Abstract
Microplastics (MPs) are a ubiquitous pollutant detected not only in marine and freshwater bodies, but also in tap and bottled water worldwide. While MPs have been extensively studied, the toxicity of their smaller counterpart, nanoplastics (NPs), is not well documented. Despite likely large-scale human and animal exposure to NPs, the associated health risks remain unclear, especially during early developmental stages. To address this, we investigated the health impacts of exposures to both 50 and 200 nm polystyrene NPs in larval zebrafish. From 6 to 120 hours post-fertilization (hpf), developing zebrafish were exposed to a range of fluorescent NPs (10-10,000 parts per billion). Dose-dependent increases in accumulation were identified in exposed larval fish, potentially coinciding with an altered behavioral response as evidenced through swimming hyperactivity. Notably, exposures did not impact mortality, hatching rate, or deformities; however, transcriptomic analysis suggests neurodegeneration and motor dysfunction at both high and low concentrations. Furthermore, results of this study suggest that NPs can accumulate in the tissues of larval zebrafish, alter their transcriptome, and affect behavior and physiology, potentially decreasing organismal fitness in contaminated ecosystems. The uniquely broad scale of this study during a critical window of development provides crucial multidimensional characterization of NP impacts on human and animal health.
Keywords: microplastics, nanoplastics, zebrafish, transcriptomics, neurobehavior
Graphical Abstract
Capsule:
The results of this study suggest that NPs can accumulate in the tissues of larval zebrafish, alter their transcriptome, and affect behavior and physiology, potentially decreasing organismal fitness in contaminated ecosystems.
1. Introduction:
Plastic pollution is ubiquitous and an emerging concern in both freshwater and marine environments. Since mass production began in the 1940s, plastic manufacturing has increased rapidly, with 348 million tons produced globally in 2018 (Plastics – the Facts 2018). Large amounts end up in the oceans, which are now predicted to contain more than five trillion individual pieces of plastic materials (equaling 250,000 tons) in the first 20 meters of the water column (Eriksen et al., 2014). Plastics have been identified virtually everywhere: from arctic sea ice to ocean sediments (Barnes et al., 2009; Obbard et al., 2014). In freshwater systems, plastics have been identified in large quantities in lakes, rivers, and basins, especially in areas near dense human populations (Eerkes-Medrana et al., 2015; Pivonsky et al., 2018). Their ubiquity has allowed for potential human exposure to plastics through the consumption of aquatic organisms and via drinking water, especially due to the inability of drinking water facilities to entirely remove anthropogenic particles sourced through freshwater environments (Kosuth et al., 2018; Wang et al., 2020). In 2019, the World Health Organization (WHO) called for a greater assessment of plastics in the environment after 90% of bottled water was found to contain small plastic particles (World Health Organization). In addition, anthropogenic particles, many of which are likely plastic fragments and fibers, have been detected in over 81% of tap water sources, allowing for an average of 5,800 particles to be ingested annually per person (Kosuth et al., 2018).
Nanoplastics (NPs), defined as synthetic plastic particles less than 1 μm in size (Gigault et al., 2018), can be classified as primary or secondary based on their origination. Primary NPs are utilized for specific industrial or domestic applications to be of certain size, texture, and composition (Auta et al., 2017), and are included in resin pellets, sunscreens, and personal care products such as toothpastes, shower gels, facial cleansers/scrubs, shampoos, conditioners, shaving creams, and deodorants (Fendall et al., 2009; Costa et al., 2010; Castaneda et al., 2014; Duis et al., 2016). Although plastic is inert, common plastics such as nylon, high-density polyethylene (HDPE), and low-density polyethylene (LDPE) can be fragmented from wave action, exposure to UV radiation, and other physical and/or digestive processes (Andrady, 2011; Gigault et al., 2016; Lambert and Wagner, 2016; Dawson et al., 2018; Ekvall et al., 2019). These degradation processes are the main source of environmental NPs. Globally, microplastics have reached high densities ranging from <1 parts per billion (ppb) to tens of thousands of ppb in both freshwater and marine ecosystems (Li et al., 2016). However, as the small size of NPs limits their ability to be measured, few studies have directly quantified environmental NP presence. Thus, the environmental burden of NPs is relatively unknown, although theorized to be higher than that of MPs (Koelmans et al, 2015; Ter Halle et al., 2017).
Due to the likelihood of ingestion or inhalation by aquatic organisms and humans, investigation into the potential negative consequences of NPs is warranted. Similar to microplastics, NPs can accumulate in the gastrointestinal tract and on the outer epithelium of aquatic organisms (Van Pomeren et al., 2017; Brun et al., 2018). Crucian carp (Carassius carassius), a freshwater fish, were found to ingest NPs both directly and indirectly through the food chain (Mattsson et al., 2017). These contaminants have the potential for trophic transfer following the consumption of algae, allowing for NP accumulation (Manabe et al., 2011; Della Torre et al., 2014; Bergami et al., 2016). Countless fish species have been used to study plastics accumulation in organ systems and tissues; in zebrafish (Danio rerio), acute exposures resulted in NP presence in the gallbladder, yolk sac, pericardium, gills, gastrointestinal tract, and liver, from where transfer to other organs via blood would be possible (Skjolding et al., 2017; Veneman et al., 2017). NPs in particular can accumulate in the heart and brain of fish causing distinct neurobehavioral effects (Mattsson et al., 2017; Pitt et al., 2018). Following their accumulation in several aquatic organisms, both MPs (Wright et al., 2013; Rochman et al., 2014; Avio et al., 2015; Cole et al., 2015b; Sussarellu et al., 2016) and NPs (Cedervall et al., 2012; Besseling et al., 2014; Della Torre et al., 2014; Mattsson et al., 2014; Greven et al., 2016; Brun et al., 2018; Lee et al., 2018; Brun et al., 2019) can induce stress responses such as oxidative stress, inhibited growth, inflammation, dysregulation of energy metabolism, and endocrine disruption. In human cerebral and epithelial cells, high concentrations of polystyrene induced oxidative stress in vitro (Schirinzi et al., 2017), indicating the potential for cytotoxicity following NP exposure in vivo. However, insufficient evidence exists regarding the toxic endpoints of NP exposures despite current literature detailing large-scale plastic accumulation in cells and tissues (von Moos et al., 2012; Della Torre et al., 2014). In addition, few studies have attempted to evaluate transcriptomic outcomes of NP exposures. In the African catfish (Clarias gariepinus), MP exposure downregulated reproductive biomarker genes (Karami et al., 2016). Transient changes were also induced in the zebrafish transcriptome following developmental MP exposure, with over 1,700 genes differentially expressed at 20 mg/L including consistent downregulation of genes associated with nervous system functioning and metabolic pathways (LeMoine et al., 2018). Brun et al. (2019) showed that NP exposure can alter gene expression related to glucose metabolism, oxidative stress, and serotonin transport in larval zebrafish as well. However, significant gaps remain in understanding the transcriptomic changes associated with NP exposure endpoints, particularly at various NP sizes and doses during developmental stages when endocrine function and the epigenome are particularly sensitive (Wilkinson et al., 2015).
To address this, we utilized the zebrafish model to identify and quantify transcriptomic changes associated with NP accumulation, developmental abnormalities, and changes in survivorship and behavior. Zebrafish are an NIH-validated model organism ideal for studies in developmental toxicology due to high conservation between the zebrafish and human genomes (Howe et al., 2013). To our knowledge, this is one of very few studies that link whole genome transcriptomic alterations with behavioral outcomes in developing zebrafish exposed to NPs. Thus, the results of this study will provide new insights into the biological fate of NPs, as well as their neurobehavioral and physiologic effects in a vertebrate model, with implications for human and animal health.
2. Materials and Methods
2.1. Zebrafish husbandry and embryo collection
Adult AB lineage zebrafish were maintained in reverse osmosis (RO) water buffered with Instant Ocean salts (600 mg/L; Aquarium Systems Inc, Mentor, OH) on a 14:10 hour light/dark cycle. Temperatures were maintained at 27°C–29°C and pH at 7-7.5. Fish were fed a mixture of Zeigler Adult Zebrafish Diet (Zeigler Bros. Inc, Gardners, PA), Spirulina Flake Fish Food (Ocean Star International, Snowville, UT), and 300-500 micron Golden Pearls (Aquatic Foods Inc, Fresno, CA) two times daily. Multiple (>10) spawning tanks were set-up in the afternoon, and dividers separating the sexes (1 male: 2 females per spawn) were removed the following morning. Embryos were collected after two hours of spawning, rinsed in a 0.6% solution of bleach for 10 minutes, and transferred to a fresh solution containing RO water and 600 mg/L Instant Ocean salts in glass petri dishes until the start of NP exposure at 6 hours post-fertilization (hpf). Larval zebrafish were euthanized at 120 hpf via an immersion bath with 0.4 g/L tricaine methanesulfonate (MS-222) and 0.66 g/L sodium bicarbonate, followed by the addition of 300 μL of 6.25% bleach as an adjunctive method of euthanasia (Leary et al., 2013). Animal use protocols were approved by the Wayne State University Institutional Animal Care and Use Committees, according to the National Institutes of Health Guide to the Care and Use of Laboratory Animals (Protocol No. 16-03-054).
2.2. Nanoplastics
50 and 200 nm sizes of polystyrene NPs (labelled with Dragon Green) were purchased from Bangs Laboratories Inc (Fishers, IN). Polystyrene was selected as it is one of the most common plastic polymers used commercially in the United States, has been frequently detected in the marine environment, and is often used in experimental microplastic exposures (Andrady, 2011). These microbeads fluoresce green under 300-550 nm wavelengths, allowing for visualization under a fluorescent microscope. Each stock solution contained 1.02 g/cm3 fluorescent beads with a mean diameter of 42 nm or 194 nm. Stock solutions were shaken vigorously to prevent agglomeration of individual particles during dosage. Additionally, the stock solutions contained 2 mM of sodium azide that acted as an antimicrobial agent, and 0.1% Tween 20 in deionized water, a surfactant to prevent particle aggregation. Stock concentrations of sodium azide and Tween 20 were diluted one thousand-fold in the control and highest polystyrene exposure solutions, and decreased further with each ten-fold dilution of exposure solution. Inclusion of Tween 20 and sodium azide did not negatively impact larval/embryonic NP accumulation, behavior, gene expression, abnormal growth, or survivorship.
2.3. Nanoplastic exposures
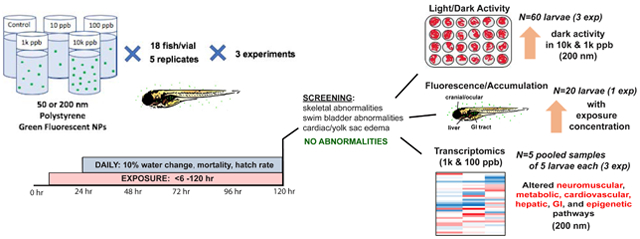
Experiments were repeated three times, each consisting of 5 replicates (1 vial = 1 biological replicate) per treatment group with 18 embryos exposed per replicate. Experimental design was identical for 50 and 200 nm microspheres, conducted independently. At least ten spawning events occurred per experiment. In a single experiment, each replicate consisted of embryos from a different spawn, but these replicates were held consistent across exposure conditions to control for parental effects. At 6 hpf, zebrafish embryos were placed randomly in 20 mL glass scintillation vials, selected to avoid the use of plastic materials and allow for gentle shaking of NP solution within vials. These contained 18 mL of a polystyrene exposure solution; this amount was selected after trials to optimize embryo survival and number of embryos per vial at a density of 1 embryo/mL. Embryos were dosed at 0 (control), 10, 100, 1000, or 10,000 parts per billion (ppb), determined as mass per volume (μg/L). A 10,000 ppb exposure was equivalent to 2.46x1014 microspheres/L for 50 nm microspheres and 2.54x1012 microspheres/L for 200 nm microspheres. Because environmentally relevant levels of NPs remain largely unknown (Ter Halle et al., 2017), this range is based on environmentally relevant concentrations of known microplastics (Koelmans et al., 2015) and is similar to what has been used in other exposure studies (Lenz et al., 2016; Pitt et al., 2018; Brun et al., 2019). Solutions were made by adding the appropriate concentration of stock solution to 18 mL of RO water with 600 mg/L Instant Ocean salts. Embryos were incubated at 28°C from 6 to 120 hpf. A 10% water change with fresh exposure solution was conducted daily and vials were shaken gently by hand twice daily for 10 seconds to ensure NP homogeneity within the vial. Mortality and hatching rates were recorded each day, and any dead eggs were immediately removed. Embryos that died before 24 hours were excluded from the mortality rate calculation. From each replicate, all surviving fish were analyzed for developmental abnormalities. Mortality and abnormality data were combined across experiments for analysis (initial n=270; 18 embryos x 5 replicates x 3 repeats). At 120 hpf, the presence of developmental abnormalities was determined under a brightfield microscope. Namely, the incidence of uninflated swim bladders, skeletal curvature, cardiac edema, and yolk sac edema was recorded. Embryos unhatched at 120 hpf were not included in downstream analyses. A subset of fish was collected for behavioral analysis; the rest were then euthanized to be either collected for transcriptomic analysis or separately imaged for the accumulation analyses post-mortem.
