Abstract
Background/Aims
Epigenetic change is one of the mechanisms that regulates the expression of microRNAs (miRNAs) and is known to play a role in Helicobacter pylori-associated gastric carcinogenesis. We aimed to evaluate the epigenetic changes of miR-200a/b in H. pylori-associated gastric carcinogenesis and restoration after eradication.
Methods
The expression and methylation levels of miR-200a/b were evaluated in gastric cancer (GC) cell lines, human gastric mucosa of H. pylori-negative and -positive controls, and H. pylori-positive GC patients. Next, the changes in the expression and methylation levels of miR-200a/b were compared between H. pylori-eradication and H. pylori-persistence groups at 6 months. Real-time reverse transcription-polymerase chain reaction was conducted to investigate the miRNA expression levels, and MethyLight was performed to assess the methylation levels.
Results
In the GC cell lines, the level of miR-200a/b methylation decreased and the level of expression increased after demethylation. In the human gastric mucosa, the miR-200a/b methylation levels increased in the following group order: H. pylori-negative control group, H. pylori-positive control group, and H. pylori-positive GC group. Conversely, the miR-200a/b expression levels decreased in the same order. In the H. pylori-persistence group, no significant changes were observed in the methylation and expression levels of miR-200a/b after 6 months, whereas the level of methylation decreased and the level of expression of miR-200a/b increased significantly 6 months in the H. pylori-eradication group.
Conclusions
Epigenetic alterations of miR-200a/b may be implicated in H. pylori-induced gastric carcinogenesis. This field defect for cancerization is suggested to be improved by H. pylori eradication.
Keywords: Helicobacter pylori, MicroRNAs, Methylation, Epigenetic alteration, Stomach neoplasm
INTRODUCTION
Gastric cancer (GC) is the fifth most common malignancy and the third leading cause of global cancer mortality.1 Although the incidence is declining globally, East Asia, including Korea and Japan, remains a region of high incidence of GC.2 It has been widely accepted that GC, especially intestinal-type GC, develops through progressive changes from chronic gastritis to gastric atrophy, intestinal metaplasia, dysplasia, and invasive carcinoma. Helicobacter pylori is thought to be crucial in the initiation of this sequence of changes in the Correa pathway.3 Despite several studies on the mechanism by which H. pylori leads to GC, this is not yet fully explained.
Recently, epigenetic changes have been attracting attention as one of the mechanisms of gastric carcinogenesis. One of the most consistent epigenetic changes in human cancer is aberrant DNA methylation, which has also been linked to gastric carcinogenesis.4 Importantly, previous reports have shown that methylation alterations of multiple genes occurs in both H. pylori infection and H. pylori-related gastric carcinogenesis.5,6 The accumulation of aberrant methylation through long-term H. pylori infection forms an epigenetic field defect that is susceptible to GC.7 Genes that are hypermethylated during H. pylori infection are not only tumor suppressor genes, but also those of non-coding RNAs, such as microRNAs (miRNAs).8 To date, more than 1,000 miRNAs have been identified in humans, and various miRNAs are implicated in the development of cancer.9 A growing number of studies point out that abnormal expression of miRNA has a crucial role in the initiation and progression of GC. For example, the expression of miR-1, -9, -34b, and -129, which have a tumor suppressor function, was downregulated in GC by hypermethylation of their promoter CpG islands.10,11 In contrast, miR-196a/b, an oncogenic miRNA, was upregulated in GC by hypomethylation of the promoter region.12,13
The miR-200 family is a group of miRNAs consisting of miR-200a, -200b, -200c, -141, and -429. Among them, miR-200b/a/429 are encoded by the gene on chromosome 1 and miR-200c/141 are encoded by the gene on chromosome 12.14 This family is closely linked to the expression of ZEB1 and ZEB2, key regulators of epithelial-mesenchymal transition, and regulates crucial processes in carcinogenesis, such as tumor initiation, progression, invasion, and metastasis of various types of cancer.14-16 The expression profiling of miR-200 family members is cancer-specific; downregulated in invasive bladder cancer and renal cell carcinoma, but overexpressed in lung, colorectal, and ovarian cancer.17-21 Several studies have demonstrated that miR-200 family were downregulated in GC, suggesting its role as a tumor suppressor in GC.16,22,23 Lower expression of miR-200 were known to be related to poor prognosis of GC: histological grade, size of tumor, invasion depth, lymphatic invasion, lymph node metastasis, intravascular cancer embolus, and disease-free survival.16,22,23 Although previous studies have shown that hypermethylation of the promoter CpG island was one of the mechanisms of miR-200c/141 downregulation, the exact mechanism of dysregulation of the remaining miR-200 members in GC has not been fully elucidated.23,24 To our knowledge, the methylation status and subsequent dysregulation of miR-200 family in the non-cancerous gastric mucosa of GC patients have not been studied. Furthermore, it has not yet been fully elucidated whether epigenetic alterations in the miR-200 family are affected by H. pylori infection and eradication.
Here, we examined whether epigenetic fields related to miR-200a/b were formed during H. pylori infection and gastric carcinogenesis, and recovered after the eradication of H. pylori.
