, the clinical pattern of disease differs from that of sporadic EMZL in the non-transplant setting. Five of seven cases of EMZL in our series presented with intestinal disease, with four presenting in the colon. In a series of 252 cases of sporadic EMZL, only 6% were reported with intestinal disease.44 We had a single case of gastric EMZL, but it was negative for H. pylori. Rare cases similar to ours have been reported previously. A literature review revealed one reported case of EBV-negative EMZL involving colon and two involving parotid gland in SOT recipients, along with one case of colonic involvement in a HSCT recipient that was donor derived (Table 4).17, 30,35
The exact pathogenesis of EBV-negative PTLD is not well understood; various hypotheses for pathogenesis include chronic inflammation,45 diminished immunosurveillance,46 allograft induced chronic immune stimulation,47 and infection with oncoviruses other than EBV.48, 49 We can invoke the hypothesis that chronic immunosuppression along with chronic antigen stimulation induced by the allograft plays a significant role in the pathogenesis of EBV-negative MZL in the posttransplant setting. In this regard, the pathogenesis may be similar to that of sporadic EMZL. For example, two major risk factors include chronic infection with H. pylori and autoimmune disease, i.e. Sjogren’s syndrome, both implicated in the pathogenesis of gastric and salivary gland EMZL, respectively. We would withhold designation of H. pylori-associated gastric MALT lymphoma as a form of monomorphic PTLD, pending further evidence that the incidence of such cases is increased in patients with SOT.
Most forms of EBV-negative monomorphic PTLDs are clinically aggressive with poor prognosis, thus requiring multiagent chemotherapy.6, 8, 50 Data on the impact of EBV status on response to therapy and patient survival has been conflicting,51 but a recent study revealed that EBV negativity did not associate with high-risk features (advanced stage, older age, high lactate dehydrogenase, central nervous system involvement), inferior response to initial therapy (including reduction of immunosuppression alone or rituximab) or inferior overall survival.2 The majority of the patients in our study underwent reduction in immunosuppression as the initial modality of treatment. Various subsequent treatment strategies including anti-CD20 therapy (rituximab), chemotherapy and surgery were utilized. The patients with available follow up in our series exhibited an indolent course with complete resolution of disease, even in the patients with Stage IV disease (cases 1 and 3). Case 1 did present with a late recurrence involving the same anatomic site (colon); however, it was clonally unrelated to the original tumor, having features of a “secondary primary”. Similar to recent reports, EBV-negative MZL patients in our study did not require aggressive chemotherapy and remained in remission at 6 to 108 months (mean, 33.4 months) after diagnosis51.
All the patients in our study presented more than a year after SOT and would be considered instances of late PTLD. The fact that the number as well as the proportion of EBV-negative PTLDs in organ transplant recipients has increased with time has been highlighted by many studies.2, 29, 52 Our study calls attention to the occurrence of MZL in this setting. As the recipients live longer, the burden of EBV-negative PTLD is increasing and we may encounter more cases of EBV-negative PTLD in coming years. Further genomic studies of these cases will be of interest and may identify further similarities or differences with sporadic lymphomas. While most cases of EBV-negative PTLD are clinically aggressive, posttransplant MZLs are clinically indolent and can be managed conservatively.
