Abstract
Environmental and occupational metal exposure poses serious global concerns. Metal exposure have severally been associated with neurotoxicity and brain damage. Furthermore, receptor for advanced glycation end products (RAGE) is also implicated in neurological disorders, particularly those with altered glucose metabolism. Here, we examine potential compounding effect of metal exposure and RAGE expression on dopamine (DA) and serotonin (SER) neurons in C. elegans. In addition, we evaluate the effect of RAGE expression on DA and SER neurons in hyperglycemic conditions. Newly generated RAGE-expressing C. elegans tagged with green fluorescent proteins (GFP) in DAergic and SERergic neurons were treated with cadmium (Cd) or manganese (Mn). Additionally, the RAGE-expressing worms were also exposed to high glucose conditions. Results showed metals induced neurodegeneration both in the presence and absence of RAGE expression, but the manner of degeneration differed between Cd and Mn treated nematodes. Furthermore, RAGE-expressing worms showed significant neurodegeneration in both DAergic and SERergic neurons. Our results indicate co-occurrence of metal exposure and RAGE expression can induce neurodegeneration. Additionally, we show that RAGE expression can exacerbate hyperglycemic induced neurodegeneration.
Keywords: metals, RAGE, cadmium, manganese, hyperglycemia, neurodegeneration
1. Introduction
The receptor for advanced glycation end products (RAGE) is a multi-ligand molecule characterized as a binder for advanced glycation end products (AGEs), one of its numerous ligands (Pinkas et al., 2019). RAGE has been implicated in hyperglycemic- and heavy metal-induced neurotoxicity (Milatovic et al., 2009; Shirdhankar et al., 2017). While AGEs, the end products of glycation exogenously originate from certain foods via Maillard’s reaction, hyperglycemic-induced endogenous formation of AGE has also been observed. They accumulate in the body, and subsequently induce oxidative stress and inflammation (Monnier, 1990; Pinkas and Aschner, 2016). AGEs bind to their multi-ligand receptors RAGE to generate reactive oxygen species (ROS) and induce up-regulation of inflammatory cytokines and transcription factors that have been implicated in a wide range of pathologies, including cardiovascular diseases, nephropathy, and diabetes, as well as Alzheimer’s disease (AD), Parkinson’s disease (PD), and other neuropathies (Juranek et al., 2015; Ott et al., 2014; Ramasamy et al., 2016). As such, RAGE expression can be targeted as both an indicator and therapeutic tool in understanding the neurodegeneration that is associated with AGE-related diseases.
The overproduction and accumulation of AGEs has been implicated as a potential cellular mechanism for neurotoxicity under high glucose conditions (Singh et al., 2001; Wendt et al., 2006). Schlotterer et al. (2009) used a C. elegans model to demonstrate the lifespan-shortening effect of hyperglycemic conditions as a result of AGE-mediated modification of mitochondrial proteins and increased ROS formation (Pinkas et al., 2018b; Schlotterer et al., 2009).
Previous studies have shown that chemical elements, such as heavy metals, can potentially affect RAGE expression, leading to the onset or exacerbation of the aforementioned pathologies (Heimfarth et al., 2018; Niño et al., 2018; Pinkas et al., 2018a). Manganese (Mn) is an essential heavy metal whose nutritional and metabolic function is paralleled by its role as a neurotoxicant upon occupational or nutritional exposure. Excessive Mn exposure is a significant non-genetic risk factor for PD, as it is implicated in DAergic neurodegeneration in the substantia nigra, causing the motor deficits. Cadmium (Cd) is a non-essential heavy metal which is known to induce oxidative stress and impair normal nervous system functioning as a result of occupational exposure (Chen et al., 2016). Both metals have been implicated in RAGE mediated hyperglycemic neurotoxicity (Pinkas and Aschner, 2016; Pinkas et al., 2018a; Zhuang et al., 2012).
Here we performed a study to examine the potential link between RAGE and metal-induced neurotoxicity, specifically focusing on RAGE activation as a potential mechanism of metal-induced neurodegeneration. This study uses a novel transgenic RAGE-expressing C. elegans model to examine the neurotoxic impact of Mn and Cd exposure on DAergic and SERergic systems in the presence of RAGE. We hypothesize that due to the contributions of both RAGE and heavy metal exposure to oxidative stress and inflammation-related neuronal death, the combination of RAGE expression and metal exposure will exacerbate the toxic effects in C. elegans, establishing RAGE activation as a mediator in the pathogenicity caused by heavy metal exposure. Furthermore, we evaluated the involvement of RAGE in hyperglycemia-induced neurodegeneration.
