Abstract
Purpose:
To assess the association between optical coherence tomography angiography (OCTA) quantified avascular areas (AA) and diabetic retinopathy (DR) severity, progression and treatment requirement in the following year.
Design:
Prospective cohort study.
Methods:
We recruited diabetic patients from tertiary academic retina practice and obtained 3×3-mm macular OCTA scans with AngioVue system and standard 7-field color photographs at baseline and 1-year visit. A masked grader determined the DR severity from the color photographs using the Early Treatment of Diabetic Retinopathy (ETDRS) scale. A custom algorithm detected extrafoveal AA (EAA) excluding the central 1mm circle in projection-resolved superficial vascular complex (SVC), intermediate capillary plexus (ICP), and deep capillary plexus (DCP).
Results:
Of 138 patients (41 males, age 26–84, mean 59.4 years), 92 completed one-year follow-up. At baseline, EAAs for SVC, ICP and DCP were all significantly correlated with retinopathy severity (p<0.0001). The DCP EAA was significantly associated with worse visual acuity (r=−0.24, P=0.02), but the SVC and ICP EAA were not. At one year, 11 eyes progressed in severity by at least one-step. Multivariate logistic regression analysis demonstrated the progression was significantly associated with baseline SVC EAA (OR=8.73, P=0.04). During the follow up, 33 eyes underwent treatment. Multivariate analysis demonstrated treatment requirement was significantly associated with baseline DCP EAA (OR=3.39, P=0.002). No baseline metric was associated with vision loss at one year.
Conclusions
EAAs detected by OCTA in diabetic eyes are significantly associated with baseline DR severity, disease progression, and treatment requirement over one year.
Introduction
Macular ischemia is a key finding in diabetic retinopathy (DR), a leading cause of blindness worldwide in working age population,1–3 correlated with visual impairment,4 treatment response5,6 and disease progression.7,8 Recently, numerous studies have demonstrated the value of optical coherence tomography angiography (OCTA) for quantification of macular vascular changes in diabetic retinopathy, correlating it to disease severity and response to treatment.9–17
The goal of clinical evaluation of diabetic retinopathy is to assess the risk of vision loss and identify the treatment threshold.18 The Diabetic Retinopathy Study and the Early Treatment of Diabetic Retinopathy Study (ETDRS) evaluated the features of modified Airlie House grading system and fluorescein angiographic features against prospective outcomes.2,7,19–21 The findings from these studies serve as the fundamentals of standard of care in diabetic retinopathy evaluation. We hypothesize that OCTA quantified macular metrics, beyond being correlated with clinical severity, can predict the risk of progression, vision loss, and treatment requirement.
To test this, we performed a prospective study with rigorous clinical procedures for visual acuity and retinopathy severity assessment.21 In addition, we have applied advanced OCTA technology that addresses key issues in OCTA evaluation of DR. First, we applied projection-resolved (PR) OCTA algorithm to remove artifacts that can interfere with the evaluation of the deeper layers of the retinal vasculature.22 Second, we adopted a 3-layer segmentation scheme instead of the conventional 2-layers, with the understanding from histology that the deep vascular complex consist of two distinct laminar capillary plexuses—intermediate capillary plexus (ICP) and deep capillary plexus (DCP).23 Our group has previously demonstrated that en face evaluation of these plexuses as individual slabs is more sensitive to vascular changes than overlapping slabs.11 PR-OCTA is critical in producing distinct 3-layered slabs that are segmented in the appropriate anatomic layer. Third, we evaluated macular ischemia by measuring avascular areas (AA) instead of vessel density. Unlike vessel density, avascular areas are less dependent on signal strength and can be measured by human graders, providing a basis for ground truth validation.10 Finally, we have employed a machine-learning algorithm for detection and segmentation of the AA, which we have recently validated across OCTA of a full range of quality and clinical severity and resistant to error due to defocusing or shadowing from floaters.24,25
Methods
This observational prospective single center study was approved by the Institutional Review Board of Oregon Health Science University, adhered to the tenets of the Declaration of Helsinki, and complied with the Health Insurance Portability and Accountability Act of 1996. Each participant gave a written informed consent.
Participants with Type I diabetes of greater than 5 years duration or Type II diabetes of any duration of age between 18 to 79 years were recruited from Casey Eye Institute, Oregon Health Science University. We excluded pregnant or lactating women, those unable to consent or cooperate with OCTA scans, or those with presence of significant non-diabetic ocular diseases or a history of intraocular surgery, except intravitreal injections or cataract surgeries, within 4 months prior to screening. One eye of each participants was included into the study.
We obtained a medical history, clinical examination and imaging at baseline and 1-year follow-up from each participant. Previous intraocular treatments, if any, including focal laser, panretinal photocoagulation, intravitreal injections, cataract surgeries or vitrectomies were recorded. The clinical examination included Early Treatment of Diabetic Retinopathy Study (ETDRS) protocol visual acuity, intraocular pressure, slit-lamp biomicroscopy and indirect binocular ophthalmoscopy. Imaging procedures included OCTA using a commercially available 70-KHz spectral domain optical coherence tomography (RTVue-XR, Optovue, Fremont, CA) with 840 nm central wavelength and standard 7-field ETDRS color fundus photography. A retinal specialist (TH) determined the severity of diabetic retinopathy based on standard 7-field ETDRS color fundus photos using ETDRS severity scale20,21 masked to other clinical information and OCTA images. The DR severity at baseline and 1-year follow-up were assessed separately in a masked fashion. Progression of DR severity was defined as at least one level increase within ETDRS severity scale.20,21 The treating clinician determined the treatment requirement according to the standard of care without reviewing OCTA images.
