Abstract
Mounting evidence points to the significance of neurovascular-related dysfunction in veterans with blast-related mTBI, which is also associated with reduced [18F]-fluorodeoxyglucose (FDG) uptake.
The goal of this study was to determine whether plasma VEGF-A is altered in veterans with blast-related mTBI and address whether VEGF-A levels correlate with FDG uptake in the cerebellum, a brain region that is vulnerable to blast-related injury 72 veterans with blast-related mTBI (mTBI) and 24 deployed control (DC) veterans with no lifetime history of TBI were studied. Plasma VEGF-A was significantly elevated in mTBIs compared to DCs. Plasma VEGF-A levels in mTBIs were significantly negatively correlated with FDG uptake in cerebellum. In addition, performance on a Stroop color/word interference task was inversely correlated with plasma VEGF-A levels in blast mTBI veterans. Finally, we observed aberrant perivascular VEGF-A immunoreactivity in postmortem cerebellar tissue and not cortical or hippocampal tissues from blast mTBI veterans.
These findings add to the limited number of plasma proteins that are chronically elevated in veterans with a history of blast exposure associated with mTBI. It is likely the elevated VEGF-A levels are from peripheral sources. Nonetheless, increasing plasma VEGF-A concentrations correlated with chronically decreased cerebellar glucose metabolism and poorer performance on tasks involving cognitive inhibition and set shifting. These results strengthen an emerging view that cognitive complaints and functional brain deficits caused by blast exposure are associated with chronic blood-brain barrier injury and prolonged recovery in affected regions.
Keywords: Neurotrauma, Neurovascular, Vascular, Angiogenic, Neurovascular unit, Endothelial
1. Introduction
Diagnostic and prognostic evaluation of individuals exposed to blast overpressures resulting in mild traumatic brain injury (TBI) relies on neurologic examination, cognitive testing, and brain imaging [1]. In spite of impressive advances [2], there remain significant gaps in our understanding about relationships between postconcussive cognitive status and central nervous system (CNS) dysfunction. Closing this knowledge gap is important for improving how latent injury progression is monitored, determining long term outcomes, and furthering mechanistic insights. Significant strides have been made in identifying molecular biomarkers found in blood, cerebrospinal fluid (CSF), and other biofluids from individuals with acute moderate to severe TBI [3]. However, less is known about candidate biofluid biomarkers capable of informing brain function in living individuals years after blast injury, although progress is accelerating rapidly [4–6]. Thus far, the many of the best characterized blood and CSF biomarkers normalize within days to weeks following a mTBI.
Blast exposure is increasingly recognized to be associated with chronic cerebrovascular pathology. A growing body of findings high-light the importance of blast-induced disturbances affecting the network of endothelial cells and perivascular astrocytes that comprise the neurovascular system of the brain [7–11]. Multiple preclinical findings indicate that blast exposure alters the level of vascular endothelial growth factor-A (VEGF-A), a key angiogenic factor regulating vascular endothelial survival, growth, and remodeling [12–15]. These findings, coupled with work showing that blast disturbs blood-brain barrier (BBB) integrity [9,10,16–18], which is also regulated by VEGF-A [19], and that a history of blast exposure disrupts regulatory networks inhibiting VEGF-A expression [18] prompted us to test the hypothesis that plasma VEGF-A is altered in veterans with chronic blast-related mTBI.
2. Materials and methods
2.1. Study participants
Studies were approved by the VA Puget Sound Human Subjects Committee and conformed to institutional regulatory guidelines and principles of human subjects protections in the Declaration of Helsinki. Written informed consent was obtained for all study participants. Lifetime history of both blast-related and impact-related mTBI was obtained using a semi-structured interview by two expert TBI clinicians described in detail elsewhere [20]. All participants were male. Females were eligible for study inclusion, but no females with blast-related mTBI enrolled. For inclusion in the mTBI group, participants must have had at least one blast exposure with acute symptoms meeting VA/DoD/American Congress of Rehabilitation Medicine criteria for mTBI. History of subconcussive blast exposure was not acquired. Study exclusion criteria included moderate-severe TBI, seizure disorder, insulin-dependent diabetes, current DSM-IV diagnosis of alcohol abuse or other substance abuse, schizophrenia or other psychotic disorders, bipolar disorder, dementia, and taking medications likely to affect cognition.
2.2. Positron emission tomography (PET)
Standard clinical PET acquisitions were performed 60 min after injection of [18F]-FDG (8–10 mCi) on either a GE Advance PET or Philips Gemini PET/CT scanner. Data from GE underwent 3D-filtered back projection reconstruction, while Philips data underwent OSEM reconstruction. Three 5 min frames were averaged following motion correction, smoothed using an 8×8×8mm Gaussian filter, then transformed directly into MNI standardized space using SPM12 [21]. Individual images were scaled for intensity using a VOI applied to parenchyma in PMOD (PMOD Technologies, Zurich), yielding unitless fractional uptake values in the images. The AAL VOI library [22] was applied to extract sub-region values. Multivariate regression analysis addressed possible confounds arising from use of two scanners with no significant effect of scan instrument confounding study interpretations.
2.3. Plasma protein measurements
Blood was collected from an intravenous catheter into sodium EDTA tubes. Blood cells were removed by centrifugation. Blood processing and freezing occurred within one hour of collection. CSF was obtained as described elsewhere [23]. Plasma proteins were measured using assays validated for human plasma with an electrochemiluminescense platform (Quickplex 120, MesoScale Discovery).
2.4. Cognitive testing
Scaled scores from the inhibition subscale of the Delis-Kaplan Executive Function Scale (DKEFS) Color Word Interference Test assessed response inhibition [24]. Time to complete the task was converted to age-adjusted scaled scores, with higher scores corresponding to better performance. Self-reported cognitive shifting abilities were assessed with the shift subscale of the Behavioral Rating Inventory for Executive Function-Adult Version (BRIEF-A) [25].
2.5. Human tissue samples
Autopsies were approved or exempt from human subjects regulations and IRB. Tissue samples were obtained from autopsies of blast-exposed veterans and age, sex-matched, non-exposed controls. Formalin-fixed, paraffin embedded sections of cerebellum with cerebellar cortex at the level (and including) dentate nucleus were received on charged microscope slides and submitted for immunostaining. Brain sections were stained using a commercially available kit (Opal Manual IHC Kit, Akoya Bioscience). Antibodies used were: anti-VEGF-A (Santa Cruz), anti-caveolin-1 (Cell Signaling) and anti-calbindin (EMD Millipore). Heat-mediated antigen retrieval was performed in AR6 buffer. Stained slides were mounted with ProLong Diamond antifade mountant (ThermoFisher). Confocal microscopy was performed using a Leica TCS SP5 II microscope. Images were acquired with the Leica Application Suite. All images are single z-plane scans. Post-acquisition image processing and figure preparation was accomplished using Leica Application Suite and Photoshop software (Adobe) that was limited to linear contrast and brightness adjustments and applied identically to mTBI and matching control images.
2.6. Statistics
As appropriate, Chi-square tests or between-subjects t-tests were used. P values denote two-tailed significance. Pearson and Spearman correlation p values correspond to two-tailed outcomes. Benjamini–Hochberg procedures corrected for multiple comparisons using a false discovery rate of 0.05 [26]. Statistical analyses were done using SPSS software (IBM).
3. Results
3.1. Study participant characteristics
72 Operation Iraqi Freedom/Operation Enduring Freedom (OIF/OEF) veterans with blast-related mTBI and 24 OIF/OEF Deployed Control (DC) veterans with no history of TBI were studied. These groups were matched for age (T [89]=0.606, n.s.), race (Chi-square = 0.157[df = 3], n.s.), and Apolipoprotein E genotype (Chi-square = 5.87[df = 4], n.s.). The mTBI group was evaluated an average of 5.5 (plasma collection) and 5.3 (imaging) years after their last reported mTBI (Table 1). As expected of this population [20,27], mTBI veterans had elevated comorbid posttraumatic stress disorder (PTSD) symptoms measured by the PTSD Checklist-Military version [28] (PCL-M:T [92]=7.13, p ≤ .0001); increased depressive symptoms measured by the Patient Health Questionaire-9 [29] (PHQ-9 total score:.T [93]=4.98, p ≤ .0001); poorer sleep quality measured by the Pittsburgh Sleep Quality Index [30] (PSQI: T [89]=6.00, p ≤ .0001); but not significantly increased alcohol use [31] (AUDIT-C:T [93]=1.37, n.s.) compared to DCs.
Table 1.
