Abstract
Risk of relapse is a major challenge in the treatment of substance use disorders. Several types of learning and memory mechanisms are involved in substance use and have implications for relapse. Associative memories form between the effects of drugs and the surrounding environmental stimuli, and exposure to these stimuli during abstinence causes stress and triggers drug craving, which can lead to relapse. Understanding the neural underpinnings of how these associations are formed and maintained will inform future advances in treatment practices. A large body of research has expanded our knowledge of how associative memories are acquired and consolidated, how they are updated through reactivation and reconsolidation, and how competing extinction memories are formed. This review will focus on the vast literature examining the mechanisms of cocaine Pavlovian associative memories with an emphasis on the molecular memory mechanisms and circuits involved in the consolidation, reconsolidation, and extinction of these memories. Additional research elucidating the specific signaling pathways, mechanisms of synaptic plasticity, and epigenetic regulation of gene expression in the circuits involved in associative learning will reveal more distinctions between consolidation, reconsolidation, and extinction learning that can be applied to the treatment of substance use disorders.
Keywords: Learning, Addiction, Consolidation, Reconsolidation, Extinction
Introduction
Memories related to obtaining positive outcomes promote continued reward-seeking behavior that is important for survival. Unfortunately, when the outcome is a drug of abuse, these memories can become abnormally strong and maladaptive, thus promoting drug use over other behaviors. Even during abstinence, exposure to environmental cues associated with drug use can trigger memories that elicit craving, cause physiological stress, and initiate drug-seeking behaviors that lead to relapse [1–4]. Uncovering the mechanisms underlying the formation and retrieval of these drug memories may reveal ways to improve treatment and prevent relapse [5]. Much of the research focused on understanding the neural underpinnings of drug-associated memories has focused on memories formed during exposure to cocaine as the drug of abuse [6]. Cocaine use disorder remains a prominent burden on society, and cocaine use continues to increase [7]. Furthermore, there has been an increase in overdose deaths involving concomitant cocaine and heroin use [7]. Finally, identification of neural mechanisms relevant to cocaine memories likely can be applied to other drugs of abuse [6, 8]. Thus, in this review, we will focus on experiments specifically addressing Pavlovian associative memories between cocaine and environmental contexts and discrete cues because of the extensive literature examining the consolidation, reconsolidation, and extinction of these memories.
Types of cocaine memories
Memories about reinforcers often involve associations between the reinforcer and contextual or discrete environmental cues, information about the interoceptive effects or the value of the reinforcer, and information about behaviors that result in seeking and obtaining the reinforcer [3, 8, 9]. Drug memories can elicit cravings that promote continued use and relapse, but different types of memories may be differentially important depending on the type of drug used and the extent of drug use [3]. Moreover, these different types of cocaine associations may develop through different mechanisms and in different brain circuits, which is important to understand in order to optimize treatment development. With a focus on cocaine, we will briefly describe some of the types of memories that can contribute to drug-seeking behavior and relapse (Fig. 1).
Fig. 1.
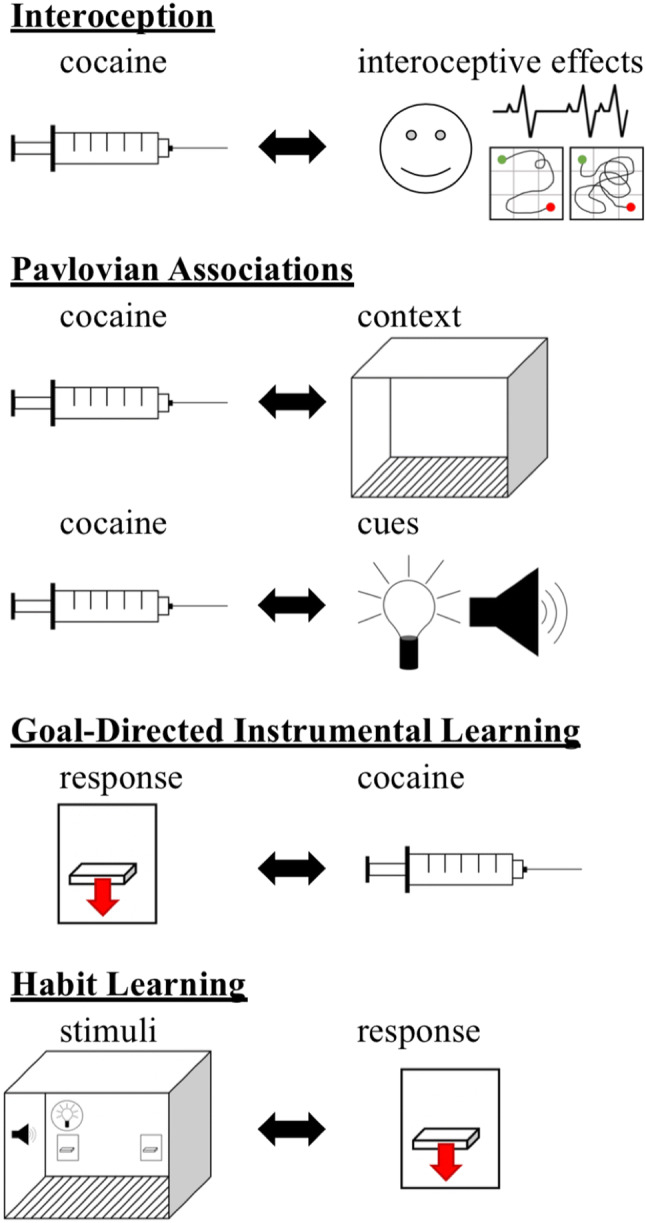
Schematic illustrating the types of associative memories involved in cocaine-seeking behavior. Interoception involves associations between cocaine and its reinforcing and interoceptive effects, including increased heart rate and blood pressure, increased locomotor activity, and activation of brain regions associated with reward. Pavlovian conditioning results in associations between cocaine and discrete cues and/or the context in which cocaine is received. Goal-directed instrumental learning concerns the association between the instrumental response and cocaine, and habit learning concerns associations between contextual and discrete environmental stimuli and the instrumental response
Interoception and reward
Due to its direct effects on synaptic dopamine levels in brain regions associated with reward, cocaine is highly reinforcing [10]. Neurons in the ventral tegmental area (VTA) of the midbrain send dopaminergic projections to the nucleus accumbens (NAc), also referred to as the ventral striatum, which are important for reward-related prediction and learning [10]. Cocaine increases synaptic dopamine in the NAc and other brain regions, including the dorsal striatum, prefrontal cortex (PFC), and limbic regions, and dopamine’s modulatory effects on neuronal activity influences both the rewarding effects of cocaine as well as reward-related learning [8, 10]. Due to its effects on the dopamine system, cocaine also produces several physiological effects, referred to as interoceptive effects, and associative memories between the use of cocaine and these rewarding/interoceptive effects are formed [11].
Pavlovian associations
In addition to interoceptive associative memories, Pavlovian associative memories can form between reinforcers and contextual or discrete environmental cues (also known as classical conditioning). Although these associations provide valuable information about the availability of natural reinforcers, environmental cues associated with cocaine use can lead to craving and relapse in individuals with substance use disorders [1]. Pavlovian associations are dependent on the basolateral amygdala (BLA), and contextual associations specifically appear to additionally rely on the hippocampus [6, 12]. These regions interact with each other, the PFC, and other sensory input regions to establish memory traces that can then promote drug using actions via outputs to the striatum when these cues and contexts are subsequently encountered [13].
Goal-directed instrumental learning (response–outcome associative learning)
When cocaine is self-administered, instrumental learning occurs alongside Pavlovian conditioning [14]. Initial learning of instrumental responses requires the formation of response–outcome associative memories that guide goal-directed behavior [15]. This process involves learning that an instrumental response or behavior results in delivery of the drug and its subsequent rewarding effects [16]. Goal-directed learning relies on cortico-striatal circuits that use contextual stimuli to detect reward availability and guide behavior [14]. The dorsomedial striatum (DMS) gets direct and indirect input from the PFC (particularly the prelimbic region, PL) and the BLA to produce context-appropriate behavioral responses [14, 17, 18].
Habit learning (stimulus–response associative learning)
Under certain conditions, usually after significant repetition of the same action, a goal-directed behavior can shift to become a stimulus–response habit [15]. Once habits form, behavior no longer relies on the value of the outcome, but instead contextual or discriminative stimuli initiate a more automated behavioral response [19]. Acquisition of stimulus–response associative learning relies on the BLA, but the maintenance of habitual behavior depends on the central nucleus of the amygdala (CeN) [18]. Similarly to goal-directed behavior, learning habitual behavior requires changes in cortico-striatal circuitry, including a shift in the brain region mediating the behavior from the DMS to the dorsolateral striatum (DLS) [20, 21]. Although interoceptive, goal-directed, and habitual associative memory formation are important aspects of cocaine use and addiction, the most extensive knowledge about cocaine memory formation, reactivation, and extinction addresses Pavlovian associative memories, which will be the focus of this review.
Cellular and molecular mechanisms of memory
There are several cellular and molecular processes that underlie the formation and maintenance of cocaine memories. Generally, memory traces are formed when a combination of neuronal input and activity triggers cellular events that ultimately result in changes in protein expression, including changes in the membrane expression of receptors, neuronal morphology, and the excitability of cells. All of these forms of neuroplasticity can lead to changes in the way the neuron responds to inputs, thus allowing for the formation of a memory trace and the process of learning. Drugs, including cocaine, influence these natural memory processes [22]. For example, strong evidence exists for plasticity in corticolimbic circuits during cocaine Pavlovian memory formation, and suggests that these circuits are also activated during cocaine cue-induced craving, making them important targets for the treatment of cocaine use disorder [2, 13, 23, 24].
Memory formation requires changes in how neurons communicate. Long-term potentiation (LTP) and long-term depression (LTD) are activity-dependent mechanisms by which synapses can be strengthened or weakened [25]. These processes result in altered expression and localization of glutamate receptors and their subunits at the synapse, amongst other neurophysiological adaptations [26, 27]. Although LTP and LTD can be regulated by modulatory neurotransmitters such as dopamine, these processes occur at glutamatergic excitatory synapses, and rely on glutamate release from the presynaptic neuron along with temporally related post-synaptic depolarization [24, 28–30]. LTP and LTD are induced by activation of particular kinases and phosphatases that regulate the phosphorylation and activation of signaling molecules [31]. Typically, activation of protein kinases such as calcium/calmodulin‐dependent protein kinase II (CaMKII), extracellular signal-regulated kinases (ERKs), and protein kinases A and C (PKA; PKC) is associated with LTP [31, 32]. Alternatively, activation of protein phosphatases like calcineurin (CaN) and protein phosphatase 1 (PP1) is associated with LTD [31, 32]. These proteins regulate the activity of downstream proteins, signaling molecules, and transcription factors [10, 28, 31, 32].
Immediate early genes (IEGs) are a subset of genes that experience increased transcription in response to neuronal activity and plasticity [33]. These genes, including c-fos, BDNF and Zif268 encode proteins that are often used as neuronal markers of plasticity because of their reliable expression in neuronal ensembles that encode memory traces [33]. Although the direct roles of each IEG in neural plasticity have not been systematically characterized, they generally encode synaptic proteins, secretory proteins, and transcription factors that have a direct impact on synaptic properties, thus contributing to the formation of long-term plasticity [33]. Synaptic plasticity is also mediated by epigenetic modifications that regulate gene expression [34, 35]. Two important epigenetic processes involved in memory include DNA methylation, regulated by DNA methyltransferases (DNMTs) and demethylases, and histone acetylation, regulated by histone acetyltransferases (HATs) and histone deacetylases (HDACs) [34, 35]. All of these learning and memory processes have been implicated in cocaine memories.
Cocaine memory acquisition, consolidation, and retrieval
The molecular and circuit mechanisms involved in the acquisition of long-term memories, including several of the mechanisms discussed above, have been extensively studied and reviewed [36]. Once memories are acquired, they can undergo consolidation, a process of stabilization that lasts for hours or days by which cellular and molecular changes create a memory trace, or a physical representation in the brain (Fig. 2) [37]. These cellular and molecular changes include gene expression, protein phosphorylation and localization, DNA methylation, and histone acetylation [10, 22, 34, 35, 38] and occur within brain circuits involved in reward, memory, and action selection [10, 39, 40]. The acquisition and consolidation of cocaine memories can differ from memories for natural reinforcers, in part because the neurobiological effects of cocaine impact mechanisms of memory acquisition and consolidation, resulting in memories that can be maladaptive and hinder abstinence [9].
Fig. 2.
