Abstract
Measles caused an estimated minimum of one million fatalities annually before vaccination. Outstanding progress towards controlling the virus has been made since the measles vaccine was introduced, but reduction of measles case-fatalities has stalled at around 100,000 annually for the last decade and a 2019 resurgence in several geographical regions threatens some of these past accomplishments. Whereas measles eradication through vaccination is feasible, a potentially open-ended endgame of elimination may loom. Other than doubling-down on existing approaches, is it worthwhile to augment vaccination efforts with antiviral therapeutics to solve the conundrum? This question is hypothetical at present, since no drugs have yet been approved specifically for the treatment of measles, or infection by any other pathogen of the paramyxovirus family. This article will consider obstacles that have hampered anti-measles and anti-paramyxovirus drug development, discuss MeV-specific challenges of clinical testing, and define drug properties suitable to address some of these problems.
Keywords: measles, antiviral, treatment, vaccination, RNA-dependent RNA polymerase
Challenges to paramyxovirus drug development
Paramyxoviruses are negative polarity RNA viruses that are collectively a major threat to human and animal health. In addition to measles virus (MeV), the family includes other highly contagious infectious agents such as mumps virus, the human parainfluenza viruses (HPIVs), and the highly pathogenic, zoonotic Hendra and Nipah viruses. The global Measles & Rubella Initiative started in 2001 was extraordinarily successful in lowering measles deaths in the first years after launch, until reaching the current status quo of approximately 100,000 case-fatalities per year in 2007. Despite the availability of effective vaccine prophylaxis, MeV therefore remains a leading cause of mortality due to respiratory infectious diseases. Overall measles case numbers have reached a decade-high in 2019 [1].
Considering the major health and economic threats posed by paramyxoviruses and lack of vaccine protection against, for instance, the parainfluenza viruses or henipaviruses, it may seem surprising that no antiviral drug has been specifically developed yet against any member of the family. However, there are several obstacles to paramyxovirus drug development: i) because the viruses replicate rapidly and cause mostly acute disease, narrow windows of opportunity for therapeutic intervention are anticipated; ii) major clinical signs often reflect immunopathogenesis and viral load is already in decline shortly after disease manifestation; iii) the size of treatable patient groups will likely be limited despite high disease prevalence, restricting economic potential; iv) the predominantly pediatric patient population of paramyxovirus diseases complicates the design of clinical efficacy trials; and v) further exacerbating the problem, a human challenge model has not yet been established for any paramyxovirus.
The recent resurgence of measles has nevertheless initiated discussion as to whether therapeutics directed specifically against MeV could augment vaccination and aid in measles case management [2,3]. Although the above developmental obstacles are in principle applicable to most paramyxoviruses, the discrete mechanism of MeV host invasion and pathogenesis must be appreciated when defining a hypothetical drug product profile.
MeV host invasion and clinical signs of measles
MeV spreads through the respiratory route, originally infecting CD150-positive alveolar macrophages and dendritic cells in the respiratory tract [4], which subsequently home to the draining lymph nodes where initial centers of viral replication are established [5]. Mediated by virus dissemination via infected lymphocytes, systemic infection through cell-associated viremia ensues that ultimately results in basolateral reentry of the virus into respiratory tissues through the Nectin-4 receptor [6,7], viral release into the lumen of the respiratory tract [8,9,10], and discharge of lipid droplets containing MeV particles. The incubation period from first host invasion to the prodromal phase characterized by increasing fever, cough, coryza or conjunctivitis is approximately 10-12 days, and the characteristic maculopapular rash develops on average 14 days after exposure [11].
First introduced in 1963, the measles vaccine is safe and efficacious, and is estimated to have prevented over 21 million measles death in the time period from 2000-2017 alone [12]. However, MeV remains responsible for major morbidity worldwide and estimated global measles deaths have hovered around 100,000 per year since 2007 [13]. Two factors in particular drive the continued health threat posed by MeV: the virus is exceptionally contagious and infection results in immunosuppression of the host that can last for several years.
Impact of high MeV infectivity
The primary basic reproduction number R0 of MeV is estimated to be 12-18 [14], although anecdotal evidence suggests that actual R0 values can be much higher [15]. This implies that a primary measles patient infects on average 12-18 contacts, making MeV one of the most contagious infectious agents identified to date [16]. For comparison, R0 numbers of other respiratory viruses range from 1.2-2.1 for respiratory syncytia virus (RSV) [17] and 1.4-2.8 for the 1918 H1N1 influenza virus that caused the Spanish flu pandemic [18]. Due to this exceptionally high infectivity, a herd immunity of approximately 95% is required to prevent sporadic measles outbreaks in a population [19]. Since this high level of immunity cannot be achieved in a population with a single dose of the vaccine, the immunocompromised are not eligible for vaccination, and vaccine failures occur, essentially every child must receive two doses of the vaccine, currently recommended to be administered in the United States at 12-15 months and 4-6 years of age [11].
Parental opposition to vaccination due to religious beliefs or safety concerns has created pockets of low herd immunity in the United States that have fueled large local measles outbreaks in 2019 [20]. Vaccination coverage has remained below eradication targets in large parts of the European region and for the first time four countries, Albania, Czechia, Greece and the United Kingdom, have lost their measles elimination status [21]. Due to limited access to vaccination, measles activity remains furthermore high in Sub-Saharan Africa and parts of the Western Pacific region. For the first half of 2019, the WHO reported a 10-fold increase of cases in the African region, 2-fold increase in the European region, and 3-fold increase in the Western Pacific region compared to 2018 [1].
MeV-induced immunosuppression
Within the paramyxovirus family, MeV is a member of the morbillivirus genus. Characteristic for morbillivirus infections is the induction of long-lasting immunosuppression that has been well documented for MeV [22,23,24] and animal morbilliviruses such as canine distemper virus (CDV) [25,26]. Almost all animal morbilliviruses recognize species homologs of human CD150, consistent with the characteristic lymphotropism of most pathogens in the genus. In addition to macrophages and dendritic cells, the CD150 receptor is highly expressed on memory T and B cells and plasma cells, and shortly after infection a phase of transient lymphocytopenia ensues [27]. Although peripheral lymphocyte counts are restored 2-4 weeks after viral clearance and overall IgG levels do not decline during measles [28], immunosuppression typically lasts for several months, but was still detectable in a subset of recoverees 5 years after the primary disease [24,29].
A paradox therefore exists between the effective adaptive anti-MeV immune response mounted by measles patients, which ultimately clears the infection and results in lasting humoral protection against reinfection, and the loss of immune memory against other pathogens. Neutralizing anti-MeV antibodies are predominantly directed against the viral attachment protein [30,31,32]. Two recent studies have concluded that a state of measles-induced humoral immune amnesia may arise from MeV depletion specifically of memory B cell populations, compromising previously acquired immunity and potentially exposing measles recoverees to unrelated future infections [24,25]. Consistent with this interpretation, assessment of population-level data from several high-income countries has revealed that measles outbreaks are linked to mortality from unrelated infectious diseases in the following 2-3 years [24,33].