Subject characteristics of living Veterans.
| Demographics | Blast-related mTBI | Deployed Controls (DC) | p value |
|---|---|---|---|
| Mean (SD), range | Mean (SD), range | ||
| Age (years) | 34.4 (9.7) 22–60 | 33.0 (7.0) 23–47 | n.s. |
| Race % | 4.2, 5.6, 76.1, 14.1 | 4.2, 4.2, 75.0, 16.6 | n.s. |
| A, B/AA, W, O | |||
| Hispanic Ethnicity % | 14.1 | 12.5 | n.s. |
| Apolipoprotein E genotype % (2,3) (2,4) (3,3) (3,4) (4,4) | 8.5, 1.4, 64.8, 22.5, 2.8 | 13, 0, 39.1, 39.1, 8.7 | n.s. |
| Blast exposure/TBI history | |||
| Number of blast-related mTBIs during military service | 29 (65) 1 > 100 Median = 11 | N.A. | |
| Number of lifetime mTBIs with LOC | 2.4 (0.3) 1–12 | N.A. | |
| Time since last blast-related mTBI to blood draw (years) | 5.5 (2.8) 0.6–12.3 | N.A. | |
| Time since last blast-related mTBI to PET scan (years) | 5.3 (2.7) 0.5–12.1 | N.A. | |
| Behavioral and neurological measures | |||
| PCL-M total score | 52.33 (16.2) 19–84 | 26.7 (11.8) 17–62 | 0.0001 |
| PHQ-9 score | 11.7 (7.1) 0–27 | 3.7 (5.7) 0–24 | 0.0001 |
| PSQI score | 11.3 (4.4) 1–20 | 5.4 (3.1) 0–11 | 0.0001 |
| AUDIT-C score | 3.5 (2.5) 0–10 | 2.7 (1.6) 0–5 | n.s. |
| BRIEF-A Cognitive Shift T-scores | 65.1 (12.3) 39–88 | 54.1 (11.3) 39–66 | 0.036 |
| Stroop word color interference test | 10.2 (3.6) 1–15 | 11.1 (2.5) 7–14 | n.s. |
For Race% A, B/AA, W, and O correspond to Asian, Black/African American, White, and Other, respectively. Percent self-identified Hispanic ethnicity indicated. All participants were male US military veterans. LOC denotes the lifetime number of reported losses of consciousness lasting less than 30 min. A LOC greater than 30 min met criterion for study exclusion. Posttraumatic stress disorder (PTSD) was evaluated by the PTSD Checklist-Military version (PCL-M). Depression was evaluated with the Patient Health Questionaire-9 (PHQ-9). Alcohol use was measured with the Alcohol Use Disorders Identification Test-Consumption (AUDIT-C). Sleep quality was evaluated using the Pittsburgh Sleep Quality Index (PSQI). Behavioral Rating Inventory for Executive Function-Adult version (BRIEF-A) cognitive shift subscale and Stroop word color interference test evaluated higher order cognitive flexibility. P-values for Apolipoprotein E genotype and Race denote results of Chi-Square analyses and Fischer’s Exact test for % Hispanic. No participants in this study had an apoE 2,2 genotype. P-values for Age, PCL-M, PHQ-9, PSQI, AUDIT-C, BRIEF-A.
3.2. VEGF-A is elevated in plasma from veterans with blast-related mTBI
Multiple preclinical reports have found that VEGF-A levels are altered by blast exposure [12,13,15,32–35]. However, in this regard little is known in humans. We tested the hypothesis that VEGF-A is elevated in plasma from veterans with blast-related mTBI using a validated electrochemiluminescent assay. Fig. 1 shows that plasma VEGF-A was significantly elevated in the mTBIs compared to DCs (T [94]= 2.60, p ≤ .011).
Fig. 1.
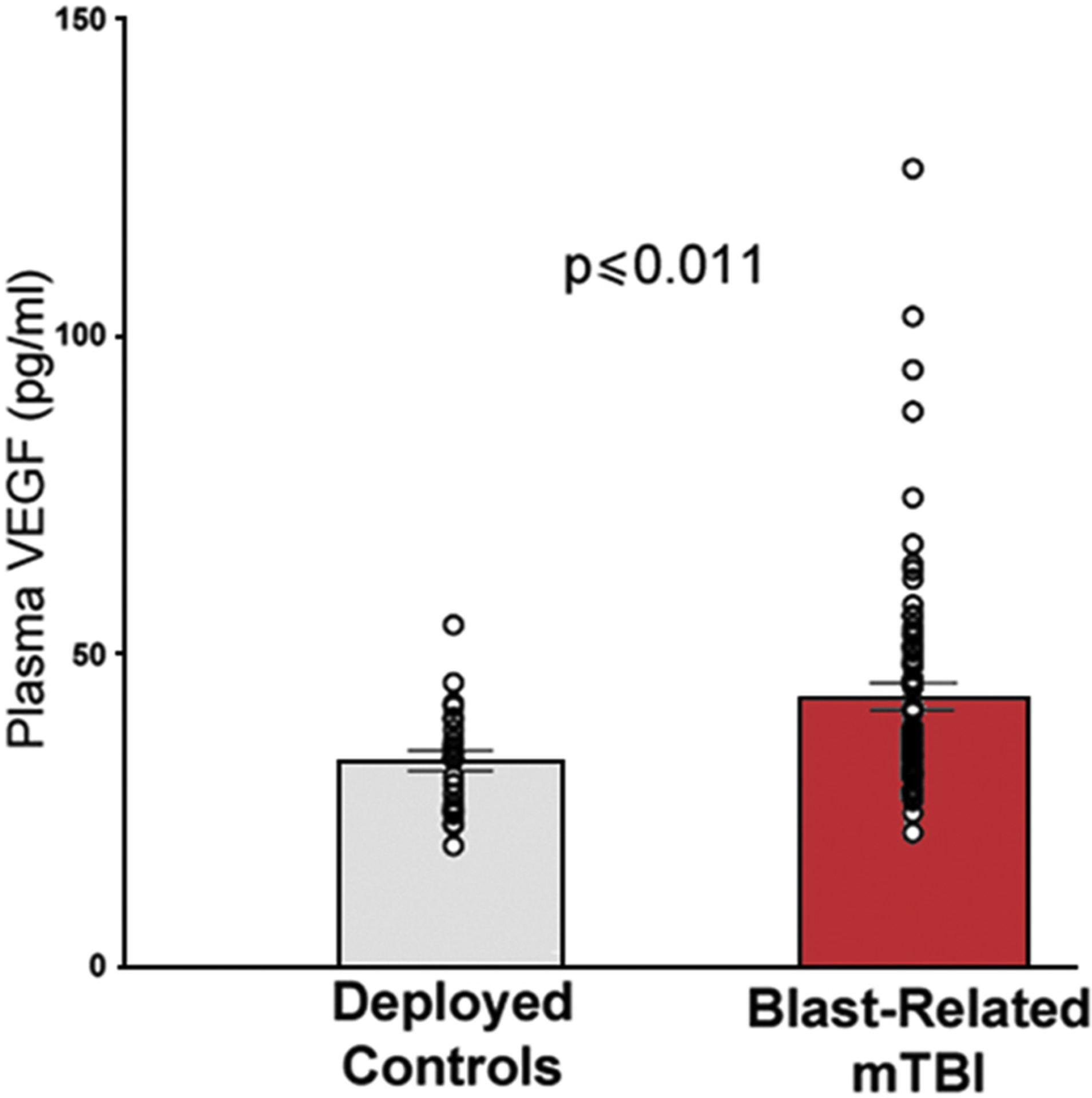
Plasma VEGF-A levels are chronically elevated in mTBI veterans with self-reported repetitive blast exposure.
VEGF-A levels measured on average 5.5 years after last reported mTBI were significantly elevated (p ≤ .011) in OIF/OEF veterans with self-reported repetitive blast exposures accompanied by acute mTBI symptoms (N = 72) compared to deployed control veterans (N = 24).
This platform also measured basic fibroblast growth factor-2 (bFGF), plasma placental growth factor (PLGF), KEK receptor tyrosine kinase (Tie2), VEGF-C, VEGF-D, and FMS-like tyrosine kinase 1 (FLK-1). In addition to VEGF-A, only bFGF levels were significantly increased in mTBIs versus DCs (bFGF:T [94]=2.95, p ≤ .004); and with no significant change in PLGF:T [94]=0.625, n.s.; Tie2:T [94]=0.297, n.s.; VEGF-C:T [86]=1.47, n.s.; VEGF-D:T [94]=0.83, n.s.; FLK-1:T [94] =0.37, n.s. Following correction for multiple comparisons (see Methods, 2.6), VEGF-A and bFGF differences remained statistically significant at the 0.05 level. In blast subjects, plasma VEGF-A and bFGF levels did not correlate with the log10 transformed number of self-reported blast exposures (Pearson r = −1.36, n.s.; r = 0.058, n.s., respectively). In addition, none of these proteins differed significantly in the CSF of mTBIs compared to DCs (VEGF-A:T [66]=0.562, n.s., N = 47, 21 mTBI and DC, respectively; bFGF:T [52]=0.848, n.s., N = 36, 18; PGIF:T [66]=0.858, n.s., N = 47, 21; VEGF-C:T [16] =0.111, n.s., N = 13, 5; VEGF-D:T [65]=0.423, n.s., N = 46, 21; FLK 1:T [66]=0.025, n.s., N = 47, 21).
