Abstract
Purpose
Rapid drug desensitization is known to be a good strategy in patients with drug hypersensitivity to chemotherapy. However, changes in maximal drug concentration and exposure time in blood through desensitization may alter other adverse reactions and efficacy of the drug. We investigated rapid desensitization for carboplatin in terms of severe adverse drug reactions (ADRs) and efficacy compared with the standard infusion.
Methods
A retrospective cohort study was conducted on patients with recurrent ovarian cancer who received carboplatin chemotherapy from 2017 to 2019. We compared serious adverse events (SAEs), ADRs according to organ classes, time to progression (TTP), and overall survival (OS).
Results
Of 108 desensitization procedures performed in 21 patients, 104 were successfully accomplished (96.3%). There were compared with 271 procedures in 41 patients who received the standard infusion method. There were 8 (7.7%) SAEs in the rapid desensitization group and 34 (12.5%) in the control group. One drug-related death occurred in the rapid desensitization group. In the rapid desensitized group, except for neutropenia, there was no statistically significant increase in SAEs and over grade 3 of ADRs according to organ classes compared with the control group. In the efficacy analysis, TTP and OS were similar in the 2 groups.
Conclusions
Rapid desensitization of carboplatin can lower the risk of immediate hypersensitivity reactions without changing the inherent effect and severe ADRs.
Keywords: Desensitization, hypersensitivity, antineoplastic agents, carboplatin, drug-related side effects and adverse reactions, drug effects, survival, ovarian neoplasm
INTRODUCTION
Immediate drug hypersensitivity reactions (HSRs), which are manifested as hives, angioedema, rhinitis, bronchospasm, and anaphylactic shock, can occur within 1-6 hours after taking a causative drug.1,2 These reactions are difficult to predict, progress rapidly, and can lead to life-threatening reactions like anaphylaxis.3 Avoidance is the best way to prevent HSR. Despite, in some cases, knowing that a chemotherapeutic agent may cause immediate drug HSR, re-administration may be considered due to the significant benefit of the drug.
Rapid drug desensitization (RDD) is a safe approach to prevent HSRs to chemotherapeutic agents.4 The concept of RDD is to induce temporary non-reactivity by administering the causative drug at a concentration of 1/100 to 1/10,000 of the normal dose depending on the degree of risk, and gradually increasing the infusion rate every 15 to 20 minutes until the target dose is achieved.5,6,7 After introduction of RDD, 90% or more patients experienced no breakthrough reaction (BTR) or only mild BTR. Overall health costs between an RDD group and a standard infusion group were not different.8,9 So, RDD is a safe and efficient method in patients who have experienced immediate HSR to chemotherapeutic agents.
However, when the drug is administered by RDD, the administration rate and duration of exposure is changed. Carboplatin is recommended to be administered for 30 minutes to 1 hour, but by RDD, carboplatin will be administered for more than 4 hours, and the final rate will also be different from the standard method.10,11,12 Adjusting the rate of drug administration changes the blood concentration and exposure time of the drug, so it can change adverse drug reactions (ADRs) and efficacy. Twenty-four-hour continuous infusion of amphotericin B reduces renal toxicity and infusion reaction without increasing mortality compared with a 4-hour infusion.13 Doxorubicin administered as a 48-hour or 96-hour continuous intravenous infusion reduced cardiac toxicity.14 Thus, continuous infusion is known as a method of lowering some toxicity by reducing the peak blood drug concentration. However, the ADRs of other chemotherapeutic drugs have been reported to increase due to continuous administration. There has been a meta-analysis of paclitaxel 3-hour and 24-hour infusions. In the 24-hour infusion group, there were more frequent side effects and severe toxicity, especially febrile neutropenia, diarrhea, and sore mouth. In contrast, 24-hour infusions caused less nerve toxicity.15,16 Mucositis and stomatitis when treated with 5-fluorouracil are also known as ADRs that require more attention in continuous infusion.17 RDD is a drug administration method that changes the infusion rate of a drug and involves a change in the blood drug concentration. Therefore, it is necessary to accumulate knowledge about the effects of these methods of administration on ADRs and efficacy of drugs. But, studies of ADRs and efficacy of RDD for chemotherapeutic agents are scarce.