2. Methods
2.1. C. elegans strains and maintenance
C. elegans were grown and maintained on OP50-seeded nematode growth media (NGM), at 20°C. Strains used in this study include the BY200 [dat-1::GFP] and GR1333 [tph-1::GFP] to visualize dopamine (DA) and serotonin (SER) neurons respectively. These were obtained from the Caenorhabditis Genetics Center (CGC), USA. We previously designed pan-neuronal RAGE-expressing C. elegans strains (Pinkas et al., 2019).
2.2. Strains crossing
The worm strains tagged with GFP in DA [dat-1::GFP] or SER [tph-1::GFP] neurons were crossed with worms expressing pan-neuronal RAGE using standard protocol to create strains of worms that simultaneously express RAGE and also have GFP labelled DA or SER neurons. Successful crossing was confirmed by routine PCR techniques using primers previously generated (Pinkas et al., 2019).
2.3. Acute metal treatments
Acute exposure of worms to cadmium (Cd) and manganese (Mn) were performed according to previous protocol (Ijomone et al., 2020). Briefly, 2500 gravid adult worms were washed in 1% sodium hypochlorite and 0.25 M NaOH solution, and the egg were isolated by floating in 30% sucrose gradient to obtain a synchronous L1 population. L1 worms were treated with CdCl2 at concentrations of 10, 25 and 50 mM or MnCl2 at concentrations of 50, 100, 200 mM for 1 hour. Metal concentrations for CdCl2 (Dalton and Curran, 2018) and MnCl2 (Au et al., 2009) were selected based on previous literatures.
2.4. Assessment of DA and SER neurons
dat-1::GFP, tph-1::GFP, RAGE+dat-1::GFP, and RAGE+tph-1::GFP worms were treated with Cd and Mn as described above. At about 1-hour post treatment, 10 worms per strain were mounted on slides with 4% agarose pads and immobilized with 2 mM levamisole. DAergic and SERergic neurons in the head (anterior) region of worms (Fig. 1) were visualized using fluorescence microscopy (Nikon Eclipse). Images were acquired and Image J software was used to analyze and measure fluorescent signal intensity. Loss or reduction of fluorescent signal in GFP-tagged neurons indicates neuronal degeneration (Tsai et al., 2017). Assay was independently repeated in 2 trials.
Fig. 1.
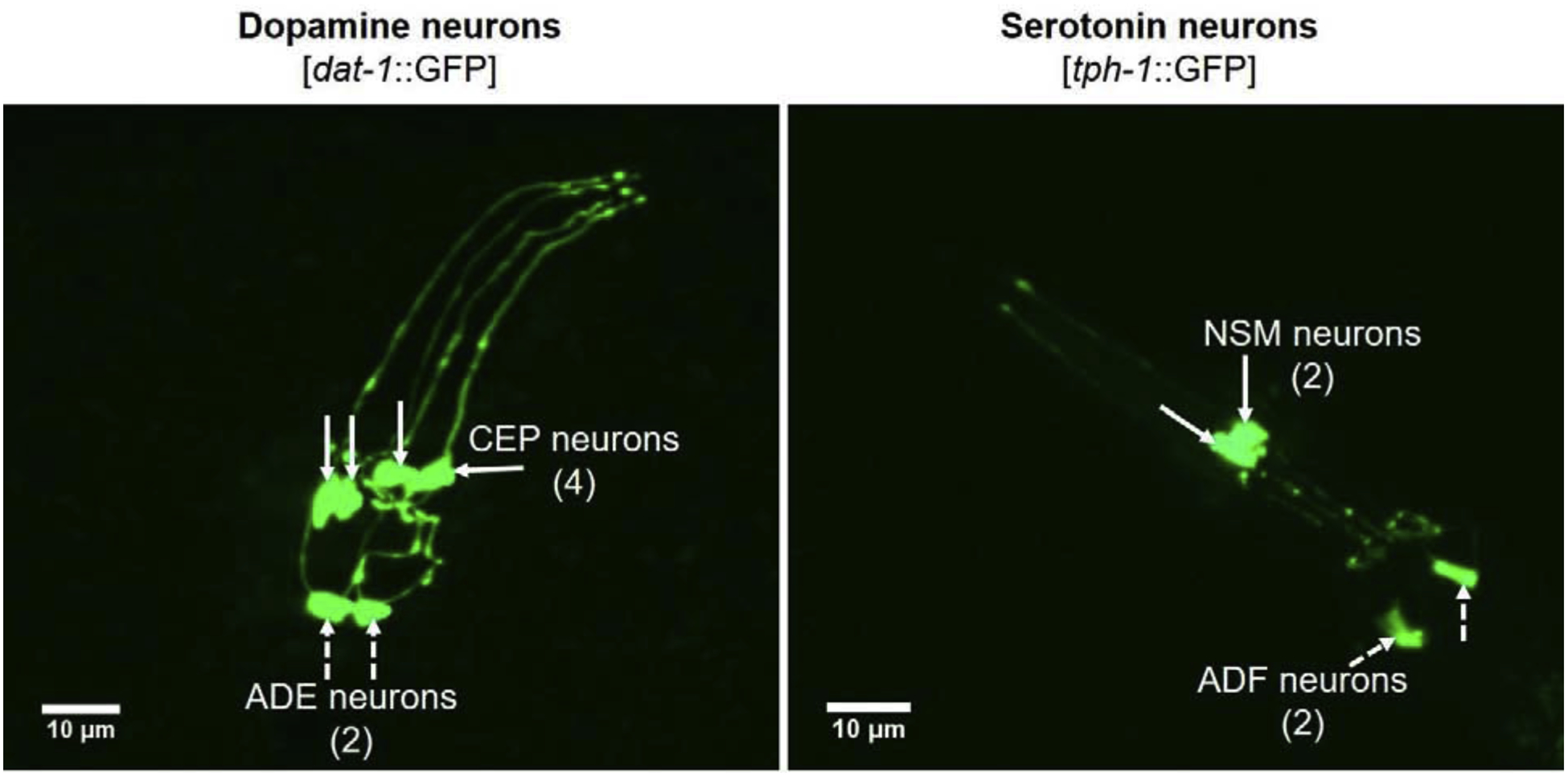