3.3. Plasma VEGF-A corresponds with cerebellar glucose uptake by FDG-PET
To investigate relationships between plasma VEGF-A and CNS function in blast mTBI veterans we quantified positron emission tomography (PET) fractional [18F]-fluorodeoxyglucose (FDG) uptake in cerebellum. We focused on the cerebellum because of its vulnerability to blast-related mTBI, which we and others have shown is associated with lower FDG uptake [9,36,37]. Based on prior findings that suggest the Crus I, Crus II and posterior aspects of the cerebellum display more robust chronic FDG-PET imaging abnormalities [9], five bilateral cerebellar volumes of interest (VOIs) were selected for study from standardized clinical PET/CT images in 68 veterans with blast-related mTBI. Fig. 2 shows that FDG uptake in cerebellar lobules VII, VIII, IX, Crus I, and Crus II were significantly negatively correlated with plasma VEGF-A levels (Pearson r = −0.301, p ≤ .013; r = −0.266, p ≤ .028; r = −0.262, p ≤ .031; r = −0.343, p ≤ .004; and r = −0.327, p ≤ .006, respectively, N = 68). All five regions remained statistically significant after correction for multiple comparisons. Collective analysis (lobules VII-IX + Crus I + Crus II) confirmed a significant correlation between increasing plasma VEGF-A levels and decreasing cerebellar FDG uptake (r = −0.325, p ≤ .007, N = 68). Linear regression analysis showed that this correlation remained significant after accounting for possible confounds arising from the use of two PET scanners (r = −0.294, p ≤ .013).
Fig. 2.
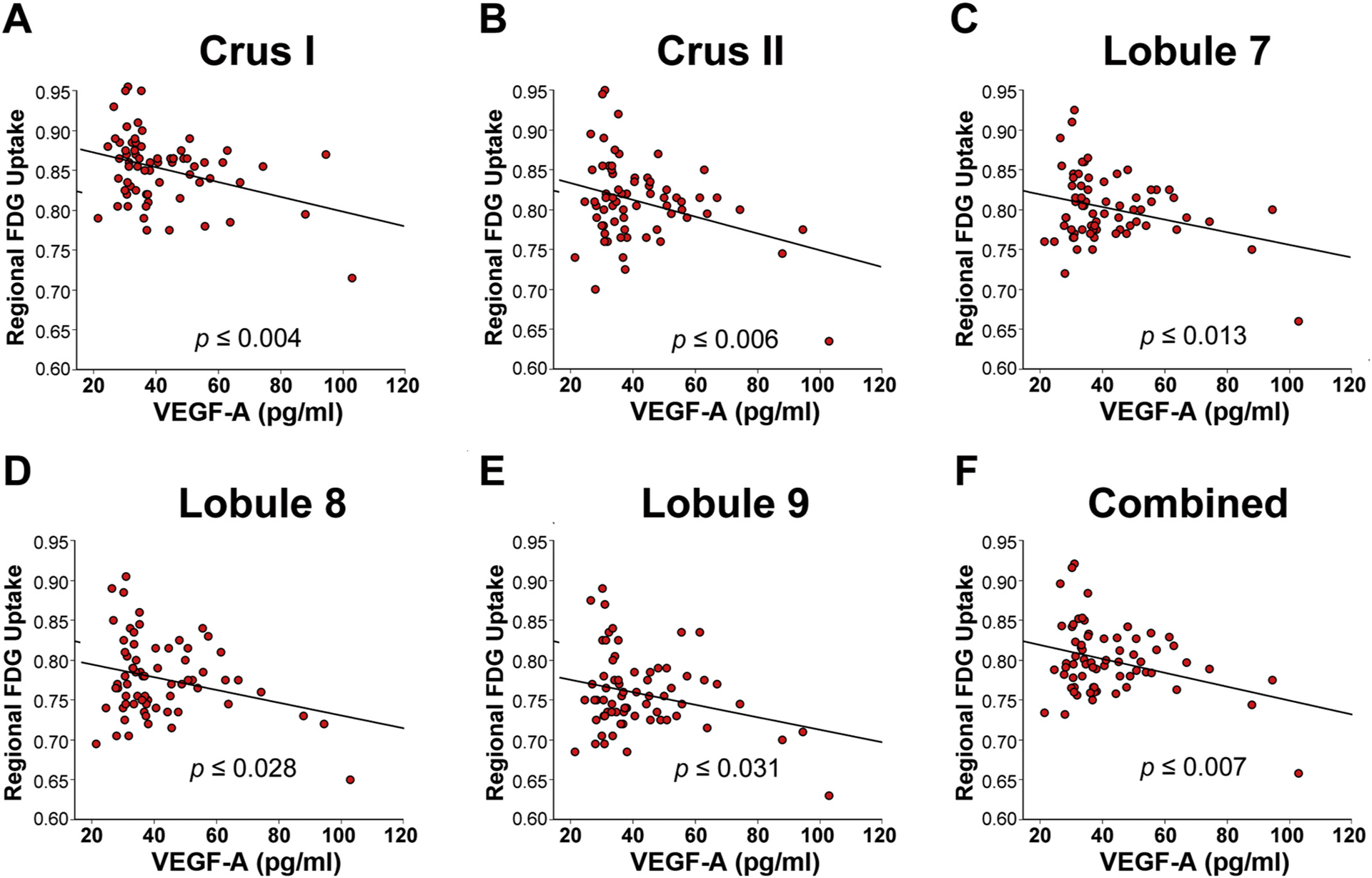
Higher plasma VEGF-A levels correspond with lower regional FDG-uptake in blast-mTBI veterans.
Resting state [18F]-fluorodeoxyglucose (FDG) PET imaging in veterans with blast-related mTBI (N = 68) was significantly negatively correlated with levels of plasma VEGF-A in cerebellar VOIs comprising left + right (A) Crus I; (B) Crus II; (C) Lobule 7; (D) Lobule 8; (E) Lobule 9; and (F) combined (Crus I, II, Lobules 7–9), indicating that increasing plasma VEGF-A levels corresponded with reduced metabolism in the cerebellum as measured by FDG-PET.
Further confirming the specificity of these findings to blast mTBI, FDG uptake in deployed controls was not correlated with plasma VEGF-A (Crus I: r = 0.134, n.s.; Crus II: r = 0.164, n.s.; VII: r = 0.257, n.s.; VIII: r = 0.158, n.s.; IX: r = 0.084, n.s.; combined Crus I, II, lobules VII-IX; r = 0.167, n.s.). Adding further to the specificity of the FDG/VEGF-A correlations in mTBI veterans, bFGF was not significantly correlated with FDG uptake after adjusting for multiple comparisons (Crus I: r = −0.251, p ≤ .039; Crus II: r = −0.194, n.s.; VII:r = −0.181, n.s.; VIII:r = −0.206, n.s.; IX r = −0.150, n.s.; combined: r = 0–0.212, n.s.).
3.4. Plasma VEGF-A corresponds with cognitive inhibition and set shifting impairment
We have previously reported correlations between blast exposure and impairments in sensorimotor integration [9]. However, among these veterans, motor-related symptoms are minor relative to complaints involving higher order cognitive and behavioral functions [38,39]. The cerebellum is part of a network that subserves a number of executive cognitive functions [40,41] that include supporting performance on the Stroop color interference test, a well-established measure of higher order cognitive inhibition of an over-learned perceptual response [24,42–44]. We identified a significant negative correlation between plasma VEGF-A levels in the blast mTBI group versus scores from the Inhibition subscale of the Delis-Kaplan Executive Function Scale (DKEFS) color/word interference test (Fig. 3A), an instrument used to assess response inhibition (Spearman r = −0.434, p ≤ .013, N = 32).
Fig. 3.
