Abstract
Background:
In Botswana, nearly two-thirds of cervical cancer patients are HIV-positive. This study examined the relationship between CD4 count and chemoradiation therapy outcomes among cervical cancer patients with HIV.
Setting:
A prospective cohort study of 231 HIV-positive women with locally invasive cervical cancer was conducted in Gaborone, Botswana from January 2015 to February 2018.
Methods:
Primary outcome was survival, defined as time from scheduled end of chemoradiation therapy to death or last contact with patient. Nadir CD4 count was defined as lowest CD4 available before cancer diagnosis. Delta CD4 count was defined as improvement from nadir CD4 to CD4 at cancer diagnosis. Hazard ratio (HR) analyses were adjusted for presenting variables (age, baseline hemoglobin, cancer stage, and performance status) and treatment variables (chemotherapy cycles and radiation dose).
Results:
231 patients were included in nadir CD4 analysis; 139 were included in delta CD4 analysis. Higher delta CD4 was significantly associated with reduced mortality after adjusting for presenting and treatment variables (CD4 100–249: HR 0.45, 95% CI 0.21–0.95; CD4 ≥ 250: HR 0.45, 95% CI 0.20–1.02) . Higher nadir CD4 showed a trend towards reduced mortality after adjusting for presenting and treatment variables (HR 0.94, 95% CI 0.84–1.06).
Conclusions:
Higher delta CD4 (greater improvement from nadir CD4 to CD4 at cervical cancer diagnosis) is significantly associated with lower mortality. While not statistically significant, data suggest that higher nadir CD4 may reduce mortality. These results reinforce the importance of early HIV diagnosis and ART initiation, as their effects impact cervical cancer outcomes years later.
Keywords: cervical cancer, chemoradiotherapy, HIV, HPV, CD4, immune reconstitution
INTRODUCTION
Cervical cancer is a major -- and preventable -- cause of morbidity and mortality worldwide.1 The burden of disease is especially high in sub-Saharan Africa, which has the highest incidence of cervical cancer in the world.2,3 In Botswana, an upper middle-income country from this region, cervical cancer is the most common gynecologic cancer and the leading cause of cancer death among women.1,4,5 The high prevalence of HIV in Botswana is an additional factor contributing to the increasing burden of cervical cancer. As of 2016, 21.9% of adults between age 15 and 49 are living with HIV in Botswana.6 Nearly two-thirds of cervical cancer cases in Botswana occur in patients who are HIV-positive.4,7
Cervical cancer is an AIDS-defining condition, yet its association with HIV-related immunosuppression is not fully understood. HIV-related immunodeficiency is known to increase the risk for acquisition and persistence of genital human papillomavirus (HPV) infection and leads to higher rates of cervical cancer.8 It is believed that HIV-induced immunosuppression leads to an inability to control HPV expression, resulting in increased risk of persistent infection, increased oncogenicity of high-risk HPV subtypes, and increased activity of low-risk HPV subtypes.9 However, contrary to other AIDS-defining illnesses which only present with severe immunosuppression, most cervical cancer cases in HIV-infected women occur in individuals with relatively preserved immune systems (CD4 cell count > 200 cells/mm3) at the time of cancer diagnosis.10 Also in contrast to other AIDS-defining illnesses in which ART-related immune reconstitution is clearly beneficial, the impact of antiretroviral therapy (ART) on progression to cervical cancer remains uncertain. Indeed, to date, registry-based data have consistently shown lack of effect of ART on cervical cancer incidence.11-15 Clinical research, on the other hand, has produced more conflicting results regarding both the effect of ART on HPV infection and persistence, as well as its impact on cervical disease progression/regression.16-22 Despite these inconsistencies, most literature shows that more advanced HIV disease is associated with increased HPV prevalence and increased rate of progression of cervical disease.23-27 HIV research has shown that nadir CD4 is a consistent predictor of functional immune reconstitution and an important predictor of prognosis in HIV infected individuals.28 Initiating ART after the immune system has been already severely damaged by HIV may result in an inability to regain functional local and systemic immune responses, even in the context of large increases in CD4 cell count and a sustained fall in viral load. While it is not known how CD4 cell count levels or the extent of immune reconstitution after ART affect survival among HIV-infected women receiving cervical cancer treatment (chemoradiation), it is possible that some of the issues mentioned above play a role in conditioning treatment response and survival during cancer treatment.
In this study, we examined the relationship between CD4 cell count and survival among HIV-infected patients with cervical cancer. We hypothesized that lower nadir CD4 cell count and smaller increases in CD4 cell counts will be associated with poorer survival after controlling for treatment- and cancer-specific variables. Accordingly, we determined the effect of both nadir CD4 cell count, defined as a patient’s lowest CD4 cell count prior to cancer diagnosis, and of delta CD4 cell count, defined as a patient’s improvement after ART from nadir CD4 to CD4 count at the time of cervical cancer diagnosis, on survival among HIV-positive cervical cancer patients who received chemoradiation therapy.