2.4. Behavioral analysis
Zebrafish larvae were gently rinsed with RO water containing 600 mg/L Instant Ocean salts three times at 120 hpf. Fish were then transferred to new 20 mL scintillation vials containing 18 mL of the RO solution. 4 larval fish from each of five replicates per condition were randomly placed (1 larvae/well) across five 24-well plates for behavioral analysis, in three separate experiments (n=60 total, 20 per experiment). Plates were placed in an incubator at 28°C for an hour to acclimate, then transferred to a DanioVision™ observation chamber (Noldus Inc., Wageningen, Netherlands) for an alternating light/dark locomotion test to screen for neurobiological alterations. The testing paradigm proceeded as follows: a 12-minute acclimation period inside the DanioVision Observation Chamber in the dark, followed by an alternating cycle of 3 minute intervals of light and dark stimuli repeated 4 times. Larval fish behavior was recorded live using a Basler Gen1 Camera (Basler acA1300-60) and EthoVision XT 13 software (Noldus Inc., Wageningen, Netherlands). Following the behavioral test, data points were analyzed at 30 second time intervals evaluating behavioral endpoints of distance traveled and velocity.
2.5. Nanoplastic accumulation
Immediately after euthanasia, zebrafish were imaged using a Nikon stereomicroscope (Melville, NY, USA) with a X-Cite fluorescent illuminator (Series 120). From one experiment, 4 fish were selected at random for accumulation analysis from each of five replicates (20 fish selected per exposure group). Each fish was individually imaged for green fluorescence with a Nikon camera lens (AF-P 70-300mm) at 3x magnification using a 700 millisecond exposure and 25.6 gain (n=20). These settings were optimized to the 10,000 ppb concentration to prevent oversaturation of images. Zebrafish larvae were rinsed with RO water containing 600 mg/L Instant Ocean salts at 120 hpf to prevent background NP fluorescence. Intensity of fluorescence was quantified in whole larvae using ImageJ software (ImageJ2, Bethesda, Maryland, USA).
2.6. RNA isolation and Quant-Seq analysis
After rinsing 3 times to remove residual NPs, larvae were euthanized at 5 dpf and collected in 300 μL of RNAlater Stabilization Solution (Invitrogen, Carlsbad, CA). For transcriptomic analysis, 3 pooled samples of 5 larvae each were collected per experiment (repeated 3 times) from each exposure group (pooled larvae were from the same replicate) for a total of 9 pooled samples per condition. Of these samples, 5 each from the control, 100, and 1,000 ppb groups were randomly selected across the three experiments for downstream RNA isolation and Quant-Seq analysis (n=5 biological replicates per concentration, 5 larvae per pool). Larvae used for transcriptomic analyses were not subjected to behavioral or accumulation studies. Larvae were stored in RNAlater solution for 7 days at 4°C to allow for tissue permeation, then RNAlater was removed and samples were transferred to −80°C for storage. Larvae were homogenized and RNA was isolated using the RNeasy® Lipid Tissue Mini Kit (Qiagen, Hilden, Germany). The Qubit® 2.0 Fluorometer and Qubit® RNA High Sensitivity Assay Kit (Invitrogen, Carlsbad, CA) were used to measure RNA concentrations. 3’ mRNA-seq libraries were prepared from isolated RNA using QuantSeq 3’ mRNA-Seq Library Prep Kit FWD for Illumina (Lexogen, Vienna, Austria). Samples were normalized to 40 ng/μL (total input of 200 ng in 5 μL) and amplified at 17 cycles. Libraries were quantified using a Qubit® 2.0 Fluorometer and Qubit® dsDNA Broad Range Assay Kit (Invitrogen, Carlsbad, CA), and run on an Agilent TapeStation 2200 (Agilent Technologies, Santa Clara, CA) for quality control. The samples were sequenced on a HiSeq 2500 (Illumina, San Diego, CA) in rapid mode (single-end 50 bp reads). Reads were aligned to D. rerio (Build danRer10) using the BlueBee Genomics Platform (BlueBee, Rijswijk, The Netherlands). Differential gene expression between the control and exposure lineage zebrafish was evaluated using DEseq2 (available through GenePattern; Broad Institute, Cambridge, Massachusetts). Details on nucleic acid concentrations, sequencing output, and read alignment are included in Supplemental Table S1. Genes with significant changes in expression in 100 and 1,000 ppb 200 nm NP-exposed fish, as defined by absolute log2 fold change value ≥ 0.75, p-value < 0.05, and Benjamini-Hochberg adjusted p-value < 0.1 (Supplemental Table S2), were uploaded into Ingenuity Pathway Analysis software (IPA; QIAGEN Bioinformatics, Redwood City, CA) for analysis using RefSeq IDs as identifiers. Complete IPA disease and function pathway output is reported in Supplemental Table S3.
2.7. Statistical analysis and reproducibility
Accumulation and behavioral data were assessed for normality using a Shapiro-Wilk test. Natural log transformation of accumulation data was performed to approximate a normal distribution. One-way ANOVAs with Tukey post-hoc tests were carried out to compare differences among treatment groups exposed to the same size NP microsphere. Abnormalities, mortality, and hatching rate data were analyzed using Chi-square analyses. All statistical analyses were performed in R (2013; version 3.3.1; R Core Team, 2019) using α = 0.05 to determine statistical significance. DESeq2 was used to analyze differential gene expression, and a Benjamini-Hochberg correction was performed to adjust p-values for multiple hypothesis testing. IPA-generated pathways were analyzed with a Right-Tailed Fisher’s Exact Test, with significantly altered pathways defined by p-values < 0.01.
3. Results
3.1. Developmental abnormalities
NPs in either size group did not have a statistically significant impact on the development of abnormalities from 0 to 120 hpf (Supplemental Table S4). The rate of swim bladder uninflation increased by ~7% in the 100 and 10,000 ppb treatment groups within the 200 nm size class compared to the control, but this change was not significant.
3.2. Mortality and hatching rates
Mortality rates were not impacted by the presence of 50 or 200 nm NPs relative to control (p>0.05) for any treatment group. NPs in either size group did not have an impact on the daily hatching rates of zebrafish from 0 to 120 hpf, and no significant results were apparent among each treatment group relative to the control (p>0.05, Supplemental Table S4). Over 80% of all fish in the exposure and control groups hatched by 72 hpf. Percent unhatched after 120 hpf increased by over 5% in 10,000 ppb exposures to 50 nm polystyrene compared to the control, but this increase was not significant.
3.3. Behavior
50 nm NP exposure did not impact the total distance travelled at any of the 48 time intervals sampled (Supplemental Figure S1, p>0.05), and no significance was reported in either light or dark cycles among each respective concentration (p>0.05). However, 200 nm NP-exposed fish displayed hyperactive behavior at the two highest concentrations during the dark cycles. The first dark photoperiod showed a significant difference between the control and 10,000 ppb exposure groups at one of the six 30 second time intervals sampled (Figure 1, Supplemental Figure S1, p=0.005). For all subsequent dark photoperiods, 10,000 ppb exposed fish displayed hyperactivity at every 30 second time interval relative to the control groups (Figure 1, Supplemental Figure S1, p<0.05). During dark cycles, fish in the 10,000 ppb exposure group were also significantly hyperactive relative to 10 and 100 ppb exposed fish in greater than 66% of sampled 30 second time periods. The 1000 ppb exposure group exhibited hyperactivity at only two of the 30 second time intervals sampled (Figure 1, Supplemental Figure S1, p=0.02 and p=0.01). In contrast, no significant difference in total distance travelled was found between the control group and NP exposure groups during the light periods. Supplemental Figure S2 and Figure 1 detail the average total distance traveled for each concentration of 50 and 200 nm exposures, respectively, with significance only indicated following 1000 and 10,000 ppb exposures to 200 nm NPs.
Figure 1:
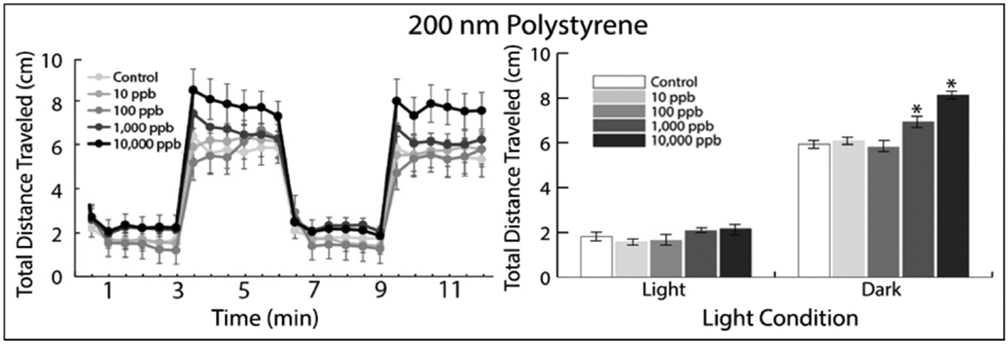
Representative sample of the total distance travelled (cm) (left) and total averaged locomotor activity of zebrafish larvae (right) after 120 hpf of exposure to 200 nm polystyrene microplastics (N=60). An asterisk (*) is used to distinguish significantly different means compared to the control (p<0.05) as determined by ANOVA with Tukey post-hoc test.
3.4. Nanoplastic accumulation
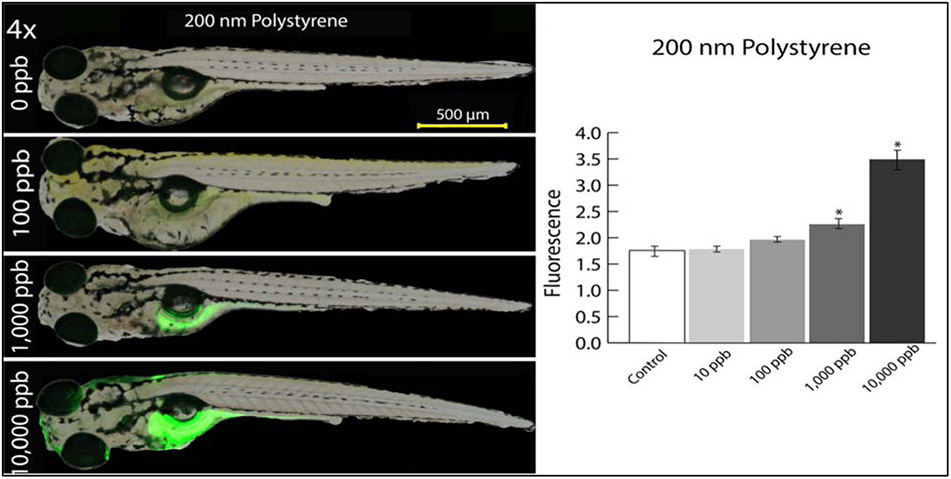
As measured by intensity of fluorescence in whole larval fish, NP accumulation in larval zebrafish increased with exposure concentration for both 50 and 200 nm NPs (Supplemental Figure S3, Figure 2, p<0.01 and p<0.01, respectively). At 10 ppb and 100 ppb, no difference in accumulation was found compared to the control for either 50 or 200 nm NP-exposed fish (p>0.05). However, NP accumulation increased significantly relative to the control for both 50 and 200 nm at 1000 ppb (p<0.01 and p<0.01, respectively) and 10,000 ppb (Supplemental Figure S3, Figure 2, p<0.01 and p<0.01, respectively). For exposure to 50 nm polystyrene at 10,000 ppb, NP fluorescence increased by approximately 38%, while 200 nm exposed fish at the same concentration displayed an increase in fluorescence of over 65%. The polystyrene microspheres accumulated primarily in the gastrointestinal tract, liver, ocular, and cranial regions of exposed fish (Figure 2, Supplemental Figures S4 and S5) following the five-day exposures.
Figure 2:
Representative samples of zebrafish exposed to nanoplastics at 0, 100, 1,000, and 10,000 ppb (left) and fluorescent values indicative of accumulation of nanoplastics in whole larval zebrafish from exposures to 0-10,000 ppb of 200 nm nanoplastics (right) at 120 hpf (N=20). An asterisk (*) is used to distinguish significantly different means compared to the control (p<0.05) as determined by ANOVA with Tukey post-hoc test. Natural log transformation of accumulation data was performed to approximate a normal distribution. Zebrafish were imaged post-mortem using a Nikon stereomicroscope (Melville, NY, USA) with a X-Cite fluorescent illuminator (Series 120).