MATERIALS AND METHODS
1. GC cell lines
Three GC cell lines, AGS, MKN-1, and MKN-45 were obtained from the Korean Cell Line Bank (Seoul, Korea) (Supplementary Table 1). Cells were incubated in RPMI-1640 medium containing 10% fetal bovine serum, L-glutamine (300 mg/L), 25 mM HEPES, and 25 mM NaHCO3, and plated on day 0. On day 1, cells were treated with 2 μM 5-Aza-2’-deoxycytidine (Sigma-Aldrich and Merck KGaA, Darmstadt, Germany), demethylating agent, and replenished daily with the demethylating agent and medium. Cells were harvested on day 4.
2. Gastric mucosa specimens
We included 40 patients with H. pylori-positive GC, 20 H. pylori-positive and 20 H. pylori-negative controls. The H. pylori-positive GC group consisted of patients who underwent endoscopic submucosal dissection for GC, and the control group consisted of those diagnosed as normal or gastritis by upper gastrointestinal endoscopy. All subjects included in the present study were older than 18 years of age, had no other malignancy, were not taking antibiotics nor proton pump inhibitors within the last 4 weeks, and did not report history of H. pylori eradication. During endoscopy, gastric mucosa specimens were harvested from two sites in the antrum and two sites in the corpus (in case of GC patients, non-cancerous tissues were collected) for histological evaluation according to the updated Sydney System: glandular atrophy, intestinal metaplasia, neutrophils and mononuclear cells infiltration, and H. pylori.25 Additional two samples of gastric mucosa were harvested from the antrum for miRNA-related analysis and restored at –80°C. H. pylori infection was considered positive if the histological (hematoxylin-eosin and modified Giemsa staining) or rapid urease test (Delta West Ltd., Bentley, Australia) was positive. The patients with H. pylori-positive GC were randomly allocated to the eradication or persistence group. The patients in the eradication group underwent 1-week of therapy (omeprazole 20 mg, amoxicillin 1 g, and clarithromycin 500 mg twice daily) 2 weeks after endoscopic resection. To determine the effect of H. pylori eradication on the epigenetic regulation of miRNA expression, we also collected gastric mucosal tissues at 6 months after endoscopic submucosal dissection. This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB number: H-1507-112-690) and was performed in accordance with the Helsinki Declaration. All participants signed written informed consent before participation.
3. RNA extraction and quantitative reverse transcription-polymerase chain reaction
miRNAs were isolated from cell lines and gastric tissues preserved at –80°C using mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA) according to the manufacturer’s instructions. Complementary DNA was synthesized from reverse transcription of the miRNAs using TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Darmstadt, Germany). Quantitative reverse transcription-polymerase chain reaction was carried out using 2 μL of complementary DNA in a total mix of 20 μL containing 10 μL of TaqMan Universal Master Mix II (Applied Biosystems) and analyzed on an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Human GAPDH gene served as an internal control and the relative expression levels of miRNAs were calculated using the comparative 2–ΔΔCt method.26 All samples were tested in triplicate.
4. DNA extraction, bisulfite conversion, and methylation analysis
DNA was isolated from the cell lines and tissues using the LaboPassTM Blood Mini kit (Cosmogenetech, Seoul, Korea) following the manufacturer’s instructions. Bisulfite conversion was performed on 1 μg of genomic DNA using the EZ DNA Methylation Kit (Zymo Research, Orange, CA, USA), which convert unmethylated cytosine to uracil.
Methylation status of the bisulfite-modified miRNA promoters was analyzed using real-time PCR-based MethyLight assay.27,28 Pairs of primers and probes were designed by the software, Beacon Designer (PREMIER Biosoft International, Palo Alto, CA, USA) (Supplementary Table 2). In both miR-200a and miR-200b, six CpG sites in the promoter region were included in the methylation analysis (Supplementary Fig. 1). Levels of DNA methylation were reported as a percentage of methylated reference (PMR), which was calculated as PMR=100×[(methylated reaction/ALU)sample/(methylated reactoin/ALU)M.SssI]. The MethyLight assay was also performed in triplicate.