In this study, we compared severe ADRs and efficacy between RDD and standard administration in patients with relapsed ovarian cancer who received carboplatin as a palliative chemotherapy.
MATERIALS AND METHODS
Informed consent statement
This study was approved by the Institutional Review Board (IRB) of Kyungpook National University Chilgok Hospital (IRB number: 2020-02-013).
Study protocol and study population
A retrospective cohort study was performed on patients with the following inclusion criteria: 1) carboplatin based chemotherapy from 2017 to 2019 at Kyungpook National University Chilgok Hospital; 2) age ≥ 20 years; 3) tubo-ovarian cancer or primary peritoneal carcinomatosis; 4) platinum sensitive relapsed cancer; and 5) chemotherapy regimen: carboplatin + gemcitabine or carboplatin + paclitaxel (± bevacizumab). Patients who did not the complete chemotherapy schedule of the cycle for any reason were excluded.
Each patient received a standard 6 to 9 cycles of carboplatin area under the curve 6 and paclitaxel 175 mg/m2 every 3 weeks as a frontline chemotherapy previously. All the patients underwent radical debulking tumor surgery. Subsequently, a second-line or later-line chemotherapy regimen was administered due to recurring ovarian cancer between 2017 and 2019. The group to which carboplatin was administered through RDD was labelled as the RDD group, and the group to which carboplatin was administered as a 1-hour infusion, which is a standard method, was labelled as the control group. We retrospectively reviewed the patients' electronic medical records and obtained the patients' baseline characteristics (such as age, sex and underling diseases), previous chemotherapy history, chemotherapy regimen, chemotherapy cycle number, carboplatin dose, whether desensitization performed, and desensitization result. The severity of the initial HSR and BTR was scored according to Brown's anaphylaxis classification as in previous studies.8,18 A grade 1 reaction (mild) was defined as involving only skin and subcutaneous tissue. Systemic involvement of respiratory, cardiovascular and gastrointestinal (GI) organs was regarded as a grade 2 reaction (moderate). Concurrent systemic organ involvement and vital sign changes (such as hypoxia, hypotension, and neurological changes) were classified as a grade 3 reaction (severe).
Rapid desensitization protocol
The 1-bag, 12-step desensitization protocol was previously reported by Lee et al.10 and Chung et al. 19 and we performed desensitization with a modified protocol (Fig. 1). The 1-bag protocol started at 1/1,500 of the final infusion rate without dilution. Each step, except the last one, consuming 15 minutes, and the dose at each step was increased to 1.6 to 2.1 times the previous step until reaching a rate of 300 mL/hr as the final step. Since the 1-bag protocol use an undiluted drug, only a very small amount of the drug enters the initial step, and 5% dextrose water was administered as a side stream at a rate of 40 mL/hr throughout the entire desensitization process. H1 blockers (fexofenadine 180 mg and chlorpheniramine 4 mg) and montelukast (10 mg) were given 1 hour before chemotherapy as premedication. Systemic steroid (dexamethasone 5 mg) was administered as an antiemetic, regardless of the RDD.
Fig. 1. The 1-bag 12-step carboplatin desensitization protocol. For example: 400 mg carboplatin, mixed with 500 mL of 5% dextrose, and 0.741 mg/mL of carboplatin is administered. Start at 1/1,500 final speed, increase speed at 15-minute intervals. The 5% dextrose water was administered as a side stream at a rate of 40 mL/hr.
NA, not available.
Outcome measures
ADRs
The primary outcome was the incidence of serious adverse events (SAEs) as compared with the RDD group and the control group. SAEs were defined as follows: 1) death; 2) life-threatening; 3) admission to hospital, prolongation of hospitalization or emergency room visit; 4) disability or permanent damage; and 5) congenital anomaly/birth defect. The secondary outcome was the incidence of over grade 3 of ADRs according to organ classes. Organ classes were classified based on Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (NCI CTCAE v5.0). The grades are classified according to the following symptoms and signs: grade 1 reactions where the patient has mild symptoms and additional interventions are not required; grade 2 reactions where the patient has moderate symptoms and requires oral medication; grade 3 reactions where the patient shows severe symptoms and requires hospitalization or intravenous medication; grade 4 reactions where the patient exhibits life-threatening; grade 5 reactions where a drug reaction was related to patient death. Laboratory tests were performed on days 8 and 22 after carboplatin administration, and the ADR grade was according to CTCAE 5.0.value.