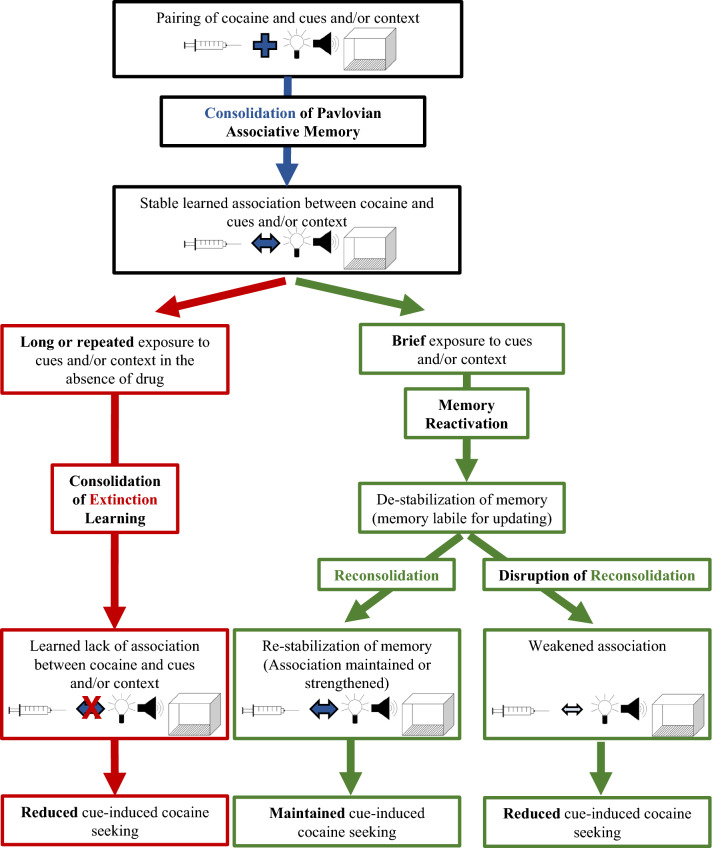
Schematic demonstrating the consolidation (blue), reconsolidation (green), and extinction (red) of cocaine Pavlovian memories. The temporally paired delivery of cocaine with discrete and contextual cues results in the consolidation of the associative memory between cocaine and these cues. Once consolidated, this memory is stable. Long or repeated exposure to these cues results in extinction learning, which involves learning about the lack of association between cocaine and these cues and reduced cue-induced cocaine seeking. Alternatively, brief exposure to these cues results in reactivation of the previously consolidated associative memory, which is de-stabilized, allowing for potential updating of the memory. Under normal conditions, memory reactivation is followed by reconsolidation, and the memory is re-stabilized, resulting in maintenance of the associative memory and cue-induced cocaine seeking. If reconsolidation is disrupted, the association between the cues and cocaine is weakened, resulting in reduced cue-induced cocaine seeking
Memory acquisition and consolidation are separate processes that can be distinguished temporally. Experimental manipulations occurring before learning address acquisition, while those occurring within minutes to hours after learning address consolidation [41]. However, it is important to note that experimental manipulations occurring before learning can still be in effect during the consolidation period, and may additionally affect consolidation.
Cocaine memory circuitry
Several brain regions interact to form cocaine-context and cocaine–cue associative memories because memory formation involves processing sensory information about contextual stimuli, detecting the rewarding effects of cocaine, directing attention, and forming reward-seeking behaviors. Corticolimbic circuitry is particularly involved in forming Pavlovian associations between stimuli and rewards, making these brain regions a nexus for cocaine–context and cocaine–cue associative learning [13, 42]. The dopaminergic reward circuitry and the striatum are also important for this type of associative learning [10, 39, 43, 44]. Below, we will discuss molecular mechanisms that occur in brain regions and circuits between these regions that contribute to Pavlovian associative memories between cocaine and contextual stimuli (Table 1).
Table 1.
Summary of experiments addressing mechanisms of cocaine Pavlovian memory acquisition, consolidation, and expression
| Paradigm | Brain region | Manipulation | Results | Species, age, sex | Citations |
|---|---|---|---|---|---|
| CPP | DHC | Inhibition with muscimol | Impaired acquisition, expression, and late-stage consolidation, but not early consolidation | Rat, adult, M; mouse, adult, M; rat, adult, M | Meyers et al. [41]; Hitchcock and Lattal [45]; Kramar et al. [46] |
| CPP | DHC | HDAC inhibitor | Enhanced acquisition and/or consolidation | Mouse, adult, M | Itzhak et al. [47] |
| CPP | DHC and PL | DNA methyltransferase inhibitor | Inhibition in the DHC restrained acquisition, inhibition in the PL blocked expression | Mouse, adult, M | Han et al. [48] |
| CPP | DHC | D1/D5 dopamine receptor antagonist | Impaired acquisition and expression, but not consolidation | Rat, adult, M | Kramar et al. [46] |
| CPP | DHC | D1 dopamine receptor agonist or antagonist | Agonist impaired and antagonist enhanced consolidation | Rat, adult, M | Kramar et al. [73] |
| CPP | DHC | β-adrenergic receptor antagonist | Impaired expression | Rat, adult, M | Otis and Mueller [76] |
| CPP | BLA | Protein synthesis, PKA, PKC, and MEK inhibitors | Inhibition of protein synthesis and PKC, but not PKA or MEK, blocked expression | Mouse, adult, M | Lai et al. [49] |
| CPP | Amygdala | Cdk5 inhibitor | Cdk5 inhibition in the BLA, but not CeA, inhibited consolidation and expression | Rat, adult, M | Li et al. [50] |
| Classical conditioning after self-administration | BLA | NMDA receptor antagonist | Inhibited acquisition and consolidation | Rat, adult, M | Feltenstein and See [51] |
| Self-admin | MGN-LA | n/a | Potentiation of projections from the MGN to the LA as associations form | Rat, adult, M | Rich et al. [52] |
| CPP | mPFC | Chemogenetic inhibition | Inhibition of glutamatergic, but not GABAergic, mPFC neurons suppressed acquisition and expression | Mouse, adult, M | Zhang et al. [55] |
| CPP | PL | Removal of perineuronal nets from interneurons | Impaired acquisition | Rat, adult, M | Slaker et al. [56] |
| CPP | mPFC | CB1 receptor antagonist | Consolidation of memory induced by high cocaine dose impaired, by low cocaine dose facilitated | Mouse, adult, M | Hu et al. [57] |
| CPP | NAc core | Rac inhibitor | Inhibited consolidation | Rat, adult, M | Ding et al. [58] |
| Self-admin | NAc | mTOR inhibitor | mTOR inhibition in the core, but not shell, impaired consolidation | Rat, adult, M | Wang et al. [59] |
| CPP | NAc | knockout of the histone acetyltransferase CBP | Prevented acquisition | Mouse, adult, M + F | Malvaez et al. [63] |
| CPP | NAc | Dephosphorylation of HDAC5 at S279 | Impaired acquisition | Mouse, adult, M | Taniguchi et al. [62] |
| CPP | NAc | HDAC3 knockout | Enhanced acquisition | Mouse; adult, M + F | Rogge et al. [61] |
| CPP | NAc | Baf53B knockout (inhibits nucleosome remodeling) | Reduced acquisition and LTP | Mouse, adult, M + F | White et al. [64] |
| CPP | NAc | Chemogenetic inhibition | Inhibition of GABAergic neurons inhibited expression | Mouse, adult, M | Zhang et al. [65] |
| CPP | VTA | NMDA receptor antagonist | Impaired acquisition and expression | Rat, adult, M | Zhou et al. [72] |
| CPP | Systemic | Stress-induced noradrenergic activity leading to increased dopamine release in the mPFC | Attenuated acquisition and expression | Rat, adult, M | Shinohara et al. [74] |
| CPP | Systemic | β1-adrenergic receptor antagonist | Impaired expression | Rat, adult, M | Fitzgerald et al. [75] |
| CPP | Systemic | M1 receptor antagonist | Impaired consolidation | Mouse, adult, M | Zacarias et al. [77] |
Hippocampus
It is widely accepted that the hippocampus plays a major role in memory acquisition, and particularly the dorsal hippocampus (DHC) in contextual Pavlovian conditioning for drug memories [12]. Local inhibition of the DHC with muscimol prior to cocaine conditioned place preference (CPP) conditioning disrupts learning, affirming that the DHC is also important for the acquisition of contextual conditioning for cocaine [41]. Additionally, inhibition of the DHC impairs retrieval of a cocaine CPP memory [45]. The role of the DHC in cocaine memory consolidation is less clear. Although evidence suggests that the DHC is necessary for the consolidation of aversive contextual conditioning, inhibition of the DHC immediately or 6 h after cocaine CPP training does not disrupt learning, providing evidence that the DHC is not immediately essential for the consolidation of cocaine-context associations [41]. In contrast, inhibition of the DHC with muscimol 12 h after cocaine CPP conditioning impairs memory retention, indicating that neuronal activity in the DHC may be important for other memory storage processes or late-stage consolidation [46].
Epigenetic mechanisms in the DHC are also important for cocaine–context associative memory. Systemic administration of the HDAC inhibitor sodium butyrate prior to cocaine CPP conditioning leads to increased histone acetylation in the hippocampus, but not the amygdala, and improves later recall of cocaine–context associative memory [47]. These results suggest that histone acetylation plays a role in the acquisition or consolidation of these memories in the hippocampus. Additionally, injection of a DNMT inhibitor into the CA1 area of the hippocampus hindered acquisition of the cocaine–context associative memory, indicating that DNA methylation in the hippocampus is also important for acquisition [48].
Amygdala
Research involving fear-related associative learning suggests that whereas the DHC is particularly important for contextual associations, the BLA is important for both contextual and cue associations [12]. Evidence supports this dichotomy in the cocaine literature as well [47, 49]. Expression of a cocaine CPP memory is impaired by inhibition of protein synthesis or PKC in the BLA, indicating that both protein synthesis and PKC-mediated protein phosphorylation in the amygdala are necessary for cocaine-context memory retrieval [49]. Similarly, the consolidation and expression of cocaine CPP memory is impaired by inhibition of the protein kinase cyclin-dependent kinase 5 (cdk5) in the BLA [50]. Additionally, the BLA plays a critical role in the acquisition and consolidation of cocaine-cue (or cocaine-conditioned stimuli) associative memories. NMDA receptor antagonism in the BLA immediately before or after a cocaine-cue classical conditioning session inhibits the acquisition and consolidation of the cocaine–cue associative memory, respectively [51]. Pairing an audiovisual cue with cocaine infusions during self-administration compared to saline also leads to potentiation of projections from the MGN of the thalamus to the lateral amygdala, indicating that potentiation of synapses carrying auditory information about conditioned stimuli to the amygdala during drug use plays an important role in acquiring these associations [52]. Further investigation is required to determine if cocaine associative memory formation depends on intracellular signaling pathways and epigenetic mechanisms in the amygdala similar to those required for other associative memories [53, 54].
Prefrontal cortex
Cocaine memory acquisition, consolidation, and retrieval also rely heavily on the PFC and its corticolimbic circuit connections to the hippocampus and amygdala [13]. The PFC is thought to be important for action selection based on the value of goals, which is an essential aspect of drug-seeking behavior [10]. Chemogenetic inhibition of glutamatergic, but not GABAergic, neurons in the medial prefrontal cortex (mPFC) suppresses both the acquisition and expression of cocaine CPP, without affecting lithium chloride-induced conditioned place aversion, suggesting a unique role of excitatory transmission in the mPFC in reward–context associative memory acquisition and retrieval [55]. Although chemogenetic inhibition of GABAergic neurons in the mPFC does not affect cocaine CPP learning, these GABAergic neurons still play an important modulatory role [56]. Specifically, the removal of perineuronal nets, which are important for neural plasticity, from parvalbumin-containing, fast-spiking GABAergic interneurons in the PL impairs the acquisition of cocaine CPP [56]. Additionally, activity at CB1 receptors in the mPFC can bidirectionally modulate consolidation of cocaine CPP learning, with CB1 antagonism in the mPFC impairing CPP memory consolidation induced by higher doses of cocaine, but facilitating consolidation induced by lower doses [57]. Finally, inhibition of DNMTs in the mPFC impairs the expression, but not acquisition, of cocaine CPP, demonstrating a role of DNA methylation in the mPFC in the retrieval of cocaine–context associative memories [48].
Nucleus accumbens (NAc)
Dopaminergic activity in the NAc, particularly the shell region, has also been implicated in the consolidation of Pavlovian learning [8]. Moreover, numerous intracellular signaling processes in the NAc have also been implicated in cocaine-associative memory. For example, Rac, a regulator of actin dynamics and neural plasticity, is essential for the consolidation of cocaine CPP conditioning, and the kinase activity of mammalian target of rapamycin (mTOR) in the NAc core regulates the expression of cocaine–cue associations in a self-administration paradigm [58, 59]. In addition, epigenetic mechanisms play a role in cocaine memory formation [60]. Local knockout of the histone acetyltransferase CREB-binding protein inhibits cocaine CPP learning, and increased activity of HDAC5 in the NAc suppresses the acquisition of cocaine CPP, while local knockout of HDAC3 in the NAc enhances CPP acquisition, providing evidence that histone acetylation in the NAc is an important mechanism for cocaine–context associative learning [61–63]. In addition to histone acetylation, nucleosome remodeling in the NAc also appears to be essential, demonstrated by reduced LTP and cocaine CPP acquisition when nucleosome remodeling is inhibited [64]. Finally, chemogenetic inhibition of GABAergic neurons in the NAc prior to CPP testing reduces place preference, suggesting that the activity of GABAergic neurons in the NAc is also important, though this experiment did not differentiate between GABAergic interneurons and medium spiny neurons (MSNs) [65].
Dorsal striatum
When Pavlovian associations between cocaine and environmental stimuli form in a paradigm where the drug is obtained by operant self-administration, these associations guide the acquisition of the drug-seeking and drug-taking behavior [44, 66, 67]. Evidence suggests that while Pavlovian conditioning relies on the ventral striatum (NAc), as drug-seeking progresses, Pavlovian associations guide the formation of goal-directed and eventually habitual instrumental response strategies aimed at obtaining the drug [44, 66, 67]. Goal-directed drug-seeking depends on input to the DMS as well as corticolimbic circuits described above [20, 44, 66]. Under certain conditions, drug-seeking can become habitual, where the behavior is initiated by environmental stimuli without relying on the value of the outcome, and this behavior is dependent on dopaminergic input to the DLS [20, 66, 68]. Goal-directed cocaine-seeking and the acquisition of habitual cocaine-seeking are also reliant on functional connectivity between the BLA and DMS, and the performance of habitual cocaine-seeking behavior relies on multisynaptic functional connectivity between the CeN and the DLS [18]. Cue-controlled goal-directed and habitual drug seeking additionally continue to rely on corticolimbic circuits described above [18, 67, 69].