5. Statistical analysis
The Kruskal-Wallis test was recruited for the overall comparison of continuous variables of the three groups (H. pylori-negative controls, H. pylori-positive controls, and H. pylori-positive GC) since the data were not normally distributed. The Mann–Whitney U test was then used for pairwise group comparisons. The chi-square or Fisher exact tests were recruited for the analysis of the categorical variables. The ranked analysis of covariance model was applied to examine differences in the level of methylation and miRNA expression after correcting for baseline imbalances. The Wilcoxon signed-rank test was used for comparison between before and after eradication for H. pylori-eradication and H. pylori-persistence groups. Differences were considered statistically significant at p<0.05. Statistical analyses were performed using IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
RESULTS
1. miR-200a/b methylation and expression in GC cell lines
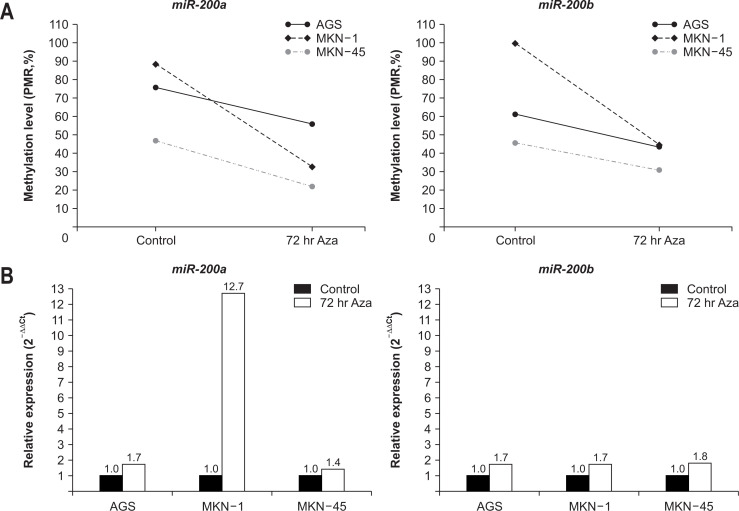
We measured the levels of miRNA expression and DNA methylation in the GC cell lines, AGS, MKN-1, and MKN-45. To assess the role of methylation in the expression of miRNAs, we examined their expression in three GC cell lines before and after the treatment with the demethylating agent, 5-Aza-2’-deoxycytidine. The levels of miR-200a methylation decreased by 25% in AGS, 63% in MKN-1, and 53% in MKN-45 after 72 hours of demethylation treatment (Fig. 1A). The expression of miR-200a increased by 1.7-fold in AGS, 12.7-fold in MKN-1, and 1.4-fold in MKN-45 after 72 hours of demethylation treatment (Fig. 1B). The promoter methylation levels of miR-200b also decreased by 29% in AGS, 55% in MKN-1, and 33% in MNK-45, and the expression of miR-200b increased by 1.7-fold in AGS and MKN-1, and 1.8-fold in MKN-45 after demethylation treatment (Fig. 1).
Fig. 1.
Changes in promoter DNA methylation and microRNA (miRNA) expression following demethylating treatment in gastric cancer (GC) cell lines. (A) Promoter DNA methylation levels. The methylation levels of the promoter region of miR-200a and miR-200b was decreased in three GC cell lines after 72 hours of treatment with the demethylating agent 5-Aza-2’-deoxycytidine. (B) Relative expression levels. After 72 hours of treatment with the demethylating agent, the expression levels of both miR-200a and miR-200b increased in three GC cell lines.
PMR, percentage of methylated reference.
2. Clinicopathological characteristics of subjects
In clinicopathological characteristics, the patients with H. pylori-positive GC were significantly older than H. pylori-positive and -negative controls (median, 66.5 vs 57.0 and 56.5, both p<0.001). The proportion of males was significantly higher in the H. pylori-positive GC group than that in the H. pylori-positive and -negative control groups (65.0% vs 20.0% and 35.0%, p=0.002 and p=0.028, respectively). In pathological characteristics, the degree of mucosal atrophy was more severe in the H. pylori-positive GC group than that in the H. pylori-negative control group (p=0.019), whereas no significant difference was seen between the H. pylori-positive GC and H. pylori-positive control groups. The degree of intestinal metaplasia was significantly more severe in the H. pylori-positive GC group than that in the H. pylori-positive and -negative control groups (p=0.002 and p=0.007, respectively) (Table 1).
Table 1.
Clinicopathological Characteristics of the Subjects
| Variable |
H. pylori-positive GCs (n=40) |
H. pylori-positive controls (n=20) |
p-value* |
H. pylori-negative controls (n=20) |
p-value† |
|---|---|---|---|---|---|
| Age, yr | 66.5 (59.3–72.0) | 57.0 (48.0–61.0) | <0.001 | 56.5 (47.3–61.8) | <0.001 |
| Male sex | 26 (65.0) | 4 (20.0) | 0.002 | 7 (35.0) | 0.028 |
| Mucosal atrophy | 0.077 | 0.019 | |||
| Absent to mild | 24 (60.0) | 17 (85.0) | 18 (90.0) | ||
| Moderate to severe | 16 (40.0) | 3 (15.0) | 2 (10.0) | ||
| Intestinal metaplasia | 0.002 | 0.007 | |||
| Absent to mild | 17 (42.5) | 17 (85.0) | 16 (80.0) | ||
| Moderate to severe | 23 (57.5) | 3 (15.0) | 4 (20.0) | ||
| Neutrophil | 1.000 | <0.001 | |||
| Absent to mild | 1 (2.5) | 1 (5.0) | 19 (95.0) | ||
| Moderate to severe | 39 (97.5) | 19 (95.0) | 1 (5.0) | ||
| Monocyte | 0.595 | <0.001 | |||
| Absent to mild | 2 (5.0) | 2 (10.0) | 18 (90.0) | ||
| Moderate to severe | 38 (95.0) | 18 (90.0) | 2 (10.0) |
Data are presented as the median (interquartile range) or number (%).
H. pylori, Helicobacter pylori; GC, gastric cancer.
*Comparison between the H. pylori-positive GCs group and the H. pylori-positive controls; †Comparison between the H. pylori-positive GCs group and the H. pylori-negative controls.