We obtained 3mm × 3mm central macular OCTA scans with 304 × 304 A-scan density. Orthogonal registration and merging of two consecutive scans were used to obtain macula volume scans.26 We excluded scans with a signal strength index <55 or scan quality index <6 or obvious motion artifacts.10 Remaining scans were exported for a custom imaging processing and analysis, the details of which have been reported previously.9,10,27–30 Briefly, a semi-automated algorithm based on directional graph search segmented the volumes into superficial vascular complex (SVC), intermediate capillary plexus (ICP), and deep capillary plexus (DCP). The SVC layer was defined from internal limiting membrane to the inner plexiform layer/inner nuclear layer interface, which included nerve fiber layer, the ganglion cell layer, and the inner plexiform layer, approximately 80% of ganglion cell complex (GCC). The ICP was defined as the outer 20% of the GCC plus the inner 50% of the inner nuclear layer (INL). The DCP was defined as the outer 50% of INL to the OPL. A senior retina fellow reviewed the segmentations and adjusted manually where necessary. In cases where significant DME or exudates caused incorrect segmentation, we manually corrected the boundaries using the adjacent B-scans without edema as reference. A custom deep-learning algorithm detected extrafoveal AA (EAA) excluding the central 1mm circle in projection-resolved OCTA as described previously.9,10,25 The convolutional neural network (CNN) based algorithm used OCTA and en face reflectance map to determine whether a low flow signal area represents a true nonperfusion area or a low signal or motion artifact.25
Statistical analysis was performed using Statistical Package for Social Sciences software (SPSS for Windows, version 25.0; IBM SPSS Inc., Chicago, USA). Descriptive statistics included mean, standard deviation (SD), range, and percentages were presented where appropriate. Analysis of variance was used to compare the EAAs of different groups. Pearson correlation was used to analyze the associations between EAA in different plexus and visual acuity. The association between DR severity and EAA at baseline was analyzed using Spearman correlation. Logistic regression analyzed the association between baseline EAA and the retinopathy progression, treatment for DME or DR, and vision loss during the 1-year follow up. All P values were 2-sided and considered statistically significant if the value was less than 0.05. Bonferroni correction was applied when performing multiple comparisons.
Results
Ninety-five out of 138 (69%) patients with diabetes were enrolled and followed for one year, three of which were excluded due to poor image quality. The specific reasons for non-follow up, when they could be identified, were a change in insurance (1), death (2), moving out of the area (5). Table 1 summarizes the baseline clinical characteristics of the participants. There were no significant differences between follow-up group and non-follow-up group in terms of mean age (59.4±12.7 vs. 55.1±12.9 years, P=0.15), gender proportion (54% vs. 50% female P=0.59), DR severity (severe NPDR/PDR proportion 46.7% vs. 48.5%, P=0.86), SVC EAA (0.72±0.51 vs. 0.60±0.52 mm2, P=0.24), ICP EAA (0.67±0.55 vs. 0.60 ±0.68 mm2, P=0.55) and DCP EAA (0.74±0.61 vs. 0.68±0.71 mm2, P=0.61) at baseline.
Table 1.
Baseline Clinical Characteristics
| Parameters | Value (range) |
|---|---|
| Age (year) | 59.4±12.7 (28–84) |
| Gender (male/female) | 41/51 |
| Diabetes type (1/2) | 26/66 |
| Diabetes duration (year) | 20.1±11.8 (1–55) |
| HbA1c(%) | 7.7±1.6 (5.2–14.0) |
| Hypertension history (with/without) | 70/22 |
| Systolic blood pressure (mmHg) | 130.4±20.1 (89–186) |
| Diastolic blood pressure (mmHg) | 70.6±13.1 (46–110) |
| Body mass index | 33.3±8.5 (21.1–66.6) |
| BCVA (ETDRS letters) | 79.8±8.5 (40–94) |
| Intraocular pressure (mmHg) | 14.6±3.6 (8–24) |
| Axial length (mm) | 23.7±1.1 (21.2–29.2) |
| ETDRS severity (scale) | Frequency (n) |
| No DR (10) | 16 |
| Microaneurysms only (20) | 3 |
| Mild NPDR (20–35) | 19 |
| Moderate NPDR (43) | 4 |
| Moderately severe NPDR (47) | 7 |
| Severe NPDR (13) | 17 |
| Mild PDR (61) | 9 |
| Moderate PDR (65) | 11 |
| High risk PDR (71) | 5 |
| High risk PDR (75) | 1 |
| Total number of patients | 92 |
ETDRS: early treatment diabetic retinopathy study; BCVA: best-corrected visual acuity; DR: diabetic retinopathy; NPDR: non-proliferative diabetic retinopathy; PDR: proliferative diabetic retinopathy.
The EAA of SVC, ICP, DCP, and the sum of all plexuses were significantly correlated (P<0.001) with retinopathy severity with Spearman coefficients of 0.42, 0.53, 0.48, and 0.62, respectively (Figures 1 and 2). For DM without DR, mild to moderate NPDR, severe NPDR and PDR group, the mean SVC EAA was 0.33 mm2, 0.64 mm2, 0.83 mm2 and 1.01 mm2 (P<0.001) respectively; ICP EAA was 0.28 mm2, 0.46 mm2, 1.01 mm2 and 0.96 mm2 (P<0.001) respectively; DCP EAA was 0.24 mm2, 0.60 mm2, 1.09 mm2 and 1.02 mm2(P<0.001) respectively; sum of EAA of all the three layers was 0.84 mm2, 1.65 mm2, 2.94 mm2 and 2.99 mm2 (P<0.001) respectively. At baseline, the DCP EAA was associated with worse visual acuity (Pearson correlation coefficient=−0.24, P=0.02) (Figure 3), but the SVC EAA (correlation coefficient=−0.05, P=0.70) and ICP EAA (correlation coefficient=−0.01, P=0.79) were not.
Figure 1.