Modulatory systems
Modulatory neurotransmitter systems, such as the dopaminergic, noradrenergic, and cholinergic systems, affect neural plasticity and influence memory acquisition, consolidation, and expression [70, 71]. Drugs of abuse, including cocaine, increase dopamine concentrations at the synapse, which then alters memory formation processes throughout the reward circuitry, including the PFC, striatum, and limbic system [10]. The role of dopaminergic projections from the midbrain in reward-related learning and memory has been extensively reviewed previously, so a few representative studies are included here [10]. For example, one study showed that NMDA receptor activity in the VTA, which contains dopaminergic cell bodies, is necessary for both cocaine CPP memory acquisition and retrieval [72]. Furthermore, several studies suggest that VTA dopaminergic projections to the DHC are particularly critical for contextual learning related to drugs of abuse [10]. For instance, infusion of a D1/D5 dopamine receptor antagonist into the DHC before cocaine CPP conditioning impairs memory acquisition, and infusion before testing impairs retrieval, suggesting that dopaminergic input to the DHC is important for both the acquisition and retrieval of cocaine–context associative memories [46]. Alternatively, this input may be less important for consolidation, because D1/D5 antagonism immediately and 12 h after cocaine CPP conditioning did not impair learning [46]. Interestingly, administering a D1 receptor antagonist in the DHC 12 h after cocaine CPP conditioning actually extends the persistence of the cocaine–context associative memory, while administering a D1 agonist at this timepoint impairs later recall [73]. Together, these experiments suggest that dopaminergic input to the DHC is initially important for cocaine–context associative memory acquisition and for later retrieval and memory expression, but may actually hinder late-stage consolidation [46, 73].
In addition to dopamine, noradrenergic projections from the locus coeruleus regulate attention and vigilance, and therefore have a role in memory acquisition and retrieval [10]. Recent evidence suggests that stress-induced noradrenergic activity interacts with the VTA reward circuitry to increase dopamine release in the mPFC, augmenting cocaine CPP memory retrieval [74]. Systemic inhibition of β1-adrenergic receptors impairs the retrieval of cocaine-CPP memory, demonstrating an influence of adrenergic activity on memory expression [75]. More specifically, adrenergic input to the DHC also plays a role, as inhibition of β-adrenergic receptors in the DHC impairs the retrieval of cocaine CPP memories [76]. Finally, the neuromodulator acetylcholine is also implicated in the regulation of appetitive contextual memories, as systemic antagonism of M1 type muscarinic receptors disrupts cocaine CPP memory consolidation [77].
Effects of cocaine on memory systems
The circuitry described above is important for memory acquisition, consolidation, and expression, and has been particularly implicated in Pavlovian associative memories between cocaine and environmental cues and contexts. Because cocaine’s effects on dopamine release influence the reward circuitry involved in endogenous reward learning and Pavlovian conditioning, it can enhance the formation of these memories and lead to aberrant learning that promotes behaviors characteristic of addiction [9]. Drugs of abuse, including cocaine, have behavioral, physiological, and molecular effects on learning and memory that have been extensively reviewed elsewhere [3, 9, 10, 22, 66]. Briefly, exposure to cocaine and other stimulants leads to persistent plastic changes in the circuits involved in reward and memory formation, and can also promote the formation of habits, which are thought to contribute to the development of the compulsive drug use characteristic of addiction [9, 78–81].
Cocaine memory reactivation and reconsolidation
After memories are acquired and undergo consolidation, they are believed to be stable and are resistant to amnestic agents [82]. However, memory retrieval, or reactivation, triggers a reconsolidation process during which memories are again labile and susceptible to manipulation (Fig. 2) [82, 83]. This reconsolidation process may serve the evolutionary purpose of allowing memories to be updated with new information, but it may also allow for the disruption of maladaptive drug-related memories [84–88]. There is considerable overlap between the molecular and circuit mechanisms that underlie original consolidation and subsequent reconsolidation, with processes including protein synthesis, transcription factors, protein kinases and phosphorylation, NMDA-receptor-mediated synaptic plasticity, and modulatory receptor activity [28, 83, 89]. However, there are distinctions between the consolidation and reconsolidation of memories, both in the molecular processes and brain regions involved [83, 90].
Disrupting reconsolidation
The period of lability a memory enters during reconsolidation has become a target for intervention in substance use disorders [91]. Because cocaine memories often promote drug craving and seeking, disrupting these memories could provide relief for people trying to maintain abstinence [87]. Much of the research about the reconsolidation of cocaine memories has investigated methods of reactivating cocaine memories, what processes are required for reconsolidation, and how to then interfere with these processes in order to develop potential clinical treatments (Table 2).
Table 2.
Summary of experiments addressing mechanisms of cocaine Pavlovian reconsolidation
| Paradigm | Brain region | Manipulation | Results | Species, age, sex | Citations |
|---|---|---|---|---|---|
| CPP or CA, context reactivation | Systemic | NMDA receptor antagonist | Impaired reconsolidation | Rat, adult, M | Alaghband et al. [92] and Brown et al. [107]; Alaghband et al. [96] |
| CA, context reactivation | Systemic | Protein synthesis inhibitor | Impaired reconsolidation | Rat, adult, M | Bernardi et al. [101] |
| CPP, context reactivation | Systemic | β-adrenergic receptor antagonist; protein synthesis inhibitor; nNOS inhibitor; GSK3 inhibitor; D3 dopamine receptor antagonist | Impaired reconsolidation | Rat, adult, M; mouse, adult, M; mouse, adult, M; mouse, adult, M; mouse, adult, M + F | Fricks-Gleason et al. [97] and Bernardi et al. [102]; Fan et al. [98]; Itzhak et al. [99]; Shi et al. [103]; Yan et al. [105] |
| CPP, context reactivation | Systemic | muscarinic receptor antagonist, NMDAR antagonist, or NMDAR partial agonist | Impaired reconsolidation (or enhanced extinction) | Mouse, adult, M | Kelley et al. [100] |
| CPP, context reactivation | DHC | Chemogenetic inhibition or Tet3 knockdown in pyramidal neurons | Impaired reconsolidation | Mouse, adult, M | Liu et al. [108] |
| Self-admin, context reactivation | DHC | Inactivation with tetrodotoxin or protein synthesis inhibitor; SFK inhibitor | Only inactivation with tetrodotoxin impaired reconsolidation; impaired reconsolidation | Rat, adult, M | Ramirez et al. [95]; Wells et al. [109] |
| Self-admin, context reactivation | DHC | SFK inhibitor | Impaired reconsolidation | Rat, adult, M | Wells et al. [109] |
| Self-admin, context reactivation | BLA | Protein synthesis inhibitor | Impaired reconsolidation | Rat, adult, M | Fuchs et al. [110] |
| CPP, context reactivation | Amygdala | Cdk5 inhibitor | Inhibition in BLA, but not CeA, impaired reconsolidation | Rat, adult, M | Li et al. [50] |
| Self-admin, context reactivation | BLA | PKA or CaMKII inhibitor; ERK inhibitor | PKA and ERK, but not CaMKII inhibition impaired reconsolidation; | Rat, adult, M | Arguello et al. [93]; Wells el. al [111] |
| CPP, context reactivation | BLA | GSK-3β inhibitor | Impaired reconsolidation | Rat, adult, M | Wu et al. [112] |
| CPP, context reactivation | BLA | β-adrenergic receptor antagonist | Impaired reconsolidation, not expression | Rat, adult, M | Otis et al. [113] |
| Self-admin, context reactivation | BLA/DHC | Unilateral protein synthesis inhibitor in BLA and contralateral inhibitor on DHC | BLA-DHC disconnection impaired reconsolidation | Rat, adult, M | Wells et al. [114] |
| CPP, context reactivation | NAc core | MEK (ERK kinase) inhibitor | Impaired reconsolidation | Rat, adult, M | Miller and Marshall [115] |
| CPP, context reactivation | NAc shell | NMDA receptor antagonist or D1 dopamine receptor antagonist | Impaired reconsolidation | Rat, adult, M | Li et al. [116] |
| CPP, context reactivation | PL | cAMP pathway inhibitors | Impaired reconsolidation | Rat, adult, M | Otis et al. [118] |
| CPP, context reactivation | mPFC | histone lysine demethylase inhibitor | Impaired reconsolidation | Mouse, adult, M | Zhang et al. [119] |
| Self-admin, cue reactivation | Systemic | HAT inhibitor; β-adrenergic receptor antagonist or protein synthesis inhibitor | Impaired reconsolidation; only protein synthesis inhibitor impaired reconsolidation | Rat, adult, M | Monsey et al. [120]; Dunbar and Taylor [121] |
| Self-admin, cue reactivation | BLA | Zif268 knockdown; DNMT inhibitor; PKA inhibitor; Epac activator; CaMKII inhibitor | Impaired reconsolidation | Rat, adult, M | Lee et al. [94, 125]; Shi et al. [122]; Sanchez et al. [123]; Wan et al. [124]; Rich et al. [126] |
| Self-admin, response-contingent cue reactivation | Systemic | β-adrenergic receptor antagonist | Impaired reconsolidation | Rat, adult, M | Milton et al. [130] |
| Self-admin, response-contingent cue reactivation | Systemic, IL, or NAc | NMDA receptor antagonists | Impaired reconsolidation | Rat, adult, M | Hafenbreidel et al. [128] |
| Self-admin, response-contingent cue reactivation | Systemic or BLA | NMDA receptor antagonist | Impaired reconsolidation | Rat, adult, M | Milton et al. [129] |
| Self-admin, response-contingent cue reactivation | Systemic | D1 or D3 dopamine receptor antagonist | Impaired reconsolidation | Mouse, adult, M + F | Yan et al. [132] |
| Self-admin, US reactivation | Systemic | HAT inhibitor | Impaired reconsolidation | Rat, adult, M | Dunbar and Taylor [135] |
Context reactivation
One method of preclinically investigating the reconsolidation of cocaine–context associative memories is by briefly re-exposing an animal to a cocaine-associated context [92]. This re-exposure can occur after either cocaine CPP conditioning or conditioned activity (CA), a paradigm in which Pavlovian associations between a psychostimulant and an environment produce increased locomotion when the animal is re-exposed to the environment without drug [92]. Although these methods can model subsequent retrieval of the cocaine-context associative memory through preference for the drug-paired chamber or presence of conditioned reactivity, they cannot evaluate subsequent drug-seeking behavior. Therefore, re-exposure to the context of drug self-administration after cocaine self-administration training has been used to evaluate both subsequent memory retrieval and drug-seeking behavior [52, 93–95]. Brief re-exposure to a cocaine-associated context triggers reactivation and reconsolidation (Fig. 2). Reconsolidation of a CPP and CA memories after context exposure are inhibited by systemic administration of NMDA receptor antagonists, β-adrenergic receptor antagonists, a muscarinic acetylcholine receptor antagonist, protein synthesis inhibitors, and kinase inhibitors, suggesting several of the processes involved in cocaine memory consolidation are also required for reconsolidation [92, 96–103]. The re-exposure to a context associated with cocaine self-administration also increases serum corticosterone concentrations, and this activation of the HPA axis may also play a role in memory reactivation and reconsolidation [104]. In addition, a role for dopamine modulation of the reward circuitry on reconsolidation is demonstrated by the observation that antagonism of dopamine D3 receptors, which are preferentially expressed in mesocortioclimbic regions, impaired reconsolidation of CPP memory [105]. Further, nitric oxide signaling, a retrograde messenger involved in the formation of memories through facilitation of synaptic plasticity, as well as NMDA receptor activity, is also important for reconsolidation after reactivation of cocaine contextual memory in the CPP paradigm [99, 106, 107].
Experiments involving region-specific interventions have revealed, unsurprisingly, that several of the brain regions important for cocaine-context memory consolidation are also involved in reconsolidation. Whereas the DHC is particularly important for the acquisition of these memories, its role in consolidation is somewhat ambiguous [41, 46–48]. However, the DHC plays a clear role in the reconsolidation of cocaine–context associative memories. Inhibition of excitatory pyramidal neurons in the DHC after cocaine-context memory reactivation impairs the reconsolidation of a cocaine CPP memory, and DNA demethylation by the methylcytosine dioxygenase tet3 plays an essential role in the epigenetic control of the post-retrieval activity of these neurons [108]. Additionally, inactivation of the DHC with tetrodotoxin after re-exposure to the context of cocaine self-administration reduced subsequent reinstatement in this context, suggesting the disruption of reconsolidation [95]. NMDA receptor-mediated plasticity in the DHC appears to be particularly important, as inhibition of SFK, a tyrosine kinase that regulates the phosphorylation of NMDA receptor subunits disrupts reconsolidation of a cocaine-self-administration contextual memory [109]. However, inhibition of protein synthesis in the hippocampus had no effect, indicating that regions other than DHC are responsible for the effects of broad protein synthesis-dependent reconsolidation events [95].
Indeed, inhibition of protein synthesis in the BLA after reactivation of the cocaine context memory blunted context-induced reinstatement of self-administration, suggesting that the BLA may be at least one region that requires protein synthesis for reconsolidation of these memories [110]. Additionally, activity of the protein kinase Cdk5 in the BLA is required for the reconsolidation of cocaine CPP memories just as it is required for consolidation, and PKA, but not CaMKII, activity is also required for the reconsolidation of the associative memory between cocaine and the context of self-administration [50, 93]. Other kinase activity in the BLA, including ERK and glycogen synthase kinase 3β (GSK-3β), are important for cocaine–context associative memory reconsolidation for self-administration and CPP, respectively, indicating that the mechanisms of plasticity regulated by these kinases play a role in reconsolidation in the BLA [111, 112]. Modulatory input to the BLA also influences reconsolidation, demonstrated by the ability of β-adrenergic receptor blockade to disrupt reconsolidation, but not retrieval, of cocaine CPP memories [113]. Interestingly, reconsolidations of cocaine–context associative memories for self-administration not only relies on the DHC and BLA individually, but functional disconnection of the two regions disrupts reconsolidation, indicating that interaction between the two regions is essential for cocaine–context associative memory reconsolidation [114].
Kinase activity in the NAc is also required for reconsolidation of cocaine–context associative memories. Inhibition of ERK in the NAc core region interferes with both the retrieval and reconsolidation of cocaine CPP memory by preventing the activation of several downstream transcription factors that regulate the expression of IEG-regulated proteins important for synaptic plasticity [115]. Additionally, expression of the IEG-regulated proteins Zif268 and Fos B is increased by cocaine CPP reactivation in several brain regions, including the NAc, amygdala, hippocampus, PFC, and VTA [116]. This upregulated expression in the NAc shell in response to contextual memory reactivation was reduced by the infusion of NMDA or dopamine D1 receptor antagonists, which also disrupted reconsolidation, suggesting the activation of these receptors and subsequent IEG protein expression in the NAc is a necessary mechanism for reconsolidation [116]. Finally, reconsolidation of CPP memories is also dependent on protein degradation in the NAc [117].