3. miR-200a/b methylation and expression in gastric mucosa according to H. pylori infection and disease state
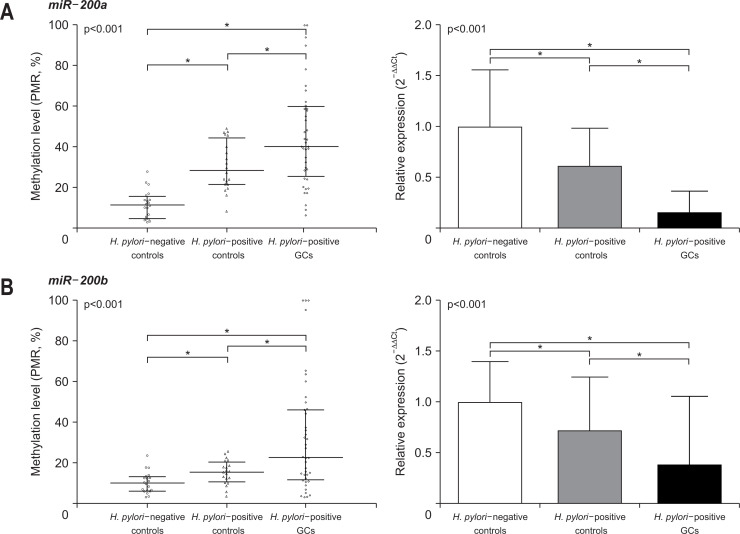
We measured the level of methylation and corresponding miRNA expression in the gastric mucosa of the three groups. In the MethyLight assay, the promoter DNA methylation level of miR-200a was lowest in the H. pylori-negative control group, followed by the H. pylori-positive control group, and then the H. pylori-positive GC group (median, 11.5 vs 28.4 vs 40.3, all p<0.001) (Fig. 2). On the other hand, the expression level of miR-200a was highest in the H. pylori-negative control group, and significantly decreased in the H. pylori-positive control (0.6-fold that of the H. pylori-negative control) and H. pylori-positive GC groups (0.1-fold that of the H. pylori-negative control). miR-200b also followed the same pattern of promoter methylation and expression as miR-200a. The promoter methylation level of miR-200b increased gradually in the H. pylori-negative control, H. pylori-positive control, and H. pylori-positive GC groups (median, 9.9 vs 15.3 vs 22.6, all p<0.001) (Fig. 2B). The expression levels of miR-200b decreased significantly in the H. pylori-negative control, H. pylori-positive control (0.7-fold that of the H. pylori-negative control), and H. pylori-positive GC groups (0.4-fold that of the H. pylori-negative control). We compared miR-200a/b promoter methylation and expression levels according to the subtypes of GC in the H. pylori-positive GC group, but failed to find any significant correlations (Supplementary Table 3).
Fig. 2.
The levels of promoter DNA methylation and corresponding miRNA expression in the study groups. The levels of miR-200a/b methylation are presented as medians and interquartile ranges. The levels of miR-200a/b expression are shown as fold changes relative to the Helicobacter pylori-negative controls and standard deviations. (A) miR-200a. The promoter levels of miR-200a (left) methylation were lowest in the H. pylori-negative controls, followed by the H. pylori-positive controls and then the H. pylori-positive GCs (all p<0.001). The miR-200a expression (right) level was highest in the H. pylori-negative controls, followed by the H. pylori-positive controls and then the H. pylori-positive GCs (all p<0.001). (B) miR-200b. miR-200b showed the same pattern as miR-200a for promoter DNA methylation and expression.
miRNA, microRNA; PMR, percentage of methylated reference; GC, gastric cancer. *p<0.001.
4. miR-200a/b methylation and expression in gastric mucosa with adjustment of baseline imbalance
Because of the significant difference in age and sex in the baseline characteristics of each group, further analysis was needed with adjustment of these variables. Promoter methylation and miRNA expression of miRNAs were analyzed using a non-parametric ranked analysis of covariance model with group as a factor, and age and sex as covariates. In this model, the levels of promoter methylation (p=0.001) and expression of miR-200a (p=0.003) were shown to be significantly different among groups after adjustment of age and sex (Table 2). Age and sex were not significantly associated with the promoter methylation levels and miR-200a expression. Similarly, the levels of promoter methylation (p<0.001) and expression of miR-200b (p=0.001) in each group were significantly different after adjustment of age and sex. Regarding miR-200b, age and sex were not significantly associated with the promoter methylation levels and miRNA expression.
Table 2.
Differences in the Promoter Methylation and Expression Levels of miRNAs among the Groups after Adjustment for Covariates
| Variable | miR-200a | miR-200b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Promoter methylation | miRNA expression | Promoter methylation | miRNA expression | |||||||||
| df | F | p-value | df | F | p-value | df | F | p-value | df | F | p-value | |
| Age | 1 | 0.482 | 0.490 | 1 | 0.016 | 0.900 | 1 | 0.004 | 0.952 | 1 | 0.565 | 0.455 |
| Sex | 1 | 0.709 | 0.402 | 1 | 1.175 | 0.282 | 1 | 0.329 | 0.568 | 1 | 1.343 | 0.250 |
| Group | 2 | 8.104 | 0.001 | 2 | 6.468 | 0.003 | 2 | 8.977 | <0.001 | 2 | 8.415 | 0.001 |
miRNA, microRNA; df, degree of freedom; F, variance ratio.