Confocal imaging of DA and SER neurons in the head region of dat-1::GFP and tph-1::GFP C. elegans. Representative images were captured by confocal microscopy (PerkinElmer Spinning disc, Inverted Nikon TE2000-S). CEP – cephalic neurons; ADE – anterior deirid neurons; NSM – neurosecretory motor neurons; ADF – amphid neurons.
2.5. Hyperglycemia assay in RAGE-expressing C. elegans
This assay was modified from a previously described protocol (Schlotterer et al., 2009). Synchronized RAGE+dat-1::GFP and RAGE+tph-1::GFP worms were grown to the L4 stage NGM plates. Fifty worms were then picked onto Fluorodeoxyuridine (FUDR) plates for each strain. Thereafter, 150 ul of 40 mM sorbitol (control) or 40 mM glucose were added to each plate daily for 2 weeks. At the end of 2 weeks, DA and SER neurons were assessed in 5 worms per strain as described above. Assays were independently repeated 4 times.
2.6. Statistical analysis
Data from metal experiments were analyzed using two-way ANOVA for strain x dose interaction effects, followed by Bonferroni post-hoc to compare mean values of various experimental groups. Data from hyperglycemia were analyzed using T-test. Data analysis were performed with GraphPad Prism 8 (GraphPad Inc., USA).
3. Results
Two-ANOVA analysis showed no significant interaction [P = 0.1425] between RAGE expression and Cd treatments on DAergic neuron mean GFP fluorescence. Additionally, there was no significant strain effect [P = 0.7473] on DAergic neurons. However, a significant dose-dependent effect [P < 0.001] was observed. Further post-hoc analysis showed significantly reduced GFP signal intensity in DAergic neurons in the absence or presence of RAGE-expression following 50 mM Cd treatment compared with respective controls. For SERergic neurons, there was no significant interaction [P = 0.1282] between RAGE expression and Cd treatments on mean GFP fluorescence. While there was a significant strain effect [P < 0.05], there was no significant dose-dependent effect [P = 0.0774]. These results indicate significant neurodegeneration in DAergic neurons following Cd treatment with or without RAGE expression, but no significant changes in SERergic neurons (Fig. 2).
Fig. 2.