METHODS
Study Design:
Prospective observational cohort.
Population:
Patients were enrolled from two tertiary hospitals in Gaborone, Botswana (Princess Marina Hospital and Gaborone Private Hospital) between July 2013 and July 2017 and followed until September 2018. All women above the age of 18 with newly diagnosed locally invasive cervical were approached for enrollment. Less than 5% of patients who were approached for enrollment declined to participate in the study. Among HIV-positive patients, only those with sufficient survival data and CD4 count data were included in this analysis. Thirty-nine patients were excluded by these criteria, resulting in a total of 231 patients that qualified for final analysis. Nadir CD4 count was defined as lowest CD4 count available before cancer diagnosis. Delta CD4 count was defined as improvement from nadir CD4 count to CD4 count at cervical cancer diagnosis. Of the HIV-positive women with documented nadir CD4 counts, changes (delta) in CD4 cell count were calculated for patients with at least two documented CD4 count measurements at least 6 months apart. Eligible patients were enrolled and treated at the gynecologic oncology multidisciplinary team clinic at Princess Marina Hospital and at Gaborone Private Hospital with radiation or chemoradiation based on their stage and performance status.
Treatment of Cervical Cancer in Botswana:
The government of Botswana provides free public healthcare to all citizens, including ART for HIV-infected individuals and treatment for cervical cancer. The standard of care for locally advanced cervical cancer (FIGO stages IB1-IVB) in Botswana and globally consists of radiation, including external beam radiation and brachytherapy, and cisplatin-based chemotherapy.29-31 The standard of care for early stage invasive disease (FIGO stages IA1-IB1) is radical hysterectomy; however, the availability of gynecologic oncology services in Botswana is extremely limited. Thus, many patients with early stage invasive disease who cannot access surgical treatment are managed with radiation with or without chemotherapy. Indications for stopping or holding chemotherapy in this patient population include low performance status, elevated creatinine, and toxicity. Because radiation therapy is not yet available in Botswana’s public sector, patients are referred to Gaborone Private Hospital, the country’s only radiation oncology facility, and treatment is fully funded by the government of Botswana.
Management of HIV in Botswana:
The prevalence of HIV in Botswana is among the highest in the world at 18% among adults.32 All patients diagnosed with cancer are screened for HIV prior to starting cancer treatment and initiated on ART, which is provided at no cost to all citizens through Botswana’s public healthcare system. Prior to 2016, ART was provided only to citizens with CD4 < 350 cells/uL or with WHO stage 3 or 4 HIV disease, with invasive cervical cancer classified as a marker of WHO stage 4 disease.33 First line treatment for HIV prior to 2016 included the following combinations of antiretroviral medications: tenofovir + emtricitabine + efavirenz, emtricitabine/tenofovir (Truvada) + nevirapine, lamivudine/zidovudine (Combivir) + efavirenz, abacavir + lamivudine + nevirapine, and abacavir + lamivudine + efavirenz.33 In 2016, Botswana adopted the “Treat All” policy, which expanded free ART access to all HIV+ citizens, regardless of CD4 count or clinical stage of disease. Per Botswana’s 2016 Integrated HIV Clinical Care Guidelines, first line treatment for HIV now consists of ART with emtricitabine/tenofovir (Truvada) + dolutegravir.34
While complete data on ART adherence and viral load is not available for this study population, ART adherence among HIV-positive individuals in Botswana has been examined in previous studies. A cross-sectional survey of 300 patients conducted at Princess Marina Hospital, the same hospital at which this study was conducted, found that 81.3% of adults on ART were adherent to prescribed treatment.35 Adherence was defined as no missed ART doses in the past 4 days, no missed ART doses in the past 1 month, and no missed ART refill visits in the past 3 months. Additionally, data from a recent PEPFAR-funded community randomized trial conducted in Botswana found that 96.5% of patients on ART achieved virologic suppression.36
Data Collection:
Demographic variables (age and distance from hospital), clinical variables (hemoglobin at cancer diagnosis, stage at diagnosis, performance status, HIV status, time on ART, nadir CD4 cell count, and CD4 cell count at time of cervical cancer treatment), and cancer treatment variables (number of chemotherapy cycles and total radiation dose) were collected from patient medical records, electronic medical records, and radiation treatment planning system. Total radiation dose was calculated in 2 Gy equivalent accounting for external beam and brachytherapy dose (EQD2). The use of EQD2 as a measure of total radiation dose combines both the external beam and brachytherapy doses in one value and accounts for variation in brachytherapy regimen between patients. Nadir CD4 cell count was defined as lowest CD4 cell count available for each patient prior to cancer therapy. Delta CD4 cell count was defined as improvement from nadir CD4 cell count to the CD4 cell count at the time of cervical cancer diagnosis.
Outcomes:
The primary outcome was cancer survival, defined as the time from scheduled end of radiation therapy +/− chemotherapy until death or until last contact with the patient in September 2018.