3.5. Transcriptomic analysis
Only control, 100, and 1,000 ppb concentrations were analyzed for transcriptomic effects, in order to compare the lowest exposure concentration that produced neurobehavioral effects with 200 nm microspheres (i.e. 1,000 ppb) to the highest concentration that produced no neurobehavioral effects (i.e. 100 ppb). Exposure to 200 nm microspheres resulted in the greatest amount of differentially expressed genes (DEGs): 734 (62% upregulated) DEGs were reported at 100 ppb, while 1,000 ppb exposures resulted in 864 (62% upregulated) DEGs (Supplemental Tables S2, S5). Considerable overlap was evident, with 53% of DEGs at 100 ppb and 45% of DEGs at 1,000 ppb present in both conditions (Supplemental Figure S6). Contrastingly, 50 nm exposures at 100 ppb resulted in no DEGs, while 1,000 ppb exposures resulted in only 2 DEGs: hsd11b2 [hydroxysteroid (11-beta) dehydrogenase 2; log2FC=1.27, q<0.1] and slc3a2b (solute carrier family 3 member 2b; 1.06, 0,) (Supplemental Tables S2, S5).
DEGs from 200 nm NP exposures were assessed with IPA software to determine enrichment of biological and disease-linked pathways. 100 and 1,000 ppb exposures reported multiple enriched pathways; namely, organismal injury and abnormalities, endocrine system disorder, and neurological disease were three of the top five general disease and disorder pathways altered in both conditions (Table 1). In 100 ppb exposures, 71 and 88 DEGs were altered in skeletal/muscular and nervous system development and function pathways, respectively (Table 1). Nervous system-related sub-pathways were affected in both concentrations, including movement disorders (78 DEGs in 1,000 ppb, 80 DEGs in 100 ppb), neuromuscular disease (64 DEGs in 1,000 ppb, 59 DEGs in 100 ppb), morphology of nervous system (56 DEGs in 1,000 ppb, 47 DEGs in 100 ppb), and development of neurons (51 DEGs in 1,000 ppb, 56 DEGs in 100 ppb) (Supplemental Table S3). DEGs altered in nervous system sub-pathways include csnk2b (casein kinase 2, beta polypeptide; 1,000 ppb log2FC= 0.83, q<.001; 100 ppb log2FC= 0.83, q<.001), eef2k (eukaryotic elongation factor 2 kinase; 1.67, q<.001; 1.13, q<.01), gmfb (glia maturation factor, beta; 1,000 ppb log2FC= 0.95, q<.001), and ntf3 (neurotrophin 3; 1,000 ppb log2FC= 0.95, q<.1) involved in neurogenesis and synaptic function, as well as chrna1 (cholinergic receptor, nicotinic, alpha 1 (muscle); 0.77, q<.05; 0.80, q<.05), atp1a2a (ATPase Na+/K+ transporting subunit alpha 2a; 0.79, q<.01; 0.80, q<.05), bicd2 (bicaudal D homolog 2; −1.08, q<.05; −1.02, q<.01), and myhb (myosin heavy chain b; −1.31, q<.001; −1.12, q<.001) implicated in neuromuscular dysfunction.
Table 1:
Ingenuity pathway analysis-generated list of overlapping pathways from exposures to 100 and 1,000 ppb polystyrene nanoplastics of the 200 nm size class. Using a Right-Tailed Fisher’s Exact Test, p-values < .01 were considered significant.
| 100 ppb | 1,000 ppb | |||
|---|---|---|---|---|
| Pathway | p-value | # of Genes changed | p-value | # of Genes changed |
| DISEASE AND DISORDERS | ||||
| Cancer | 3.75 × 10−3 | 441 | 3.53 × 10−3 | 513 |
| Organismal Injury and Abnormalities | 3.75 × 10−3 | 445 | 3.53 × 10−3 | 523 |
| Endocrine System Disorder | 2.37× 10−3 | 353 | 3.52 × 10−3 | 427 |
| Hereditary Disorder | 3.31 × 10−3 | 129 | 3.53 × 10−3 | 135 |
| Neurological Disease | 3.32 × 10−3 | 160 | 3.53 × 10−3 | 182 |
| MOLECULAR AND CELLULAR FUNCTION | ||||
| Cellular Function and Maintenance | 3.83 × 10−3 | 153 | 3.33 × 10−3 | 168 |
| Cellular Assembly and Organization | 3.83 × 10−3 | 138 | 3.33 × 10−3 | 130 |
| Cellular Growth and Proliferation | 3.46 × 10−3 | 132 | 3.52 × 10−3 | 164 |
| PHYSIOLOGICAL SYSTEM DEVELOPMENT | ||||
| Organismal Development | 3.90 × 10−3 | 162 | 3.35 × 10−3 | 216 |
| Organismal Survival | 2.66 × 10−3 | 136 | 2.78 × 10−3 | 168 |
| Nervous System Development and Function | 3.46 × 10−3 | 88 | - | - |
| Muscular System Development and Function | 3.75 × 10−3 | 71 | - | - |
| CARDIOTOXICITY | ||||
| Cardiac Enlargement | 2.24 × 10−5 | 29 | 1.39 × 10−4 | 35 |
| Cardiac Dysfunction | 2.37 × 10−5 | 19 | 1.93 × 10−4 | 20 |
| Cardiac Arrythmia | 3.90 × 10−4 | 17 | 1.80 × 10−3 | 18 |
| HEPATOTOXICITY | ||||
| Liver Hyperplasia/Hyperproliferation | 3.48 × 10−5 | 194 | 7.83 × 10−5 | 212 |
| Liver Necrosis/Cell Death | 2.77 × 10−3 | 14 | 6.57 × 10−3 | 11 |
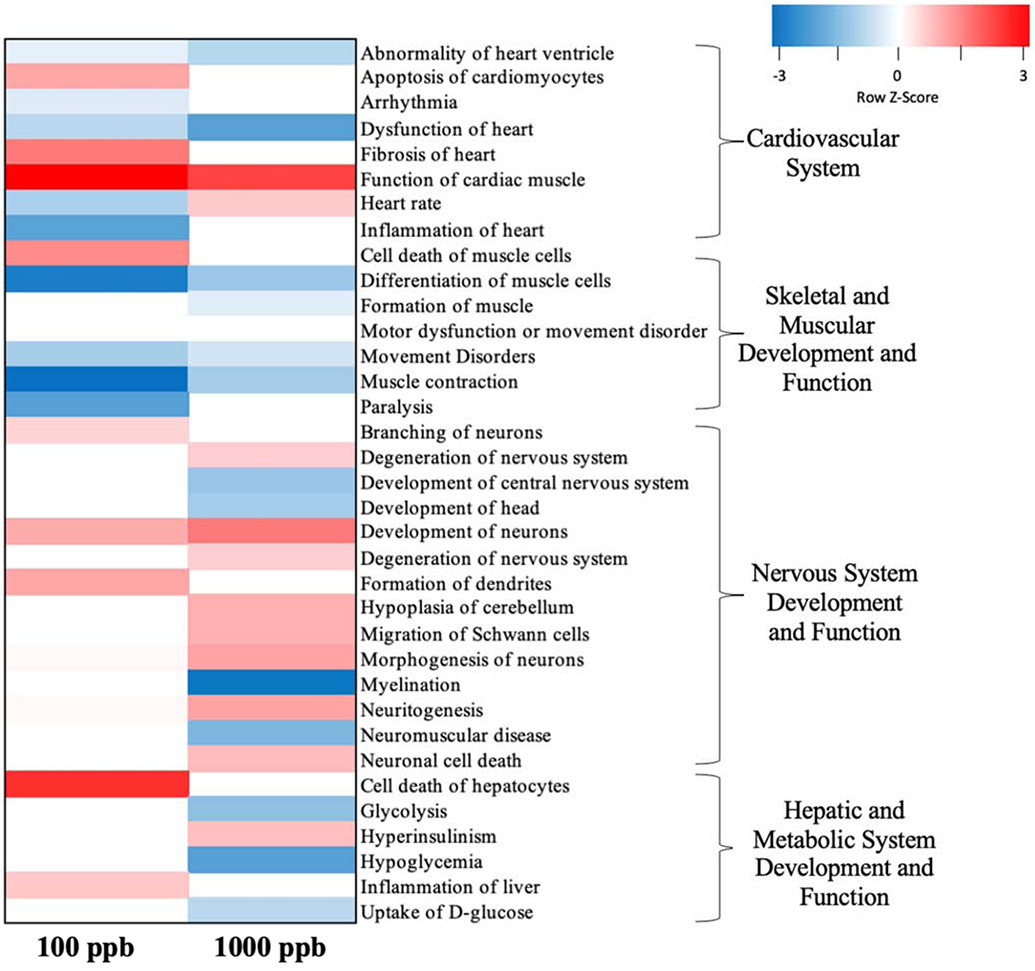
In addition to nervous system and neuromuscular pathways, both 100 and 1,000 ppb exposures dysregulated metabolic, cardiac, and hepatic pathways; Figure 3 details a heatmap of the predicted activation state of pathways from each of these categories, while Table 2 indicates the log2 fold change and q-value of specific DEGs across all pathways of interest for 100 and 1,000 ppb exposures to 200 nm NPs. Several DEGs that were implicated in metabolic dysfunction included pdk2a (pyruvate dehydrogenase kinase 2a; 0.91, q<.01; 0.85, q<.05), apoa4b.3 (apolipoprotein A-IV b, tandem duplicate 3; 1.23, q<.01; 0.88, q<.1), and insrb (insulin receptor b; 1,000 ppb log2FC= −0.85, q<.1). DEGs related to cardiovascular function included slc8a1b (solute carrier family 8 member 1b; 1,000 ppb log2FC= −0.78, q<.05), myh6 (myosin, heavy chain 6, cardiac muscle, alpha; −0.75, q<.1; −0.84, q<.05) and amot (angiomotin; −1.20, q<.01; −1.34, q<.001)]. DEGs involved in hepatic metabolism included cyp3a65 (cytochrome p450, family 3, subfamily a, polypeptide 65; 1,000 ppb log2FC= −0.86, q<.01) and cyp7a1 (cytochrome p450, family 7, subfamily a, polypeptide 1; 100 ppb log2FC= 0.84, q<.05), while altered gastrointestinal homeostasis genes included aqp1a.1 (aquaporin 1a (Colton blood group), tandem duplicate 1; 1.13, q<.001; 0.96, q<.01) and mfge8b (milk fat globule EGF and factor V/VIII domain containing b; 1.01, q<.01; 0.81, q<.05). Finally, a subset of DEGs were also involved in epigenetic modification [kdm2aa (lysine (K)-specific demethylase 2Aa; 1,000 ppb log2FC= 0.82, q<.05), smyd3 (SET and MYND domain containing 3; 100 ppb log2FC= −1.01, q<.05), and hdac3 (histone deacetylase 3; 1,000 ppb log2FC= 0.89, q<.1)].
Figure 3:
Heatmap indicating predicted upregulation or downregulation in subpathways based on z-scores. Subpathways from 200 nm NP exposures to 100 and 1,000 ppb were selected and categorized.
Table 2:
Genes in pathways of interest (neuromuscular, hepatic, gastrointestinal, metabolic, cardiovascular, and epigenetic) among zebrafish exposed to 100 and 1000 ppb of 200 nm polystyrene microplastics. Bolded categories display genes found to be differentially expressed (absolute log2 fold change value ≥ 0.75, p-value < 0.05, and BH adjusted p-value < 0.1). p-values were determined using DESeq2.
