Abstract
Purpose of Review
In this report, we review the need for point-of-care (POC) or near real-time testing for antiretrovirals, progress in the field, evidence for guiding implementation of these tests globally, and future directions in objective antiretroviral therapy (ART) or pre-exposure prophylaxis (PrEP) adherence monitoring.
Recent Findings
Two cornerstones to end the HIV/AIDS pandemic are ART, which provides individual clinical benefits and eliminates forward transmission, and PrEP, which prevents HIV acquisition with high effectiveness. Maximizing the individual and public health benefits of these powerful biomedical tools requires high and sustained antiretroviral adherence. Routine monitoring of medication adherence in individuals receiving ART and PrEP may be an important component in interpreting outcomes and supporting optimal adherence. Existing practices and subjective metrics for adherence monitoring are often inaccurate or unreliable, and therefore, are generally ineffective for improving adherence. Laboratory measures of antiretroviral concentrations using liquid chromatography tandem mass spectrometry have been utilized in research settings to assess medication adherence, although these are too costly and resource-intensive for routine use.
Summary
Newer, less costly technologies such as antibody-based methods can provide objective drug level measurement and may allow for POC or near-patient adherence monitoring in clinical settings. When coupled with timely and targeted counseling, POC drug level measures can support adherence clinic-based interventions to ART or PrEP in near real-time.
Keywords: HIV, adherence, antiretroviral therapy (ART), pre-exposure prophylaxis (PrEP), point-of-care test, tenofovir
INTRODUCTION
Over 20 million people living with HIV (PLHIV) are receiving antiretroviral therapy (ART), and millions more are expected to start in the coming years (1). Newer ART regimens that include potent HIV integrase strand transfer inhibitors (INSTIs) with a high barrier to resistance may reduce the prevalence and impact of HIV drug resistance (2). The Joint United Nations Programme on HIV/AIDS (UNAIDS) has set an ambitious goal of initiating 95% of all PLHIV on ART by 2030 (3). The feasibility of ending the HIV/AIDS epidemic will in part depend on achieving near-universal coverage of knowledge of HIV status, ART for all PLHIV, and maintaining suppressed HIV viral load (VL), which requires ongoing adherence, but prevents onward HIV transmission (4–6).
Recent treatment as prevention trials, however, highlight the challenges of reaching near universal coverage of ART and the continued importance of primary prevention (7). When taken with good adherence, either daily or on an event-driven basis (8), PrEP significantly reduces the risk of HIV acquisition and is an important tool for HIV prevention (9–11). The World Health Organization (WHO) recommends all HIV-uninfected persons in areas with high risk of HIV acquisition (defined as ≥3% HIV incidence) receive pre-exposure prophylaxis (PrEP) to prevent HIV acquisition (12). Globally, millions of people at risk of HIV acquisition, including approximately 1.2 million individuals in the United States (U.S.), qualify for PrEP (13). However, the estimated number of actual PrEP users may be far less.
The efficacy of both ART and oral PrEP are highly dependent on patients maintaining adequate adherence (14). Ending HIV/AIDS will require improvements in HIV medication adherence from current levels to prevent further acquisition and transmission and to improve HIV-related outcomes. Therefore, routine monitoring of medication adherence may have a crucial role in supporting individuals receiving ART or PrEP. All PrEP and approximately 90% of ART regimens include tenofovir disoproxil fumarate (TDF), or tenofovir alafenamide (TAF), co-formulated with lamivudine or emtricitabine (FTC). In this review, we present the need for point-of-care (POC) or near real-time adherence testing, technologies currently available for real-time, objective monitoring of tenofovir-based ART and PrEP adherence, evidence for implementing these technologies globally, and future directions in objective HIV adherence measurement.
NON-PHARMACOLOGIC MEASURES OF ADHERENCE
For the millions of PLHIV receiving ART and those at risk of HIV acquisition who are on PrEP, adequate adherence remains critical to effectiveness (15,16). An accurate assessment of adherence that is available during a clinical encounter allows for immediate clinical decision-making and provides an opportunity for timely, accurate, and strategic provider-patient communication around medication-taking. Appropriate counseling and interventions that can be conducted in real-time during the patient’s visit may help address adherence barriers and lead to more optimal treatment outcomes (17,18).
In routine clinical practice, however, obtaining an accurate account of real-time adherence to HIV treatment or PrEP has proven challenging. Clinicians often rely on clinical measures of therapeutic impact of ART, most often HIV RNA VL, or decreasingly, CD4 lymphocyte counts or presence of opportunistic infections. However, the latter indicators are not relevant for PrEP, are more often indicative of long-standing adherence problems to ART, and are not often available in real-time in routine care. Delayed recognition of adherence problems can mean missed opportunities for more timely counseling and interventions prior to the onset of prevention failure, virologic failure, or immunosuppression. Moreover, waiting for these indicators can be particularly problematic since adherence patterns are established early in the course of treatment, and early attrition in ART programs remains high (19).
Several subjective measures of adherence, such as self-reported adherence, clinician assessments, pill counts, pharmacy refill records, and clinic visit attendance, have been widely used to assess adherence at the time of a clinical encounter. These measures are convenient, inexpensive, and offer flexibility in the mode of administration and timing of assessment to provide detailed data on patterns of medication-taking (20). However, they all have serious shortcomings for both validity and reliability. Self-reports are susceptible to social desirability and affirmation biases, as well as inaccurate recall, and concerns about their tendency to overestimate adherence in comparison to more direct measures, such as drug levels, are well-documented (20–24). Clinician assessment is notoriously flawed and generally not better than chance predictions (25). Pill counts have been associated with other measures of adherence, including electronic monitoring devices, self-report, and concurrent (yet not reliably correlated) viral suppression (26). However, pill counting is subject to inaccuracies from sharing of medications, forgetting to bring pills to clinics, and intentional pill dumping, and therefore, has been poorly correlated with drug levels (27,28). Pharmacy refill records, which need to be comprehensive, systematically recorded, readily retrievable, and facilitated by a universal health care system, do not reliably correlate with viral suppression, especially in low- and middle-income countries (LMICs) and adolescents (29–32). Clinical attendance, especially timeliness of attendance, can be an indicator of self-reported medication adherence and immune function, but existing studies have not demonstrated reliable associations with HIV VL (33–35).
Electronic drug monitoring, such as the medication event monitoring system (MEMS), WisePill, along with newer technologic advances, offer more objective estimates of adherence, yet they are expensive, cumbersome, and subject to non-use, which is difficult to distinguish from non-adherence (17,36). With the rapid increase of PLHIV initiating ART globally and the desired uptake in PrEP, there is an urgent need to redesign models of antiretroviral monitoring and management, while not increasing the burden to HIV providers, laboratories, and health systems (37–40), especially in LMICs. Objective data and counseling on recent medication adherence provided at regular intervals and in real-time may open the window for more accurate and strategic communication about medication-taking during routine ART and PrEP visits.
ANTIRETROVIRAL DRUG LEVELS IN BIOLOGICAL SAMPLES AS AN OBJECTIVE ADHERENCE MEASURE
Pharmacologic measurements of antiretroviral drugs or their metabolites have been routinely used to assess adherence in multiple PrEP studies and clinical trials (41). Biological samples for adherence assessments are generally not timed samples at “peaks” or “troughs” after dosing, but rather, obtained at “random” times after dosing during a clinical visit. However, pharmacologic measurements for PrEP and ART adherence assessment can be grouped into “recent dosing” and “cumulative dosing” metrics (Table 1) (41). Because TDF and TAF are commonly used for both ART and PrEP, and co-formulated with lamivudine or FTC, we will focus mostly on tenofovir (TFV)-based regimens and their metrics.
Table 1.
