Abstract
The prolonged impairment in muscle strength, power and fatigue resistance following eccentric exercise has been ascribed to a plethora of mechanisms, including delayed muscle refueling and microvascular and mitochondrial dysfunction. This review will explore the hypothesis that local heat therapy (HT) hastens functional recovery following strenuous eccentric exercise by facilitating glycogen resynthesis, reversing vascular derangements, augmenting mitochondrial function and stimulating muscle protein synthesis.
Keywords: Heat therapy, Eccentric exercise, Recovery, Muscle function, Muscle damage
SUMMARY for TOC:
Local heat therapy hastens recovery after eccentric exercise.
INTRODUCTION
Unaccustomed exercise as well as major changes in the frequency, intensity and volume of training may result in muscle damage and consequently in a myriad of symptoms, including muscle soreness and a prolonged loss of muscle function (1). These manifestations are particularly evident following activities involving lengthening (eccentric) contractions. For example, isolated eccentric exercise leads to histological alterations of muscle fibers and the surrounding extracellular matrix, decreased muscle strength, increased muscle swelling, and increased stiffness (2). Similar effects may be elicited by prolonged whole-body exercise with an eccentric contraction component or bias such as downhill running or downward-stepping exercise (3). Although temporary, these symptoms might result in poor adherence to exercise regimens and ultimately impair competitive performance and the adaptations to training.
The marked and prolonged reductions in maximal strength and power are thought to be the most reliable indirect markers of exercise-induced muscle damage (4). A single bout of repeated maximal eccentric exercise of the knee extensors has been shown to promote a 40–50% reduction in maximal strength from baseline levels that persists for several days or even weeks (5, 6). Competitive team-sports events such as basketball (7) and soccer (8), which entail sudden changes in direction and speed, also elicit temporary (24–48 h) reductions in muscle strength. Exercise-induced muscle damage reduces performance in endurance time trials and time-to-exhaustion tests (9). This marked and prolonged decline in muscle function, along with other debilitating symptoms such as local pain, prompted the widespread use of a number of recovery strategies after intense exercise, such as cold water immersion, compression garments, foam rolling, massage and others. Unfortunately, the available evidence reveals that the vast majority of these methods fail to mitigate the detrimental consequences of intense exercise on muscle strength, power and work capacity (10–13).
Local heat therapy (HT) modalities (e.g. shortwave diathermy, water-circulating garments, heat wraps, whirlpool therapy, etc) are commonly employed in the management of chronic musculoskeletal conditions associated with pain, increased tissue stiffness and reduced range of motion (14). These methods allow for specific muscle groups or body parts to be heated with minimal or no changes in body core temperature. Building upon the finding that HT hastens the acute recovery of contractile function following fatiguing arm exercise (15) and reports that pre-exercise HT protects against eccentric exercise-induced muscle damage (16, 17), we recently investigated the impact of repeated local HT on the recovery of muscle function after a bout of maximal, unaccustomed eccentric contractions of the knee extensors (5). One randomly selected thigh was treated with local HT for 90 min immediately after and 24, 48, 72 and 96h following the exercise bout, whereas the opposite thigh received a thermoneutral intervention. Fatigue resistance, as determined by the total work amount completed during 28 consecutive maximal isokinetic contractions at 180°/s, recovered faster in the thigh exposed to HT as compared with the control thigh (Figure 1). Perceived muscle soreness also tended to decrease faster following repeated exposure to HT relative to the control treatment (5).
FIGURE 1:
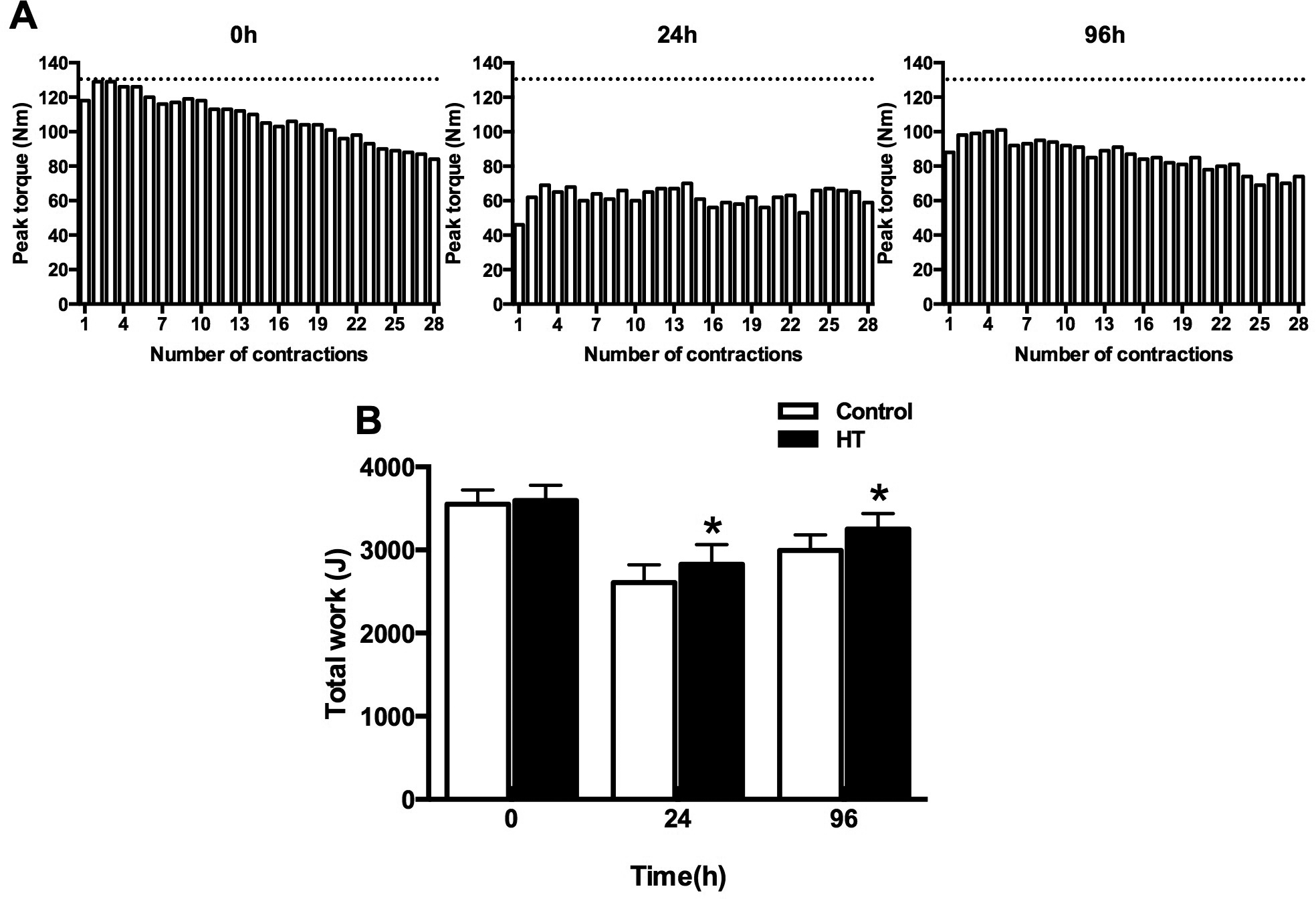
Local heat therapy (HT) hastens the recovery of work capacity of the knee-extensors in humans following maximal eccentric exercise. Young individuals performed unaccustomed maximal eccentric exercise and had one thigh treated with local HT for 90 min immediately after the exercise bout and during 4 subsequent days. Fatigue resistance of the knee-extensors was assessed by quantifying the total work completed during a bout of 28 maximal isokinetic contractions at 180°/s. The upper panels show examples of the temporal changes in torque during the fatigue bout at baseline and at 1 and 4 days following the completion of eccentric exercise. As anticipated, maximal lengthening contractions elicited a marked and persistent decline in fatigue resistance of the knee extensors. Of note, however, exposure to local HT abrogated this effect as revealed by the lower reduction in total work relative to baseline (lower panel). *Main effect for HT (p=0.02). Based on data from (5).