| 100 ppb | 1000 ppb | ||||||
|---|---|---|---|---|---|---|---|
| Gene name | Gene Symbol |
log2 FC | p-value | p-adj | log2 FC | p-value | p-adj |
| CARDIOVASCULAR SYSTEM | |||||||
| Angiotensin I converting enzyme 2 | ace2 | −0.86 | 1.14 x 10−4 | 6.10 x 10−3 | −0.87 | 4.18 x 10−7 | 6.28 x 10−5 |
| Angiomotin | amot | −1.34 | 4.66 x 10−6 | 9.26 x 10−4 | −1.20 | 3.11 x 10−5 | 1.61 x 10−3 |
| Cholinergic receptor muscarinic 2a | chrm2a | −1.10 | 7.71 x 10−4 | 1.79 x 10−2 | −0.52 | 1.25 x 10−1 | 3.79 x 10−1 |
| Cytochrome P450 family 2 subfamily N polypeptide 13 | cyp2n13 | 1.03 | 8.52 x 10−5 | 4.99 x 10−3 | 0.94 | 3.16 x 10−4 | 8.32 x 10−3 |
| Myosin heavy chain 6 cardiac muscle alpha | myh6 | −0.84 | 1.61 x 10−3 | 2.78 x 10−2 | −0.75 | 7.08 x 10−3 | 6.64 x 10−2 |
| Solute carrier family 8 member 1b | slc8a1b | −0.48 | 8.74 x 10−2 | 2.86 x 10−1 | −0.78 | 7.84 x 10−4 | 1.54 x 10−2 |
| Myosin heavy chain 7B cardiac muscle beta b | myh7bb | −0.88 | 2.12 x 10−6 | 6.26 x 10−4 | −0.94 | 3.74 x 10−9 | 2.39 x 10−6 |
| Titin-cap (telethonin) | tcap | 1.50 | 6.02 x 10−7 | 3.04 x 10−4 | 1.06 | 1.37 x 10−3 | 2.25 x 10−2 |
| GENE EXPRESSION AND EPIGENETICS | |||||||
| Bromodomain adjacent to zinc finger domain 1A | baz1a | 1.26 | 1.09 x 10−4 | 5.92 x 10−3 | 1.19 | 4.11 x 10−4 | 9.97 x 10−3 |
| Bromodomain adjacent to zinc finger domain 1B | baz1b | −0.81 | 8.15 x 10−6 | 1.22 x 10−3 | −0.85 | 1.31 x 10−8 | 5.84 x 10−6 |
| Chromodomain helicase DNA binding protein 1 | chd1 | −0.45 | 4.09 x 10−3 | 4.74 x 10−2 | −0.76 | 1.81 x 10−6 | 1.95 x 10−4 |
| Chromodomain helicase DNA binding protein 4a | chd4a | −0.53 | 6.08 x 10−3 | 5.94 x 10−2 | −0.89 | 1.99 x 10−7 | 3.91 x 10−5 |
| Chromodomain helicase DNA binding protein 6 | chd6 | −0.71 | 3.68 x 10−5 | 3.05 x 10−3 | −0.87 | 2.63 x 10−12 | 7.03 x 10−9 |
| Lysine (K)-specific demethylase 2aa | kdm2aa | 0.43 | 7.87 x 10−2 | 2.70 x 10−1 | 0.82 | 1.23 x 10−3 | 2.08 x 10−2 |
| SET and MYND domain containing 3 | smyd3 | −1.01 | 1.91 x 10−3 | 3.06 x 10−2 | −0.43 | 1.84 x 10−1 | 4.61 x 10−1 |
| Histone deacetylase 3 | hdac3 | 0.90 | 6.65 x 10−3 | 6.28 x 10−2 | 0.89 | 1.15 x 10−2 | 9.09 x 10−2 |
| GASTROINTESTINAL DISEASE | |||||||
| Aquaporin 1a (Colton blood group) tandem duplicate 1 | aqp1a.1 | 0.96 | 6.04 x 10−5 | 3.98 x 10−3 | 1.13 | 1.64 x 10−8 | 5.84 x 10−6 |
| Aquaporin 3a | aqp3a | 0.81 | 6.14 x 10−6 | 1.03 x 10−3 | 0.93 | 1.02 x 10−7 | 2.43 x 10−5 |
| Aquaporin 8a tandem duplicate 1 | aqp8a.1 | 0.88 | 5.24 x 10−6 | 9.92 x 10−4 | 0.66 | 1.89 x 10−3 | 2.78 x 10−2 |
| Milk fat globule EGF and factor V/VIII domain-containing B | mfge8b | 0.81 | 3.13 x 10−3 | 4.05 x 10−2 | 1.01 | 3.05 x 10−4 | 8.15 x 10−3 |
| HEPATIC AND METABOLIC FUNCTIONING | |||||||
| Apolipoprotein A-1b | apoa1b | 1.09 | 5.84 x 10−4 | 1.50 x 10−2 | 0.65 | 1.68 x 10−2 | 1.14 x 10−1 |
| Apolipoprotein A-IV B tandem duplicate 3 | apoa4b.3 | 0.88 | 1.19 x 10−2 | 9.00 x 10−2 | 1.23 | 1.05 x 10−4 | 3.81 x 10−3 |
| Cytochrome P450 family 3 subfamily A polypeptide 65 | cyp3a65 | −0.57 | 3.99x10−3 | 4.69x10−2 | −0.86 | 9.57x10−5 | 3.52x10−3 |
| Cytochrome P450 family 7 subfamily A polypeptide 1 | cyp7a1 | 0.84 | 4.13 x 10−3 | 4.77 x 10−2 | −0.15 | 6.57 x 10−1 | 8.56 x 10−1 |
| Si:ch211-160o17.2 (Inositol Polyphosphate-1-Phosphatase) |
si:ch211- 160o17.2 (INPP1) |
0.26 | 3.80 x 10−1 | 6.37 x 10−1 | 0.92 | 2.10 x 10−3 | 2.98 x 10−2 |
| Insulin receptor b | insrb | −0.48 | 1.46 x 10−1 | 3.79 x 10−1 | −0.85 | 1.24 x 10−2 | 9.47 x 10−2 |
| Phosphoenolpyruvate carboxykinase 1 (soluble) | pck1 | 0.46 | 5.56 x 10−2 | 2.22 x 10−1 | 0.81 | 7.48 x 10−4 | 1.49 x 10−2 |
| Pyruvate dehydrogenase kinase 2a | pdk2a | 0.85 | 1.49 x 10−3 | 2.66 x 10−2 | 0.91 | 3.45 x 10−4 | 8.91 x 10−3 |
| Suppressor of cytokine signaling 3b | socs3b | 1.28 | 1.34 x 10−4 | 6.79 x 10−3 | 1.13 | 8.45 x 10−4 | 1.62 x 10−2 |
| Serine/threonine kinase 25b | stk25b | 1.05 | 5.71 x 10−4 | 1.48 x 10−2 | 0.79 | 1.71 x 10−2 | 1.16 x 10−1 |
| NEUROMUSCULAR FUNCTIONING | |||||||
| Abelson helper integration site 1 | ahi1 | −0.48 | 5.07 x 10−2 | 2.11 x 10−1 | −0.98 | 1.17 x 10−6 | 1.32 x 10−4 |
|
Adaptor related protein complex 1 subunit
sigma 2 |
ap1s2 | 0.95 | 3.40 x 10−3 | 4.28 x 10−2 | 0.64 | 3.23 x 10−2 | 1.73 x 10−1 |
| ATPase Na+/K+ transporting subunit alpha 2a | atp1a2a | 0.80 | 2.74 x 10−4 | 1.02 x 10−2 | 0.79 | 2.74 x 10−4 | 7.51 x 10−3 |
| ATPase Na+/K+ transporting subunit alpha 3a | atp1a3a | 0.89 | 3.69 x 10−4 | 1.17 x 10−2 | 0.27 | 2.46 x 10−1 | 5.33 x 10−1 |
| Bicaudal D homolog 2 (Drosophila) | bicd2 | −1.02 | 2.54 x 10−4 | 9.98 x 10−3 | −1.08 | 7.28 x 10−4 | 1.46 x 10−2 |
| Cholinergic receptor nicotinic alpha 1 (muscle) | chrna1 | 0.80 | 1.98 x 10−3 | 3.11 x 10−2 | 0.77 | 1.94 x 10−3 | 2.82 x 10−2 |
| Casein kinase 2 beta polypeptide | csnk2b | 0.83 | 2.18 x 10−7 | 1.56 x 10−4 | 0.83 | 3.81 x 10−8 | 1.16 x 10−5 |
| Eukaryotic elongation factor 2 kinase | eef2k | 1.14 | 1.86 x 10−4 | 8.31 x 10−3 | 1.67 | 6.15 x 10−7 | 8.57 x 10−5 |
| Fibronectin leucine-rich transmembrane protein 2 | flrt2 | 0.38 | 2.59 x 10−1 | 1.00 | 0.91 | 6.47 x 10−3 | 6.28 x 10−2 |
| Glia maturation factor beta | gmfb | 0.71 | 6.72 x 10−4 | 1.64 x 10−2 | 0.95 | 1.93 x 10−6 | 2.01 x 10−4 |
| HCLS1 associated protein X-1 | hax1 | 0.86 | 1.32 x 10−3 | 2.52 x 10−2 | 1.12 | 6.37 x 10−5 | 2.58 x 10−3 |
| Kinesin family member 1Ab | kif1ab | −0.63 | 2.82 x 10−3 | 3.84 x 10−2 | −0.80 | 5.02 x 10−4 | 1.14 x 10−2 |
| Myosin heavy chain b | myhb | −1.12 | 1.99 x 10−7 | 1.56 x 10−4 | −1.31 | 2.64 x 10−21 | 3.53 x 10−17 |
| Neurotrophin 3 | ntf3 | 0.32 | 3.70 x 10−1 | 1.00 | 0.95 | 7.57 x 10−3 | 6.90 x 10−2 |
| Peripherin | prph | −0.74 | 8.51 x 10−3 | 7.37 x 10−2 | −1.12 | 1.41 x 10−4 | 4.61 x 10−3 |
| Solute carrier family 6 member 1a | slc6a1a | 0.90 | 1.01 x 10−2 | 8.11 x 10−2 | 0.34 | 3.20 x 10−1 | 6.11 x 10−1 |
| Synaptosome-associated protein 91a | snap91a | 0.73 | 6.32 x 10−4 | 1.58 x 10−2 | 0.96 | 6.50 x 10−6 | 5.22 x 10−4 |
4. Conclusions
NPs are an ubiquitous pollutant in the environment and bioavailable to a wide range of species (Cedervall et al., 2012; Della Torre et al., 2014; Cole et al., 2015a; Greven et al., 2016). Here, we demonstrate that embryonic and early larval NP exposure in zebrafish alters gene expression in neurological disease and movement disorder pathways, coinciding with hyperactivity and large-scale accumulation in the gastrointestinal tract, liver, cranial, and ocular regions, in a dose- and size-dependent manner. Our results correspond with previous studies indicating the end organs for NP accumulation in fish, particularly following developmental NP exposure (Mattsson et al., 2017; Skjolding et al., 2017; Pitt et al., 2018). Nanopolystrene can penetrate through the zebrafish chorion, resulting in developmental immersion in NPs (Pitt et al., 2018). Exposure via ingestion and/or the respiratory system at the early larval stage is also possible (Lu et al., 2016; Van Pomeren et al., 2017; Parenti et al., 2019). We did not observe increased mortality or developmental abnormalities due to developmental NP exposure. These findings are similar to Pitt et al. (2018) and Brun et al. (2019), who, respectively, found only decreased larval heart rate and decreased swim bladder inflation in NP-exposed fish, and LeMoine et al. (2018), who found no changes in hatch rate or mortality in MP-exposed fish.
Overall, studies detailing size-dependent toxicity vary widely, with no clear pattern of outcomes. While Lee et al. (2018) detailed neurotoxicity due to larger sizes of MPs (1-5μm), but not from NPs, many have determined that NPs have a more significant impact on health than larger sized plastics (Wu et al., 2019; Yi et al., 2019). In the current study, 200 nm NP exposures had significantly higher accumulation that was associated with profound transcriptomic and neurobehavioral consequences, while 50 nm did not. Only 2 genes were dysregulated compared to control in fish exposed to 50 nm NPs. (Supplemental Tables S2, S5). 200 nm NPs have potentially greater disruptive or occlusive effect on tissues and/or blood vessels, causing higher levels of inflammation and stress response, which are reported mechanisms of plastics-induced toxicity (Mohr et al., 2014; Lu et al., 2016; Gopinath et al. 2019; Yong et al., 2020). However, closer histological and pathway analysis will be needed to confirm this in future studies.
Similar to Brun et al. (2019), we found that NP-exposed larval zebrafish presented with hyperactivity during dark periods at the two highest levels of exposure (1,000 and 10,000 ppb). Contrastingly, Pitt et al. (2018) and Chen et al. (2017) observed hypoactivity during dark periods in NP-exposed fish. No difference in activity was present in light periods; however, this is reasonable considering the innate tendencies of larval zebrafish to move less during light intervals and more during dark intervals (MacPhail et al. 2008). Limonta et al. (2019) reports hyperactivity in dark periods in adult zebrafish chronically exposed to MPs, but this study assayed daily locomotor activity long-term in a 12:12h light/dark cycle, suggesting that behavioral outcomes in this case may rather indicate changes in adult circadian rhythms. In adult zebrafish exposed to NPs, Sarasamma et al. (2020) found hyperactivity in a novel tank test, as well as alterations in more complex social behaviors; future studies raising exposed larval fish to adulthood will allow us to determine whether hyperactivity persists into adulthood and to characterize a larger repertoire of behavioral phenotypes than is possible at early stages of development. Discrepancies in developmental stage, NP size, NP type, length of exposure, and behavioral assay design may account for the conflicting behavioral outcomes across studies, highlighting the need for expansion of behavioral testing methods as well as comprehensively designed studies exploring multiple experimental factors.
