Abstract
Background:
Patients with eosinophilic esophagitis have increased numbers of mucosal mast cells. Administration of the proton pump inhibitor omeprazole can reduce both esophageal mast cell and eosinophil numbers and attenuate type 2 inflammation in these subjects.
Objective:
Given that maintenance of an acidic environment within granules is important for mast cell homeostasis, we sought to evaluate the effects of omeprazole on mast cell functions including development, IgE:FcεRI-mediated activation and responses to food allergen.
Methods:
Mast cell degranulation, cytokine secretion and early signaling events in the FcεRI pathway, including protein kinase phosphorylation and Ca2+ flux, were measured following IgE crosslinking in murine bone marrow-derived mast cells and human cord blood-derived mast cells. The effects of omeprazole on these responses were investigated as was its impact on mast cell-dependent anaphylaxis and food allergy phenotypes in vivo.
Results:
Murine and human mast cells treated with omeprazole exhibited diminished degranulation and release of cytokines and histamine in response to allergen. In murine mast cells, phosphorylation of protein kinases, ERK and SYK, was decreased. Differentiation of mast cells from bone marrow progenitors was also inhibited. IgE-mediated passive anaphylaxis was blunted in mice treated with omeprazole as was allergen-induced mast cell expansion and mast cell activation in the intestine in a model of food allergy.
Conclusion:
Our findings suggest that omeprazole targets pathways important for the differentiation and activation of murine mast cells and for the manifestations of food allergy and anaphylaxis.
Keywords: food allergy, anaphylaxis, mast cell, omeprazole, proton pump inhibitor
Capsule Summary:
Omeprazole inhibits IgE-mediated mast cell activation and allergic responses to food allergen.
Introduction
Food allergy is a major health problem in industrialized countries and its prevalence has been increasing over the past decades1, 2. There is a lack of understanding of the mechanisms leading to increased immunological sensitivity to foods in affected subjects and there are no cures for the disease. Patients are advised to practice allergen avoidance and manage acute reactions that arise after accidental exposures using injectable epinephrine2, 3. Insight into the cellular and molecular pathways regulating hypersensitivity reactions to foods are badly needed and may lead to innovative strategies for treatment. In its most common and severe form, food allergy is mediated by the recognition of food antigens by food-specific IgE antibodies. Reactions are triggered upon IgE:allergen-mediated activation of mast cells which harbor the high affinity IgE receptor, FcεRI, on their cell surface4. Crosslinking of the receptor leads to degranulation of mast cells and release of inflammatory mediators that lead to clinical phenotypes including gastrointestinal responses (oral pruritus, abdominal pain, vomiting, diarrhea) and systemic anaphylaxis including vasodilation and vascular leakage with tissue edema. Together, these physiological changes can lead to decreased blood pressure with eventual shock and to respiratory failure3.
The prevalence of chronic inflammatory forms of immunologically-mediated food sensitivity is increasing. Many of these can belong to the category of eosinophilic gastrointestinal disorders. The most common is eosinophilic esophagitis (EoE), which is characterized by an elevated number of eosinophils in the esophagus and is one of the leading causes of food impaction and esophageal strictures5–7. There is clearly overlap between the two types of food sensitivity. For instance, in patients affected by EoE, tissue mast cell homeostasis is dysregulated, and IgE-mediated food allergies are often also present8. Increased mast cell-associated transcripts, including, for example, CPA3 and TPSB2, and mast cell numbers are typically present in the esophageal tissue of EoE patients9–12. In a subset of EoE patients, symptoms of esophageal dysfunction and eosinophilia are relieved following a course of PPI therapy, giving rise to the term “PPI-responsive EoE”13, 14. Although PPIs are used primarily to treat esophagitis and gastritis, they have also been shown to ameliorate symptoms, reverse tissue inflammation, and normalize the allergic transcriptome this subset of patients. Moreover, PPI treatment of subjects with PPI-responsive EoE results in a decrease in mast cell numbers with RNA analysis showing a reduction in mast cell transcripts 15.
By covalently binding to the cysteine residues of H+/K+ ATPases of the parietal cells, PPIs block the secretion of gastric acid, subsequently changing the pH of the cell and stomach lumen16. Mast cells are known to be sensitive to changes in pH. The granules of the cell are acidic compartments analogous to lysosomes17. Inhibition of proton pumps in mast cells by bafilomycin A1, a selective inhibitor of vacuolar ATPase, affects the acidification of the granules and therefore the processing and activity of granule contents18. Based on these observations, we reasoned that PPIs might similarly affect the pH of mast cell compartments and, consequently, the effector functions of the cells. We tested our hypothesis in in vitro and in vivo, analyzing the effects of PPIs on the functions of cultured mast cells and in mast cell-dependent mouse models of anaphylaxis and food allergy, respectively. Our results provide evidence that PPIs interact with targets that mediate activating signals provided by FcεRI ligation in mast cells, inhibit mediator release and attenuate the physiologic and inflammatory responses to mast cell activation in vivo.
Methods
Murine mast cell culture.
Bone marrow-derived mast cells (BMMCs) were cultured as previously described19. Briefly, BMMCs were derived from bone marrow precursor cells of BALB/c mice. Cells were cultured in RPMI-1640 medium supplemented with 10% FCS (Gibco), 100U/ml penicillin (Gibco), 100μg/ml streptomycin (Gibco), 1% Minimum Essential Medium nonessential amino acids (Gibco), 10mM HEPES buffer (Gibco), 55μM 2-mercaptoethanol (Gibco), 10μg/ml gentamicin (Life Technologies), and 20ng/mL each of IL-3 and SCF (complete media). Once cultures were mature (>90% of cells c-Kit+ FcεRIα+) cytokines were reduced to 10ng/ml (gating strategy for mast cells is provided in Fig E1A). IgE-induced degranulation (LAMP-1 expression), cytokine release, calcium flux, protein tyrosine phosphorylation and changes in granule and cytosolic pH were determined as detailed in the Online Repository.
Cord blood-derived human mast cell culture.
