Abstract
Background/Aims
Ghrelin agonists are emerging prokinetic agents for treating gastroparesis. Although recent clinical trials have demonstrated their efficacy in patients with diabetic gastroparesis (DG), the impact of such agents on symptoms and gastric dysmotility remains unclear. We performed a systematic review and meta-analysis to evaluate the efficacy and safety of ghrelin agonists in patients with DG.
Methods
A search of common electronic databases (MEDLINE, Embase, and Cochrane Central Register of Controlled Trials) was preformed, using keyword combinations that referenced ghrelin and DG and retrieving all eligible randomized controlled trials (RCTs) of ghrelin agonists versus placebo in patients with DG. The primary outcome measure was the change in patient-reported overall gastroparesis symptom scores. Secondary outcomes included the change in gastric emptying time, specific symptoms related to gastroparesis, and adverse events. A random-effects model was applied to all study outcomes. Heterogeneity among studies was determined by the chi-square test and I2 statistics.
Results
We selected six RCTs of patients with DG (n=557) for meta-analysis. Ghrelin agonist administration (vs placebo) significantly improved overall gastroparesis symptoms (standardized mean difference, –0.34; 95% confidence interval, –0.56 to –0.13) and significantly improved symptoms related to gastroparesis, including nausea, vomiting, early satiety, and abdominal pain. Adverse events recorded for ghrelin agonists and placebo did not differ significantly. There was no significant heterogeneity among eligible studies.
Conclusions
Compared with placebo, ghrelin agonists are effective and well-tolerated for the treatment of DG.
Keywords: Diabetes mellitus, Gastroparesis, Ghrelin, Meta-analysis, Systematic review
INTRODUCTION
Diabetic gastroparesis (DG) is a serious complication of long-standing diabetes mellitus, resulting in malnutrition, poor glycemic control, and poor quality of life.1,2 In a U.S. population-based study, 10-year cumulative incidences of DG for patients with type 1 and 2 diabetes mellitus were 5.2% and 1.0%, respectively; and the risk of DG was >30-fold in those with type 1 diabetes mellitus, relative to age-/sex-matched controls.3 Although various prokinetic agents, namely dopamine D2 receptor antagonists (metoclopramide and domperidone) and motilin receptor agonists (erythromycin), are current mainstays in the treatment of DG,4,5 their long-term use is hampered by adverse events (AEs) (i.e., potential tardive dyskinesia) or waning efficacy due to tachyphylaxis.4 Consequently, novel agents with differing mechanisms of action must be developed to ensure long-term efficacy and safety in the treatment of DG.
Ghrelin is a peptide hormone released from gastric mucosal endocrine cells that serves as a ligand for growth hormone secretagogue receptor 1a.6 In vitro studies have shown the expression of ghrelin receptor in enteral nervous system of human and animal intestine.7,8 Preclinical studies have shown that ghrelin enhances gastrointestinal (GI) motility via stimulation of vagal signaling, thus prompting efforts at therapeutic targeting.6 Ghrelin can also affect GI motility directly within the enteric nerve system or within the central nerve system via crossing the blood-brain barrier.6 The prokinetic effects of ghrelin on GI motility have been demonstrated in postsurgical, opioid-induced as well as diabetic models of rodents with various routes of administration.9
Synthetic selective ghrelin receptor agonists, including TZP-101 (ulimorelin), TZP-102, and RM-131 (relamorelin), are under development and actually surpass native ghrelin in half-life.10,11 TZP-101 is a first-in-class ghrelin agonist with a potent binding affinity for ghrelin receptor.12 In the first phase I human study of ghrelin agonists, parenteral TZP-101 was well tolerated with a promising pharmacokinetic and pharmacodynamic profile for use in healthy volunteers.11 The volume of distribution is approximately 114 mL/kg and half-life values of approximately 13 hours, which were independent of dose.11 However, several prospective randomized controlled trials (RCTs) showed inconsistent efficacy of ghrelin agonists for the treatment of DG.13-18 In a phase 2b, randomized, double-blind 12-week placebo-controlled trial, oral TZP-102 was not superior to placebo for the treatment of DG, but there was substantial improvement of symptoms in both ghrelin agonist and placebo groups.15 In a recent phase 2b randomized, placebo-controlled trial among the largest number of patients with moderate to severe gastroparesis symptoms related to diabetes, RM-131 significantly reduced gastroparesis symptoms compared to placebo with acceleration of gastric emptying.18 Therefore, we conducted this systematic review and meta-analysis to better assess the efficacy and safety of synthetic ghrelin agonists (compared with placebo) in the treatment of DG.
MATERIALS AND METHODS
This systematic review and meta-analysis were conducted in accord with Preferred Reporting Items for Systematic and Meta-analysis (PRISMA) report guidelines.19
1. Search strategy and study selection
Using common electronic databases (MEDLINE, Embase, and Cochrane Central Register of Controlled Trials), we searched the medical literature (prior to June 2018) for the following terms: ghrelin AND (diabetic OR diabetes) AND (gastroparesis OR gastropathy). Two independent authors (S.W.H. and J.K.) reviewed and selected pertinent studies, all restricted to English language. Eligible publications met the following criteria: (1) any patient with DG; (2) ghrelin agonist intervention; (3) placebo as comparator; (4) gastroparesis symptoms, gastric emptying time (GET), and AEs as outcomes; and (5) prospective comparative study design (Supplementary Material 1). There were no restrictions on drug regimens or durations of treatment. Abstracts, case reports, review articles, non-comparative studies, and preclinical studies were excluded from this meta-analysis.