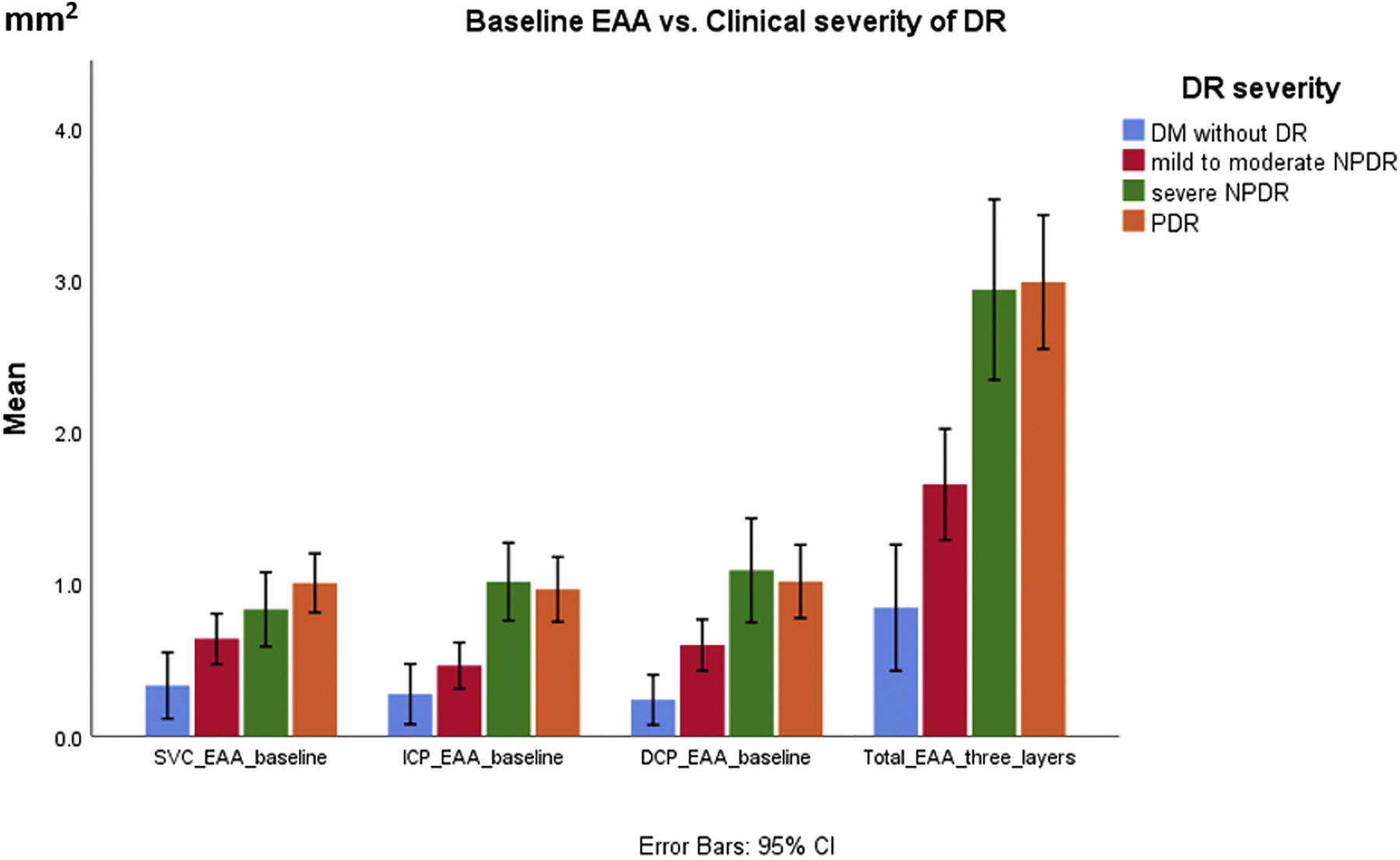
Baseline Extrafoveal Avascular Area (EAA) of Individual Plexuses vs. Clinical Diabetic Retinopathy Severity. EAA: extrafoveal avascular area; SVC: superficial vascular complex; ICP: intermediate capillary plexus; DCP: deep capillary plexus; DM: diabetes mellitus; DR: diabetic retinopathy; NPDR: non-proliferative diabetic retinopathy; PDR: proliferative diabetic retinopathy.
Figure 2:
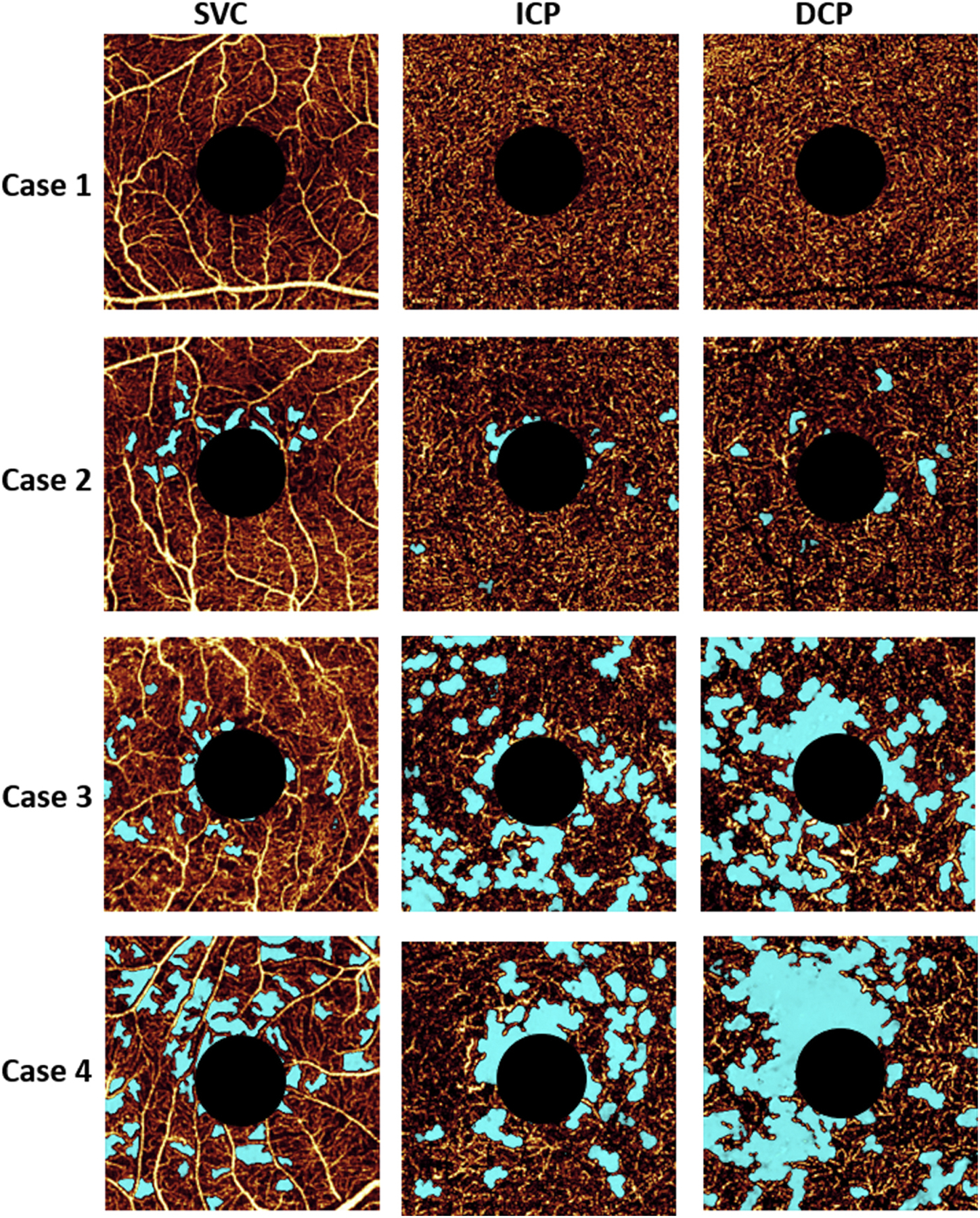
Optical coherence tomography angiography of a diabetic eye without retinopathy (case 1), mild nonproliferative diabetic retinopathy (case 2), severe nonproliferative diabetic retinopathy (case 3), and proliferative diabetic retinopathy (case 4). Light blue indicates avascular area. Superficial vascular complex (SVC), intermediate capillary plexus (ICP) and deep capillary plexus (DCP) are presented in separate en face angiograms.