Antiretroviral drug concentration thresholds and interpretations.
| Drug Metabolite | Threshold Concentration | Interpretation | Limitations |
|---|---|---|---|
| Short-term metrics of adherence | |||
| TFV urine (44,78) | 1,000–1,500 ng/mL | Ingested dose in the preceding 24 hours | Recent dose only |
| FTC urine (45) | 1,844 ng/mL | Ingested dose in the preceding 24 hours | Recent dose only |
| TFV plasma (42) | 35 ng/mL | Ingested dose in the preceding 24 hours at steady state | Recent dose only; requires obtaining and processing venous blood; requires LC-MS/MS* |
| FTC plasma (42) | 49 ng/mL | Ingested dose in the preceding 24 hours at steady state | Recent dose only; requires obtaining and processing venous blood; requires LC-MS/MS* |
| FTC-TP (DBS) from F/TDF (63) | 0.125 pmol/3 mm punch | Ingested dose in the preceding 24 hours at steady state | Recent dose only; requires obtaining and processing venous blood; requires LC-MS/MS* |
| Long-term metrics of adherence | |||
| TFV-DP (freshly lysed PBMC) (14) | 40 fmol/106 cells | 2–3 doses/week on average; 90% effective concentration for MSM | Specialty test; impractical and too costly for widespread use; requires venipuncture; requires LC-MS/MS* |
| TFV-DP (DBS) from TDF (62,65) | 700fmol/3mm punch | ≥4 doses/week on average; >90% efficacy in MSM | Cumulative dosing only; requires LC-MS/MS* |
| TFV-DP (DBS) from TAF (58) | 950 fmol/two 7 mm punches | ≥4 doses/week on average | Cumulative dosing only; requires LC-MS/MS* |
| TFV scalp hair (59,69) | 0.023 ng/mg | ≥4 doses/week on average | Requires LC-MS/MS* |
LC-MS/MS limitations include expense and need for proper equipment and laboratory personnel
Short-term metrics of adherence
Recent dosing assessment includes measuring TFV and/or FTC in plasma (42), urine (43–45), and saliva samples (46), as well as emtricitabine-triphosphate (FTC-TP) in red blood cells (RBCs) (47). The interpretation of recent dosing adherence is a function of the drug half-life and/or pharmacokinetic variability of the drug moiety and can provide useful information about the timing of the last dose. For example, TFV has a plasma terminal half-life of approximately 15 hours, which results in minimal accumulation with repeated daily doses (42,48). Thus, TFV concentrations following a single dose are similar to concentrations following repeated daily doses, which is a major limitation of short-term metrics since recent dosing may not reflect typical patterns of dosing. Sensitive TFV plasma assays (e.g., with lower limit of quantification [LLOQ] of 0.3 ng/mL) can detect TDF ingestion within the prior seven days, whereas less sensitive TFV plasma assays (e.g., with LLOQ of 10 ng/mL) detect TDF dosing within the preceding two days (9,42). However, these short-term metrics are subject to “white coat” adherence bias, where an individual may take a dose or doses just prior to a clinic visit or drug level testing (49). Nevertheless, placebo-controlled PrEP efficacy trials demonstrated a strong relationship between plasma TFV levels and PrEP efficacy (14,50), and a recent study in South Africa found plasma TFV levels to be good predictors of viral suppression among women receiving ART (51,52).
Other biological samples, including urine, fingerprick whole blood, and oral swabs, may be more accessible and less invasive for a POC and near real-time test. Urine TFV concentrations are much higher and more variable than plasma TFV concentrations, but TFV exhibits a similar half-life in urine to plasma (43–45). Sensitive urine assays (e.g., 50 ng/mL) can detect TDF ingestion as far back as 10–14 days following drug cessation (43,44). Saliva and oral fluid TFV concentrations are lower and more variable compared with plasma, but are easier to collect and have potential for self-testing, as demonstrated by several HIV self-tests (53). In two studies, testing saliva and oral fluid was preferred by both adult and adolescent patients over the collection of fingerprick blood, but these results may not be generalizable (54,55). Detection of TFV in saliva poses the additional challenge that TFV concentrations are only 2–3% of the plasma TFV concentration, or roughly 0.5 ng/mL to 5 ng/mL (45,53). However, this is not beyond the limits of detection for small molecule drugs by POC diagnostic immunoassays since several drugs of abuse are detectable at these low levels with existing technologies (56).
Finally, FTC-TP in dried blood spots (DBS) is a metabolite with a short half-life (~31 hours) and is highly correlated with recent dosing of TDF/FTC and TAF/FTC (47,57). However, blood from fingerprick or venipuncture must be spotted onto cards to form a DBS within 60 minutes after collection to allow for the accurate measurement of intracellular FTC-TP (47). Blood resting at room temperature prior to spotting will allow any FTC from plasma (if present) to load RBCs during this time, causing a spuriously high result for FTC-TP (58).
Long-term metrics of adherence
Longer-term metrics of adherence include tenofovir-diphosphate (TFV-DP) in peripheral blood mononuclear cells (PBMCs) and RBCs (DBS), and TFV and FTC in hair (59–61). The longer half-life of these drugs or metabolites in their respective biological samples results in drug accumulation, which can provide an indication of averaged adherence over longer time periods. Long-term metrics, therefore, are less susceptible to “white coat” adherence (61–63). To date, directly-observed therapy (DOT) trials have shown strong linear relationships between patterns of TFV-based drug dosing (e.g., two, four, and seven doses per week) and TFV or TFV-DP concentrations and a reduced risk of HIV acquisition in the context of PrEP (14,62,64,65). Intracellular TFV-DP in PBMCs has a half-life of approximately four days, and accumulation of approximately five- to eight-fold with daily TDF dosing (42,48). Efficacy thresholds or ‘benchmarks’ were estimated for TFV-DP in PBMCs, including 40 fmol/106 cells and 83 fmol/106 cells (freshly lysed cells) as the EC90 and EC99 for adults receiving PrEP, respectively (62). These concentrations represented approximately two to three TDF doses per week and seven TDF doses per week on average over the preceding weeks. However, PBMC collection is laborious and expensive, and therefore, not practical for routine clinical settings. TFV-DP in DBS exhibits a 17-day half-life and 25-fold accumulation, which provides a measure of cumulative daily TDF adherence over one to two months (61). In the iPrEx open label extension (OLE) study of daily TDF/FTC in 1,225 men who have sex with men (MSM), none of the 28 observed HIV infections occurred in PrEP-taking participants who had TFV-DP ≥700 fmol/punch, corresponding with 100% efficacy (95% confidence interval [CI]: 84–100%) (65). An equivalent threshold for TFV-DP and effectiveness among women has yet to be established, although directly-observed dosing of TDF/FTC has established comparability and dose-proportionality of DBS TFV-DP measurements in women and men (62). TFV-DP ≥700 fmol/3mm punch is commensurate with ≥4 doses per week on average according to DOT studies with TDF and ≥950 fmol/two 7mm punches for TAF (61,62). Strong associations were also observed between TFV-DP in DBS and virologic outcomes in those receiving ART (66,67).
Hair samples have been tested for TFV and FTC as another measure of cumulative dosing (59). Testing can be conducted on small hair samples (100–200 strands) using hair strands proximal to the scalp and cut down to 1 to 1.5 cm, representing about four to six weeks of hair growth. Hair levels can reflect dosing out to many weeks, depending on how long the hair sample is and how the hair is cut (68). TFV and FTC hair concentrations are highly correlated with TFV-DP and FTC-TP levels in DBS, respectively, and higher TFV concentrations in hair are associated with greater increases in creatinine on PrEP (69,70). Drug concentrations can be assayed along the length of the hair shaft, providing an estimate of adherence over different time periods in the past (71–73). Segmental hair analysis, therefore, can provide granularity in assessing patterns of adherence, which is helpful to overcoming some of the limitations of longer-term metrics. Finally, concentrations of antiretroviral drugs in hair have been associated with virologic outcomes on ART (74). Hair collection is noninvasive, and hair can be stored and shipped at room temperature and without biohazard. As with other long-term metrics of adherence, development of POC tests have been hampered by the need for relatively complex measurement by tandem mass spectrometry (75).