Possible benefits of therapeutics for measles control
Because measles is predominantly an immunopathogenic disease and virus load drops rapidly with the onset of rash, the window of opportunity for the treatment of established disease must be extremely narrow, if existent at all. The greatest health benefit can consequently be expected from post-exposure prophylaxis (PEP), especially since administration of the measles vaccine after infection is largely ineffective unless given within the first hours after exposure (figure 1) [34].
Figure 1.
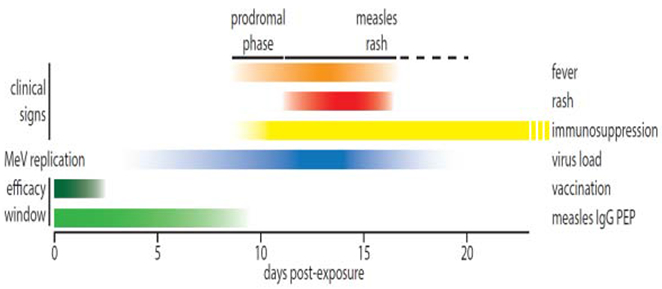
Timeline of MeV load, clinical signs (fever and rash), and immunosuppression phase during acute measles based on [59]. Approximate efficacy cut-offs for post-exposure vaccination and measles IgG PEP are shown.
Providing proof-of-concept for PEP efficacy, passive immunization with measles immunoglobulins (measles IgG) prevents measles when given within six days of contact [35], and administration up to nine days post infection still reduces subsequent measles symptoms [35,36]. Since no alternative therapies currently exist, the German Standing Committee on Vaccination (STIKO), for instance, recommends that measles IgG PEP is administered intravenously to high-risk groups such as infants below 6 months of age, susceptible pregnant women, and immunocompromised patients ineligible for measles vaccination, preferentially within 6 days after the alleged exposure [37]. However, the availability of measles IgG is limited, especially since MeV neutralizing antibody titers have declined since measles immunity of human plasma donors has become predominantly vaccine acquired [38]. Individual IgG batches are not standardized, the product is cost-intensive, deployment requires a cold chain, and intravenous treatment must be administered by trained healthcare professionals under sterile conditions. These limitations compromise application to lower risk groups in high-income countries and essentially exclude IgG-based PEP for low and middle-income countries, in which measles case numbers are highest.
To respond to the needs of high- and low-income country populations alike, a viable replacement of the problematic IgG component of measles PEP cannot compromise on four fundamental demands: the drug product must be amenable to cost-effective manufacture, shelf-stable at ambient temperature, orally bioavailable, and safe for prophylactic use in children. Of the three major therapeutic classes – biopharmaceuticals, synthetic therapeutic peptides, and chemically synthesized small-molecule (SMOL) pharmaceuticals – only SMOLs, which currently comprise over 80% of licensed therapeutics, have the potential to meet each of these requirements [39].
As a second conceivable indication in addition to use in PEP, a measles therapeutic may be beneficial for improved management of validated, rare measles complications such as acute demyelinating encephalomyelitis (ADEM) [40], measles inclusion body encephalitis (MIBE) [41], and subacute sclerosing panencephalitis (SSPE) [42,43]. Of these, MIBE and SSPE are associated with prolonged phases of viral replication in the brain, and both are ultimately fatal due to neurological damage. Whereas MIBE develops within two to six months of primary measles [44,45], SSPE typically occurs years after measles and disease progression after first manifestation varies among patients from rapid advance to chronic encephalitis. Approximately 95% of patients die within three years of SSPE diagnosis [46], although aggressively advancing SSPE is fatal on average within three months. MIBE is concentrated in the immunocompromised. In contrast, no specific conditions predisposing for SSPE have been identified, although males are affected with a 3:1-ratio over females [46]. Experimental treatments of SSPE with broad-spectrum antivirals such as ribavirin with or without α-interferon have been attempted. However, results were mixed leaving the ultimate impact on disease outcome unclear [47,48,49,50,51,52,53,54,55] and side effects of the regimen are severe [56,57]. Provided effective drug concentrations can be reached in the CNS, a blood-brain barrier permissive MeV-specific pharmaceutical may better slow SSPE and MIBE disease progression and prolong life than the tested broad-spectrum candidates that have shown only moderate activity against MeV in cell culture tests [58].
Lastly, the overproportional health benefit of the measles vaccine beyond suppression of direct measles deaths has highlighted the major impact of measles immunosuppression on heightened population susceptibility to other infectious agents [24,33]. Accordingly, the health impact of measles drugs would expand tremendously if the degree of MeV-induced immunosuppression could be alleviated or its duration shortened when therapy is started after first appearance of clinical signs. Reflecting a knowledge gap in therapeutic measles management, efficacy evaluation of measles IgG treatment has predominantly concentrated on suppression of primary measles and little information is available concerning alleviation of long-term measles complications. With a better appreciation of the importance of measles immunosuppression for public health, determining the latest time point after infection at which measles immunosuppression can be successfully modulated has emerged as an important question in morbillivirus pathogenesis and drug development.
Developmental obstacles define a desirable measles drug product profile
A viable path to cost-effective manufacture must be paramount to measles drug development, in particular since measles PEP in low-income countries with high measles prevalence or local outbreaks is anticipated to be a major application of the product. SMOLs best respond to this request, since they can typically be produced in existing facilities, using established methods and available starting materials. Shelf stability and oral bioavailability can furthermore often be optimized if necessary through synthetic chemotype and/or formulation development [39], addressing three of the discussed fundamental measles drug profile requests. By comparison, biopharmaceuticals and synthetic peptide-type drugs are high cost, often require dedicated production facilities, and are typically not orally available.
However, the concerns remain that realistically the economic opportunity of a measles drug will be limited and that clinical testing will be challenging. In particular, clinical trial design may pose a major obstacle due to geographical disease prevalence and age distribution of measles patients. Phase 1 safety trials of drug candidates can certainly be conducted at established trial sites enrolling healthy adult volunteers and following well-developed procedures. However, no human volunteer challenge model can be established for measles due to disease severity and widespread anti-measles immunity in adults. Natural disease occurs even in Western European regions with continued endemic transmission in irregular sweeps of MeV through a population, infecting a large portion of the immunologically naive and then retreating until a sufficiently large pool of susceptible people has been restored [59]. Outbreaks originating from virus importation into susceptible local communities, as experienced in the United States since the interruption of endemic transmission, are even harder to predict. A suitable site with predictable patient population for phase 2 efficacy testing can therefore not easily be identified in Western Europe or North America, but rather would need to be located in an area with low measles herd immunity such as Sub-Saharan Africa.
Exacerbating the problem, measles is a pediatric disease and disease burden in Sub-Saharan countries such as the DRC is highest in the one to nine years of age group [60]. Because minors cannot volunteer for a clinical trial or be enrolled by their guardians, the only ethically acceptable format of trial design would be that participation has some possibility of providing a health benefit to the enrollee. If candidates comprise children with confirmed, symptomatic measles at the time of enrollment, alleviation of standard clinical signs such as fever or rash cannot reasonably be used as primary endpoints for the reasons discussed, leaving objectives undefined. Testing a clinical candidate for performance in measles PEP would solve the endpoint problem, but actual infection with MeV would not be confirmed at the time of enrollment, and measles vaccination rather than experimental treatment should first be offered to all without measles immunity seeking medical care after a possible exposure.