5. Effect of H. pylori eradication on the methylation and expression of miR-200a/b
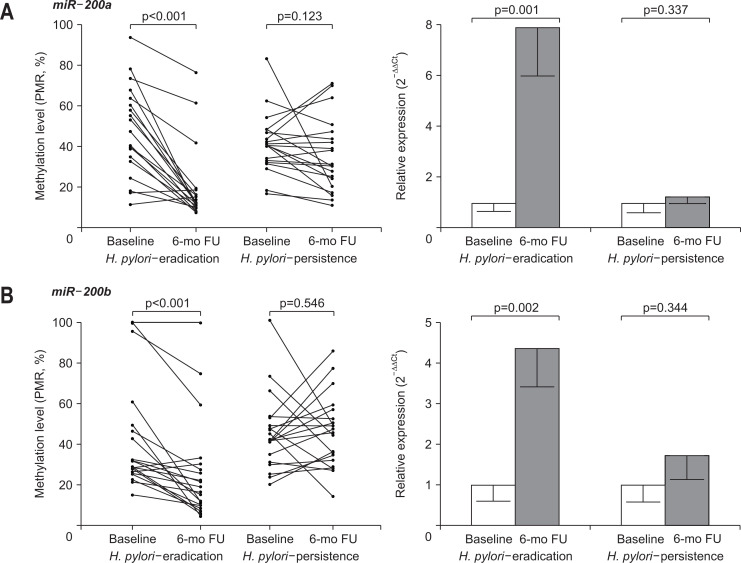
In 40 patients with H. pylori-positive GC, half of them received H. pylori eradication therapy (H. pylori-eradication group), and the other half did not receive eradication therapy (H. pylori-persistence group). In all 20 patients in the H. pylori-eradication group, H. pylori was confirmed to have been successfully eradicated. No significant difference was observed in baseline clinicopathological characteristics between the two groups (Table 3). Promoter methylation and expression levels in the non-cancerous gastric mucosa of H. pylori-positive GC patients were compared before and after 6 months of H. pylori eradication. In the H. pylori-eradication group, the promoter methylation level of miR-200a decreased significantly, and the expression level of miR-200a increased significantly (7.9-fold of baseline) at 6 months after H. pylori eradication, whereas the methylation and expression levels of miR-200a in the H. pylori-persistence group were not significantly different during follow-up at 6 months (Fig. 3A). In the H. pylori-eradication group, there was a significant decrease in the promoter methylation level and a significant increase in the expression level (4.4-fold of baseline) of miR-200b after 6 months compared with baseline. In the H. pylori-persistence group, there were no significant changes in the promoter methylation and expression levels of miR-200b between baseline and 6 months (Fig. 3B).
Table 3.
Clinicopathological Characteristics of the Helicobacter pylori-Eradiation and -Persistence Groups at Baseline and the 6-Month Follow-up
| Characteristic | Baseline | 6-Month follow-up | ||||
|---|---|---|---|---|---|---|
|
H. pylori-eradication (n=20) |
H. pylori-persistence (n=20) |
H. pylori-eradication (n=20) |
H. pylori-persistence (n=20) |
p-value* | ||
| Age, yr | 70.0 (56.5–72.8) | 65.0 (60.3–70.8) | - | - | ||
| Male sex | 12 (60.0) | 14 (70.0) | - | - | ||
| Mucosal atrophy | 0.327 | |||||
| Absent to mild | 10 (50.0) | 14 (70.0) | 14 (70.0) | 11 (55.0) | ||
| Moderate to severe | 10 (50.0) | 6 (30.0) | 6 (30.0) | 9 (45.0) | ||
| Intestinal metaplasia | 0.519 | |||||
| Absent to mild | 6 (30.0) | 11 (55.0) | 7 (35.0) | 9 (45.0) | ||
| Moderate to severe | 14 (70.0) | 9 (45.0) | 13 (65.0) | 11 (55.0) | ||
| Neutrophil | <0.001 | |||||
| Absent to mild | 1 (5.0) | 0 | 17 (85.0) | 2 (10.0) | ||
| Moderate to severe | 19 (95.0) | 20 (100.0) | 3 (15.0) | 18 (90.0) | ||
| Monocyte | 0.001 | |||||
| Absent to mild | 1 (5.0) | 1 (5.0) | 9 (45.0) | 0 | ||
| Moderate to severe | 19 (95.0) | 19 (95.0) | 11 (55.0) | 20 (100.0) | ||
Data are presented at the median (interquartile range) or number (%).
*Comparison between the 6-month follow-up results in the H. pylori-eradication and H. pylori-persistence groups.
Fig. 3.
Change in promoter DNA methylation and corresponding microRNA (miRNA) expression according to the Helicobacter pylori eradication therapy. (A) miR-200a and (B) miR-200b. In the H. pylori-eradication group, there was a significant difference in the promoter methylation and expression levels of miR-200a/b at 6 months after eradication.
FU, follow-up.
DISCUSSION
In this study, the level of DNA methylation might influence the level of miR-200a/b expression in GC cell lines. In addition, aberrant DNA methylation of miR-200a/b was observed in H. pylori-infected gastric tissue, which was also found in non-cancerous gastric tissue of patients with H. pylori-positive GC. The results indicate that H. pylori infection might affect the promoter methylation of miR-200a/b, alter the level of expression of miRNAs, and eventually form an epigenetic field for cancerization. H. pylori eradication has been shown to have the potential to improve these epigenetic changes and recover the expression of miR-200a/b. To our best knowledge, this research is the first to show that the regulation of miR-200a/b expression via promoter methylation may be an important mechanism for H. pylori to form the epigenetic field of GC, which can be recovered after eradication therapy.