Other mechanisms appear to regulate the reconsolidation of cocaine–context associative memories in the PFC. Particularly in the PL, cocaine CPP training increases the intrinsic excitability of pyramidal neurons [118]. This plasticity is positively correlated with memory retrieval, and prevention of this plasticity interferes with the reconsolidation of these memories [118]. Additionally, reconsolidation of CPP memories involves histone methylation-mediated regulation of synaptic plasticity in the mPFC [119].
Cue reactivation
Associative memories between cocaine and discrete cues, such as an audiovisual cue presented during infusion of cocaine in a self-administration paradigm, undergo similar consolidation and reconsolidation processes as contextual cues, though in some instances the brain regions and molecular mechanisms regulating cocaine-cue memories do differ from cocaine–context memories. Cocaine cue memories can also be reactivated with brief re-exposure to the cues, and disrupting the reconsolidation of these cocaine–cue associations learned during self-administration leads to reduced cue-induced cocaine-seeking behavior, which is thought to model a reduction in cue-induced drug craving [5, 28, 120]. The reconsolidation of these cocaine-cue associative memories is disrupted by both inhibition of protein synthesis or inhibition of HATs, but contrary to consolidation, reconsolidation does not appear to rely on the activity of β-adrenergic receptors [120, 121]. Just as in cocaine-cue associative memory consolidation, the BLA is the main site of reconsolidation of these memories. Several molecular substrates of plasticity are required in the BLA for reconsolidation of cocaine–cue associative memories, including protein synthesis, DNA methyltransferase, calcium-calmodulin dependent kinase II (CaMKII), and cAMP signaling that regulates downstream PKA and exchange protein activated by cAMP (Epac) activity [94, 122–126]. Additionally, reactivation of cocaine-cue associative memories results in increased phosphorylation of several proteins in the BLA and NAc, providing further evidence that mechanisms of plasticity involving phosphorylation cascades are required [127].
In addition to simply using brief cue re-exposure to reactivate cocaine–cue associative memories, some experiments have used response-contingent cue presentations in the context of self-administration, in which cues, but no drug, are presented in response to active lever presses in the self-administration context [128–132]. Although it is difficult to differentiate between effects of cocaine–context memory reactivation, cocaine-cue memory reactivation, and instrumental responding in these experiments, they still provide additional information about the molecular mechanisms and circuits involved in reconsolidation of reactivated cocaine memories. When cocaine memories are reactivated in this way, β-adrenergic receptor blockade disrupts reconsolidation [130]. This effect is likely primarily due to disruption of the cocaine–context associative memory, because evidence suggests that β-adrenergic receptor blockade effectively disrupts reconsolidation of cocaine-context, but not cocaine-cue, associative memories [97, 102, 121]. In addition, NMDA receptor blockade in the infralimbic region of the mPFC or in the BLA disrupts reconsolidation after memory reactivation by this brief, response-contingent cue exposure paradigm, further implicating these regions in cocaine memory reconsolidation [128, 129]. Furthermore, both dopamine D1 and D3 receptor blockade can disrupt reconsolidation and reduce subsequent cue-induced reinstatement, supporting a role of the dopamine modulatory system in the reconsolidation of cocaine-cue memories [132].
Unconditioned stimulus (US) reactivation
Due to the fact that brief exposure to a CS selectively destabilizes the memory of the association between the US and that CS, disruption of reconsolidation only affects the reactivated cue [120, 133]. Thus, cue reactivation may have limited clinical potential because of the vast number of cocaine-associated cues and contexts that likely exist for human substance users [120, 134, 135]. However, evidence from both the fear conditioning and the drug self-administration literature suggest that reactivation of the unconditioned stimulus, or US, can destabilize multiple US–CS associations, making this a potentially more effective target for disrupting a sufficient number of cocaine-associated memories to reduce relapse [134–137]. As previously described, inhibition of HATs after reactivation of cocaine-cue associative memory by brief cue presentation selectively reduces subsequent reinstatement mediated by the reactivated cue [120, 135]. Alternatively, systemic HAT inhibition after cocaine memory reactivation with a priming dose of cocaine disrupts the reconsolidation of cocaine’s association to multiple cues, resulting in reduced cue-induced reinstatement to non-reactivated cues [135]. To date, little other research has investigated mechanisms for disrupting cocaine memories after US reactivation, leaving this as an important for future studies.
Reconsolidation and distinctions from consolidation
Overall, several of the molecular mechanisms and neural circuits involved in memory consolidation are also important for the reconsolidation of cocaine–context Pavlovian associative memories, including kinase activity, NMDA-receptor mediated plasticity, expression of IEG proteins, neuromodulation, and epigenetic modifications [92–94, 102, 135]. However, there are some distinctions in the mechanisms of plasticity involved between the two processes and in their reliance on different brain regions. For example, although the role of DHC activity is not immediately necessary for consolidation of cocaine–context associations, DHC inhibition does impair reconsolidation of cocaine–context associations [41, 108]. Although there are few direct comparisons between consolidation and reconsolidation of cocaine associative memories, evidence from literature examining the consolidation and reconsolidation of other associative memories suggests that older memories are more resistant to disruption of reconsolidation than disruption of consolidation and that memories being consolidated may take longer to stabilize than memories being reconsolidated [83]. Additionally, evidence suggests that in the hippocampus, BDNF is required for consolidation, but not reconsolidation, of fear conditioning [90]. Alternatively, Zif268 is required for reconsolidation, but not consolidation [90]. Taken together, these results support dissociable mechanisms regulating consolidation and reconsolidation.
Clinical applications of reconsolidation disruption
Many of the studies investigating reactivation of cocaine memories and the disruption of their reconsolidation have identified several plasticity mechanisms, signaling cascades, and neurotransmitter systems that may be potential targets for use in the treatment of substance use disorders, which have been extensively reviewed [91, 138–141]. For example, a study showing that systemic inhibition of β-adrenergic receptors with propranolol could somewhat disrupt reconsolidation following reactivation of cocaine-cue associative memory provides evidence that this may be an effective treatment option [142]. Additionally, a meta-analysis of the few clinical experiments examining the disruption of memory reconsolidation to treat substance use disorders produced moderately encouraging results [91]. In these experiments, participants dependent on or currently using substances of abuse including alcohol, cocaine, heroin, or nicotine underwent memory reactivation by exposure to drug-associated cues [91, 142]. Following reactivation, pharmacological, and behavioral interventions were performed, such as delivery of NMDA receptor antagonists, counter-conditioning, or extinction [91]. Findings from these clinical experiments are modest, indicating that these methods may need to be improved upon to effectively treat substance use disorders in humans [91, 142]. Preclinical evidence suggests that during reconsolidation of reactivated cocaine–context associative memories, these memories may additionally be more susceptible to counter-conditioning, providing another avenue for improving clinical treatments [138]. Still, several reasonable concerns have been raised about the ethical challenges posed by these types of treatments, which could cause stress, harm, or increased risk of relapse if treatment is not effective, and these concerns will need to be carefully considered as knowledge is gained from preclinical examination of reconsolidation and is translated into clinical practice [141].
Cocaine memory extinction
Memory retrieval can not only initiate reconsolidation of that memory, but can also initiate a process known as extinction. Extinction learning occurs when a memory is repeatedly retrieved in the absence of the expected outcome. After extinction learning, the previously formed associative memories are less likely to guide behavior [143]. Although extinction was initially interpreted as erasure of learning, ample memory phenomena contradict this idea, including spontaneous recovery (the recovery of an extinguished memory with time), renewal (the re-expression of the original memory outside of the extinction context, and reinstatement (the re-expression of the original memory when the outcome is experienced again) [143]. Therefore, extinction of associative memories is generally accepted as a process that involves new learning about a lack of association, and these new memories compete with previously learned associative memories to guide behavior (Fig. 2) [5, 87, 143]. Because extinction involves the formation of new memories, it is not surprising that many of the mechanisms involved in initial memory consolidation and reconsolidation are also required for extinction learning [144], but there are also several distinctions between these processes [145, 146].
Extinction can involve several types of learning. The molecular mechanisms of instrumental extinction, which describes the learning of a lack of association between a behavioral response and its outcome, have been thoroughly investigated in preclinical models of drug self-administration [3, 39]. Although it is a valuable tool for investigating subsequent cue-induced and cocaine-primed reinstatement of drug-seeking behavior, it is outside the scope of this review’s focus on Pavlovian cocaine memories. Therefore, we will focus on the molecular and circuit mechanisms of the extinction of Pavlovian associations (Table 3).
Table 3.
Summary of experiments addressing mechanisms of cocaine Pavlovian extinction
| Paradigm | Brain region | Manipulation | Results | Species, age, sex | Citations |
|---|---|---|---|---|---|
| CPP, context extinction | Systemic | Partial NMDA receptor agonist | Enhanced extinction | Rat, adult, M; mouse, adolescent, M | Hammond et al. [151]; Paolone et al. [153]; Thanos et al. [147] |
| Self-admin, context extinction | Systemic | Partial NMDA receptor agonist | Enhanced extinction | Mouse, adolescent, M | Thanos et al. [154] |
| Self-admin, context extinction | Systemic | mGlu5 glutamate receptor negative allosteric modulator | Impaired extinction | Rat, adult, M | Kim et al. [148] |
| CPP, context extinction | Systemic | mGlu5 glutamate receptor positive allosteric modulator | Enhanced extinction | Rat, adult, M | Gass and Olive [155] |
| CPP, context extinction | DHC | Inactivation with muscimol | Impaired extinction and expression of extinction | Mouse, adult, M | Hitchcock and Lattal [45] |
| CPP, context extinction | Systemic or BLA | Partial NMDA receptor agonist | Enhanced extinction | Rat, adult, M | Botreau et al. [152] |
| CPP, context extinction | Systemic or mPFC | NMDA receptor activation | Enhanced extinction | Mouse, adult, M | Tsai et al. [156] |
| CPP, context extinction | vmPFC | Optogenetic inhibition | Impaired extinction | Mouse, adult, M | Van den Oever et al. [157] |
| CPP, context extinction | IL | TrkB agonist | Enhanced extinction | Rat, adult, M | Otis et al. [158] |
| CPP or self-admin, context extinction | IL | β-adrenergic receptor antagonist | Impaired extinction | Mouse, adult, M | Huang et al. [159] |
| CPP, context extinction | PL | D1 dopamine receptor overexpression | Enhanced extinction | Rat, juvenile, M + F | Brenhouse et al. [160] |
| CPP, context extinction | Systemic | D3 dopamine receptor antagonist | Enhanced extinction | Mouse, adult, M | Ashby et al. [161]; Galaj et al. [162] |
| CPP, context extinction | Global | D4 dopamine receptor antagonist | Impaired extinction in females | Mouse, adult, M + F | Ananth et al. [163] |
| CPP, context extinction | NAc | Optogenetic stimulation or inhibition of ChAT interneurons | Activation enhanced extinction, inhibition suppressed extinction | Mouse | Lee et al. [165] |
| CPP, context extinction | Systemic | NPY inhibitor | Enhanced extinction | Mouse, M | Sørensen et al. [166] |
| CPP, context extinction | Systemic | HDAC or HDAC3 inhibitor; HDAC inhibitor | HDAC3 and HDAC inhibition at a low dose enhanced extinction; HDAC inhibition at a high dose impaired extinction | Mouse, adult, M | Malvaez et al. [167, 168]; Raybuck et al. [169] |
| Self-admin, cue extinction | n/a | n/a | Cue extinction reduces cue-induced reinstatement | Rat, adolescent and adult, M | Madsen et al. [149] |
| Self-admin, cue extinction | Systemic; NAc | mGluR5 antagonist; NMDA receptor antagonist | Impaired extinction | Rat, adult, M | Perry et al. [172]; Torregrossa et al. [171] |
| Self-admin, cue extinction | MGN-LA | n/a | Cue extinction induces LTD | Rat, adult, M | Rich et al. [52] |
| Self-admin, contingent extinction | rBLA, dSUB, or IL | Protein synthesis inhibitor | rBLA and dSUB, but not IL, inhibition impaired extinction | Rat, adult, M | Szalay et al. [174] |
| Self-admin, contingent extinction | rBLA and dSUB | Disconnection (unilateral, contralateral inhibition with lidocaine) | Impaired extinction | Rat, adult, M | Szalay et al. [175] |
| Self-admin, contingent extinction | BLA and PL | n/a | Increased c-Fos expression after contingent extinction | Rat, M | Dhonnchadha et al. [176] |
| Self-admin, contingent extinction | Systemic | Partial NMDA agonist | Enhanced extinction | Mouse, adult, M; rat, adult, M; rat and squirrel monkey, adult, M | Thanos et al. [154]; Thanos et al. [178]; Dhonnchadha et al. [177] |
| Self-admin, contingent extinction | vmPFC, BLA, NAc, DHC | n/a | Total expression of GluA1 increased in the vmPFC and decreased in the BLA, and GluA1 phosphorylation increased in the vmPFC and NAc | Rat, adult, M | Dhonnchadha et al. [179] |
| Self-admin, contingent extinction | NAc shell, core, and DLS | n/a | Altered synaptosomal GluR1, NMDAR1, PICK1, and PSD95; altered synaptosomal mGluR5 | Rat, adult, M; Rat | Ghasemzadeh et al. [180]; Ghasemzadeh et al. [181] |
Pavlovian extinction
Although brief re-exposure to a cocaine-paired context or discrete cue reactivates a memory and triggers reconsolidation, extended or repeated exposure to these stimuli without cocaine reinforcement initiates extinction learning [5, 87]. The cocaine–context association can be extinguished in a cocaine CPP model by extended exposure to the cocaine-paired compartment without cocaine, which reduces preference for the cocaine-paired chamber [45, 87, 147]. Similarly, for cocaine self-administration, extended exposure to the context of self-administration can extinguish the cocaine–context association and lead to subsequently reduced drug-seeking behavior [148]. Additionally, in self-administration paradigms, repeated exposure to the cocaine-paired discrete cue results in a learned lack of association between cocaine and the cue [52, 87, 149]. Subsequently, reinstatement of drug-seeking behavior in response to cue exposure is attenuated [52, 149, 150]. Investigation of extinction learning has revealed several molecular mechanisms necessary for this memory process.