Conflicts of Interest and Source of Funding:
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Natkunam Y, Gratzinger D, Chadburn A, et al. 2018. ‘Immunodeficiency-associated lymphoproliferative disorders: time for reappraisal?’, Blood, 132: 1871–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luskin MR, Heil DS, Tan KS, et al. The Impact of EBV Status on Characteristics and Outcomes of Posttransplantation Lymphoproliferative Disorder. Am J Transplant. 2015;15:2665–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dharnidharka VR, Webster AC, Martinez OM, et al. Post-transplant lymphoproliferative disorders. Nat Rev Dis Primers. 2016;2:15088. [DOI] [PubMed] [Google Scholar]
- 4.Knight JS, Tsodikov A, Cibrik DM, et al. Lymphoma after solid organ transplantation: risk, response to therapy, and survival at a transplantation center. J Clin Oncol. 2009;27:3354–3362. [DOI] [PubMed] [Google Scholar]
- 5.Ghobrial IM, Habermann TM, Maurer MJ, et al. Prognostic analysis for survival in adult solid organ transplant recipients with post-transplantation lymphoproliferative disorders. J Clin Oncol. 2005;23:7574–7582. [DOI] [PubMed] [Google Scholar]
- 6.Leblond V, Davi F, Charlotte F, et al. Posttransplant lymphoproliferative disorders not associated with Epstein-Barr virus: a distinct entity? J Clin Oncol. 1998;16:2052–2059. [DOI] [PubMed] [Google Scholar]
- 7.Leblond V, Dhedin N, Mamzer Bruneel MF, et al. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol. 2001;19:772–778. [DOI] [PubMed] [Google Scholar]
- 8.Nelson BP, Nalesnik MA, Bahler DW, et al. Epstein-Barr virus-negative post-transplant lymphoproliferative disorders: a distinct entity? Am J Surg Pathol. 2000;24:375–385. [DOI] [PubMed] [Google Scholar]
- 9.Dharnidharka VR. Comprehensive review of post-organ transplant hematologic cancers. Am J Transplant. 2018;18:537–549. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues Lyon, France: International Agency for Research on Cancer (IARC) 2017. [Google Scholar]
- 11.Gibson SE, Swerdlow SH, Craig FE, et al. EBV-Positive Extranodal Marginal Zone Lymphoma of Mucosa-associated Lymphoid Tissue in the Posttransplant Setting: A Distinct Type of Posttransplant Lymphoproliferative Disorder? Am J Surg Pathol. 2011;35:807–815. [DOI] [PubMed] [Google Scholar]
- 12.Gong S, Crane GM, McCall CM, et al. Expanding the Spectrum of EBV-positive Marginal Zone Lymphomas: A Lesion Associated With Diverse Immunodeficiency Settings. Am J Surg Pathol. 2018;42:1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke CA, Morton LM, Lynch C, et al. Risk of lymphoma subtypes after solid organ transplantation in the United States. Br J Cancer. 2013;109:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinlan SC, Morton LM, Pfeiffer RM, et al. Increased risk for lymphoid and myeloid neoplasms in elderly solid-organ transplant recipients. Cancer Epidemiol Biomarkers Prev. 2010;19:1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wotherspoon AC, Diss TC, Pan L, et al. Low grade gastric B-cell lymphoma of mucosa associated lymphoid tissue in immunocompromised patients. Histopathology. 1996;28:129–134. [DOI] [PubMed] [Google Scholar]
- 16.Le Meur Y, Pontoizeau-Potelune N, Jaccard A, et al. Regression of a gastric lymphoma of mucosa-associated lymphoid tissue after eradication of Helicobacter pylori in a kidney graft recipient. Am J Med. 1999;107:530. [PubMed] [Google Scholar]
- 17.Hsi ED, Singleton TP, Swinnen L, et al. Mucosa-associated lymphoid tissue-type lymphomas occurring in post-transplantation patients. Am J Surg Pathol. 2000;24:100–106. [DOI] [PubMed] [Google Scholar]
- 18.Shehab TM, Hsi ED, Poterucha JJ, et al. Helicobacter pylori-associated gastric MALT lymphoma in liver transplant recipients. Transplantation. 2001;71:1172–1175. [DOI] [PubMed] [Google Scholar]