POINT-OF-CARE URINE TENOFOVIR TESTING
Several POC tests intended to measure TFV for monitoring adherence are either in development, have been developed, or are currently being evaluated in clinical studies. Recently, several groups have been developing and validating POC lateral flow immunoassays to detect TFV in urine (76–79). These assays use antibody-based technology and provide results within minutes, similar to a urine-based pregnancy test. This type of POC test can provide information on the actual ingestion of a TFV-based compound directly, allowing for objective and real-time measurement of adherence during a routine clinical visit. Currently, however, these tests have not yet been approved for routine clinical use.
The existing POC TFV tests are single antibody inhibitive immunoassays that utilize an immunoglobulin G (IgG) or M (IgM) antibody that selectively binds to the phosphonate or amine group of the TFV molecule. A POC antibody-based TFV test can be developed as a semi-quantitative test or as a qualitative (yes/no) test for a pre-specified drug concentration threshold. Determining a threshold for a qualitative test will introduce differences in diagnostic sensitivity and specificity in relation to actual pill ingestion and clinical adherence. Setting a higher drug concentration threshold will increase diagnostic sensitivity and decrease specificity, which can lead to more false positive results. For example, one POC antibody-based TFV test set a detection threshold of 1,500 ng/mL, which was estimated to provide 98% accuracy for detecting TDF ingestion within the preceding 24 hours (78,80). Another immunoassay set a detection threshold of 1,000 ng/mL, based on clinical data from observed cross-sectional mass spectrometry data (43). While the test may be as simple to use as an over-the-counter urine pregnancy test, all short-term metrics of adherence will remain susceptible to issues of “white coat” adherence.
The development of these novel POC TFV-based metrics of adherence will present several challenges and opportunities for delivering ART or PrEP, particularly in LMICs. An ideal test to monitor ART or PrEP adherence would be low cost, have a fast turnaround time, and be simple to both administer and interpret results (Table 2). The POC tests in development are low cost and easy to administer. However, while urine collection is generally more acceptable than blood collection, its collection also requires some personal privacy, which is not always achievable in a busy clinical setting. Several studies (detailed below) are currently being planned to determine the feasibility, acceptability, impact, and cost-effectiveness of implementing urine POC TFV testing for monitoring ART or PrEP adherence in LMICs.
Table 2.
Target product profile for a POC TFV test.
| Test Attributes | Minimum Acceptable | Ideal |
|---|---|---|
| Qualitative vs. quantitative | Qualitative | Semi-Quantitative |
| Specimen | Plasma or whole blood from venipuncture | Urine, saliva, or fingerprick blood |
| Specific analyte | Short-term metabolite (TFV) | Long-term metabolite (TFV-DP) |
| Power | Simple battery | None required |
| Cost | <$20 per test | <$2 per test |
| Capacity | 1 sample | Multiple samples |
| Analysis time | <60 minutes | <10 minutes |
| Stability of result | ≥30 minutes after analysis | ≥180 minutes after analysis |
| Equipment/reader | Plate reader (if quantitative results) | Lateral flow readout (visual) |
| Sample preparation required | Low technological level that could be completed by a health worker | None required |
| Shelf life/stability | ≥1 year at room temperature | ≥2 years at room temperature |
| Sensitivity | ≥90% | ≥98% |
| Specificity | ≥90% | ≥98% |
POINT-OF-CARE TESTING FOR ADHERENCE MONITORING IN HIV CARE
As new technologies for HIV care emerge, the potential for improvements in the HIV care cascade and HIV-related outcomes may broaden. POC tests intended for use in HIV care settings include HIV diagnostic tests, CD4 cell counts, and HIV VL tests. Since 2007, the U.S. Centers for Disease Control and Prevention (CDC) and the WHO have recommended that primary care settings integrate POC diagnostic tests into routine HIV screening and diagnostics (81–83). Newer POC TFV tests may improve HIV outcomes by allowing timely, actionable counseling and interventions for PLHIV who present with poor ART adherence. Therefore, integrating POC TFV testing into HIV care could have a significant impact on HIV-related outcomes.
Many studies have demonstrated the positive impacts of POC HIV self-testing platforms, as well as POC VL and CD4 testing, in the context of HIV screening and care, but the potential impact of POC urine TFV-based adherence testing on care outcomes for PLHIV is currently unknown. Real-time, POC urine TFV testing may improve adherence monitoring and offers the opportunity for earlier, better-informed adherence counseling and support for PLHIV receiving ART (23). The STREAM-HIV (Clinicaltrials.gov NCT04341779) and Standing Tall studies are planned to assess the feasibility and acceptability of POC urine TFV monitoring in varying settings in South Africa, as well as its effect on ART adherence, retention in care, viral suppression, drug resistance, and cost of care (84). Additionally, these studies will evaluate the potential effects of integrating POC urine TFV testing into care with other POC tests, such as a POC VL test. A qualitative study of providers, PLHIV receiving ART, and people receiving PrEP in Seattle found that POC urine TFV testing was acceptable and potentially useful for better informed clinical decision-making (85).
Overall, POC tests in the context of HIV screening and care may identify new infections earlier, link newly diagnosed PLHIV to care more rapidly, improve HIV-related outcomes, reduce costs, and improve health care efficiencies by reducing turnaround time for test results and reducing time in clinic for PLHIV receiving ART. Rapid HIV screening at the point of care has the potential to yield higher volumes of HIV diagnoses (86), while HIV self-testing in various settings has been found to increase uptake and frequency of testing (87).
A meta-analysis of studies that integrated POC CD4 cell count into care found improvements in projected life expectancy and reduced costs when CD4 count was conducted in real-time at the clinic as compared to laboratory-based testing (88). With more recent universal test-and-treat guidelines, countries have shifted away from routine CD4 testing in favor of treating all PLHIV regardless of CD4 count and have instead placed more emphasis on VL testing to monitor ART adherence (89). However, POC CD4 count at diagnosis may continue to play a role in HIV care, as WHO guidelines recommend intensive follow-up and care for those with a low CD4 count (90).
Several POC tests for measuring HIV VL are currently available, and have proven to be effective for facilitating rapid ART initiation and improving retention in care for infants, as well as increasing viral suppression rates and improving retention in care for adults (91–93). POC HIV VL test results may also prompt earlier interventions, such as ART regimen switches, when needed (92,94). POC VL monitoring costs are similar to laboratory-based VL testing, but POC VL monitoring may be more cost-effective when accounting for improvements in clinical care (95–97).
Integrating POC urine TFV testing into HIV care with other POC tests may prove effective in earlier identification of those at risk of defaulting from care, stopping treatment, viremia, or developing ART drug resistance. POC adherence testing could lead to targeted and expedited enhanced adherence counseling or necessary ART regimen switches. More research is still needed to elucidate the role of POC urine TFV monitoring in care and how it may be used alongside other POC tests (Figure 1), such as a VL test, to improve outcomes along the HIV continuum of care and efficiencies within the health care system.
Figure 1. Integration of POC testing in the HIV continuum of care.
