Abstract
This review provides an update on the characteristics of nonalcoholic fatty liver disease (NAFLD), with a focus on the effects of age, sex, and body mass index. Age is a risk factor for NAFLD progression; however, extremely old patients have unique features, namely, the associations between metabolic comorbidities and NAFLD are weaker and NAFLD is not a risk factor for mortality. The prevalence of NAFLD is higher in men than in premenopausal women, whereas the reverse is true after menopause. Thus, before menopause, estrogen may have protective effects against NAFLD. Our hospital data showed that over 25% of male patients with NAFLD and almost 40% of female patients with NAFLD, especially elderly patients, were nonobese. Although histological steatosis and activity were associated with body mass index, the prevalence of nonalcoholic steatohepatitis was not. The prevalence of advanced fibrosis showed a significant sex difference. Advanced fibrosis was significantly more frequent among severely obese men but the prevalence was lower among severely obese women. This difference could be because a substantial proportion of severely obese women were premenopausal; thus, estrogen may have much stronger effects on the development of fibrosis than on obesity. Further studies are required to develop tailored management strategies.
Keywords: Non-alcoholic fatty liver disease; Age, sex difference; Lean NAFLD; Obesity; Body mass index
INTRODUCTION
The obesity pandemic has become a major public health problem and has resulted in a dramatic increase of metabolic syndrome, type 2 diabetes, hyperlipidemia, hypertension, and nonalcoholic fatty liver disease (NAFLD). NAFLD can be both the result and cause of metabolic syndrome and these diseases, due to the existence of a vicious cycle linking these conditions. NAFLD encompasses a broad spectrum of hepatic injury that ranges from simple steatosis (nonalcoholic fatty liver [NAFL]) to steatohepatitis (nonalcoholic steatohepatitis [NASH]). NAFL is generally benign and non-progressive, while NASH can progress to cirrhosis and even hepatocellular carcinoma (HCC). Currently, NAFLD is the leading cause of chronic liver disease worldwide, as well as the most rapidly increasing cause of cirrhosis and HCC.1-8 Individuals with NAFLD have a higher standardized mortality ratio (hazard ratio of around 1.6) compared with the general population due to elevation of both liver-related and cardiovascular mortality.9-17 The most common causes of death among NAFLD patients are malignancy or cardiovascular disease, followed by liver-related disease. It is thought that NAFLD generally shows slow progression, with liver-related morbidity and mortality occurring in a small proportion of patients. A strong association of the prevalence and severity of NAFLD with age, sex, and ethnicity is well known.1-5,18,19
Obesity is a major determinant of the prevalence of NAFLD.1-5 Previous studies have clearly demonstrated that Asian populations have a higher risk of insulin resistance, type 2 diabetes, NAFLD/NASH, and cardiovascular disease than populations of European descent at any given body mass index (BMI).20 This difference in the level of risk may be explained by the presence of more visceral fat and subcutaneous fat in Asians than in Europeans at any given BMI, as well as earlier arrest of adipocyte maturation and development of insulin resistance in Asians during weight gain.21-24 Based on these considerations, obesity is defined as a BMI ≥25 kg/m2 for the Japanese population instead of a BMI ≥30 kg/m2, as it is for Europeans.25 The severity of obesity in a population parallels the incidence of NAFLD. However, occurrence of NAFLD in lean or nonobese individuals has also been attracting attention, because lean NAFLD is not rare and is generally not a benign condition.26,27
This review provides an update on the characteristics of NAFLD/NASH, focusing on the influence of age, sex, and BMI.
EPIDEMIOLOGY
A very large meta-analysis (8,515,431 subjects from 22 countries) performed by Younossi et al.15 showed that the global prevalence of NAFLD among adults is 25.24% (95% confidence interval [CI], 22.10 to 28.65), with the highest prevalence being found in the Middle East (31.79%), followed by South America (30.45%) and Asia (27.37%). The higher prevalence of NAFLD in these geographic areas seems to be mainly explained by genetic factors. A single nucleotide polymorphism of patatin-like phospholipase domain–containing protein 3 (PNPLA3) rs738409 is known to be the most important risk allele for the onset and progression of NAFLD, as well as for development of HCC, with the G allele increasing the risk of these outcomes.28-30 The PNPLA3 risk allele is most common among Hispanics, who show the highest susceptibility to NAFLD, followed by Asians, and it is more frequent in nonobese NAFLD patients.31 NAFLD is associated with various metabolic comorbidities, including obesity (51.34%; 95% CI, 41.38 to 61.20), type 2 diabetes (22.51%; 95% CI, 17.92 to 27.89), hyperlipidemia (69.16%; 95% CI, 49.91 to 83.46), hypertension (39.34%; 95% CI, 33.15 to 45.88), and metabolic syndrome (42.54%; 95% CI, 30.06 to 56.05).
According to data obtained from annual health check, the prevalence of NAFLD in Japan is estimated to be around 10% to 30%,1,32-34 suggesting that the prevalence of NASH may fall within the range of 2% to 6%. The prevalence of NAFLD generally increases with BMI, being 5% to 20% in nonobese individuals, around 50% in persons with a BMI exceeding 25 kg/m2 and less than 30 kg/m2, and around 80% in those with a BMI over 30 kg/m2. It has been reported that the prevalence of NAFLD is around 50% in patients with type 2 diabetes and 40% among those with dyslipidemia. Obesity and metabolic comorbidities have a synergistic effect on the incidence of NAFLD.