Recently, epigenetic changes in cancer-related genes, such as tumor suppressor genes, have been proposed to be one of the important processes in H. pylori-associated gastric carcinogenesis.5,6 DNA methylation is one of the epigenetic mechanisms that has been known to occur not only in the promoters of protein-coding genes but also non-coding genes, such as miRNAs.10-13 When aberrant DNA methylation accumulates in normal-appearing gastric mucosal tissues due to prolonged H. pylori infection, these epigenetic alterations form an epigenetic field for cancerization that is susceptible to GC.7,29 In previous studies, miR-133a and Wnt antagonist genes were epigenetically silenced by promoter methylation in patients with H. pylori-associated GC.30 Considering that endoscopic resection has been a standard treatment for early type of GC, the importance of epigenetic field formed in residual stomach has been highlighted.31 To date, most of the studies on the expression of miRNA in GC patients have investigated the expression levels of miRNA in GC tissues, compared to the matched non-cancerous tissues of the same patient.32,33 There have been a few studies comparing the epigenetic status of non-cancerous gastric tissues of GC patients and normal gastric tissues of non-predisposed subjects, which might explore the risk of second primary GC after endoscopic resection of the primary one.
The miR-200 family is one of the most studied miRNA family in many carcinomas. The miR-200 family expression has been well known to be dysregulated in various cancers, including renal cell carcinoma, bladder cancer, colorectal cancer, and ovarian cancer.17,18,20,21 In GC, miR-200 family acts as tumor suppressor and lower expression of these members has been reported to be related to poor prognosis of GC.16,22,23 Although the mechanisms controlling the expression of the miR-200 family have been partially explained by DNA methylation and histone changes, they have not yet been fully understood.23,34,35
The present study revealed that miR-200a/b expression might be epigenetically regulated both in vitro and in vivo. The level of promoter methylation of miR-200a/b decreased and the expression of miR-200a/b increased after demethylating treatment in three GC cell lines. In human gastric mucosa, promoter methylation increased gradually in the order of H. pylori-negative control, H. pylori-positive control, and H. pylori-positive GC group, while miR-200a/b expression was gradually downregulated in the same order. These changes were significant even after adjustment of the covariates, such as age and sex. The present study highlights the role of miR-200a/b as a potential tumor suppressor in the initiation of H. pylori-related GC and suggests that methylation-dependent regulation may be an important mechanism to control its expression.
In this study, the eradication of H. pylori resulted in a decrease in the promoter methylation levels of miR-200a/b and an increase in the expression of these miRNAs in the H. pylori-positive GC group. Also, H. pylori eradication could prevent further progression of an epigenetic field for cancerization and restore the epigenetic changes that have already occurred during chronic infection. These findings might include one of the molecular mechanisms that explain the results of previous studies that the eradication of H. pylori reduced the incidence of metachronous GC and GC-related deaths.36,37 It is remarkable that the promoter methylation levels of miR-200a/b in the H. pylori-eradication group were still higher than those in the H. pylori-negative control at 6 months follow-up, which might be explained by the fact that aberrant methylation in H. pylori-infected gastric epithelial cells decreases via cell turnover after the successful eradication, while that in H. pylori-infected gastric stem cells might persist even after eradicating H. pylori.38
We demonstrated that the degree of neutrophil and monocyte infiltration decreased significantly at 6 months after eradication in the H. pylori-eradication group, but remained at a similar level in the H. pylori-persistence group. Previous studies that analyzed the mechanism of aberrant DNA methylation caused by the infection of H. pylori have suggested that infection-associated inflammatory response was a critical factor. The infection of H. pylori has been known to influence the release of many pro-inflammatory cytokines, including tumor necrosis factor-α, interleukin-1, 6, and 8, and nuclear factor κB, and also trigger a T1 helper cell inflammatory response.39 These inflammatory dysregulations are considered to have a critical role in gastric inflammation and carcinogenesis.38 In this study, the eradication of H. pylori led to a decrease in inflammatory response in gastric epithelial cells and further might reduce aberrant DNA methylation. Further researches are required to confirm the direct effect of H. pylori eradication on the cellular level, i.e., expression and methylation changes of miR-200a/b in the gastric epithelial cell itself.
In this study, the methylation levels of miR-200a/b were highly variable in the H. pylori-positive GC group, whereas those in the H. pylori-negative and -positive control groups were limited to a relatively narrow range (Supplementary Table 4). Similar results were obtained in previous studies that quantitatively analyzed the level of DNA methylation.5,30 It has been suggested that aberrant promoter methylation may occur only in a fraction of cells in non-cancerous tissues.5 Additional studies are needed to determine whether the levels of methylation vary depending on the location within the same individual.