Transcriptomic analysis revealed dysregulation in nervous system development and neurological disease pathways at 1,000 and 100 ppb NP exposures. These results are similar to several other studies detailing MP- and NP-induced neurotoxicity, as determined by reduced activity of the neurotransmission biomarker acetylcholinesterase (AChE) in aquatic bivalves, the common goby (Pomatoschistus microps), and larval zebrafish (Oliveira et al., 2013; Avio et al., 2015; Chen et al., 2017; Ribeiro et al., 2017). LeMoine et al. (2018) also reported dysregulation of genes involved in neural development and function in MP-exposed larval zebrafish. We found a large subset of DEGs involved in neurogenesis and synaptic function; for example, exposure to both NP concentrations upregulated the serine/threonine kinase csnk2b, a modulator of neuron and oligodendrocyte proliferation, neuronal morphology, and synaptic transmission (Huillard et al., 2010; Yang et al., 2018), hax1, which suppresses neuronal apoptosis (Chao et al., 2008), and eef2k, a Ca2+-regulated kinase involved in synaptic plasticity (Park et al., 2008). Heise et al. (2017) reported that eEF2K strongly inhibits GABAergic signaling in rodents; therefore, increased expression can enhance neuronal excitation. Likewise, slc6a1a, which was upregulated in 100 ppb-exposed fish, is critical for GABA reuptake from the synaptic cleft. Dysregulation of its orthologue SLC6A1 has been shown in several studies to be involved in ADHD susceptibility in humans by modulating the balance of neuronal excitation/inhibition (Yuan et al., 2017; Yuan et al., 2020). In 1,000 ppb exposures, neural and glial growth factors were upregulated, including ntf3, which promotes neuronal differentiation and sensory neuronal survival (Usui et al., 2012; Lin et al. 2018), and gmfb, which is known to play both a pro-developmental and neuroinflammatory role in the CNS (Fan et al., 2018). Synaptic genes snap91a, which regulates clathrin-mediated synaptic vesicle recycling, and flrt2, also important in neuronal migration, were also upregulated in 1,000 ppb-exposed fish (Yao et al., 1999; Cicvaric et al., 2018). Overall, the upregulation of a subset of neurogenesis and synaptic plasticity genes suggests NP exposure dysregulates a variety of neurodevelopmental pathways, one outcome of which may be neuronal excitation, increased network activity, and subsequent hyperactive behavioral outcomes. Supporting this, hyperactivity and ADHD symptoms have been linked to increased functional connectivity in human cerebellar networks (Mostert et al., 2016). Such disorders are thought to be triggered by alterations in brain formation and neurotransmitter signaling during early development via processes such as neural migration, morphogenesis, and cell signaling, all of which are likely disrupted by NP exposure, as evidenced by our transcriptomic findings.
Changes in neuromotor/neuromuscular pathways likely also contribute to neurobehavioral outcomes. Following NP exposure, movement disorder and neuromuscular disease pathways were altered in both exposure groups, including disorder of basal ganglia (58 DEGs in 1,000 ppb; 57 DEGs in 100 ppb), dyskinesia (47 DEGs in 1,000 ppb; 44 in 100 ppb), and hypoplasia of cerebellum in 1,000 ppb-exposed fish alone (7 DEGs). The basal ganglia, which are homologous to the subpallium in zebrafish, are responsible for voluntary and involuntary motor activity, thus disruption can result in impaired motor activity (Palmér et al., 2017). In fact, in 100 ppb-exposed fish, we found upregulation of the membrane protein trafficking gene ap1s2, which is implicated in basal ganglia disease in humans (Saillour et al., 2007). In 1,000 ppb-exposed fish, we also found downregulation of ahi1, which is associated with cerebellar development in humans and mice (Louie and Gleeson, 2005; Lancaster et al., 2011). As in mammals, the zebrafish cerebellum is a locus of motor control (Ahrens et al., 2012); therefore, dysregulation of cerebellar development can result in neuromotor consequences. Multiple neuromuscular genes were downregulated in both exposure groups, including skeletal muscle subunit myhb (Wang et al., 2012) and axonal transport gene bicd2, while cytoskeletal gene prph and axonal transport gene kif1ab were downregulated in 1,000 ppb-exposed fish alone. Although BICD2 and KIF1A regulate axonal transport in opposite directions (retrograde vs anterograde, respectively), impairment of either can affect motor neuron survival and brain development, leading to neuromuscular disease (Yonekawa et al., 1998; Rossor et al., 2020). Likewise, downregulation of PRPH, which is involved in cytoskeletal development and maintenance of PNS neurons, increases the likelihood of peripheral neuropathy (Bjornsdottir et al., 2019). Another subset of neuromuscular genes was upregulated in both exposure groups, including the nicotinic acetylcholine receptor subunit chrna and the Na+/K+-ATPase atp1a2a; atp1a3a was also upregulated in 100 ppb-exposed fish. As CHRNA1 activity regulates synaptic signaling at neuromuscular junctions, increases in AChR signaling can induce excitotoxicity and various myopathies (Lefebvre et al., 2004). Na+/K+-ATPases also regulate skeletal muscle and neuronal excitability through maintaining the Na+ and K+ electrochemical gradient, the disruption of which is also known to cause neuromuscular disease (Holm et al., 2016; Castaneda et al., 2018). Taken together, our transcriptomic findings suggest that NP exposure dysregulates pathways important to neuromotor control, motor neuron survival, and neuromuscular excitability, promoting neurological and neuromuscular disorders.
While our neurobehavioral findings of hyperactivity in 1,000 and 10,000 ppb NP-exposed fish correspond with transcriptomic findings in 100 and 1,000 ppb exposures suggesting increased neuronal activity and excitability, we also observed changes in pathways suggesting neuromotor impairment. One possibility is that other indications of neuromuscular impairment are present in our NP-exposed fish but are not discerned by our methods. While the comparison of total distance travelled in light and dark periods is an excellent neurobehavioral screening tool, it has limitations in detecting subtle motor outcomes. The evaluation of tap-elicited startle response, for example, may be a useful future direction for determining changes in rapid escape-driven motor reflexes (Levin and Cerutti, 2009). Alternatively, other indications of neuromuscular impairment may present later in life, affecting movement, food intake, and survival. The neuromuscular system undergoes large-scale structural and functional development as fish dramatically increase in size between 5 dpf and adulthood (Johnston et al., 2011). Additionally, deficits in active food seeking behavior in the water column do not affect survival until yolk sac depletion, several days after our testing period (Hernandez et al., 2018).
Finally, transcriptomic dysregulation of stress response and energy regulation networks, in conjunction with hyperactivity-linked neurological pathways, may be counteracting potential motor dysfunction and neurodegenerative effects to promote a hyperactive phenotype. 200 nm NPs generally upregulated glucose and lipid metabolism genes in 100 and 1,000 ppb-exposed fish, including the lipid metabolism genes apoa4b.3 and apoa1b, respectively altered in both exposure groups and in 100 ppb alone (Gomez et al., 2010), the glycolytic regulator si:ch211-160o17.2 (INPP1) and rate-limiting gluconeogenesis enzyme pck1 in 1,000 ppb (Burgess et al., 2007; Benjamin et al., 2014), and the glycolytic enzyme pdk2a in both groups (Kumar et al., 2016). Supporting our findings, Cedervall et al. (2012) reported that 24 nm NPs can bind to apoA-I in fish serum and alter muscle/liver cholesterol distribution when consumed through the food chain, disrupting fat metabolism. Likewise, Brun et al. (2019) found increased pck1 promotor activity in NP-exposed zebrafish, concluding that plastic-induced stress responses following 72-120 hpf exposures to 25 nm polystyrene microspheres may be related to disruption in glucose metabolism and increased cortisol, another reported mechanism for hyperactivity and neurological disruption in fish (Wendelaar, 1997; Joäls et al., 2012; Joäls et al., 2018). In 1,000 ppb-exposed fish, downregulation of the insulin receptor insrb suggests further dysregulation of glucose metabolism patterns (Aronoff et al., 2004; Noguchi et al., 2013), and the upregulation of socs3b (both exposure groups) and stk25b (100 ppb only) is known to impair mitochondrial function and increase leptin and insulin resistance, another adverse metabolic consequence of increased cortisol (Chursa et al., 2017; Pedroso et al., 2018). Wu et al. (2019) further links MP exposure to mitochondrial dysfunction in human intestine cells, and zebrafish models of epilepsy exhibiting hyperactivity also implicate glycolysis and mitochondrial respiration in neuronal excitation (Kumar et al., 2016). Thus, dysregulation of glucose and lipid homeostasis and overall energy metabolism may contribute to developmental outcomes of NP-induced hyperactivity and neuronal dysfunction.
Stress-induced dysregulation of energy metabolism also has large-scale consequences for overall organismal health and physiology, including long-term outcomes such as metabolic syndrome, depression, osteoporosis, immunosuppression, and hypertension (Chrousos et al., 2000). In fish, stress events can cause energy relocation from investment processes such as reproduction and development towards survival-oriented processes such as locomotion (Wendelaar, 1997). Although early growth and development did not appear to be impacted in the current study, endocrine system disease and organismal development pathways were significantly altered by both NP exposure conditions. Others have also reported dysregulation of lipid metabolism, oxidative stress, immune response, and inflammatory pathways in larval zebrafish exposed to NPs (Veneman et al., 2017; Brun et al., 2018). Together, these findings suggest potential later-life consequences of early-life NP exposure on reproductive health and/or organismal fitness. Chronic exposures may have a stronger impact on such relocation processes if hyperactivity persists.
Corresponding with the ability of NPs to travel through and occlude vasculature and accumulate in the heart (Mohr et al., 2014; Mattsson et al., 2017; Pitt et al., 2018; Gopinath et al., 2019), we observed that NP exposure altered cardiovascular development and function pathways, important for maintaining metabolic and overall physiological health. In both exposure groups, several cardiac muscle genes involved in contraction and development were dysregulated, including sarcomere assembly gene tcap and myosin subunits myh7bb and myh6, orthologues of which are implicated in various forms of cardiomyopathy (Hayashi et al., 2004; Carniel et al., 2005; Chen et al., 2020). A subset of genes involved in blood vessel development and regulation was also affected in both groups, with downregulation of VEGF-regulated angiogenesis gene amot (Aase et al., 2007) and vasodilator ace2 (Burrell et al., 2012), as well as upregulation of cyp2n13, an orthologue of which can produce cardioprotective epoxyeicosatrienoic acids that can stimulate vasodilation and angiogenesis and protect against hypoxia (Solanki et al., 2018). slc8a1b, a Na+/Ca2+ transporter that regulates cardiac excitability and contractility (Ebert et al., 2005), and chrm2a, an M2 muscarinic receptor that modulates heart rate (Steele et al., 2009) were downregulated, respectively, in 100 and 1,000 ppb fish. Overall, dysregulation of cardiac muscle and vasculature formation and function may lead to later-life survival deficits and cardiovascular and/or metabolic disease outcomes. Although developmental assessment of cardiac edema did not reveal NP-induced cardiotoxicity, assessment of outcomes such as heart rate or mitochondrial bioenergetics may reveal more subtle effects. If NP exposure alters vasculature in the brain, this may contribute to neurodegenerative or neuroinflammatory outcomes; thus, future studies will explore NP presence in larval brain vasculature.
Primarily in 1,000 ppb-exposed fish, we found altered expression of a subset of chromatin modifying enzymes, histone demethylases and methyltransferases, and histone deacetylases. The dysregulation of genes involved in epigenetic modification can both alter the dynamics of developmental transcriptional networks, and potentially induce epimutations that persist into adulthood, mediating later-life physiological outcomes of early NP exposure. baz1a and baz1b were upregulated and downregulated, respectively, in both exposure groups; these accessory subunit members of the ISWI family of chromatin remodeling proteins regulate nucleosome mobilization and transcription and have also been implicated in neurodevelopment and behavioral regulation (Sun et al., 2016; Zaghlool et al., 2016). In 1,000 ppb-exposed fish, three members of the chromodomain-helicase-DNA binding protein family (chd1, chd4a, chd6) were downregulated. This ATP-dependent chromatin remodeling family interacts with a variety of histone modifying enzymes (including HDACs and HATs) to regulate chromatin accessibility, and disruption of human orthologues CHD1 and CHD4 has also been linked to neurodevelopmental disorders (Tai et al., 2003; Pray-Grant et al., 2005; Low et al., 2016; Weiss et al., 2016; Pilarowksi et al., 2017). Several enzymes modifying histone methylation were also dysregulated. The H3K36 histone demethylase kdm2aa, associated with transcriptional silencing and CpG island promoter regulation, was upregulated in 1,000 ppb-exposed fish (Frescas et al., 2008; Blackledge et al., 2010), whereas the H3K4 histone methyltransferase smyd3, a promoter of transcriptional activation, was downregulated in 100 ppb-exposed fish (Kim et al., 2009). Finally, the histone deacetylase hdac3 was upregulated in both exposure groups. HDAC3, generally associated with a repressive transcriptional profile, is a known neurotoxic factor highly expressed in neurons and neuronal support cells (Thomas and D’Mello, 2018). Taken together, changes in these epigenetic modifiers suggest altered regulation of transcriptional networks, possibly indicating increased transcriptional silencing as well as neurodegenerative outcomes in both exposure groups. Future exploration of both developmental and later-life NP-induced epigenetic modifications and associated phenotype will provide valuable information to the field, as there has been limited assessment of the epigenetic outcomes of either MP or NP exposure to date.