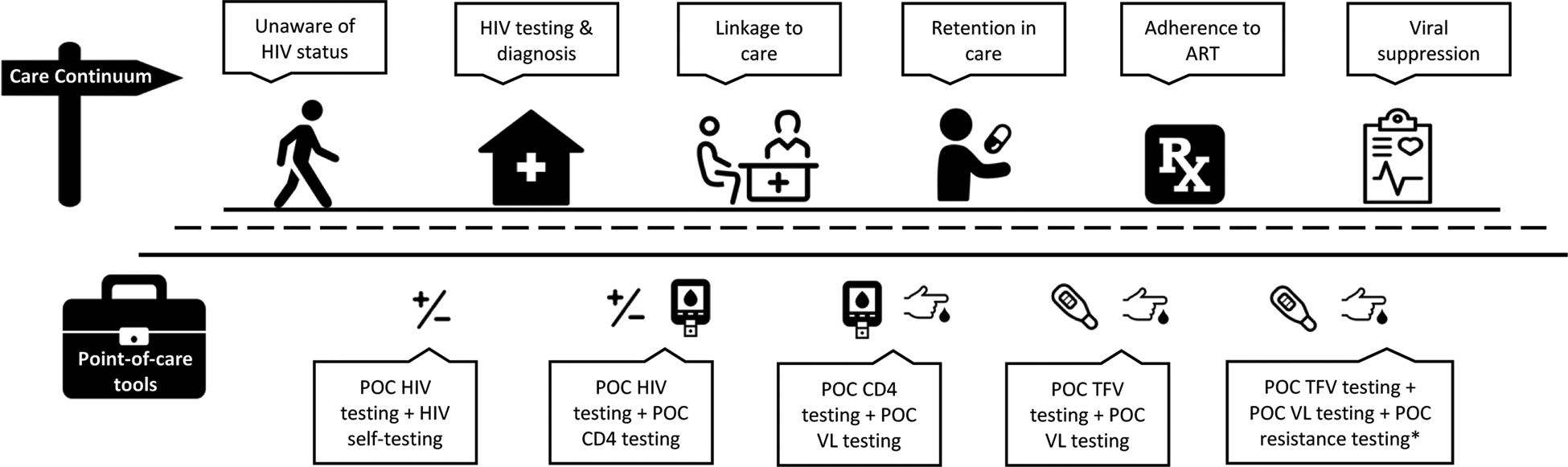
*Should a POC resistance test become available
TENOFOVIR AND TENOVOFIR-DIPHOSPHATE DRUG LEVELS FOR PrEP ADHERENCE COUNSELING
The use of antiretroviral drug levels was essential to understanding a strong dose-response relationship between PrEP dosing (as measured by plasma TFV levels) and observed efficacy in several PrEP clinical trials (51). In the era of PrEP implementation, using drug levels to guide PrEP adherence counseling is a nascent field, and few studies have used TFV-DP levels to provide feedback. Qualitative research among 59 MSM who participated in the iPrEX open label extension study indicated that detectable/undetectable drug level feedback was highly acceptable, and half the participants considered the information useful (98). In the VOICE PrEP trial, a subset of women who received feedback about their drug levels found the information to be useful and suggested that real-time drug level monitoring and feedback would promote more honest discussions about PrEP use (99). In a single-arm longitudinal study, a brief intervention based on results from drug level monitoring may have increased PrEP adherence among self-identified MSM and transgender women in Los Angeles (100).
The HIV Prevention Trials Network (HPTN) 082 study was designed to evaluate initiation and adherence to PrEP among African adolescent girls and young women (AGYW). AGYW were randomized to either a standard adherence support package (two-way SMS, adherence clubs, and brief counseling) versus enhanced adherence support, which also included antiretroviral drug level monitoring (via TFV-DP levels in DBS) with feedback and appropriate counseling. The drug level categories used a threshold of 500 fmol/punch at week four and 700 fmol/punch at week eight for the high adherence category, based on the threshold of TFV-DP that was associated with 100% effectiveness of PrEP among MSM in iPrEx OLE (65). At study conclusion, adherence at six months was similar between AGYW in the two adherence intervention arms (101). However, almost 40% of AGYW in the intervention arm did not receive drug level counseling because of shipping and laboratory turnaround time, missed visits, or incorrect counseling about adherence due to transcription errors (101).
Two studies will soon be starting to evaluate the acceptability, feasibility, and impact of implementing POC urine TFV adherence testing and counseling for PrEP. First, the PUMA trial will be a pilot randomized clinical trial to determine whether POC TFV adherence testing with subsequent feedback improves long-term PrEP adherence (measured via TFV levels in hair) among Kenyan women (Clinicaltrials.gov NCT03935464) (102). Second, the PrEP SMART study will compare the effectiveness of a scaled approach to PrEP adherence support strategies among South African AGYW (Clinicaltrials.gov NCT04038060) (103). In the PrEP SMART trial, young women initiating PrEP will be randomized to either two-way SMS or WhatsApp peer support groups, and those who have moderate or low PrEP drug levels after two months of use (intracellular TFV-DP levels <500 fmol/punch) will undergo a secondary randomization to more intensive adherence support, either quarterly drug level feedback based on detectable urine TFV via the POC assay or monthly adherence counseling sessions. These studies will inform the effectiveness of real-time adherence counseling based on urine TFV monitoring on subsequent PrEP adherence. Comparative acceptability, effectiveness, and cost-effectiveness studies of TFV assays that provide a measure of a longer window of PrEP use, such as TFV-DP levels in DBS or TFV levels in hair, are needed in diverse populations of PrEP users.
FUTURE DIRECTIONS IN POC ANTIRETROVIRAL TESTING
Existing POC urine TFV immunoassays are limited by the half-life of TFV in urine, which limits information on adherence to the prior week or less, informing drug level feedback and counseling accordingly. A POC test in development for measuring intracellular TFV-DP (which allows for longer-term adherence monitoring) may be an important tool in the future of adherence and therapeutic drug monitoring for ART and PrEP. New technologies for near real-time measurement of TFV-DP using benchtop platforms would provide timely, informative, and actionable information for patients and providers in a range of clinical settings. Drug measurements may also be useful for newer long-acting drug formulations, particularly during the long pharmacokinetic tail of long-acting drugs (104).
Benchtop liquid chromatography-mass spectrometry for near real-time drug testing
Liquid chromatography tandem mass spectrometry (LC-MS/MS) is widely used to measure TFV and TFV-DP to determine ART and PrEP adherence in research settings (44,62). Portable mass spectrometers have been developed for applications in forensics, environmental monitoring, and clinical diagnostics (105–108). LC-MS/MS technology may be possible to apply for near real-time drug level testing, and instruments could be adapted to detect a wide variety of biomarkers (109). While complex sample preparation and throughput are major barriers, newer portable instruments are compatible with complex sample matrices, easy-to-use disposable cartridges, and improved software for automated analysis (107). Perhaps the largest obstacle to widespread deployment of these instruments for HIV treatment and PrEP adherence monitoring are the unit and per-sample costs, which are still not competitive with POC antibody-based diagnostic tests. Nevertheless, clinical use of LC-MS/MS-based technology continues to increase for laboratory medicine and for diagnosing infections in clinical settings (109–111). The DBS assay developed by Anderson et al is now in development for near real-time use (62). Increasing miniaturization, as well as broader incorporation of LC-MS/MS technology into different areas of medicine, may converge to enable near real-time adherence testing in the setting of HIV treatment and prevention (112).
Detection of tenofovir-diphosphate by competitive immunoassay
A competitive immunoassay to measure TFV-DP could be developed into a POC lateral flow assay, but poses some additional challenges. Unlike TFV, which is freely circulating in urine or serum, measurement of intracellular TFV-DP requires cell lysis before detection. The clinical concentration range of TFV-DP in blood lysate is 15–170 fmol/106 RBCs or about 75 nM-850 nM in whole blood, which is two- to five-fold higher than the clinical range of TFV in plasma (17 nM-170 nM), and 20-fold lower than TFV in urine (1.7 μM-170 μM) (44,61). In addition, cell lysate contains high concentrations (0.1–1.5 mM) of adenosine triphosphate, which has a similar molecular structure to TFV and may lead to non-specific binding to the detection antibody (113). Cell lysis solutions can be integrated into the lateral flow consumable for POC diagnostic tests (114,115). A POC diagnostic test for TFV-DP will be more complex, but will also provide more clinical information than a POC TFV test alone. New immunoassay formats may also be able to use fingerprick blood samples since they are compatible with small sample volumes (<5 μL), and previous studies have established correlations between HIV drug metabolites in venous and fingerprick blood draws (116).