Cord blood-derived mast cells (CBMCs) were generously provided by the laboratory of Dr. J. Boyce (Brigham and Women’s Hospital, Boston, MA). Human mast cells were derived from cord blood mononuclear cells as previously described20. Mature human mast cells were always maintained and cultured in the presence of SCF (100ng/mL). For activation experiments, human mast cells were primed with IL-4 (10ng/mL) for three days in upregulate their surface expression of FcεRI. On the third day of IL-4 treatment cells were incubated with human myeloma IgE (100ng/ml; Chemicon International, Tecaluma, CA). Unbound IgE was washed away and cells were stimulated with rabbit anti-human IgE (100ng/ml). Details quantification of degranulation by measuring LAMP-1 expression, and release of histamine, prostaglandin D2 and cytokines are detailed in the Online Repository.
Treatment of mast cell cultures with omeprazole.
Omeprazole solid (Sigma, catalog number O104) was first activated for 30 minutes in PBS at a pH of 4 to convert it to the active sulfonamide form. An equal volume of complete media was added to the activated omeprazole to neutralize the pH before adding it to cell cultures. Cells were treated with omeprazole at a final concentration of 50μM as previously reported21–23. Drug vehicle (DMSO) was prepared in the same manner, including acidification and neutralization, at equal volumes.
Mouse studies.
All work was performed under protocols reviewed and approved by Boston Children’s Hospital Institutional Animal Care and Use Committee. All mice used in this study were bred and maintained under specific pathogen-free conditions in individually ventilated cages. To induce food allergy, 6–8-week-old BALB/c mice were sensitized for three consecutive weeks by intraperitoneal injection of 100μg of ovalbumin (OVA; Sigma, catalog number A5503) adsorbed to 1.5mg of aluminum hydroxide (Imject Alum, Pierce, Rockford, IL) in 0.2mL of sterile PBS. Following sensitization, animals were divided into two groups: omeprazole- or vehicle-treated. Omeprazole treatment was administered intragastrically at a dose of 12.5mg/kg per mouse (prepared in sterile PBS), as previously described23, and untreated animals were given equal volumes of vehicle (DMSO). Two hours post treatment, the mice were intragastrically challenged with 50mg of ovalbumin. Animals were re-challenged two days later and sacrificed two hours following the second challenge. Tissues were collected for follow-up experiments as described in the Online Repository. For passive systemic anaphylaxis experiments, female BALB/c mice were treated with 1.6mg esomeprazole in 0.2mL (a formulation of the drug for intravenous injection in humans) or with vehicle (PBS) for 4 consecutive days by intraperitoneal injection. On the third day, mice were intraperitoneally sensitized with 10μg IgE anti-DNP (clone SPE-7, Sigma-Aldrich, St. Louis, MO). Two hours after the final treatment mice were challenged intraperitoneally with 75μg DNP-BSA. Body temperature was recorded every 5 minutes for 60 minutes via implanted transponders24.
Mast cell differentiation assay.
To study the effects of omeprazole on the differentiation of progenitor cells to BMMCs, cells were treated with 50μM of omeprazole. Media were changed every three days and differentiation was assessed every six days by measuring the percentage of c-Kit and FcεRIα double-positive cells by flow cytometry.
Interleukin 4 (IL-4)-induced mast cell expansion in the small intestine.
Mice were injected with complexes of 2μg of IL-4 (Peprotech) and 10μg of anti-IL-4 (Biolegend) on days 0, 3 and 6. Mice were treated with esomeprazole daily from day −1 to day 6 by intraperitoneal injection. Blood and small intestine were collected on day 7.
Results
Omeprazole blocks the degranulation of bone marrow-derived murine mast cells and cord blood-derived human mast cells
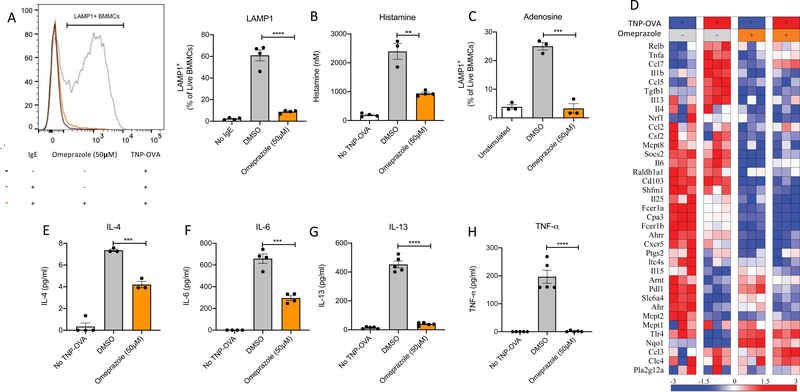
To test the effects of omeprazole on mast cells, we first examined its effects on IgE-mediated mast cell degranulation and cytokine production using cultured cells. Surface expression of lysosomal-associated membrane protein 1 (LAMP-1) following stimulation with antigen for 10 minutes was used as an indicator of the degree of mast cell degranulation. At baseline, in the absence of antigen-specific IgE (IgE anti-TNP), exposure of BMMCs to antigen (TNP-OVA) had no effect, and LAMP-1 expression was minimal (2.293% ± 0.5031%) (Fig 1A). As expected, upon stimulation with TNP-OVA, mast cells sensitized with IgE anti-TNP exhibited nearly a 30-fold increase in LAMP-1 expression on their surface (60.95% ± 5.118%). In contrast, IgE-sensitized BMMCs preincubated with 50μM omeprazole for two hours prior to stimulation with TNP-OVA exhibited markedly suppressed IgE-induced degranulation (8.775% ± 0.7180% LAMP-1+). and histamine release (Fig 1B). A dose response analysis revealed that suppression of degranulation by omeprazole was exerted in a dose-dependent manner over a range of 10 to 50μM in BMMCs (Fig E1B). The concentration of omeprazole used did not affect the viability of murine mast cells (Fig E1C).
Figure 1. Omeprazole blocks IgE-induced mast cell degranulation, cytokine secretion and transcriptional changes.