Surveys on the etiology of liver cirrhosis and HCC in Japan have shown that hepatitis C virus infection is the leading cause of HCC (53%), followed by hepatitis B (13%) virus infection, while NAFLD accounts for 5.8% of cirrhosis and 4.3% of HCC.35,36 There is no doubt that NAFLD will increase in the future.
There has been an increase of reports about HCC in patients with NAFLD/NASH.37-48 As with other liver diseases, the most important risk factors for HCC are cirrhosis, age and male sex. In addition, obesity, type 2 diabetes, and PNPLA3 increase the risk of HCC. Their mean age at diagnosis of HCC is around 70 years old and they show male predominance. In comparison with patients whose HCC is due to other etiologies, patients with NAFLD/NASH-related HCC are more likely to have comorbidities such as obesity, type 2 diabetes, hypertension, and cardiovascular disease. The most problematic aspect of NAFLD/NASH-related HCC is development of a substantial number of tumors in patients without cirrhosis.
Hepatitis B virus infection is the predominant etiology of HCC in Korea as well as in China and Taiwan. HCC cases from etiologies other than viral hepatitis infection, or alcohol ranged from 6.8% to 15.1%. NAFLD-related HCC falls in this category. Since Korea is a hepatitis B virus-endemic area, prior hepatitis B virus infection is considered to play a role even in the development of NAFLD-related HCC.49
In the United States, NAFLD has been recognized as one of the leading causes of cirrhosis in adults and NAFLD-related cirrhosis is currently the second most frequent indication for liver transplantation.5,15,50,51
AGE
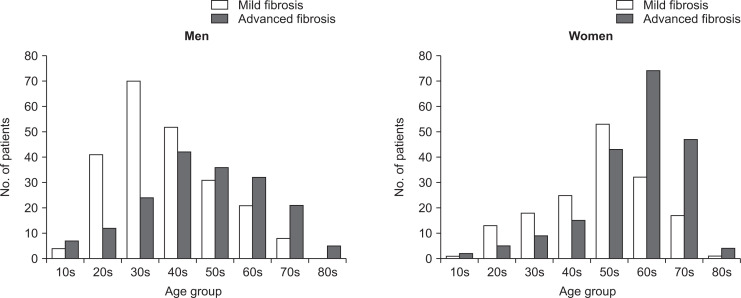
The most important histological feature of NAFLD/NASH associated with mortality is the severity of fibrosis.14,52-54 Risk factors for the development of advanced fibrosis or cirrhosis are reported to be age, type 2 diabetes, morbid obesity, PNPLA3 and elevation of transaminases.55-62 Fig. 1 is a distribution of NAFLD patients diagnosed at our university hospital by age and sex from severity of fibrosis. In older generation advanced fibrosis was more common than mild fibrosis in both genders. Aging is associated with multidimensional functional decline. The age-associated decline of mitochondrial function and anti-oxidant mechanisms contributes to development of metabolic syndrome and metabolic comorbidities.63 Aging also affects liver morphology and physiology, with nearly one third of hepatic volume and perfusion being lost between the ages of 30 and 100 years.64 Histological examination reveals enlargement of hepatocytes, an increase of binucleated hepatocytes, and a decrease of mitochondria with aging.65,66 Age is also a risk factor for hepatic steatosis due to the onset of multiple abnormalities of hepatic lipid metabolism. A gradual decline in the production of sex hormones, growth hormone, and insulin-like growth factor 1 with aging might be one reason for development of hepatic steatosis.67 In addition, older age is associated with reduced physical activity, which leads to sarcopenia, that is, a decrease of muscle mass and function. Recent studies have shown that sarcopenia is a risk factor for the development and progression of NAFLD independently of obesity, insulin resistance, and metabolic syndrome.68-70 All of these factors may contribute to the increasing prevalence and severity of metabolic syndrome and NAFLD/NASH with advancing age.
Fig. 1.
Distribution of patients with nonalcoholic fatty liver disease by mild or advanced fibrosis status with respect to age and sex. In the older generation, advanced fibrosis was more common than mild fibrosis in both sexes. Data was obtained from Tokyo Women’s Medical University in 1991 to 2018 (n=811: 406 men and 359 women).
We previously analyzed the influence of age, sex, and lifestyle-related diseases in 193 patients with biopsy-proven NASH.57 To assess the effect of menopause, we separated the patients into groups younger and older than 55 years. Women were predominant in the older group (23.8% in the younger group vs 67.4% in the older group, p<0.001). The prevalence of severe obesity (defined as a BMI >30 kg/m2) and hyperlipidemia was higher in the younger group (36.6% vs 16.3%, p=0.002 and 73.3% vs 50.5%, p=0.001, respectively). In contrast, type 2 diabetes and hypertension were significantly more common in the older group (30.7% vs 53.3%, p=0.001 and 14.9% vs 45.7%, p<0.001, respectively). Liver biopsy showed that the older group had a significantly higher prevalence of advanced fibrosis (23.8% vs 54.3%, p<0.001). Multivariate analysis of risk factors for advanced fibrosis revealed that age (odds ratio, 1.089; 95% CI, 1.024 to 1.159) and BMI (odds ratio, 1.101; 95% CI, 1.011 to 1.199) were independent predictors in the younger group. In contrast, absence of hyperlipidemia (odds ratio, 0.389; 95% CI, 0.157 to 0.966) was the only significant independent predictor of advanced fibrosis in the older group. Our study showed that NASH was infrequent among young women, while women (probably postmenopausal) were predominant among the subjects older than 55 years. The metabolic comorbidities associated with NASH differed with age, since a higher prevalence of severe obesity and hyperlipidemia was noted in the younger group, while type 2 diabetes and hypertension were more frequent in the older group. Advanced liver fibrosis showed an increase with aging, while age and BMI (younger group) or the absence of hyperlipidemia (older group) were predictors for advanced fibrosis. These results are consistent with other reports.