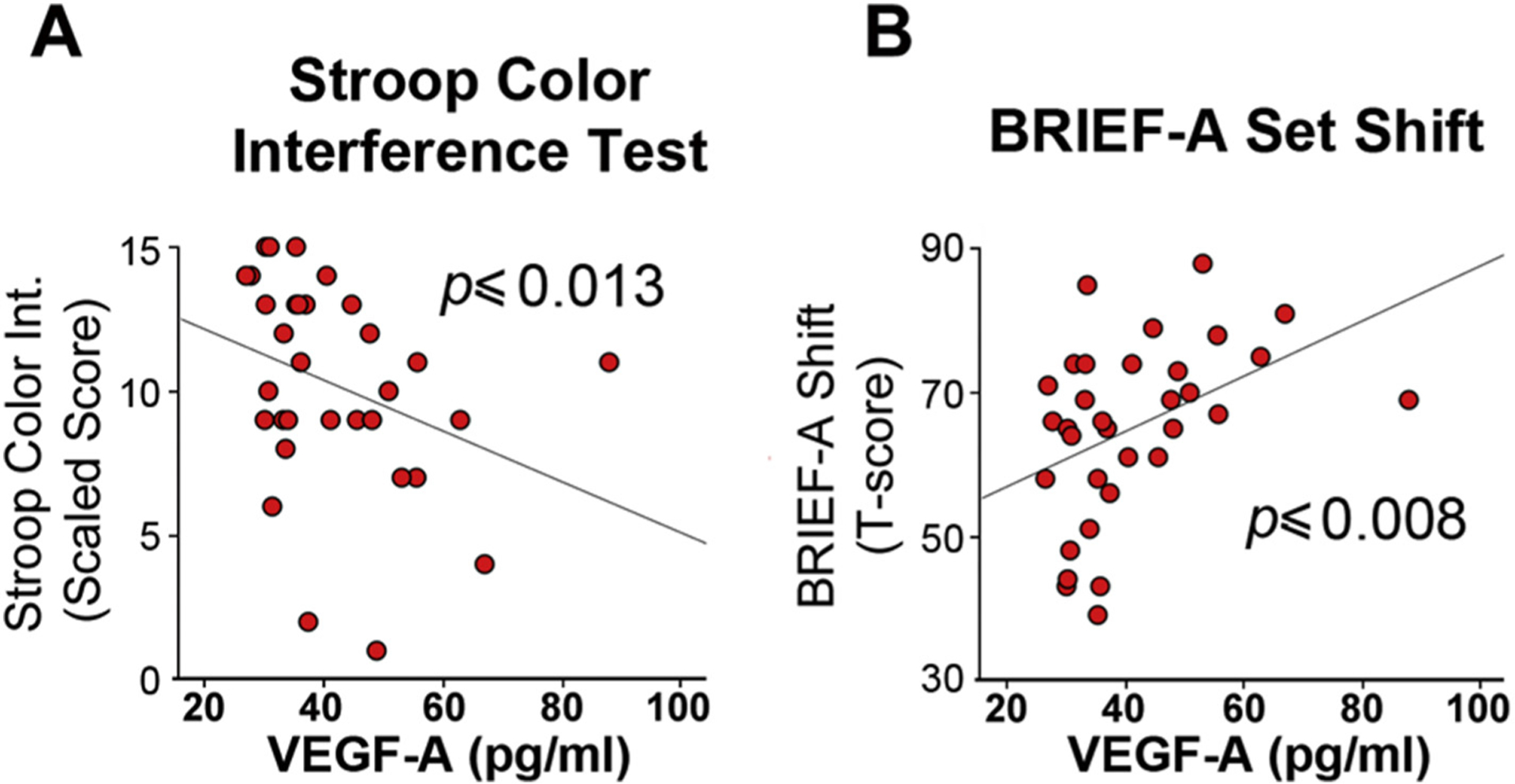
Objective and self-reported measures of cognitive executive functional impairment correspond with increasing plasma VEGF-A in blast-mTBI veterans.
(A) Scaled scores on the Stroop Color Word Interference Test, which is used to assess cognitive inhibition of over-learned word reading responses were significantly negatively correlated plasma VEGF-A levels in veterans with blast-related mTBI (N = 32). Lower scores correspond with worse performance. (B) Self-reported difficulties with shifting from one cognitive task or situation to another measured by the Behavioral Rating Inventory for Executive Function-Adult version (BRIEF-A) was significantly correlated with plasma VEGF-A levels in veterans with blast-related mTBI (N = 33). Higher BRIEF-A shift subscale T-scores correspond with greater difficulty with cognitive shifting.
These data indicate that lower performance on the Stroop task corresponded with higher plasma VEGF-A levels. To further test this idea we analyzed T-scores on the Behavioral Rating Inventory for Executive Function-Adult version (BRIEF-A), a self-report inventory that rates clinically significant difficulties in ability to cognitively set shift in different situations, including between tasks, from a way of thinking, or from an anticipated schedule [25]. Fig. 3B shows that plasma VEGF-A levels in the mTBI veterans were positively correlated with BRIEF-A shift subscale T-scores (Spearman’s r = 0.453, p ≤ .008), indicating that greater difficulty with cognitive shifting corresponded with higher plasma VEGF-A levels.
Executive cognitive functions engaged by the Stroop color/word test are commonly associated with prefrontal cortical brain regions [45]. Uniformly, plasma VEGF-A levels in the blast mTBIs did not correlate significantly with FDG uptake in superior frontal orbital, medial frontal, and inferior frontal orbital cortex (r = 0.097, n.s.; r = 0.152, n.s.; and r = −0.058, n.s, respectively) even though well-established cerebro-cerebellar connectivity networks functionally link a number of frontal cortical brain regions to the cerebellum [46,47].
3.5. Perivascular VEGF-A is increased in cerebellum of veterans with blast-related mTBI
Endothelial cells express luminal and abluminal VEGF receptors [48,49] that internalize VEGF-A [50]. In addition, astrocyte-associated extracellular matrix compartments can complex with and sequester VEGF-A in the brain [51]. Even though VEGF-A was not elevated in CSF of veterans with mTBI, it is still possible that VEGF-A could accumulate and become sequestered in the cerebellum.
To test this idea, we examined cerebellar VEGF-A immunoreactivity in a rare set of three postmortem specimens from veterans with blast-related mTBI and two comparable non-TBI veteran controls. TBI1 was an active duty 46 year old male Navy SEAL who died from a self-inflicted gunshot to the head. Military blast exposure included multiple improvised explosive devices (IEDs) and other ordnance. Contact sports history consisted of high school, college, semi-professional football, and mixed martial arts. Medical/psychiatric history included diagnoses of PTSD, alcohol use disorder, hearing loss, and chronic pain. Neuropathologic findings were CTE (Stage 1) and interface astroglial scarring (IAS). TBI2 was an active duty male Navy SEAL who died at age 35 by drowning. Military blast exposure included multiple IEDs and other ordnance. There was no history of participation in contact sports. Medical/psychiatric diagnoses included PTSD, alcohol/substance use disorder, chronic pain, paranoid ideation, and manic episodes. Neuropathological findings were IAS without tau pathology. TBI3 was a male Army veteran who died at age 46 following complications of elective back surgery. This case had participated in Dr. Peskind’s study of blast mTBI and had extensive clinical characterization including lifetime history of mTBI as for the living Veterans reported here. Military blast exposure consisted of > 50 IEDs and other ordnance with acute symptoms consistent with VA/DoD criteria for mTBI and no history of contact sports or impact TBIs. Medical/psychiatric diagnoses included PTSD, alcohol use disorder, migraine headaches, chronic back pain, hearing loss, and tinnitus. Neuropathologic diagnoses included CTE (Stage 1) and meningitis. Control1 was a male active duty Navy officer with no known TBIs who died at 43 due to cardiac arrest. Medical history included hypertension, hypercholesterolemia, and chronic obstructive pulmonary disease. There were no significant neuropathological abnormalities, including no evidence of tauopathy or neurodegeneration. Control2 was a male Army veteran who died at age 57 due to cardiac arrest. He had no lifetime history of TBI. However, he reported falling down the stairs and bumping his head twice at the ages of 4 and 7 years without loss of consciousness or requiring any urgent care or emergency room evaluation. Medical/psychiatric diagnoses included headaches and remote history of a “mental breakdown.”
Fig. 4 shows VEGF-A immunoreactivity (green) was associated with microvascular profiles immunostained with the endothelial cell marker caveolin (blue), which were more readily detected in the blast-related mTBI cases than controls. Calbindin immunoreactivity (red) was used to label Purkinje cells. It is well established that cerebellar Purkinje cells express VEGF-A [52,53]. In keeping with this we observed a number of VEGF-A positive Purkinje cells, which supported the specificity of the immunostaining, but there was no apparent distinction in Purkinje cell VEGF-A expression in the mTBI versus control cases. We also examined VEGF-A expression in cortex and hippocampus from the same postmortem cases (Supplementary Fig. 1). Occasional VEGF-A positive peri-microvessel profiles were also observed in cortex and hippocampus, but unlike in the cerebellum there was no apparent difference between the control and TBI cases. As previously reported [54,55], neuronal VEGF-A immunoreactivity was evident in cortical and hippocampal neurons, but as with the cerebellum there was no notable distinction between the control and TBI cases.
Fig. 4.