Detection of tenofovir diphosphate by enzymatic assay
In contrast to an immunoassay, TFV-DP could also be detected by an enzymatic assay that measures the drug’s pharmacological activity (117). DNA synthesis by reverse transcriptase (RT), the enzyme targeted by TFV-DP, can be measured using DNA templates, nucleotides, and fluorescent dyes to estimate TFV-DP concentration in a patient sample. A theoretical model to predict fluorescence output of the assay as a function of TFV-DP levels was developed and validated using TFV-DP spiked in whole blood to demonstrate the assay operates in clinically relevant samples (118). The assay distinguished TFV-DP levels corresponding to low and high PrEP adherence. The approach may be suitable as a POC test, since it is fast (30-minute assay time), requires a small volume of blood that can be collected from a fingerprick, and has a simple sample preparation step (dilution in water). Enzymatic assays monitoring activity of HIV drugs and their metabolites are currently being validated with clinical samples and benchmarked against drug level measurement by LC-MS/MS.
CONCLUSIONS
Newer technologies and assays are emerging that allow for POC and near real-time antiretroviral drug level testing for monitoring adherence to HIV treatment and prevention. These novel POC tests may improve real-time adherence counseling and interventions to reduce barriers and improve outcomes. Short-term metrics of adherence (such as TFV levels in urine) are more amenable to POC, but are more fallible to “white coat” dosing than long-term adherence metrics (80). Several POC urine TFV tests have been developed and will be evaluated in upcoming clinical trials to determine acceptability, feasibility, costs, and impact on subsequent ART or PrEP adherence. Furthermore, measurement of drug pharmacokinetics from POC tests may allow personalized assessment of drug metabolism and enable patients and providers to tailor ART dosing schedules and PrEP delivery.
As the antiretroviral drug landscape evolves for both ART and PrEP, identifying and assessing other potential drugs/metabolites to assess recent and/or cumulative adherence will be important. In addition, more research will be needed on the methodology transfer from sophisticated lab-based scientific instruments to quantify drug concentrations to less costly POC tests that enable providers to counsel about adherence at the same visit. Future POC and near real-time tests should be suitable for newer formulations of TFV, such as tenofovir alafenamide (TAF), but may need to be adapted for newer drug classes, including INSTIs (119). As efforts accelerate to end the HIV/AIDS epidemic, POC adherence monitoring may be an essential tool for both achieving viral suppression for PLHIV and preventing new infections with PrEP.
Footnotes
Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
Human and Animal rights and Informed Consent
This article does not contain any data from studies with human or animal subjects that was performed by any of the authors and has not already been published elsewhere.
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS). Global Report 2019: Report on the Global AIDS Epidemic. Geneva; 2019. [Google Scholar]
- 2.Walmsley SL, Antela A, Clumeck N, Duiculescu D, Eberhard A, Gutieŕrez F, et al. Dolutegravir plus Abacavir-Lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–18. [DOI] [PubMed] [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS (UNAIDS). Understanding Fast-Track Targets: Accelerating Action to End the AIDS Epidemic by 2030. 2015;12 Available from: https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Understanding_FastTrack_en.pdf [Google Scholar]
- 4.US Department of Health and Human Services (DHHS). What is ‘Ending the HIV Epidemic: A Plan for America’? [Internet]. HIV.gov. [cited 2019 Nov 7]. Available from: https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview
- 5.Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90 An ambitious treatment target to help end the AIDS epidemic [Internet]. Geneva; 2014. Available from: http://unaids.org/sites/default/files/media_asset/90-90-90_en.pdf [Google Scholar]
- 6.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med. 2016;375(9):830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brault MA, Spiegelman D, Hargreaves J, Nash D, Vermund SH. Treatment as Prevention: Concepts and Challenges for Reducing HIV Incidence. J Acquir Immune Defic Syndr. 2019. Dec 1;82:S104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molina JM, Charreau I, Chidiac C, Pialoux G, Cua E, Delaugerre C, et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect Dis. 2018. March 1;18(3):308–17. [DOI] [PubMed] [Google Scholar]
- 9.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010. December 30;363(27):2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012. August 2;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–90. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization (WHO). Policy Brief: WHO Expands Recommendation on Oral Pre-Exposure Prophylaxis of HIV Infection (PrEP) [Internet]. Geneva; 2015. Available from: http://apps.who.int/iris/bitstream/handle/10665/197906/WHO_HIV_2015.48_eng.pdf;jsessionid=FD090759CBB747974F6F77E7F020202D?sequence=1 [Google Scholar]
- 13.Smith Dawn K.; Van Handel Michelle; Wolitski Richard J.; Stryker Jo Ellen; Hall H. Irene; Prejean Joseph; Koenig Linda J.; Valleroy LA Vital Signs: Estimated Percentages and Numbers of Adults with Indications for Preexposure Prophylaxis to Prevent HIV Acquisition — United States, 2015. Morb Mortal Wkly Rep. 2015;64(46):1291–5. [DOI] [PubMed] [Google Scholar]
- 14.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012. September 12;4(151). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang C-J, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001. October;33(8):1417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haberer JE, Musinguzi N, Boum Y, Siedner MJ, Mocello AR, Hunt PW, et al. Duration of Antiretroviral Therapy Adherence Interruption Is Associated With Risk of Virologic Rebound as Determined by Real-Time Adherence Monitoring in Rural Uganda. J Acquir Immune Defic Syndr. 2015;70(4):386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell KM, Haberer JE. Actionable Adherence Monitoring: Technological Methods to Monitor and Support Adherence to Antiretroviral Therapy. Curr HIV/AIDS Rep. 2018;15(5):388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Bärnighausen T. Interventions to improve adherence to antiretroviral therapy: A rapid systematic review. AIDS. 2014;28(Suppl 2). [DOI] [PubMed] [Google Scholar]
- 19.Fox MP, Rosen S. Retention of Adult Patients on Antiretroviral Therapy in Low- and Middle-Income Countries: Systematic Review and Meta-analysis 2008–2013. J Acquir Immune Defic Syndr. 2015. May 1;69(1):98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stirratt MJ, Dunbar-Jacob J, Crane HM, Simoni JM, Czajkowski S, Hilliard ME, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015. December 1;5(4):470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearson CR, Simoni JM, Hoff P, Kurth AE, Martin DP. Assessing antiretroviral adherence via electronic drug monitoring and self-report: an examination of key methodological issues. AIDS Behav. 2007. March;11(2):161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav. 2006. May;10(3):227–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castillo-Mancilla JR, Haberer JE. Adherence Measurements in HIV: New Advancements in Pharmacologic Methods and Real-Time Monitoring. Curr HIV/AIDS Rep. 2018. February;15(1):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orrell C, Cohen K, Leisegang R, Bangsberg DR, Wood R, Maartens G. Comparison of six methods to estimate adherence in an ART-naïve cohort in a resource-poor setting: which best predicts virological and resistance outcomes? AIDS Res Ther. 2017. April 4;14(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walshe L, Saple DG, Mehta SH, Shah B, Bollinger RC, Gupta A. Physician estimate of antiretroviral adherence in India: Poor correlation with patient self-report and viral load. AIDS Patient Care STDS. 2010. March 1;24(3):189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haberer JE, Robbins GK, Ybarra M, Monk A, Ragland K, Weiser SD, et al. Real-time electronic adherence monitoring is feasible, comparable to unannounced pill counts, and acceptable. AIDS Behav. 