Context extinction
Because of the divergent mechanisms involved in Pavlovian cocaine–context and cocaine–cue associations, we will separately discuss the processes involved in the extinction learning of these associative memories. Given the DHC’s well-established role in cocaine-context associations, it is not surprising that inactivation of the DHC inhibits both CPP extinction learning and retrieval of a previously learned extinction memory [45]. Glutamate-dependent neuroplasticity also plays a clear role, as evidenced by the ability of systemically administered d-serine and DCS, which both facilitate NMDA receptor signaling through activation of the glycine modulatory site [151–154], to facilitate the extinction of cocaine-context associative memories in both CPP and self-administration paradigms [147, 152–154]. Additionally, the mGluR5 receptor (which is coupled to NMDA receptors) plays a role in extinction of cocaine–context associations in both a CPP and self-administration paradigm [148, 155].
More specifically, NMDA receptor activity in the PFC appears to play an important role in the extinction of cocaine–context associative memories. Systemic or mPFC-specific activation of NMDA receptors enhances extinction [156], and inhibition of vmPFC pyramidal neurons inhibits CPP extinction learning [157]. In the infralimbic (IL) region of the PFC, enhancement of GluN2B NMDA receptor activity by brain-derived neurotrophic factor (BDNF) activation of the tropomyosin-related kinase B (TrkB) signaling cascade facilitates extinction learning [158]. Furthermore, IL activation of β-adrenergic signaling and the β-arrestin cascade, which facilitates ERK activation, promotes extinction learning [159].
In addition to the IL, the dorsally located prelimbic (PL) PFC has also been shown to regulate the extinction of cocaine–context memories. For example, overexpression of dopamine D1 receptors in the PL in juvenile male rats facilitates CPP extinction learning, and because the D1 receptor increases neuronal activity through the activation of PKA, these results suggest that increased excitability of PL neurons promotes extinction learning [160]. However, the dopamine system may regulate extinction differentially based on brain region, as systemic selective D3 antagonism can also enhance CPP extinction [161, 162]. Moreover, there may be sex differences in how the dopamine system regulates extinction, as knockout of the D4 receptor in females inhibits extinction learning, while knockout in males reduces cocaine-primed reinstatement of CPP memory [163]. These results indicate subtleties in the effects of dopamine on extinction learning and suggest that dopamine receptors positively coupled to adenylyl cyclase (i.e., D1) promote extinction, while receptors negatively coupled to adenylyl cylcase (i.e., D3 and D4) generally oppose extinction learning [164].
In addition to dopamine, other modulatory neurotransmitters are involved in extinction of cocaine–context associations. First, cholinergic interneurons regulate the plasticity of excitatory synapses onto NAc MSNs that occurs during CPP extinction learning [165]. Additionally, systemic inhibition of neuropeptide Y (NPY) facilitates extinction learning and protects against cocaine-priming induced reinstatement without affecting CPP learning [166]. Finally, epigenetic mechanisms of cocaine–context extinction learning have been identified. For example, nonspecific HDAC and specific HDAC3 inhibition enhance extinction [167, 168]. However, higher doses of HDAC inhibitors may inhibit extinction, suggesting possible dose-dependent effects of histone acetylation or off target effects of the inhibitors [169].
Cue extinction
Cue extinction involves repeated exposure to drug-associated discrete cues in order to extinguish drug–cue associations. This procedure allows for the subsequent evaluation of cue-induced drug-seeking behavior in self-administration paradigms. Interestingly, compound cue extinction, or the presentation of multiple cocaine-associated discrete cues simultaneously, leads to reduced spontaneous recovery of cocaine seeking, suggesting there may be an advantage of simultaneously extinguishing multiple drug–cue associations [170]. In both adolescent and adult rats, cue extinction after cocaine self-administration or 1 week into abstinence reduces cue-induced reinstatement for up to 30 days of abstinence [149].
Several studies have shown that glutamate receptor activity-dependent plasticity is necessary for cue extinction learning. Inhibition of NMDA receptors in the NAc inhibits cue extinction learning [171]. Additionally, systemic inhibition of the mGlu5 receptor inhibits cue extinction learning [172]. Furthermore, plasticity in the lateral amygdala, which has been repeatedly implicated in Pavlovian cue associations, appears to be a critical locus for cue extinction learning [52]. Specifically, cue extinction induces LTD at thalamo-lateral amygdala synapses, and this LTD accompanies reduced cue-induced reinstatement of cocaine-seeking behavior [52]. To further support the role of thalamo-amygdala synaptic plasticity in cue extinction, optogenetic induction of depotentiation of synapses in the LA receiving inputs from the MGN of the thalamus was found to mimic the physiological and behavioral effects of cue extinction [52].
Response-contingent cue extinction
In several experiments, extinction sessions occur in which cues are presented contingently in response to lever presses without the delivery of cocaine. Although it is difficult to disentangle the effects of cue extinction learning and instrumental extinction learning in these experiments, they may provide additional insights into the extinction of cocaine–cue associations. Not surprisingly, evidence suggests that response-contingent cue extinction may be more effective than cue extinction alone [173].
Inhibition of protein synthesis in the rostral BLA and dorsal subiculum (dSUB) of the hippocampus immediately after contingent cue extinction reduces extinction learning [174]. Additionally, functional disconnection of these regions by contralateral unilateral inhibition also reduces extinction learning, suggesting a role of protein synthesis in and interaction between the BLA and hippocampus during extinction learning [174, 175]. Additionally, response-contingent cue extinction increases c-Fos expression in the BLA and PL, suggesting increased neuronal activity in these areas during extinction learning [176]. NMDA receptor partial agonism with DCS augments consolidation of response-contingent cue extinction learning in mice, rats, and squirrel monkeys [154, 177, 178]. Contingent cue extinction also causes changes in the expression of the GluA1 AMPA receptor in the BLA and ventromedial PFC (vmPFC) and changes in phosphorylation in the vmPFC and NAc, but no changes in the DHC [179]. Contingent cue extinction leads to altered synaptic localization of glutamate receptor subunits and scaffolding proteins in the NAc and DLS [180, 181]. Overall, these results implicate the BLA, hippocampus, PFC, and NAc in response-contingent cue extinction. Particularly, glutamate-related neural plasticity in several of these regions appears to be important for response-contingent cue extinction learning [179].
Clinical applications of Pavlovian extinction
There are multiple challenges in the application of Pavlovian extinction to clinical practice [182]. Despite strong preclinical support for the use of Pavlovian extinction to reduce cue-induced reinstatement, cue exposure therapy has shown mixed results for reducing relapse to cocaine and other drug use [182, 183]. In these experiments, participants with histories of drug use were exposed to drug-associated cues repeatedly, often in multiple sessions, in an attempt to replicate preclinical findings that these exposures can extinguish drug–cue associations [182, 183]. Meta-analyses indicate that cue exposure treatments have only been modestly successful and point to potential areas of improvement [182, 183]. One prominent challenge is that its affects appear to be context specific, limiting the efficacy of cue extinction outside the clinic [150, 184, 185]. Systemic and intra-NAc core partial NMDA receptor agonism with DCS after cue extinction reduces the context specificity of cue extinction for cocaine-associated cues in rodents [150]. This is likely due to enhanced extinction, and indicates a possible avenue for overcoming the context specificity of cue extinction [150, 171]. Still, the use of DCS to enhance the effects of cue exposure therapy has not yielded promising results, suggesting other or additional strategies may be necessary [186–188].
Several other obstacles exist, including targeting extinction without inducing and enhancing reconsolidation, particularly because the two processes rely on very similar mechanisms [87]. Evidence from preclinical research suggests that compounding a cocaine-associated cue with a food-associated cue in cue extinction may interfere with cue extinction, so care should be taken to prevent this effect in clinical studies [189]. Finally, sex differences in these mechanisms are largely understudied. 17β-estradiol is important for extinction learning in females, suggesting sex differences and hormone levels may be important considerations for clinical application [190].
Conclusions and future directions
Memory mechanisms that allow for natural reward-related learning can become maladaptive in the formation of substance use disorders [3, 9]. Not only do drugs affect normal memory processes, but drug-associated memories can contribute to craving and relapse [6, 9, 22]. A large body of research has focused on the molecular and circuit mechanisms involved in cocaine-related Pavlovian associations. This research has uncovered memory mechanisms involving activity-dependent neural plasticity, phosphorylation and signaling molecules, expression of IEGs, and epigenetics that occur in various brain regions associated with reward-related learning, particularly corticostriatolimbic circuitry. These findings have contributed toward advances in the clinical treatment of substance use disorders, but many challenges still impede the efficacy and development of treatment options.
Age and sex considerations
Almost all of the preclinical experiments reviewed above were conducted exclusively in adult, male rodents. Therefore, little is known about how consolidation, reconsolidation, and extinction of drug–cue associative memories changes throughout development and differs between sexes. Evidence suggests that adolescents are more sensitive to cocaine reward and less susceptible to extinction [191–193]. Adolescent rats are quicker to acquire, take more, and have more inelastic economic demand for cocaine during self-administration than adults [191, 194]. Additionally, adolescent rats are less sensitive to negative consequences when self-administering cocaine compared to adults [192]. In both adolescents and adults, females acquire self-administration more quickly and show a higher demand for cocaine under progressive ratios [194, 195].
These differences in the rewarding effects of cocaine may result in altered formation of Pavlovian associations between cocaine and cues. Age and sex have a clear effect on cocaine–context associations formed in CPP learning, where females and adolescents tend to be more sensitive to CPP than males and adults, and adolescents are more resistant to extinction and adolescents and females are more susceptible to reinstatement [193, 196–198]. There are also established sex differences in cocaine-cue-induced fos expression and cue-induced reinstatement of cocaine-seeking, and some of these sex differences may be attributed at least in part to differential effects of gonadal hormones [199, 200]. Despite extensive knowledge of sex and age effects on the reinforcing properties of cocaine and the formation and persistence of cocaine-context associations, little is known about their impact on the cellular and molecular mechanisms of Pavlovian memory consolidation and reconsolidation, making this an important avenue for future research.
Combining strategies for treatment
As discussed above, there are several similarities between the molecular mechanisms and neural circuits involved in the consolidation, reconsolidation, and extinction of cocaine memories. Targeting these processes may provide novel strategies for the treatment of cocaine use disorders as well as other substance use disorders. Particularly, both the disruption of reconsolidation and facilitation of extinction learning have shown promising results preclinically. However, applying these concepts to clinical practice has been more challenging. As with treatments for other psychiatric disorders, it is likely that the most effective treatments for substance use disorders will involve a combination of strategies.
One interesting proposal is the combination of extinction learning with reconsolidation disruption in a paradigm termed retrieval-extinction. The effects of performing extinction during the reconsolidation window after retrieval of drug–cue and other associative memories have been extensively reviewed [201–203]. Preclinical evidence shows that cocaine CPP context extinction following reactivation of the cocaine–context associative memory further inhibits cocaine-primed CPP reinstatement, and retrieval-extinction can also decrease reinstatement, spontaneous recovery, and renewal in animals that self-administered cocaine or methamphetamine [204–206]. However, some preclinical evidence indicates that retrieval-extinction does not enhance extinction for cocaine, and may even inhibit extinction for nicotine [207]. Although these discrepancies could be due to methodological differences, there is significant evidence against extinction-retrieval for other types of memory as well [201, 208]. However, clinical applications of retrieval-extinction for substance use disorders have yielded moderately promising results [91, 205]. Overall, retrieval-extinction should be further investigated as a potential strategy to improve treatment outcomes.
Additionally, combining pharmacological manipulations with behavioral interventions could further enhance effects. The possibility of pharmacologically enhancing extinction learning while simultaneously disrupting reconsolidation of the original drug memory has been proposed [28]. Because of the mechanistic overlap between reconsolidation and extinction, many pharmacological interventions, such as the partial NMDA receptor agonist DCS, would simultaneously enhance extinction and reconsolidation, producing potentially deleterious effects [28, 146, 209]. However, there are distinctions between reconsolidation and extinction [145, 146]. Proteomic analysis of protein phosphorylation has been used to identify a phosphorylation site on calcium-calmodulin-dependent kinase II α (CaMKIIα) that is bidirectionally regulated by reconsolidation and extinction [126]. Furthermore, inhibition of CaMKII in the BLA facilitates extinction and disrupts reconsolidation, providing evidence that these processes can be inversely regulated by a single intervention [126]. Further investigation into regulators of reconsolidation and extinction learning would likely provide a basis for enhancing the combination of these strategies when applied clinically.
Expansion of animal models to model more cocaine memories
Along with the challenges of context specificity of extinction and the difficulty in identifying biological differences between reconsolidation and extinction, other factors may complicate the treatment of cocaine use disorders. Extensive cocaine use can result in weakened executive control, impaired working memory, and the development of habitual behavior [210–213]. Unlike human cocaine use disorder, much of the preclinical research described involves experimenter-administered cocaine or short-term drug self-administration that facilitates goal-directed drug-seeking behavior, which cannot fully encompass the long-term effects of cocaine on memory functions and behavior [212]. Therefore, continued research should evaluate how Pavlovian associations may guide the development of increasingly habitual or compulsive drug use. Additionally, the formation and extinction of cocaine-associated Pavlovian memories should be examined in animal models that focus on under-studied aspects of addiction, such as extended drug self-administration and habitual and compulsive drug use.