Figure 3.
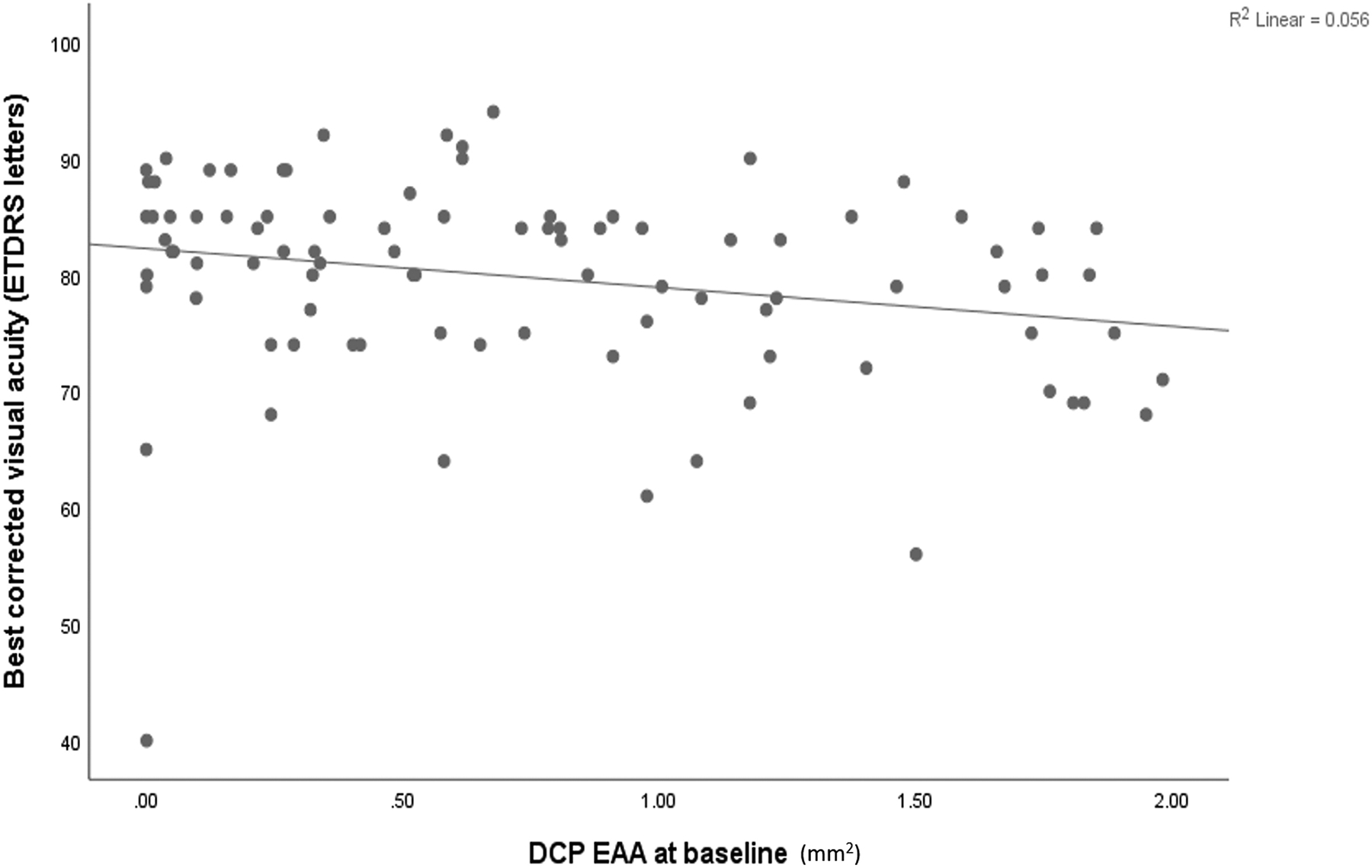
Correlation plot of the deep capillary plexus extrafoveal avascular area (DCP EAA) and best-corrected visual acuity in ETDRS letters.
At one year, 11 eyes progressed in severity by at least one-step. The baseline severity for these 11 eyes were mild to moderate NPDR in 4 eyes, severe NPDR in 3 eyes and PDR in 4 eyes. The baseline EAA for the eyes that progressed vs. those that did not were 1.08±0.36 mm2 and 0.67±0.51 mm2 (P=0.01) for SVC, 1.01±0.64 mm2 and 0.64±0.53 mm2 (P=0.04) for ICP, and 0.99±0.73 mm2 and 0.73±0.59 mm2 (P=0.19) for DCP respectively. In univariate logistic regression analysis, the progression was significantly associated with SVC EAA (OR=6.10, P=0.02), ICP EAA (OR=2.26, P=0.04), but not with DCP EAA (P=0.19). The progression was borderline associated with axial length (P=0.07) and HbA1C level (P=0.09), but not associated with age (P=0.44), gender (P=0.57), diabetes mellitus type (P=0.22), hypertension history (P=0.64) or baseline DR severity (P=0.20). (Table 2) A multivariate logistic regression model with the progression as the dependent variable, and the variables with a P value smaller than 0.10, including SVC EAA, ICP EAA, axial length and HbA1C level as covariates demonstrated progression was significantly associated with SVC EAA only (OR=8.73, β=2.17, P=0.04), with the estimated probability of progression = (e2.17x − 4.09)/(1- e2.17x − 4.09), where x is SVC EAA in mm2.
Table 2.