2012;16(2):375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agot K, Taylor D, Corneli AL, Wang M, Ambia J, Kashuba ADM, et al. Accuracy of Self-Report and Pill-Count Measures of Adherence in the FEM-PrEP Clinical Trial: Implications for Future HIV-Prevention Trials. AIDS Behav. 2015. May 1;19(5):743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saberi P, Chakravarty D, Ming K, Legnitto D, Gandhi M, Johnson MO, et al. Moving Antiretroviral Adherence Assessments to the Modern Era: Correlations Among Three Novel Measures of Adherence. AIDS Behav. 2019;24(1):284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genn L, Chapman J, Okatch H, Abell N, Marukutira T, Tshume O, et al. Pharmacy Refill Data are Poor Predictors of Virologic Treatment Outcomes in Adolescents with HIV in Botswana. AIDS Behav. 2019. August;23(8):2130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acri T, TenHave TR, Chapman JC, Bogner HR, Gross R. Lack of association between retrospectively collected pharmacy refill data and electronic drug monitoring of antiretroviral adherence. AIDS Behav. 2010. August;14(4):748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grossberg R, Gross R. Use of pharmacy refill data as a measure of antiretroviral adherence. Curr HIV/AIDS Rep. 2007. December;4(4):187–91. [DOI] [PubMed] [Google Scholar]
- 32.McMahon JH, Jordan MR, Kelley K, Bertagnolio S, Hong SY, Wanke CA, et al. Pharmacy adherence measures to assess adherence to antiretroviral therapy: review of the literature and implications for treatment monitoring. Clin Infect Dis. 2011. February 15;52(4):493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luma HN, Mbatchou Ngahane BH, Mapoure YN, Mengjo NB, Temfack E, Joko HA, et al. Cross-sectional assessment of three commonly used measures of adherence to combination antiviral therapy in a resource limited setting. Int J STD AIDS. 2015;28(1):69–76. [DOI] [PubMed] [Google Scholar]
- 34.Kunutsor S, Walley J, Katabira E, Muchuro S, Balidawa H, Namagala E, et al. Clinic Attendance for Medication Refills and Medication Adherence amongst an Antiretroviral Treatment Cohort in Uganda: A Prospective Study. AIDS Res Treat. 2010;2010:872396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bastard M, Pinoges L, Balkan S, Szumilin E, Ferreyra C, Pujades-Rodriguez M. Timeliness of Clinic Attendance Is a Good Predictor of Virological Response and Resistance to Antiretroviral Drugs in HIV-Infected Patients. PLoS One. 2012. November 7;7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garrison LE, Haberer JE. Technological methods to measure adherence to antiretroviral therapy and preexposure prophylaxis. Curr Opin HIV AIDS. 2017. September;12(5):467–74. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization (WHO), US President’s Emergency Plan for AIDS Relief (PEPFAR), Joint United Nations Programme on HIV/AIDS (UNAIDS). Task shifting: rational redistribution of tasks among health workforce teams: global recommendations and guidelines. Geneva; 2007. [Google Scholar]
- 38.Grimsrud A, Kaplan R, Bekker L-G, Myer L. Outcomes of a nurse-managed service for stable HIV-positive patients in a large South African public sector antiretroviral therapy programme. Trop Med Int Heal. 2014. September;19(9):1029–39. [DOI] [PubMed] [Google Scholar]
- 39.Sanne I, Orrell C, Fox MP, Conradie F, Ive P, Zeinecker J, et al. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): A randomised non-inferiority trial. Lancet. 2010;376(9734):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherr K, Pfeiffer J, Mussa A, Vio F, Gimbel S, Micek M, et al. The role of nonphysician clinicians in the rapid expansion of HIV care in Mozambique. J Acquir Immune Defic Syndr. 2009. November;52 Suppl 1:S20–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brooks KM, Anderson PL. Pharmacologic-Based Methods of Adherence Assessment in HIV Prevention. Clin Pharmacol Ther. 2018. December 1;104(6):1056–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hendrix CW, Andrade A, Bumpus NN, Kashuba AD, Marzinke MA, Moore A, et al. Dose Frequency Ranging Pharmacokinetic Study of Tenofovir-Emtricitabine After Directly Observed Dosing in Healthy Volunteers to Establish Adherence Benchmarks (HPTN 066). AIDS Res Hum Retroviruses. 2016. January;32(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koenig H, Mounzer K, Daughtridge G, Sloan C, Lalley-Chareczko L, Moorthy G, et al. Urine assay for tenofovir to monitor adherence in real time to tenofovir disoproxil fumarate/emtricitabine as pre-exposure prophylaxis. HIV Med. 2017. July;18(6):412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drain PK, Kubiak RW, Siriprakaisil O, Klinbuayaem V, Quame-Amaglo J, Sukrakanchana P-O, et al. Urine Tenofovir Concentrations Correlate with Plasma and Relates to TDF Adherence: A Randomized Directly-observed Pharmacokinetic Trial (TARGET Study). Clin Infect Dis. 2019;69(9):1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haaland RE, Martin A, Livermont T, Fountain J, Dinh C, Holder A, et al. Brief Report: Urine Emtricitabine and Tenofovir Concentrations Provide Markers of Recent Antiretroviral Drug Exposure Among HIV-Negative Men Who Have Sex With Men. J Acquir Immune Defic Syndr. 2019. Nov 1;82(3):252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fonsart J, Saragosti S, Taouk M, Peytavin G, Bushman L, Charreau I, et al. Single-dose pharmacokinetics and pharmacodynamics of oral tenofovir and emtricitabine in blood, saliva and rectal tissue: a sub-study of the ANRS IPERGAY trial. J Antimicrob Chemother. 2017;72(2):478–85. [DOI] [PubMed] [Google Scholar]
- 47.Castillo-Mancilla JR, Bushman LR, Meditz A, Seifert SM, Zheng J-H, Guida LA, et al. Emtricitabine-Triphosphate in Dried Blood Spots (DBS) as a Marker of Recent Dosing In: Conference on Retroviruses and Opportunistic Infections. Seattle; 2015. [Google Scholar]
- 48.Seifert SM, Chen X, Meditz AL, Castillo-Mancilla JR, Gardner EM, Predhomme JA, et al. Intracellular Tenofovir and Emtricitabine Anabolites in Genital, Rectal, and Blood Compartments from First Dose to Steady State. AIDS Res Hum Retroviruses. 2016. November 1;32(10–11):981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. “White coat compliance” limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV Clin Trials. 2008. July;9(4):238–46. [DOI] [PubMed] [Google Scholar]
- 50.Donnell D, Baeten JM, Bumpus NN, Brantley J, Bangsberg DR, Haberer JE, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014. July 1;66(3):340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O’Reilly KR, Koechlin FM, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016. July 31;30(12):1973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips TK, Sinxadi P, Abrams EJ, Zerbe A, Orrell C, Hu N-C, et al. A Comparison of Plasma Efavirenz and Tenofovir, Dried Blood Spot Tenofovir-Diphosphate, and Self-Reported Adherence to Predict Virologic Suppression Among South African Women. J Acquir Immune Defic Syndr. 2019. July 1;81(3):311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Lastours V, Fonsart J, Burlacu R, Gourmel B, Molina J-M. Concentrations of tenofovir and emtricitabine in saliva: implications for preexposure prophylaxis of oral HIV acquisition. Antimicrob Agents Chemother. 2011. October;55(10):4905–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krause J, Subklew-Sehume F, Kenyon C, Colebunders R. Acceptability of HIV self-testing: a systematic literature review. BMC Public Health. 2013. Dec 8;13(1):735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kowalczyk Mullins TL, Braverman PK, Dorn LD, Kollar LM, Kahn JA. Adolescent Preferences for Human Immunodeficiency Virus Testing Methods and Impact of Rapid Tests on Receipt of Results. J Adolesc Heal. 2010. Feb;46(2):162–8. [DOI] [PubMed] [Google Scholar]