However, the situation is completely different among very old patients with NAFLD. Kagansky et al.71 evaluated 91 octogenarians admitted to the rehabilitation department of a geriatric hospital and found NAFLD (diagnosed by ultrasonography criteria) in 42 of them (46.2%). There were no significant differences of the following parameters between the patients with or without NAFLD: age, sex, chronic illnesses, anthropometric parameters, lipid profile, fasting glucose, prevalence of metabolic syndrome, and the levels of transaminases, ferritin, and iron. Conversely, NAFLD patients younger than 70 years had a significantly higher prevalence of obesity, glucose intolerance, and metabolic syndrome compared with the elderly NAFLD patients. According to a population-based study of 2,811 participants (mean age: 76.4±6.0 years) performed in Rotterdam, the prevalence of NAFLD diagnosed by ultrasonography decreased with advancing age (p<0.001), declining from 35.8% among participants <70 years old to 21.1% among those aged ≥85 years.72 Similarly the association between metabolic syndrome and NAFLD became weaker with advancing age.
Golabi et al.73 assessed the prevalence, risk factors, and mortality of NAFLD in 3,271 individuals older than 60 years by using data from the Third National Health and Nutrition Examination Survey linked with mortality information. They reported that the prevalence rate of NAFLD was 40.3% among subjects aged 60 to 74 years and 39.2% among those aged >74 years. Among the subjects aged 60 to 74 years, the risk of 5-year all-cause mortality was associated with the presence of NAFLD (adjusted hazard ratio, 1.60; 95% CI, 1.24 to 1.96) and cardiovascular mortality was also higher in this group. In contrast, NAFLD was not associated with all-cause mortality or cardiovascular mortality among individuals aged >74 years.
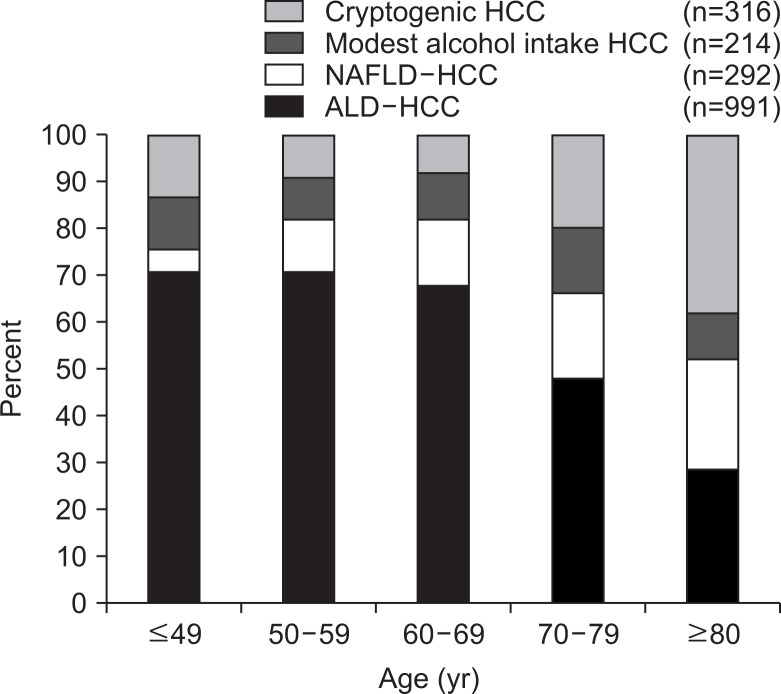
According to our nationwide survey of HCC due to nonviral chronic liver disease in Japan, most HCC patients aged 80 years or older did not have any known etiology, such as alcoholic liver disease or NAFLD, and their condition was classified as cryptogenic (Fig. 2).41 Among HCC patients aged 80 years or older, the prevalence of obesity, type 2 diabetes, and cirrhosis was also significantly lower than in younger patients. These findings were similar to those for NAFLD patients in the same age groups.
Fig. 2.
Etiology of nonviral hepatocellular carcinoma (HCC) with respect to age based on a nationwide survey in 2009.41 Among patients aged over 80 years, the percentage of cryptogenic HCC was increasing, and the prevalence of obesity, type 2 diabetes, and cirrhosis was significantly lower in this patient group.
NAFLD, nonalcoholic fatty liver disease; ALD, alcoholic liver disease.
Thus, the association between metabolic comorbidities and NAFLD becomes weaker in the very old population. In addition, NAFLD is not associated with an increased risk of mortality in this age group. The age defined as “elderly” varies among studies and this may lead to conflicting results.
GENDER DIFFERENCE
As we mentioned, the prevalence of NAFLD is higher in men than in premenopausal women, while the reverse is true after menopause.8,18,19,32,34,74-79 This difference has generally been explained by the influence of sex hormones. Estrogen regulates energy homeostasis by controlling mitochondrial structure and function, as well as by enhancing insulin release and modulating the secretion/action of growth hormone.80 Postmenopausal women tend to gain weight along with a shift in its distribution to an increase of visceral fat.8,81-85 Most women reach their maximum body weight after menopause. Experimental studies have shown that estrogen decreases the generation of reactive oxygen species, down-regulates the expression of transforming growth factor beta-1, and inhibits activation and fibrogenesis by stellate cells.86,87 Accordingly, women are protected from the development and progression of NAFLD/NASH by estrogen before menopause. In addition, it has been reported that hormone replacement therapy has a protective effect against metabolic syndrome and NAFLD after menopause.88 Recent studies have demonstrated that testosterone is also closely associated with metabolic syndrome, type 2 diabetes, and NAFLD.89-94 Production of testosterone gradually decreases with aging, and the prevalence of these diseases increases at an older age.