Logistic Regression Analysis (univariate) of the Baseline Predictors of Diabetic Retinopathy Progression at One-year Visit.
| Parameters | B | Sig. | Odds ratio | 95% CI for odds ratio |
|---|---|---|---|---|
| SVC EAA (mm2) | 1.807 | 0.02 | 6.10 | 1.29–28.80 |
| ICP EAA (mm2) | 1.181 | 0.04 | 2.26 | 1.04–10.24 |
| DCP EAA (mm2) | 0.680 | 0.19 | 1.97 | 0.72–5.44 |
| Axial length (mm) | −0.659 | 0.07 | 0.52 | 0.25–1.06 |
| HbAIC | −0.722 | 0.09 | 0.49 | 0.21–1.12 |
| DR severity | 0.411 | 0.20 | 1.51 | 0.81–2.82 |
| Body mass index | −0.100 | 0.11 | 0.91 | 0.80–1.02 |
| Diabetes type (1 or 2) | −0.799 | 0.22 | 0.45 | 0.12–1.63 |
| Age | −0.019 | 0.44 | 0.98 | 0.94–1.03 |
| Sex | 0.377 | 0.57 | 1.46 | 0.39–5.39 |
| Hypertension history | −0.388 | 0.64 | 0.68 | 0.14–3.42 |
| Diastolic blood pressure | 0.010 | 0.69 | 1.01 | 0.96–1.06 |
| Systolic blood pressure | −0.005 | 0.78 | 1.00 | 0.96–1.03 |
| Intraocular pressure | 0.016 | 0.86 | 1.02 | 0.85–1.21 |
EAA: extrafoveal avascular area; SVC: superficial vascular complex; ICP: intermediate capillary plexus; DCP: deep capillary plexus; DR: diabetic retinopathy; ETDRS: early treatment diabetic retinopathy study grading scheme; CI: confidence interval.
At baseline, 46 eyes were treatment naïve. The other 46 eyes had undergone treatments including focal laser in 22 eyes, panretinal photocoagulation in 17 eye, intravitreal anti-VEGF injections in 29 eyes, intravitreal steroids in two eyes and cataract surgeries in 22 eyes. During the follow up, 33 eyes (including 6 baseline treatment naïve eyes and 27 previously treated eyes) underwent treatment for diabetic macular edema or vitreous hemorrhage, including intravitreal injection of anti-VEGF agents (28), intravitreal steroids (3), focal laser (2), panretinal photocoagulation (5) and vitrectomy (2). The baseline EAA was significantly larger in the eyes that required treatment during the 1-year follow-up than in those that did not in SVC, (0.90±0.49 vs. 0.63±0.50 mm2, P=0.02), ICP (0.90±0.57 vs. 0.54±0.49 mm2, P=0.002) and DCP (1.02±0.67 vs. 0.59±0.51 mm2, P=0.001). In univariate logistic regression model, the treatment requirement was significantly associated with the presence of DME (OR=6.99, P<0.001); clinical DR severity (OR=2.82, P<0.001); DCP EAA (OR=3.39, P=0.002); ICP EAA (OR=3.54, P=0.002); and SVC EAA (OR=2.95, P=0.017). The treatment requirement was not significantly associated with age, gender, Body mass index, HbA1C level, and axial length. (See Table 3). The multivariate model demonstrated that the treatment requirement was significantly associated with DCP EAA (OR=3.39, β=1.22, P=0.002), but not with SVC EAA (P=0.13) or ICP EAA (P=0.19), with the probability of treatment = (e1.22x – 1.55)/(1- e1.22x – 1.55) where x is DCP EAA in mm2. Separate analysis on the treatment naïve eyes demonstrated the treatment requirement was significantly associated with ICP EAA (OR=6.58, P=0.039) and borderline associated with DCP EAA (OR=5.14, P=0.065), but not associated with SVC EAA (P=0.21).
Table 3.
Logistic Regression (univariate) Analysis of Baseline Predictors of Treatment Requirement in 1 year.
| Parameters | B | P value | Odds ratio | 95% CI for odds ratio |
|---|---|---|---|---|
| Clinical diabetic retinopathy severity | 1.037 | <0.001 | 2.82 | 1.72–4.68 |
| Diabetic macular edema or not | 1.945 | <0.001 | 6.99 | 2.46–19.85 |
| DCP EAA | 1.221 | 0.002 | 3.39 | 1.58–7.29 |
| ICP EAA | 1.263 | 0.003 | 3.54 | 1.52–8.22 |
| SVC EAA | 1.081 | 0.017 | 2.95 | 1.21–7.16 |
| Axial length | −0.359 | 0.104 | 0.70 | 0.45–1.08 |
| HbA1C | 0.202 | 0.218 | 1.22 | ^ 0.89–1.69 |
| Age | −0.015 | 0.368 | 0.99 | 0.95–1.02 |
| Body mass index | −0.018 | 0.507 | 0.98 | 0.93–1.04 |
| Sex | −0.247 | 0.572 | 0.78 | 0.33–1.84 |
EAA: extrafoveal avascular area; SVC: superficial vascular complex; ICP: intermediate capillary plexus; DCP: deep capillary plexus; DR: diabetic retinopathy; ETDRS: early treatment diabetic retinopathy study grading scheme; NPDR: non-proliferative diabetic retinopathy; PDR: proliferative diabetic retinopathy.
Considering the potential impact of DME on EAA quantification, we did a separate analysis on those eyes without DME (n=69) after excluding eyes with DME (n=23) at baseline. The results were similar to those described above. At baseline, the EAA of SVC, ICP and DCP increased significantly with severity of DR. For DM without DR, mild to moderate NPDR, severe NPDR and PDR group, the mean SVC EAA was 0.29 mm2, 0.74 mm2, 0.67 mm2 and 0.99 mm2 (P=0.001) respectively; ICP EAA was 0.29 mm2, 0.44 mm2, 0.98 mm2 and 0.98 mm2 (P<0.001) respectively; DCP EAA was 0.25 mm2, 0.56 mm2, 0.82 mm2 and 0.94 mm2(P=0.001) respectively. Compared to eyes without progression at one-year follow-up visit, those progressed had a significant larger baseline SVC EAA (1.21 vs. 0.66 mm2, P=0.025), larger but not statistically significant ICP EAA (1.03 vs. 0.64 mm2, P=0.13) and DCP EAA (0.88 vs. 0.64 mm2, P=0.39). Eyes that required treatment during the 1-year follow-up, compared to those that did not require treatment, had a significantly larger baseline EAA in SVC (0.96 vs. 0.62 mm2, P=0.02), ICP (0.95 vs. 0.56 mm2, P=0.01) and DCP (0.87 vs. 0.57 mm2, P=0.02). In eyes without DME, there was no significant association between visual acuity and SVC EAA (r=−0.02, P=0.88), ICP EAA (r=−0.09, P=0.48), DCP EAA (r=−0.19, P=0.18) at baseline.
At one-year visit, there were 4 eyes that lost 15 letters of vision or more. The cause of vision loss was diabetic macular edema (3 eyes) and cataract (1 eye). No baseline OCTA metric was associated with vision loss of 15 letters or more at one year (all P>0.05).