A) Representative histogram and bar plots for LAMP-1 expression by BMMCs following sensitization with IgE anti-TNP, treatment with drug vehicle (DMSO) or omeprazole, and challenge with TNP-OVA for 10 minutes. B) Histamine release into culture supernatant from IgE anti-TNP sensitized BMMCs following activation with TNP-OVA for 5 minutes. C) LAMP-1 expression of BMMCs following activation with adenosine. D) Heatmap of transcriptional changes in IgE sensitized BMMCs pre-treated with and without omeprazole followed by antigen stimulation for two hours. Direct mRNA counts as measured by the nCounter® Digital Analyzer System (NanoString) were normalized to internal positive, negative and housekeeping gene controls and presented as standard deviation from the row mean. E-H) Release of cytokines (IL-4, IL-6, IL-13, TNF-α) by IgE-sensitized BMMCs following omeprazole treatment and antigen stimulation for six hours. Statistical analysis by one-way analysis of variance. Data are shown for one experiment representative of two independent experiments for LAMP1, histamine and cytokine secretion assay; transcriptional profiling by NanoString was performed once. *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001
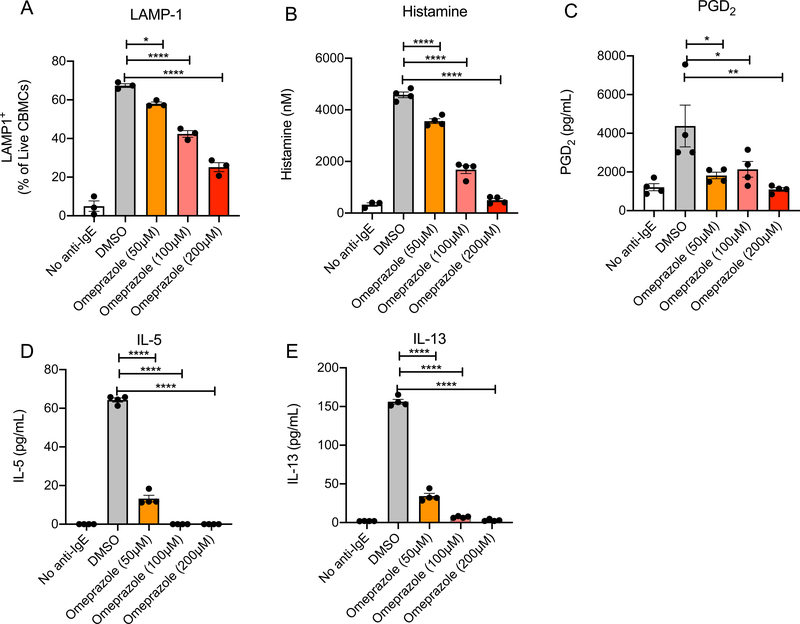
In order to test whether omeprazole similarly affects activation of human mast cells we took advantage of human cord blood-derived mast cells. These were sensitized with myeloma IgE and activated with anti-IgE in the presence or absence of omeprazole. As observed with murine BMMC, LAMP-1 expression and histamine release were suppressed by omeprazole in a dose dependent manner (Fig 2 A and B).
Figure 2. Omeprazole blocks IgE-induced human mast cell degranulation and cytokine secretion.
Bar plots showing LAMP-1 expression (A) and histamine release (B) by human mast cells following sensitization with IgE, treatment with drug vehicle (DMSO) or omeprazole, and challenge with anti-IgE for 10 and 5 minutes, respectively. Secretion of PGD2 (C), IL-5 (D) and IL-13 (E) by IgE-sensitized human mast cells following omeprazole treatment and stimulation with anti-IgE for six hours. Statistical analysis by one-way analysis of variance. *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001.
Though crosslinking of the IgE receptor is the best-known trigger of mast cell degranulation and the one likely to mediate immunologically specific responses to foods, stimulation through other receptors can also activate mast cells. We next tested if omeprazole can block non-IgE receptor-mediated mast cell activation, such as through G-protein coupled adenosine receptors. Stimulation of BMMCs with 100μM adenosine caused a 6-fold increase in LAMP-1 expression compared to unstimulated cells (25.03% ± 1.313% vs 3.847% ± 0.777%, Fig 1C). Treatment with omeprazole prior to adenosine stimulation restrained surface LAMP-1 at levels similar to those observed in unstimulated cells (3.310% ± 1.640%).
Upon IgE activation, transcription and translation of pro-inflammatory cytokines that contribute to allergic inflammation also occurs in mast cells. To assess these transcriptional changes, we used a predesigned NanoString panel composed of probes for the assessment of genes related to Th2 inflammation25. Transcriptional profiling of allergen-stimulated, IgE-sensitized BMMCs performed two hours following antigen exposure revealed the induction of a number of genes, including some encoding cytokines associated with allergic inflammation (Fig 1D). Pretreatment with omeprazole at 50μM for two hours attenuated the IgE:FcεRI-induced expression in BMMCs of pro-inflammatory genes such as, CCL5, IL-1β, IL-18, TGFβ, and IL-6 as well as IL-13 which is specifically related to type 2 inflammation. We further investigated the effect of omeprazole on select mast-cell-specific cytokines that are known to be synthesized de novo and released from the cells. Six hours after allergen challenge, IgE-sensitized BMMCs produced significantly greater amounts of IL-4, IL-6, IL-13 and TNF-α than unstimulated cells (Fig 1E–H). In cells pretreated with omeprazole, the production and release of these cytokines was reduced. Likewise, in human mast cell cultures we observed a dose responsive decrease in the secretion of PGD2 and cytokines, IL-5 and IL-13 (Fig 2C–E). Taken together, these results show that omeprazole treatment blocks the immediate release of preformed mediators contained in mast cell granules, the FcεRI-induced transcriptional program and the eventual production and secretion of cytokines.