Therapeutic candidates with a broadened anti-paramyxovirus activity spectrum could solve the conundrum, provided antiviral activity extends to a member of the family that causes predictable disease in adult patients. Paramyxoviruses of the respirovirus genus such as the HPIVs type 1 and 3 (HPIV1 and HPIV3), for instance, are currently not vaccine-preventable and are associated with major morbidity [61,62]. HPIV3 specifically infects up to 18% of hematopoietic stem cell transplant patients [63], of which 20-40% progress to lower respiratory infection within a median time of 78 days. Case fatality rates are devastatingly high, comprising approximately 10% after upper respiratory infection and increasing to 27% when viral pneumonia is acquired [64,65,66,67]. In addition to major clinical need and a perceived larger window of opportunity for treatment, transplant recipients are subject to close monitoring, maximizing the prospect of early detection of infection. Although no HPIV3-specific treatment is currently available, novel drug candidates such as DAS181 [68] and off-label use of ribavirin [69] have been explored, establishing proof-of-concept that clinical tests can be conducted in this patient group. A broadened antiviral activity spectrum that promises to benefit different patient groups may furthermore better offset the cost of drug development and licensing.
Traditionally, broad-spectrum activity is predominantly associated with two classes of antivirals, host-directed drugs that interfere with host pathways or factors commonly exploited by a number of viruses for their replication [70,71,72,73,74,75], and broad-spectrum ribonucleoside analogs or their precursors such as ribavirin or T-705 [76,77,78]. In fact, off-label use of ribavirin alone or in combination with pegylated IFNα against measles was explored clinically, but outcomes were mixed [48,49,51,79,80] and ribavirin suffers from serious toxicity liabilities such as hemolytic anemia [56,57]. For use in measles PEP, broad-spectrum inhibitors are extremely unlikely to ultimately meet the safety margins demanded by a pediatric patient population. Better suited for this indication are well-behaved direct-acting antivirals that specifically block a distinct viral protein or function and therefore do not inherently interfere with a host activity (in contrast to host-directed antivirals) or affect an analogous host activity (i.e. incorporation of ribonucleoside analogs by host polymerases).
Status of experimental small-molecule measles therapeutics
A number of experimental SMOLs have been implicated with anti-measles activity in cell culture. Chemically, these compounds represent diverse classes such as fully synthetic molecules, natural product-derived substances, and antisense sequence molecules. The states of individual candidates has been reviewed before in detail [81,82]. To summarize the previous discussion, most of these substances were either only moderately active, cytotoxic, inactive when added post-infection in cell culture, or the active ingredient in natural extracts remained uncharacterized. Over the last 15 years, my group has applied structure-informed drug discovery and high-throughput screening approaches to the identification of anti-measles SMOLs. Based on high-resolution structural insight into the docking pose and confirmed oral efficacy in anti-morbillivirus PEP, respectively, I consider the resulting AS-48 entry [83,84,85] and ERDRP-0519 polymerase [86,87,88] inhibitor classes among the most advanced to date.
MeV Entry Inhibition:
The entry inhibitor class stabilizes an intracellular transport-competent prefusion conformation of the MeV fusion (F) protein [89], which mediates merger of the viral lipid envelope with target cell membranes for cell invasion [90]. Docking into a microdomain located near the base of the prefusion F head domain [85] (figure 2) that is involved in regulating conformational stability of the metastable prefusion F structure [91], AS-48 prevents refolding of the F protein into a postfusion conformation and thus viral entry. The compound is reportedly well-behaved, highly target-specific, and has gained considerable traction in the literature as a molecular probe used in characterization of F function [92,93,94,95,96,97,98,99,100].
Figure 2.
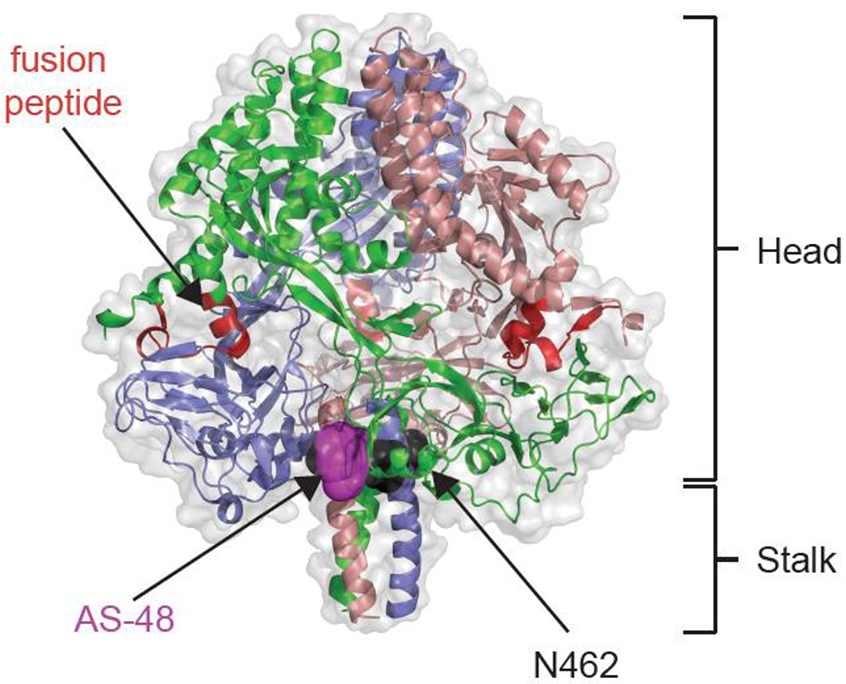
Docking pose of the MeV entry inhibitor AS-48 to MeV F. A ribbon model of the of the prefusion F trimer, colored by monomer, in complex with AS-48 is shown (PDB 5YZC). The prefusion head and stalk domains are highlighted. Docked AS-48 is depicted in magenta, resistance hot-spot N462 [89] is shown in black. The target membrane attack domain, the fusion peptides [90] are highlighted in red.
Despite these apparent strengths, entry inhibitors have in my opinion limited translational potential against the measles indication for three reasons. Firstly, resistance to SMOL inhibitors of paramyxovirus and structurally closely related pneumovirus F proteins emerges rapidly [89,101] and does not necessarily coincide with a reduction in viral fitness and pathogenesis [101]. Fusion inhibitors may secondly have limited potential against MeV infection of the brain, which affects their usefulness for the treatment of persistent MeV infections. SSPE viruses in particular morph essentially into replication units during the multi-year incubation period before onset of neurological symptoms, accumulating a large number of mutations in the viral envelope proteins that greatly impair the formation of infectious viral particles and traditional routes of virion spread [102,103,104]. Thirdly, membrane fusion constitutes a unique, rapidly completed step in the viral replication cycle, creating a single short-lived opportunity for inhibitor action. Since the drug-target kinetics of non-covalently acting well-behaved SMOLs is typically characterized by high on and off rates, target occupancy is often intermittently low under the dynamic conditions present in vivo [105,106], providing transient opportunity for a virion to bypass the block and complete membrane merger. Once the pharmacological entry bottleneck has been negotiated successfully, the viral replication cycle continues uninhibited until release of progeny virions. Indeed, recent results of phase 2 clinical trials with the RSV entry inhibitor presatovir were sobering, since the compound lacked clinical benefit when administered to hematopoietic-cell transplant recipients, but resistant viruses emerged in approximately 11% of the treatment group compared to 0% of the placebo group [107].