Discussion
Photographic grading of diabetic retinopathy severity, particularly 7-field grading using the ETDRS scale, has been the gold-standard in the management of diabetic retinopathy. It has been the standard way of reporting the retinopathy severity in virtually all major clinical trials. Although it has the singular advantage of being backed by prospective data on the risk of progression of disease and vision loss on a large cohort of patients, its place in everyday practice has been challenged.31 In 2003, Wilkinson et al proposed the International Clinical Diabetic Retinopathy Scale (ICDRS) as a more practical alternative to the ETDRS Scale, which many clinicians find cumbersome and impractical.32 While ICDRS has gained some acceptance, it still relies on qualitative interpretation of subtle clinical findings, such as venous beading or intraretinal microvascular abnormalities. In addition, clinical trials for diabetic macular edema using anti-VEGF treatments have found that the clinical features used for clinical grading are altered by anti-VEGF medications, while noting that macular ischemia may be useful in predicting progression to proliferative disease.8 This study explored the potential of OCTA avascular areas as an objective alternative to photographic grading scale to assess the risk of progression and treatment requirement.
In this prospective longitudinal study, we found OCTA-quantified avascular areas are significantly associated with clinical diabetic retinopathy severity grading and treatment requirement and disease progression at one year, demonstrating potential prognostic value of OCTA in DR management. This confirms previous studies based on fluorescein angiography suggesting that macular ischemia is associated with disease progression7,8. This finding suggests that eyes with larger macular avascular area on PR-OCTA may need closer monitoring for disease progression and treatment requirement.
The association between EAAs and DR severity was consistent with our previous studies, which demonstrated that segmented EAA is closely associated with DR severity.9–11 Our study has shown the strongest association between SVC EAA and DR severity10, but the literature is divergent on which vascular plexus and which parameter is the most closely associated with DR severity.12–15,17 Durbin et al demonstrated that the superficial retinal layer vessel density had the highest area under receiver operating characteristic curve for differentiating DR from healthy eyes compared to foveal avascular zone (FAZ) area and vessel density in deep retinal layer.17 Bhanushali et al. found spacing between the large vessels in the deep retinal layers had the highest diagnostic power for differentiating DR from normal controls compared to other parameters including spacing between large and small vessels in the superficial plexus, FAZ area, and vessel density.13 Comparing the vessel density and FAZ area of superficial and deep layer in 3mm × 3mm and 6mm × 6mm scans for differentiating DR severity, Binotti reported vessel density on deep plexus in 3mm × 3mm scans has the highest area under the receiver operating characteristic curve for detecting high-risk DR.14 Ashraf et al reported that FAZ area in superficial plexus, vessel density in deep layer and FAZ acircularity were the best parameters for distinguishing DR severity.12 There may be important methodological differences leading to these discrepancies. One is the segmentation scheme. Many studies, while reporting that they are segmenting SVC from DVC, the segmentation scheme actually included the ICP along with the SVC, creating an overlapping slab, which may decrease the sensitivity of detecting capillary loss in the SVC. Another is projection artifacts, which may influence not only the measured vessel density, but also the segmentation scheme.33
In addition to carefully dealing with projection artifacts and using anatomically correct boundaries for segmentation, we chose to use avascular areas instead of vessel densities (skeletonized or binarized) to assess macular ischemia. Studies have shown the dependence of vessel density on OCTA signal strength and age,10,34 while avascular areas are less dependent on those potential confounders10,25. Furthermore, vascular metrics in OCTA is subject to artifacts caused by vitreous opacities and vignetting, which can cause false capillary dropouts. Using a deep-learning algorithm that can distinguish false low perfusion areas due to low signal artifacts25, we excluded these false capillary drop outs, improving the performance of the metric.
We found that a larger DCP EAA was significantly associated with worse baseline BCVA, but not SVC or ICP. DCP located at the outer border of the inner nuclear layer.23,35 Experimental studies demonstrated that the DCP contributes 10–15% of photoreceptor inner segment oxygen requirement.36 In hypoxia, the retinal vascular contribution to the metabolic needs of the outer retina becomes more significant, as the choroidal vasculature fails to autoregulate its blood supply in the setting of hypoxia37. Recent studies with OCTA demonstrated co-localization of photoreceptor disruption and DCP non-perfusion, highlighting the importance of the DCP to the oxygen requirement of the photoreceptor in diabetic retinopathy.38–40 Previous structural OCT studies have demonstrated the impact of disruption of photoreceptors on visual acuity in DR.41,42 Our findings further support the role of DCP ischemia in photoreceptor loss in DR. After excluding DME eyes, in eyes without DME, the association between a larger DCP EAA and worse visual acuity was not significant (r=−0.19, P=0.18). Although this may be due to a relatively small sample size (not powerful to reach a significant result), this result suggests that DME may play a more important role than DCP ischemia in vision loss.
The association of avascular areas and one-year disease progression is in agreement with previous studies based on FA. ETDRS Report No. 13 found that FA-graded macular capillary nonperfusion is a risk factor for progression to proliferative diabetic retinopathy. One-year risk of development of PDR was 18.2% in eyes without macular ischemia and 41.3% in eyes with severe macular ischemia.7 Sim et al. reported a greater macular ischemia grade on FA was independently predictive for 27-month progression, and diabetic macular ischemia progression itself was predictive of the loss of visual function.4 The results of RISE and RIDE trials also demonstrated patients with diabetic macular ischemia progressed to neovascular complications of DR earlier than those without macular ischemia.8 Interestingly, DCP EAA, which is not visualized with FA43, is not associated with 1-year disease progression in the current study.
In the current study, we did not find significant association between systemic factors such as HbA1C level, duration of diabetes, hypertension and DR progression, although these were reported associated with development and progression of DR in other studies44,45. We speculate that in our relatively small group of patients, the macular ischemia contributes more to DR worsening than systemic factors, particularly over a relatively short period of 1 year. It is noteworthy that in all the parameters we tested (table 3), SVC and ICP EAA were the only parameters predictive of one-step or more DR progression. Similar finding was also found in RIDE and RISE study, presence of macular capillary non-perfusion on FA was the only parameter predicting progression to PDR8.
About one third of the participants underwent treatment for DME or proliferative diabetic retinopathy, with the majority receiving intraocular injections of anti-VEGF agents. In addition to presence of DME and higher DR severity, larger SVC, ICP and DCP EAAs increased the possibility of treatment requirement. In our multivariate model, after adjustment of DME, every increase of 1-mm DCP EAA increased 2.6 times possibility of the one-year treatment requirement. This finding may be of practical significance for clinicians when scheduling follow-up visits and treatment plans.