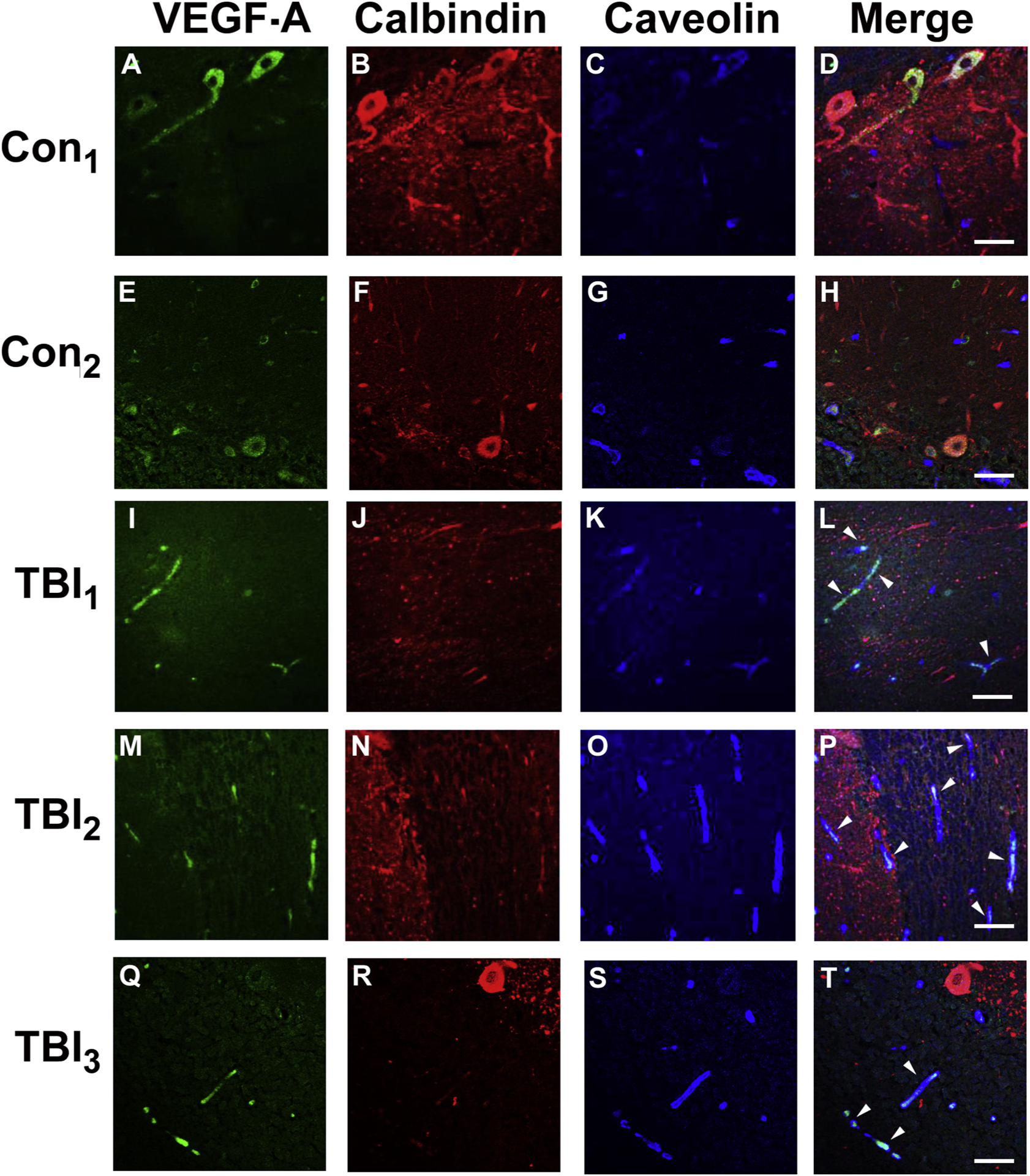
VEGF-A accumulates in cerebellum of veterans with blast-related mTBI.
Cerebellar postmortem specimens from veterans and active duty servicemembers with blast-related (TBI1–3) and two controls with no known TBI history (see Results, 3.5) were immunostained for VEGF-A (green), calbindin (red) that stains Purkinje cell bodies/processes, and caveolin (blue), which stains vascular endothelial cells. In distinction to controls (A-H), in the TBI cases (I-T), VEGF-A immunostaining was associated with microvascular profiles (caveolin-positive) (white arrow heads). In keeping with previous findings [52], prominent Purkinje cell body VEGF-A expression was observed, thereby supporting the specificity of the VEGF-A immunostaining, but did not reliably distinguish the mTBI and control cases. Scale bars = 50 μm.
4. Discussion
4.1. Elevated VEGF-A implicates chronic vascular dysfunction following blast-related mTBI
The intensive effort to identify chronic blood-borne biomarkers associated with repetitive mTBI in servicemembers and veterans has proven quite challenging. Recent advances indicate that altered protein expression of ubiquitination proteins [56], neurofilament light chain (NfL) [5], amyloid-β peptides [57,58] and tau species [58–60] are chronically elevated in blood and/or blood-derived exosomes from military service members with mTBI. Further, several epigenetic processes are altered in veterans with chronic mTBI [4,6,18]. Herein we report that VEGF-A is significantly elevated in plasma from blast mTBI veterans an average of 5.3 years after their last reported mTBI.
Whether aberrant VEGF-A levels contribute to sustained pathological processes or reflect ongoing compensatory mechanisms promoting vascular recovery cannot be addressed by these data. Increased VEGF-A levels could be part of the adaptive vascular responses to chronic injury, an idea consistent with recent findings of chronic neurovasculature thinning in blast-exposed animals [7]. Alternately, over time vascular remodeling may reach a stable homeostatic state, yet after multiple cycles of mTBI-induced activation, processes that promote VEGF-A secretion could become chronically hyperactive.
The initiating hypothesis of this study focused on VEGF-A, not bFGF, which we also found is elevated in mTBI veterans. Interestingly, bFGF interacts with VEGF-A signaling pathways [61,62]. How bFGF is involved in chronic blast-related mTBI cannot be addressed by these findings and is beyond the scope of the current report. Nonetheless, elevated plasma bFGF in blast mTBI veterans further supports the idea that vascular disturbances are an important feature of the chronic pathophysiology attending blast exposure. Importantly, the results herein are strongly supported by our recent report that microRNAs that directly suppress VEGF-A and bFGF expression are significantly reduced in plasma exosomes from veterans with blast-related mTBI [18].
4.2. Blast exposure is a unique method of inflicting trauma
VEGF-A is expressed throughout the body. In the CNS, VEGF-A expression is especially prominent in cerebellum and choroid plexus [63,64]. In searching for blood-borne TBI biomarkers, emphasis is often placed on molecules expressed selectively in brain. However, blast presents a more complex injury than blunt impact head trauma. The intense shock waves generated by high explosives propagate through the entire body, imparting simultaneous insults to multiple interacting organ systems, even when the victim is wearing protective body armor [65]. Blast-induced shock waves can induce biomechanical tissue injury throughout the body by multiple means that include shear stress, tensile stress, and formation of micro-cavitation bubbles that can damage cells [66] and may play important roles in mediating injury to small vessels [67,68] such as those in brain tissue. The fact that peripheral blast exposure of the torso in an animal model with the head shielded from blast forces still causes CNS pathology [69] lends importantly to the idea that blast overpressure injuries induce a form of polytrauma [34,69]. From this perspective, it is possible that VEGF-A disturbances may reflect blast-induced systemic insults with multiple functional consequences affecting both the brain and periphery together. The findings in this report necessarily leave open questions regarding the potential mechanisms and consequences of chronically elevated plasma VEGF-A. Nonetheless, it is possible that such elevations may reflect angiogenic regulatory mechanisms attempting to counteract chronically thinning cerebral vascular structures, which has been observed in blast-exposed rats [7]. Elevated plasma VEGF-A could also be indicative of dyshomeostasis of the dynamic interplay between endothelial cells and perivascular astrocytes of the BBB that is mediated, in part by VEGF-A [19] and which would be consistent with evidence of blast-induced BBB dysfunction [9,10,16–19].
4.3. Plasma VEGF-A association with cerebellar hypometabolism
Although there has long been evidence that the cerebellum is vulnerable to mild impact neurotrauma [70,71], this brain region has nonetheless received comparatively less attention than some other brain regions. We and others have reported that the cerebellum is vulnerable to blast-induced mTBI [9,10,20,37,65,72–85] [86]. Interestingly, a very recent report of tau PET imaging in veterans with blast TBI detected significant tau tracer uptake in the cerebellum and other brain regions that have not typically been associated with CTE tau pathology [87].
Using FDG-PET we found that glucose hypometabolism correlations with blast exposure were most prominent in the inferior/posterior cerebellum [9]. Therefore, we reasoned that if plasma VEGF-A levels could serve as a peripheral indicator of CNS function, such associations might more easily be ascertained by examining FDG uptake in cerebellum. Our findings support this idea. Plasma VEGF-A levels did not correlate with the frontal cortical areas examined. This does not mean these cortical regions are unaffected by blast mTBI nor does it suggest aberrant VEGF-A levels could not affect other brain regions. It is possible the cerebellum may be vulnerable to mTBI due to a number of factors that include: (i) very high neuronal density, in which approximately 69 billion of the 86 billion total neurons in adult human brain are in cerebellum [88]; (ii) high metabolic demand partially accounted for by high cell density; (iii) a cerebellar microvascular structure that is vulnerable to injury [89]; and (iv) the anatomical location of the cerebellum with respect to the posterior fossa.
4.4. Limitations
The correspondence between decreasing FDG uptake and increasing VEGF-A are well in keeping with multiple reports of blast-related neurovascular dysfunction [7–16]. Nonetheless, these data do not rule out other possibilities involving neuronal/synaptic loss or additional non-vascular functions of VEGF-A [90]. For example, VEGF-A complexed in extracellular matrices around Purkinje cells is a chemo-attractant that mediates granule cell/Purkinje cell connections [53]. It is not possible to determine if the VEGF-A immunoreactivity we observed in the blast mTBI cases is from peripheral or central sources. Importantly, because of the limited number of postmortem blast mTBI cerebellum cases available, these data must be interpreted cautiously to suggest only the possibility that elevated VEGF-A may localize in proximity to parenchymal perivascular and peri-astrocytic compartments. Similarly, our finding that VEGF-A accumulates in perivascular domains of the cerebellum, but not cortex or hippocampus, does not speak to the issue of where in the brain plasma VEGF-A levels may influence function.