MeV Polymerase Inhibition:
Synthetic development of the AS-48 chemotype was therefore discontinued in favor of advance of inhibitors of the viral RNA-dependent RNA polymerase (RdRP) complex. Structurally and functionally unique to members of the mononegavirus order, the RdRP complex consists of a hetero-oligomer of the viral polymerase (L) protein complexed with the phospho- (P) protein as its obligatory molecular chaperone [108]. This P-L complex interacts with the protein-encapsidated viral genome, acting either as transcriptase for viral protein expression or replicase for the production of progeny viral genomes [108].
A number of strategic advantages suggest that RdRP inhibitors may be particularly effective: i) anticipated opportunity for inhibition is plentiful, because all polymerase complex activities – initiation at the promoter, polymerization, mRNA capping, and cap methylation – must be performed numerous times to complete a single viral replication cycle; ii) the inhibitory effect very likely does not need to be absolute, since a reduction of polymerization rate alone should result in virus attenuation; and iii) probably most importantly, polymerase inhibitors should experience an efficacy boost in vivo due to a two-pronged antiviral effect. Firstly, they directly suppress the expression of viral proteins and/or production of progeny genomes. Secondly, they should impair the ability of the virus to counteract the innate antiviral response of the host. Paramyxoviruses express non-structural immunomodulatory proteins, the V and C proteins in the case of MeV [109], that suppress the type I interferon response [110]. Not surprisingly, morbillivirus recombinants engineered to be defective in the expression of these non-structural proteins were viable, but attenuated in vivo [111,112]. In contrast to viral entry inhibitors, blockers of RdRP activity can therefore be expected to trigger pharmacological virus attenuation.
The ERDRP-0519 chemotype was first discovered in a high-throughput screen, blocking polymerase activity of all currently circulating MeV genotypes [88]. Synthetic hit-to-lead development of the scaffold has yielded the orally bioavailable lead ERDRP-0519, which inhibits morbilliviruses with nanomolar potency [86,87]. Pan-morbillivirus inhibition in particular represents a major asset of the compound, since MeV is human-tropic and no animal model other than non-human primates recapitulates hallmarks of human measles. Activity of ERDRP-0519 against closely related CDV, however, opened a path to establish CDV infection of one of its natural hosts, ferrets, as a surrogate efficacy assay. Reportedly, the CDV/ferret system accurately recapitulates the complex pathogenesis of human infection by MeV, including host invasion through the respiratory tract and mediastinal lymph nodes, primary cell-associated viremia, lymphocytopenia, fever, rash, and virus shedding from the respiratory tract [113]. With the exception of non-human primates, these hallmarks of measles are not reproduced by laboratory animal models of MeV infection [114]. Unlike MeV, however, ferrets succumb to pathogenic CDV within two weeks of infection [115], providing a decisive primary efficacy marker.
When tested in the CDV/ferret system, PEP with oral ERDRP-0519 resulted in complete survival of all treated animals, reduced viral burden by over two orders of magnitude, and almost completely alleviated all clinical signs of disease [86]. Prolonged twice-daily treatment was well tolerated without signs of adverse effects. The CDV/ferret model furthermore confirmed the anticipated pharmacological attenuation benefit associated with viral polymerase inhibitors, since ERDRP-0519 PEP resulted in a massive increase of the expression of effector interferon-stimulated genes four days after the initiation of treatment compared to control animals [86].
Resistance profiles of the chemotype against MeV and CDV closely overlapped [86,116], identifying hot-spots in direct proximity of the polymerase catalytic site for phosphodiester bond formation (figure 3). Importantly, resistance was in all cases associated with reduced viral fitness, attenuation, and/or reduced transmission success of the resistant variant compared to the genetic parent virus [86]. In addition, ERDRP-0519 was found to be shelf-stable at ambient conditions for over one year and a cost-effective scalable chemical synthesis strategy was established [86]. This compound thus meets the central requests of a measles drug product profile. The CDV/ferret efficacy model furthermore provides a viable system for final de-risking of the asset prior to formal development.
Figure 3.
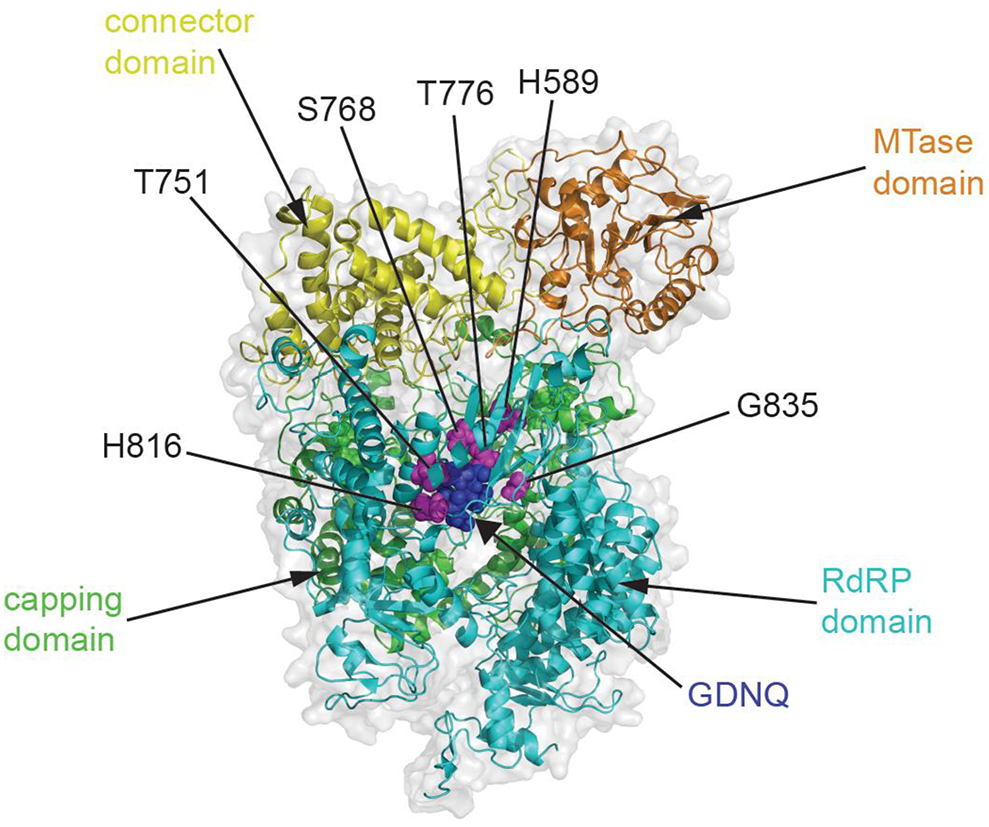
Target site mapping of the MeV polymerase inhibitor ERDRP-0519. Homology model of the MeV L protein based on the coordinates reported for VSV L (PDB 5A22) and colored by functional domain. The catalytic center for phosphodiester bond formation (GDNQ) is shown with blue spheres, confirmed resistance sites are specified and shown in magenta.