Omeprazole treatment dampens activating signaling pathways in mast cells
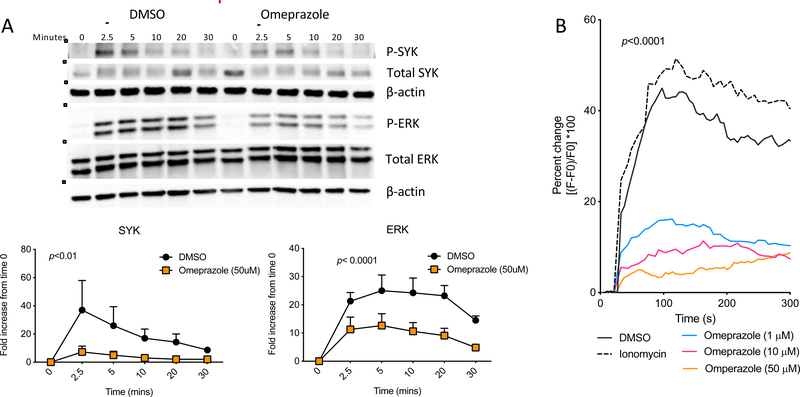
To obtain a better understanding of how omeprazole attenuates mast cell activation, we investigated early events in the well-characterized signaling cascade activated by FcεRI crosslinking. This pathway drives both degranulation with release of preformed mediators of anaphylaxis and the upregulation of inflammatory cytokine transcription and secretion4. To test the effect of omeprazole on signaling, we stimulated IgE anti-TNP bound BMMCs via FcεRI crosslinking with TNP-OVA for 2.5 to 30-minutes with or without pretreatment with omeprazole. The degree of phosphorylation of upstream (SYK) and downstream (ERK) protein kinases was measured to assess the strength of activation of the pathway. As previously extensively described by others26, 27, crosslinking of the IgE receptor resulted in phosphorylation of both SYK and ERK detectable as early as 2.5 minutes after stimulation (Fig 3A). Quantification of the ratio in signal intensities of phosphorylated to total protein showed that the IgE-induced phosphorylation was significantly weaker at all time points in omeprazole-treated cells following antigen stimulation.
Figure 3. Effect of omeprazole on calcium flux and signaling pathways downstream of FcεRI.
A) Representative SYK and ERK phosphorylation blots and compiled ratios of phospho protein/total protein intensities in IgE-sensitized BMMCs treated or untreated (DMSO) with omeprazole for two hours. Statistics calculated following two-way ANOVA. B) Mobilization of calcium from intracellular stores following antigen stimulation of IgE-sensitized BMMCs treated with omeprazole for two hours or untreated (DMSO). P-value calculated by two-way ANOVA between DMSO and omeprazole (50 μM). Data are shown for one experiment representative of at least 2 independent experiments.
Another early signal critical for degranulation and cytokine production by mast cells is increased cytosolic calcium. FcεRI crosslinking is associated with the release of calcium from endoplasmic reticulum stores into the cytosol. This is necessary for granule fusion with the plasma membrane and exocytosis of the contents28. Blocking calcium increase can disrupt the full range of mast cell activation phenotypes following IgE receptor crosslinking. Using the Ca2+chelating fluorophore, Fluo 4, we observed a robust increase in cytosolic Ca2+ ([Ca2+]i) immediately after antigen stimulation (Fig 3B). Compared to IgE-sensitized untreated BMMCs, omeprazole-treated mast cells exhibited a dramatic suppression of increase in [Ca2+]i in a dose responsive manner. These results show that omeprazole exerts suppressive effects on several of the key signaling events elicited by cross-linking of FcεRI.
Omeprazole dampens passive IgE-mediated systemic anaphylaxis
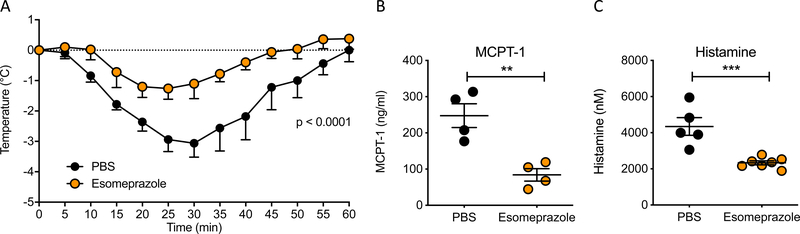
Systemic anaphylaxis is the most dramatic clinical outcome of an allergic reaction and is caused by the multi-tissue effects of mediators released by activated mast cells and basophils following crosslinking of the cell surface IgE receptor24, 29–31. Our in vitro data showed that treatment of mast cells with omeprazole blocked their IgE:FcεRI-induced degranulation. Using a model of passive systemic anaphylaxis, we assessed if the inhibition observed in vitro is recapitulated in vivo and whether diminished mast cell activation in the presence of omeprazole is accompanied by attenuation of the physiologic manifestations of anaphylaxis. Mice were passively sensitized with anti-DNP IgE and challenged intraperitoneally with DNP-BSA. Changes in core body temperature were measured as an indicator of vasodilation (with increased cutaneous blood flow and cooling) and shock. As expected, untreated BALB/c mice exhibited a significant drop in temperature following antigen challenge (Fig 4A). To investigate the effects of proton pump inhibitors on anaphylaxis, we used esomeprazole which is a single (S) enantiomer of omeprazole and available as an injectable formulation. Esomeprazole treatment for 4 days prior to challenge significantly decreased the drop in core body temperature to less than half of vehicle treated animals. Release of the mast cell granule constituent protease, MCPT-1, and histamine were also reduced to less than 50% that observed in PBS-treated animals (Fig 4B and C).
Figure 4. Effect of omeprazole on passive systemic anaphylaxis.
A) Change in core body temperature of mice treated with omeprazole or drug vehicle, sensitized with SPE-7, and challenged with DNP-BSA. Statistical analysis by two-way analysis of variance. B) Serum concentration of MCPT-1 in animals from PSA experiment at endpoint. C) Plasma histamine concentration in animals 5 minutes after allergen challenge. Statistical analysis by unpaired t-test. Data are from one experiment representative of 2 independent experiments with n = 4–5 for temperature drop and MCPT1 levels; histamine levels are from one experiment with n = 3–4 animals. **P<0.01 and ***P<0.001
Treatment with omeprazole alters the pH in the cytosol and subcellular compartments of the mast cell
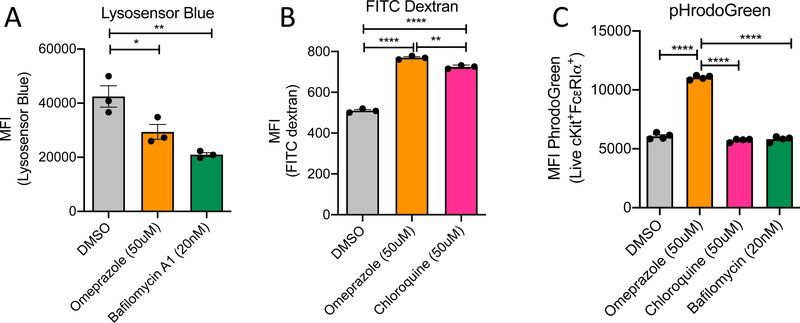
Based on the known action of omeprazole as a proton pump inhibitor in gastric parietal cells, we sought to determine whether it affects the pH of the acidic storage compartments and cytosol of mast cells. Using Lysosensor Blue, a dye that accumulates in acidic intracellular compartments, we assessed the effects of omeprazole on pH in mast cell granules. As a control we treated BMMCs with bafilomycin, a blocker of V-ATPase that has previously been shown to increase the pH of mast cell granules18. In comparison to vehicle-treated cells, the fluorescence intensity of Lysosensor Blue in both omeprazole- and bafilomycin A1-treated cells was significantly decreased, indicating that the intracellular granule compartment pH was increased (Fig 5A).