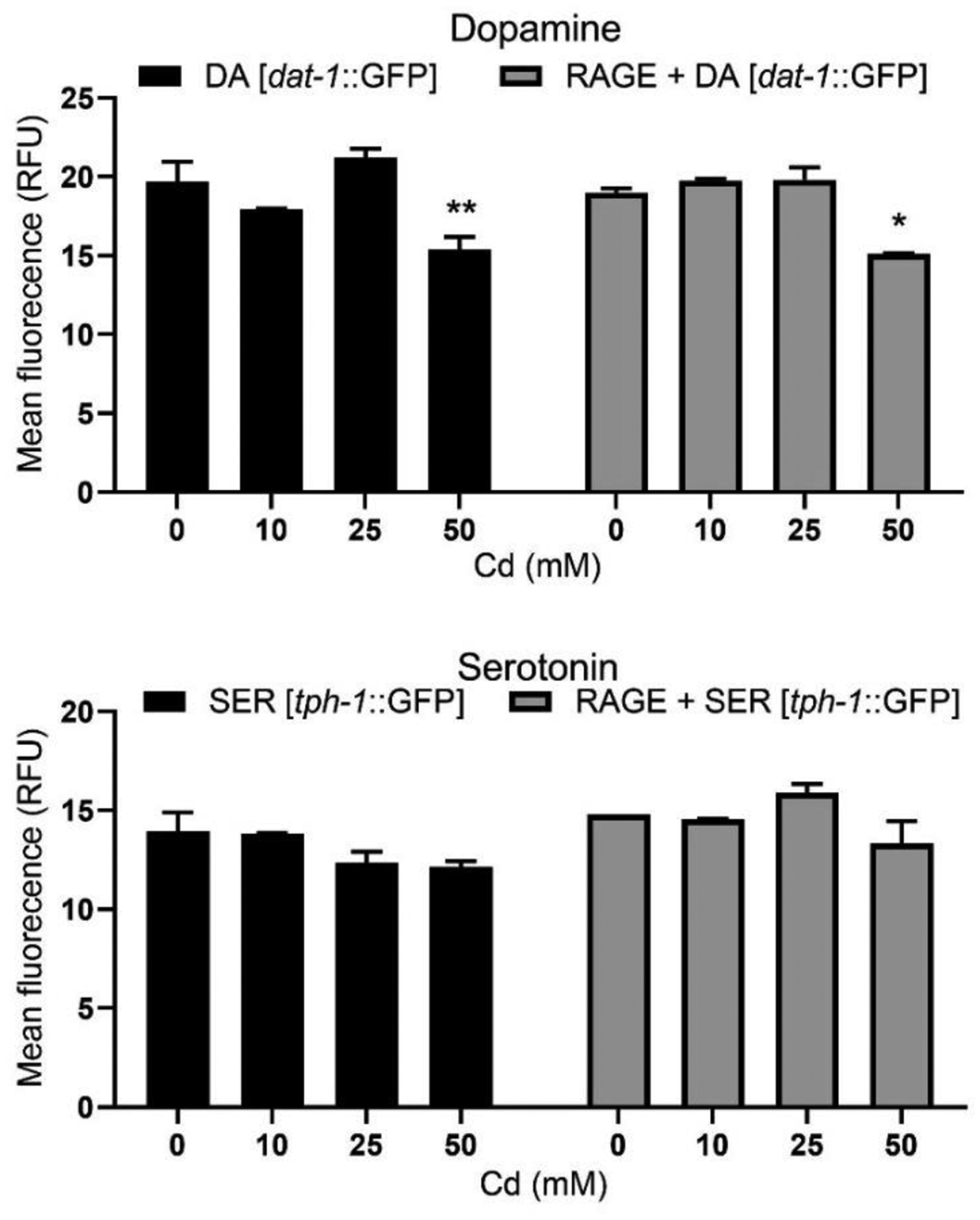
Cd toxicity on DA and SER neurons with or without pan-neuronal RAGE expression. Ten worms per strain were imaged by confocal microscopy and fluorescent signal intensity was measured. Assay was repeated for 2 trials. Data were analyzed by two-way ANOVA followed by Bonferroni post-test. * P < 0.05, ** P < 0.01 compared to respective strain control.
Two-ANOVA analysis showed significant interaction [P < 0.05] between RAGE expression and Mn treatment DAergic neurons. Similarly, there was significant strain effect [P < 0.01] and dose-dependent effect [P < 0.001]. Further post-hoc analysis showed significantly reduced GFP signal intensity in DAergic neurons only in the presence of RAGE expression following 200 mM Mn treatment compared with respective control. For SERergic neurons, there was no significant interaction [P = 0.1340] between RAGE expression and Mn treatments. However, there was significant strain effect [P < 0.05] and dose-dependent effect [P < 0.01]. Further post-hoc analysis showed significant reduction in GFP signal intensity is observed in RAGE-expressing worms following 200 mM Mn treatment only. In addition, significantly reduced GFP signal intensity in SER neurons was observed only in the absence of RAGE expression at 50, 100 and 200 mM Mn treatments compared with respective control. These results indicate significant neurodegeneration in DAergic neurons following Mn treatment in RAGE-expressing worms, as well as significant neurodegeneration in SERergic neurons following Mn treatment in the presence or absence of RAGE expression (Fig. 3).
Fig. 3.