- 19.Aull MJ, Buell JF, Peddi VR, et al. MALToma: a Helicobacter pylori-associated malignancy in transplant patients: a report from the Israel Penn International Transplant Tumor Registry with a review of published literature. Transplantation. 2003;75:225–228. [DOI] [PubMed] [Google Scholar]
- 20.Boissonnat P, El Bekkali Y, Salles G, et al. Regression of gastric lymphoma of mucosa associated with lymphoid tissue (MALT) following cardiac transplantation. J Heart Lung Transplant. 2002;21:1044–1045. [DOI] [PubMed] [Google Scholar]
- 21.Bakanay SM, Kaygusuz G, Topcuoglu P, et al. Epstein-barr virus-negative post-transplant lymphoproliferative diseases: three distinct cases from a single center. Turk J Haematol. 2014;31:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashrafi F, Shahidi S, Ebrahimi Z, et al. Outcome of rapamycin therapy for post-transplant-lymphoproliferative disorder after kidney transplantation: case series. Int J Hematol Oncol Stem Cell Res. 2015;9:26–32. [PMC free article] [PubMed] [Google Scholar]
- 23.Dojcinov SD, Venkataraman G, Raffeld M, et al. EBV positive mucocutaneous ulcer--a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98–3936. Leukemia. 2003;17:2257–2317. [DOI] [PubMed] [Google Scholar]
- 25.Chaffin JM, Savage NM, Sharma S, et al. Persistent indolent pancolonic marginal zone lymphoma of MALT-type with plasmacytic differentiation - A rare post-transplant lymphoma? Hum Pathol (N Y). 2017;10:74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noone AM, Pfeiffer RM, Dorgan JF, et al. Cancer-attributable mortality among solid organ transplant recipients in the United States: 1987 through 2014. Cancer. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank D, Cesarman E, Liu YF, et al. Posttransplantation lymphoproliferative disorders frequently contain type A and not type B Epstein-Barr virus. Blood. 1995;85:1396–1403. [PubMed] [Google Scholar]
- 28.Nalesnik MA, Jaffe R, Starzl TE, et al. The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988;133:173–192. [PMC free article] [PubMed] [Google Scholar]
- 29.Peters AC, Akinwumi MS, Cervera C, et al. The Changing Epidemiology of Posttransplant Lymphoproliferative Disorder in Adult Solid Organ Transplant Recipients Over 30 Years: A Single-center Experience. Transplantation. 2018;102:1553–1562. [DOI] [PubMed] [Google Scholar]
- 30.Kim MJ, Yun SH, Chun HK, et al. Post-transplant lymphoproliferative disorder localized in the colon after liver transplantation: report of a case. Surg Today. 2009;39:1076–1079. [DOI] [PubMed] [Google Scholar]
- 31.Oertel SH, Verschuuren E, Reinke P, et al. Effect of anti-CD 20 antibody rituximab in patients with post-transplant lymphoproliferative disorder (PTLD). Am J Transplant. 2005;5:2901–2906. [DOI] [PubMed] [Google Scholar]
- 32.Shoji F, Yano T, Soejima Y, et al. Multiple pulmonary mucosa-associated lymphoid tissue lymphomas after living donor liver transplantation. Liver Transpl. 2009;15:1891–1893. [DOI] [PubMed] [Google Scholar]
- 33.Verma S, Frambach GE, Seilstad KH, et al. Epstein--Barr virus-associated B-cell lymphoma in the setting of iatrogenic immune dysregulation presenting initially in the skin. J Cutan Pathol. 2005;32:474–483. [DOI] [PubMed] [Google Scholar]
- 34.Cassidy DP, Vega F, Chapman JR. Epstein-Barr Virus-Positive Extranodal Marginal Zone Lymphoma of Bronchial-Associated Lymphoid Tissue in the Posttransplant Setting: An Immunodeficiency-Related (Posttransplant) Lymphoproliferative Disorder? Am J Clin Pathol. 2017;149:42–49. [DOI] [PubMed] [Google Scholar]
- 35.Sundararajan S, Chen H, Kumar A, et al. Donor-derived marginal zone lymphoma following reduced-intensity allogeneic peripheral blood stem cell transplant. Leuk Lymphoma. 2016;57:1735–1738. [DOI] [PubMed] [Google Scholar]