It is difficult to assess the potential neurodegenerative specificity of elevated plasma VEGF-A to chronic blast-related mTBI. In distinction to blast-related mTBI, there are reports that VEGF-A levels are decreased in the serum of Alzheimer’s disease (AD) patients [91,92], as well as in peripheral mononuclear cells in Huntington’s disease patients [93], but is elevated in serum in multiple sclerosis patients [94]. Interestingly, serum VEGF levels are elevated in AD patients with cerebral microbleeds [95] and higher plasma VEGF levels correspond to lower Mini-Mental State Examination (MMSE) scores [96] [4,6,17,18].
Finally, it is important to recognize the limitations of history by retrospective recollection of blast mTBIs, as well as not acquiring history of subconcussive blast exposure. Both mTBI and DC veterans may have had at least some exposure to subconcussive blast, at the least in military training in preparation for deployment to the Iraq/Afghanistan combat theaters.
5. Conclusions
Plasma VEGF-A is chronically elevated in veterans with a history of repeated blast exposure causing mTBI. These findings add to the small number of blood-borne molecules thus far identified in this population during the chronic injury phase. This may reflect vascular dysregulation in the periphery and perhaps the CNS. Regardless of its source, increasing plasma VEGF-A levels correlate with decreasing glucose metabolism in the cerebellum, poorer performance on the Stoop color/word interference test, and increased difficulty in subjective cognitive set shifting. Overall, these findings significantly strengthen the idea that chronic focal vascular dysfunction may be an important pathophysiological consequence of repetitive blast exposure.
Supplementary Material
Acknowledgements
We thank Ms. Kim Howard, Ms. Lisa Keene, and Ms. Molly Chinn for outstanding technical support and Ms. Allison Beller for assistance with case selection and coordination of materials. The authors affirm that they have no conflicts of interest or relevant financial interests, activities, relationships, and affiliations pertaining to this study.
Funding/support
This work was supported by research grants from the Departments of Veterans Affairs Rehabilitation Research and Development Service Merit Review Grant IO1 RX001612 (Peskind) and Research and Development Medical Research Service I01 BX002311-05 (Cook); NIH R01AG046619 (Banks); NIA T32AG052354 (Logsdon); VA Puget Sound Health Care System Seed Grant (Meabon); NIA Alzheimer’s Disease Research Center AG005136 (Keene, Marshall); Friends of Alzheimer’s Research Endowment (Peskind); Nancy and Buster Alvord Endowment (Keene); Department of Defense W81XWH-16-1-0301 (Wang), W911NF-17-2-0086 (Wang), DTRA HDTRA1-13-C-0055 (Wang); NIH U01HL126496-02 (Wang), R56HL133887 (Wang), U01CA213330 (Wang) and R01DA040395 (Wang); R50CA211270 (Muzi); IK2 BX003258-01A1 (Schindler); VA CSR&D CX-001787 (Terry).
Abbreviations:
- bFGF
basic fibroblast growth factor, FGF2
- BBB
blood-brain barrier
- BRIEF-A
Behavioral Rating Inventory for Executive Function-Adult Version
- CNS
central nervous system
- CSF
cerebrospinal fluid
- CTE
chronic traumatic encephalopathy
- DKEFS
Delis-Kaplan Executive Function Scale
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- FDG
[18F]-fluorodeoxyglucose
- IAS
interface astroglial scarring
- mTBI
mild traumatic brain injury
- OEF/OIF/
Operation Enduring Freedom/Operation Iraqi Freedom
- PET
positron emission tomography
- PHQ-9
Patient Health Questionaire-9
- PSQI
Pittsburgh Sleep Quality Index
- PTSD
posttraumatic stress disorder
- VA/DoD
Veterans Affairs/Department of Defense
- VEGF-A
vascular endothelial growth factor-A)
- VOI
volume of interest
Footnotes
Notification
The opinions expressed herein are those of the authors and are not necessarily representative of those of the Uniformed Services University, the United States Department of Defense or the United States Army, Navy, Marines or Air Force.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jns.2020.117049.
References
- [1].Blennow K, Brody DL, Kochanek PM, Levin H, McKee A, Ribbers GM, et al. , Traumatic brain injuries, Nat. Rev. Dis. Primers 2 (2016) 16084. [DOI] [PubMed] [Google Scholar]
- [2].Koerte IK, Lin AP, Willems A, Muehlmann M, Hufschmidt J, Coleman MJ, et al. , A review of neuroimaging findings in repetitive brain trauma, Brain Pathol. 25 (2015) 318–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Agoston DV, Shutes-David A, Peskind ER, Biofluid biomarkers of traumatic brain injury, Brain Inj. 31 (2017) 1195–1203. [DOI] [PubMed] [Google Scholar]
- [4].Edwards KA, Motamedi V, Osier ND, Kim HS, Yun S, Cho YE, et al. , A moderate blast exposure results in dysregulated gene network activity related to cell death, survival, structure, and metabolism, Front. Neurol 11 (2020) 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dickstein DL, De Gasperi R, Gama Sosa MA, Perez-Garcia G, Short JA, Sosa H, et al. , Brain and blood biomarkers of tauopathy and neuronal injury in humans and rats with neurobehavioral syndromes following blast exposure, Mol. Psychiatry (2020) 3138–3167, 10.1038/s41380-020-0674-z In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang Z, Wilson CM, Mendelev N, Ge Y, Galfalvy H, Elder G, et al. , Acute and chronic molecular signatures and associated symptoms of blast exposure in military Breachers, J. Neurotrauma 37 (2020) 1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gama Sosa MA, De Gasperi R, Perez Garcia GS, Perez GM, Searcy C, Vargas D, et al. , Low-level blast exposure disrupts gliovascular and neurovascular connections and induces a chronic vascular pathology in rat brain, Acta Neuropathol. Commun 7 (2019) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Elder GA, Gama Sosa MA, De Gasperi R, Stone JR, Dickstein DL, Haghighi F, et al. , Vascular and inflammatory factors in the pathophysiology of blast-induced brain injury, Front. Neurol 6 (2015) 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Meabon JS, Huber BR, Cross DJ, Richards TL, Minoshima S, Pagulayan KF, et al. , Repetitive blast exposure in mice and combat veterans causes persistent cerebellar dysfunction, Sci. Transl. Med 8 (2016) 321ra326. [DOI] [PubMed] [Google Scholar]
- [10].Logsdon AF, Meabon JS, Cline MM, Bullock KM, Raskind MA, Peskind ER, et al. , Blast exposure elicits blood-brain barrier disruption and repair mediated by tight junction integrity and nitric oxide dependent processes, Sci. Rep 8 (2018) 11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Abutarboush R, Gu M, Kawoos U, Mullah SH, Chen Y, Goodrich SY, et al. , Exposure to blast overpressure impairs cerebral microvascular responses and alters vascular and Astrocytic structure, J. Neurotrauma 36 (2019) 3138–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ahmed F, Plantman S, Cernak I, Agoston DV, The temporal pattern of changes in serum biomarker levels reveals complex and dynamically changing pathologies after exposure to a single low-intensity blast in mice, Front. Neurol 6 (2015) 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sajja VS, Tenn C, McLaws LJ, Vandevord PJ, A temporal evaluation of cytokines in rats after blast exposure, Biomed. Sci. Instrum 48 (2012) 374–379. [PubMed] [Google Scholar]
- [14].Kwon SK, Kovesdi E, Gyorgy AB, Wingo D, Kamnaksh A, Walker J, et al. , Stress and traumatic brain injury: a behavioral, proteomics, and histological study, Front. Neurol 2 (2011) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kovesdi E, Gyorgy AB, Kwon SK, Wingo DL, Kamnaksh A, Long JB, et al. , The effect of enriched environment on the outcome of traumatic brain injury; a behavioral, proteomics, and histological study, Front. Neurosci 5 (2011) 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huber BR, Meabon JS, Hoffer ZS, Zhang J, Hoekstra JG, Pagulayan KF, et al. , Blast exposure causes dynamic microglial/macrophage responses and micro-domains of brain microvessel dysfunction, Neuroscience 319 (2016) 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Logsdon AF, Schindler AG, Meabon JS, Yagi M, Herbert MJ, Banks WA, et al. , Nitric oxide synthase mediates cerebellar dysfunction in mice exposed to repetitive blast-induced mild traumatic brain injury, Sci. Rep (2020), 10.1038/s41598-020-66113-7 In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ghai V, Fallen S, Baxter D, Scherler K, Kim TK, Zhou Y, et al. , Alterations in plasma microRNA and protein levels in war veterans with chronic mild traumatic brain injury, J. Neurotrauma 37 (2020) 1418–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, et al. , Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease, J. Clin. Invest 122 (2012) 2454–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Petrie EC, Cross DJ, Yarnykh VL, Richards T, Martin NM, Pagulayan K, et al. , Neuroimaging, Behavioral, and psychological Sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans, J. Neurotrauma 31 (2014) 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Della Rosa PA, Cerami C, Gallivanone F, Prestia A, Caroli A, Castiglioni I, et al. , A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia, Neuroinformatics 12 (2014) 575–593. [DOI] [PubMed] [Google Scholar]