- 56.Manchikanti L, Malla Y, Wargo BW, Cash KA, Pampati V, Damron KS, et al. Protocol for accuracy of point of care (POC) or in-office urine drug testing (immunoassay) in chronic pain patients: A prospective analysis of immunoassay and liquid chromatography tandem mass spectometry (LC/MS/MS). Pain Physician. 2010. January;13(1). [PubMed] [Google Scholar]
- 57.Yager J, Castillo-Mancilla JR, Ibrahim ME, Brooks KM, McHugh C, MaWhinney S, et al. Tenofovir-Diphosphate in Dried Blood Spots Following Escalating TAF/FTC Dosing In: Conference on Retroviruses and Opportunistic Infections Seattle; 2019. [Google Scholar]
- 58.Zheng J-H, Rower C, McAllister K, Castillo-Mancilla J, Klein B, Meditz A, et al. Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal. 2016. April 15;122:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu AY, Yang Q, Huang Y, Bacchetti P, Anderson PL, Jin C, et al. Strong Relationship between Oral Dose and Tenofovir Hair Levels in a Randomized Trial: Hair as a Potential Adherence Measure for Pre-Exposure Prophylaxis (PrEP). PLoS One. 2014. January 8;9(1):e83736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adams JL, Sykes C, Menezes P, Prince HMA, Patterson KB, Fransen K, et al. Tenofovir diphosphate and emtricitabine triphosphate concentrations in blood cells compared with isolated peripheral blood mononuclear cells: a new measure of antiretroviral adherence? J Acquir Immune Defic Syndr. 2013. March 1;62(3):260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castillo-Mancilla JR, Zheng J-H, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013. February;29(2):384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anderson PL, Liu AY, Castillo-Mancilla JR, Gardner EM, Seifert SM, McHugh C, et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother. 2018. January 1;62(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: Clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43(9):595–612. [DOI] [PubMed] [Google Scholar]
- 64.Koss CA, Bacchetti P, Hillier SL, Livant E, Horng H, Mgodi N, et al. Differences in Cumulative Exposure and Adherence to Tenofovir in the VOICE, iPrEx OLE, and PrEP Demo Studies as Determined via Hair Concentrations. AIDS Res Hum Retroviruses. 2017. August;33(8):778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: A cohort study. Lancet Infect Dis. 2014;14(9):820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Castillo-Mancilla JR, Morrow M, Coyle RP, Coleman SS, Gardner EM, Zheng J-H, et al. Tenofovir Diphosphate in Dried Blood Spots Is Strongly Associated With Viral Suppression in Individuals With Human Immunodeficiency Virus Infections. Clin Infect Dis. 2019. April 8;68(8):1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morrow M, MaWhinney S, Coyle RP, Coleman SS, Gardner EM, Zheng J-H, et al. Predictive Value of Tenofovir Diphosphate in Dried Blood Spots for Future Viremia in Persons Living With HIV. J Infect Dis. 2019. April 3; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koren G, Bellaish E, Maman K. Hair Analysis for Drug-Facilitated Crime: The Critical Role of Hair Growth Rate. J Forensic Sci. 2019. September;64(5):1574–5. [DOI] [PubMed] [Google Scholar]
- 69.Gandhi M, Glidden DV., Liu A, Anderson PL, Horng H, Defechereux P, et al. Strong Correlation Between Concentrations of Tenofovir (TFV) Emtricitabine (FTC) in Hair and TFV Diphosphate and FTC Triphosphate in Dried Blood Spots in the iPrEx Open Label Extension: Implications for Pre-exposure Prophylaxis Adherence Monitoring. J Infect Dis. 2015. November 1;212(9):1402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gandhi M, Murnane PM, Bacchetti P, Elion R, Kolber MA, Cohen SE, et al. Hair levels of preexposure prophylaxis drugs measure adherence and are associated with renal decline among men/transwomen. AIDS. 2017. October 23;31(16):2245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thaden JT, Gandhi M, Okochi H, Hurt CB, McKellar MS. Seroconversion on preexposure prophylaxis: a case report with segmental hair analysis for timed adherence determination. AIDS. 2018;32(9):F1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gilliland WM, Prince HMA, Poliseno A, Kashuba ADM, Rosen EP. Infrared Matrix-Assisted Laser Desorption Electrospray Ionization Mass Spectrometry Imaging of Human Hair to Characterize Longitudinal Profiles of the Antiretroviral Maraviroc for Adherence Monitoring. Anal Chem. 2019. August 20;91(16):10816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen SE, Sachdev D, Lee SA, Scheer S, Bacon O, Chen MJ, et al. Acquisition of tenofovir-susceptible, emtricitabine-resistant HIV despite high adherence to daily pre-exposure prophylaxis: a case report. Lancet HIV. 2019. January 1;6(1):e43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gandhi M, Ameli N, Bacchetti P, Anastos K, Gange SJ, Minkoff H, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis. 2011. May;52(10):1267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tüdos AJ, Besselink GAJ, Schasfoort RBM. Trends in miniaturized total analysis systems for point-of-care testing in clinical chemistry. Lab Chip. 2001. December 1;1(2):83–95. [DOI] [PubMed] [Google Scholar]
- 76.Pratt GW, Fan A, Melakeberhan B, Klapperich CM. A competitive lateral flow assay for the detection of tenofovir. Anal Chim Acta. 2018. August 9;1017:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gandhi M, Bacchetti P, Rodrigues WC, Spinelli M, Koss CA, Drain PK, et al. Development and Validation of an Immunoassay for Tenofovir in Urine as a Real-Time Metric of Antiretroviral Adherence. EClinicalMedicine. 2018. August;34(2):255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gandhi M, Wang G, King R, Rodrigues WC, Vincent M, Glidden DV., et al. Development and Validation of the First Point-of-Care Assay to Objectively Monitor Adherence to HIV Treatment and Prevention in Real-Time in Routine Settings. AIDS. 2019. October;34(2):255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Daughtridge G, Hebel S, Larabee L, Patani H, Cohen A, Fischl M, et al. Development and Clinical Use Case of a Urine Tenofovir Adherence Test In: HIV Diagnostics Conference. Atlanta; 2019. [Google Scholar]
- 80.Gandhi M, Bacchetti P, Spinelli MA, Okochi H, Baeten JM, Siriprakaisil O, et al. Brief Report: Validation of a Urine Tenofovir Immunoassay for Adherence Monitoring to PrEP and ART and Establishing the Cutoff for a Point-of-Care Test. J Acquir Immune Defic Syndr. 2019. May 1;81(1):72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.US Centers for Disease Control and Prevention (CDC). Screening in Clinical Settings | Screening for HIV [Internet]. 2019. [cited 2019 Dec 1]. Available from: https://www.cdc.gov/hiv/clinicians/screening/clinical-settings.html
- 82.World Health Organization (WHO). Consolidated guidelines on HIV testing services 2015 [Internet]. 2015. [cited 2019 Dec 1]. Available from: http://apps.who.int/iris/bitstream/10665/179870/1/9789241508926_eng.pdf?ua=1&ua=1 [PubMed]
- 83.World Health Organization (WHO). WHO recommends HIV self-testing: Policy brief [Internet]. World Health Organization (WHO) Geneva: World Health Organization; 2016. [cited 2019 Jul 23]. Available from: https://www.who.int/hiv/pub/vct/who-recommends-hiv-self-testing/en/ [Google Scholar]
- 84.Clinicaltrials.gov. Identifier NCT04341779, Simplifying Treatment and Monitoring for HIV. Bethesda: National Library of Medicine (US); 2020. [Google Scholar]