Figure 5. pH of cellular compartments following omeprazole treatment of BMMCs.
A) Measurement of changes in pH by of intracellular vesicles by Lysosensor blue following treatment with omeprazole and bafilomycin. B) Loss of quenching of FITC-dextran in BMMCs treated with omeprazole or chloroquine. C) Measurement of changes in cytosolic pH by pHrodoGreen following treatment with omeprazole, chloroquine or bafilomycin. Statistical analysis by one-way analysis of variance. Data are shown from one representative of two experiments. MFI, mean fluorescence intensity. *P<0.05, **P<0.01, ***P<0.001 , and ****P<0.0001
In an alternate approach to detecting changes in the pH of intracellular vesicles, we performed a FITC-dextran quenching experiment. FITC-dextran is taken up into endosomes which fuse with acidic intracellular vesicles. The FITC signal is quenched by the acidic pH within those compartments. Chloroquine, a blocker of endosome-lysosome fusion, was included as a control for this experiment. Compared to untreated cells, both chloroquine- and omeprazole-treated cells exhibited higher FITC fluorescence intensity than did untreated cells, consistent with an increased pH in those normally acidic organelles (Fig 5B). As granules represent a significant fraction of mast cell volume, we reasoned that inhibition of proton accumulation in those compartments might be accompanied by an opposite effect, namely reduction of pH, in the cytosol. We tested this hypothesis using pHrodoGreen, a dye that becomes trapped in the cytosol and whose fluorescence is inversely proportional to the pH of the cytosol. The pHrodoGreen assay showed that BMMCs treated with omeprazole had a more acidic cytosol environment compared to untreated cells (Fig 5C). Notably, the cytosolic pH was unaffected by pretreatment with bafilomycin. Furthermore, mast cells treated with bafilomycin have previously been characterized as having altered morphology with swollen granules that have diminished staining with May Grünwald/Giemsa (MGG) 18. In our hands, we observed similar changes (Fig E2). This dramatic phenotype was not observed in omeprazole treated cells. However, unlike the DMSO-treated control cells, they did exhibit a few punctate MGG negative regions (Fig E2). These findings suggest that the effects of omeprazole on mast cell function might involve the target of bafilomycin, the V-ATPase or a related proton pump.
Omeprazole blocks mast cell differentiation in vitro and in vivo
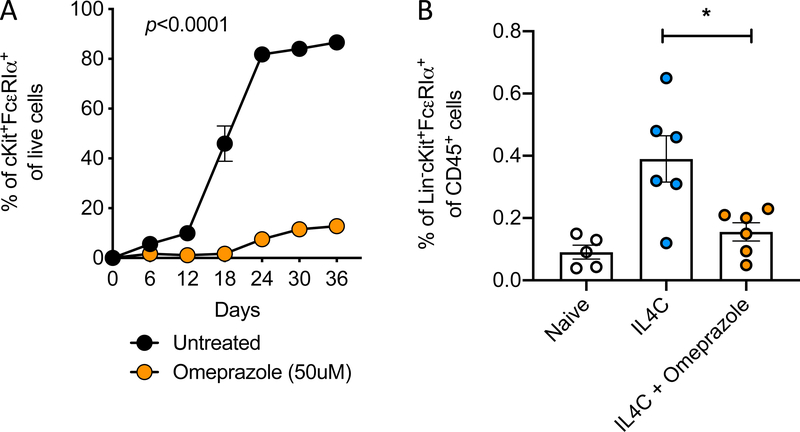
As our findings had shown that treatment with omeprazole affects the signaling pathways and activation of mature mast cells, we next evaluated whether they might affect the differentiation of precursor cells into mature mast cells. Culturing murine bone marrow stem cells in the presence of IL-3 and SCF induces differentiation into mature mast cells. Mast cell maturation in such cultures can be assessed by monitoring the appearance of c-Kit and FcεRIα double-positive cells (Fig E1A). Under normal growth conditions, more than 80% (86.65% ± 1.350%) of cells in such cultures acquire the double-positive mast cell phenotype after five weeks (Fig 6A). However, less than 15% (12.75% ± 0.75%) of cells cultured in the presence of 50μM of omeprazole were mature. In order to determine whether omeprazole might similarly affect mast cell differentiation in vivo, we took advantage of an IL-4-driven model. We and others have reported that exogenous administration of IL-4 as an immune complex with anti-IL4 (IL4C) to prolong the cytokine’s half-life (three injections over one week), elicits an expansion of intestinal mast cells (Fig 6B)19, 32. Using this approach along with flow cytometric enumeration of intestinal mast cells, we found that daily treatment with esomeprazole by intraperitoneal injection decreased IL-4-induced mast cell expansion (Fig 6B). These in vivo data corroborate our tissue culture findings in showing that omeprazole can block the maturation of precursor cells into mast cells.
Figure 6. Omeprazole inhibits differentiation of myeloid precursor cells to mast cells.