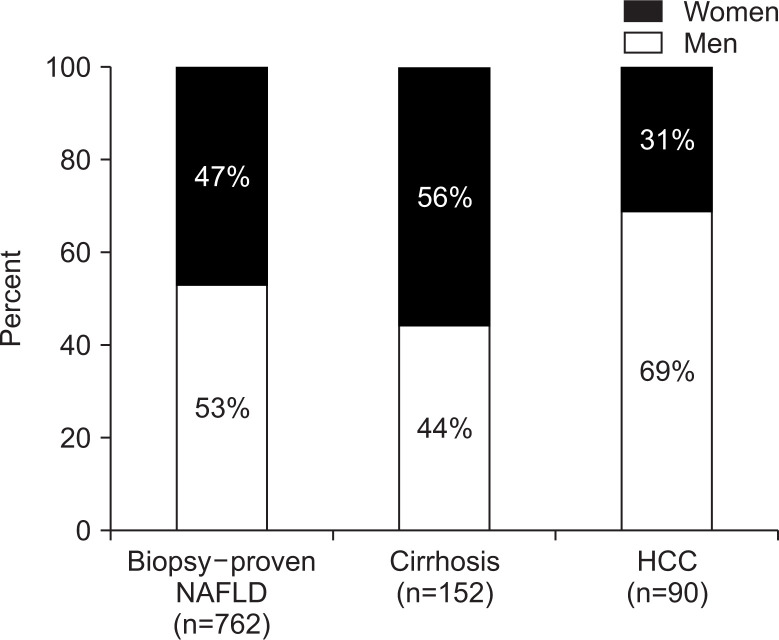
According to data from annual health checks performed in Japan, the prevalence of NAFLD among men is around 30% in all age groups above 30 years old.32 In contrast, it gradually increases from 7% among women in their 30s to 23% for women above 60 years old. In a series of 762 patients with biopsy-proven NAFLD diagnosed at our university hospital, males were predominant. The percentage of cirrhotic NAFLD was higher in women than in men (56% vs 44%), while the percentage of HCC was higher in men than in women (69% vs 31%) (Fig. 3). This difference in cirrhosis is probably due to the women being about 10 years older than men. As we mentioned, age is a risk factor for progression of fibrosis. The gender difference of HCC may be partly attributable to differences in exposure to the risk factors for this cancer, such as less alcohol consumption and smoking among women.37,95,96 However, it was reported that estrogen-mediated inhibition of interleukin 6 production explained the gender disparity of HCC in mouse models,97 so estrogen may also influence the pathogenesis of human HCC.
Fig. 3.
Percentage of male and female patients with a diagnosis of nonalcoholic fatty liver disease (NAFLD), cirrhosis, or hepatocellular carcinoma (HCC). Our hospital data showed a male predominance in all patients with NAFLD, although a female predominance was noted in patients with cirrhosis. A male predominance was noted in patients with HCC.
Yang et al.98 performed a cross-sectional study that investigated the influence of sex and menopause on the severity of liver fibrosis using a large single-center prospective database of 541 patients with a histologic diagnosis of NASH. After adjusting for covariates, the adjusted cumulative odd ratio for more severe fibrosis was 1.4 (95% CI, 0.9 to 2.1; p=0.17) among postmenopausal women and 1.6 (95% CI, 1.0 to 2.5; p=0.03) among men, using premenopausal women as the reference. Thus, they found that men had a higher risk of more severe fibrosis compared to women before menopause, while the severity of liver fibrosis was similar between postmenopausal women and men.
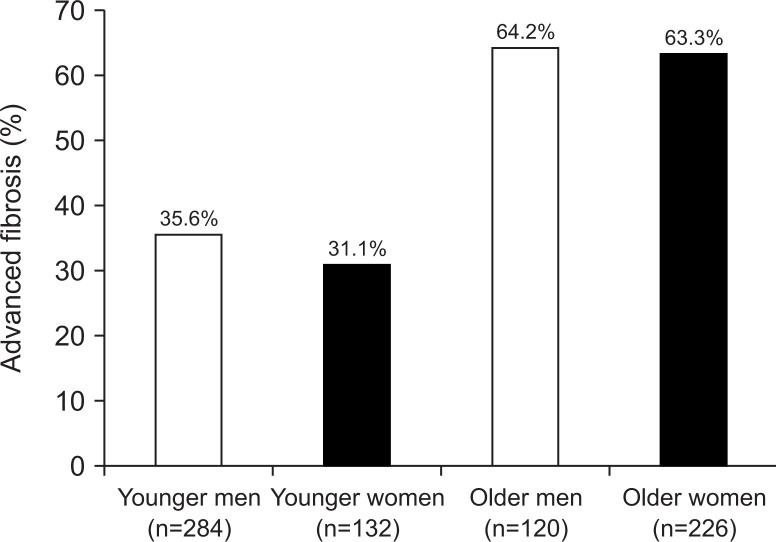
However, our data showed that sex had no influence on the severity of fibrosis when NASH patients were classified into younger or older groups for multivariate analysis.57 In fact, recent our data of NAFLD patients showed that advanced fibrosis in 36% of 284 younger men and 31% of 132 younger women, while advanced fibrosis existed in 64% of 120 older men and 63% of 226 older women (Fig. 4) These were very similar frequencies for both sexes. Therefore, further studies are needed to better understand the influence of sex on progression of fibrosis in NAFLD/NASH.