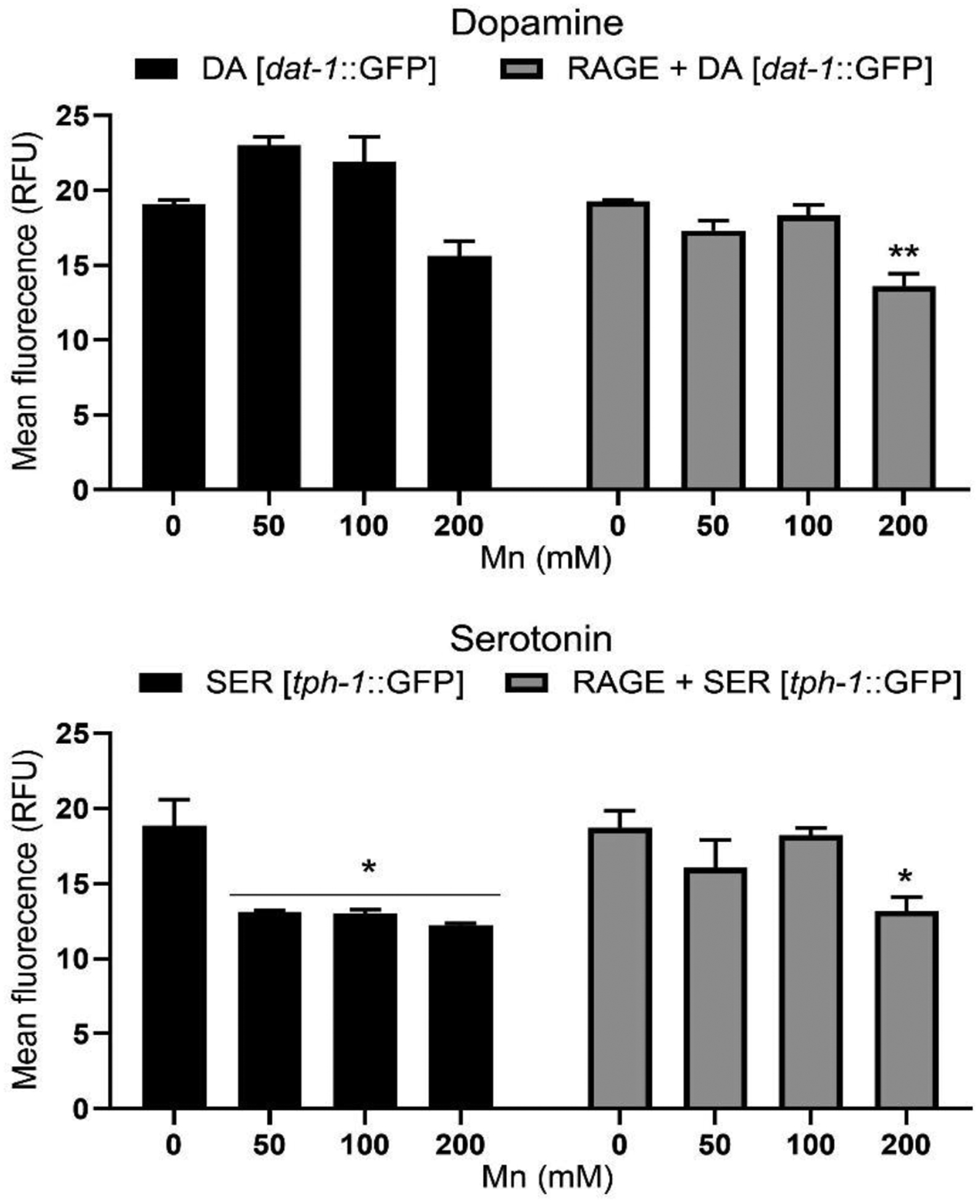
Mn toxicity on DA and SER neurons with or without pan-neuronal RAGE expression. Ten worms per strain were imaged by confocal microscopy and fluorescent signal intensity was measured. Assay was repeated for 2 trials. Data were analyzed by two-way ANOVA followed by Bonferroni post-test. * P < 0.05, ** P < 0.01 compared to respective strain control.
Hyperglycemic assay in RAGE-expressing worms, revealed that high glucose exposure induced significant neurodegeneration in both DAergic and SERergic neurons in presence of RAGE expression (Fig. 4).
Fig. 4.