2. Data extraction and quality assessment
Two authors (S.W.H. and J.K.) independently extracted data from eligible studies, resolving any disagreement by consensus. Extracted data included the following: named author(s); trial location; year of publication; drug regimen and duration of treatment; number of enrollees in each treatment arm; posttreatment change in DG symptom scores and scale applied in symptom assessment; change in GET and method of measurement; and AEs. In dose-dependent RCTs, the regimen with the greatest clinical efficacy in terms of the change in overall gastroparesis symptoms was preferred for extracting data (Supplementary Table 1). In studies with varying assessment scales, data related to primary outcome measures were chiefly extracted. We also contacted corresponding authors to clarify or remedy confusing or missing information. The risk of bias tool of the Cochrane Group served to gauge quality of analysis.20
3. Study outcomes and measurement scales
The primary outcome measure was change in severity of overall gastroparesis symptoms, based on patient-reported scales. Secondary outcomes were change in GET, specific gastroparesis-related symptoms gauged before and after treatment, and AEs. There are a variety of patient-reported scales for measuring symptoms related to gastroparesis. The Gastroparesis Cardinal Symptom Index (GCSI)21 and Patient Assessment of Upper Gastrointestinal Symptom Severity Index (PAGI-SYM) have been widely used.22 Recently, GCSI was revised as a daily diary,23 and the Diabetic Gastroparesis Symptom Severity Diary (DGSSD) was also developed to score symptoms of DG. In this regard, we made no restrictions on patient-reported scales when selecting study outcomes for meta-analysis. GET was equated with gastric emptying half-time after ingesting an isotope-labeled diet. A breath test or scintigraphy served to measure half-times of gastric emptying. Data on GET were likewise extracted without regard to measurement methods. We also extracted data on AEs and serious adverse events (SAEs), based on results presented in each study, detailing events and numbers of patients affected.
4. Statistical methods
Dichotomous outcomes were calculated as odds ratios (ORs), with 95% confidence intervals (CIs). Continuous data were each expressed as standardized mean difference (SMD) or mean difference (MD), with 95% CI. Given the diversity in scoring of gastroparesis symptoms, we used SMD to report pooled treatment effects, whereas results of same-scale GET analytics were expressed as MDs. A random-effects model was ultimately invoked, applying inverse-variance method for all study outcomes. Some data proved insufficient to calculate standard deviations of changes occurring, so we imputed values (correlation coefficients) derived from other studies.20 A p<0.05 was considered statistically significant. Heterogeneity among studies was estimated via chi-square test and I2 statistics. Significance in chi-square testing was set at p<0.10, gauging heterogeneity among studies by I2 statistics as follows: 0% to 40%, marginal; 30% to 60%, moderate; 50% to 90%, substantial; and 75% to 100%, immense. We assessed potential causes of substantial heterogeneity and conducted subgroup analyses according to type of ghrelin agonists, route of administration and treatment duration. All computations relied on Review Manager freeware (RevMan v5.3; Cochrane Community, London, UK).
RESULTS
1. Search selection
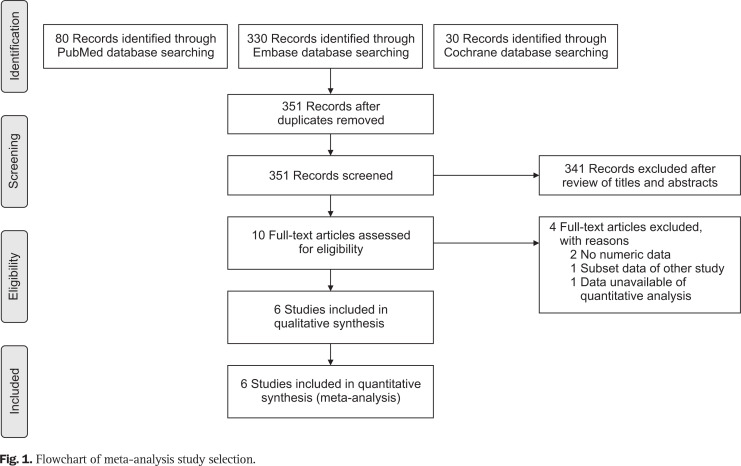
Our literature search returned a total of 438 articles. Discounting duplicates, 351 articles remained for title and abstract screening. The latter yielded 10 articles for full text assessment, but only six met our inclusion criteria and advanced to data extraction/synthesis (Fig. 1).13-18 A study by Shin et al.24 fulfilled the conditions for enrollment in this meta-analysis, but the values were only presented with median and interquartile range. The values with median and interquartile range could not be converted to those with mean and standard deviation in the absence of raw data. Hence, the study was excluded from the qualitative synthesis stage.
Fig. 1.
Flowchart of meta-analysis study selection.
2. Study characteristics and risk of bias assessment
All publications selected for analysis (n=557) were prospective RCTs. Each subject with DG had been stratified to test agent (n=263) or placebo (n=294) groups. Five studies were parallel investigations,13-15,17,18 and the remaining trial was a cross-over study.16 Four were multinational efforts,13-15,18 and two were conducted in the United States.16,17 Patient-reported scales for assessment of gastroparesis symptoms were distributed as follows: GCSI, three RCTs;13-15 GCSI daily diary, four RCTs;15-18 PAGI-SYM, three RCTs;14,15,17 and DGSSD, two RCTs (Table 1).17,18 In all study populations, prolongation of GET was stipulated in screening phases of those studies eligible for meta-analysis, five of them designating change in GET as a study outcome.14-18 With exception of one RCT (using scintigraphy), GET was determined by breath test.16 Characteristics and study outcomes of RCTs selected for meta-analysis are summarized in Table 1.