Statistical Analysis:
Nadir CD4 cell count was analyzed as a continuous variable (by counts of 100 cells/mm3) and delta CD4 cell count was analyzed as a categorical variable with the following categories: < 100, 100–249, ≥ 250 cells/mm3. Hazard ratios (HR) were estimated separately with Cox regression models for nadir CD4 and delta CD4 cell count. HR analyses were adjusted for the following variables: age, baseline hemoglobin, stage of cancer at diagnosis, performance status, chemotherapy cycles, and radiation dose received. Additionally, nadir CD4 cell count was included in the final adjusted model for the analyses using delta CD4 cell count as the main exposure. Kaplan-Meier survival curves were constructed for delta CD4 cell count categories as defined previously.
Ethical Approval:
All participants provided written informed consent (English or Setswana). This study was reviewed and approved by the institutional review board at the University of Pennsylvania and by the Ministry of Health in Botswana.
RESULTS
Patient Characteristics
Overall, 231 (85.6%) of 270 eligible patients were included in the study. Of these patients, 139/231 had at least two measurements of CD4 cell count and were included in the appropriate analyses (Table 1). Median nadir CD4 cell count was 318 cells/mm3 (interquartile range [IQR] 192–486 cells/mm3), and median delta CD4 cell count was 187 cells/ mm3 (IQR 99–364 cells/mm3). The most common stage of presentation was FIGO IIA/IIB, and over 40% of patients received 4 or more cycles of chemotherapy. The median total radiation dose in 2 Gy equivalent accounting for external beam radiation and brachytherapy was (EQD2) 78 Gy. Over 90% of patients in both groups were on ART. Median survival was 633 days for nadir CD4 patients and 569 days for delta CD4 patients (Table 1). Cause of death data was available for 33 patients, of which 30 (90.9%) had cancer as the cause of death.
Table 1:
Characteristics of HIV-positive patients
| Characteristic | Patients for nadir CD4 analysis (N = 231) |
Patients for delta CD4 analysis (N = 139) |
|
|---|---|---|---|
| Age, years, median (IQR) | 43.0 (37.0–49.0) | 42.0 (36.5–48.0) | |
| Nadir CD4 cells/mm3 median (IQR) | 318.0 (192.0–486.0) | 260.3 (152.0–393.0) | |
| Nadir CD4 cells/mm3 (categorical) | < 200 | 60 (26.0%) | 52 (37.4%) |
| ≥ 200 | 171 (74.0%) | 87 (62.6%) | |
| Delta CD4 cells/mm3, median (IQR) | N/A | 187.0 (99.0–364.0) | |
| Delta CD4 cells/mm3 (categorical) | < 100 | N/A | 36 (25.9%) |
| 100–249 | N/A | 51 (36.7%) | |
| ≥ 250 | N/A | 52 (37.4%) | |
| Stage | Stage IA, IB | 27 (11.7%) | 16 (11.5%) |
| Stage IIA, IIB | 113 (48.9%) | 66 (47.5%) | |
| Stage IIIA, IIIB | 78 (33.8%) | 48 (34.5%) | |
| Stage IVA, IVB | 11 (4.8%) | 8 (5.8%) | |
| Missing | 2 (0.9%) | 1 (0.7%) | |
| Number of chemo cycles | 0 | 48 (20.8%) | 33 (23.7%) |
| 1 | 18 (7.8%) | 11 (7.9%) | |
| 2 | 16 (6.9%) | 7 (5.0%) | |
| 3 | 30 (13.0%) | 20 (14.4%) | |
| 4 | 62 (26.8%) | 34 (24.5%) | |
| 5 | 43 (18.6%) | 24 (17.3%) | |
| Missing | 14 (6.1%) | 10 (7.2%) | |
| Performance status (KPS*) | ≤ 80 | 50 (21.7%) | 33 (23.8%) |
| > 80 | 147 (63.6%) | 89 (64.0%) | |
| Missing | 34 (14.7%) | 17 (12.2%) | |
| Hemoglobin at baseline, gm/dl, median (IQR) | 10.5 (9.1–12.1) | 10.4 (9.1–12.1) | |
| RT dose, median EQD2 (IQR) | 78.0 (68.0–80.0) | 78.0 (64.0–80.0) | |
| Treatment modality | Chemo only | 1 (0.4%) | 1 (0.7%) |
| RT only | 62 (26.8%) | 43 (30.9%) | |
| Chemo & RT | 168 (72.7%) | 95 (68.3%) | |
| Treatment intent | Curative | 81 (35.1%) | 45 (32.4%) |
| Palliative | 149 (64.5%) | 94 (67.6%) | |
| Missing | 1 (0.4%) | 0 | |
| On ART# | 216 (93.5%) | 138 (99.3%) | |
| Median survival in days (IQR) | 633.0 (329.0–866.0) | 569.0 (313.0–863.0) | |
| Baseline CD4 cells/mm3** | 484.0 (335.5–629.5) | 494.0 (343.0–626.0) | |
| Baseline viral load*** | ≤ 400 | 137 (59.3) | 105 (75.5) |
| > 400 | 10 (4.3) | 5 (3.6) | |
| Missing | 84 (36.4) | 29 (20.9) |
ART: Antiretroviral therapy
KPS: Karnofsky Performance Status
CD4 cell count at the time closest to treatment initiation
Viral load at the time closest to cancer diagnosis
Nadir CD4 cell count analysis
By the end of study follow up, 75 (32.5%) patients had died. Median survival for patients with nadir CD4 < 200 was 550 days (IQR 343.5–830), while median survival for patients with nadir CD4 ≥ 200 was 647 days (IQR 328–873). There was no significant difference in median survival between patients with CD4 < 200 and CD4 ≥ 200 (HR 0.99, p = 0.97). Higher nadir CD4 cell counts showed a trend toward reduced mortality in the unadjusted (HR 0.93, 95% confidence interval [CI] 0.83–1.03) and adjusted (HR 0.94, 95% CI 0.84–1.06) analyses but without reaching statistical significance (Table 2). Receipt of chemotherapy was significantly associated with reduced mortality in unadjusted analysis (HR 0.38, 95% CI 0.23–0.62). Higher baseline hemoglobin and higher radiotherapy doses were significantly associated with reduced mortality in both unadjusted and adjusted analysis (Table 2).