A) Maturation of precursor cells to BMMCs in the presence or absence of omeprazole. Statistical analysis by two-way analysis of variance. B) Percentage of small intestinal mast cells among CD45+ cells in mice intraperitoneally injected with immune complexes of IL-4 and anti-IL-4 (IL4C) to induce mastocytosis and intragastrically treated with omeprazole. For in vitro mast cell differentiation, data is from one experiment involving three independent cultures; the in vivo experiment was repeated twice, and data are shown for one experiment. *P<0.05
Treatment with omeprazole blocks allergic responses in a mast cell-dependent mouse model of food allergy
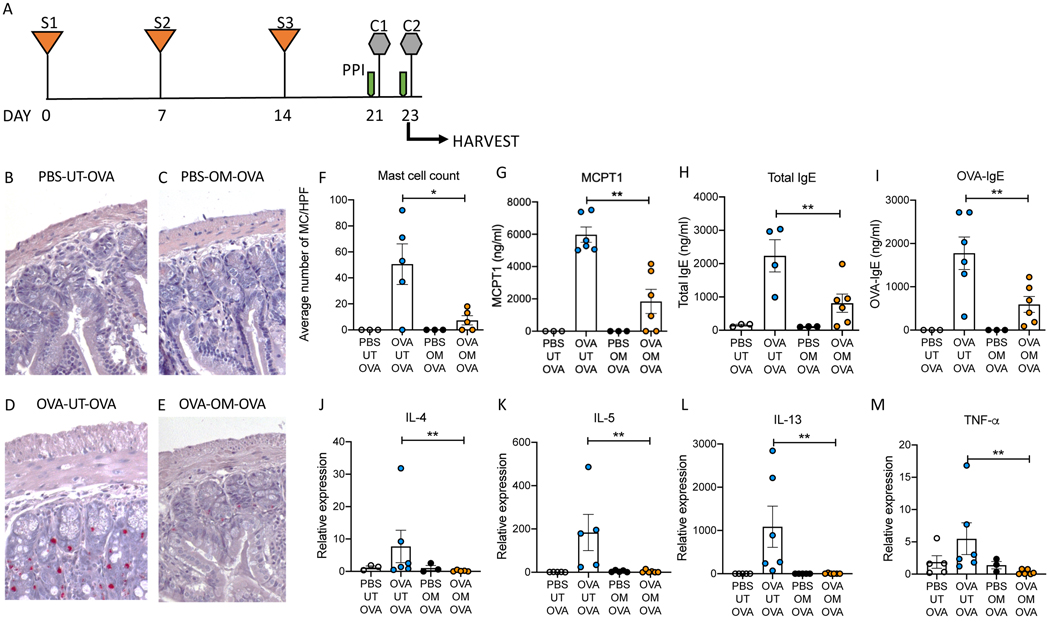
In addition to driving immediate hypersensitivity reactions, mast cells are known to play key roles in initiating and sustaining allergic inflammatory responses19, 31, 33, 34. They provide a critical tissue source of cytokines that activate mucosal antigen presenting cells, as well as IL-4 which primes and consolidates T-helper 2 responses. Our analysis of the effects of omeprazole on cultured mast cells showed a dramatic suppression of cytokine production following IgE-mediated activation by allergen (Fig 1C–D). Our final aim was to investigate whether this effect would be reflected in vivo by an altered response to food allergen. To test this possibility, we adapted a well-characterized mast cell-dependent model of food allergy initially described by Rothenberg and colleagues (Fig 7A)31. In this model, mice sensitized intraperitoneally with allergen and then repeatedly enterally challenged to develop strong IgE responses and food sensitivity. The response to repeated allergen challenge was accompanied by intestinal mast cell expansion and a correlation has been demonstrated between the number of mast cells in the small intestine and the severity of allergic reactions29.
Figure 7. Treatment with omeprazole blocks inflammation in a mouse model of ovalbumin-induced food allergy.
A) Experimental design (S: sensitization, C: challenge, PPI: proton pump inhibitor). B-E) Representative CAE-stained histological sections of small intestine from mice from each experimental group. F) Summary of average counts of mast cells per high power field. G) Serum concentrations of MCPT-1 at the experimental endpoint. H and I) Serum total and OVA-specific IgE concentrations at experimental endpoint. J-M) Expression of cytokine transcripts in the small intestines of mice from each experimental group at study endpoint. UT: Untreated, OM: Omeprazole, OVA: Ovalbumin. Data are shown are from one experiment representative of three independent experiments with n = 3–6 per group. Statistical analysis by one-way analysis of variance. *P<0.05, **P<0.01
Examination of chloroacetate esterase (CAE)-stained jejunal sections revealed a robust expansion of mast cells following ovalbumin challenge in mice that had been sensitized to the antigen (Fig 7D), but not in unsensitized controls (Fig 7B and C). This was reduced by omeprazole treatment (Fig 7E). Enumeration of mast cells in sections from multiple mice confirmed these observations (Fig 7F). Following allergen challenge by gavage, omeprazole-treated mice also exhibited greatly reduced release of MCPT-1 into the serum (Fig 7G). The increase in total and allergen specific IgE (OVA-IgE) in the serum following antigen challenge in sensitized mice was attenuated in omeprazole treated mice (Fig 7 H and I). mRNA expression of several cytokines associated with allergic inflammation, IL-4, IL-5, IL-13 and TNF-α, was also reduced in omeprazole-treated, allergen-sensitized and challenged animals compared to untreated animals (Fig 7 J–M). These findings indicate that omeprazole affects pathways involved in mast cell differentiation and activation in response to ingested allergen and alters the intestinal cytokine environment.
Discussion
Our study provides strong evidence that the proton pump inhibitor omeprazole exerts significant effects on pathways regulating mast cell homeostasis and function and can modulate mast cell-dependent allergic disease phenotypes. We demonstrate that omeprazole attenuates mast cell activation induced by FcεRI crosslinking and by adenosine, with decreased degranulation and cytokine responses. Suppression occurs early in the signaling cascade with decreased phosphorylation of the FcεRI-proximal tyrosine protein kinase SYK, as well as the downstream serine protein kinase ERK, along with attenuation of increased [Ca2+]i. The physiologic impact of the inhibitory effects of omeprazole on mast cell function were clearly evident in in vivo models of anaphylaxis and mast cell-dependent food allergy. Furthermore, we observe that omeprazole at 200μM can also block the activation of basophils, another major cell involved in allergic phenotypes and anaphylaxis (Fig E3).