Table 1.
Characteristics of Included Studies
| Author | Year | Country | Study design | Intervention group | Placebo group | Outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age | HbA1c | No. | Age | HbA1c | ||||||
| Ejskjaer et al.13 | 2010 | Multinational | Double blinded RCT, parallel | 13 | 44.0±11.0 | 8.6±1.7 | 19 | 45.7±12.6 | 8.0±1.6 | GSA, GCSI | |
| Ejskjaer et al.14 | 2013 | Multinational | Double blinded RCT, parallel | 21 | 49.8±12.3 | 7.9±1.5 | 26 | 50.2±12.1 | 8.3±1.6 | GCSI, PAGI-SYM GET (BT and SG) |
|
| McCallum et al.15 | 2013 | Multinational | Double blinded RCT, parallel | 69 | 54.0±10.9 | 7.8±1.5 | 66 | 54.0±12.0 | 7.8±1.5 | GCSI, GSDD PAGI-SYM, GET (BT) |
|
| Shin et al.16 | 2013 | United States | Double blinded RCT, cross-over | 10 | 51.8±7.9 | 7.2±1.3 | 10 | 51.8±7.9 | 7.2±1.3 | GCSI-DD, GET (SG) | |
| Lembo et al.17 | 2016 | United States | Double blinded RCT, parallel | 68 | 53.5±10.7 | NR | 69 | 55.2±11.1 | NR | DGSSD, GCSI-DD PAGI-SYM, GET (BT) |
|
| Camilleri et al.18 | 2017 | Multinational | Double blinded RCT, parallel | 82 | 57.1 | 8.1 | 104 | 55.7 | 7.8 | DGSSD, GCSI-DD, GET (BT) | |
Data are presented as mean±SD or mean value.
HbA1c, hemoglobin A1c; RCT, randomized controlled study; GSA, Gastroparesis Symptom Assessment; GCSI, Gastroparesis Cardinal Symptom Index; PAGI-SYM, Patient Assessment of Upper Gastrointestinal Symptom Severity Index; GET, gastric emptying time; BT, breath test; SG, scintigraphy; GSDD, Daily Diary of Gastroparesis Symptoms Questionnaire; GCSI-DD, Gastroparesis Cardinal Symptom Index-Daily Diary; NR, not reported; DGSSD, Diabetic Gastroparesis Symptom Severity Diary.
These studies differed in terms of drugs used, dosages, methods of administration, and treatment durations (Table 2). One study was aimed at TZP-101,13 another two tested TZP-102,14,15 and the final three evaluated RM-131,16-18 showing wide variation in duration of treatment (range, 1 day to 12 weeks). The report of McCallum et al.15 provided results of two clinical trials with different dosing schedules (once daily and three times daily). Because the researchers allowed dual enrollment, we selected one of the regimens by the selection criteria according to the greatest clinical efficacy on overall gastroparesis symptoms.15
Table 2.
Regimen and Treatment Duration of Ghrelin Agonists in Each Study
| Author | Medication name | Regimen | Selected regimen for analysis | Treatment duration |
|---|---|---|---|---|
| Ejskjaer et al.13 | TPZ-101* | 20, 40, 80 160, 320, and 600 μg/kg single daily IV infusion |
80 μg/kg single daily infusion |
4 Days |
| Ejskjaer et al.14 | TPZ-102 | 10, 20, and 40 mg qd p.o. | 20 mg qd p.o. | 28 Days |
| McCallum et al.15 | TPZ-102 | 10, 20 mg qd p.o., 10 mg tid p.o. | 10 mg qd p.o. | 12 Weeks |
| Shin et al.16 | RM-131† | 100 μg qd s.c. | 100 μg qd s.c. | 1 Days |
| Lembo et al.17 | RM-131 | 10 μg qd, bid s.c. | 10 μg bid s.c. | 28 Days |
| Camilleri et al.18 | RM-131 | 10, 30, and 100 μg bid s.c. | 100 μg bid s.c. | 12 Weeks |
IV, intravenous; qd, once daily; p.o., per oral; tid, three times daily; s.c., subcutaneous; bid, twice daily.
*TPZ101 referred to as ulimorelin; †RM-131 referred to as relamorelin.