- [22].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. , Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain, Neuroimage 15 (2002) 273–289. [DOI] [PubMed] [Google Scholar]
- [23].Peskind ER, Riekse R, Quinn JF, Kaye J, Clark CM, Farlow MR, et al. , Safety and acceptability of the research lumbar puncture, Alzheimer Dis. Assoc. Disord 19 (2005) 220–225. [DOI] [PubMed] [Google Scholar]
- [24].Delis KE, Kramer JHDC, Delis-Kaplan Executive Function System, Psychological Corp., San Antonio, TX, 2001. [Google Scholar]
- [25].Roth R IP, Gioia G, Behavioral Rating Inventory for Executive Function-Adult Version, Psychological Assessment Resources, Lutz, FL, 2005. [Google Scholar]
- [26].McDonald JH, Handbook of Biological Statistics, 3rd ed, Sparky House Publishing, Baltimore, MD USA, 2014. [Google Scholar]
- [27].Ryan-Gonzalez C, Kimbrel NA, Meyer EC, Gordon EM, DeBeer BB, Gulliver SB, et al. , Differences in post-traumatic stress disorder symptoms among Post-9/11 veterans with blast- and non-blast mild traumatic brain injury, J. Neurotrauma 36 (2019) 684–694. [DOI] [PubMed] [Google Scholar]
- [28].Forbes D, Creamer M, Biddle D, The validity of the PTSD checklist as a measure of symptomatic change in combat-related PTSD, Behav. Res. Ther 39 (2001) 977–986. [DOI] [PubMed] [Google Scholar]
- [29].Kroenke K, Spitzer RL, Williams JB, The PHQ-9: validity of a brief depression severity measure, J. Gen. Intern. Med 16 (2001) 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ, The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research, Psychiatry Res. 28 (1989) 193–213. [DOI] [PubMed] [Google Scholar]
- [31].Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA, The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test, Arch. Intern. Med 158 (1998) 1789–1795. [DOI] [PubMed] [Google Scholar]
- [32].Cetin A, Deveci E, Expression of VEGF and GFAP in a rat model of traumatic brain injury treated with Honokiol: a biochemical and immunohistochemical study, Folia Morphol. (Warsz) 78 (2019) 684–694. [DOI] [PubMed] [Google Scholar]
- [33].Ozevren H, Deveci E, Tuncer MC, Histopathological changes in the choroid plexus after traumatic brain injury in the rats: a histologic and immunohistochemical study, Folia Morphol. (Warsz) 77 (2018) 642–648. [DOI] [PubMed] [Google Scholar]
- [34].Hubbard WB, Greenberg S, Norris C, Eck J, Lavik E, VandeVord P, Distinguishing the unique Neuropathological profile of blast Polytrauma, Oxidative Med. Cell. Longev 2017 (2017) 5175249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Michinaga S, Kimura A, Hatanaka S, Minami S, Asano A, Ikushima Y, et al. , Delayed administration of BQ788, an ETB antagonist, after experimental traumatic brain injury promotes recovery of blood-brain barrier function and a reduction of cerebral Edema in mice, J. Neurotrauma 35 (2018) 1481–1494. [DOI] [PubMed] [Google Scholar]
- [36].Buchsbaum MS, Simmons AN, DeCastro A, Farid N, Matthews SC, Clusters of low (18)F-Fluorodeoxyglucose uptake voxels in combat veterans with traumatic brain injury and post-traumatic stress disorder, J. Neurotrauma 32 (2015) 1736–1750. [DOI] [PubMed] [Google Scholar]
- [37].Peskind ER, Petrie EC, Cross DJ, Pagulayan K, McCraw K, Hoff D, et al. , Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war veterans with persistent post-concussive symptoms, Neuroimage 54 (Suppl. 1) (2011) S76–S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pagulayan KF, Rau H, Madathil R, Werhane M, Millard SP, Petrie EC, et al. , Retrospective and prospective memory among OEF/OIF/OND veterans with a self-reported history of blast-related mTBI, J. Int. Neuropsychol. Soc 24 (2018) 324–334. [DOI] [PubMed] [Google Scholar]
- [39].Pagulayan KF, Petrie EC, Cook DG, Hendrickson RC, Rau H, Reilly M, et al. , Effect of blast-related mTBI on the working memory system: a resting state fMRI study, Brain Imaging and Behavior 14 (2018) 949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Stoodley CJ, The cerebellum and cognition: evidence from functional imaging studies, Cerebellum 11 (2012) 352–365. [DOI] [PubMed] [Google Scholar]
- [41].Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, et al. , Consensus paper: the cerebellum’s role in movement and cognition, Cerebellum 13 (2014) 151–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rentiya Z, Khan NS, Ergun E, Ying SH, Desmond JE, Distinct cerebellar regions related to motor and cognitive performance in SCA6 patients, Neuropsychologia 107 (2017) 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Braga-Neto P, Pedroso JL, Alessi H, Dutra LA, Felicio AC, Minett T, et al. , Cerebellar cognitive affective syndrome in Machado Joseph disease: core clinical features, Cerebellum 11 (2012) 549–556. [DOI] [PubMed] [Google Scholar]
- [44].Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Nagase T, et al. , Regional gray and white matter volume associated with Stroop interference: evidence from voxel-based morphometry, Neuroimage 59 (2012) 2899–2907. [DOI] [PubMed] [Google Scholar]
- [45].Audenaert K, Lahorte P, Brans B, van Laere K, Goethals I, van Heeringen K, et al. , The classical stroop interference task as a prefrontal activation probe: a validation study using 99Tcm-ECD brain SPECT, Nucl. Med. Commun 22 (2001) 135–143. [DOI] [PubMed] [Google Scholar]
- [46].Middleton FA, Strick PL, Cerebellar projections to the prefrontal cortex of the primate, J. Neurosci 21 (2001) 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Buckner RL, The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging, Neuron 80 (2013) 807–815. [DOI] [PubMed] [Google Scholar]
- [48].Stefanini MO, Wu FT, Mac Gabhann F, Popel AS, The presence of VEGF receptors on the luminal surface of endothelial cells affects VEGF distribution and VEGF signaling, PLoS Comput. Biol 5 (2009) e1000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hudson N, Powner MB, Sarker MH, Burgoyne T, Campbell M, Ockrim ZK, et al. , Differential apicobasal VEGF signaling at vascular blood-neural barriers, Dev. Cell 30 (2014) 541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Simons M, Gordon E, Claesson-Welsh L, Mechanisms and regulation of endothelial VEGF receptor signalling, Nat. Rev. Mol. Cell Biol 17 (2016) 611–625. [DOI] [PubMed] [Google Scholar]
- [51].Egervari K, Potter G, Guzman-Hernandez ML, Salmon P, Soto-Ribeiro M, Kastberger B, et al. , Astrocytes spatially restrict VEGF signaling by polarized secretion and incorporation of VEGF into the actively assembling extracellular matrix, Glia 64 (2016) 440–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sentilhes L, Michel C, Lecourtois M, Catteau J, Bourgeois P, Laudenbach V, et al. , Vascular endothelial growth factor and its high-affinity receptor (VEGFR-2) are highly expressed in the human forebrain and cerebellum during development, J. Neuropathol. Exp. Neurol 69 (2010) 111–128. [DOI] [PubMed] [Google Scholar]
- [53].Ruiz de Almodovar C, Coulon C, Salin PA, Knevels E, Chounlamountri N, Poesen K, et al. , Matrix-binding vascular endothelial growth factor (VEGF) iso forms guide granule cell migration in the cerebellum via VEGF receptor Flk1, J. Neurosci 30 (2010) 15052–15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Boer K, Troost D, Spliet WG, van Rijen PC, Gorter JA, Aronica E, Cellular distribution of vascular endothelial growth factor A (VEGFA) and B (VEGFB) and VEGF receptors 1 and 2 in focal cortical dysplasia type IIB, Acta Neuropathol. 115 (2008) 683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rigau V, Morin M, Rousset MC, de Bock F, Lebrun A, Coubes P, et al. , Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy, Brain 130 (2007) 1942–1956. [DOI] [PubMed] [Google Scholar]