The mechanisms by which local HT facilitates the recovery of muscle function following exercise-induced muscle damage remains undefined. The functional decline following intense eccentric exercise, particularly the ability to do work and resist fatigue, stems from a plethora of mechanisms, including alterations in excitation-contraction coupling (9), impaired muscle refueling (18, 19), vascular derangements (20–22) and mitochondrial dysfunction (23–25). This review will explore the hypothesis that local HT abrogates the negative effects of intense exercise on skeletal muscle metabolism and on the vasculature, and as a result, accelerates the recovery of muscle performance. Specifically, we postulate that heat-induced increases in intramuscular temperature and the consequent elevation in muscle blood flow initiate a cascade of events that culminate with improved replenishment of the muscle energy stores, improved vascular and mitochondrial function and reduced muscle soreness (Figure 2). We first review evidence implicating elevated tissue temperature and muscle hyperemia as important mediators of the therapeutic effects of local HT. Subsequently we discuss putative specific mechanisms by which repeated exposure to local HT restores muscle work capacity and fatigue resistance following strenuous eccentric exercise. The readers are referred to other excellent reviews that address the potential for HT to hasten recovery following muscle injuries (26) and to augment the adaptations to endurance (27) and strength training (26).
FIGURE 2:
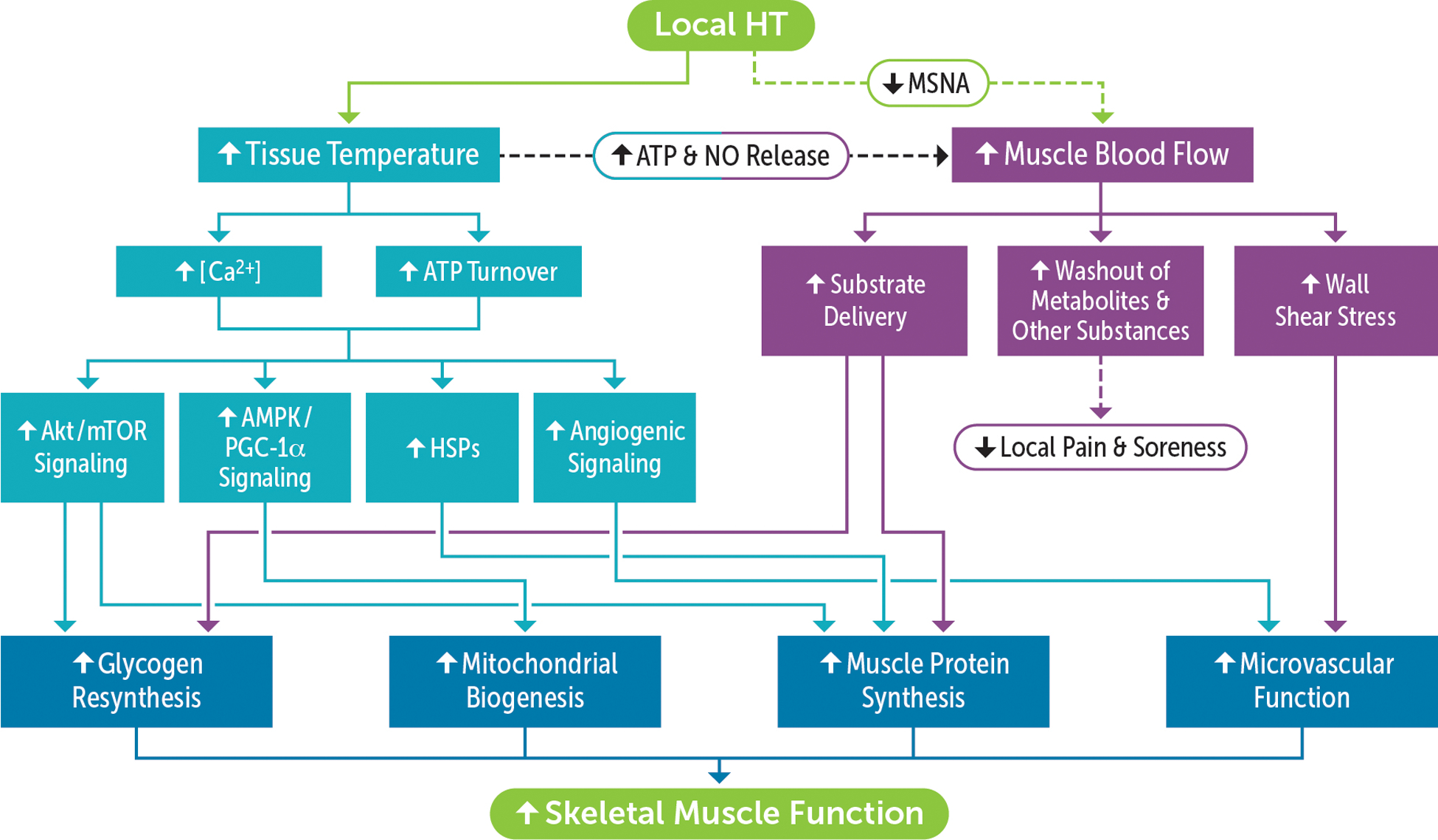
Conceptual framework for the mechanistic basis underlying the therapeutic effects of local heat therapy (HT) on post-exercise recovery. Elevated muscle temperature during exposure to HT perturbs cellular homeostasis and stimulates multiple signaling pathways involved, among others, in muscle hypertrophy, mitochondrial biogenesis and angiogenesis. In addition, activation of temperature-sensitive mechanisms evokes an increase in local muscle blood flow, thereby facilitating the delivery of substrates needed for muscle refueling and repair and the removal of substances that sensitize muscle nociceptors. Repeated increases in vascular shear rate during HT sessions may also enhance vasomotor function and increase the expression of angiogenic factors. Combined, local hyperthermia and hyperemia may reduce muscle soreness and induce a cluster of responses that favor the recovery of contractile function, including accelerated glycogen resynthesis and reversal of the detrimental impact of strenuous exercise on the vasculature as well as the mitochondria.