Fig. 4.
Percentage of nonalcoholic fatty liver disease patients with advanced fibrosis in the younger and older groups with respect to sex. The frequencies for both sexes were similar.
LEAN NAFLD
The prevalence of lean NAFLD varies due to differences in the definition of lean or nonobese NAFLD and differences in the method of diagnosis (imaging modalities or histology), with the reported prevalence ranging from approximately 10% to 30%.26,27,99-104 Previous studies have shown that patients with lean NAFLD usually have insulin resistance and higher plasma triglyceride levels compared to matched controls without NAFLD, although both of these changes are usually smaller than in patients with obese NAFLD. Histologically the prevalence of NASH and the severity of fibrosis do not differ significantly between nonobese and obese patients with NAFLD, but nonobese NAFLD patients have less severe steatosis. Unfortunately, no analyses stratified by sex were done in these studies, despite the features of NAFLD/NASH showing gender differences as mentioned above.
To investigate the features of male and female patients with nonobese NAFLD, we performed a cross-sectional single-center study of 762 patients with biopsy-proven NAFLD.27 The patients were classified into three groups according to the Japanese criteria for obesity, which were a nonobese group (BMI <25 kg/m2), an obese group (25 to 30 kg/m2), and a severely obese group (≥30 kg/m2).
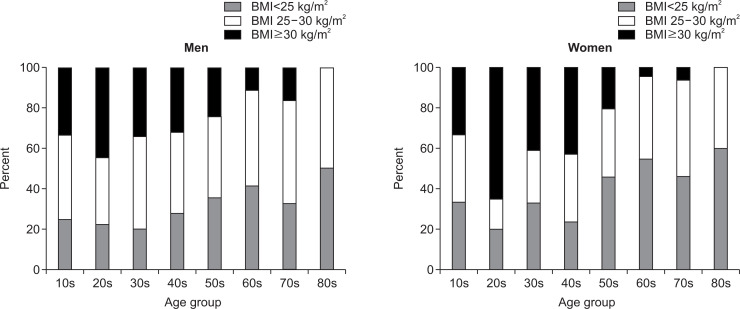
We found that 28.7% of the men and 39.1% of the women had nonobese NAFLD. The percentage of nonobese, obese and severely obese NAFLD patients clearly showed that severely obese patients were more common in the younger generation and nonobese patients were more common in the older generation in both genders (Fig. 5). Visceral fat obesity measured by computed tomography was found in 80% of the male patients and 87% of the female patients with nonobese NAFLD. Only 1.9% of male NAFLD patients and 0.6% of female NAFLD patients had no risk factors for NAFLD. The median age of the nonobese, obese, and severely obese men was 49.9 years, 46.8 years, and 40.5 years (p<0.01), respectively, while the women were aged 60.2 years, 59.6 years, and 48.5 years (p<0.01), respectively. Interestingly, the prevalence of metabolic comorbidities and PNPLA3 risk alleles did not differ among the three BMI groups in both genders. Also, the prevalence of NASH did not differ significantly among these groups, although nonobese patients of both sexes were more likely to had mild steatosis and low histological activity. Most of these findings were consistent with previous reports.26,99-103 Surprisingly, the prevalence of advanced fibrosis showed a marked difference between men and women. In men, advanced fibrosis was significantly more common among severely obese NAFLD patients (nonobese, 31.0%; obese, 41.6%; severely obese, 60.9%; p<0.01), even though the severely obese group was the youngest. However, the opposite was seen in women, and the prevalence of advanced fibrosis was significantly lower in severely obese female NAFLD patients than in obese or nonobese patients (nonobese, 51.4%; obese, 62.9%; severely obese, 33.7%; p<0.01). As in men, the severely obese group was youngest among women. Thus, obesity was associated with advanced liver fibrosis in men, but not in women. This difference may have arisen because a substantial proportion of severely obese women were premenopausal, and suggests that estrogen may have a much stronger influence on the development of fibrosis than obesity among females.
Fig. 5.
Percentage of nonobese, obese, and severely obese patients with nonalcoholic fatty liver disease with respect to age and sex.27 For both sexes, severely obese patients were more common in the younger generation, and nonobese patients were more common in the older generation. Data was obtained from Tokyo Women’s Medical University in 1991 to 2018 (n=762).
BMI, body mass index.
The nonobese group was the oldest of the three groups among both men and women. It is well known that elderly persons lose muscle mass (sarcopenia) and gain weight due to accumulation of fat. We clearly showed that the limb skeletal muscle mass and skeletal muscle index (skeletal muscle mass of the four limbs/height2 [kg/m2]) were lower in patients with nonobese NAFLD, suggesting that sarcopenia may be a risk factor for NAFLD among nonobese persons.
NAFLD/NASH occurred in nonobese subjects, especially in elderly persons, and was not milder than in obese subjects. While histological steatosis and activity were associated with BMI, the prevalence of NASH and advanced fibrosis were not. Prevalence of advanced fibrosis showed a significant sex difference.