Summary and perspective
Considering the precedent set by successful measles IgG PEP, an effective anti-MeV drug can reasonably be anticipated to provide clinical benefit when used for PEP in cases when vaccination is declined, unavailable, or contraindicated. In order to provide viable replacement of the measles IgG component especially in low-income countries where the clinical need is greatest, the successful therapeutic must be amenable to cost-effective manufacture, shelf-stable at ambient temperature, orally bioavailable, and safe for prophylactic use in pediatric patients. A direct-acting anti-MeV SMOL provides the best perspective to address these fundamental requirements and the SMOL ERDRP-0519 has indeed emerged as a promising developmental candidate. Targeting of the viral polymerase complex specifically promises to provide high anti-MeV impact in vivo, resulting from a combination of direct antiviral activity and pharmacological viral attenuation. However, the problem remains that the path to clinical advance beyond phase I safety trials in adult volunteers is unclear. Drug candidates with broadened activity spectrum could mitigate the obstacle, provided they ultimately meet the stringent safety profile required for PEP use in pediatric patients.
When entertaining the development of a SMOL for measles PEP, it must also be considered whether a therapeutic could actually interfere with vaccination drives by providing vaccine-skeptics with a perceived alternative option. Of course, the current trivalent MMR vaccine provides protection not only against measles, but also against mumps and rubella. Even if that added benefit is ignored, all evidence suggests that the risk of discouraging vaccination is minimal for a drug that is designed predominantly for measles PEP. The German recommendation to make measles IgG available to the unvaccinated for PEP, for instance, has not triggered a decline in vaccination rates in Germany. In fact, it appears that the availability of measles PEP has little, if any, relevance for the decision-making process of vaccine skeptics, and the same can reasonably be expected to apply to a therapeutic replacing the measles PEP IgG component. Almost 20 years after launch of the global Measles & Rubella Initiative and despite best efforts, global measles elimination has lost ground in 2019 in several geographical regions. Rather than pursuing a more-of-the-same strategy and viewing a therapeutic as a potential threat to viral eradication plans, a drug suitable for cost-effective measles PEP could actually provide a fresh opportunity to expand our antiviral arsenal and in conjunction with vaccination contribute to breaking the threat of a prolonged eradication endgame stalemate.
Acknowledgements
I thank R.M. Cox for assistance with figure preparation and R.M. Cox, J. Sourimant, and A.L. Hammond for helpful discussion and critical reading of the manuscript. Work in my laboratory is supported, in part, by Public Health Service grants AI071002 and AI141222 from the NIH/NIAID and HD079327 from the NIH/NICHD. The funders had no role in manuscript preparation or the decision to submit the work for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The author is a co-inventor on respective United States patent US20160310511A1 “Myxovirus Therapeutics, Compounds, and Uses Related Thereto” covering composition of matter and method of use of ERDRP-0519 and application No. 62/935,896 “Small Molecules Polymerase Inhibitors” covering method of use of GRP-88309 for anti-MeV therapy. This publication could affect his personal financial status.
References
- [1].Immunization, Vaccines and Biologicals. New measles surveillance data from WHO. https://www.who.int/immunization/newsroom/new-measles-data-august-2019/en/. 2019.
- [2].NIAID Measles Workshop. (http://www.cvent.com/events/niaid-measles-workshop/event-summary-2ebe538915984d0493d249306dd0ed12.aspx?i=a3538844-58a5-4d64-b184-4e08324dd12a). 2019.*NIH workshop discussing possible profile and use of measles antivirals
- [3].NIAID Council Minutes: June 3, 2019 (https://www.niaid.nih.gov/about/niaid-council-minutes-june-3-2019). 2019.
- [4].Tatsuo H, Ono N, Tanaka K, Yanagi Y: SLAM (CDw150) is a cellular receptor for measles virus. Nature 2000, 406:893–897. [DOI] [PubMed] [Google Scholar]
- [5].Laksono BM, de Vries RD, McQuaid S, Duprex WP, de Swart RL: Measles Virus Host Invasion and Pathogenesis. Viruses 2016, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Noyce RS, Bondre DG, Ha MN, Lin LT, Sisson G, et al. : Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog 2011, 7:e1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Muhlebach MD, Mateo M, Sinn PL, Prufer S, Uhlig KM, et al. : Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 2011, 480:530–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ludlow M, Rennick LJ, Sarlang S, Skibinski G, McQuaid S, et al. : Wild-type measles virus infection of primary epithelial cells occurs via the basolateral surface without syncytium formation or release of infectious virus. J Gen Virol 2010, 91:971–979. [DOI] [PubMed] [Google Scholar]
- [9].Singh BK, Li N, Mark AC, Mateo M, Cattaneo R, et al. : Cell-to-Cell Contact and Nectin-4 Govern Spread of Measles Virus from Primary Human Myeloid Cells to Primary Human Airway Epithelial Cells. J Virol 2016, 90:6808–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tahara M, Takeda M, Shirogane Y, Hashiguchi T, Ohno S, et al. : Measles virus infects both polarized epithelial and immune cells by using distinctive receptor-binding sites on its hemagglutinin. J Virol 2008, 82:4630–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Epidemiology and Prevention of Vaccine-Preventable Diseases. Chapter 13: Measles. (https://www.cdc.gov/vaccines/pubs/pinkbook/meas.html). 2015.
- [12].Word Health Organization. Measles (https://www.who.int/news-room/fact-sheets/detail/measles). 2013.
- [13].Dabbagh A, Laws RL, Steulet C, Dumolard L, Mulders MN, et al. : Progress Toward Regional Measles Elimination - Worldwide, 2000-2017. MMWR Morb Mortal Wkly Rep 2018, 67:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Anderson RM, May RM: Directly transmitted infections diseases: control by vaccination. Science 1982, 215:1053–1060. [DOI] [PubMed] [Google Scholar]
- [15].Christensen PE, Schmidt H, Bang HO, Andersen V, Jordal B, et al. : An epidemic of measles in southern Greenland, 1951; measles in virgin soil. II. The epidemic proper. Acta Med Scand 1953, 144:430–449. [DOI] [PubMed] [Google Scholar]
- [16].Moss WJ, Griffin DE: Global measles elimination. Nat Rev Microbiol 2006, 4:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Weber A, Weber M, Milligan P: Modeling epidemics caused by respiratory syncytial virus (RSV). Math Biosci 2001, 172:95–113. [DOI] [PubMed] [Google Scholar]
- [18].Mills CE, Robins JM, Lipsitch M: Transmissibility of 1918 pandemic influenza. Nature 2004, 432:904–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Holzmann H, Hengel H, Tenbusch M, Doerr HW: Eradication of measles: remaining challenges. Med Microbiol Immunol 2016, 205:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Centers for Disease Control and Prevention. Measles Cases and Outbreaks. https://www.cdc.gov/measles/cases-outbreaks.html. 2019.
- [21].World Health Organization. Regional Office for Europe. European Region loses ground in effort to eliminate measles. http://www.euro.who.int/en/media-centre/sections/press-releases/2019/european-region-loses-ground-in-effort-to-eliminate-measles. 2019.