Table 2:
Factors associated with death among HIV-infected cervical cancer patients (Nadir CD4, n=231)
| Category | Crude HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|---|
| Nadir CD4 cells/mm3 (continuous by 100) | 0.93 (0.83–1.03) | 0.94 (0.84–1.06) | |
| Age (years) | 1.00 (0.97–1.03) | 1.01 (0.98–1.04) | |
| Number of chemo cycles | None | 1.00 | 1.00 |
| 1–5 | 0.38 (0.23–0.62) | 0.63 (0.35–1.14) | |
| Missing | 0.74 (0.32–1.72) | 0.96 (0.37–2.53) | |
| Hemoglobin (g/dl) at baseline | 1st quartile (3.7–9.0) | 1.00 | 1.00 |
| 2nd quartile (9.1–10.4) | 1.17 (0.64–2.15) | 1.14 (0.59–2.21) | |
| 3rd quartile (10.5–12.1) | 0.57 (0.29–1.12) | 0.56 (0.26–1.21) | |
| 4th quartile (12.2–15.3) | 0.35 (0.16–0.78) | 0.41 (0.18–0.91) | |
| Missing | 1.68 (0.78–3.59) | 1.25 (0.55–2.80) | |
| Stage | Stage I | 1.00 | 1.00 |
| Stage II | 1.81 (0.76–4.30) | 1.22 (0.49–3.05) | |
| Stage III | 2.05 (0.85–4.95) | 0.84 (0.31–2.25) | |
| Stage IV | 3.16 (0.96–10.38) | 0.85 (0.23–3.19) | |
| Missing | 2.34 (0.28–19.46) | 0.60 (0.06–5.87) | |
| RT dose (total EQD2) | 1st quartile (9.3–67.1) | 1.00 | 1.00 |
| 2nd quartile (67.2–77.9) | 0.41 (0.23–0.74) | 0.50 (0.25–0.99) | |
| 3rd quartile (78.0–79.8) | 0.32 (0.17–0.60) | 0.30 (0.14–0.62) | |
| 4th quartile (79.9–90.0) | 0.32 (0.17–0.60) | 0.33 (0.16–0.67) | |
| Performance status (KPS*) | ≤ 80 | 1.00 | 1.00 |
| > 80 | 0.63 (0.38–1.06) | 0.61 (0.35–1.06) | |
| Missing | 0.55 (0.26–1.17) | 0.43 (0.19–0.97) |
KPS: Karnofsky Performance Status
Delta CD4 cell count analysis
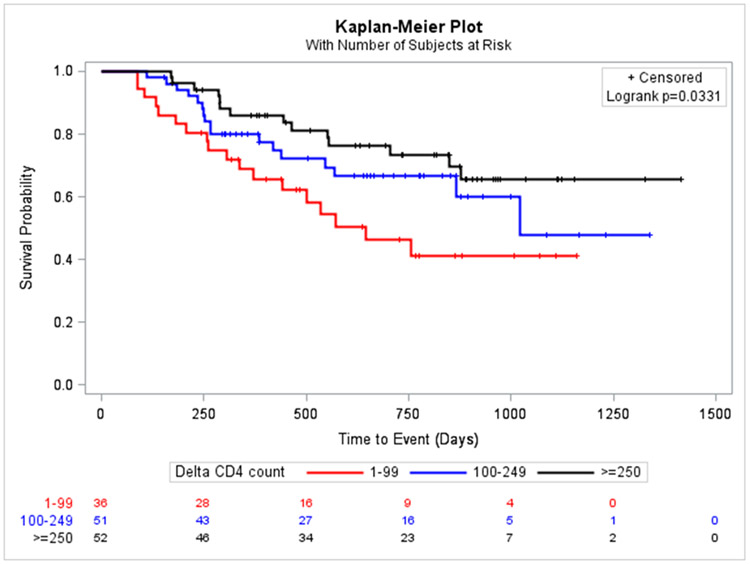
By the end of study follow up, 49 (35.2%) patients in this subpopulation had died. Larger delta CD4 cell count was significantly associated with reduced mortality in the unadjusted analysis (CD4 ≥ 250 HR 0.41, 95% CI 0.20–0.82) and after adjusting for confounders (CD4 100–249 HR 0.45, 95% CI 0.21–0.95; CD4 ≥ 250 HR 0.45, 95% CI 0.20–1.02; Table 3). The protective effect of larger delta CD4 cell counts on mortality remained significant in analyses not accounting for nadir CD4 cell count in the model. Higher radiotherapy doses and increased number of chemotherapy cycles were significantly associated with reduced mortality in unadjusted and adjusted analyses (Table 3 and Figure 1).