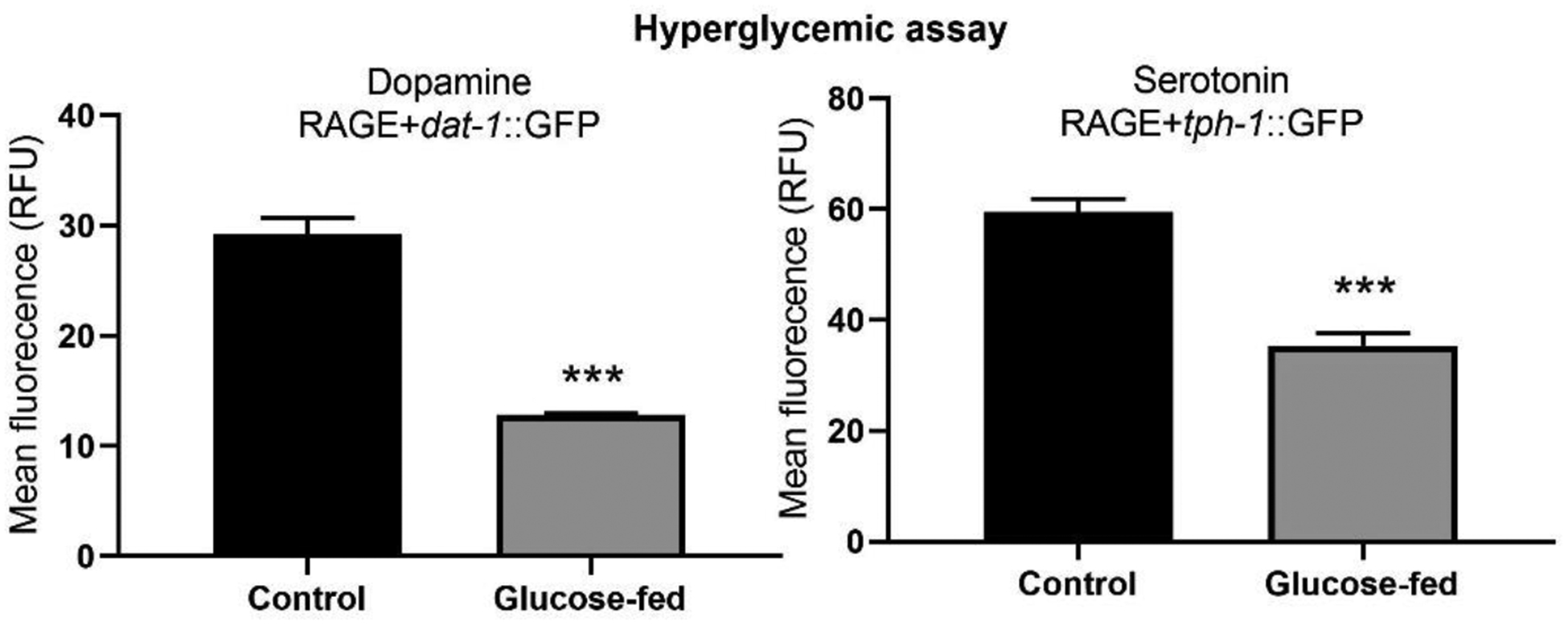
Hyperglycemic treatment induced toxicity on DA and SER neurons of RAGE-expressing C. elegans. Five worms per strain were imaged by confocal microscopy and fluorescent signal intensity was measured. Assay was repeated for 4 trials. Data were analyzed by two-way ANOVA followed by Bonferroni post-test. * P < 0.05, ** P < 0.01 compared to respective strain control.
4. Discussion
The neurotoxic effect of hyperglycemia and heavy metal exposure has been demonstrated (Bishak et al., 2015; Chen et al., 2016; Pinkas and Aschner, 2016; Pinkas et al., 2018b). In this report, we have used a novel transgenic RAGE-expressing C. elegans model to examine the neurodegenerative impact of Mn and Cd exposure on DAergic and SERergic neurons in the presence of RAGE, concomitantly highlighting the role of hyperglycemia in neuronal loss. We took advantage of the transparent body of C. elegans to study morphological changes in neurons subsets using GFP expression to identify different neuronal subtypes. Changes in GFP expression firmly represent the real morphological changes in the neuron structure as previously confirmed by the electron micrograph (Nass et al., 2002). Loss or reduction of fluorescent signal in GFP-tagged worms indicates neuronal degeneration (Tsai et al., 2017).