- [22].von Pirquet C: Das Verhalten der kutanen Tuberkulinreaktion während der Masern. . Dtsch Med Wochenschr 1908, 34:1297–1300. [Google Scholar]
- [23].de Vries RD, de Swart RL: Measles immune suppression: functional impairment or numbers game? PLoS Pathog 2014, 10:e1004482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mina MJ, Metcalf CJ, de Swart RL, Osterhaus AD, Grenfell BT: Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science 2015, 348:694–699.*demonstration of the major long-term impact of measles immunosuppression on publich health
- [25].Petrova VN, Sawatsky B, Han AX, Laksono BM, Walz L, et al. : Incomplete genetic reconstitution of B cell pools contributes to prolonged immunosuppression after measles. Sci Immunol 2019, 4.**mechanistic evaluation of MeV-induced immune amnesia
- [26].von Messling V, Springfeld C, Devaux P, Cattaneo R: A ferret model of canine distemper virus virulence and immunosuppression. J Virol 2003, 77:12579–12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Griffin DE: Measles virus-induced suppression of immune responses. Immunol Rev 2010, 236:176–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mina MJ, Kula T, Leng Y, Li M, de Vries RD, et al. : Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science 2019, 366:599–606.**mechanistic evaluation of MeV-induced immune amnesia
- [29].Gadroen K, Dodd CN, Masclee GMC, de Ridder MAJ, Weibel D, et al. : Impact and longevity of measles-associated immune suppression: * matched cohort study using data from the THIN general practice database in the UK. BMJ Open 2018, 8:e021465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hashiguchi T, Kajikawa M, Maita N, Takeda M, Kuroki K, et al. : Crystal structure of measles virus hemagglutinin provides insight into effective vaccines. Proc Natl Acad Sci U S A 2007, 104:19535–19540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tahara M, Ito Y, Brindley MA, Ma X, He J, et al. : Functional and structural characterization of neutralizing epitopes of measles virus hemagglutinin protein. J Virol 2013, 87:666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lech PJ, Tobin GJ, Bushnell R, Gutschenritter E, Pham LD, et al. : Epitope dampening monotypic measles virus hemagglutinin glycoprotein results in resistance to cocktail of monoclonal antibodies. PLoS One 2013, 8:e52306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mina MJ, Grenfell BT, Metcalf CJE: Response to Comment on "Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality". Science 2019, 365. [DOI] [PubMed] [Google Scholar]
- [34].McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS, Centers for Disease C, et al. : Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013, 62:1–34. [PubMed] [Google Scholar]
- [35].Young MK, Nimmo GR, Cripps AW, Jones MA: Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev 2014:CD010056.*benefit assessment of measles IgG PEP
- [36].Zingher A, Mortimer P: Convalescent whole blood, plasma and serum in the prophylaxis of measles: JAMA, 12 April, 1926; 1180-1187. Rev Med Virol 2005, 15:407–418; discussion 418-421. [DOI] [PubMed] [Google Scholar]
- [37].Matysiak-Klose D, Santibanez S, Schwerdtfeger C, Koch J, von Bernuth H, et al. : Post-exposure prophylaxis for measles with immunoglobulins revised recommendations of the standing committee on vaccination in Germany. Vaccine 2018, 36:7916–7922.*benefit assessment of measles IgG PEP
- [38].Christenson B, Bottiger M: Measles antibody: comparison of long-term vaccination titres, early vaccination titres and naturally acquired immunity to and booster effects on the measles virus. Vaccine 1994, 12:129–133. [DOI] [PubMed] [Google Scholar]
- [39].Ganellin CR, Jefferis R, Roberts SM: Introduction to Biological and Small Molecule Drug Research and Development Theory and Case Studies Preface. Introduction to Biological and Small Molecule Drug Research and Development: Theory and Case Studies 2013:Xix–Xx. [Google Scholar]
- [40].Nasr JT, Andriola MR, Coyle PK: ADEM: literature review and case report of acute psychosis presentation. Pediatr Neurol 2000, 22:8–18. [DOI] [PubMed] [Google Scholar]
- [41].Rima BK, Duprex WP: Morbilliviruses and human disease. J Pathol 2006, 208:199–214. [DOI] [PubMed] [Google Scholar]
- [42].ter Meulen V, Stephenson JR, Kreth HW (1983) Subacute sclerosing panencephalitis In: Fraenkel-Conrat H, Wagner RR, editors. Comprehensive Virology. New York: Plenum Press; pp. 105–159. [Google Scholar]
- [43].Modlin JF, Jabbour JT, Witte JJ, Halsey NA: Epidemiologic studies of measles, measles vaccine, and subacute sclerosing panencephalitis. Pediatrics 1977, 59:505–512. [PubMed] [Google Scholar]
- [44].Turner A, Jeyaratnam D, Haworth F, Sinha MD, Hughes E, et al. : Measles-associated encephalopathy in children with renal transplants. Am J Transplant 2006, 6:1459–1465. [DOI] [PubMed] [Google Scholar]
- [45].Freeman AF, Jacobsohn DA, Shulman ST, Bellini WJ, Jaggi P, et al. : A new complication of stem cell transplantation: measles inclusion body encephalitis. Pediatrics 2004, 114:e657–660. [DOI] [PubMed] [Google Scholar]
- [46].National Institute of Neurological Disorders and Stroke. Subacute Sclerosing Panencephalitis Information Page (https://www.ninds.nih.gov/disorders/all-disorders/subacute-sclerosing-panencephalitis-information-page). 2019.