Despite potential NP accumulation in the liver and gastrointestinal tract of exposed fish, effects on hepatic and gastrointestinal functioning have been largely unexplored. Pathway analysis revealed that 1,000 and 100 ppb exposures resulted in 212 and 194 DEGs, respectively, in liver hyperplasia/hyperproliferation pathways. cyp7a1, a hepato-biliary enzyme controlling cholesterol catabolism and bile acid synthesis, was upregulated in 100 ppb exposures (Shin et al., 2003) and cyp3a65, a hepatic and gastrointestinal phase I xenobiotic metabolizing enzyme, was downregulated in 1,000 ppb exposures (Jackson et al., 2017), suggesting altered liver cell metabolic function and impaired drug metabolism due to NP exposure. Several aquaporin genes, which play a role in regulating gastrointestinal transmembrane water transport and fluid secretion (Liao et al., 2020), and mfge8b, involved in maintaining intestinal epithelial homeostasis and maintenance of mucosal integrity (Bu et al., 2007), were upregulated in both exposure groups (aqp1a.1, aqp3a, mfge8b) or in 100 ppb alone (aqp8a.1), suggesting altered gut fluid transport and barrier function in NP-exposed fish. Similarly, others have seen NP-induced effects on cytochrome p450 enzyme activity in insect cells (Fröhlich et al., 2010), and PS MP exposure in mice also resulted in gut MP accumulation, gut barrier dysfunction, and upregulation of bile acid metabolic enzymes (Jin et al., 2019). Further investigation into NP-induced hepatic and gastrointestinal endpoints and functioning is warranted. Recent studies have indicated the potential for fluorophores to leach from labeled plastics and accumulate in tissues, resulting in false positive NP accumulation (Catarino et al., 2019; Schur et al., 2019). As these findings present a limitation for this study, future directions will include exposure to unlabeled polystyrene NPs as well as measuring polystyrene body burden to address this confound.
Overall, the present study demonstrates that exposure to polystyrene NPs can induce size- and dose-dependent effects on neurobehavior, as well as potential hepatic, cardiovascular, gastrointestinal, epigenetic, and metabolic dysfunction, based on transcriptomic outcomes observed in NP-exposed zebrafish. Although we only found hyperactivity to be elicited at 1,000 ppb or above in 200 nm NP-exposed fish, the large amount of overlap in both DEGs and pathways of interest between 100 and 1,000 ppb exposure conditions suggests that, on the whole, these exposure groups share similar physiological outcomes of early NP exposure. IPA analysis indicates two potential mechanisms for hyperactivity in 200 nm NP-exposed zebrafish: dysregulation of gene expression related to energy metabolism with implications for neurological development and stress responses, as well as induction of motor dysfunction and neurodegenerative processes with potential impacts on locomotion. Furthermore, this study establishes a link between three interdependent outcomes: NP accumulation within the cranial region, alterations in specific genes and pathways essential in neurological development and function, and hyperactivity. Epigenetic, endocrine, and metabolic transcriptomic dysregulation also suggests later-life physiological outcomes, but further research is needed to determine the long-term consequences of early life NP exposure.
Supplementary Material
Highlights:
Health impacts of exposures to both 50 and 200 nm polystyrene nanoplastics in larval zebrafish were investigated.
Dose-dependent increases in plastic accumulation were identified, as measured by whole body fluorescence.
Altered behavioral response was evidenced by swimming hyperactivity.
Transcriptomic analysis suggests neurodegeneration and motor dysfunction at both high and low concentrations.
Acknowledgements
We acknowledge Emily Crofts, Kim Bauman, and all members of the Warrior Aquatic, Translational, and Environmental Research (WATER) lab at Wayne State University for help with zebrafish care and husbandry. Funding was provided from the Richard Barber Interdisciplinary program [to AMP, AFP, YZ and TRB]. Additional funding was provided by the National Center for Advancing Translational Sciences [K01 OD01462 to TRB], the WSU Center for Urban Responses to Environmental Stressors [P30 ES020957 to DNM, AMP, AFP and TRB], the National Institute of Environmental Health Sciences [F31 ES030278 to DNM]. Additional funding was provided by the National Institute of General Medicine Sciences [R25 GM 058905 to ALS] and the National Science Foundation (Grant No. 1735038 to CA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Bibliography
- Aase K, Ernkvist M, Ebarasi L, Jakobsson L, Majumdar A, Yi C, Birot O, Ming Y, Kvanta A, Edholm D, Aspenstrom P, Kissil J, Claesson-Welsh L, Shimono A, Holmgren L, 2007. Angiomotin regulates endothelial cell migration during embryonic angiogenesis. Genes & Dev. 21, 2055–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens MB, Li JM, Orger MB, Robson DN, Schier AF, Engert F, Portugues R, 2012. Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature. 485, 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrady AL, 2011. Microplastics in the marine environment. Mar. Pollut. Bull 62,1596–1605. [DOI] [PubMed] [Google Scholar]
- Aronoff SL, Berkowitz K, Shreiner B, Want L, 2004. Glucose metabolism and regulation: Beyond insulin and glucagon. Diabetes Spectr. 17, 183–190. [Google Scholar]
- Auta HS, Emenike CU, Fauziah SH, 2017. Distribution and importance of microplastics in the marine environment: a review of sources, fate, effects, and potential solutions. Environ. Int 102, 165–176. [DOI] [PubMed] [Google Scholar]
- Avio CG, Gorbi S, Milan M, Benedetti M, Fattorini D, d’Errico G, Pauletto M, Bargelloni L, Regoli F, 2015. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut 198, 211–222. [DOI] [PubMed] [Google Scholar]
- Barnes DKA, Galgani F, Thompson RC, Barlaz M, 2009. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. London Ser 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin DI, Louie SM, Mulvihill MM, Kohnz RA, Li DS, Chan LG, Sorrentino A, Bandyopadhyay S, Cozzo A, Ohiri A, Goga A, Ng S-W, Nomura DK, 2014. Inositol phosphate recycling regulates glycolytic and lipid metabolism that drives cancer aggressiveness. ACS Chem. Biol 9, 1340–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergami E, Bocci E, Vannuccini ML, Monopoli M, Salvati A, Dawson KA, Corsi I, 2016. Nano-sized polystyrene affects feeding, behavior, and physiology of brine shrimp Artemia franciscana larvae. Ecotox. Environ. Safe 123, 18–25. [DOI] [PubMed] [Google Scholar]
- Besseling E, Wang B, Lürling M, Koelmans AA, 2014. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ. Sci. Technol 48, 12336–12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsdottir G, Ivarsdottir EV, Bjarnadottir K, Benonisdottir S, Gyfladottir SS, Arnadottir GA, Benediktsson R, Halldorsson GH, Helgadottir A, Jonasdottir A, Jonasdottir A, Jonsdottir I, Kristindottir AM, Magnussin OT, Masson G, Melsted P, Rafner T, Sigurdsson A, Sigurdsson G, Skuladottir A, Steinthorsdottir V, Styrkarsdottir U, Thorgeirsson G, Thorleifsson G, Vikingsson A, Gudbiartsson DF, Holm H, Stefansson H, Thorsteindottir U, Norddahl GL, Sulem P, Thorgeirsson TE, Stefansson K, 2019. A PRPH splice-donor variant associates with reduced sural nerve amplitude and risk of peripheral neuropathy. Nat. Commun 10, 1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackledge NP, Zhou JC, Tolstorukov MY, Farcas AM, Park PJ, Klose RJ, 2010. CpG islands recruit a histone H3 lysine 36 demethylase. Mol. Cell 38, 179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun NR, Koch BEV, Varela M, Peijnenburg., Spaink HP, Vilver MG, 2018. Nanoparticles induce dermal and intestinal innate immune system responses in zebrafish embryos. Environ. Sci. Nano 5, 904–916. [Google Scholar]
- Brun NR, van Hage P, Hunting ER, Haramis APG, Vink SC, Vijver MG, Schaaf MJM, Tudorache C, 2019. Polystyrene nanoplastics disrupt glucose metabolism and cortisol levels with a possible link to behavioural changes in larval zebrafish. Commun. Biol 2, UNSP 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu H-F, Zuo X-L, Wang X, Ensslin MA, Koti V, Hsueh W, Raymond AS, Shur BD, Tan X-D, 2007. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J. Clin. Invest 117, 3673–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess SC, He TT, Yan Z, Lindner J, Sherry AD, Malloy CR, Browning JD, Magnuson MA, 2007. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 5, P313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell LM, Harrap SB, Velkoska E, Patel SK, 2012. The ACE2 gene: its potential as a functional candidate for cardiovascular disease. Clin. Sci. (Lond.) 124, 65–76. [DOI] [PubMed] [Google Scholar]
- Carniel E, Taylor MRG, Sinagra G, Di Lenarda A, Ku L, Fain PR, Boucek MM, Cavanaugh J, Miocic S, Slavov D, Graw SL, Feiger J, Zu XZ, Dao D, Ferguson DA, Bristow MR, Mestroni L, 2005. Alpha-myosin heavy chain: A sarcomeric gene associated with dilated and hypertrophic phenotypes of cardiomyopathy. Circulation. 112, 54–9. [DOI] [PubMed] [Google Scholar]
- Castaneda RA, Avlijas S, Simard MA, Ricciardi A, 2014. Microplastic pollution in St. Lawrence river sediments. Can. J. Fish. Aquat. Sci 71 (12), 1767–1771. [Google Scholar]
- Castaneda MS, Zanoteli E, Scalco RS, Scaramuzzi V, Caldas VM, Reed UC, da Silva AMS, O’Callaghan B, Phadke R, Bugiardini E, Sud R, McCall S, Hanna MG, Poulsen H, Mannikko R, Matthews E, 2018. A novel ATP1A2 mutation in a patient with hypokalaemic periodic paralysis and CNS symptoms. Brain. 141, 3308–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarino AI, Frutos A, Henry TB, 2019. Use of fluorescent-labelled nanoplastics (NPs) to demonstrate NP absorption is inconclusive without adequate controls. Sci. Total. Environ 670, 915–920. [DOI] [PubMed] [Google Scholar]
- Cedervall T, Hansson LA, Lard M, Frohm B, Linse S, 2012. Food chain transport of nanoparticles affects behaviour and fat metabolism in fish. PLoS ONE 7, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J-R, Parganas E, Boyd K, Hong CY, Opferman JT, Ihle JN, 2008. Hax1-mediated processing of HtrA2 by Parl allows survival of lymphocytes and neurons. Nature. 452, 98–102. [DOI] [PubMed] [Google Scholar]
- Chen Q, Gundlach M, Yang S, Jiang J, Velki M, Yin D, Hollert H, 2017. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Tot. Environ 584, 1022–1031. [DOI] [PubMed] [Google Scholar]
- Chen P, Li Z, Nie J, Wang H, Yu B, Wen Z, Sun Y, Shi X, Jin L, Wang D-W, 2020. MYH7B variants cause hypertrophic cardiomyopathy by activating the CaMK-signaling pathway. Sci. China Life Sci 10.1007/s11427-019-1627-y. [DOI] [PubMed] [Google Scholar]
- Chrousos GP 2000. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. International Journal of Obesity. 24, S50–S55. [DOI] [PubMed] [Google Scholar]
- Chursa U, Nunez-Duran E, Cansby E, Amrutkar M, Sutt S, Stahlman M, Olsson B-M, Boren J, Johansson ME, Backhed F, Johansson BR, Sihlbom C, Mahlapuu M, 2017. Overexpression of protein kinase STK25 in mice exacerbates ectopic lipid accumulation, mitochondrial dysfunction and insulin resistance in skeletal muscle. Diabetologia. 60, 553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicvaric A, Yang J, Bulat T, Zambon A, Dominguez-Rodriguez M, Kuhn R, Sadowicz MG, Siwert A, Egea J, Pollak DD, Moeslinger T, Monje FJ, 2018. Enhanced synaptic plasticity and spatial memory in female but not male FLRT2-haplodeficient mice. Sci. Rep 8, 3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M, Galloway TS, 2015a. Ingestion of nanoplastics and microplastics by pacific oyster larvae. Environ. Sci. Technol 49, 14625–14632. [DOI] [PubMed] [Google Scholar]
- Cole M, Lindeque P, Fileman E, Halsband C, Galloway TS, 2015b. The impact of polystyrene microplastics on feeding, function, and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol 49 (2), 1130–1137. [DOI] [PubMed] [Google Scholar]
- Costa MF, do Sul JAI, Silva-Cavalcanti JS, Araujo MCB, Spengler A, Tourinho PS, Tourinho PS, 2010. On the importance of size of plastic fragments and pellets on the strandline: a snapshot of a Brazilian beach. Environ. Monit. Assess 168, 299–304. [DOI] [PubMed] [Google Scholar]