In this novel study, we observed a significant reduction in GFP signal intensity in DAergic neurons following Cd treatment. As shown, the neurodegeneration observed in Cd-induced DA neurons is independent of RAGE expression. Furthermore, Mn treatment resulted in significant neurodegeneration in DAergic cells of RAGE expressing worms. The neurotoxic impact of Mn treatment observed in this study is consistent with reports by Seo et al. (2013) where Mn treatment led increased synthesis of apoptotic markers and subsequently resulted in neuronal loss (Chen et al., 2016; Milatovic et al., 2009). On the other hand, neurodegeneration is not observed in both RAGE and non-RAGE expressing SER neurons following Cd treatment. However, Mn treatment induced significant neurodegeneration in SER neurons both in the presence and absence of RAGE expression. Curiously, only at the highest dose of Mn treatment does RAGE expression induced SERergic degeneration. Lower doses which induced SERergic degeneration in non-RAGE expressing worms showed no such corresponding effect in RAGE expressing worms. The observation is confounding, as it suggests a potential attenuating response upon RAGE expression in SERergic neurons and warrants further study. Finally, we initially hypothesized that due to the contributions of both RAGE and heavy metal exposure on oxidative stress and inflammation-related neuronal death (Bishak et al., 2015; Teismann et al., 2012), the combination of RAGE expression and metal exposure will exacerbate toxic effects in C. elegans. However, no exacerbating effect was observed as RAGE expression did not induce further neurodegeneration beyond already observed in non-RAGE-expressing worms. Furthermore, the study implicates RAGE-expression in hyperglycemic-induced neurodegeneration in both DA and SER neurons. This is consistent with reports by other authors (Juranek et al., 2015; Pinkas and Aschner, 2016). In an earlier preliminary report, we showed that high glucose exposure induced DAergic but not SERergic degeneration (Pinkas et al., 2018b). Compared to the earlier report, here we observe that RAGE expression further increases DAergic degeneration and induces SERergic degeneration. These suggest that RAGE expression exacerbates glucose induced neurodegeneration in C. elegans. Shirdhankar et al. (2017) also reported the loss of motor neurons and reduction in acetylcholinesterase activity following hyperglycemic-induced neurotoxicity in C. elegans. Furthermore, metal imbalance has been implicated in hyperglycemic disorders (Chen et al., 2009; Khan and Awan, 2014). It is however unclear how RAGE expression comorbid with metal dysregulation or overexposure may contribute to pathogenesis of hyperglycemic disorders. Further investigations of RAGE and its ligands using the C. elegans model are warranted. Assays evaluating the effects of ligand-receptor interactions using C. elegans can provide insight into the role of RAGE in various pathologies. Treatments of hyperglycemia while RAGE is expressed can be evaluated using this C. elegans model. Potential inhibitors of RAGE activation can be tested using C. elegans to alleviate hyperglycemic-induced pathologies.
Overall, metal exposure and RAGE expression can induce neurodegeneration both independently and possible inter-dependently, and this may have implications in hyperglycemic-induced neurological consequence. It is noteworthy that the study is limited particularly in number of experimental trails, hence further studies are essential.
Acknowledgements
MA is supported by National Institute of Health (NIH), USA grants; NIEHS R01 10563, NIEHS R01 07331 and NIEHS R01 020852.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Au C, Benedetto A, Anderson J, Labrousse A, Erikson K, Ewbank JJ, Aschner M, 2009. SMF-1, SMF-2 and SMF-3 DMT1 orthologues regulate and are regulated differentially by manganese levels in C. elegans. PloS one 4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishak YK, Payahoo L, Osatdrahimi A, Nourazarian A, 2015. Mechanisms of cadmium carcinogenicity in the gastrointestinal tract. Asian Pac J Cancer Prev 16(1), 9–21. [DOI] [PubMed] [Google Scholar]
- Chen P, Miah MR, Aschner M, 2016. Metals and neurodegeneration. F1000Research 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Yang CY, Huang CF, Hung DZ, Leung YM, Liu SH, 2009. Heavy metals, islet function and diabetes development. Islets 1(3), 169–176. [DOI] [PubMed] [Google Scholar]
- Dalton HM, Curran SP, 2018. Hypodermal responses to protein synthesis inhibition induce systemic developmental arrest and AMPK-dependent survival in Caenorhabditis elegans. PLoS genetics 14(7), e1007520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimfarth L, Delgado J, Mignori MR, Gelain DP, Moreira JCF, Pessoa-Pureur R, 2018. Developmental neurotoxicity of the hippocampus following in utero exposure to methylmercury: Impairment in cell signaling. Archives of toxicology 92(1), 513–527. [DOI] [PubMed] [Google Scholar]