Table 3.
Factors associated with death among HIV-infected cervical cancer patients (Delta CD4, n=139)
| Category | Crude HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|---|
| Delta CD4 (cells/mm3) (Categorical) | < 100 | 1.00 | 1.00 |
| 100–249 | 0.59 (0.31–1.15) | 0.45 (0.21–0.95) | |
| ≥ 250 | 0.41 (0.20–0.82) | 0.45 (0.20–1.02) | |
| Age (years) | 1.00 (0.96–1.03) | 1.02 (0.98–1.06) | |
| Hemoglobin at baseline (g/dl) | 1st quartile (4.1–9.0) | 1.00 | 1.00 |
| 2nd quartile (9.1–10.4) | 1.43 (0.66–3.10) | 1.72 (0.71–4.19) | |
| 3rd quartile (10.5–12.1) | 0.72 (0.28–1.82) | 0.60 (0.21–1.76) | |
| 4th quartile (12.2–15.3) | 0.45 (0.18–1.15) | 0.46 (0.16–1.30) | |
| Missing | 1.34 (0.46–3.94) | 1.29 (0.39–4.27) | |
| Stage | Stage I | 1.00 | 1.00 |
| Stage II | 1.89 (0.66–5.47) | 1.12 (0.34–3.66) | |
| Stage III | 1.73 (0.59–5.12) | 0.59 (0.18–2.00) | |
| Stage IV | 2.21 (0.49–9.92) | 0.62 (0.11–3.64) | |
| RT dose (EQD2) | 1st quartile (9.3–63.9) | 1.00 | 1.00 |
| 2nd quartile (64.0–77.9) | 0.52 (0.25–1.08) | 0.70 (0.29–1.71) | |
| 3rd quartile (78.0–80.5) | 0.38 (0.17–0.82) | 0.28 (0.12–0.70) | |
| 4th quartile (80.6–89.5) | 0.38 (0.17–0.85) | 0.41 (0.15–1.09) | |
| Number of chemo cycles | None | 1.00 | 1.00 |
| 1–5 | 0.32 (0.18–0.59) | 0.43 (0.21–0.88) | |
| Missing | 0.62 (0.23–1.66) | 1.46 (0.46–4.61) | |
| Performance status (KPS*) | ≤ 80 | 1.00 | 1.00 |
| > 80 | 0.82 (0.44–1.55) | 0.69 (0.34–1.41) | |
| Missing | 0.31 (0.09–1.07) | 0.16 (0.04–0.64) | |
| Nadir CD4 cells/mm3 (continuous by 100) | 1.07 (0.92–1.25) | 1.03 (0.86–1.25) |
KPS: Karnofsky Performance Status
Figure 1:
Kaplan-Meier Survival Curves by Delta CD4 categories
Exclusion of Stage IA Patients
Because chemoradiation is not the global standard of care for early stage invasive disease, both nadir CD4 and delta CD4 analyses were repeated after removal of the 5 patients with stage IA disease. For nadir CD4 and delta CD4 analyses, results remained relatively unchanged. Higher nadir CD4 cell counts showed a trend toward reduced mortality in the unadjusted (HR 0.93, 95% CI 0.84–1.04) and adjusted (HR 0.95, 95% CI 0.84–1.07) analyses but did not reach statistical significance (Appendix Table 1). Larger delta CD4 cell count was significantly associated with reduced mortality in the unadjusted (CD4 ≥ 250 HR 0.39 95% CI 0.19–0.80) and adjusted (CD4 100–249 HR 0.46, 95% CI 0.22–0.97; CD4 ≥ 250 HR 0.43, 95% CI 0.19–0.96) analyses (Appendix Table 2).