KEY STIMULI UNDERPINNING THE PHYSIOLOGICAL ADAPTATIONS TO HT
Perturbations in cellular homeostasis during exposure to heat stress, including increases in myocellular calcium and enhanced ATP turnover, elicit the activation of a multitude of intracellular signaling networks. Below we provide an overview of the pioneering studies in cultured cells that revealed the potent effects of heat stress on signaling pathways involved in the regulation of vascular function and remodeling, protein synthesis and mitochondrial biogenesis. Subsequently we summarize the studies that examined the impact of local HT on muscle blood flow. We focus on HT-induced muscle hyperemia, as opposed to other physiological adjustments, because of its critical role in mitigating the negative impact of intense exercise on muscle refueling and vascular function.
Signalling Responses to Elevated Tissue Temperature
Discerning the isolated contribution of elevated tissue temperature to the adaptations to HT is difficult to achieve using in vivo models, in which other important mechanisms (e.g. heat-induced hyperemia) are present. One approach that overcomes this barrier is the use of cultured cells subjected to brief episodes of heat stress. Experiments in cultured murine C2C12 myotubes and rat myoblast cells (L6) revealed that even in the absence of increased blood flow and other cofounding factors, increased temperature elicits marked effects on signaling pathways involved in the regulation of muscle mass, cellular stress management and mitochondrial biogenesis. Goto and co-workers were among the first to demonstrate that incubation of L6 cells at 41°C for 60 min stimulates protein synthesis, as revealed by an increase in protein content (28). These findings were later confirmed in C2C12 cells (29, 30) and appear to be mediated in part by heat-induced stimulation of the mammalian target of rapamycin (mTOR) pathway (31). Moon and colleagues reported that heat shock at 45°C for 1 h in L6 cells induced a rapid activation of the phosphoinositide 3-kinase (PI 3-kinase)/Akt signaling pathway and a consequent increase in glycogen synthesis (32). More recently, Liu and Brooks reported that incubation of C2C12 myotubes at 40°C for 1 h activated the energy-sensing factors 5’ AMP-activated protein kinase (AMPK) and sirtuin 1 (SIRT1) as well as their downstream targets such as peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) (33). Most importantly, repeated exposure to heat stress for 5 days (1 h/day) increased the expression of several oxidative phosphorylation proteins and mitochondrial DNA copy number (33). These changes occur in conjunction with increased expression of heat shock proteins (HSPs), a large family of molecular chaperones that play diverse cellular roles, including cell protection from stressors.
In cultured endothelial cells, episodic exposures to heat stress activate signaling pathways involved in angiogenesis as well as the control of vasomotor function. Harris and colleagues first showed that incubation of bovine aortic endothelial cells at 42°C for 1 h promoted an increase in the content of endothelial nitric oxide synthase (eNOS) expression and augmented endothelial nitric oxide (NO) release following stimulation with bradykinin (34). This observation is relevant because NO is an important vasodilator and also a key regulator of vascular growth (35). In a co-culture system of human endothelial cells and primary osteoblasts, exposure to 41°C for 1 h elevated the expression of pivotal angiogenesis mediators, including vascular endothelial growth factor (VEGF), angiopoietin-1 (ANGPT1) and angiopoietin-2 (ANGPT2) (36). Altogether, these findings reveal that heat stress prompts a comprehensive cell signaling response that favors energy storage, hypertrophy, augmented oxidative capacity and vascular growth.
The mechanisms linking elevated temperature and the activation of the aforementioned signaling pathways are unknown. One possibility is the perturbation in energy homeostasis (37), which activates energy sensing molecules, including AMPK. Activation of AMPK has been proposed to mediate, at least in part, HT-induced mitochondrial biogenesis (33) and elevated glucose uptake (37). Another potential signal is the increase in intramyocyte calcium concentration ([Ca2+]) promoted by heat stress. Obi and colleagues first reported that hyperthermia promoted a temperature-dependent increase in intracellular calcium [Ca2+] in C2C12 cells that is mediated in part by the activation of transient receptor potential vanilloid 1 (TRPV1) channels (31). These findings were recently replicated in the rat spinotrapezius muscle. Ikegami and colleagues reported that heat stress at 40°C for 20 min induced a ~18% increase in intracellular [Ca2+] in concert with TRPV1 phosphorylation (38). Among other targets, elevated [Ca2+] may elicit the activation of mTOR (39) and conceivably contribute to the effects of HT on muscle protein synthesis (28).