- Ijomone OM, Miah MR, Akingbade GT, Bucinca H, Aschner M, 2020. Nickel-Induced Developmental Neurotoxicity in C. elegans Includes Cholinergic, Dopaminergic and GABAergic Degeneration, Altered Behaviour, and Increased SKN-1 Activity. Neurotoxicity Research 37, 1010–1028. [DOI] [PubMed] [Google Scholar]
- Juranek J, Ray R, Banach M, Rai V, 2015. Receptor for advanced glycation end-products in neurodegenerative diseases. Reviews in the Neurosciences 26(6), 691–698. [DOI] [PubMed] [Google Scholar]
- Khan AR, Awan FR, 2014. Metals in the pathogenesis of type 2 diabetes. Journal of Diabetes & Metabolic Disorders 13(1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M, 2009. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicology and applied pharmacology 240(2), 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier VM, 1990. Nonenzymatic glycosylation, the Maillard reaction and the aging process. Journal of Gerontology 45(4), B105–B111. [DOI] [PubMed] [Google Scholar]
- Nass R, Hall DH, Miller DM, Blakely RD, 2002. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proceedings of the National Academy of Sciences 99(5), 3264–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niño SA, Martel-Gallegos G, Castro-Zavala A, Ortega-Berlanga B, Delgado JM, Hernández-Mendoza H.c., Romero-Guzmán E, Ríos-Lugo J, Rosales-Mendoza S, Jimenez-Capdeville ME, 2018. Chronic Arsenic Exposure Increases Aβ (1–42) Production and Receptor for Advanced Glycation End Products Expression in Rat Brain. Chemical research in toxicology 31(1), 13–21. [DOI] [PubMed] [Google Scholar]
- Ott C, Jacobs K, Haucke E, Santos AN, Grune T, Simm A, 2014. Role of advanced glycation end products in cellular signaling. Redox biology 2, 411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkas A, Aschner M, 2016. Advanced glycation end-products and their receptors: Related pathologies, recent therapeutic strategies, and a potential model for future neurodegeneration studies. Chemical research in toxicology 29(5), 707–714. [DOI] [PubMed] [Google Scholar]
- Pinkas A, Cunha Martins A, Aschner M, 2018a. C. elegans—An Emerging Model to Study Metal-Induced RAGE-Related Pathologies. International journal of environmental research and public health 15(7), 1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkas A, Lawes M, Aschner M, 2018b. System-specific neurodegeneration following glucotoxicity in the C. elegans model. NeuroToxicology 68, 88–90. [DOI] [PubMed] [Google Scholar]
- Pinkas A, Lee KH, Chen P, Aschner M, 2019. A C. elegans Model for the Study of RAGE-Related Neurodegeneration. Neurotoxicity research 35(1), 19–28. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Shekhtman A, Schmidt AM, 2016. The multiple faces of RAGE–opportunities for therapeutic intervention in aging and chronic disease. Expert opinion on therapeutic targets 20(4), 431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotterer A, Kukudov G, Bozorgmehr F, Hutter H, Du X, Oikonomou D, Ibrahim Y, Pfisterer F, Rabbani N, Thornalley P, 2009. C. elegans as model for the study of high glucose–mediated life span reduction. Diabetes 58(11), 2450–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo YA, Li Y, Wessling-Resnick M, 2013. Iron depletion increases manganese uptake and potentiates apoptosis through ER stress. Neurotoxicology 38, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirdhankar R, Sorathia N, Rajyadhyaksha M, 2017. Short-term hyperglycaemia induces motor defects in C. elegans. BioRxiv, 240473. [Google Scholar]
- Singh R, Barden A, Mori T, Beilin L, 2001. Advanced glycation end-products: a review. Diabetologia 44(2), 129–146. [DOI] [PubMed] [Google Scholar]
- Teismann P, Sathe K, Bierhaus A, Leng L, Martin HL, Bucala R, Weigle B, Nawroth PP, Schulz JB, 2012. Receptor for advanced glycation endproducts (RAGE) deficiency protects against MPTP toxicity. Neurobiology of aging 33(10), 2478–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C-W, Tsai R-T, Liu S-P, Chen C-S, Tsai M-C, Chien S-H, Hung H-S, Lin S-Z, Shyu W-C, Fu R-H, 2017. Neuroprotective effects of betulin in pharmacological and transgenic Caenorhabditis elegans models of Parkinson’s disease. Cell transplantation 26(12), 1903–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt T, Harja E, Bucciarelli L, Qu W, Lu Y, Rong LL, Jenkins DG, Stein G, Schmidt AM, Yan SF, 2006. RAGE modulates vascular inflammation and atherosclerosis in a murine model of type 2 diabetes. Atherosclerosis 185(1), 70–77. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Pang X, Zhang W, Wu W, Zhao J, Yang H, Qu W, 2012. Effects of zinc and manganese on advanced glycation end products (AGEs) formation and AGEs-mediated endothelial cell dysfunction. Life sciences 90(3–4), 131–139. [DOI] [PubMed] [Google Scholar]