PPIs have been reported to have clinical benefit in eosinophilic esophagitis35. It was initially postulated that the omeprazole responsiveness of some subjects with distal esophageal eosinophilia indicated that their esophageal inflammation was triggered by acid reflux and not food-induced type 2 inflammation. However, there is evidence that the mechanism of action of omeprazole can be attributed to anti-type 2 inflammatory effects. For instance, oral administration of omeprazole prior to allergen instillation into mouse airways reduced the recruitment of inflammatory cells, including eosinophils21. In PPI-responsive EoE, there is now general agreement, based largely on transcriptomic analyses, that omeprazole treatment attenuates type 2 inflammation11. There is evidence that omeprazole impacts a range of signaling intermediates. For instance, studies on epithelial cells have shown that omeprazole decreases IL-4-, IL-13-, and IFN-ß-induced phosphorylation of STAT621.
The mechanism whereby omeprazole exerts its effects on mast cells remains to be determined. On gastric parietal cells, omeprazole binds to and inhibits the gastric ATPase. However, we have found that expression of this proton pump is quite low in mast cells. We speculate that omeprazole could be binding to the V-ATPase, the target of bafilomycin A1. Treatment with bafilomycin has previously been shown to alter mast cell morphology, inhibit the maturation of mast cell granules and processing granule contents and impair mast cell activation18. Furthermore, it has been reported that omeprazole binds to the catalytic sites of V-ATPaess purified from adrenal chromaffin granules and reconstituted into liposomes36. In our hands, bafilomycin and omeprazole exerted similar effects on mast cell granule pH (Fig 5 A). Yet, we have reason to speculate that additional targets for omeprazole might exist in mast cells as omeprazole, but not bafilomycin, affects cytosolic pH (Fig 5C) and changes observed in granule morphology are not identical by both drugs (Fig E2).
Several studies describe a link between the use of acid suppression and the development of food allergies37. Elevating the pH of the stomach can decrease the activity of stomach enzymes, such as pepsin, that are needed for protein digestion. It is hypothesized that incomplete digestion might allow larger peptides harboring allergenic epitopes to enter the small intestine and induce antigen sensitization38. In our studies, omeprazole had an anti-allergic effect, but it was only administered once sensitization was established. Animals are sensitized to either ovalbumin or with anti-DNP IgE via the intraperitoneal route, and so our experimental design allows us to study the effects of omeprazole on allergen challenge-induced inflammation and avoid its effects on sensitization.
Other research groups have also described anti-inflammatory effects of PPIs. For example, omeprazole has been shown to decrease the migration of polymorphonuclear neutrophils (PMN) towards the chemoattractant IL-839. Correlating with this decrease in migration, a decrease in the expression of adhesion molecules CD11b and CD18 on PMNs has also been observed40. Furthermore, production of IL-8 by epithelial cells following stimulation with IL-1β is also inhibited39. These effects are beneficial in attenuating the inflammation observed in infections, such as from Helicobacter pylori. In models of sepsis, injection with omeprazole pre- or post-endotoxic shock can increase survival time by limiting cytokine production23. Treatment of monocytes and animals with omeprazole inhibits TLR agonist-induced secretion of IL-1β and TNF-α23.
Our studies establish that omeprazole can interfere with pathways involved in mast cell differentiation and activation as well as allergic inflammation. These observations provide insight into the beneficial effects of this agent in some patients with EoE. In future studies, it will be of great interest to identify the specific pharmacologic target(s) of omeprazole in mast cells, and to consider the development of higher affinity and more specific small molecule inhibitors.
Supplementary Material
Key Messages:
Omeprazole blocks IgE-mediated mast cell degranulation, and prostaglandin D2 and cytokine production in response to allergen as well as IgE-mediated hypersensitivity in vivo.
Pretreatment with omeprazole results in decreased phosphorylation of protein kinases and inhibits calcium flux into the cytosol.
In a food allergy model, omeprazole inhibits mast cell expansion, type 2 allergic inflammation and hypersensitivity responses to ingested allergen.
Acknowledgements
The authors are grateful to Dr. Joshua Boyce and Dr. Chunli (Lily) Feng for providing human cord blood derived- mast cells. We are also grateful to Dr. Takashi Fujimura, Ms. Catherine Lavallee for advice and assistance with animal studies, and to Ms. Yvonne Nguyen for technical assistance.
Research in this report was funded by NIH, grants 1R01AI119918-05 (HCO), DK097112-05 (RR) and 5T32AI007512-33 (SCM). CK is supported by a postdoctoral fellowship from the Fonds de recherche du Québec - Santé. EF was supported by a Bridge Grant from the Research Council of Boston Children’s Hospital, an Emerging Investigator Award from FARE, a Senior Research Award from the Crohn’s and Colitis Foundation, and an unrestricted gift from the Mead Johnson Nutrition Company. This work was further supported by an NIH grant of the Harvard Digestive Diseases Center (P30DK034854, Core C). Additional generous support was provided by a Sean N. Parker Center Seed Grant, the Bunning Family Foundation, the Nanji Family Fund for Food Allergy Research, the Rao Chakravorti Family Fund, and the Christine Olsen and Robert Small Food Allergy Research Fund.