- 85.Bardon AR, Simoni JM, Layman LM, Stekler JD, Drain PK. Utility and acceptability of a point-of-care urine tenofovir test for adherence to HIV pre-exposure prophylaxis and antiretroviral therapy: a qualitative assessment among U.S. clients and providers. 2019;Manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu G, Zaman MH. Low-cost tools for diagnosing and monitoring HIV infection in low-resource settings. Bull World Health Organ. 2012. December;90(12):914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson CC, Kennedy C, Fonner V, Siegfried N, Figueroa C, Dalal S, et al. Examining the effects of HIV self-Testing compared to standard HIV testing services: A systematic review and meta-Analysis. J Int AIDS Soc. 2017;20(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vojnov L, Markby J, Boeke C, Harris L, Ford N, Peter T. POC CD4 Testing Improves Linkage to HIV Care and Timeliness of ART Initiation in a Public Health Approach: A Systematic Review and Meta-Analysis Roques P, editor. PLoS One. 2016. May 13;11(5):e0155256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rice B, Boulle A, Schwarcz S, Shroufi A, Rutherford G, Hargreaves J. The continuing value of CD4 cell count monitoring for differential HIV care and surveillance. J Med Internet Res. 2019. March 1;21(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.World Health Organization (WHO). Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy [Internet]. Geneva; 2017. [cited 2020 Jan 12]. Available from: https://www.who.int/hiv/pub/guidelines/advanced-HIV-disease/en/ [PubMed] [Google Scholar]
- 91.Drain PK, Dorward J, Bender A, Lillis L, Marinucci F, Sacks J, et al. Point-of-Care HIV Viral Load Testing: an Essential Tool for a Sustainable Global HIV/AIDS Response. Clin Microbiol Rev. 2019. June 19;32(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Agutu CA, Ngetsa CJ, Price MA, Rinke de Wit TF, Omosa-Manyonyi G, Sanders EJ, et al. Systematic review of the performance and clinical utility of point of care HIV-1 RNA testing for diagnosis and care. PLoS One. 2019;14(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Drain PK, Dorward J, Violette LR, Quame-Amaglo J, Thomas KK, Samsunder N, et al. Point-of-care HIV viral load testing combined with task shifting to improve treatment outcomes: an open-label non-inferiority randomized controlled trial to Simplify HIV TREAtment and Monitoring (STREAM Study). Lancet HIV. 2020;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nicholas S, Poulet E, Wolters L, Wapling J, Rakesh A, Amoros I, et al. Point-of-care viral load monitoring: outcomes from a decentralized HIV programme in Malawi. J Int AIDS Soc. 2019;22(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Estill J, Egger M, Blaser N, Vizcaya LS, Garone D, Wood R, et al. Cost-effectiveness of point-of-care viral load monitoring of antiretroviral therapy in resource-limited settings: Mathematical modelling study. AIDS. 2013. June 1;27(9):1483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Necker M, de Beer JC, Stander MP, Connell CD, Mwai D. Economic and public health impact of decentralized HIV viral load testing: A modelling study in Kenya. PLoS One. 2019. February 1;14(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Simeon K, Sharma M, Dorward J, Naidoo J, Dlamini N, Moodley P, et al. Comparative cost analysis of point-of-care versus laboratory-based testing to initiate and monitor HIV treatment in South Africa. PLoS One. 2019;14(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koester KA, Liu A, Eden C, Amico KR, McMahan V, Goicochea P, et al. Acceptability of drug detection monitoring among participants in an open-label pre-exposure prophylaxis study. AIDS Care. 2015. October 3;27(10):1199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Montgomery ET, Mensch B, Musara P, Hartmann M, Woeber K, Etima J, et al. Misreporting of Product Adherence in the MTN-003/VOICE Trial for HIV Prevention in Africa: Participants’ Explanations for Dishonesty. AIDS Behav. 2017. February 1;21(2):481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Landovitz RJ, Beymer M, Kofron R, Amico KR, Psaros C, Bushman L, et al. Plasma Tenofovir Levels to Support Adherence to TDF/FTC Preexposure Prophylaxis for HIV Prevention in MSM in Los Angeles, California. J Acquir Immune Defic Syndr [Internet]. 2017. [cited 2019 May 17];76(5):501–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28902074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Celum C, Mgodi N, Bekker L-G, Hosek S, Donnell D, Anderson PL, et al. PrEP Adherence and Effect of Drug Level Feedback Among Young African Women in HPTN 082. In: International AIDS Society. Mexico City; 2019. [Google Scholar]
- 102.Clinicaltrials.gov. Identifier NCT03935464, Point-of-care Urine Monitoring of Adherence (PUMA): Testing a Real-Time Urine Assay of Tenofovir in PrEP (PUMA) [Internet]. Bethesda: National Library of Medicine (US); 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT03935464 [Google Scholar]
- 103.Clinicaltrials.gov. Identifer NCT04038060, The PrEP (Pre-exposure Prophylaxis) SMART Study [Internet]. Bethesda: National Library of Medicine (US); 2019. Available from: https://clinicaltrials.gov/ct2/show/study/NCT04038060 [Google Scholar]
- 104.Spreen WR, Margolis DA, Pottage JC. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS. 2013. November;8(6):565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Devereaux ZJ, Reynolds CA, Fischer JL, Foley CD, DeLeeuw JL, Wager-Miller J, et al. Matrix-Assisted Ionization on a Portable Mass Spectrometer: Analysis Directly from Biological and Synthetic Materials. Anal Chem. 2016;88(22):10831–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Da Silva LC, Pereira I, De Carvalho TC, Allochio Filho JF, Romão W, Vaz BG. Paper spray ionization and portable mass spectrometers: A review. Anal Methods. 2019;11(8):999–1013. [Google Scholar]
- 107.Li L, Chen TC, Ren Y, Hendricks PI, Cooks RG, Ouyang Z. Mini 12, miniature mass spectrometer for clinical and other applications - Introduction and characterization. Anal Chem. 2014;86(6):2909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gao L, Sugiarto A, Harper JD, Cooks RG, Ouyang Z. Design and characterization of a multisource hand-held tandem mass spectrometer. Anal Chem. 2008. October 1;80(19):7198–205. [DOI] [PubMed] [Google Scholar]
- 109.Jannetto PJ, Fitzgerald RL. Effective use of mass spectrometry in the clinical laboratory. Clin Chem. 2016. January 1;62(1):92–8. [DOI] [PubMed] [Google Scholar]
- 110.Patel TS, Kaakeh R, Nagel JL, Newton DW, Stevenson JG. Cost analysis of implementing matrix- assisted laser desorption ionization-time of flight mass spectrometry plus real-time antimicrobial stewardship intervention for bloodstream infections. J Clin Microbiol. 2017. January 1;55(1):60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heaney LM, Jones DJ, Suzuki T. Mass spectrometry in medicine: A technology for the future? Futur Sci OA. 2017;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pu F, Pandey S, Bushman LR, Anderson PL, Ouyang Z, Cooks RG. Direct quantitation of tenofovir diphosphate in human blood with mass spectrometry for adherence monitoring. Anal Bioanal Chem. 2020. January 2; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ataullakhanov FI, Vitvitsky VM. What determines the intracellular ATP concentration. Biosci Rep. 2002. October;22(5–6):501–11. [DOI] [PubMed] [Google Scholar]
- 114.Seok Y, Jang H, Oh J, Joung HA, Kim MG. A handheld lateral flow strip for rapid DNA extraction from staphylococcus aureus cell spiked in various samples. Biomed Phys Eng Express. 2019. April 17;5(3). [Google Scholar]
- 115.VanDine RW, Mahesh Babu U, Sambursky RP. US8614101B2 - In situ lysis of cells in lateral flow immunoassays [Internet]. US; 2009. [cited 2019 Dec 10]. Available from: https://patents.google.com/patent/US8614101B2/en
- 116.Castillo-Mancilla J, Seifert S, Campbell K, Coleman S, McAllister K, Zheng JH, et al. Emtricitabine-Triphosphate in dried blood spots as a marker of recent dosing. Antimicrob Agents Chemother. 2016. November 1;60(11):6692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Olanrewaju AO, Posner JD. An enzymatic assay to measure long-term adherence to pre-exposure prophylaxis and antiretroviral therapy. bioRxiv Bioeng. [Google Scholar]
- 118.Olanrewaju A, Sullivan B, Bender A, Zhang J, Lo T, Bardon A, et al. Development of an enzymatic assay for quantitative measurement of adherence to antiretroviral therapy and pre-exposure prophylaxis In: The 14th International Conference on HIV Treatment and Prevention Adherence. Miami; 2019. [Google Scholar]
- 119.Ruane PJ, DeJesus E, Berger D, Markowitz M, Bredeek UF, Callebaut C, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013. Aug 1;63(4):449–55. [DOI] [PubMed] [Google Scholar]