Clinicians make treatment decisions in DR based on multiple factors, such as the severity of DR, DME, and visual acuity. Since DCP EAA is significantly associated with disease severity and worse visual acuity, it is not surprising that we find a significant association between DCP EAA and treatment requirement for the overall study population. For treatment naïve eyes, the treatment requirement is significantly associated with ICP EAA. Except that ICP EAA is associated with DR severity, it is unclear why the strongest relationship with treatment requirement was seen in the ICP.
In this study, OCTA-quantified avascular areas were not associated with vision loss of 15 letters or more at 1 year. This may be due to the fact that vision loss in diabetic retinopathy can occur over a longer timeline than 1 year. In addition, there were only four eyes lost 15 letters or more in our cohort, limiting the power to detect a significant result. Furthermore, as the patients received sight-saving treatments according to standard of care, we did not observe the natural history of these eyes.
Limitations of the study included a relatively small size of cohort with a relatively low follow-up rate of 69%. However, the non-follow up group had similar baseline demographic characteristics, DR severity and avascular areas compared to the group that completed the follow up. The 1-year follow-up period is short, especially considering the time course of diabetic retinopathy. The patients received the standard-of-care treatments but the specific strategy in delivering the standard of care treatment was inconsistent. Another limitation of the study is the small field of view of the OCTA scans (3×3-mm). The currently available OCTA technology obtains the most reliable capillary-level resolution images with 3×3-mm field of view.47 However, a good correlation between central macular ischemia and peripheral ischemia has been reported46, and numerous studies showed excellent correlation between OCTA metrics from 3×3-mm scans and DR clinical severity9–15,17. It is, then, a reasonable hypothesis that the OCTA-derived metric from the central macula can predict DR progression and treatment requirement. The strengths of the study include rigorous clinical evaluation including ETDRS vision, masked photographic grading; advanced image processing with PR-OCTA and 3-layer segmentation, and machine-learning aided avascular area detection that is robust over a wide range of image quality.25 A study with a larger cohort and a longer follow-up period may further validate the predictive value of OCTA-measured metrics in the clinical management of DR.
In conclusion, avascular areas detected by projection resolved OCTA in diabetic eyes are significantly associated with baseline DR severity, disease progression, and treatment requirement over one year, providing clinically useful information based on objective metrics. A larger prospective study with longer follow up period is necessary to further validate the potential of OCTA avascular areas as a practical and objective biomarker in the management of diabetic retinopathy.
Supplementary Material
Acknowledgment
a. Funding/Support: The study was supported by grants R01 EY024544, R01 EY027833, and P30 EY010572 from the National Institutes of Health, an unrestricted departmental funding grant and William & Mary Greve Special Scholar Award from Research to Prevent Blindness, New York.
b. Financial Disclosures: Drs Jia, Bailey (financial support), and Huang have a significant financial interest in Optovue Inc.
c. Other Acknoledgements: The authors would like to thank Prof. Dongseok Choi from Oregon Health Science University- Portland State University School of Public Health for his help with logistic regression analysis.
Biography
Biosketch of Qi Sheng You
Dr. Qi Sheng You earned his MD, PhD in Capital Medical University, Beijing, China. He is currently a senior research associate at Casey Eye Institute, Oregon Healthy & Science University. His research interests include retina imaging and ophthalmic epidemiology.

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Wong TY, Sun J, Kawasaki R, et al. Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology 2018; 125(10): 1608–22. [DOI] [PubMed] [Google Scholar]
- 2.Classification of diabetic retinopathy from fluorescein angiograms. ETDRS report number 11. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991; 98(5 Suppl): 807–22. [PubMed] [Google Scholar]
- 3.Bresnick GH, Condit R, Syrjala S, Palta M, Groo A, Korth K. Abnormalities of the Foveal Avascular Zone in Diabetic Retinopathy. Arch Ophthalmol 1984; 102(9): 1286–93. [DOI] [PubMed] [Google Scholar]
- 4.Sim DA, Keane PA, Zarranz-Ventura J, et al. The effects of macular ischemia on visual acuity in diabetic retinopathy. Invest Ophthalmol Vis Sci 2013; 54(3): 2353–60. [DOI] [PubMed] [Google Scholar]
- 5.Chung EJ, Roh MI, Kwon OW, Koh HJ. Effects of macular ischemia on the outcome of intravitreal bevacizumab therapy for diabetic macular edema. Retina 2008; 28(7): 957–63. [DOI] [PubMed] [Google Scholar]
- 6.Manousaridis K, Talks J. Macular ischaemia: a contraindication for anti-VEGF treatment in retinal vascular disease? Br J Ophthalmol 2012; 96(2): 179–84. [DOI] [PubMed] [Google Scholar]
- 7.Group ETDRSR. Fluorescein angiographic risk factors for progression of diabetic retinopathy: ETDRS report number 13. Ophthalmology 1991; 98(5): 834–40. [PubMed] [Google Scholar]
- 8.Ip MS, Domalpally A, Sun JK, Ehrlich JS. Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology 2015; 122(2): 367–74. [DOI] [PubMed] [Google Scholar]
- 9.Hwang TS, Gao SS, Liu L, et al. Automated Quantification of Capillary Nonperfusion Using Optical Coherence Tomography Angiography in Diabetic Retinopathy. JAMA Ophthalmol 2016; 134(4): 367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang TS, Hagag AM, Wang J, et al. Automated Quantification of Nonperfusion Areas in 3 Vascular Plexuses With Optical Coherence Tomography Angiography in Eyes of Patients With Diabetes. JAMA Ophthalmol 2018; 136(8): 929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang TS, Zhang M, Bhavsar K, et al. Visualization of 3 Distinct Retinal Plexuses by Projection-Resolved Optical Coherence Tomography Angiography in Diabetic Retinopathy. JAMA Ophthalmol 2016; 134(12): 1411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashraf M, Nesper PL, Jampol LM, Yu F, Fawzi AA. Statistical Model of Optical Coherence Tomography Angiography Parameters That Correlate With Severity of Diabetic Retinopathy. Invest Ophthalmol Vis Sci 2018; 59(10): 4292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhanushali D, Anegondi N, Gadde SG, et al. Linking Retinal Microvasculature Features With Severity of Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci 2016; 57(9): Oct519–25. [DOI] [PubMed] [Google Scholar]