- Dawson AL, Kawaguchi S, King CK, Townsend KA, King R, Huston WM, Nash SMB, 2018. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun 9, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Torre C, Bergami E, Salvati A, Faleri C, Cirino P, Dawson KA, Corsi I, 2014. Accumulation and embryotoxicity of polystyrene nanoparticles at early stage of development of sea urchin embryos Paracentrotus lividus. Environ. Sci. Technol 48, 12302–12311. [DOI] [PubMed] [Google Scholar]
- Duis K, Coors A, 2016. Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fates, and effects. Environ. Sci. Eur 28, UNSP 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AM, Hume GL, Warren KS, Cook NP, Burns CG, Mohideen MA, Siegal G, Yelon D, Fishman MC, Garrity DM, 2005. Calcium extrusion is critical for cardiac morphogenesis and rhythm in embryonic zebrafish hearts. Proc. Natl. Acad. Sci. USA 102, 17705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eerkes-Medrana D, Thompson RC, Aldridge DC, 2015. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritization of research needs. Water Res. 75, 63–82. [DOI] [PubMed] [Google Scholar]
- Ekvall MT, Lundqvist M, Kelpsiene E, Šileikis E, Gunnarsson SB, Cedervall T, 2019. Nanoplastics formed during the mechanical breakdown of daily-use polystyrene products. Nanoscale Advances. 1(3), 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen M, Lebreton LCM, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, Reisser J, 2014. Plastic pollution in the world’s oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS One. 9(12), e111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Fong T, Chen X, Chen C, Luo P, Xie H, 2018. Glia maturation factor-beta: a potential therapeutic target in neurodegeneration and neuroinflammation. Neuropsychiatr. Dis. Treat 14, 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendall LS, Sewell MA, 2009. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull 58 (8), 1225–1228. [DOI] [PubMed] [Google Scholar]
- Frescas D, Guardavaccaro D, Kuchay SM, Kato H, Poleshko A, Basrur V, Elenitoba-Johnson KS, Katz RA, Pagano M, 2008. KDM2A represses transcription of centromeric satellite repeats and maintains the heterochromatic state. Cell Cycle. 7, 3539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich E, Kueznik T, Samberger C, Roblegg E, Wrighton C, and Pieber TR 2010. Size-dependent effects of nanoparticles on the activity of cytochrome P450 isoenzymes. Toxicol. Appl. Pharmacol 242(3), 326–32. [DOI] [PubMed] [Google Scholar]
- Gigault J, Pedrono B, Maxit B, ter Halle A, 2016. Marine plastic litter: the unanalyzed nano-fraction. Environ. Sci. Nano 3, 346–350. [Google Scholar]
- Gigault J, ter Halle A, Baudrimont M, Pascal P-Y, Gauffre F, Phi T-L, El Hadri H, Grassl B, Reynaud S, 2018. Current opinion: what is a nanoplastic? Environ. Pollut 235, 1030–1034. [DOI] [PubMed] [Google Scholar]
- Gomez P, Perez-Martinez P, Marin C, Camargo A, Yubero-Serrano EM, Garcia-Rios A, Rodriguez F, Delgado-Lista J, Perez-Jimenez F, Lopez-Miranda J, 2010. APOA1 and APOA4 gene polymorphisms influence the effects of dietary fat on LDL particle size and oxidation in healthy young adults. J. Nutr 140, 773–8. [DOI] [PubMed] [Google Scholar]
- Gopinath PM, Saranya V, Vijayakumar S, Meera MM, Ruprekha S, Kunal R, Pranay A, Thomas J, Mukherjee A, Chandrasekaran N, 2019. Assessment on interactive prospectives of nanoplastics with plasma proteins and the toxicological impacts of virgin, coronated and environmentally released-nanoplastics. Sci Rep. 9, 8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greven AC, Merk T, Karagoz F, Mohr K, Klapper M, Jovanovic B, Palic D, 2016. Polycarbonate and polystyrene nanoplastic particles act as stressors to the innate immune system of fathead minnow (Pimephales promelas). Environ. Toxicol. Chem 35, 3093–3100. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Arimura T, Itoh-Satoh M, Ueda K, Hohda S, Inagaki N, Takahashi M, Hori H, Yasunami M, Nishi H, Koga Y, Nakamura H, Matsuzaki M, Choi BY, Bae SW, You CW, Han KH, Park JE, Knoll R, Hoshijima M, Chien KR, Kimura A, 2004. Tcap gene mutations in hypertrophic cardiomyopathy and dilated cardiomyopathy. J. Am. Coll. Cardiol 44, 2192–201. [DOI] [PubMed] [Google Scholar]
- Heise C, Taha E, Murru L, Ponzoni L, Cattaneo A, Guarnieri FC, Montani C, Mossa A, Vezzoli E, Ippolito G, Zapata J, Barrera I, Ryazanov AG, Cook J, Poe M, Stephen MR, Kopanitsa M, Benfante R, Rusconi F, Braida D, Francolini M, Proud CG, Valtorta F, Passafaro M, Sala M, Bachi A, Verpeli C, Rosenblum K, Sala C, 2017. eEF2K/eEF2 pathway controls the excitation/inhibition balance and susceptibility to epileptic seizures. Cereb. Cortex 27, 2226–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez RE, Galitan L, Cameron J, Goodwin N, Ramakrishnan L, 2018. Delay of initial feeding of zebrafish larvae until 8 days postfertilization has no impact on survival or growth through the juvenile stage. Zebrafish. 15(5), 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm TH, Isaksen TJ, Glerup S, Heuck A, Bottger P, Fuchtbauer E-M, Nedergaard S, Nyengaard JR, Andreasen M, Nissen P, Lykke-Hartmann K, 2016. Cognitive deficits caused by a disease-mutation in the alpha3 Na+/K+-ATPase isoform. Sci. Rep 6, 31972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroia CF, Torrance J, Berthelot C, 2013. The zebrafish reference genome sequences and its relationship to the human genome. Nature. 496, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huillard E, Ziercher L, Blond O, Wong M, Deloulme J-C, Souchelnytskyi S, Baudier J, Cochet C, Buchou T, 2010. Disruption of CK2beta in embryonic neural stem cells compromises proliferation and oligodendrogenesis in the mouse telencephalon. Mol. Cell Biol 30, 2737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JS, Kennedy CJ 2017. Regulation of hepatic abcb4 and cyp3a65 gene expression and multidrug/multixenobiotic resistance (MDR/MXR) functional activity in the model teleost, Danio rerio (zebrafish). Comp. Biochem. Physiol. C. Toxicol. Pharmacol 200, 34–41. [DOI] [PubMed] [Google Scholar]
- Jin Y, Lu L, Tu W, Luo T, Fu Z, 2019. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ 649, 308–317. [DOI] [PubMed] [Google Scholar]
- Joäls M, Sarabdjitsingh R, Karst H, 2012. Unraveling the time domains of corticosteroid hormone influences on brain activity: Rapid, slow, and chronic modes. Pharmacol. Rev 64, 901–938. [DOI] [PubMed] [Google Scholar]
- Joäls M, 2018. Corticosteroids and the brain. J. Endocrinol 238, R121–R130. [DOI] [PubMed] [Google Scholar]
- Johnston IA, Bower NI, Macqueen DJ, 2011. Growth and the regulation of myotomal muscle mass in teleost fish. J. Exp. Biol 214, 1617–1628. [DOI] [PubMed] [Google Scholar]
- Karami A, Romano N, Galloway T, Hamzah H, 2016. Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environ. Res 151, 58–70. [DOI] [PubMed] [Google Scholar]
- Kim H, Heo K, Kim JH, Kim K, Choi J, An W, 2009. Requirement of histone methyltransferase SMYD3 for estrogen receptor-mediated transcription. J. Biol. Chem 284, 19867–19877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelmans AA, Nesseling E, Shim WJ, 2015. Nanoplastics in the aquatic environment. Critical Review In: Bergmann M, Gutow L, Klages M (eds) Marine Anthropogenic Litter. Springer, Cham. [Google Scholar]
- Kosuth M, Mason SA, Wattenberg EV, 2018. Anthropogenic contamination of tap water, beer, and sea salt. Plos One. 13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MG, Rowley S, Fulton R, Dinday MT, Baraban SC, Patel M, 2016. Altered glycolysis and mitochondrial respiration in a zebrafish model of Dravet Syndrome. eNeuro. 3, e0008–16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Wagner M, 2016. Characterization of nanoplastics during the degradation of polystyrene. Chemo. 145, 265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Gopal DJ, Kim J, Saleem SN, Silhavy JL, Louie CM, Thacker BE, Williams Y, Zaki MS, Gleeson JG, 2011. Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert Syndrome. Nat. Med 17, 726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre CB, Gwaltney-Bran S, McCrackin MA, Meyer R, 2013. AVMA Guidelines for the Euthanasia of Animals (2013 Edition). American Veterinary Medical Association, Schaumberg, IL. [Google Scholar]
- Lee LL, Liu MT, Song Y, Lu SB, Hu JN, Cao CJ, Xie B, Shi HH, He DF, 2018. Polystyrene (nano)microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environ. Sci-Nano 5(8), 2009–2020. [Google Scholar]
- Lefebvre JL, Ono F, Puglielli C, Seidner G, Franzini-Armstrong C, Brehm P, Granato M, 2004. Increased neuromuscular activity causes axonal defects and muscular degeneration. Development. 131, 2605–2618. [DOI] [PubMed] [Google Scholar]
- LeMoine CMR, Kelleher BM, Lagarde R, Northam C, Elebute OO, Cassone BJ, 2018. Transcriptional effects of polyethylene microplastics ingestion in developing zebrafish (Danio rerio). Environ. Pollut 243(A), 591–600. [DOI] [PubMed] [Google Scholar]
- Lenz R, Enders K, Nielsen TG, 2016. Microplastic exposure studies should be environmentally realistic. Proceedings of the National Academy of Sciences, 113(29), E4121–E4122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Cerutti DT, 2009. Behavioral neuroscience of zebrafish In Buccafusco JJ (Eds.), Methods of Behavior Analysis in Neuroscience (2nd ed.). CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- Li WC, Tse HF, Fok L, 2016. Plastic waste in the marine environment: a review of sources, occurrences and effects. Sci. Tot. Environ 566, 333–349. [DOI] [PubMed] [Google Scholar]
- Liao S, Gan L, Lv L, Mei Z, 2020. The regulatory roles of aquaporins in the digestive system. Genes & Diseases. In press. 10.1016/j.gendis.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limonta G, Mancia A, Benkhalqui A, Bertolucci C, Abelli L, Fossi MC, Panti C, 2019. Microplastics induce transcriptional changes, immune response and behavioral alterations in adult zebrafish. Sci Rep. 9(1),15775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-MJ, Hsin I-L, Sun HS, Lin S, Lai Y-L, Chen H-Y, Chen T-Y, Chen Y-P, Shen Y-T, Wu H-M, 2018. NTF3 is a novel target gene of the transcription factor POU3F2 and is required for neuronal differentiation. Mol. Neurobiol 55, 8403–8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie CM, Gleeson JG, 2005. Genetic basis of Joubert Syndrome and related disorders of cerebellar development. Hum. Mol. Genet 14, R235–42. [DOI] [PubMed] [Google Scholar]
- Low JKK, Webb SR, Silva APG, Saathoff H, Ryan DP, Torrado M, Brofelth M, Parker BL, Shepherd NE, Mackay JP, 2016. CHD4 is a peripheral component of the nucleosome remodeling and deacetylase complex. J. Biol. Chem 291, 15853–15866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YF, Zhang Y, Deng YF, Jiang W, Zhao Y, Gend JJ, Ding LL, Ren HQ, 2016. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Tech 50(7), 4054–4060. [DOI] [PubMed] [Google Scholar]
- MacPhail RC, Brooks J, Hunter DL, Padnos B, Irons T, Padilla S, 2008. Locomotion in larval zebrafish: influence of time of day, lighting and ethanol. Neurotoxicology. 30, 52–58. [DOI] [PubMed] [Google Scholar]
- Manabe M, Tatarazako N, Kinoshita M, 2011. Uptake, excretion and toxicity of nano-sized latex particles on medaka (Oryzias latipes) embryos and larvae. Aquat. Toxicol 105 (3), 576–581. [DOI] [PubMed] [Google Scholar]
- Mattsson K, Ekvall MT, Hannsson LA, Linse S, Malmendal A, Cedervall T, 2014. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Env. Sci. Technol 49, 553–561. [DOI] [PubMed] [Google Scholar]
- Mattsson K, Johnson EV, Malmendal A, Linse S, Hansson LA, Cedervall T, 2017. Nature. Brain damage and behavioral disorders in fish induced by plastic nanoparticles delivered through the food chain. Nature. 7, 11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr K, Sommer M, Baier G, Schottler S, Okwieka P, Tenzer S, Landfester K, Mailander V, Schmidt MDP, Meyer RG, 2014. Aggregation behavior of polystyrene-nanoparticles in human blood serum and its impacts on the in vivo distribution in mice. J. Nanomed. Nanotechnol 5, 2. [Google Scholar]