CONCLUSION
Age and sex are important determinants of the clinical characteristics of NAFLD/NASH. Because the factors contributing to development and progression of NAFLD are complex, further large-scale studies of patients stratified by age, sex, ethnicity, and genetic status will be required to elucidate its pathogenesis and clinical features, in order to develop evidence-based tailored management strategies.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
References
- 1.Watanabe S, Hashimoto E, Ikejima K, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. J Gastroenterol. 2015;50:364–377. doi: 10.1007/s00535-015-1050-7. [DOI] [PubMed] [Google Scholar]
- 2.Wong VW, Chan WK, Chitturi S, et al. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33:70–85. doi: 10.1111/jgh.13857. [DOI] [PubMed] [Google Scholar]
- 3.Chitturi S, Wong VW, Chan WK, et al. The Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 2: management and special groups. J Gastroenterol Hepatol. 2018;33:86–98. doi: 10.1111/jgh.13856. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver (EASL), author; European Association for the Study of Diabetes (EASD), author; European Association for the Study of Obesity (EASO), author EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 6.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Kim HK, Park JY, Lee KU, et al. Effect of body weight and lifestyle changes on long-term course of nonalcoholic fatty liver disease in Koreans. Am J Med Sci. 2009;337:98–102. doi: 10.1097/MAJ.0b013e3181812879. [DOI] [PubMed] [Google Scholar]
- 8.Park SH, Jeon WK, Kim SH, et al. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21(1 Pt 1):138–143. doi: 10.1111/j.1440-1746.2005.04086.x. [DOI] [PubMed] [Google Scholar]
- 9.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 11.Dam-Larsen S, Becker U, Franzmann MB, Larsen K, Christoffersen P, Bendtsen F. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44:1236–1243. doi: 10.1080/00365520903171284. [DOI] [PubMed] [Google Scholar]
- 12.Söderberg C, Stål P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 13.Haflidadottir S, Jonasson JG, Norland H, et al. Long-term follow-up and liver-related death rate in patients with non-alcoholic and alcoholic related fatty liver disease. BMC Gastroenterol. 2014;14:166. doi: 10.1186/1471-230X-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 16.Tada T, Kumada T, Toyoda H, et al. Progression of liver fibrosis is associated with non-liver-related mortality in patients with nonalcoholic fatty liver disease. Hepatol Commun. 2017;1:899–910. doi: 10.1002/hep4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rafiq N, Bai C, Fang Y, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto E, Tokushige K. Prevalence, gender, ethnic variations, and prognosis of NASH. J Gastroenterol. 2011;46 Suppl 1:63–69. doi: 10.1007/s00535-010-0311-8. [DOI] [PubMed] [Google Scholar]
- 19.Lonardo A, Nascimbeni F, Ballestri S, et al. Sex differences in NAFLD: state of the art and identification of research gaps. Hepatology. 2019;70:1457–1469. doi: 10.1002/hep.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO Expert Consultation, author. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 21.Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372–379. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 22.Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001;9:381–387. doi: 10.1038/oby.2001.49. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson RN., Jr Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr. 1994;60:23–28. doi: 10.1093/ajcn/60.1.23. [DOI] [PubMed] [Google Scholar]
- 24.Abate N, Chandalia M. Risk of obesity-related cardiometabolic complications in special populations: a crisis in Asians. Gastroenterology. 2017;152:1647–1655. doi: 10.1053/j.gastro.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 25.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group, author. The metabolic syndrome: a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 26.Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15:474–485. doi: 10.1016/j.cgh.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 27.Tobari M, Hashimoto E, Taniai M, et al. Characteristics of non-alcoholic steatohepatitis among lean patients in Japan: not uncommon and not always benign. J Gastroenterol Hepatol. 2019;34:1404–1410. doi: 10.1111/jgh.14585. [DOI] [PubMed] [Google Scholar]
- 28.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 29.Singal AG, Manjunath H, Yopp AC, et al. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109:325–334. doi: 10.1038/ajg.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen J, Wong GL, Chan HL, et al. PNPLA3 gene polymorphism accounts for fatty liver in community subjects without metabolic syndrome. Aliment Pharmacol Ther. 2014;39:532–539. doi: 10.1111/apt.12609. [DOI] [PubMed] [Google Scholar]
- 31.Wei JL, Leung JC, Loong TC, et al. Prevalence and severity of nonalcoholic fatty liver disease in non-obese patients: a population study using proton-magnetic resonance spectroscopy. Am J Gastroenterol. 2015;110:1306–1314. doi: 10.1038/ajg.2015.235. [DOI] [PubMed] [Google Scholar]
- 32.Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003;38:954–961. doi: 10.1007/s00535-003-1178-8. [DOI] [PubMed] [Google Scholar]
- 33.Jimba S, Nakagami T, Takahashi M, et al. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005;22:1141–1145. doi: 10.1111/j.1464-5491.2005.01582.x. [DOI] [PubMed] [Google Scholar]
- 34.Eguchi Y, Hyogo H, Ono M, et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586–595. doi: 10.1007/s00535-012-0533-z. [DOI] [PubMed] [Google Scholar]
- 35.The Japan Society of Hepatology, author. Kankohennoseiinbetujittai 2018. Igakutoshoshuppan; Tokyo: 2012. [Google Scholar]
- 36.Tateishi R, Uchino K, Fujiwara N, et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011-2015 update. J Gastroenterol. 2019;54:367–376. doi: 10.1007/s00535-018-1532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: growing evidence of an epidemic? Hepatol Res. 2012;42:1–14. doi: 10.1111/j.1872-034X.2011.00872.x. [DOI] [PubMed] [Google Scholar]