- 14.Binotti WW, Romano AC. Projection-Resolved Optical Coherence Tomography Angiography Parameters to Determine Severity in Diabetic Retinopathy. Invest Ophthalmol Vis Sci 2019; 60(5): 1321–7. [DOI] [PubMed] [Google Scholar]
- 15.Nesper PL, Roberts PK, Onishi AC, et al. Quantifying Microvascular Abnormalities With Increasing Severity of Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci 2017; 58(6): Bio307–bio15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Moon BG, Cho AR, Yoon YH. Optical Coherence Tomography Angiography of DME and Its Association with Anti-VEGF Treatment Response. Ophthalmology 2016; 123(11): 2368–75. [DOI] [PubMed] [Google Scholar]
- 17.Durbin MK, An L, Shemonski ND, et al. Quantification of Retinal Microvascular Density in Optical Coherence Tomographic Angiography Images in Diabetic Retinopathy. JAMA Ophthalmol 2017; 135(4): 370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei J, Durbin MK, Shi Y, et al. Repeatability and Reproducibility of Superficial Macular Retinal Vessel Density Measurements Using Optical Coherence Tomography Angiography En Face Images. JAMA Ophthalmol 2017; 135(10): 1092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Color photography vs fluorescein angiography in the detection of diabetic retinopathy in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Arch Ophthalmol 1987; 105(10): 1344–51. [DOI] [PubMed] [Google Scholar]
- 20.Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991; 98(5 Suppl): 823–33. [PubMed] [Google Scholar]
- 21.Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991; 98(5 Suppl): 786–806. [PubMed] [Google Scholar]
- 22.Zhang M, Hwang TS, Campbell JP, et al. Projection-resolved optical coherence tomographic angiography. Biomed Opt Express 2016; 7(3): 816–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell JP, Zhang M, Hwang TS, et al. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci Rep 2017; 7: 42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Y, Camino A, Wang J, Huang D, Hwang TS, Jia Y. MEDnet, a neural network for automated detection of avascular area in OCT angiography. Biomed Opt Express 2018; 9(11): 5147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Hormel TT, You Q, et al. Robust non-perfusion area detection in three retinal plexuses using convolutional neural network in OCT angiography. Biomed Opt Express 2020; 11(1): 330–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 2012; 20(4): 4710–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Camino A, Hua X, et al. Invariant features-based automated registration and montage for wide-field OCT Angiography. Biomed Opt Express 2018; 10(1):120–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zang P, Liu G, Zhang M, et al. Automated three-dimensional registration and volume rebuilding for wide-field angiographic and structural optical coherence tomography. J Biomed Opt 2017; 22(2): 26001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Hwang TS, Dongye C, Wilson DJ, Huang D, Jia Y. Automated Quantification of Nonperfusion in Three Retinal Plexuses Using Projection-Resolved Optical Coherence Tomography Angiography in Diabetic Retinopathy. Invest Ophthalmol Vis Sci 2016; 57(13): 5101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang M, Wang J, Pechauer AD, et al. Advanced image processing for optical coherence tomographic angiography of macular diseases. Biomed Opt Express 2015; 6(12): 4661–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon SD, Goldberg MF. ETDRS Grading of Diabetic Retinopathy: Still the Gold Standard? Ophthalmic Res 2019; 62(4): 190–5. [DOI] [PubMed] [Google Scholar]
- 32.Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003; 110(9): 1677–82. [DOI] [PubMed] [Google Scholar]
- 33.Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina 2015; 35(11): 2163–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu JJ, Camino A, Liu L, et al. Signal Strength Reduction Effects in OCT Angiography. Ophthalmol Retina 2019; 3(10): 835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Max Snodderly D, Weinhaus RS. Retinal vasculature of the fovea of the squirrel monkey, Saimiri sciureus: Three-dimensional architecture, visual screening, and relationships to the neuronal layers. J Comp Neurol 1990; 297(1): 145–63. [DOI] [PubMed] [Google Scholar]
- 36.Birol G, Wang S, Budzynski E, Wangsa-Wirawan ND, Linsenmeier RA. Oxygen distribution and consumption in the macaque retina. Am J Physiol Heart Circ Physiol 2007; 293(3): H1696–704. [DOI] [PubMed] [Google Scholar]
- 37.Yi J, Liu W, Chen S, et al. Visible light optical coherence tomography measures retinal oxygen metabolic response to systemic oxygenation. Light Sci Appl 2015; 4: e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byeon SH, Chung H. Deep Retinal Capillary Nonperfusion Is Associated With Photoreceptor Disruption in Diabetic Macular Ischemia? Am J Ophthalmol 2017; 174: 179–80. [DOI] [PubMed] [Google Scholar]
- 39.Scarinci F, Jampol LM, Linsenmeier RA, Fawzi AA. Association of Diabetic Macular Nonperfusion With Outer Retinal Disruption on Optical Coherence Tomography. JAMA Ophthalmol 2015; 133(9): 1036–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scarinci F, Nesper PL, Fawzi AA. Deep Retinal Capillary Nonperfusion Is Associated With Photoreceptor Disruption in Diabetic Macular Ischemia. Am J Ophthalmol 2016; 168 (8): 129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim K, Kim ES, Kim Y, Yu SY, Kwak HW. Correlation between preoperative en face optical coherence tomograpjy of photoreceptor layer and visual prognosis after macular hole surgery. Retina 2018; 38(6): 1220–30. [DOI] [PubMed] [Google Scholar]
- 42.Mathew R, Richardson M, Sivaprasad S. Predictive value of spectral-domain optical coherence tomography features in assessment of visual prognosis in eyes with neovascular age-related macular degeneration treated with ranibizumab. Am J Ophthalmol 2013; 155(4): 720–6, 726.e1. [DOI] [PubMed] [Google Scholar]
- 43.Spaide RF, Klancnik JM Jr., Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol 2015; 133(1): 45–50. [DOI] [PubMed] [Google Scholar]
- 44.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes care 2012; 35(3): 556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui J, Ren JP, Chen DN, et al. Prevalence and associated factors of diabetic retinopathy in Beijing, China: a cross-sectional study. BMJ open 2017; 7(8): e015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sim DA, Keane PA, Rajendram R, Karampelas M, Selvam S, Powner MB, Fruttiger M, Tufail A, Egan CA. Patterns of peripheral retinal and central macula ischemia in diabetic retinopathy as evaluated by ultra-widefield fluorescein angiography. Am J Ophthalmol 2014. July;158(1):144–153. [DOI] [PubMed] [Google Scholar]
- 47.Ho J, Dans K, You Q, Nudleman ED, Freeman WR. Comparison of 3 mm × 3 mm versus 6 mm × 3 mm optical coherence tomography angiography scan sizes in the evaluation of non-proliferative diabetic retinopathy. Retina 2019;39 (2):259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