- [47].Hosoya M: [Therapy and prognosis in subacute sclerosing panencephalitis]. Nippon Rinsho 2007, 65:1483–1486. [PubMed] [Google Scholar]
- [48].del Toro-Riera M, Macaya-Ruiz A, Raspall-Chaure M, Tallada-Serra M, Pasqual-Lopez I, et al. : [Subacute sclerosing panencephalitis: combined treatment with interferon alpha and intraventricular ribavirin]. Rev Neurol 2006, 42:277–281. [PubMed] [Google Scholar]
- [49].Campbell C, Levin S, Humphreys P, Walop W, Brannan R: Subacute sclerosing panencephalitis: results of the Canadian Paediatric Surveillance Program and review of the literature. BMC Pediatr 2005, 5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tomoda A, Nomura K, Shiraishi S, Miike T, Hamada A, et al. : [Trial of intraventricular ribavirin and interferon-alpha combination therapy for subacute sclerosing panencephalitis (SSPE) in Japan]. No To Hattatsu 2003, 35:321–326. [PubMed] [Google Scholar]
- [51].Hara S, Kimura H, Hoshino Y, Hayashi N, Negoro T, et al. : Combination therapy with intraventricular interferon-alpha and ribavirin for subacute sclerosing panencephalitis and monitoring measles virus RNA by quantitative PCR assay. Brain Dev 2003, 25:367–369. [DOI] [PubMed] [Google Scholar]
- [52].Forni AL, Schluger NW, Roberts RB: Severe measles pneumonitis in adults: evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin Infect Dis 1994, 19:454–462. [DOI] [PubMed] [Google Scholar]
- [53].Stogner SW, King JW, Black-Payne C, Bocchini J: Ribavirin and intravenous immune globulin therapy for measles pneumonia in HIV infection. South Med J 1993, 86:1415–1418. [DOI] [PubMed] [Google Scholar]
- [54].Gururangan S, Stevens RF, Morris DJ: Ribavirin response in measles pneumonia. J Infect 1990, 20:219–221. [DOI] [PubMed] [Google Scholar]
- [55].Fernandez H, Banks G, Smith R: Ribavirin: a clinical overview. Eur J Epidemiol 1986, 2:1–14. [DOI] [PubMed] [Google Scholar]
- [56].Manns MP, Wedemeyer H, Cornberg M: Treating viral hepatitis C: efficacy, side effects, and complications. Gut 2006, 55:1350–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, et al. : Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001, 358:958–965. [DOI] [PubMed] [Google Scholar]
- [58].Wyde PR, Moore-Poveda DK, De Clercq E, Neyts J, Matsuda A, et al. : Use of cotton rats to evaluate the efficacy of antivirals in treatment of measles virus infections. Antimicrob Agents Chemother 2000, 44:1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Moss WJ, Griffin DE: Measles. Lancet 2012, 379:153–164. [DOI] [PubMed] [Google Scholar]
- [60].World Health Organization. Global Measles and Rubella Update December 2019. (https://nam03.safelinks.protection.outlook.com/?url=https%3A%2F%2Fwww.who.int%2Fimmunization%2Fmonitoring_surveillance%2Fburden%2Fvpd%2Fsurveillance_type%2Factive%2FGlobal_MR_Update_December_2019.pptx%3Fua%3D1&data=02%7C01%7Crplemper%40gsu.edu%7C18770802b57840c041db08d7af9af8b1%7C515ad73d8d5e4169895c9789dc742a70%7C0%7C1%7C637170952443138665&sdata=ogys1PLl9pw6C6QB7EPzuB8L%2FXfakw53GVOjenytsWM%3D&reserved=0). 2019.
- [61].Hall CB: Respiratory syncytial virus and parainfluenza virus. N Engl J Med 2001, 344:1917–1928. [DOI] [PubMed] [Google Scholar]
- [62].Abedi GR, Prill MM, Langley GE, Wikswo ME, Weinbei Ga, et al. : Estimates of Parainfluenza Virus-Associated Hospitalizations and Cost Among Children Aged Less Than 5 Years in the United States, 1998-2010. J Pediatric Infect Dis Soc 2016, 5:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Peck AJ, Englund JA, Kuypers J, Guthrie KA, Corey L, et al. : Respiratory virus infection among hematopoietic cell transplant recipients: eviden. e for asymptomatic parainfluenza virus infection. Blood 2007, 110:1681–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Shah DP, Shah PK, Azzi JM, Chemaly RF: Parainfluenza virus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: A systematic review. Cancer Lett 2016, 370:358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Seo S, Xie H, Campbell AP, Kuypers JM, Leisenring WM, et al. : Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome. Clin Infect Dis 2014, 58:1357–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ustun C, Slaby J, Shanley RM, Vydra J, Smith AR, et al. : Human parainfluenza virus infection after hematopoietic stem cell transplantation: risk factors, management, mortality, and changes over time. Biol Blood Marrow Transplant 2012, 18:1580–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Nichols WG, Corey L, Gooley T, Davis C, Boeckh M: Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood 2001, 98:573–578. [DOI] [PubMed] [Google Scholar]
- [68].Salvatore M, Satlin MJ, Jacobs SE, Jenkins SG, Schuetz AN, et al. : DAS181 for Treatment of Parainfluenza Virus Infections in Hematopoietic Stem Cell Transplant Recipients at a Single Center. Biol Blood Marrow Transplant 2016, 22:965–970. [DOI] [PubMed] [Google Scholar]
- [69].Falsey AR: Current management of parainfluenza pneumonitis in immunocompromised patients: a review. Infect Drug Resist 2012, 5:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Rothan HA, Zhong Y, Sanborn MA, Teoh TC, Ruan J, et al. : Small molecule grp94 inhibitors block dengue and Zika virus replication. Antiviral Res 2019, 171:104590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Enkirch T, Sauber S, Anderson DE, Gan ES, Kenanov D, et al. : Identification and in vivo Efficacy Assessment of Approved Orally Bioavailable Human Host Protein-Targeting Drugs With Broad Anti-influenza A Activity. Front Immunol 2019, 10:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Adcock RS, Chu YK, Golden JE, Chung DH: Evaluation of anti-Zika virus activities of broad-spectrum antivirals and NIH clinical collection compounds using a cell-based, high-throughput screen assay. Antiviral Res 2017, 138:47–56. [DOI] [PubMed] [Google Scholar]
- [73].Ma C, Li F, Musharrafieh RG, Wang J: Discovery of cyclosporine A and its analogs as broad-spectrum anti-influenza drugs with a high in vitro genetic barrier of drug resistance. Antiviral Res 2016, 133:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Spickler C, Lippens J, Laberge MK, Desmeules S, Bellavance E, et al. : Phosphatidylinositol 4-kinase III beta is essential for replication of human rhinovirus and its inhibition causes a lethal phenotype in vivo. Antimicrob Agents Chemother 2013, 57:3358–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Supko JG, Malspeis L: Pharmacokinetics of michellamine B, a naphthylisoquinoline alkaloid with in vitro activity against human immunodeficiency virus types 1 and 2, in the mouse and dog. Antimicrob Agents Chemother 1995, 39:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Furuta Y, Komeno T, Nakamura T: Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci 2017, 93:449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF. et al. T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res 2009, 82:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Snell NJ: Ribavirin--current status of a broad spectrum antiviral agent. Expert Opin Pharmacother 2001, 2:1317–1324. [DOI] [PubMed] [Google Scholar]
- [79].Oshiro S, Minema H, Shiroma N, Hirayasu K, Nakada Y: [Five patients with subacute sclerosing panencephalitis treated with intraventricular alpha-interferon and inosinpranobex]. No To Hattatsu 2004, 36:70–74. [PubMed] [Google Scholar]
- [80].Gagnon A, Bouchard RW: Fulminating adult-onset subacute sclerosing panencephalitis in a 49-year-old man. Arch Neurol 2003, 60:1160–1161. [DOI] [PubMed] [Google Scholar]
- [81].Plemper RK, Hammond AL: Synergizing vaccinations with therapeutics for measles eradication. Expert Opin Drug Discov 2014, 9:201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Plemper RK, Snyder JP: Measles control--can measles virus inhibitors make a difference? Curr Opin Investig Drugs 2009, 10:811–820. [PMC free article] [PubMed] [Google Scholar]