Manipulation of cocaine memories
The finding that optogenetic induction of depotentiation in thalamo-amygdala synapses mimics the effects of cue extinction provides evidence that more specific interventions may be possible [52]. Although optogenetic and chemogenetic tools are currently only suitable for answering preclinical questions, expansion in the fields of genetics and medical technology suggests that similar tools may eventually be suitable for clinical applications. Continued preclinical research expanding our understanding of the circuits underlying how cocaine memories are consolidated, reactivated, and extinguished will allow for the continued application of this knowledge to clinical practice.
Acknowledgements
Research was supported by National Institute of Health Grants T32NS007433 (BNB) and R01DA042029 (MMT).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. doi: 10.1046/j.1360-0443.1999.9433273.x. [DOI] [PubMed] [Google Scholar]
- 2.Grant S, London ED, Newlin DB, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milton AL, Everitt BJ. The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev. 2012;36:1119–1139. doi: 10.1016/J.NEUBIOREV.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Wang GJ, Volkow ND, Fowler JS, et al. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/S0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- 5.Torregrossa MM, Taylor JR. Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology. 2013;226:659–672. doi: 10.1007/s00213-012-2750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 7.Kerridge BT, Chou SP, Pickering RP, et al. Changes in the prevalence and correlates of cocaine use and cocaine use disorder in the United States, 2001–2002 and 2012–2013. Addict Behav. 2019;90:250–257. doi: 10.1016/J.ADDBEH.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Di Chiara G, Bassareo V, Fenu S, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 9.Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol Learn Mem. 2011;96:609–623. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyman SE, Malenka RC, Nestler EJ. Neural mechanism of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 11.Mihindou C, Vouillac C, Koob GF, Ahmed SH. Preclinical validation of a novel cocaine exposure therapy for relapse prevention. Biol Psychiatry. 2011;70:593–598. doi: 10.1016/j.biopsych.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037/0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 13.Bergstrom HC, Pinard CR. Corticolimbic circuits in learning, memory, and disease. J Neurosci Res. 2017;95:795–796. doi: 10.1002/jnr.24006. [DOI] [PubMed] [Google Scholar]
- 14.Shiflett MW, Balleine BW. Molecular substrates of action control in cortico-striatal circuits. Prog Neurobiol. 2011;95:1–13. doi: 10.1016/j.pneurobio.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith RJ, Laiks LS. Behavioral and neural mechanisms underlying habitual and compulsive drug seeking. Prog Neuro-Psychopharmacol Biol Psychiatry. 2017;87:11–21. doi: 10.1016/j.pnpbp.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Hart G, Bradfield LA, Fok SY, et al. The bilateral Prefronto-striatal pathway is necessary for learning new goal-directed actions. Curr Biol. 2018;28:2218–2229.e7. doi: 10.1016/j.cub.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Murray JE, Belin-Rauscent A, Simon M, et al. Basolateral and central amygdala differentially recruit and maintain dorsolateral striatum-dependent cocaine-seeking habits. Nat Commun. 2015;6:10088. doi: 10.1038/ncomms10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Hare J, Calakos N, Yin HH. Recent insights into corticostriatal circuit mechanisms underlying habits. Curr Opin Behav Sci. 2018;20:40–46. doi: 10.1016/J.COBEHA.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray JE, Belin D, Everitt BJ. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology. 2012;37:2456–2466. doi: 10.1038/npp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodhead S, Robbins T. The relative contribution of goal-directed and habit systems to psychiatric disorders. Psychiatr Danub. 2017;29:203–213. [PubMed] [Google Scholar]
- 22.Kutlu MG, Gould TJ. Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: contributions to development and maintenance of addiction. Learn Mem. 2016;23:515–533. doi: 10.1101/lm.042192.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basu J, Siegelbaum SA. The corticohippocampal circuit, synaptic plasticity, and memory. Cold Spring Harb Perspect Biol. 2015;7:a021733. doi: 10.1101/cshperspect.a021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurden H, Takita M, Rè Se T, Jay M. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci. 2000;20(22):RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;78:637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- 26.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 27.Baez MV, Cercato MC, Jerusalinsky DA. NMDA receptor subunits change after synaptic plasticity induction and learning and memory acquisition. Neural Plast. 2018;2018:1–11. doi: 10.1155/2018/5093048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rich MT, Torregrossa MM. Molecular and synaptic mechanisms regulating drug-associated memories: towards a bidirectional treatment strategy. Brain Res Bull. 2018;141:58–71. doi: 10.1016/J.BRAINRESBULL.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z, Kujawa SG, Sewell WF. Auditory sensitivity regulation via rapid changes in expression of surface AMPA receptors. Nat Neurosci. 2007;10:1238–1240. doi: 10.1038/nn1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collin C, Miyaguchi K, Segal M. Dendritic Spine density and LTP Induction in cultured hippocampal slices. J Neurophysiol. 1997;77:1614–1623. doi: 10.1152/jn.1997.77.3.1614. [DOI] [PubMed] [Google Scholar]
- 31.D’Alcantara P, Schiffmann SN, Swillens S. Bidirectional synaptic plasticity as a consequence of interdependent Ca2+-controlled phosphorylation and dephosphorylation pathways. Eur J Neurosci. 2003;17:2521–2528. doi: 10.1046/j.1460-9568.2003.02693.x. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Stefan MI, Le Novère N. Calcium input frequency, duration and amplitude differentially modulate the relative activation of calcineurin and CaMKII. PLoS ONE. 2012;7:e43810. doi: 10.1371/journal.pone.0043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minatohara K, Akiyoshi M, Okuno H. Role of immediate-early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. Front Mol Neurosci. 2016;8:78. doi: 10.3389/fnmol.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alaghband Y, Bredy TW, Wood MA. The role of active DNA demethylation and Tet enzyme function in memory formation and cocaine action. Neurosci Lett. 2016;625:40–46. doi: 10.1016/j.neulet.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peixoto L, Abel T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology. 2013;38:62–76. doi: 10.1038/npp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris RGM, Moser EI, Riedel G, et al. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond Ser B Biol Sci. 2003;358:773–786. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bisaz R, Travaglia A, Alberini CM. The neurobiological bases of memory formation: from physiological conditions to psychopathology. Psychopathology. 2014;47:347–356. doi: 10.1159/000363702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cervo L, Mukherjee S, Bertaglia A, Samanin R. Protein kinases A and C are involved in the mechanisms underlying consolidation of cocaine place conditioning. Brain Res. 1997;775:30–36. doi: 10.1016/S0006-8993(97)00866-4. [DOI] [PubMed] [Google Scholar]
- 39.Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- 40.See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/S0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- 41.Meyers RA, Zavala AR, Speer CM, Neisewander JL. Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci. 2006;120:401–412. doi: 10.1037/0735-7044.120.2.401. [DOI] [PubMed] [Google Scholar]
- 42.Arguello AA, Richardson BD, Hall JL, et al. Role of a lateral orbital frontal cortex-basolateral amygdala circuit in cue-induced cocaine-seeking behaviour. Neuropsychopharmacology. 2017;42:727–735. doi: 10.1038/npp.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saunders BT, Richard JM, Margolis EB, Janak PH. Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat Neurosci. 2018;21:1072–1083. doi: 10.1038/s41593-018-0191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liljeholm M, O’Doherty JP. Contributions of the striatum to learning, motivation, and performance: an associative account. Trends Cogn Sci. 2012;16:467–475. doi: 10.1016/J.TICS.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hitchcock LN, Lattal KM. Involvement of the dorsal hippocampus in expression and extinction of cocaine-induced conditioned place preference. Hippocampus. 2018;28:226–238. doi: 10.1002/hipo.22826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kramar CP, Flavia Barbano M, Medina JH. Dopamine D1/D5 receptors in the dorsal hippocampus are required for the acquisition and expression of a single trial cocaine-associated memory. Neurobiol Learn Mem. 2014;116:172–180. doi: 10.1016/j.nlm.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Itzhak Y, Liddie S, Anderson KL. Sodium butyrate-induced histone acetylation strengthens the expression of cocaine-associated contextual memory. Neurobiol Learn Mem. 2013;102:34–42. doi: 10.1016/j.nlm.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han J, Li Y, Wang D, et al. Effect of 5-aza-2-deoxycytidine microinjecting into hippocampus and prelimbic cortex on acquisition and retrieval of cocaine-induced place preference in C57BL/6 mice. Eur J Pharmacol. 2010;642:93–98. doi: 10.1016/J.EJPHAR.2010.05.050. [DOI] [PubMed] [Google Scholar]
- 49.Lai Y-T, Fan H-Y, Cherng CG, et al. Activation of amygdaloid PKC pathway is necessary for conditioned cues-provoked cocaine memory performance. Neurobiol Learn Mem. 2008;90:164–170. doi: 10.1016/j.nlm.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Li F-Q, Xue Y-X, Wang J-S, et al. Basolateral amygdala Cdk5 activity mediates consolidation and reconsolidation of memories for cocaine cues. J Neurosci. 2010;30:10351–10359. doi: 10.1523/JNEUROSCI.2112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feltenstein M, See R. NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus-reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiol Learn Mem. 2007;88:435–444. doi: 10.1016/j.nlm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rich MT, Huang YH, Torregrossa MM. Plasticity at thalamo-amygdala synapses regulates cocaine-cue memory formation and extinction. Cell Rep. 2019;26:1010–1020. doi: 10.1016/j.celrep.2018.12.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC6. doi: 10.1523/JNEUROSCI.20-18-J0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siddiqui SA, Singh S, Ugale R, et al. Regulation of HDAC1 and HDAC2 during consolidation and extinction of fear memory. Brain Res Bull. 2019;150:86–101. doi: 10.1016/j.brainresbull.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Zhang T, Yanagida J, Kamii H, et al. Glutamatergic neurons in the medial prefrontal cortex mediate the formation and retrieval of cocaine-associated memories in mice. Addict Biol. 2019 doi: 10.1111/adb.12723. [DOI] [PubMed] [Google Scholar]
- 56.Slaker M, Churchill L, Todd RP, et al. Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory. J Neurosci. 2015;35:4190–4202. doi: 10.1523/JNEUROSCI.3592-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu SS-J, Liu Y-W, Yu L. Medial prefrontal cannabinoid CB1 receptors modulate consolidation and extinction of cocaine-associated memory in mice. Psychopharmacology. 2015;232:1803–1815. doi: 10.1007/s00213-014-3812-y. [DOI] [PubMed] [Google Scholar]
- 58.Ding Z-B, Wu P, Luo Y-X, et al. Region-specific role of Rac in nucleus accumbens core and basolateral amygdala in consolidation and reconsolidation of cocaine-associated cue memory in rats. Psychopharmacology. 2013;228:427–437. doi: 10.1007/s00213-013-3050-8. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Luo Y, He Y, et al. Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2010;30:12632–12641. doi: 10.1523/JNEUROSCI.1264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar A, Choi K-H, Renthal W, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 61.Rogge GA, Singh H, Dang R, Wood MA. HDAC3 is a negative regulator of cocaine-context-associated memory formation. J Neurosci. 2013;33:6623–6632. doi: 10.1523/JNEUROSCI.4472-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taniguchi M, Carreira MB, Smith LN, et al. Histone deacetylase 5 limits cocaine reward through cAMP-induced nuclear import. Neuron. 2012;73:108–120. doi: 10.1016/j.neuron.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malvaez M, Mhillaj E, Matheos DP, et al. CBP in the nucleus accumbens regulates cocaine-induced histone acetylation and is critical for cocaine-associated behaviours. J Neurosci. 2011;31:16941–16948. doi: 10.1523/JNEUROSCI.2747-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.White AO, Kramár EA, López AJ, et al. BDNF rescues BAF53b-dependent synaptic plasticity and cocaine-associated memory in the nucleus accumbens. Nat Commun. 2016;7:11725. doi: 10.1038/ncomms11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang T, Deyama S, Domoto M, et al. Activation of GABAergic neurons in the nucleus accumbens mediates the expression of cocaine-associated memory. Biol Pharm Bull. 2018;41:1084–1088. doi: 10.1248/bpb.b18-00221. [DOI] [PubMed] [Google Scholar]
- 66.Everitt BJ, Belin D, Economidou D, et al. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanderschuren LJMJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30:15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gourley SL, Taylor JR. Going and stopping: dichotomies in behavioral control by the prefrontal cortex. Nat Neurosci. 2016;19:656–664. doi: 10.1038/nn.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roozendaal B, McGaugh JL. Memory modulation. Behav Neurosci. 2011;125:797–824. doi: 10.1037/a0026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bacciottini L, Passani MB, Mannaioni PF, Blandina P. Interactions between histaminergic and cholinergic systems in learning and memory. Behav Brain Res. 2001;124:183–194. doi: 10.1016/S0166-4328(01)00230-3. [DOI] [PubMed] [Google Scholar]
- 72.Zhou S, Xue L, Wang X, et al. NMDA receptor glycine modulatory site in the ventral tegmental area regulates the acquisition, retrieval, and reconsolidation of cocaine reward memory. Psychopharmacology. 2012;221:79–89. doi: 10.1007/s00213-011-2551-6. [DOI] [PubMed] [Google Scholar]
- 73.Kramar CP, Chefer VI, Wise RA, et al. Dopamine in the dorsal hippocampus impairs the late consolidation of cocaine-associated memory. Neuropsychopharmacology. 2014;39:1645–1653. doi: 10.1038/npp.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shinohara F, Asaoka Y, Kamii H, et al. Stress augments the rewarding memory of cocaine via the activation of brainstem-reward circuitry. Addict Biol. 2019;24:509–521. doi: 10.1111/adb.12617. [DOI] [PubMed] [Google Scholar]