- 38.Tokushige K, Hashimoto E, Horie Y, Taniai M, Higuchi S. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease, alcoholic liver disease, and chronic liver disease of unknown etiology: report of the nationwide survey. J Gastroenterol. 2011;46:1230–1237. doi: 10.1007/s00535-011-0431-9. [DOI] [PubMed] [Google Scholar]
- 39.Tokushige K, Hyogo H, Nakajima T, et al. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease and alcoholic liver disease: multicenter survey. J Gastroenterol. 2016;51:586–596. doi: 10.1007/s00535-015-1129-1. [DOI] [PubMed] [Google Scholar]
- 40.Yasui K, Hashimoto E, Komorizono Y, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428–433. doi: 10.1016/j.cgh.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 41.Tokushige K, Hashimoto E, Horie Y, Taniai M, Higuchi S. Hepatocellular carcinoma based on cryptogenic liver disease: the most common non-viral hepatocellular carcinoma in patients aged over 80 years. Hepatol Res. 2015;45:441–447. doi: 10.1111/hepr.12372. [DOI] [PubMed] [Google Scholar]
- 42.Taniai M, Hashimoto E, Tobari M, et al. Clinicopathological investigation of steatohepatitic hepatocellular carcinoma: a multicenter study using immunohistochemical analysis of adenoma-related markers. Hepatol Res. 2018;48:947–955. doi: 10.1111/hepr.13203. [DOI] [PubMed] [Google Scholar]
- 43.Kawada N, Imanaka K, Kawaguchi T, et al. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J Gastroenterol. 2009;44:1190–1194. doi: 10.1007/s00535-009-0112-0. [DOI] [PubMed] [Google Scholar]
- 44.Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14:124–131. doi: 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology. 2016;63:827–838. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 46.Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155:1828–1837. doi: 10.1053/j.gastro.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stine JG, Wentworth BJ, Zimmet A, et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther. 2018;48:696–703. doi: 10.1111/apt.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156:477–491. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim BH, Park JW. Epidemiology of liver cancer in South Korea. Clin Mol Hepatol. 2018;24:1–9. doi: 10.3350/cmh.2017.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 51.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149:1471–1482. doi: 10.1053/j.gastro.2015.07.056. [DOI] [PubMed] [Google Scholar]
- 52.Younossi ZM, Stepanova M, Rafiq N, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874–1882. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- 53.Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 54.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155:443–457. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 55.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 56.Hashimoto E, Tokushige K, Ludwig J. Diagnosis and classification of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: current concepts and remaining challenges. Hepatol Res. 2015;45:20–28. doi: 10.1111/hepr.12333. [DOI] [PubMed] [Google Scholar]
- 57.Yatsuji S, Hashimoto E, Tobari M, Tokushige K, Shiratori K. Influence of age and gender in Japanese patients with non-alcoholic steatohepatitis. Hepatol Res. 2007;37:1034–1043. doi: 10.1111/j.1872-034X.2007.00156.x. [DOI] [PubMed] [Google Scholar]
- 58.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 59.Ratziu V, Giral P, Charlotte F, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/S0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 60.Fan JG, Saibara T, Chitturi S, et al. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J Gastroenterol Hepatol. 2007;22:794–800. doi: 10.1111/j.1440-1746.2007.04952.x. [DOI] [PubMed] [Google Scholar]
- 61.Okanoue T, Umemura A, Yasui K, Itoh Y. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in Japan. J Gastroenterol Hepatol. 2011;26 Suppl 1:153–162. doi: 10.1111/j.1440-1746.2010.06547.x. [DOI] [PubMed] [Google Scholar]
- 62.Hashimoto E, Taniai M, Tokushige K. Characteristics and diagnosis of NAFLD/NASH. J Gastroenterol Hepatol. 2013;28 Suppl 4:64–70. doi: 10.1111/jgh.12271. [DOI] [PubMed] [Google Scholar]
- 63.Bechtold M, Palmer J, Valtos J, Iasiello C, Sowers J. Metabolic syndrome in the elderly. Curr Diab Rep. 2006;6:64–71. doi: 10.1007/s11892-006-0054-3. [DOI] [PubMed] [Google Scholar]
- 64.Wynne HA, Cope LH, Mutch E, Rawlins MD, Woodhouse KW, James OF. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology. 1989;9:297–301. doi: 10.1002/hep.1840090222. [DOI] [PubMed] [Google Scholar]
- 65.Premoli A, Paschetta E, Hvalryg M, Spandre M, Bo S, Durazzo M. Characteristics of liver diseases in the elderly: a review. Minerva Gastroenterol Dietol. 2009;55:71–78. [PubMed] [Google Scholar]
- 66.Gan L, Chitturi S, Farrell GC. Mechanisms and implications of age-related changes in the liver: nonalcoholic fatty liver disease in the elderly. Curr Gerontol Geriatr Res. 2011;2011:831536. doi: 10.1155/2011/831536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Floreani A. Liver diseases in the elderly: an update. Dig Dis. 2007;25:138–143. doi: 10.1159/000099478. [DOI] [PubMed] [Google Scholar]
- 68.Lee YH, Kim SU, Song K, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008-2011) Hepatology. 2016;63:776–786. doi: 10.1002/hep.28376. [DOI] [PubMed] [Google Scholar]
- 69.Bhanji RA, Narayanan P, Allen AM, Malhi H, Watt KD. Sarcopenia in hiding: the risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology. 2017;66:2055–2065. doi: 10.1002/hep.29420. [DOI] [PubMed] [Google Scholar]
- 70.Koo BK, Kim D, Joo SK, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123–131. doi: 10.1016/j.jhep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 71.Kagansky N, Levy S, Keter D, et al. Non-alcoholic fatty liver disease: a common and benign finding in octogenarian patients. Liver Int. 2004;24:588–594. doi: 10.1111/j.1478-3231.2004.0969.x. [DOI] [PubMed] [Google Scholar]