The risk of bias is shown in Supplementary Figs 1 and 2. Methods of randomization and allocation concealment were clearly detailed in all studies having low risks of performance and detection bias, except the study by Lembo et al.17 with insufficient information on the risk of allocation concealment. Attrition rates in two RCTs were high.17,18
3. Overall gastroparesis symptoms
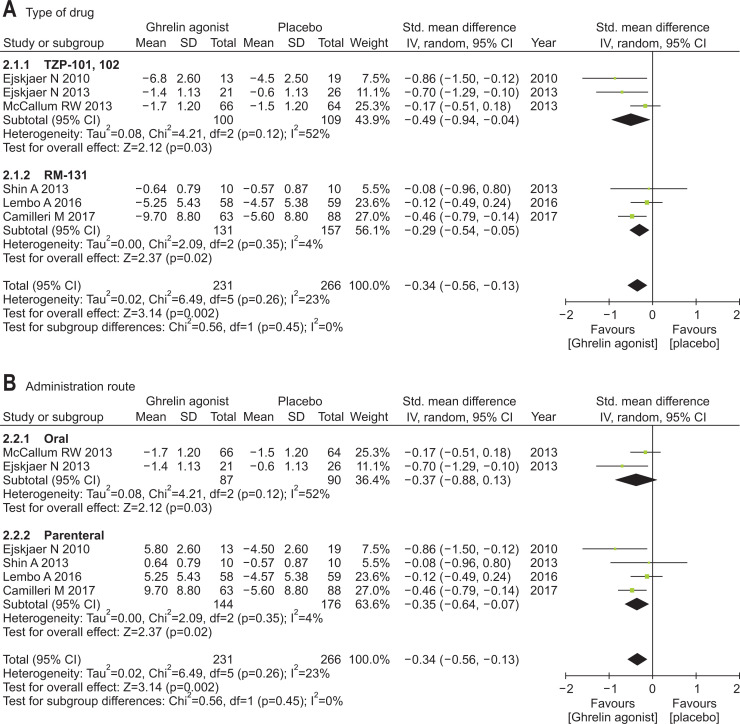
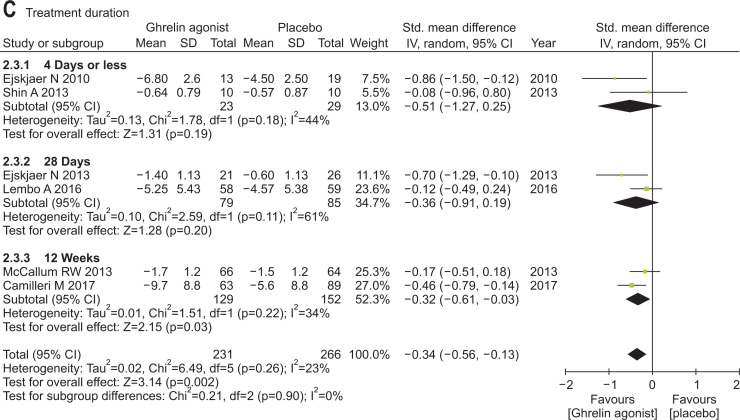
Our meta-analysis confirmed a significantly better performance by ghrelin agonists (vs placebo) regarding change in overall gastroparesis symptoms scores (SMD, –0.34; 95% CI, –0.56 to –0.13). There was no significant heterogeneity among studies (p=0.26; I2=23%). RM-131 differed significantly, compared with placebo (SMD, –0.29; 95% CI, –0.54 to –0.05), and so did TZP-101,102 (SMD, –0.49; 95% CI, –0.94 to –0.04). Ghrelin agonist via parenteral route differed significantly, compared with placebo (SMD, –0.35; 95% CI, –0.64 to –0.07), although orally administered ghrelin agonist did not (SMD, –0.37; 95% CI, –0.88 to 0.13). The efficacy of ghrelin agonists for improving overall gastroparesis symptoms was only found at 12-week treatment duration (Fig. 2).
Fig. 2.
Efficacy of ghrelin agonists (vs placebo) for treating gastroparesis symptoms overall: (A) subgroup analysis by agent (TZP-101, 102, or RM-131), (B) subgroup analysis by route of administration (parenteral vs oral), and (C) subgroup analysis by treatment duration.
Std., standardized; IV, inverse variance; CI, confidence interval.
4. Specific gastroparesis symptoms
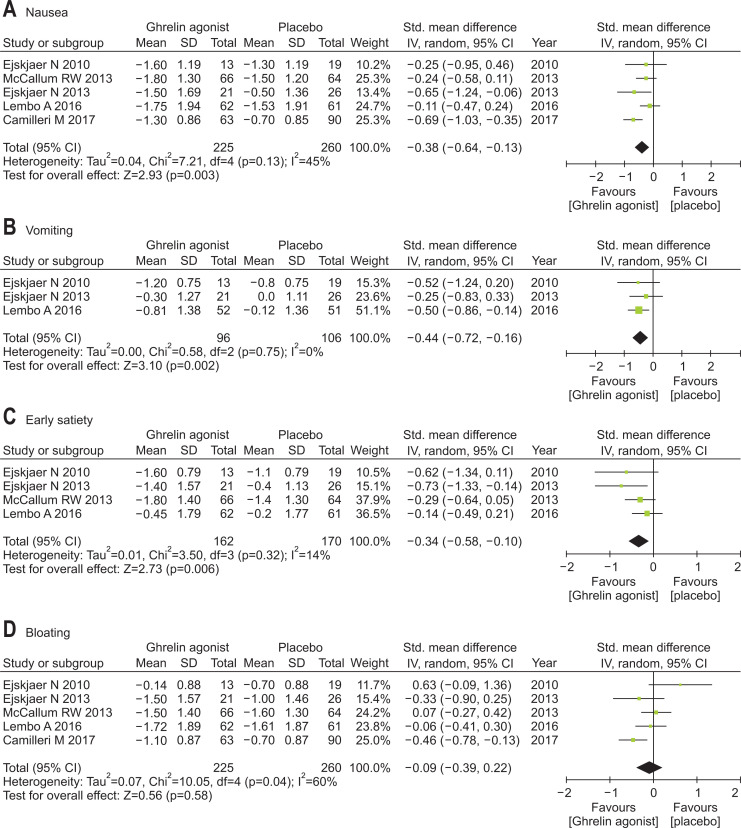
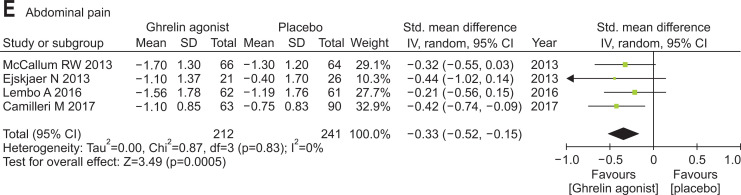
Ghrelin agonists (vs placebo) significantly improved nausea (SMD, –0.38; 95% CI, –0.64 to –0.13), vomiting (SMD, –0.44; 95% CI, –0.72 to –0.16), early satiety (SMD, –0.34; 95% CI, –0.58 to –0.10) and abdominal pain (SMD, –0.33; 95% CI, –0.52 to –0.15). However, bloating was not improved by ghrelin agonist (i.e., similar to placebo) (Fig. 3).