- [56].Heinzelmann M, Reddy SY, French LM, Wang D, Lee H, Barr T, et al. , Military personnel with chronic symptoms following blast traumatic brain injury have differential expression of neuronal recovery and epidermal growth factor receptor genes, Front. Neurol 5 (2014) 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lejbman N, Olivera A, Heinzelmann M, Feng R, Yun S, Kim HS, et al. , Active duty service members who sustain a traumatic brain injury have chronically elevated peripheral concentrations of Abeta40 and lower ratios of Abeta42/40, Brain Inj. 30 (2016) 1436–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gill J, Mustapic M, Diaz-Arrastia R, Lange R, Gulyani S, Diehl T, et al. , Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel, Brain Inj. 32 (2018) 1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Olivera A, Lejbman N, Jeromin A, French LM, Kim HS, Cashion A, et al. , Peripheral Total tau in military personnel who sustain traumatic brain injuries during deployment, JAMA Neurol. 72 (2015) 1109–1116. [DOI] [PubMed] [Google Scholar]
- [60].Kenney K, Qu BX, Lai C, Devoto C, Motamedi V, Walker WC, et al. , Higher exosomal phosphorylated tau and total tau among veterans with combat-related repetitive chronic mild traumatic brain injury, Brain Inj. 32 (2018) 1276–1284. [DOI] [PubMed] [Google Scholar]
- [61].Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, et al. , Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis, J. Cell Biol 141 (1998) 1659–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M, Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis, Cytokine Growth Factor Rev. 16 (2005) 159–178. [DOI] [PubMed] [Google Scholar]
- [63].Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR, Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors, Mol. Biol. Cell 3 (1992) 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Maharaj AS, Saint-Geniez M, Maldonado AE, D’Amore PA, Vascular endothelial growth factor localization in the adult, Am. J. Pathol 168 (2006) 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cernak I, Noble-Haeusslein LJ, Traumatic brain injury: an overview of pathobiology with emphasis on military populations, J. Cereb. Blood Flow Metab 30 (2010) 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Nakagawa A, Manley GT, Gean AD, Ohtani K, Armonda R, Tsukamoto A, et al. , Mechanisms of primary blast-induced traumatic brain injury: insights from shock-wave research, J. Neurotrauma 28 (2011) 1101–1119. [DOI] [PubMed] [Google Scholar]
- [67].Zhong P, Xi X, Zhu S, Cocks FH, Preminger GM, Recent developments in SWL physics research, J. Endourol 13 (1999) 611–617. [DOI] [PubMed] [Google Scholar]
- [68].Zhong P, Zhou Y, Zhu S, Dynamics of bubble oscillation in constrained media and mechanisms of vessel rupture in SWL, Ultrasound Med. Biol 27 (2001) 119–134. [DOI] [PubMed] [Google Scholar]
- [69].Cernak I, The importance of systemic response in the pathobiology of blast-induced neurotrauma, Front. Neurol 1 (2010) 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Fukuda K, Aihara N, Sagar SM, Sharp FR, Pitts LH, Honkaniemi J, et al. , Purkinje cell vulnerability to mild traumatic brain injury, J. Neurotrauma 13 (1996) 255–266. [DOI] [PubMed] [Google Scholar]
- [71].Corsellis JA, Bruton CJ, Freeman-Browne D, The aftermath of boxing, Psychol. Med 3 (1973) 270–303. [DOI] [PubMed] [Google Scholar]
- [72].Mayorga MA, The pathology of primary blast overpressure injury, Toxicology 121 (1997) 17–28. [DOI] [PubMed] [Google Scholar]
- [73].Huber BR, Meabon JS, Martin TJ, Mourad PD, Bennett R, Kraemer BC, et al. , Blast exposure causes early and persistent aberrant phospho- and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury, J. Alzheimers Dis 37 (2013) 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Cernak I, Blast injuries of the lung: development, prognosis and possible therapy, Vojnosanit. Pregl 54 (1997) 91–102. [PubMed] [Google Scholar]
- [75].Cernak I, Savic J, Malicevic Z, Zunic G, Radosevic P, Ivanovic I, et al. , Involvement of the central nervous system in the general response to pulmonary blast injury, J. Trauma 40 (1996) S100–S104. [DOI] [PubMed] [Google Scholar]
- [76].Cernak I, Wang Z, Jiang J, Bian X, Savic J, Ultrastructural and functional characteristics of blast injury-induced neurotrauma, J. Trauma 50 (2001) 695–706. [DOI] [PubMed] [Google Scholar]
- [77].Murthy JM, Chopra JS, Gulati DR, Subdural hematoma in an adult following a blast injury. Case report, J. Neurosurg 50 (1979) 260–261. [DOI] [PubMed] [Google Scholar]
- [78].Kamnaksh A, Budde MD, Kovesdi E, Long JB, Frank JA, Agoston DV, Diffusion tensor imaging reveals acute subcortical changes after mild blast-induced traumatic brain injury, Sci. Rep 4 (2014) 4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, et al. , Detection of blast-related traumatic brain injury in U.S. military personnel, N. Engl. J. Med 364 (2011) 2091–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mac Donald C, Johnson A, Cooper D, Malone T, Sorrell J, Shimony J, et al. , Cerebellar white matter abnormalities following primary blast injury in US military personnel, PLoS One 8 (2013) e55823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Warden DL, French LM, Shupenko L, Fargus J, Riedy G, Erickson ME, et al. , Case report of a soldier with primary blast brain injury, Neuroimage 47 (Suppl. 2) (2009) T152–T153. [DOI] [PubMed] [Google Scholar]
- [82].Liu W, Wang B, Wolfowitz R, Yeh PH, Nathan DE, Graner J, et al. , Perfusion deficits in patients with mild traumatic brain injury characterized by dynamic susceptibility contrast MRI, NMR Biomed. 26 (2013) 651–663. [DOI] [PubMed] [Google Scholar]
- [83].Fischer BL, Parsons M, Durgerian S, Reece C, Mourany L, Lowe MJ, et al. , Neural activation during response inhibition differentiates blast from mechanical causes of mild to moderate traumatic brain injury, J. Neurotrauma 31 (2014) 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yeh PH, Wang B, Oakes TR, French LM, Pan H, Graner J, et al. , Postconcussional disorder and PTSD symptoms of military-related traumatic brain injury associated with compromised neurocircuitry, Hum. Brain Mapp 35 (2014) 2652–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Koliatsos VE, Cernak I, Xu L, Song Y, Savonenko A, Crain BJ, et al. , A mouse model of blast injury to brain: initial pathological, neuropathological, and behavioral characterization, J. Neuropathol. Exp. Neurol 70 (2011) 399–416. [DOI] [PubMed] [Google Scholar]
- [86].Lu J, Ng KC, Ling G, Wu J, Poon DJ, Kan EM, et al. , Effect of blast exposure on the brain structure and cognition in Macaca fascicularis, J. Neurotrauma 29 (2012) 1434–1454. [DOI] [PubMed] [Google Scholar]
- [87].Robinson ME, McKee AC, Salat DH, Rasmusson AM, Radigan LJ, Catana C, et al. , Positron emission tomography of tau in Iraq and Afghanistan veterans with blast neurotrauma, NeuroImage. Clin 21 (2019) 101651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, et al. , Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain, J. Comp. Neurol 513 (2009) 532–541. [DOI] [PubMed] [Google Scholar]
- [89].Nonaka H, Akima M, Hatori T, Nagayama T, Zhang Z, Ihara F, The micro-vasculature of the human cerebellar meninges, Acta Neuropathol. 104 (2002) 608–614. [DOI] [PubMed] [Google Scholar]
- [90].Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P, Role and therapeutic potential of VEGF in the nervous system, Physiol. Rev 89 (2009) 607–648. [DOI] [PubMed] [Google Scholar]
- [91].Huang L, Jia J, Liu R, Decreased serum levels of the angiogenic factors VEGF and TGF-beta1 in Alzheimer’s disease and amnestic mild cognitive impairment, Neurosci. Lett 550 (2013) 60–63. [DOI] [PubMed] [Google Scholar]
- [92].Mateo I, Llorca J, Infante J, Rodriguez-Rodriguez E, Fernandez-Viadero C, Pena N, et al. , Low serum VEGF levels are associated with Alzheimer’s disease, Acta Neurol. Scand 116 (2007) 56–58. [DOI] [PubMed] [Google Scholar]
- [93].Cesca F, Bregant E, Peterlin B, Zadel M, Dubsky de Wittenau G, Siciliano G, et al. , Evaluating the SERCA2 and VEGF mRNAs as potential molecular biomarkers of the onset and progression in Huntington’s disease, PLoS One 10 (2015) e0125259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Su JJ, Osoegawa M, Matsuoka T, Minohara M, Tanaka M, Ishizu T, et al. , Upregulation of vascular growth factors in multiple sclerosis: correlation with MRI findings, J. Neurol. Sci 243 (2006) 21–30. [DOI] [PubMed] [Google Scholar]
- [95].Zhang JB, Li MF, Zhang HX, Li ZG, Sun HR, Zhang JS, et al. , Association of serum vascular endothelial growth factor levels and cerebral microbleeds in patients with Alzheimer’s disease, Eur. J. Neurol 23 (2016) 1337–1342. [DOI] [PubMed] [Google Scholar]
- [96].Yu S, Liu YP, Liu YH, Jiao SS, Liu L, Wang YJ, et al. , Diagnostic utility of VEGF and soluble CD40L levels in serum of Alzheimer’s patients, Clin. Chim. Acta 453 (2016) 154–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