Heat-Induced Muscle Hypermia
The effects of elevated tissue temperature are likely amplified by the accompanying changes in muscle blood flow, which favors the delivery of energetic substrates and stimulates signaling mechanisms involved in vascular remodeling. The therapeutic effects of local HT have long been ascribed to increased muscle perfusion, but surprisingly, few studies have comprehensively examined the impact of heat stress on the magnitude and distribution of skeletal muscle blood flow. Earlier studies in which muscle blood flow during local or whole-body heat stress was determined by clearance of radiolabeled compounds (125I-labeled antipyrine or 133xenon) yielded conflicting results (40–43). The discrepant findings can be partially explained by important differences in the HT modality and regimen and the consequent magnitude of tissue hyperthermia. For example, perfusion of warm water through a tube-lined suit raised calf muscle temperature from ~34°C to 37.5°C and elicited a ~1.5-fold increase in muscle blood flow during single-leg heating in healthy young men (44). Conversely, Sekins and colleagues reported that rapid and large increases in muscle blood flow during local thigh heating with microwave diathermy were particularly evident when intramuscular temperatures were above 42°C (43). Along the same lines, studies on rats and pigs revealed that HT-induced changes in muscle blood flow, as determined by radiolabeled microspheres, were mostly apparent when the muscle was heated above 42°C (45, 46). Song and colleagues reported that muscle blood flow increased by 3.5 and 6-fold when the legs of rats were exposed to a water bath heated to 43°C and 44°C, respectively (46). Akyurekli and co-workers utilized microwave diathermy to examine the impact of progressive hyperthermia to a target temperature of 44.5°C on the magnitude and distribution of blood flow in porcine skeletal muscle (45). On average, muscle blood flow increased by a factor of 4 within 15–30 min of heating, but the greatest increase in blood flow occurred in the regions where the temperature was between ~42°C and 44.5°C (45).
The exact local temperature-sensitive mechanisms that explain the changes in muscle blood flow during hyperthermia remain poorly defined. One attractive possibility is the release by erythrocytes of the potent vasodilator adenosine 5’-triphosphate (ATP) as they travel through heated skeletal muscle (47, 48). This hypothesis is supported by the close relationship between the increases in blood flow and deep venous plasma ATP concentrations during local limb heating as well evidence derived from in vitro experiments that ATP is progressively released from red blood cells rises with increasing temperature from 33 to 42°C (47, 48). As previously mentioned, heat stress has also been shown to enhance the content and activity of eNOS and elicit a consequent increase in the release of NO (34). The consistent increase in eNOS expression across several different tissues and preparations, including cultured endothelial cells (34), rat arteries (34), isolated human arteries (49) and more recently, the human skeletal muscle microcirculation (50), implicates endothelial-derived NO as a pivotal mediator of the vascular responses to heat stress. In addition to these vasoactive agents, local HT may increase muscle blood flow by also evoking a reduction in muscle sympathetic nerve activity (MSNA) (51). Takahashi and co-workers showed that a hydrocollator pack applied to the shin and anterior foot for 15 min reduced MSNA burst rate by ~43% (51).
Heat-induced increases in muscle blood flow aid in the recovery from intense, muscle-damaging exercise through several different possible mechanisms: First, the delivery of substrates (e.g. glucose, amino acids) that are required for refueling and tissue remodeling is enhanced because of increased tissue perfusion (52). Second, the removal of by-products of metabolism as well as other substances (e.g. nerve growth factor) that sensitize muscle nociceptors and contribute to the development of pain may be accelerated (53). Third, increased blood flow may also facilitate the arrival and distribution of cells (e.g. blood-borne macrophages) that are important for proper remodeling (54). Fourth, increases in vascular wall shear-stress, the viscous drag exerted by flowing blood on the vessel wall, might conceivably help mitigate the detrimental consequences of lengthening contractions on the skeletal muscle microcirculation (21, 22).
PUTATIVE MECHANISMS UNDERLYING THE EFFECTS OF HT ON MUSCLE RECOVERY FOLLOWING EXERCISE
Glycogen Resynthesis
Intramuscular glycogen is a pivotal fuel source during high-intensity exercise and it is firmly established that glycogen depletion is associated with premature fatigue (55). Reduced work capacity following intense eccentric exercise results in part from impaired glycogen resynthesis (9). While the replenishment of glycogen stores is typically completed within 24 h after strenuous endurance exercise, muscle refueling can be delayed for days after exercise involving lengthening contractions (19). For example, glycogen content in type 2 fibers remains below resting levels for as much as 48 h after a soccer match, even when a diet rich in carbohydrates and whey protein is provided (56). This impairment is the result of a reduction in muscle glucose uptake due to lower membrane content of GLUT-4 glucose transporters as well as transient insulin resistance (18, 19). Local HT has the potential to overcome these abnormalities by: 1) enhancing glucose delivery, 2) stimulating muscle glucose uptake and 3) increasing the activity of glycogen synthase (15, 37). Along these lines, accumulating evidence indicates that, when coupled with optimal nutrition, local HT accelerates glycogen resynthesis following a bout of exhaustive arm (15) and leg (57) cycling in recreationally active young individuals. Although compelling, it is worth highlighting that the aforementioned studies focused on recovery following cycling and it is unclear whether similar findings would be obtained after a bout of heavy eccentric exercise.
Skeletal Muscle Microvascular Function
Another mechanism that may contribute to eccentric exercise-induced prolonged impairment in muscle function, particularly the ability to resist fatigue, is the increasingly well-documented dysfunction of the skeletal muscle microcirculation. Unaccustomed eccentric exercise slows and abrogates the skeletal muscle blood flow responses to a single contraction in humans (21, 22). The sluggish hyperemic response at the onset of contractions lowers the driving pressure for oxygen diffusion from capillary to myocyte, as demonstrated in the rat spinotrapezius muscle (20), and ultimately may contribute to the development of premature fatigue. Structural changes in the capillary network induced by lengthening contractions explain, at least in part, the abnormal hyperemic responses to contractions. In both rat (58) and human skeletal muscle (5, 59), intense eccentric exercise has been shown to reduce skeletal muscle capillarization. Local HT can combat these derangements by elevating tissue temperature and evoking increases in wall-shear stress, both of which may up-regulate eNOS expression (50). Confirming earlier observations in a model of whole-body heat stress (50), we reported that repeated local thigh heating for 8 weeks (5 days/wk) increases skeletal muscle eNOS expression and skeletal muscle capillarization in humans. We first demonstrated that a single session of local HT promotes the expression of pivotal angiogenic mediators vascular endothelial growth factor (VEGF) and ANGPT1 in human skeletal muscle (60). Furthermore, other groups have shown that repeated increases in shear stress during local HT elicit marked increases in conduit artery (61) and cutaneous (62) vascular function. These studies provide firm evidence that local HT may remedy the detrimental effects of intense eccentric exercise on the microcirculation by promoting a pro-angiogenic milieu in skeletal muscle and enhancing vasomotor function.