Fig. 3.
Efficacies of ghrelin agonists (vs placebo) in treating specific gastroparesis symptoms: (A) nausea, (B) vomiting, (C) early satiety, (D) bloating, and (E) abdominal pain.
Std., standardized; IV, inverse variance; CI, confidence interval.
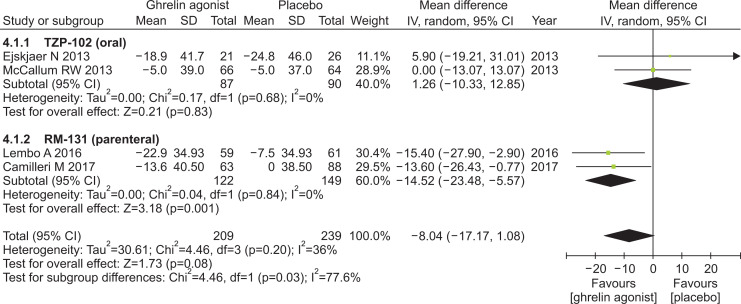
5. Gastric emptying time
In a study by Shin et al.,16 there were no available data on GET at baseline, even upon contacting authors for information. This study was subsequently excluded from meta-analysis. Ghrelin agonists showed a tendency to improve GET compared with placebo (MD, –8.04; 95% CI, –17.17 to 1.08). RM-131 (parenteral) significantly shortened GET, compared with placebo (MD, –14:52; 95% CI, –23.48 to –5.57), but TZP-102 (oral) did not (Fig. 4).
Fig. 4.
Effects of ghrelin agonists (vs placebo) on gastric emptying time.
IV, inverse variance; CI, confidence interval.
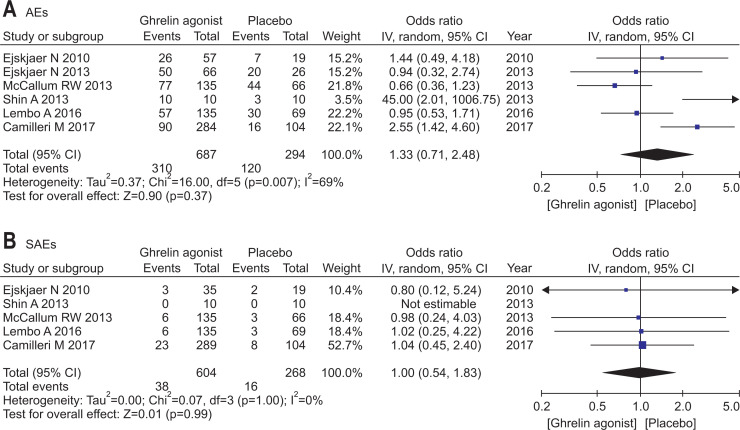
6. Adverse events
The pooled effects of AEs were based on the number of patients with AEs in all eligible studies, regardless of dosing regimen. The proportion of patients with AEs or SAEs in each study and the relevance to treatment were summarized in Table 3. Accordingly, no significant difference between ghrelin agonist and placebo was evident (OR, 1.33; 95% CI, 0.71 to 2.48). Similarly, there was no significant difference in pooled SAEs (OR, 1.00; 95% CI, 0.54 to 1.83) (Fig. 5). AEs common to each ghrelin agonist were GI symptoms, such as nausea, vomiting, diarrhea, and abdominal pain. AEs related to glycemic control were also frequently reported. SAEs were rare, including coronary heart disease, atrial fibrillation, diabetic ketoacidosis, and serious infectious (i.e., pneumonia, urinary tract infection, and sepsis). Most studies under investigation did not clearly document associations between ghrelin agonists and AEs, and no definitive dose-response relations were discerned. Although AEs linked to hyperglycemia were not significantly increased, blood glucose trended higher in the ghrelin agonist group (Supplementary Fig. 3). In a study by Camilleri et al.,18 some recipients of RM-131 displayed dose-dependent glycemic increases.
Table 3.