DISCUSSION
HIV infection is known to be associated with an increased risk of progression to cervical cancer but its role in survival among HIV-infected patients who have already progressed to cervical cancer is less clear. As cervical cancer treatment with curative intent becomes more available and accessible in settings with high prevalence of HIV, identifying factors associated with survival in this population becomes increasingly important for prognosis, treatment, and follow up. In this study, we followed HIV-infected women with cervical cancer to determine the effect of nadir and longitudinal changes in CD4 cell count on survival. We demonstrated that the degree of immune reconstitution (as indicated by the increase in CD4 cell count in response) by the time of cancer treatment was associated with cervical cancer survival. Similarly, higher levels of maximal historical immunosuppression (as indicated by lower CD4 cell count nadir) were associated with decreased survival, although the upper limit of the 95% confidence interval crossed over 1.
The association between the change (delta) in CD4 cell count in response to ART between nadir and the time of cancer treatment and increased cancer survival is aligned with our knowledge of the effects of immune reconstitution over functional immune surveillance for pathogens and cancers. While the introduction of ART has been revolutionary in the management of HIV, not all patients reconstitute CD4+ T cells at the same rate or to the same extent in response to ART. Numerous studies have found nadir CD4 count to be the most important determinant of immune reconstitution following ART initiation.28,37-39 Further, even in patients with a robust CD4 cell response, different degrees of local and systemic immune suppression remain. Impaired immunologic response after ART is associated with progression of HIV infection and death.37 Our results suggest that impaired immunological responses, as indicated by a less robust increase in CD4 cell count, are also associated with worse survival among cervical cancer patients receiving treatment. The partial immune reconstitution afforded by ART might be expected to decrease susceptibility to HPV infection and cervical disease. However, the local effects of improved local immune surveillance are uncertain. Several studies have shown that mucosal CD4+ cells are destroyed early in the course of HIV infection and do not significantly recover with ART, despite increased circulating CD4 count.40,41 The resulting deficiency in cellular immunity could explain poorer responses to cervical cancer therapy in women with lower nadir CD4 counts, and our results provide further evidence for this hypothesis. Interestingly, the relationship between immunosuppression and HPV disease is least pronounced for HPV16, suggesting that certain subtypes have different efficiencies avoiding immune surveillance and are less dependent on a weakened immune system to cause cervical disease. The high prevalence of HPV16 in our population may have led to an apparently lower effect of ART over cervical cancer outcomes.
Higher nadir CD4 showed a trend towards reduced mortality but did not reach statistical significance. Higher CD4 cell count was associated with reduced mortality, suggesting that the patient’s historical level of maximal immune suppression might be associated with decreased functional immune response to decrease cancer progression or improve treatment response. Previous data has shown that there is an association between ART and decreased incidence of precancerous lesions, supporting the hypothesis that even with lower CD4 count, ART can help improve immune function to a degree to reduce risk of infection.18,42,43 In a recent systematic review of studies from sub-Saharan Africa looking at ART, CD4, cell count, and CIN, Menon et al. found that the greatest treatment effect of ART was seen with women starting at the lowest CD4 count, since higher CD4 counts would already be associated with a lower incidence of CIN2+.44 Further, in a another systematic review by Kelly et al. in 2018, researchers found that in studies that took into account nadir or current CD4 cell count or ART duration, ART was associated with a reduction in high risk HPV and cervical lesion outcome.45
The finding that radiotherapy dose and number of chemotherapy cycles were associated with lower risk of death increases our confidence in our results, as these are two well-known factors associated with survival.5 Therefore, we expected to find such associations in our study. These findings also provide additional evidence for the critical role of chemoradiotherapy in cervical cancer treatment.
This study is not without limitations. As this was an uncontrolled, observational study, the association between CD4 cell count nadir and the change in CD4 cell count with survival may be confounded by unmeasured factors. CD4 cell counts were not collected at regular intervals, and data were not available for all participants. Data on ART adherence was not documented in this study population, and viral load data was only available for 50% of study participants. The 95% confidence intervals for the effect estimate of the association between nadir CD4 and survival were wide and extended over null (OR of 1.0), which may be due to lack of power.
Additionally, in determining mortality, the authors assumed that deaths in this cohort were due to cervical cancer, rather than HIV, but this assumption was not verified through comparison to a cohort of HIV-positive patients without cervical cancer. The overall mortality rate from HIV in Botswana is 22% among hospitalized patients; thus, it is possible that some women in this study may have died of HIV-related complications.46 However, among the patients with known cause of death in this study, the vast majority (90.9%) of deaths were due to cancer. Further studies examining cause of death may help clarify trends.
In conclusion, our results identify the degree of severity of immunosuppression and the degree of immune reconstitution after ART as significant predictors of mortality among HIV-infected women with cervical cancer. This reinforces the importance of early HIV diagnosis and ART initiation, as its effects could impact cervical cancer outcomes years later.
Supplementary Material
Acknowledgments
Funding: None.
Sources of support: Mentored Patient Oriented Career Research Development Award (1-K08CA230170–01A1), Department of Radiation Oncology, University of Pennsylvania, Sub-Saharan African Collaborative HIV and Cancer Consortia-U54 (1U54 CA190158–01)
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest.