Abbreviations:
- OVA
ovalbumin
- MCPT-1
mast cell protease-1
- EoE
eosinophilic esophagitis
- PPI
proton pump inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sicherer SH, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med 2009; 60:261–77. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 2014; 133:291–307; quiz 8. [DOI] [PubMed] [Google Scholar]
- 3.Renz H, Allen KJ, Sicherer SH, Sampson HA, Lack G, Beyer K, et al. Food allergy. Nat Rev Dis Primers 2018; 4:17098. [DOI] [PubMed] [Google Scholar]
- 4.Oettgen HC, Burton OT. IgE receptor signaling in food allergy pathogenesis. Curr Opin Immunol 2015; 36:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuta GT, Katzka DA. Eosinophilic Esophagitis. N Engl J Med 2015; 373:1640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Shea KM, Aceves SS, Dellon ES, Gupta SK, Spergel JM, Furuta GT, et al. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology 2018; 154:333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spergel J, Aceves SS. Allergic components of eosinophilic esophagitis. J Allergy Clin Immunol 2018; 142:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelz BJ, Wechsler JB, Amsden K, Johnson K, Singh AM, Wershil BK, et al. IgE-associated food allergy alters the presentation of paediatric eosinophilic esophagitis. Clin Exp Allergy 2016; 46:1431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu Blatman KS, Gonsalves N, Hirano I, Bryce PJ. Expression of mast cell-associated genes is upregulated in adult eosinophilic esophagitis and responds to steroid or dietary therapy. J Allergy Clin Immunol 2011; 127:1307–8 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niranjan R, Mavi P, Rayapudi M, Dynda S, Mishra A. Pathogenic role of mast cells in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 2013; 304:G1087–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen T, Dellon ES, Moawad FJ, Furuta GT, Aceves SS, Rothenberg ME. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump inhibitor-reversible allergic inflammation. J Allergy Clin Immunol 2015; 135:187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abe Y, Sasaki Y, Yagi M, Yaoita T, Nishise S, Ueno Y. Diagnosis and treatment of eosinophilic esophagitis in clinical practice. Clin J Gastroenterol 2017; 10:87–102. [DOI] [PubMed] [Google Scholar]
- 13.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128:3–20 e6; quiz 1–2. [DOI] [PubMed] [Google Scholar]
- 14.Lucendo AJ, Arias A, Molina-Infante J. Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remission in Patients With Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2016; 14:13–22 e1. [DOI] [PubMed] [Google Scholar]
- 15.Dohil R, Newbury RO, Aceves S. Transient PPI responsive esophageal eosinophilia may be a clinical sub-phenotype of pediatric eosinophilic esophagitis. Dig Dis Sci 2012; 57:1413–9. [DOI] [PubMed] [Google Scholar]
- 16.Shin JM, Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil 2013; 19:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson RG, Carty SE, Fingerhood BJ, Scarpa A. The internal pH of mast cell granules. FEBS Lett 1980; 120:75–9. [DOI] [PubMed] [Google Scholar]
- 18.Pejler G, Hu Frisk JM, Sjostrom D, Paivandy A, Ohrvik H. Acidic pH is essential for maintaining mast cell secretory granule homeostasis. Cell Death Dis 2017; 8:e2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burton OT, Darling AR, Zhou JS, Noval-Rivas M, Jones TG, Gurish MF, et al. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal Immunol 2013; 6:740–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochi H, Hirani WM, Yuan Q, Friend DS, Austen KF, Boyce JA. T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J Exp Med 1999; 190:267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortes JR, Rivas MD, Molina-Infante J, Gonzalez-Nunez MA, Perez GM, Masa JF, et al. Omeprazole inhibits IL-4 and IL-13 signaling signal transducer and activator of transcription 6 activation and reduces lung inflammation in murine asthma. J Allergy Clin Immunol 2009; 124:607–10, 10 e1. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Cheng E, Huo X, Yu C, Zhang Q, Pham TH, et al. Omeprazole blocks STAT6 binding to the eotaxin-3 promoter in eosinophilic esophagitis cells. PLoS One 2012; 7:e50037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balza E, Piccioli P, Carta S, Lavieri R, Gattorno M, Semino C, et al. Proton pump inhibitors protect mice from acute systemic inflammation and induce long-term cross-tolerance. Cell Death Dis 2016; 7:e2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathias CB, Hobson SA, Garcia-Lloret M, Lawson G, Poddighe D, Freyschmidt EJ, et al. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. J Allergy Clin Immunol 2011; 127:795–805 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platzer B, Baker K, Vera MP, Singer K, Panduro M, Lexmond WS, et al. Dendritic cellbound IgE functions to restrain allergic inflammation at mucosal sites. Mucosal Immunol 2015; 8:516–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev 2009; 228:149–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki R, Scheffel J, Rivera J. New insights on the signaling and function of the high-affinity receptor for IgE. Curr Top Microbiol Immunol 2015; 388:63–90. [DOI] [PubMed] [Google Scholar]
- 28.Holowka D, Wilkes M, Stefan C, Baird B. Roles for Ca2+ mobilization and its regulation in mast cell functions: recent progress. Biochem Soc Trans 2016; 44:505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahrens R, Osterfeld H, Wu D, Chen CY, Arumugam M, Groschwitz K, et al. Intestinal mast cell levels control severity of oral antigen-induced anaphylaxis in mice. Am J Pathol 2012; 180:1535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin RY, Schwartz LB, Curry A, Pesola GR, Knight RJ, Lee HS, et al. Histamine and tryptase levels in patients with acute allergic reactions: An emergency department-based study. J Allergy Clin Immunol 2000; 106:65–71. [DOI] [PubMed] [Google Scholar]
- 31.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest 2003; 112:1666–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF Jr., Katona IM, et al. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol 2002; 169:4417–22. [DOI] [PubMed] [Google Scholar]
- 33.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med 2012; 18:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev 2018; 282:121–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohm M, Richter JE. Treatment of eosinophilic esophagitis: overview, current limitations, and future direction. Am J Gastroenterol 2008; 103:2635–44; quiz 45. [DOI] [PubMed] [Google Scholar]
- 36.Moriyama Y, Patel V, Ueda I, Futai M. Evidence for a common binding site for omeprazole and N-ethylmaleimide in subunit A of chromaffin granule vacuolar-type H(+)-ATPase. Biochem Biophys Res Commun 1993; 196:699–706. [DOI] [PubMed] [Google Scholar]
- 37.Jordakieva G, Kundi M, Untersmayr E, Pali-Scholl I, Reichardt B, Jensen-Jarolim E. Country-wide medical records infer increased allergy risk of gastric acid inhibition. Nat Commun 2019; 10:3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pali-Scholl I, Jensen-Jarolim E. Anti-acid medication as a risk factor for food allergy. Allergy 2011; 66:469–77. [DOI] [PubMed] [Google Scholar]
- 39.Handa O, Yoshida N, Fujita N, Tanaka Y, Ueda M, Takagi T, et al. Molecular mechanisms involved in anti-inflammatory effects of proton pump inhibitors. Inflamm Res 2006; 55:476–80. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida N, Yoshikawa T, Tanaka Y, Fujita N, Kassai K, Naito Y, et al. A new mechanismfor anti-inflammatory actions of proton pump inhibitors--inhibitory effects on neutrophil-endothelial cell interactions. Aliment Pharmacol Ther 2000; 14 Suppl 1:74–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.