Adverse Events and Severe Adverse Events in Each Study
| Symptom | Author | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ejskjaer et al.13 | Ejskjaer et al.14 | McCallum et al.15 | Shin et al.16 | Lembo et al.17 | Camilleri et al.18 | |||||||||
| I | C | I | C | I | C | I | C | I | C | I | C | I | C | |
| GI symptoms | 4/57 (7) | 1/19 (5) | 9/66 (14) | 4/26 (15) | 13/135 (7) | 9/66 (14) | NR | NR | NR | NR | NR | NR | 26/258 (10) | 14/111 (13) |
| Nausea | 3/57 (5) | 1/19 (5) | 4/66 (6) | 4/26 (15) | 6/135 (4) | 7/66 (10) | NR | NR | NR | NR | NR | NR | 13/258 (5) | 12/111 (10) |
| Vomiting | 2/57 (4) | 1/19 (5) | 5/66 (8) | 0 | 4/135 (3) | 4/66 (6) | 2/10 (20) | 0 | NR | NR | NR | NR | 13/268 (5) | 5/111 (5) |
| Abdominal pain | 3/57 (5) | 1/19 (5) | 7/66 (11) | 0 | 5/135 (4) | 4/66 (6) | NR | NR | NR | NR | 17/289 (6) | 0 | 32/547 (6) | 5/85 (6) |
| Diarrhea | 2/57 (4) | 1/19 (5) | 2/66 (3) | 0 | 6/135 (4) | 5/66 (8) | NR | NR | 4/135 (3) | 4/69 (6) | NR | NR | 14/393 (4) | 10/154 (6) |
| UTI | 2/57 (4) | 1/19 (5) | 4/66 (6) | 2/26 (8) | 7/135 (5) | 4/66 (6) | NR | NR | 5/135 (4) | 2/69 (3) | NR | NR | 18/393 (5) | 9/180 (5) |
| Headache | 6/57 (11) | 1/19 (5) | 4/66 (6) | 0 | 49/135 (36) | 29/66 (44) | NR | NR | 5/135 (4) | 2/69 (3) | 25/289 (9) | 2/104 (2) | 89/682 (13) | 34/284 (12) |
| AEs | 26/57 (46) | 7/19 (37) | 50/66 (76) | 20/26 (77) | 77/135 (57) | 44/66 (67) | 10/10 (100) | 3/10 (30) | 57/135 (42) | 30/69 (44) | 90/284 (32) | 16/104 (15) | 26/258 (10) | 14/111 (13) |
| SAEs | 3/57 (5) | 2/19 (10) | 6/66 (9) | NR | 6/135 (4) | 3/66 (5) | 0 | 0 | 6/135 (4) | 3/69 (4) | 23/289 (8) | 8/104 (8) | 13/258 (5) | 12/111 (10) |
| Relationship between treatment and SAEs | Possible | None | None | Possible | None | Possible | ||||||||
Data are presented as number/total number (%).
I, intervention; C, control; GI, gastrointestinal; UTI, urinary tract infection; AEs, adverse events; SAEs, serious adverse events; NR, not reported.
Fig. 5.
Ghrelin agonist and placebo group comparison: (A) adverse events and (B) serious adverse events.
IV, inverse variance; CI, confidence interval.
DISCUSSION
This meta-analysis is seemingly the first to evaluate the efficacy and safety of ghrelin agonists (vs placebo) in the treatment of DG. We found that such agents significantly improve overall gastroparesis symptoms and show a tendency to reduce GET. Although the relation between symptom improvement and GET is still controversial, both parameters are current standard outcome measures of drugs used to treat gastroparesis.25 Hence, our data support the therapeutic potential of ghrelin agonists in managing patients with DG.
Although all types of ghrelin agonists significantly outperformed placebo in patients with DG in terms of improving overall DG symptoms, there was a significant difference of the effects on GET between parenteral RM-131 and oral TZP-102. Preclinical data on RM-131 indicates a much greater potency (600- to 1,800-fold) for ghrelin receptors than TZP-102, offering a plausible explanation for the differing efficacy observed in patients with DG.10 Among the 3 studies using RM-131 on the treatment of patients with DG, only study by Camilleri et al.18 reported the significant efficacy of RM-131 on improvement of overall gastroparesis symptoms following the long-term administration of the highest dose (100 μg twice daily for 12 weeks), compared to placebo. Therefore, various dosage and treatment duration of RM-131 may affect the inconsistent results among the studies. However, the route of administration might also be related to the efficacy of ghrelin agonists. Compared with oral TZP-102 intake for more than 4 weeks, short-term parenteral administration of TZP-101 brought significantly greater improvement in overall symptoms. Unfortunately, there is no published data as yet on the bioavailability of orally administered ghrelin agonists, and our preliminary evidence is inconclusive. As further validation, oral formulations of ghrelin agonists more potent than TZP-102 must be evaluated in patients with DG.
In terms of specific DG-related GI symptoms, ghrelin agonists significantly improved abdominal pain, nausea, early satiety and vomiting, but not bloating. Although the pathophysiology of symptoms related to DG remains elusive,26 various sources maintain that chief etiologic factors of given GI symptoms may differ as follows: (1) visceral hypersensitivity to gastric distension leads to epigastric pain; (2) delayed gastric emptying results in postprandial fullness, nausea, and vomiting; and (3) impaired accommodation promotes early satiety.27-30 Thus, the differing efficacies displayed in these trials for each symptom of DG are explainable. To date, the major role defined for ghrelin agonists is an enhancement of GI motility via vagal stimulation.6 The ghrelin infusion reduced gastric accommodation in the clinical trial for healthy volunteers and at least one preclinical study suggests that ghrelin has antinociceptive effects, which counter visceral hypersensitivity.31,32 Indeed, ghrelin agonists act to reduce GET, visceral hypersensitivity and gastric accommodation, thereby improving nausea, vomiting, early satiety, and abdominal pain. On the other hand, bloating may be a heterogeneous condition involving multiple pathophysiologic factors.33 Consequently ghrelin agonists may offer no benefits for bloating related to DG.
One of the major concerns when using ghrelin agonists is the adverse effect on glycemic control. Tight glycemic control is paramount in patients with DG, so it is essential to address this issue.4 Earlier efforts have shown that the infusion of ghrelin agonists increases plasma growth hormone concentration, thus opposing the action of insulin.34,35 In a literature review, however, strong evidence of significant blood glucose elevations due to ghrelin agonists was lacking in patients with DG.24,34-36 Herein, the risk of AEs related to hyperglycemia only trended higher in our ghrelin agonist group, failing to dispel such fears. Additional prospective studies are needed to clarify this aspect of long-term ghrelin agonist use and other related complications.