This study has several strengths. To date, this is the first to demonstrate promoter methylation and subsequent dysregulation of miR-200a/b in non-cancerous gastric mucosa of patients with H. pylori-positive GC. In addition, we showed that the epigenetic alteration of gastric tissues could be recovered through H. pylori eradication even after the development of GC, which might suggest the need for re-discussion of the concept of “point of no return” in H. pylori eradication. Second, we used reverse transcription-polymerase chain reaction and MethyLight techniques to analyze miRNA expression and promoter methylation, which enabled sensitive and accurate quantitative analysis.
This study also has some limitations. First, a small number of patients were included for analysis. Nevertheless, the differences between the groups were large enough to reach statistical significance. In subgroup analysis of H. pylori-positive GC group, no significant association was found between characteristics of GC and levels of miR-200a/b expression and methylation. Further studies with large numbers of patients may be warranted. Second, there were differences in baseline characteristics, such as age and sex among the groups. We overcame the mismatches by performing further analysis with correction of these variables. Third, we did not include GC tissues in this study, which did not allow to compare between paired GC tissues and non-cancerous gastric mucosal tissues. Lastly, we did not include a study of target genes or functional analysis of miR-200a/b. ZEB1 and ZEB2 are one of potential target genes of miR-200a/b, which are known to be closely related to tumor growth and metastasis by engaging in epithelial-mesenchymal transition processes.15,16 Subsequent studies are needed on the association between methylation changes in miR-200a/b and the expression and function changes in target genes, and, further, significance in the gastric carcinogenesis process.
In conclusion, aberrant DNA methylation of miR-200a/b might be associated with the infection of H. pylori and contributed to the formation of an epigenetic field for GC, which could be recovered by H. pylori eradication. Therefore, the eradication of H. pylori should be emphasized to prevent the development of metachronous tumor in non-cancerous gastric mucosa even in patients with GC.
Supplemental Materials
ACKNOWLEDGEMENTS
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A1B03036304); and the SNUH research fund (grant number: 03-2016-0310).
The authors thank Ji Hee Kim (Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea) for technical assistance.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study conception and design, data acquisition/analysis/interpretation, manuscript drafting: J.M.C., S.G.K., N.Y.C. Statistical analysis: J.M.C., H.J.Y. Critical revision of the manuscript: H.J.Y., J.H.L., N.Y.C., W.H.K., J.S.K., H.C.J. Obtained funding, study supervision: S.G.K. All authors read and approved the final manuscript.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Shin A, Kim J, Park S. Gastric cancer epidemiology in Korea. J Gastric Cancer. 2011;11:135–140. doi: 10.5230/jgc.2011.11.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process: First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 4.Ushijima T, Sasako M. Focus on gastric cancer. Cancer Cell. 2004;5:121–125. doi: 10.1016/S1535-6108(04)00033-9. [DOI] [PubMed] [Google Scholar]
- 5.Maekita T, Nakazawa K, Mihara M, et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12(3 Pt 1):989–995. doi: 10.1158/1078-0432.CCR-05-2096. [DOI] [PubMed] [Google Scholar]
- 6.D'Angelo E, Vicentini C, Agostini M, et al. MicroRNAs as tools and effectors for patient treatment in gastrointestinal carcinogenesis. Curr Drug Targets. 2015;16:383–392. doi: 10.2174/1389450116666141210091454. [DOI] [PubMed] [Google Scholar]
- 7.Ramachandran K, Singal R. DNA methylation and field cancerization. Epigenomics. 2012;4:243–245. doi: 10.2217/epi.12.12. [DOI] [PubMed] [Google Scholar]
- 8.Ando T, Yoshida T, Enomoto S, et al. DNA methylation of microRNA genes in gastric mucosae of gastric cancer patients: its possible involvement in the formation of epigenetic field defect. Int J Cancer. 2009;124:2367–2374. doi: 10.1002/ijc.24219. [DOI] [PubMed] [Google Scholar]
- 9.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Chen WS, Leung CM, Pan HW, et al. Silencing of miR-1-1 and miR-133a-2 cluster expression by DNA hypermethylation in colorectal cancer. Oncol Rep. 2012;28:1069–1076. doi: 10.3892/or.2012.1899. [DOI] [PubMed] [Google Scholar]
- 11.Tsai KW, Liao YL, Wu CW, et al. Aberrant hypermethylation of miR-9 genes in gastric cancer. Epigenetics. 2011;6:1189–1197. doi: 10.4161/epi.6.10.16535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng S, Kuang Z, Sheng C, Zhang Y, Xu H, Cheng Q. Association of microRNA-196a-2 gene polymorphism with gastric cancer risk in a Chinese population. Dig Dis Sci. 2010;55:2288–2293. doi: 10.1007/s10620-009-1007-x. [DOI] [PubMed] [Google Scholar]
- 13.Tsai KW, Hu LY, Wu CW, et al. Epigenetic regulation of miR-196b expression in gastric cancer. Genes Chromosomes Cancer. 2010;49:969–980. doi: 10.1002/gcc.20804. [DOI] [PubMed] [Google Scholar]