- 75.Fitzgerald MK, Otis JM, Mueller D. Dissociation of β1- and β2-adrenergic receptor subtypes in the retrieval of cocaine-associated memory. Behav Brain Res. 2016;296:94–99. doi: 10.1016/j.bbr.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Otis JM, Mueller D. Inhibition of β-adrenergic receptors induces a persistent deficit in retrieval of a cocaine-associated memory providing protection against reinstatement. Neuropsychopharmacology. 2011;36:1912–1920. doi: 10.1038/npp.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zacarias MS, Ramos AC, Alves DR, Galduróz JCF. Biperiden (an M1 antagonist) reduces memory consolidation of cocaine-conditioned place preference. Neurosci Lett. 2012;513:129–131. doi: 10.1016/j.neulet.2012.01.073. [DOI] [PubMed] [Google Scholar]
- 78.Furlong TM, Corbit LH, Brown RA, Balleine BW. Methamphetamine promotes habitual action and alters the density of striatal glutamate receptor and vesicular proteins in dorsal striatum. Addict Biol. 2018;23:857–867. doi: 10.1111/adb.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nelson A, Killcross S. Amphetamine exposure enhances habit formation. J Neurosci. 2006;26:3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nordquist RE, Voorn P, de Mooij-van Malsen JG, et al. Augmented reinforcer value and accelerated habit formation after repeated amphetamine treatment. Eur Neuropsychopharmacol. 2007;17:532–540. doi: 10.1016/j.euroneuro.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 81.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 82.Sorg BA. Reconsolidation of drug memories. Neurosci Biobehav Rev. 2012;36:1400–1417. doi: 10.1016/j.neubiorev.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 84.Lee JLC, Nader K, Schiller D. An update on memory reconsolidation updating. Trends Cogn Sci. 2017;21:531–545. doi: 10.1016/j.tics.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goodman J, Ressler RL, Packard MG. The dorsolateral striatum selectively mediates extinction of habit memory. Neurobiol Learn Mem. 2016;136:54–62. doi: 10.1016/J.NLM.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 86.Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 87.Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56(Suppl 1):186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Milton A. Drink, drugs and disruption: memory manipulation for the treatment of addiction. Curr Opin Neurobiol. 2013;23:706–712. doi: 10.1016/J.CONB.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 89.Brown TE, Forquer MR, Cocking DL, et al. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn Mem. 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee JLC, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science (80–) 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 91.Walsh KH, Das RK, Saladin ME, Kamboj SK. Modulation of naturalistic maladaptive memories using behavioural and pharmacological reconsolidation-interfering strategies: a systematic review and meta-analysis of clinical and ‘sub-clinical’ studies. Psychopharmacology. 2018;235:2507–2527. doi: 10.1007/s00213-018-4983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alaghband Y, O’Dell SJ, Azarnia S, et al. Retrieval-induced NMDA receptor-dependent Arc expression in two models of cocaine-cue memory. Neurobiol Learn Mem. 2014;116:79–89. doi: 10.1016/j.nlm.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arguello AA, Hodges MA, Wells AM, et al. Involvement of amygdalar protein kinase A, but not calcium/calmodulin-dependent protein kinase II, in the reconsolidation of cocaine-related contextual memories in rats. Psychopharmacology. 2014;231:55–65. doi: 10.1007/s00213-013-3203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee JLC, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramirez DR, Bell GH, Lasseter HC, et al. Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30:901–912. doi: 10.1111/j.1460-9568.2009.06889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alaghband Y, Marshall JF. Common influences of non-competitive NMDA receptor antagonists on the consolidation and reconsolidation of cocaine-cue memory. Psychopharmacology. 2013;226:707–719. doi: 10.1007/s00213-012-2793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fricks-Gleason AN, Marshall JF. Post-retrieval -adrenergic receptor blockade: effects on extinction and reconsolidation of cocaine-cue memories. Learn Mem. 2008;15:643–648. doi: 10.1101/lm.1054608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fan H-Y, Cherng CG, Yang F-Y, et al. Systemic treatment with protein synthesis inhibitors attenuates the expression of cocaine memory. Behav Brain Res. 2010;208:522–527. doi: 10.1016/j.bbr.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 99.Itzhak Y, Anderson KL. Memory reconsolidation of cocaine-associated context requires nitric oxide signaling. Synapse. 2007;61:1002–1005. doi: 10.1002/syn.20446. [DOI] [PubMed] [Google Scholar]
- 100.Kelley JB, Anderson KL, Itzhak Y. Long-term memory of cocaine-associated context: disruption and reinstatement. NeuroReport. 2007;18:777–780. doi: 10.1097/WNR.0b013e3280c1e2e7. [DOI] [PubMed] [Google Scholar]
- 101.Bernardi RE, Lattal KM, Berger SP. Anisomycin disrupts a contextual memory following reactivation in a cocaine-induced locomotor activity paradigm. Behav Neurosci. 2007;121:156–163. doi: 10.1037/0735-7044.121.1.156. [DOI] [PubMed] [Google Scholar]
- 102.Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. NeuroReport. 2006;17:1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- 103.Shi X, Miller JS, Harper LJ, et al. Reactivation of cocaine reward memory engages the Akt/GSK3/mTOR signaling pathway and can be disrupted by GSK3 inhibition. Psychopharmacology. 2014;231:3109–3118. doi: 10.1007/s00213-014-3491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stringfield SJ, Higginbotham JA, Wang R, et al. Role of glucocorticoid receptor-mediated mechanisms in cocaine memory enhancement. Neuropharmacology. 2017;123:349–358. doi: 10.1016/j.neuropharm.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yan Y, Kong H, Wu EJ, et al. Dopamine D3 receptors regulate reconsolidation of cocaine memory. Neuroscience. 2013;241:32–40. doi: 10.1016/j.neuroscience.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Itzhak Y. Role of the NMDA receptor and nitric oxide in memory reconsolidation of cocaine-induced conditioned place preference in mice. Ann NY Acad Sci. 2008;1139:350–357. doi: 10.1196/annals.1432.051. [DOI] [PubMed] [Google Scholar]
- 107.Brown TE, Lee BR, Sorg BA. The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learn Mem. 2008;15:857–865. doi: 10.1101/lm.1152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu C, Sun X, Wang Z, et al. Retrieval-induced upregulation of Tet3 in pyramidal neurons of the dorsal hippocampus mediates cocaine-associated memory reconsolidation. Int J Neuropsychopharmacol. 2018;21:255–266. doi: 10.1093/ijnp/pyx099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wells AM, Xie X, Higginbotham JA, et al. Contribution of an SFK-mediated signaling pathway in the dorsal hippocampus to cocaine-memory reconsolidation in rats. Neuropsychopharmacology. 2016;41:675–685. doi: 10.1038/npp.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fuchs RA, Bell GH, Ramirez DR, et al. Basolateral amygdala involvement in memory reconsolidation processes that facilitate drug context-induced cocaine seeking. Eur J Neurosci. 2009;30:889–900. doi: 10.1111/j.1460-9568.2009.06888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wells AM, Arguello AA, Xie X, et al. Extracellular signal-regulated kinase in the basolateral amygdala, but not the nucleus accumbens core, is critical for context-response-cocaine memory reconsolidation in rats. Neuropsychopharmacology. 2013;38:753–762. doi: 10.1038/npp.2012.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu P, Xue Y, Ding Z, et al. Glycogen synthase kinase 3β in the basolateral amygdala is critical for the reconsolidation of cocaine reward memory. J Neurochem. 2011;118:113–125. doi: 10.1111/j.1471-4159.2011.07277.x. [DOI] [PubMed] [Google Scholar]
- 113.Otis JM, Dashew KB, Mueller D. Neurobiological dissociation of retrieval and reconsolidation of cocaine-associated memory. J Neurosci. 2013;33:1271–1281. doi: 10.1523/JNEUROSCI.3463-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wells AM, Lasseter HC, Xie X, et al. Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem. 2011;18:693–702. doi: 10.1101/lm.2273111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 116.Li Y, Ge S, Li N, et al. NMDA and dopamine D1 receptors within NAc-shell regulate IEG proteins expression in reward circuit during cocaine memory reconsolidation. Neuroscience. 2016;315:45–69. doi: 10.1016/j.neuroscience.2015.11.063. [DOI] [PubMed] [Google Scholar]
- 117.Ren Z-Y, Liu M-M, Xue Y-X, et al. A critical role for protein degradation in the nucleus accumbens core in cocaine reward memory. Neuropsychopharmacology. 2013;38:778–790. doi: 10.1038/npp.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Otis JM, Fitzgerald MK, Yousuf H, et al. Prefrontal neuronal excitability maintains cocaine-associated memory during retrieval. Front Behav Neurosci. 2018;12:119. doi: 10.3389/fnbeh.2018.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang Y-X, Akumuo RC, España RA, et al. The histone demethylase KDM6B in the medial prefrontal cortex epigenetically regulates cocaine reward memory. Neuropharmacology. 2018;141:113–125. doi: 10.1016/j.neuropharm.2018.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Monsey MS, Sanchez H, Taylor JR. The naturally occurring compound Garcinia Indica selectively impairs the reconsolidation of a cocaine-associated memory. Neuropsychopharmacology. 2017;42:587–597. doi: 10.1038/npp.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dunbar AB, Taylor JR. Inhibition of protein synthesis but not β-adrenergic receptors blocks reconsolidation of a cocaine-associated cue memory. Learn Mem. 2016;23:391–398. doi: 10.1101/lm.042838.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shi H-S, Luo Y-X, Yin X, et al. Reconsolidation of a cocaine associated memory requires DNA methyltransferase activity in the basolateral amygdala. Sci Rep. 2015;5:13327. doi: 10.1038/srep13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR. Reconsolidation of a cocaine-associated stimulus requires amygdalar protein kinase A. J Neurosci. 2010;30:4401–4407. doi: 10.1523/JNEUROSCI.3149-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wan X, Torregrossa MM, Sanchez H, et al. Activation of exchange protein activated by cAMP in the rat basolateral amygdala impairs reconsolidation of a memory associated with self-administered cocaine. PLoS ONE. 2014;9:e107359. doi: 10.1371/journal.pone.0107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee JLC, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behaviour. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 126.Rich MT, Abbott TB, Chung L, et al. Phosphoproteomic analysis reveals a novel mechanism of CaMKIIα regulation inversely induced by cocaine memory extinction versus reconsolidation. J Neurosci. 2016;36:7613–7627. doi: 10.1523/JNEUROSCI.1108-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Torregrossa MM, MacDonald M, Stone KL, et al. Phosphoproteomic analysis of cocaine memory extinction and reconsolidation in the nucleus accumbens. Psychopharmacology. 2019;236:531–543. doi: 10.1007/s00213-018-5071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hafenbreidel M, Rafa Todd C, Mueller D. Infralimbic GluN2A-containing NMDA receptors modulate reconsolidation of cocaine self-administration memory. Neuropsychopharmacology. 2017;42:1113–1125. doi: 10.1038/npp.2016.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Milton AL, Lee JLC, Butler VJ, et al. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviours. J Neurosci. 2008;28:8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Milton AL, Lee JLC, Everitt BJ. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on β-adrenergic receptors. Learn Mem. 2008;15:88–92. doi: 10.1101/lm.825008. [DOI] [PubMed] [Google Scholar]
- 131.Sorg BA, Todd RP, Slaker M, Churchill L. Anisomycin in the medial prefrontal cortex reduces reconsolidation of cocaine-associated memories in the rat self-administration model. Neuropharmacology. 2015;92:25–33. doi: 10.1016/j.neuropharm.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yan Y, Newman AH, Xu M. Dopamine D1 and D3 receptors mediate reconsolidation of cocaine memories in mouse models of drug self-administration. Neuroscience. 2014;278:154–164. doi: 10.1016/j.neuroscience.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Debiec J, Doyere V, Nader K, LeDoux JE. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc Natl Acad Sci. 2006;103:3428–3433. doi: 10.1073/pnas.0507168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Luo Y, Xue Y, Liu J, et al. A novel UCS memory retrieval-extinction procedure to inhibit relapse to drug seeking. Nat Commun. 2015;6:7675. doi: 10.1038/ncomms8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dunbar AB, Taylor JR. Garcinol blocks the reconsolidation of multiple cocaine-paired cues after a single cocaine-reactivation session. Neuropsychopharmacology. 2017;42:1884. doi: 10.1038/NPP.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Debiec J, Díaz-Mataix L, Bush DEA, et al. The amygdala encodes specific sensory features of an aversive reinforcer. Nat Neurosci. 2010;13:536–537. doi: 10.1038/nn.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Doyère V, Debiec J, Monfils M-H, et al. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci. 2007;10:414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- 138.Goltseker K, Bolotin L, Barak S. Counterconditioning during reconsolidation prevents relapse of cocaine memories. Neuropsychopharmacology. 2017;42:716–726. doi: 10.1038/npp.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kroes MCW, Schiller D, LeDoux JE, Phelps EA. Translational approaches targeting reconsolidation. Curr Top Behav Neurosci. 2016;28:197–230. doi: 10.1007/7854_2015_5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Elsey J, Kindt M. Manipulating human memory through reconsolidation: ethical implications of a new therapeutic approach. AJOB Neurosci. 2016;7:225–236. doi: 10.1080/21507740.2016.1218377. [DOI] [Google Scholar]
- 141.Lázaro-Muñoz G, Diaz-Mataix L. Manipulating human memory through reconsolidation: stones left unturned. AJOB Neurosci. 2016;7:244–247. doi: 10.1080/21507740.2016.1251989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Saladin ME, Gray KM, McRae-Clark AL, et al. A double blind, placebo-controlled study of the effects of post-retrieval propranolol on reconsolidation of memory for craving and cue reactivity in cocaine dependent humans. Psychopharmacology. 2013;226:721–737. doi: 10.1007/s00213-013-3039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 144.Fuchs RA, Feltenstein MW, See RE. The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci. 2006;23:2809–2813. doi: 10.1111/j.1460-9568.2006.04806.x. [DOI] [PubMed] [Google Scholar]