This study has several acknowledged limitations. Although significant improvement was shown at the 12-week treatment duration, the efficacy of ghrelin agonists for improving overall gastroparesis symptoms could not be fully evaluated based on treatment duration due to lack of studies enrolled in this meta-analysis. The long-term efficacy of ghrelin agonists in managing DG could not be ascertained as well, because the maximum study period among studies included was a mere 12 weeks. In addition, there was no data on the enduring efficacy of these agents after their withdrawal. Another weakness is that the rare AEs of ghrelin agonists in the setting of DG could not be properly assessed in the small number of available patients. There is concern that ghrelin agonist may heighten cancer risks due to the carcinogenic effects of growth hormone.37 Although in vivo study findings indicate that serum ghrelin level has a null or inverse association with cancer risks,38 the role of ghrelin/ghrelin agonists in the development and progression of cancer is still in doubt. Ultimately, large-scale clinical studies will be needed to evaluate the long-term efficacy and safety profile of ghrelin agonists during treatment of DG. Finally, it remains elusive whether the inconsistent results regarding the efficacy of ghrelin agonists on overall symptoms related to DG were due to administration route or dosage. The efficacy of ghrelin agonists for the treatment of DG may be valid only for specific regimen although overall efficacy of ghrelin agonists for improvement of gastroparesis symptoms in the meta-analysis. Further studies are required to determine oral bioavailability and comparative efficacy of ghrelin agonists between drug types in patients with DG.
In conclusion, ghrelin agonists effectively improve symptoms related to gastroparesis in patients with DG, more so than placebo. Despite concerns over blood glucose levels during long-term ghrelin agonist use, no significant treatment-related safety issues emerged in the course of this meta-analysis.
Supplemental Materials
ACKNOWLEDGEMENTS
The authors wish to thank Myoung-jin Jang, the Medical Research Collaborating Center, and Seoul National University Hospital for their assistance with statistical aspects of this meta-analysis.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Literature search and data extraction: S.W.H., J.K. Statistical analysis: S.W.H., H.J.L. Manuscript draft: S.W.H., J.L., J.C. Critical review: S.J.C., H.C., J.P.I. Contribution to discussion: J.C., S.G.K., J.S.K.
References
- 1.Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:5–12. doi: 10.1016/j.cgh.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu N, Abell T. Gastroparesis updates on pathogenesis and management. Gut Liver. 2017;11:579–589. doi: 10.5009/gnl16336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choung RS, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Melton LJ, 3rd, Talley NJ. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol. 2012;107:82–88. doi: 10.1038/ajg.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Gastroenterology, author. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37. doi: 10.1038/ajg.2012.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L. The investigation and treatment of diabetic gastroparesis. Clin Ther. 2018;40:850–861. doi: 10.1016/j.clinthera.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri M, Papathanasopoulos A, Odunsi ST. Actions and therapeutic pathways of ghrelin for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. 2009;6:343–352. doi: 10.1038/nrgastro.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dass NB, Munonyara M, Bassil AK, et al. Growth hormone secretagogue receptors in rat and human gastrointestinal tract and the effects of ghrelin. Neuroscience. 2003;120:443–453. doi: 10.1016/S0306-4522(03)00327-0. [DOI] [PubMed] [Google Scholar]
- 8.Xu L, Depoortere I, Tomasetto C, et al. Evidence for the presence of motilin, ghrelin, and the motilin and ghrelin receptor in neurons of the myenteric plexus. Regul Pept. 2005;124:119–125. doi: 10.1016/j.regpep.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Avau B, Carbone F, Tack J, Depoortere I. Ghrelin signaling in the gut, its physiological properties, and therapeutic potential. Neurogastroenterol Motil. 2013;25:720–732. doi: 10.1111/nmo.12193. [DOI] [PubMed] [Google Scholar]
- 10.Van der Ploeg L, Laken H, Sharma S, et al. Preclinical gastrointestinal prokinetic efficacy and endocrine effects of the ghrelin mimetic RM-131. Life Sci. 2014;109:20–29. doi: 10.1016/j.lfs.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Lasseter KC, Shaughnessy L, Cummings D, et al. Ghrelin agonist (TZP-101): safety, pharmacokinetics and pharmacodynamic evaluation in healthy volunteers: a phase I, first-in-human study. J Clin Pharmacol. 2008;48:193–202. doi: 10.1177/0091270007310380. [DOI] [PubMed] [Google Scholar]
- 12.Fraser GL, Hoveyda HR, Tannenbaum GS. Pharmacological demarcation of the growth hormone, gut motility and feeding effects of ghrelin using a novel ghrelin receptor agonist. Endocrinology. 2008;149:6280–6288. doi: 10.1210/en.2008-0804. [DOI] [PubMed] [Google Scholar]
- 13.Ejskjaer N, Dimcevski G, Wo J, et al. Safety and efficacy of ghrelin agonist TZP-101 in relieving symptoms in patients with diabetic gastroparesis: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2010;22:e1069–e1281. doi: 10.1111/j.1365-2982.2010.01519.x. [DOI] [PubMed] [Google Scholar]