Meetings: This research was previously presented at the American Society for Radiation Oncology (ASTRO) 2019 Annual Meeting in Chicago, IL, USA on September 18, 2019.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.De Vuyst H, Alemany L, Lacey C, et al. The burden of human papillomavirus infections and related diseases in sub-saharan Africa. Vaccine. 2013;31 Suppl 5:F32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black E, Richmond R. Prevention of Cervical Cancer in Sub-Saharan Africa: The Advantages and Challenges of HPV Vaccination. Vaccines (Basel). 2018;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Correction: Cancer Incidence following Expansion of HIV Treatment in Botswana. PLoS One. 2015;10(9):e0138742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grover S, Bvochora-Nsingo M, Yeager A, et al. Impact of Human Immunodeficiency Virus Infection on Survival and Acute Toxicities From Chemoradiation Therapy for Cervical Cancer Patients in a Limited-Resource Setting. Int J Radiat Oncol Biol Phys. 2018;101(1):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organization WH. Botswana HIV Country Profile: 2016. 2017; https://www.who.int/hiv/data/Country_profile_Botswana.pdf.
- 7.Grover S, Raesima M, Bvochora-Nsingo M, et al. Cervical Cancer in Botswana: Current State and Future Steps for Screening and Treatment Programs. Front Oncol. 2015;5:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clifford GM, Tully S, Franceschi S. Carcinogenicity of Human Papillomavirus (HPV) Types in HIV-Positive Women: A Meta-Analysis From HPV Infection to Cervical Cancer. Clin Infect Dis. 2017;64(9):1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowhani-Rahbar A, Hawes SE, Sow PS, et al. The impact of HIV status and type on the clearance of human papillomavirus infection among Senegalese women. J Infect Dis. 2007;196(6):887–894. [DOI] [PubMed] [Google Scholar]
- 10.De Vuyst H, Franceschi S. Human papillomavirus vaccines in HIV-positive men and women. Curr Opin Oncol. 2007;19(5):470–475. [DOI] [PubMed] [Google Scholar]
- 11.International Collaboration on HIV, Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000;92(22):1823–1830. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101(16):1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187–194. [DOI] [PubMed] [Google Scholar]
- 14.Dal Maso L, Polesel J, Serraino D, et al. Pattern of cancer risk in persons with AIDS in Italy in the HAART era. Br J Cancer. 2009;100(5):840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clifford GM, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97(6):425–432. [DOI] [PubMed] [Google Scholar]
- 16.Heard I, Schmitz V, Costagliola D, Orth G, Kazatchkine MD. Early regression of cervical lesions in HIV-seropositive women receiving highly active antiretroviral therapy. AIDS. 1998;12(12):1459–1464. [DOI] [PubMed] [Google Scholar]
- 17.Lillo FB, Ferrari D, Veglia F, et al. Human papillomavirus infection and associated cervical disease in human immunodeficiency virus-infected women: effect of highly active antiretroviral therapy. J Infect Dis. 2001;184(5):547–551. [DOI] [PubMed] [Google Scholar]
- 18.Minkoff H, Ahdieh L, Massad LS, et al. The effect of highly active antiretroviral therapy on cervical cytologic changes associated with oncogenic HPV among HIV-infected women. AIDS. 2001;15(16):2157–2164. [DOI] [PubMed] [Google Scholar]
- 19.Heard I, Tassie JM, Kazatchkine MD, Orth G. Highly active antiretroviral therapy enhances regression of cervical intraepithelial neoplasia in HIV-seropositive women. AIDS. 2002;16(13):1799–1802. [DOI] [PubMed] [Google Scholar]
- 20.Moore AL, Sabin CA, Madge S, Mocroft A, Reid W, Johnson MA. Highly active antiretroviral therapy and cervical intraepithelial neoplasia. AIDS. 2002;16(6):927–929. [DOI] [PubMed] [Google Scholar]
- 21.Uberti-Foppa C, Ferrari D, Lodini S, et al. Long-term effect of highly active antiretroviral therapy on cervical lesions in HIV-positive women. AIDS. 2003;17(14):2136–2138. [DOI] [PubMed] [Google Scholar]
- 22.Sirera G, Videla S, Lopez-Blazquez R, et al. Highly active antiretroviral therapy and incidence of cervical squamous intraepithelial lesions among HIV-infected women with normal cytology and CD4 counts above 350 cells/mm3. J Antimicrob Chemother. 2008;61(1):191–194. [DOI] [PubMed] [Google Scholar]
- 23.Palefsky JM, Minkoff H, Kalish LA, et al. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. J Natl Cancer Inst. 1999;91(3):226–236. [DOI] [PubMed] [Google Scholar]
- 24.Massad LS, Ahdieh L, Benning L, et al. Evolution of cervical abnormalities among women with HIV-1: evidence from surveillance cytology in the women’s interagency HIV study. J Acquir Immune Defic Syndr. 2001;27(5):432–442. [DOI] [PubMed] [Google Scholar]
- 25.Levi JE, Fernandes S, Tateno AF, et al. Presence of multiple human papillomavirus types in cervical samples from HIV-infected women. Gynecol Oncol. 2004;92(1):225–231. [DOI] [PubMed] [Google Scholar]