- [83].Plemper RK, Doyle J, Sun A, Prussia A, Cheng LT, et al. : Design of a small-molecule entry inhibitor with activity against primary measles virus strains. Antimicrob Agents Chemother 2005, 49:3755–3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Plemper RK, Erlandson KJ, Lakdawala AS, Sun A, Prussia A, et al. : A target site for template-based design of measles virus entry inhibitors. Proc Natl Acad Sci U S A 2004, 101:5628–5633.*first description of an MeV-specific small-molecule virus entry inhibitor
- [85].Hashiguchi T, Fukuda Y, Matsuoka R, Kuroda D, Kubota M, et al. : Structures of the prefusion form of measles virus fusion protein in complex with inhibitors. Proc Natl Acad Sci U S A 2018, 115:2496–2501.*high resolution co-crystal structure of the MeV entry inhibitor with the F protein target
- [86].Krumm SA, Yan D, Hovingh ES, Evers TJ, Enkirch T, et al. : An orally available, small-molecule polymerase inhibitor shows efficacy against a lethal morbillivirus infection in a large animal model. Sci Transl Med 2014, 6:232ra252.**proof-of-concept that PEP with an allosteric pan-morbillivirus polymerase inhibitor prevents lethal morbillivirus infection and alleviates clinical signs of morbillivirus disease
- [87].Ndungu JM, Krumm SA, Yan D, Arrendale RF, Reddy GP, et al. : Non-nucleoside Inhibitors of the Measles Virus RNA-Dependent RNA Polymerase: Synthesis, Structure-Activity Relationships, and Pharmacokinetics. Journal of medicinal chemistry 2012, 55:4220–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].White LK, Yoon JJ, Lee JK, Sun A, Du Y, et al. : Nonnucleoside inhibitor of measles virus RNA-dependent RNA polymerase complex activity. Antimicrob Agents Chemother 2007, 51:2293–2303.*first description of the pan-morbillivirus small-molecule polymerase inhibitor chemotype
- [89].Doyle J, Prussia A, White LK, Sun A, Liotta DC, et al. : Two domains that control prefusion stability and transport competence of the measles virus fusion protein. J Virol 2006, 80:1524–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Plemper RK: Cell Entry of Enveloped Viruses. Curr Opin Virol 2011, 1:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lee JK, Prussia A, Snyder JP, Plemper RK: Reversible inhibition of the fusion activity of measles virus F protein by an engineered intersubunit disulfide bridge. J Virol 2007, 81:8821–8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Mathieu C, Ferren M, Jurgens E, Dumont C, Rybkina K, et al. : Measles Virus Bearing Measles Inclusion Body Encephalitis-Derived Fusion Protein Is Pathogenic after Infection via the Respiratory Route. J Virol 2019, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kalbermatter D, Shrestha N, Ader-Ebert N, Herren M, Moll P, et al. : Primary resistance mechanism of the canine distemper virus fusion protein against a small-molecule membrane fusion inhibitor. Virus Res 2019, 259:28–37. [DOI] [PubMed] [Google Scholar]
- [94].Ha MN, Delpeut S, Noyce RS, Sisson G, Black KM, et al. : Mutations in the Fusion Protein of Measles Virus That Confer Resistance to the Membrane Fusion Inhibitors Carbobenzoxy-d-Phe-l-Phe-Gly and 4-Nitro-2-Phenylacetyl Amino-Benzamide. J Virol 2017, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Avila M, Khosravi M, Alves L, Ader-Ebert N. Bringolf F, et al. : Canine distemper virus envelope protein interactions modulated by hydrophobic residues in the fusion protein globular head. J Virol 2015, 89:1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ader-Ebert N, Khosravi M, Herren M, Avila M, Alves L, et al. : Sequential conformational changes in the morbillivirus attachment protein initiate the membrane fusion process. PLoS Pathog 2015, 11:e1004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Avila M, Alves L, Khosravi M, Ader-Ebert N, Origgi F, et al. : Molecular determinants defining the triggering range of prefusion F complexes of canine distemper virus. J Virol 2014, 88:2951–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Ader N, Brindley M, Avila M, Orvell C, Horvat B, et al. : Mechanism for active membrane fusion triggering by morbillivirus attachment protein. J Virol 2013, 87:314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ader N, Brindley MA, Avila M, Origgi FC, Langedijk JP, et al. : Structural rearrangements of the central region of the morbillivirus attachment protein stalk domain trigger f protein refolding for membrane fusion. J Biol Chem 2012, 287:16324–16334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Prussia AJ, Plemper RK, Snyder JP: Measles virus entry inhibitors: a structural proposal for mechanism of action and the development of resistance. Biochemistry 2008, 47:13573–13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Yan D, Lee S, Thakkar VD, Luo M, Moore ML, et al. : Cross-resistance mechanism of respiratory syncytial virus against structurally diverse entry inhibitors. Proc Natl Acad Sci U S A 2014, 111:E3441–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Cathomen T, Naim HY, Cattaneo R: Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J Virol 1998, 72:1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Cathomen T, Mrkic B, Spehner D, Drillien R, Naef R, et al. : A matrix-less measles virus is infectious and elicits extensive cell fusion: consequences for propagation in the brain. Embo J 1998, 17:3899–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Cattaneo R, Schmid A, Eschle D, Baczko K, ter Meulen V, et al. : Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell 1988, 55:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Tonge PJ: Drug-Target Kinetics in Drug Discovery. ACS Chem Neurosci 2018, 9:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Copeland RA, Pompliano DL, Meek TD: Drug-target residence time and its implications for lead optimization. Nat Rev Drug Discov 2006, 5:730–739. [DOI] [PubMed] [Google Scholar]
- [107].Chemaly RF, Dadwal SS, Bergeron A, Ljungman P, Kim YJ, et al. : A phase 2, randomized, double-blind, placebo-controlled trial of presatovir for the treatment of respiratory syncytial virus upper respiratory tract infection in hematopoietic-cell transplant recipients. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Fearns R, Plemper RK: Polymerases of paramyxoviruses and pneumoviruses. Virus Res 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lamb RA, Parks GD (2013) Paramyxoviridae In: Knipe DM, Howley PM, edito. s. Fields Virology. 6 ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; pp. 957–995. [Google Scholar]
- [110].Parks GD, Alexander-Miller MA: Paramyxovirus activation and inhibition of innate immune responses. J Mol Biol 2013, 425:4872–4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Svitek N, Gerhauser I, Goncalves C, Grabski E, Doring M, et al. : Morbillivirus control of the interferon response: relevance of STAT2 and mda5 but not STAT1 for canine distemper virus virulence in ferrets. J Virol 2014, 88:2941–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Devaux P, Hudacek AW, Hodge G, Reyes-Del Valle J, McChesney MB, et al. : A recombinant measles virus unable to antagonize STAT1 function cannot control inflammation and is attenuated in rhesus monkeys. J Virol 2011, 85:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].da Fontoura Budaszewski R, von Messling V: Morbillivirus Experimental Animal Models: Measles Virus Pathogenesis Insights from Canine Distemper Virus. Viruses 2016, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].de Swart RL: Measles: What we have learned from non-human primate models. Drug Discovery Today: Disease Models 2017, 23:31–34. [Google Scholar]
- [115].von Messling V, Milosevic D, Catteneo R: Tropism illuminated: lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc Natl Acad Sci U S A 2004, 101:14216–14221.*establishes the CDV ferret system as an animal model that recapitulates hallmarks of human measles disease
- [116].Yoon JJ, Krumm SA, Ndungu JM, Hoffman V, Bankamp B, et al. : Target analysis of the experimental measles therapeutic AS-136A. Antimicrob Agents Chemother 2009, 53:3860–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]