- 14.Ejskjaer N, Wo JM, Esfandyari T, et al. A phase 2a, randomized, double-blind 28-day study of TZP-102 a ghrelin receptor agonist for diabetic gastroparesis. Neurogastroenterol Motil. 2013;25:e140–e150. doi: 10.1111/nmo.12064. [DOI] [PubMed] [Google Scholar]
- 15.McCallum RW, Lembo A, Esfandyari T, et al. Phase 2b, randomized, double-blind 12-week studies of TZP-102, a ghrelin receptor agonist for diabetic gastroparesis. Neurogastroenterol Motil. 2013;25:e705–e717. doi: 10.1111/nmo.12184. [DOI] [PubMed] [Google Scholar]
- 16.Shin A, Camilleri M, Busciglio I, et al. Randomized controlled phase Ib study of ghrelin agonist, RM-131, in type 2 diabetic women with delayed gastric emptying: pharmacokinetics and pharmacodynamics. Diabetes Care. 2013;36:41–48. doi: 10.2337/dc12-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lembo A, Camilleri M, McCallum R, et al. Relamorelin reduces vomiting frequency and severity and accelerates gastric emptying in adults with diabetic gastroparesis. Gastroenterology. 2016;151:87–96. doi: 10.1053/j.gastro.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri M, McCallum RW, Tack J, Spence SC, Gottesdiener K, Fiedorek FT. Efficacy and safety of relamorelin in diabetics with symptoms of gastroparesis: a randomized, placebo-controlled study. Gastroenterology. 2017;153:1240–1250. doi: 10.1053/j.gastro.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revicki DA, Rentz AM, Dubois D, et al. Gastroparesis Cardinal Symptom Index (GCSI): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res. 2004;13:833–844. doi: 10.1023/B:QURE.0000021689.86296.e4. [DOI] [PubMed] [Google Scholar]
- 22.Rentz AM, Kahrilas P, Stanghellini V, et al. Development and psychometric evaluation of the Patient Assessment of Upper Gastrointestinal Symptom Severity Index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res. 2004;13:1737–1749. doi: 10.1007/s11136-004-9567-x. [DOI] [PubMed] [Google Scholar]
- 23.Revicki DA, Camilleri M, Kuo B, Szarka LA, McCormack J, Parkman HP. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD) Neurogastroenterol Motil. 2012;24:456–463. doi: 10.1111/j.1365-2982.2012.01879.x. [DOI] [PubMed] [Google Scholar]
- 24.Shin A, Camilleri M, Busciglio I, et al. The ghrelin agonist RM-131 accelerates gastric emptying of solids and reduces symptoms in patients with type 1 diabetes mellitus. Clin Gastroenterol Hepatol. 2013;11:1453–1459. doi: 10.1016/j.cgh.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janssen P, Harris MS, Jones M, et al. The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am J Gastroenterol. 2013;108:1382–1391. doi: 10.1038/ajg.2013.118. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A, Attaluri A, Hashmi S, Schulze KS, Rao SS. Visceral hypersensitivity and impaired accommodation in refractory diabetic gastroparesis. Neurogastroenterol Motil. 2008;20:635–642. doi: 10.1111/j.1365-2982.2008.01081.x. [DOI] [PubMed] [Google Scholar]
- 27.Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 2004;127:1239–1255. doi: 10.1053/j.gastro.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 28.Khayyam U, Sachdeva P, Gomez J, et al. Assessment of symptoms during gastric emptying scintigraphy to correlate symptoms to delayed gastric emptying. Neurogastroenterol Motil. 2010;22:539–545. doi: 10.1111/j.1365-2982.2009.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enck P, Azpiroz F, Boeckxstaens G, et al. Functional dyspepsia. Nat Rev Dis Primers. 2017;3:17081. doi: 10.1038/nrdp.2017.81. [DOI] [PubMed] [Google Scholar]
- 30.Tack J, Van den Houte K, Carbone F. Gastroduodenal motility disorders. Curr Opin Gastroenterol. 2018;34:428–435. doi: 10.1097/MOG.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 31.Ang D, Nicolai H, Vos R, et al. Influence of ghrelin on the gastric accommodation reflex and on meal-induced satiety in man. Neurogastroenterol Motil. 2009;21:528–533. doi: 10.1111/j.1365-2982.2008.01239.x. [DOI] [PubMed] [Google Scholar]
- 32.Mao Y, Li Z, Chen K, et al. Antinociceptive effect of ghrelin in a rat model of irritable bowel syndrome involves TRPV1/Opioid systems. Cell Physiol Biochem. 2017;43:518–530. doi: 10.1159/000480478. [DOI] [PubMed] [Google Scholar]
- 33.Iovino P, Bucci C, Tremolaterra F, Santonicola A, Chiarioni G. Bloating and functional gastro-intestinal disorders: where are we and where are we going? World J Gastroenterol. 2014;20:14407–14419. doi: 10.3748/wjg.v20.i39.14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray CD, Martin NM, Patterson M, et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut. 2005;54:1693–1698. doi: 10.1136/gut.2005.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ejskjaer N, Vestergaard ET, Hellström PM, et al. Ghrelin receptor agonist (TZP-101) accelerates gastric emptying in adults with diabetes and symptomatic gastroparesis. Aliment Pharmacol Ther. 2009;29:1179–1187. doi: 10.1111/j.1365-2036.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 36.Wo JM, Ejskjaer N, Hellström PM, et al. Randomised clinical trial: ghrelin agonist TZP-101 relieves gastroparesis associated with severe nausea and vomiting--randomised clinical study subset data. Aliment Pharmacol Ther. 2011;33:679–688. doi: 10.1111/j.1365-2036.2010.04567.x. [DOI] [PubMed] [Google Scholar]
- 37.Swerdlow AJ, Cooke R, Beckers D, et al. Cancer risks in patients treated with growth hormone in childhood: the SAGhE European Cohort Study. J Clin Endocrinol Metab. 2017;102:1661–1672. doi: 10.1210/jc.2016-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sever S, White DL, Garcia JM. Is there an effect of ghrelin/ghrelin analogs on cancer? A systematic review. Endocr Relat Cancer. 2016;23:R393–R409. doi: 10.1530/ERC-16-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.