- 26.Sun XW, Kuhn L, Ellerbrock TV, Chiasson MA, Bush TJ, Wright TC Jr., Human papillomavirus infection in women infected with the human immunodeficiency virus. N Engl J Med. 1997;337(19):1343–1349. [DOI] [PubMed] [Google Scholar]
- 27.Heard I, Tassie JM, Schmitz V, Mandelbrot L, Kazatchkine MD, Orth G. Increased risk of cervical disease among human immunodeficiency virus-infected women with severe immunosuppression and high human papillomavirus load(1). Obstet Gynecol. 2000;96(3):403–409. [DOI] [PubMed] [Google Scholar]
- 28.McKinnon LR, Kimani M, Wachihi C, et al. Effect of baseline HIV disease parameters on CD4+ T cell recovery after antiretroviral therapy initiation in Kenyan women. PLoS One. 2010;5(7):e11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chuang LT, Temin S, Camacho R, et al. Management and Care of Women With Invasive Cervical Cancer: American Society of Clinical Oncology Resource-Stratified Clinical Practice Guideline. J Glob Oncol. 2016;2(5):311–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90–01. J Clin Oncol. 2004;22(5):872–880. [DOI] [PubMed] [Google Scholar]
- 31.Nyongesa C, Ruff P, Donde B, Kotzen J. A phase I study of concurrent cisplatin chemotherapy in patients with carcinoma of the cervix receiving pelvic radiotherapy. Int J Gynecol Cancer. 2006;16(4):1614–1619. [DOI] [PubMed] [Google Scholar]
- 32.Botswana S Botswana AIDS Impact Survey 2013. 2016; http://www.statsbots.org.bw/sites/default/files/publications/BOTSWANA%20AIDS%20IMPACT%20SURVEY%20IV%202013.pdf. Accessed March 12, 2019. [Google Scholar]
- 33.Ministry of Health RoB. 2012 Botswana National HIV & AIDS Treatment Guidelines. 2012; https://aidsfree.usaid.gov/sites/default/files/tx_botswana_2012.pdf. Accessed May 15, 2020.
- 34.Ministry of Health RoB. Handbook of the Botswana 2016 Integrated HIV Clinical Care Guidelines. 2016; https://aidsfree.usaid.gov/sites/default/files/botswana_art_2016.pdf. Accessed May 15, 2020.
- 35.Do NT, Phiri K, Bussmann H, Gaolathe T, Marlink RG, Wester CW. Psychosocial factors affecting medication adherence among HIV-1 infected adults receiving combination antiretroviral therapy (cART) in Botswana. AIDS Res Hum Retroviruses. 2010;26(6):685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaolathe T, Wirth KE, Holme MP, et al. Botswana’s progress toward achieving the 2020 UNAIDS 90–90-90 antiretroviral therapy and virological suppression goals: a population-based survey. Lancet HIV. 2016;3(5):e221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robbins GK, Spritzler JG, Chan ES, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis. 2009;48(3):350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siddique MA, Hartman KE, Dragileva E, et al. Low CD4+ T cell nadir is an independent predictor of lower HIV-specific immune responses in chronically HIV-1-infected subjects receiving highly active antiretroviral therapy. J Infect Dis. 2006;194(5):661–665. [DOI] [PubMed] [Google Scholar]
- 39.Anthony KB, Yoder C, Metcalf JA, et al. Incomplete CD4 T cell recovery in HIV-1 infection after 12 months of highly active antiretroviral therapy is associated with ongoing increased CD4 T cell activation and turnover. J Acquir Immune Defic Syndr. 2003;33(2):125–133. [DOI] [PubMed] [Google Scholar]
- 40.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77(21):11708–11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nkwanyana NN, Gumbi PP, Roberts L, et al. Impact of human immunodeficiency virus 1 infection and inflammation on the composition and yield of cervical mononuclear cells in the female genital tract. Immunology. 2009;128(1 Suppl):e746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Firnhaber C, Westreich D, Schulze D, et al. Highly active antiretroviral therapy and cervical dysplasia in HIV-positive women in South Africa. J Int AIDS Soc. 2012;15(2):17382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Omar T, Schwartz S, Hanrahan C, et al. Progression and regression of premalignant cervical lesions in HIV-infected women from Soweto: a prospective cohort. AIDS. 2011;25(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menon S, Rossi R, Zdraveska N, et al. Associations between highly active antiretroviral therapy and the presence of HPV, premalignant and malignant cervical lesions in sub-Saharan Africa, a systematic review: current evidence and directions for future research. BMJ Open. 2017;7(8):e015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly H, Weiss HA, Benavente Y, et al. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. Lancet HIV. 2018;5(1):e45–e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barak T, Neo DT, Tapela N, et al. HIV-associated morbidity and mortality in a setting of high ART coverage: prospective surveillance results from a district hospital in Botswana. J Int AIDS Soc. 2019;22(12):e25428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.