- 36.de Jong D, Roemer MG, Chan JK, et al. B-Cell and Classical Hodgkin Lymphomas Associated With Immunodeficiency: 2015 SH/EAHP Workshop Report-Part 2. Am J Clin Pathol. 2017;147:153–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu S, Zhong D, Wu X, et al. Gastric Mucosa-associated Lymphoid Tissue Lymphoma: Posttransplant Lymphopoliferative Disorder. Am J Med Sci. 2016;352:439–441. [DOI] [PubMed] [Google Scholar]
- 38.Bata BM, Pulido JS, Patel SV, et al. Combined intraocular and systemic rituximab for ocular lymphoproliferative disorder with extranodal marginal zone lymphoma-type morphology after heart transplant. J aapos. 2018;22:159–161. [DOI] [PubMed] [Google Scholar]
- 39.Bertoni F, Conconi A, Luminari S, et al. Lack of CD95/FAS gene somatic mutations in extranodal, nodal and splenic marginal zone B cell lymphomas. Leukemia. 2000;14:446–448. [DOI] [PubMed] [Google Scholar]
- 40.Spina V, Khiabanian H, Messina M, et al. The genetics of nodal marginal zone lymphoma. Blood. 2016;128:1362–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maurus K, Appenzeller S, Roth S, et al. Panel Sequencing Shows Recurrent Genetic FAS Alterations in Primary Cutaneous Marginal Zone Lymphoma. J Invest Dermatol. 2018;138:1573–1581. [DOI] [PubMed] [Google Scholar]
- 42.Isaacson PG, Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev Cancer. 2004;4:644–653. [DOI] [PubMed] [Google Scholar]
- 43.Ferreiro JF, Morscio J, Dierickx D, et al. EBV-Positive and EBV-Negative Posttransplant Diffuse Large B Cell Lymphomas Have Distinct Genomic and Transcriptomic Features. Am J Transplant. 2016;16:414–425. [DOI] [PubMed] [Google Scholar]
- 44.Streubel B, Simonitsch-Klupp I, Mullauer L, et al. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia. 2004;18:1722–1726. [DOI] [PubMed] [Google Scholar]
- 45.Ponce RA, Gelzleichter T, Haggerty HG, et al. Immunomodulation and lymphoma in humans. J Immunotoxicol. 2014;11:1–12. [DOI] [PubMed] [Google Scholar]
- 46.Morscio J, Dierickx D, Ferreiro JF, et al. Gene expression profiling reveals clear differences between EBV-positive and EBV-negative posttransplant lymphoproliferative disorders. Am J Transplant. 2013;13:1305–1316. [DOI] [PubMed] [Google Scholar]
- 47.Hussain SK, Makgoeng SB, Everly MJ, et al. HLA and Risk of Diffuse Large B cell Lymphoma After Solid Organ Transplantation. Transplantation. 2016;100:2453–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Capello D, Gaidano G. Post-transplant lymphoproliferative disorders: role of viral infection, genetic lesions and antigen stimulation in the pathogenesis of the disease. Mediterr J Hematol Infect Dis. 2009;1:e2009018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manez R, Breinig MC, Linden P, et al. Posttransplant lymphoproliferative disease in primary Epstein-Barr virus infection after liver transplantation: the role of cytomegalovirus disease. J Infect Dis. 1997;176:1462–1467. [DOI] [PubMed] [Google Scholar]
- 50.Johnson LR, Nalesnik MA, Swerdlow SH. Impact of Epstein-Barr virus in monomorphic B-cell posttransplant lymphoproliferative disorders: a histogenetic study. Am J Surg Pathol. 2006;30:1604–1612. [DOI] [PubMed] [Google Scholar]
- 51.Perry AM, Aoun P, Coulter DW, et al. Early onset, EBV(−) PTLD in pediatric liver-small bowel transplantation recipients: a spectrum of plasma cell neoplasms with favorable prognosis. Blood. 2013;121:1377–1383. [DOI] [PubMed] [Google Scholar]
- 52.Caillard S, Lamy FX, Quelen C, et al. Epidemiology of posttransplant lymphoproliferative disorders in adult kidney and kidney pancreas recipients: report of the French registry and analysis of subgroups of lymphomas. Am J Transplant. 2012;12:682–693. [DOI] [PubMed] [Google Scholar]