Mitochondrial Function
Eccentric exercise in rodents impairs mitochondrial respiration and calcium handling and downregulates the expression of factors involved in mitochondrial biogenesis (63–65). This decline in muscle oxidative capacity might explain the well-documented alterations in muscle energetics following eccentric exercise, including an increased reliance on anaerobic metabolism (66). In humans, a single bout of unaccustomed resistance exercise reduces the expression of mitochondrial transcripts, proteins and mitochondrial DNA copy number in young and old adults (25). Muscle damage produced by isometric neuromuscular electrical stimulation in young men resulted in impaired mitochondrial function during submaximal exercise, as revealed by a slower rate of phosphocreatine recovery and reduced oxidative ATP production (24). In addition, ultraendurance exercise has been shown to reduce mitochondrial efficiency (The phosphate/oxygen ratio measured in vitro) in elite athletes (23). A growing number of studies indicate that exposure to HT may possibly avert this temporary decline in mitochondrial content and function. Hafen and colleagues recently reported that repeated heating of the vastus lateralis muscle via pulsed shortwave diathermy (2 h daily for 6 consecutive days) in young individuals augmented maximal coupled and uncoupled respiratory capacity, measured via high-resolution respirometry, and elicited an increase in the content mitochondrial electron transport protein complexes I and V expression (67). Local HT was also recently shown to prevent immobilization-induced loss of myofiber respiratory capacity and the reduction in the content of mitochondrial respiratory complexes in human skeletal muscle (68). Tamura and co-workers reported that mice that were placed in a hot environmental chamber immediately after treadmill running (5 days/wk for 3 wk) had superior mitochondrial adaptations when compared to animals that did not undergo the heat treatment (69). These findings indicate that regular exposure to whole-body HT potentiates the effects of regular exercise on muscle oxidative function (69). It remains to be determined whether a similar additive effect occurs when local HT is applied regularly during recovery from activities that entail lengthening contractions.
Protein Synthesis and Degradation
A single bout of endurance or resistance exercise has a profound positive impact on muscle protein turnover that can persist for up to 72 h (70). This response enables adaptive muscle remodeling, including removal of damaged proteins and synthesis of new myofibrillar and mitochondrial proteins (70). Postexercise feeding potentiates the increase in muscle protein synthesis and attenuates the rise in muscle protein breakdown, thus resulting in a positive net protein balance. Accumulating evidence indicates that local HT may also act in synergy with exercise to turn on anabolic signaling and potentially inhibit the major proteolytic pathways (71, 72). In rodent skeletal muscle, both a single and repeated exposure to HT activates mTOR signaling, a key pathway involved in the regulation of protein synthesis and hypertrophy (69, 71, 73). Kakigi and colleagues showed that local HT prior to and during a bout of maximal isokinetic concentric contractions boosted the activation of mTOR signaling in human skeletal muscle (74). Indeed, local heating of the vastus lateralis increased Akt and mTOR phosphorylation and magnified the effect of exercise on rpS6 phosphorylation (74). These later findings suggest that local HT may possibly facilitate recovery from intense resistance exercise by enhancing muscle protein synthesis (74). Contrary to this notion, Fuchs and colleagues recently showed that single leg immersion in water at 46°C following a bout of resistance type-exercise did not increase myofibrillar protein synthesis rates nor the capacity of the muscle to incorporate protein-derived amino acids in young males (75). Of note, the treatment was applied for only 20 min, causing the vastus lateralis muscle temperature to increase to ~37.5°C, while in the control thigh the temperature remained at ~35.2°C (75). This rather brief exposure to HT was insufficient to elicit the activation of HSPs (75) and likely induced small increases in muscle blood flow and metabolism. As detailed above, HT-induced hyperemia and the consequent delivery of amino acids and other substrates required for muscle remodeling is particularly evident at intramuscular temperatures above 42°C (43, 45, 46). Additional studies are therefore required to define whether greater tissue hyperthermia achieved through exposure to diathermy (43) or prolonged hot-water immersion (76) impacts skeletal muscle protein synthesis following exercise.
Delayed-Onset Muscle Soreness
One of the most debilitating manifestations of eccentric exercise-induced muscle damage is delayed-onset muscle soreness (DOMS), characterized by tenderness and movement-induced pain that peaks at 24–72 h after the insult (16). Using a rat model of DOMS, Tsuboshima and colleagues recently provided unique insights into the effects of local HT and icing on mechanical hyperalgesia induced by lengthening contractions (77). The use of an animal model is particularly advantageous in this case because blinding humans to thermal treatments is difficult and the potential for bias when assessing subjective parameters, such as pain, is elevated. This study revealed that, contrary to icing, local HT applied to the gastrocnemius muscle for 20 min using a gel pack markedly attenuated mechanical hyperalgesia (77). Further, the levels of several metabolites, including nicotinamide and N⁵-ethyl-L-glutamine, were altered by HT, indicating that the analgesic effects of local heat stress may be partially derived from changes in metabolism (77).
CONCLUSIONS AND FUTURE DIRECTIONS
The multifactorial genesis of the impairment in skeletal muscle function following intense eccentric exercise represents a key challenge in the development of simple, yet effective recovery therapies. Mounting evidence indicates that localized muscle heating modalities, which are commonly encountered in sports rehabilitation settings, may be potent tools to accelerate post-exercise recovery of contractile function and endurance. Indeed, local heat stress provokes a comprehensive signaling response that may reverse some of the detrimental consequences of strenuous exercise on muscle metabolic and vascular function. Nonetheless, the impact of HT on a number of other important mechanisms involved in the reparative response after muscle damage remains unexplored. For example, it is unknown how repeated local HT affects the connective tissue regeneration in skeletal muscle. It is also imperative to recognize that most of the evidence supporting a beneficial role of local HT comes from studies performed in exercise-naive humans or animals as well as from models of severe muscle injury and disuse. It is yet unclear if the findings from these experiments translate to recreationally active individuals and in particular competitive athletes. Lastly, a growing number of studies indicate that exposure to local HT prior to eccentric exercise may protect against muscle damage and enhance muscle remodeling (16, 17). Future efforts should be direct to defining which strategy (pre or post-exercise treatment or a combination of both) is optimal to ameliorate the symptoms of exercise-induced muscle damage.
KEY POINTS.
A cardinal manifestation of prolonged intense exercise, most notably activities that entail eccentric muscle contractions, is a marked decline in muscle function. Muscle strength and power as well as endurance performance can be diminished for several days or even weeks depending on the magnitude and duration of the insult.
Exposure to local heat therapy (HT) has been shown to accelerate the recovery of contractile function and endurance following both exhaustive endurance as well maximal eccentric exercise.
Emerging evidence suggests that the therapeutic effects of local HT may derive from reduced muscle soreness, increased muscle refueling, stimulation of muscle protein synthesis and the reversal of exercise-induced vascular and mitochondrial dysfunction.