- 14.Bojmar L, Karlsson E, Ellegård S, et al. The role of microRNA-200 in progression of human colorectal and breast cancer. PLoS One. 2013;8:e84815. doi: 10.1371/journal.pone.0084815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song F, Yang D, Liu B, et al. Integrated microRNA network analyses identify a poor-prognosis subtype of gastric cancer characterized by the miR-200 family. Clin Cancer Res. 2014;20:878–889. doi: 10.1158/1078-0432.CCR-13-1844. [DOI] [PubMed] [Google Scholar]
- 16.Kurashige J, Kamohara H, Watanabe M, et al. MicroRNA-200b regulates cell proliferation, invasion, and migration by directly targeting ZEB2 in gastric carcinoma. Ann Surg Oncol. 2012;19 Suppl 3:S656–S664. doi: 10.1245/s10434-012-2217-6. [DOI] [PubMed] [Google Scholar]
- 17.Wiklund ED, Bramsen JB, Hulf T, et al. Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int J Cancer. 2011;128:1327–1334. doi: 10.1002/ijc.25461. [DOI] [PubMed] [Google Scholar]
- 18.Duns G, van den Berg A, van Dijk MC, et al. The entire miR-200 seed family is strongly deregulated in clear cell renal cell cancer compared to the proximal tubular epithelial cells of the kidney. Genes Chromosomes Cancer. 2013;52:165–173. doi: 10.1002/gcc.22016. [DOI] [PubMed] [Google Scholar]
- 19.Liu XG, Zhu WY, Huang YY, et al. High expression of serum miR-21 and tumor miR-200c associated with poor prognosis in patients with lung cancer. Med Oncol. 2012;29:618–626. doi: 10.1007/s12032-011-9923-y. [DOI] [PubMed] [Google Scholar]
- 20.Cristóbal I, Rincón R, Manso R, et al. Deregulation of miR-200b, miR-200c and miR-429 indicates its potential relevant role in patients with colorectal cancer liver metastasis. J Surg Oncol. 2014;110:484–485. doi: 10.1002/jso.23661. [DOI] [PubMed] [Google Scholar]
- 21.Bendoraite A, Knouf EC, Garg KS, et al. Regulation of miR-200 family microRNAs and ZEB transcription factors in ovarian cancer: evidence supporting a mesothelial-to-epithelial transition. Gynecol Oncol. 2010;116:117–125. doi: 10.1016/j.ygyno.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang L, Guo F, Huo B, Lv Y, Wang Y, Liu W. Expression and clinical significance of the microRNA-200 family in gastric cancer. Oncol Lett. 2015;9:2317–2324. doi: 10.3892/ol.2015.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou X, Wang Y, Shan B, et al. The downregulation of miR-200c/141 promotes ZEB1/2 expression and gastric cancer progression. Med Oncol. 2015;32:428. doi: 10.1007/s12032-014-0428-3. [DOI] [PubMed] [Google Scholar]
- 24.Batista L, Bourachot B, Mateescu B, Reyal F, Mechta-Grigoriou F. Regulation of miR-200c/141 expression by intergenic DNA-looping and transcriptional read-through. Nat Commun. 2016;7:8959. doi: 10.1038/ncomms9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis: the updated Sydney System: International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Trinh BN, Long TI, Laird PW. DNA methylation analysis by MethyLight technology. Methods. 2001;25:456–462. doi: 10.1006/meth.2001.1268. [DOI] [PubMed] [Google Scholar]
- 28.Eads CA, Danenberg KD, Kawakami K, et al. MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000;28:E32. doi: 10.1093/nar/28.8.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ushijima T. Epigenetic field for cancerization. J Biochem Mol Biol. 2007;40:142–150. doi: 10.5483/BMBRep.2007.40.2.142. [DOI] [PubMed] [Google Scholar]
- 30.Lim JH, Kim SG, Choi JM, Yang HJ, Kim JS, Jung HC. Helicobacter pylori is associated with miR-133a expression through promoter methylation in gastric carcinogenesis. Gut Liver. 2018;12:58–66. doi: 10.5009/gnl17263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331–336. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 32.Yang Q, Zhang RW, Sui PC, He HT, Ding L. Dysregulation of non-coding RNAs in gastric cancer. World J Gastroenterol. 2015;21:10956–10981. doi: 10.3748/wjg.v21.i39.10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan HW, Li SC, Tsai KW. MicroRNA dysregulation in gastric cancer. Curr Pharm Des. 2013;19:1273–1284. doi: 10.2174/138161213804805621. [DOI] [PubMed] [Google Scholar]
- 34.Davalos V, Moutinho C, Villanueva A, et al. Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene. 2012;31:2062–2074. doi: 10.1038/onc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enkhbaatar Z, Terashima M, Oktyabri D, et al. KDM5B histone demethylase controls epithelial-mesenchymal transition of cancer cells by regulating the expression of the microRNA-200 family. Cell Cycle. 2013;12:2100–2112. doi: 10.4161/cc.25142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi JM, Kim SG, Choi J, et al. Effects of Helicobacter pylori eradication for metachronous gastric cancer prevention: a randomized controlled trial. Gastrointest Endosc. 2018;88:475–485. doi: 10.1016/j.gie.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Tsuda M, Asaka M, Kato M, et al. Effect on Helicobacter pylori eradication therapy against gastric cancer in Japan. Helicobacter. 2017;22:e12415. doi: 10.1111/hel.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. doi: 10.1016/j.canlet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Lamb A, Chen LF. Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer. J Cell Biochem. 2013;114:491–497. doi: 10.1002/jcb.24389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.