- 72.Koehler EM, Schouten JN, Hansen BE, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in the elderly: results from the Rotterdam study. J Hepatol. 2012;57:1305–1311. doi: 10.1016/j.jhep.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 73.Golabi P, Paik J, Reddy R, Bugianesi E, Trimble G, Younossi ZM. Prevalence and long-term outcomes of non-alcoholic fatty liver disease among elderly individuals from the United States. BMC Gastroenterol. 2019;19:56. doi: 10.1186/s12876-019-0972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong VW, Chu WC, Wong GL, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409–415. doi: 10.1136/gutjnl-2011-300342. [DOI] [PubMed] [Google Scholar]
- 75.Wang Z, Xu M, Hu Z, Hultström M, Lai E. Sex-specific prevalence of fatty liver disease and associated metabolic factors in Wuhan, south central China. Eur J Gastroenterol Hepatol. 2014;26:1015–1021. doi: 10.1097/MEG.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 76.Long MT, Pedley A, Massaro JM, et al. A simple clinical model predicts incident hepatic steatosis in a community-based cohort: the Framingham Heart Study. Liver Int. 2018;38:1495–1503. doi: 10.1111/liv.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou YJ, Li YY, Nie YQ, et al. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol. 2007;13:6419–6424. doi: 10.3748/wjg.v13.i47.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suzuki A, Abdelmalek MF. Nonalcoholic fatty liver disease in women. Womens Health (Lond) 2009;5:191–203. doi: 10.2217/17455057.5.2.191. [DOI] [PubMed] [Google Scholar]
- 79.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 80.Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014;35:8–30. doi: 10.1016/j.yfrne.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwartz RS. Obesity in the elderly: handbook of obesity. In: Bray GA, Bouchard C, James WPT, editors. Handbook of obesity. Marcel Dekker; New York: 1998. pp. 103–114. [Google Scholar]
- 82.Kotani K, Tokunaga K, Fujioka S, et al. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int J Obes Relat Metab Disord. 1994;18:207–212. [PubMed] [Google Scholar]
- 83.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55:950–954. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- 84.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Link JC, Reue K. Genetic basis for sex differences in obesity and lipid metabolism. Annu Rev Nutr. 2017;37:225–245. doi: 10.1146/annurev-nutr-071816-064827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29:719–727. doi: 10.1002/hep.510290307. [DOI] [PubMed] [Google Scholar]
- 87.Itagaki T, Shimizu I, Cheng X, et al. Opposing effects of oestradiol and progesterone on intracellular pathways and activation processes in the oxidative stress induced activation of cultured rat hepatic stellate cells. Gut. 2005;54:1782–1789. doi: 10.1136/gut.2004.053728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McKenzie J, Fisher BM, Jaap AJ, Stanley A, Paterson K, Sattar N. Effects of HRT on liver enzyme levels in women with type 2 diabetes: a randomized placebo-controlled trial. Clin Endocrinol (Oxf) 2006;65:40–44. doi: 10.1111/j.1365-2265.2006.02543.x. [DOI] [PubMed] [Google Scholar]
- 89.Fujihara Y, Hamanoue N, Yano H, et al. High sex hormone-binding globulin concentration is a risk factor for high fibrosis-4 index in middle-aged Japanese men. Endocr J. 2019;66:637–645. doi: 10.1507/endocrj.EJ18-0505. [DOI] [PubMed] [Google Scholar]
- 90.Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab. 1981;53:58–68. doi: 10.1210/jcem-53-1-58. [DOI] [PubMed] [Google Scholar]
- 91.Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol. 2009;5:673–681. doi: 10.1038/nrendo.2009.212. [DOI] [PubMed] [Google Scholar]
- 92.Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96:2341–2353. doi: 10.1210/jc.2011-0118. [DOI] [PubMed] [Google Scholar]
- 93.Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol. 2013;9:479–493. doi: 10.1038/nrendo.2013.122. [DOI] [PubMed] [Google Scholar]
- 94.Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361:1152–1163. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu EM, Wong LL, Hernandez BY, et al. Gender differences in hepatocellular cancer: disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma Res. 2018;4:66. doi: 10.20517/2394-5079.2018.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 97.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 98.Yang JD, Abdelmalek MF, Pang H, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:1406–1414. doi: 10.1002/hep.26761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cusi K. Nonalcoholic steatohepatitis in nonobese patients: not so different after all. Hepatology. 2017;65:4–7. doi: 10.1002/hep.28839. [DOI] [PubMed] [Google Scholar]
- 100.Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of nonalcoholic steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease and low visceral adiposity. J Hepatol. 2011;54:1244–1249. doi: 10.1016/j.jhep.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 101.Alam S, Gupta UD, Alam M, Kabir J, Chowdhury ZR, Alam AK. Clinical, anthropometric, biochemical, and histological characteristics of nonobese nonalcoholic fatty liver disease patients of Bangladesh. Indian J Gastroenterol. 2014;33:452–457. doi: 10.1007/s12664-014-0488-5. [DOI] [PubMed] [Google Scholar]
- 102.Akyuz U, Yesil A, Yilmaz Y. Characterization of lean patients with nonalcoholic fatty liver disease: potential role of high hemoglobin levels. Scand J Gastroenterol. 2015;50:341–346. doi: 10.3109/00365521.2014.983160. [DOI] [PubMed] [Google Scholar]
- 103.Leung JC, Loong TC, Wei JL, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65:54–64. doi: 10.1002/hep.28697. [DOI] [PubMed] [Google Scholar]
- 104.Kim D, Kim W, Joo SK, et al. Predictors of nonalcoholic steatohepatitis and significant fibrosis in non-obese nonalcoholic fatty liver disease. Liver Int. 2019;39:332–341. doi: 10.1111/liv.13983. [DOI] [PubMed] [Google Scholar]