ACKNOWLEDGMENTS
Funding disclosure: This work was supported by the American Heart Association (16SDH27600003), the National Institute on Aging (1R21AG053687-01A1), the American College of Sports Medicine Foundation and the Gatorade Sports Science Institute/Pepsico.
Conflicts of interest: None
REFERENCES
- 1.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. American journal of physical medicine & rehabilitation. 2002;81(11 Suppl):S52–69. [DOI] [PubMed] [Google Scholar]
- 2.Hyldahl RD, Hubal MJ. Lengthening our perspective: morphological, cellular, and molecular responses to eccentric exercise. Muscle & nerve. 2014;49(2):155–70. [DOI] [PubMed] [Google Scholar]
- 3.Warren GL, Palubinskas LE. Human and animal experimental muscle injury models In: Tidus PM editor. Skeletal muscle damage and repair: Human Kinetics; 2008, pp. 13–35. [Google Scholar]
- 4.Warren GL, Lowe DA, Armstrong RB. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999;27(1):43–59. [DOI] [PubMed] [Google Scholar]
- 5.Kim K, Kuang S, Song Q, Gavin TP, Roseguini BT. Impact of heat therapy on recovery after eccentric exercise in humans. J Appl Physiol (1985). 2019;126(4):965–76. [DOI] [PubMed] [Google Scholar]
- 6.Mackey AL, Donnelly AE, Turpeenniemi-Hujanen T, Roper HP. Skeletal muscle collagen content in humans after high-force eccentric contractions. Journal of applied physiology. 2004;97(1):197–203. [DOI] [PubMed] [Google Scholar]
- 7.Chatzinikolaou A, Draganidis D, Avloniti A et al. The microcycle of inflammation and performance changes after a basketball match. Journal of sports sciences. 2014;32(9):870–82. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen J, Krustrup P, Nybo L et al. Skeletal muscle glycogen content and particle size of distinct subcellular localizations in the recovery period after a high-level soccer match. Eur J Appl Physiol. 2012;112(10):3559–67. [DOI] [PubMed] [Google Scholar]
- 9.Byrne C, Twist C, Eston R. Neuromuscular function after exercise-induced muscle damage: theoretical and applied implications. Sports Med. 2004;34(1):49–69. [DOI] [PubMed] [Google Scholar]
- 10.Barnett A Using recovery modalities between training sessions in elite athletes: does it help? Sports Med. 2006;36(9):781–96. [DOI] [PubMed] [Google Scholar]
- 11.Peake JM, Neubauer O, Della Gatta PA, Nosaka K. Muscle damage and inflammation during recovery from exercise. Journal of applied physiology. 2017;122(3):559–70. [DOI] [PubMed] [Google Scholar]
- 12.Torres R, Ribeiro F, Alberto Duarte J, Cabri JM. Evidence of the physiotherapeutic interventions used currently after exercise-induced muscle damage: systematic review and meta-analysis. Physical therapy in sport : official journal of the Association of Chartered Physiotherapists in Sports Medicine. 2012;13(2):101–14. [DOI] [PubMed] [Google Scholar]
- 13.Dupuy O, Douzi W, Theurot D, Bosquet L, Dugue B. An Evidence-Based Approach for Choosing Post-exercise Recovery Techniques to Reduce Markers of Muscle Damage, Soreness, Fatigue, and Inflammation: A Systematic Review With Meta-Analysis. Front Physiol. 2018;9:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giombini A, Giovannini V, Di Cesare A et al. Hyperthermia induced by microwave diathermy in the management of muscle and tendon injuries. British medical bulletin. 2007;83:379–96. [DOI] [PubMed] [Google Scholar]
- 15.Cheng AJ, Willis SJ, Zinner C et al. Post-exercise recovery of contractile function and endurance in humans and mice is accelerated by heating and slowed by cooling skeletal muscle. J Physiol. 2017;595(24):7413–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nosaka K, Muthalib M, Lavender A, Laursen PB. Attenuation of muscle damage by preconditioning with muscle hyperthermia 1-day prior to eccentric exercise. Eur J Appl Physiol. 2007;99(2):183–92. [DOI] [PubMed] [Google Scholar]
- 17.Touchberry CD, Gupte AA, Bomhoff GL, Graham ZA, Geiger PC, Gallagher PM. Acute heat stress prior to downhill running may enhance skeletal muscle remodeling. Cell Stress Chaperones. 2012;17(6):693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asp S, Daugaard JR, Kristiansen S, Kiens B, Richter EA. Eccentric exercise decreases maximal insulin action in humans: muscle and systemic effects. J Physiol. 1996;494 (Pt 3):891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asp S, Daugaard JR, Richter EA. Eccentric exercise decreases glucose transporter GLUT4 protein in human skeletal muscle. J Physiol. 1995;482 (Pt 3):705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kano Y, Padilla DJ, Behnke BJ, Hageman KS, Musch TI, Poole DC. Effects of eccentric exercise on microcirculation and microvascular oxygen pressures in rat spinotrapezius muscle. J Appl Physiol (1985). 2005;99(4):1516–22. [DOI] [PubMed] [Google Scholar]
- 21.Larsen RG, Hirata RP, Madzak A, Frokjaer JB, Graven-Nielsen T. Eccentric exercise slows in vivo microvascular reactivity during brief contractions in human skeletal muscle. J Appl Physiol (1985). 2015;119(11):1272–81. [DOI] [PubMed] [Google Scholar]
- 22.Larsen RG, Thomsen JM, Hirata RP et al. Impaired microvascular reactivity after eccentric muscle contractions is not restored by acute ingestion of antioxidants or dietary nitrate. Physiol Rep. 2019;7(13):e14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernstrom M, Bakkman L, Tonkonogi M et al. Reduced efficiency, but increased fat oxidation, in mitochondria from human skeletal muscle after 24-h ultraendurance exercise. J Appl Physiol (1985). 2007;102(5):1844–9. [DOI] [PubMed] [Google Scholar]
- 24.Foure A, Wegrzyk J, Le Fur Y et al. Impaired mitochondrial function and reduced energy cost as a result of muscle damage. Med Sci Sports Exerc. 2015;47(6):1135–44. [DOI] [PubMed] [Google Scholar]
- 25.Ogborn DI, McKay BR, Crane JD et al. Effects of age and unaccustomed resistance exercise on mitochondrial transcript and protein abundance in skeletal muscle of men. Am J Physiol Regul Integr Comp Physiol. 2015;308(8):R734–41. [DOI] [PubMed] [Google Scholar]
- 26.McGorm H, Roberts LA, Coombes JS, Peake JM. Turning Up the Heat: An Evaluation of the Evidence for Heating to Promote Exercise Recovery, Muscle Rehabilitation and Adaptation. Sports Med. 2018;48(6):1311–28. [DOI] [PubMed] [Google Scholar]