- 145.Suzuki A, Josselyn SA, Frankland PW, et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Merlo E, Milton AL, Goozee ZY, et al. Reconsolidation and extinction are dissociable and mutually exclusive processes: behavioral and molecular evidence. J Neurosci. 2014;34:2422–2431. doi: 10.1523/JNEUROSCI.4001-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thanos PK, Bermeo C, Wang G-J, Volkow ND. d-Cycloserine accelerates the extinction of cocaine-induced conditioned place preference in C57bL/c mice. Behav Brain Res. 2009;199:345–349. doi: 10.1016/j.bbr.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim JH, Perry C, Luikinga S, et al. Extinction of a cocaine-taking context that protects against drug-primed reinstatement is dependent on the metabotropic glutamate 5 receptor. Addict Biol. 2015;20:482–489. doi: 10.1111/adb.12142. [DOI] [PubMed] [Google Scholar]
- 149.Madsen HB, Zbukvic IC, Luikinga SJ, et al. Extinction of conditioned cues attenuates incubation of cocaine craving in adolescent and adult rats. Neurobiol Learn Mem. 2017;143:88–93. doi: 10.1016/J.NLM.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 150.Torregrossa MM, Sanchez H, Taylor JR. d-cycloserine reduces the context specificity of pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J Neurosci. 2010;30:10526–10533. doi: 10.1523/JNEUROSCI.2523-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hammond S, Seymour CM, Burger A, Wagner JJ. d-serine facilitates the effectiveness of extinction to reduce drug-primed reinstatement of cocaine-induced conditioned place preference. Neuropharmacology. 2013;64:464–471. doi: 10.1016/j.neuropharm.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 153.Paolone G, Botreau F, Stewart J. The facilitative effects of d-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology. 2009;202:403–409. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- 154.Thanos PK, Subrize M, Lui W, et al. D-cycloserine facilitates extinction of cocaine self-administration in c57 mice. Synapse. 2011;65:1099–1105. doi: 10.1002/syn.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry. 2009;65:717–720. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tsai Y, Tzeng W-Y, Cherng CG, et al. Effects of sodium benzoate treatment in combination with an extinction training on the maintenance of cocaine-supported memory. Chin J Physiol. 2016;59:56–61. doi: 10.4077/CJP.2016.BAE378. [DOI] [PubMed] [Google Scholar]
- 157.Van den Oever MC, Rotaru DC, Heinsbroek JA, et al. Ventromedial prefrontal cortex pyramidal cells have a temporal dynamic role in recall and extinction of cocaine-associated memory. J Neurosci. 2013;33:18225–18233. doi: 10.1523/JNEUROSCI.2412-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Otis JM, Fitzgerald MK, Mueller D. Infralimbic BDNF/TrkB enhancement of GluN2B currents facilitates extinction of a cocaine-conditioned place preference. J Neurosci. 2014;34:6057–6064. doi: 10.1523/JNEUROSCI.4980-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huang B, Li Y, Cheng D, et al. β-Arrestin–biased β-adrenergic signaling promotes extinction learning of cocaine reward memory. Sci Signal. 2018;11:eaam5402. doi: 10.1126/scisignal.aam5402. [DOI] [PubMed] [Google Scholar]
- 160.Brenhouse HC, Thompson BS, Sonntag KC, Andersen SL. Extinction and reinstatement to cocaine-associated cues in male and female juvenile rats and the role of D1 dopamine receptor. Neuropharmacology. 2015;95:22–28. doi: 10.1016/j.neuropharm.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ashby CR, Rice OV, Heidbreder CA, Gardner EL. The selective dopamine D3 receptor antagonist SB-277011A significantly accelerates extinction to environmental cues associated with cocaine-induced place preference in male sprague-dawley rats. Synapse. 2015;69:512–514. doi: 10.1002/syn.21839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Galaj E, Haynes J, Nisanov R, et al. The dopamine D3 receptor antagonist, SR 21502, facilitates extinction of cocaine conditioned place preference. Drug Alcohol Depend. 2016;159:263–266. doi: 10.1016/j.drugalcdep.2015.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ananth M, Hetelekides EM, Hamilton J, Thanos PK. Dopamine D4 receptor gene expression plays important role in extinction and reinstatement of cocaine-seeking behavior in mice. Behav Brain Res. 2019;365:1–6. doi: 10.1016/j.bbr.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 164.Heidbreder CA, Gardner EL, Xi Z-X, et al. The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee J, Finkelstein J, Choi JY, Witten IB. Linking cholinergic interneurons, synaptic plasticity, and behaviour during the extinction of a cocaine-context association. Neuron. 2016;90:1071–1085. doi: 10.1016/j.neuron.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sørensen G, Wörtwein G, Fink-Jensen A, Woldbye DPD. Neuropeptide Y Y5 receptor antagonism causes faster extinction and attenuates reinstatement in cocaine-induced place preference. Pharmacol Biochem Behav. 2013;105:151–156. doi: 10.1016/j.pbb.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 167.Malvaez M, Sanchis-Segura C, Vo D, et al. Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biol Psychiatry. 2010;67:36–43. doi: 10.1016/j.biopsych.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Malvaez M, McQuown SC, Rogge GA, et al. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci. 2013;110:2647–2652. doi: 10.1073/pnas.1213364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Raybuck JD, McCleery EJ, Cunningham CL, et al. The histone deacetylase inhibitor sodium butyrate modulates acquisition and extinction of cocaine-induced conditioned place preference. Pharmacol Biochem Behav. 2013;106:109–116. doi: 10.1016/j.pbb.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kearns DN, Tunstall BJ, Weiss SJ. Deepened extinction of cocaine cues. Drug Alcohol Depend. 2012;124:283–287. doi: 10.1016/j.drugalcdep.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Torregrossa MM, Gordon J, Taylor JR. Double dissociation between the anterior cingulate cortex and nucleus accumbens core in encoding the context versus the content of Pavlovian cocaine cue extinction. J Neurosci. 2013;33:8370–8377. doi: 10.1523/JNEUROSCI.0489-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Perry CJ, Reed F, Zbukvic IC, et al. The metabotropic glutamate 5 receptor is necessary for extinction of cocaine-associated cues. Br J Pharmacol. 2016;173:1085–1094. doi: 10.1111/bph.13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Buffalari DM, Feltenstein MW, See RE. The effects of varied extinction procedures on contingent cue-induced reinstatement in Sprague-Dawley rats. Psychopharmacology. 2013;230:319–327. doi: 10.1007/s00213-013-3156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Szalay JJ, Jordan CJ, Kantak KM. Neural regulation of the time course for cocaine-cue extinction consolidation in rats. Eur J Neurosci. 2013;37:269–277. doi: 10.1111/ejn.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Szalay JJ, Morin ND, Kantak KM. Involvement of the dorsal subiculum and rostral basolateral amygdala in cocaine cue extinction learning in rats. Eur J Neurosci. 2011;33:1299–1307. doi: 10.1111/j.1460-9568.2010.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nic Dhonnchadha BÁ, Lovascio BF, Shrestha N, et al. Changes in expression of c-Fos protein following cocaine-cue extinction learning. Behav Brain Res. 2012;234:100–106. doi: 10.1016/j.bbr.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nic Dhonnchadha BÁ, Szalay JJ, Achat-Mendes C, et al. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology. 2010;35:357–367. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Thanos PK, Bermeo C, Wang G-J, Volkow ND. d-cycloserine facilitates extinction of cocaine self-administration in rats. Synapse. 2011;65:938–944. doi: 10.1002/syn.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nic Dhonnchadha BÁ, Lin A, Leite-Morris KA, et al. Alterations in expression and phosphorylation of GluA1 receptors following cocaine-cue extinction learning. Behav Brain Res. 2013;238:119–123. doi: 10.1016/j.bbr.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ghasemzadeh MB, Vasudevan P, Mueller CR, et al. Region-specific alterations in glutamate receptor expression and subcellular distribution following extinction of cocaine self-administration. Brain Res. 2009;1267:89–102. doi: 10.1016/j.brainres.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 181.Ghasemzadeh MB, Vasudevan P, Mueller C, et al. Neuroadaptations in the cellular and postsynaptic group 1 metabotropic glutamate receptor mGluR5 and Homer proteins following extinction of cocaine self-administration. Neurosci Lett. 2009;452:167–171. doi: 10.1016/j.neulet.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 183.Mellentin AI, Skøt L, Nielsen B, et al. Cue exposure therapy for the treatment of alcohol use disorders: a meta-analytic review. Clin Psychol Rev. 2017;57:195–207. doi: 10.1016/j.cpr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 184.Bechard AR, Knackstedt LA. The effects of Pavlovian cue extinction and ceftriaxone on cocaine relapse after abstinence. Drug Alcohol Depend. 2019;197:83–86. doi: 10.1016/j.drugalcdep.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Parker LA, Limebeer CL, Slomke J. Renewal effect: context-dependent extinction of a cocaine- and a morphine-induced conditioned floor preference. Psychopharmacology. 2006;187:133–137. doi: 10.1007/s00213-006-0422-3. [DOI] [PubMed] [Google Scholar]
- 186.Prisciandaro JJ, Myrick H, Henderson S, et al. Impact of DCS-facilitated cue exposure therapy on brain activation to cocaine cues in cocaine dependence. Drug Alcohol Depend. 2013;132:195–201. doi: 10.1016/j.drugalcdep.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Santa Ana EJ, Prisciandaro JJ, Saladin ME, et al. D-cycloserine combined with cue exposure therapy fails to attenuate subjective and physiological craving in cocaine dependence. Am J Addict. 2015;24:217–224. doi: 10.1111/ajad.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Das RK, Kamboj SK. Maintaining clinical relevance: considerations for the future of research into d-cycloserine and cue exposure therapy for addiction. Biol Psychiatry. 2012;72:e29–e30. doi: 10.1016/j.biopsych.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 189.Tunstall BJ, Verendeev A, Kearns DN. Outcome specificity in deepened extinction may limit treatment feasibility: co-presentation of a food cue interferes with extinction of cue-elicited cocaine seeking. Drug Alcohol Depend. 2013;133:832–837. doi: 10.1016/j.drugalcdep.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Twining RC, Tuscher JJ, Doncheck EM, et al. 17-Estradiol is necessary for extinction of cocaine seeking in female rats. Learn Mem. 2013;20:300–306. doi: 10.1101/lm.030304.113. [DOI] [PubMed] [Google Scholar]
- 191.Wong WC, Ford KA, Pagels NE, et al. Adolescents are more vulnerable to cocaine addiction: behavioural and electrophysiological evidence. J Neurosci. 2013;33:4913–4922. doi: 10.1523/JNEUROSCI.1371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Holtz NA, Carroll ME. Cocaine self-administration punished by intravenous histamine in adolescent and adult rats. Behav Pharmacol. 2015;26:393–397. doi: 10.1097/FBP.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology. 2008;197:237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- 195.Algallal H, Allain F, Ndiaye NA, Samaha AN. Sex differences in cocaine self-administration behaviour under long access versus intermittent access conditions. Addict Biol. 2019 doi: 10.1111/adb.12809. [DOI] [PubMed] [Google Scholar]
- 196.Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bobzean SAM, Dennis TS, Addison BD, Perrotti LI. Influence of sex on reinstatement of cocaine-conditioned place preference. Brain Res Bull. 2010;83:331–336. doi: 10.1016/j.brainresbull.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 199.Fuchs RA, Evans KA, Mehta RH, et al. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2005;179:662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- 200.Zhou L, Pruitt C, Shin CB, et al. Fos expression induced by cocaine-conditioned cues in male and female rats. Brain Struct Funct. 2014;219:1831–1840. doi: 10.1007/s00429-013-0605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Auber A, Tedesco V, Jones CE, et al. Post-retrieval extinction as reconsolidation interference: methodological issues or boundary conditions? Psychopharmacology. 2013;226:631–647. doi: 10.1007/s00213-013-3004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Beckers T, Kindt M. Memory reconsolidation interference as an emerging treatment for emotional disorders: strengths, limitations, challenges, and opportunities. Annu Rev Clin Psychol. 2017;13:99–121. doi: 10.1146/annurev-clinpsy-032816-045209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hutton-Bedbrook K, McNally GP. The promises and pitfalls of retrieval-extinction procedures in preventing relapse to drug seeking. Front Psychiatry. 2013;4:14. doi: 10.3389/fpsyt.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sartor GC, Aston-Jones G. Post-retrieval extinction attenuates cocaine memories. Neuropsychopharmacology. 2014;39:1059–1065. doi: 10.1038/npp.2013.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xue YX, Luo YX, Wu P, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science (80–) 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chen YY, Zhang LB, Li Y, et al. Post-retrieval extinction prevents reconsolidation of methamphetamine memory traces and subsequent reinstatement of methamphetamine seeking. Front Mol Neurosci. 2019 doi: 10.3389/fnmol.2019.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Struik RF, De Vries TJ, Peters J. Detrimental effects of a retrieval-extinction procedure on nicotine seeking, but not cocaine seeking. Front Behav Neurosci. 2019 doi: 10.3389/fnbeh.2019.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Goode TD, Holloway-Erickson CM, Maren S. Extinction after fear memory reactivation fails to eliminate renewal in rats. Neurobiol Learn Mem. 2017;142:41–47. doi: 10.1016/j.nlm.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lee JLC, Gardner RJ, Butler VJ, Everitt BJ. D-cycloserine potentiates the reconsolidation of cocaine-associated memories. Learn Mem. 2009;16:82–85. doi: 10.1101/lm.1186609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mittenberg W, Motta S. Effects of chronic cocaine abuse on memory and learning. Arch Clin Neuropsychol. 1993;8:477–483. doi: 10.1093/arclin/8.6.477. [DOI] [PubMed] [Google Scholar]
- 211.Gobin C, Shallcross J, Schwendt M. Neurobiological substrates of persistent working memory deficits and cocaine-seeking in the prelimbic cortex of rats with a history of extended access to cocaine self-administration. Neurobiol Learn Mem. 2019;161:92–105. doi: 10.1016/j.nlm.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 212.Belin-Rauscent A, Fouyssac M, Bonci A, Belin D. How preclinical models evolved to resemble the diagnostic criteria of drug addiction. Biol Psychiatry. 2016;79:39–46. doi: 10.1016/j.biopsych.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]