- Mostert JC, Shumskaya E, Mennes M, Onnink AMH, Hoogman M, Kan CC, Vasquez AA, Buitelaar J, Franke B, Norris DG, 2016. Characterising resting-state functional connectivity in a large sample of adults with ADHD. Prog. Neuropsychopharmacol. Biol. Psychiatry 67, 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi R, Kubota H, Yugi K, Toyoshima Y, Komori Y, Soga T, Kuroda S, 2013. The selective control of glycolysis, gluconeogenesis, and glycogenesis by temporal insulin patterns. Mol. Syst. Biol 9, 664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obbard RW, Sadri S, Wong YQ, Khitin AA, Baker I, Thompson RC, 2014. Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth’s Future. 2, 315–320. [Google Scholar]
- Oliveira M, Ribeiro A, Hylland K, Guilhermino L, 2013. Single and combined effects of microplastics and pyrene on juveniles (0+group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecological Indicators 34, 641–647. [Google Scholar]
- Palmér T, Ek F, Enqvist O, Olsson R, Astrom K, Petersson P, 2017. Action sequencing in the spontaneous swimming behavior of zebrafish larvae - implications for drug development. Sci Rep. 7, 3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti CC, Ghilardi A, Della Torre C, Magni S, Del Giacco L, Binelli A 2019. Evaluation of the infiltration of polystyrene nanobeads in zebrafish embryo tissues after short-term exposure and the related biochemical and behavioural effects. Environ. Pollut 254, 112947. [DOI] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, Huganir RL, Linden DJ, Worley PF, 2008. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 59, 70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso JAB, Ramos-Lobo AM, Donato J Jr., 2019. SOCS3 as a future target to treat metabolic disorders. Hormones (Athens). 18, 127–136. [DOI] [PubMed] [Google Scholar]
- Pilarowski GO, Vernon HJ, Applegate CD, Boukas L, Cho MT, Gurnett CA, Benke PJ, Beaver E, Heeley JM, Medne L, Krantz ID, Azage M, Niyazov D, Henderson LB, Wentzensen IM, Baskin B, Guillen Sacoto MJ, Bowman GD, Bjornsson HT, 2018. Missense variants in the chromatin remodeler CHD1 are associated with neurodevelopmental disability. J. Med. Genet 55, 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt JA, Kozal JS, Jayasundara N, Massarsk A, Trevisan R, Geitner N, Wiesner M, Levin ED, Di Giulio RT, 2018. Uptake, tissue distribution, and toxicity of polystyrene nanoplastics in developing zebrafish (Danio rerio). Aquat. Tox 194, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivonsky M, Cermakova L, Novotna K, Peer P, Cajthaml T, Janda V, 2018. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ 643, 1644–1651. [DOI] [PubMed] [Google Scholar]
- Plastics – the Facts 2018 An Analysis of European Plastics Production, Demand and Waste Data. 2018. PlasticsEurope; URL https://www.plasticseurope.org/application/files/6315/4510/9658/Plastics_the_facts_2018_AF_web.pdf. [Google Scholar]
- Pray-Grant MG, Daniel JA, Schieltz D, Yates JR 3rd, Grant PA, 2005. Chd1 chromodomain links histone H3 methylation with SAGA- and SLIK-dependent acetylation. Nature. 433, 434–8. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2019. R: A language and environmental for statistical computing. R foundation for statistical computing, Vienna, Austria: URL http://www.R-project.org/. [Google Scholar]
- Ribeiro F, Garcia AR, Pereira BP, Fonseca M, Mestre NC, Fonseca TG, Ilharco LM, Bebianno MJ, 2017. Microplastics effects in Scrobicularia plana. Mar. Poll. Bull 122(1-2), 379–391. [DOI] [PubMed] [Google Scholar]
- Rochman CM, Kurobe T, Flores I, Teh SJ, 2014. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ 493, 656–661. [DOI] [PubMed] [Google Scholar]
- Rossor AM, Sleigh JN, Groves M, Muntoni F, Reilly MM, Hoogenraad CC, Schiavo G, 2020. Loss of BICD2 in muscle drives motor neuron loss in a developmental form of spinal muscular atrophy. Acta Neuropathol. Commun 8, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saillour Y, Zanni G, Des Portes V, Heron D, Guibaud L, Iba-Zizen MT, Pedespan JL, Poirier K, Castelnau L, Julien C, Franconnet C, Bonthron D, Porteous ME, Chelly J, Bienvenu T, 2007. Mutations in the AP1S2 gene encoding the sigma 2 subunit of the adaptor protein 1 complex are associated with syndromic X-linked mental retardation with hydrocephalus and calcifications in basal ganglia. J. Med. Genet 44, 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasamma S, Audira G, Siregar P, Malhotra N, Lai Y-H, Liang S-T, Chen J-R, Chen KH-C, Hsiao C-D, 2020. Nanoplastics cause neurobehavioral impairments, reproductive and oxidative damages, and biomarker responses in zebrafish: Throwing up alarms of wide spread health risk of exposure. Int. J. Mol Sci 21(4), 1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirinzi GF, Pérez-Pomeda I, Sanchís J, Rossini C, Farré Marinella, Barceló Damià, 2017. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res 159, 579–587. [DOI] [PubMed] [Google Scholar]
- Schur C, Rist S, Baun A, Mayer P, Hartmann NB, Wagner M, 2019. When fluorescence is not a particle: The tissue translocation of microplastics in Daphnia magna seems an artifact. Environ. Toxicol. Chem 38, 1495–1503. [DOI] [PubMed] [Google Scholar]
- Shin D-J, Campos JA, Gil G, Osborne TF, 2003. PGC-1alpha activates CYP7A1 and bile acid biosynthesis. J. Biol. Chem 278, 50047–50052. [DOI] [PubMed] [Google Scholar]
- Skjolding LM, Ašmonaitė’ G, Jølck RI, Andresen TL, Selck H, Baun A, Sturve J, 2017. An assessment of the importance of exposure routes to the uptake and internal localization of fluorescent nanoparticles in zebrafish (Danio rerio), using light sheet microscopy. Nanotoxicology 11 (3), 351–359. [DOI] [PubMed] [Google Scholar]
- Solanki M, Pointon A, Jones B, Herbert K, 2018. Cytochrome P450 2J2: Potential role in drug metabolism and cardiotoxicity. Drug Metab. Dispos 46, 1053–1065. [DOI] [PubMed] [Google Scholar]
- Steele SL, Lo KHA, Li VWT, Cheng SH, Ekker M, Perry SF, 2009. Loss of M2 muscarinic receptor function inhibits development of hypoxic bradycardia and alters cardiac β-adrenergic sensitivity in larval zebrafish (Danio rerio). Am. J. Physiol. Regul. Integr. Comp. Physiol 297, R412–20. [DOI] [PubMed] [Google Scholar]
- Sun HS, Martin JA, Werner CT, Wang Z-J, Damez-Werno DM, Scobie KN, Shao N-Y, Dias C, Rabkin J, Koo JW, Gancarz AM, Mouzon EA, Neve RL, Shen L, Dietz DM, Nestler EJ, 2016. BAZ1B in nucleus accumbens regulates reward-related behaviors in response to distinct emotional stimuli. J. Neurosci 36, 3954–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussarellu R, Suquet M, Thomas Y, Lambert C, Fabioux C, Pernet MEJ, Le Goic N, Quillien V, Mingrant C, Epelboin Y, Corporeau C, Guyomarch J, Robbens J, Paul-Pont I, Soudant P, Huvent A, 2016. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. U.S.A 113 (9), 2430–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai HH, Geisterfer M, Bell JC, Moniwa M, Davie JR, Boucher L, McBurney MW, 2003. CHD1 associates with NCoR and histone deacetylase as well as with RNA splicing proteins. Biochem. Biophys. Res. Commun 308, 170–6. [DOI] [PubMed] [Google Scholar]
- Ter Halle A, Jenneau L, Martignac M, Jarde E, Pedrono B, Brach L, Gigault J, 2017. Nanoplastics in the North Atlantic Subtropical Gyre. Environ. Sci. Tech 51, 13689–13697. [DOI] [PubMed] [Google Scholar]
- Thomas EA, D’Mello SR, 2018. Complex neuroprotective and neurotoxic effects of histone deacetylases. J. Neurochem 145, 96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui N, Watanabe K, Ono K, Tomita K, Tamamaki N, Ikenaka K, Takebayashi H, 2012. Role of motoneuron-derived neurotrophin 3 in survival and axonal projection of sensory neurons during neural circuit formation. Development. 139, 1125–1132. [DOI] [PubMed] [Google Scholar]
- Van Pomeren M, Brun NR, Peijnenburg WJGM, Vijver MG, 2017. Exploring uptake and biodistribution of polystyrene (nano)particles in zebrafish embryos at different developmental stages. Aquat. Toxicol 190, 40–45. [DOI] [PubMed] [Google Scholar]
- Veneman WJ, Spaink HP, Brun NR, Bosker T, Vijver MG, 2017. Pathway analysis of systemic transcriptome responses to injected polystyrene particles in zebrafish larvae. Aquat. Toxicol 190, 112–120. [DOI] [PubMed] [Google Scholar]
- von Moos N, Burkhardt-Holm P, Kohler A, 2012. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis after an experimental exposure. Environ. Sci.Technol 46, 11327–11335. [DOI] [PubMed] [Google Scholar]
- Wang M, Yu H, Kim YS, Bidwell CA, Kuang S, 2012. Myostatin facilitates slow and inhibits fast myosin heavy chain expression during myogenic differentiation. Biochem. Biophys. Res. Commun 426, 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZF, Lin T, Chen W, 2020. Occurrence and removal of microplastics in advanced drinking water treatment plant. Sci. Tot. Environ 700, UNSP 124529. [DOI] [PubMed] [Google Scholar]
- Weiss K, Terhal PA, Cohen L, Bruccoleri M, Irving M, Martinez AF, Rosenfeld JA, Machol K, Yang Y, Liu P, Walkiewicz M, Beuten J, Gomez-Ospina N, Haude K, Fong C-T, Enns GM, Bernstein JA, Fan J, Gotway G, Ghorbani M, DDD Study, van Gassen K, Monroe GR, van Haaften G, Basel-Vanagaite L, Yang X-J, Campeau PM, Muenke M, 2016. De novo mutations in CHD4, an ATP-dependent chromatin remodeler gene, cause an intellectual disability syndrome with distinctive dysmorphisms. Am. J. Hum. Genet 99, 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelaar Bonga SE, 1997. The stress response in fish. Physiol. Rev 77, 591–625. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Hooda PS, Barker J, Barton S, Swiden J, 2015. Ecotoxic pharmaceuticals, personal care products, and other emerging contaminants: a review of environmental, report-mediated, developmental, and epigenetic toxicity with discussion proposed toxicity to humans. Crit. Rev. Env. Sci. Tec 46, 336–381. [Google Scholar]
- World Health Organization (WHO). 2019. Microplastics in drinking water Licence: CCBY-NC-SA3.0IGO;ISBN 978-92-4-151619-8.
- Wright SL, Rowe D, Thompson RC, Galloway TS, 2013. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol 23, R1031–R1033. [DOI] [PubMed] [Google Scholar]
- Wu B, Wu XM, Liu S, Wang ZZ, Chen L, 2019. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells. Chemosphere. 221, 333–341. [DOI] [PubMed] [Google Scholar]
- Yang C-P, Li X, Wu Y, Shen Q, Zeng Y, Xiong Q, Wei M, Chen C, Liu J, Huo Y, Li K, Xue G, Yao Y-G, Zhang C, Li M, Chen Y, Luo X-J, 2018. Comprehensive integrative analyses identify GLT8D1 and CSNK2B as schizophrenia risk genes. Nat. Commun 9, 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PJ, Morsch R, Callahan LM, Coleman PD, 1999. Changes in synaptic expression of clathrin assembly protein AP180 in Alzheimer’s disease analyzed by immunohistochemistry. Neuroscience. 94, 389–94. [DOI] [PubMed] [Google Scholar]
- Yi X, Chi T, Li Z, Wang J, Yu M, Wu M, Zhou H, 2019. Combined effects of polystyrene plastics and triphenyltin chloride on the green algae Chlorella pyrenoidosa. Environ. Sci. Pollut. R 26(15), 15011–15018. [DOI] [PubMed] [Google Scholar]
- Yonekawa Y, Harada A, Okada Y, Funakoshi T, Kanai Y, Takei Y, Terada S, Noda T, Hirokawa N, 1998. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J. Cell. Biol 141, 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong CQY, Valiyaveetill S, Tang BL, 2020. Toxicity of microplastics and nanoplastics in mammalian systems. Int. J. Environ. Res. Public Health 17(5), 1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F-F, Gu X, Huang X, Zhong Y, Wu J, 2017. SLC6A1 gene involvement in susceptibility to attention-deficit/hyperactivity disorder: A case-control study and gene environment interaction. Prog. Neuropsychopharmacol. Biol. Psychiatry 77, 202–208. [DOI] [PubMed] [Google Scholar]
- Yuan H, Wang Q, Li Y, Cheng S, Liu J, Liu Y, 2020. Concurrent pathogenic variants in SLC6A1/NOTCH1/PRIMPOL genes in a Chinese patient with myoclonic-atonic epilepsy, mild aortic valve stenosis and high myopia. BMC Med. Genet 21, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghlool A, Halvardson J, Zhao JJ, Etemadikhah M, Kalushkova A, Konska K, Jernberg-Wiklund H, Thuresson A-C, Feuk L, 2016. A role for the chromatin-remodeling factor BAZ1A in neurodevelopment. Hum. Mutat 37, 964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.