- 27.Hawley JA, Lundby C, Cotter JD, Burke LM. Maximizing Cellular Adaptation to Endurance Exercise in Skeletal Muscle. Cell Metab. 2018;27(5):962–76. [DOI] [PubMed] [Google Scholar]
- 28.Goto K, Okuyama R, Sugiyama H et al. Effects of heat stress and mechanical stretch on protein expression in cultured skeletal muscle cells. Pflugers Arch. 2003;447(2):247–53. [DOI] [PubMed] [Google Scholar]
- 29.Goto K, Kojima A, Morioka S et al. Geranylgeranylaceton induces heat shock protein 72 in skeletal muscle cells. Biochemical and biophysical research communications. 2007;358(1):331–5. [DOI] [PubMed] [Google Scholar]
- 30.Ohno Y, Yamada S, Sugiura T, Ohira Y, Yoshioka T, Goto K. Possible role of NF-kB signals in heat stress-associated increase in protein content of cultured C2C12 cells. Cells, tissues, organs. 2011;194(5):363–70. [DOI] [PubMed] [Google Scholar]
- 31.Obi S, Nakajima T, Hasegawa T et al. Heat induces myogenic transcription factors of myoblast cells via transient receptor potential vanilloid 1 (Trpv1). FEBS open bio. 2019;9(1):101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon B, Duddy N, Ragolia L, Begum N. Stimulation of glycogen synthesis by heat shock in L6 skeletal-muscle cells: regulatory role of site-specific phosphorylation of glycogen-associated protein phosphatase 1. The Biochemical journal. 2003;371(Pt 3):857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu CT, Brooks GA. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. Journal of applied physiology. 2012;112(3):354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris MB, Blackstone MA, Ju H, Venema VJ, Venema RC. Heat-induced increases in endothelial NO synthase expression and activity and endothelial NO release. Am J Physiol Heart Circ Physiol. 2003;285(1):H333–40. [DOI] [PubMed] [Google Scholar]
- 35.Baum O, Da Silva-Azevedo L, Willerding G et al. Endothelial NOS is main mediator for shear stress-dependent angiogenesis in skeletal muscle after prazosin administration. Am J Physiol Heart Circ Physiol. 2004;287(5):H2300–8. [DOI] [PubMed] [Google Scholar]
- 36.Li M, Fuchs S, Bose T et al. Mild heat stress enhances angiogenesis in a co-culture system consisting of primary human osteoblasts and outgrowth endothelial cells. Tissue engineering. Part C, Methods 2014;20(4):328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koshinaka K, Kawamoto E, Abe N, Toshinai K, Nakazato M, Kawanaka K. Elevation of muscle temperature stimulates muscle glucose uptake in vivo and in vitro. The journal of physiological sciences : JPS. 2013;63(6):409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikegami R, Eshima H, Mashio T et al. Accumulation of intramyocyte TRPV1-mediated calcium during heat stress is inhibited by concomitant muscle contractions. Journal of applied physiology. 2019;126(3):691–8. [DOI] [PubMed] [Google Scholar]
- 39.Ito N, Ruegg UT, Takeda S. ATP-Induced Increase in Intracellular Calcium Levels and Subsequent Activation of mTOR as Regulators of Skeletal Muscle Hypertrophy. International journal of molecular sciences. 2018;19(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Detry JM, Brengelmann GL, Rowell LB, Wyss C. Skin and muscle components of forearm blood flow in directly heated resting man. J Appl Physiol. 1972;32(4):506–11. [DOI] [PubMed] [Google Scholar]
- 41.Johnson JM, Brengelmann GL, Rowell LB. Interactions between local and reflex influences on human forearm skin blood flow. J Appl Physiol. 1976;41(6):826–31. [DOI] [PubMed] [Google Scholar]
- 42.Sekins KM, Dundore D, Emery AF, Lehmann JF, McGrath PW, Nelp WB. Muscle blood flow changes in response to 915 MHz diathermy with surface cooling as measured by Xe133 clearance. Archives of physical medicine and rehabilitation. 1980;61(3):105–13. [PubMed] [Google Scholar]
- 43.Sekins KM, Lehmann JF, Esselman P et al. Local muscle blood flow and temperature responses to 915MHz diathermy as simultaneously measured and numerically predicted. Arch Phys Med Rehabil. 1984;65(1):1–7. [PubMed] [Google Scholar]
- 44.Keller DM, Sander M, Stallknecht B, Crandall CG. alpha-Adrenergic vasoconstrictor responsiveness is preserved in the heated human leg. J Physiol. 2010;588(Pt 19):3799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akyurekli D, Gerig LH, Raaphorst GP. Changes in muscle blood flow distribution during hyperthermia. Int J Hyperthermia. 1997;13(5):481–96. [DOI] [PubMed] [Google Scholar]
- 46.Song CW. Effect of local hyperthermia on blood flow and microenvironment: a review. Cancer Res. 1984;44(10 Suppl):4721s–30s. [PubMed] [Google Scholar]
- 47.Kalsi KK, Chiesa ST, Trangmar SJ, Ali L, Lotlikar MD, Gonzalez-Alonso J. Mechanisms for the control of local tissue blood flow during thermal interventions: influence of temperature-dependent ATP release from human blood and endothelial cells. Experimental physiology. 2017;102(2):228–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalsi KK, Gonzalez-Alonso J. Temperature-dependent release of ATP from human erythrocytes: mechanism for the control of local tissue perfusion. Experimental physiology. 2012;97(3):419–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ives SJ, Andtbacka RH, Kwon SH et al. Heat and alpha1-adrenergic responsiveness in human skeletal muscle feed arteries: the role of nitric oxide. Journal of applied physiology. 2012;113(11):1690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hesketh K, Shepherd SO, Strauss JA et al. Passive heat therapy in sedentary humans increases skeletal muscle capillarization and eNOS content but not mitochondrial density or GLUT4 content. Am J Physiol Heart Circ Physiol. 2019;317(1):H114–H23. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi N, Nakamura T, Kanno N et al. Local heat application to the leg reduces muscle sympathetic nerve activity in human. Eur J Appl Physiol. 2011;111(9):2203–11. [DOI] [PubMed] [Google Scholar]
- 52.Chiesa ST, Trangmar SJ, Kalsi KK et al. Local temperature-sensitive mechanisms are important mediators of limb tissue hyperemia in the heat-stressed human at rest and during small muscle mass exercise. Am J Physiol Heart Circ Physiol. 2015;309(2):H369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakagawa T, Hiraga SI, Mizumura K, Hori K, Ozaki N, Koeda T. Topical thermal therapy with hot packs suppresses physical inactivity-induced mechanical hyperalgesia and up-regulation of NGF. The journal of physiological sciences : JPS. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeuchi K, Hatade T, Wakamiya S, Fujita N, Arakawa T, Miki A. Heat stress promotes skeletal muscle regeneration after crush injury in rats. Acta histochemica. 2014;116(2):327–34. [DOI] [PubMed] [Google Scholar]
- 55.Burke LM, van Loon LJC, Hawley JA. Postexercise muscle glycogen resynthesis in humans. Journal of applied physiology. 2017;122(5):1055–67. [DOI] [PubMed] [Google Scholar]
- 56.Gunnarsson TP, Bendiksen M, Bischoff R et al. Effect of whey protein- and carbohydrate-enriched diet on glycogen resynthesis during the first 48 h after a soccer game. Scandinavian journal of medicine & science in sports. 2013;23(4):508–15. [DOI] [PubMed] [Google Scholar]
- 57.Slivka D, Tucker T, Cuddy J, Hailes W, Ruby B. Local heat application enhances glycogenesis. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2012;37(2):247–51. [DOI] [PubMed] [Google Scholar]
- 58.Rizo-Roca D, Rios-Kristjansson JG, Nunez-Espinosa C et al. Intermittent hypobaric hypoxia combined with aerobic exercise improves muscle morphofunctional recovery after eccentric exercise to exhaustion in trained rats. Journal of applied physiology. 2017;122(3):580–92. [DOI] [PubMed] [Google Scholar]
- 59.Yu JG, Liu JX, Carlsson L, Thornell LE, Stal PS. Re-evaluation of sarcolemma injury and muscle swelling in human skeletal muscles after eccentric exercise. PLoS One. 2013;8(4):e62056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuhlenhoelter AM, Kim K, Neff D et al. Heat therapy promotes the expression of angiogenic regulators in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2016;311(2):R377–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naylor LH, Carter H, FitzSimons MG, Cable NT, Thijssen DH, Green DJ. Repeated increases in blood flow, independent of exercise, enhance conduit artery vasodilator function in humans. Am J Physiol Heart Circ Physiol. 2011;300(2):H664–9. [DOI] [PubMed] [Google Scholar]
- 62.Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DH, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol. 2010;588(Pt 9):1571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rattray B, Caillaud C, Ruell PA, Thompson MW. Heat exposure does not alter eccentric exercise-induced increases in mitochondrial calcium and respiratory dysfunction. Eur J Appl Physiol. 2011;111(11):2813–21. [DOI] [PubMed] [Google Scholar]
- 64.Rattray B, Thompson M, Ruell P, Caillaud C. Specific training improves skeletal muscle mitochondrial calcium homeostasis after eccentric exercise. Eur J Appl Physiol. 2013;113(2):427–36. [DOI] [PubMed] [Google Scholar]
- 65.Rizo-Roca D, Rios-Kristjansson JG, Nunez-Espinosa C et al. Modulation of mitochondrial biomarkers by intermittent hypobaric hypoxia and aerobic exercise after eccentric exercise in trained rats. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2017;42(7):683–93. [DOI] [PubMed] [Google Scholar]
- 66.Tee JC, Bosch AN, Lambert MI. Metabolic consequences of exercise-induced muscle damage. Sports Med. 2007;37(10):827–36. [DOI] [PubMed] [Google Scholar]
- 67.Hafen PS, Preece CN, Sorensen JR, Hancock CR, Hyldahl RD. Repeated exposure to heat stress induces mitochondrial adaptation in human skeletal muscle. Journal of applied physiology. 2018. [DOI] [PubMed] [Google Scholar]
- 68.Hafen PS, Abbott K, Bowden J, Lopiano R, Hancock CR, Hyldahl RD. Daily heat treatment maintains mitochondrial function and attenuates atrophy in human skeletal muscle subjected to immobilization. Journal of applied physiology. 2019;127(1):47–57. [DOI] [PubMed] [Google Scholar]
- 69.Tamura Y, Matsunaga Y, Masuda H et al. Postexercise whole body heat stress additively enhances endurance training-induced mitochondrial adaptations in mouse skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2014;307(7):R931–43. [DOI] [PubMed] [Google Scholar]
- 70.Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. Journal of applied physiology. 2009;106(5):1692–701. [DOI] [PubMed] [Google Scholar]
- 71.Ohira T, Higashibata A, Seki M et al. The effects of heat stress on morphological properties and intracellular signaling of denervated and intact soleus muscles in rats. Physiological reports. 2017;5(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshihara T, Sugiura T, Yamamoto Y, Shibaguchi T, Kakigi R, Naito H. The response of apoptotic and proteolytic systems to repeated heat stress in atrophied rat skeletal muscle. Physiological reports. 2015;3(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoshihara T, Naito H, Kakigi R et al. Heat stress activates the Akt/mTOR signalling pathway in rat skeletal muscle. Acta Physiol (Oxf). 2013;207(2):416–26. [DOI] [PubMed] [Google Scholar]
- 74.Kakigi R, Naito H, Ogura Y et al. Heat stress enhances mTOR signaling after resistance exercise in human skeletal muscle. The journal of physiological sciences : JPS. 2011;61(2):131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fuchs CJ, Smeets JSJ, Senden JM et al. Hot-water immersion does not increase postprandial muscle protein synthesis rates during recovery from resistance-type exercise in healthy, young males. J Appl Physiol (1985). 2020;128(4):1012–22. [DOI] [PubMed] [Google Scholar]
- 76.Morton JP, Maclaren DP, Cable NT et al. Elevated core and muscle temperature to levels comparable to exercise do not increase heat shock protein content of skeletal muscle of physically active men. Acta Physiol (Oxf). 2007;190(4):319–27. [DOI] [PubMed] [Google Scholar]
- 77.Tsuboshima K, Urakawa S, Takamoto K et al. Distinct effects of thermal treatments after lengthening contraction on mechanical hyperalgesia and exercise-induced physiological changes in rat muscle. J Appl Physiol (1985). 2020;128(2):296–306. [DOI] [PubMed] [Google Scholar]