Efficacy
The primary outcome was time to progression (TTP) as compared with the RDD group and the control group. TTP was defined as the period from the start of the chemotherapy regimen to the determination of cancer progression. The secondary outcome was 12-month survival, 24-month survival and overall survival (OS). OS was defined as the time of death for any reason from the onset of the second-line chemotherapy due to cancer recurrences.
Statistical analysis
When we performed univariate analysis of ADRs, we analyzed the same chemotherapy cycles separately. Categorical variables are presented as number and percentages, and they were analyzed using Pearson's χ2 test and Fisher's exact test. Continuous variables are presented as mean ± standard deviation ranges and were analyzed using Student's t test or the Mann-Whitney U test with a 95% confidence interval. A multivariable logistic regression test for ADRs was performed between the RDD group and the control group. Age, carboplatin dose, chemotherapy regimen, and previous chemotherapy history were corrected by logistic regression. TTP and survival analysis were compared between groups using Kaplan-Meier survival curves and a log-rank test. We assessed the multivariate effects of covariates with Cox regression analysis to estimate the efficacy adjusted by the following variables: age; time to relapse; previous chemotherapy history; Eastern Cooperative Oncology Group (ECOG) performance status (PS), concurrent chemotherapeutic drug. All results with a P value of < 0.05 were considered statistically significant. Statistical analyzes were performed with IBM SPSS Statistics (version 24.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Patient selection flow and baseline characteristics
From 2017 to 2019, 21 patients received a total of 108 desensitization procedures. Four procedures were excluded from the ADR analysis because the administration of carboplatin was not completed due to BTR symptoms. One patient was excluded from the analysis because excluded RDD procedure was the only carboplatin containing chemotherapy (Supplementary Fig. S1). Accordingly, there were 104 cycles in 20 patients included in the ADR analysis as the RDD group. The control group comprised 271 cycles in a total of 41 patients (Table 1). The mean ages of patients in the RDD group and the control group were 61.10 ± 9.79 years and 62.51 ± 9.95 years, respectively. There were no significant differences between clinical and demographic characteristics of the RDD and the control groups. All patients were diagnosed as female relapsed ovarian cancer who received palliative chemotherapy, including carboplatin. The RDD group had the mean cycle of 7.20 ± 5.54 in the same-line chemotherapy regimen, of which 5.35 ± 4.64 were administered through RDD. The control group had the mean cycle of 6.29 ± 3.79 in the same-line chemotherapy regimen. The time to the first relapse, number of previous total chemotherapy cycles and number of total carboplatin cycles tended to be higher in the RDD group, but this was statistically insignificant. ECOG PS 2, hypertension, diabetes, and cardiovascular disease were more prevalent in the control group, but there was no significant difference between the 2 groups. In the efficacy analysis, 4 patients in the RDD group who had to change regimen due to BTR were excluded and 17 RDD and 41 control patients were analyzed (Supplementary Fig. S1).
Table 1. Clinical characteristics of the study subjects.
| Characteristics | RDD group (n = 20) | Control group (n = 41) | P value | |
|---|---|---|---|---|
| Age (yr) | 61.10 ± 9.79 | 62.51 ± 9.95 | 0.603 | |
| Sex:female | 20 (100) | 41 (100) | 1.000 | |
| Total cycle | 104 | 271 | ||
| Average cycle per patient | ||||
| Cycle in the same-line chemotherapy regimen | 7.20 ± 5.54 | 6.29 ± 3.79 | 0.456 | |
| Rapid desensitization | 5.35 ± 4.64 | - | ||
| Time to first relapse after initial diagnosis (mon) | 30.35 ± 17.75 | 24.46 ± 15.46 | 0.189 | |
| Treatment course before current chemotherapy regimen | ||||
| Previous total chemotherapy cycle | 15.40 ± 9.09 | 13.54 ± 7.77 | 0.437 | |
| Previous total carboplatin cycle | 12.15 ± 6.11 | 9.34 ± 5.18 | 0.066 | |
| Initial ECOG PS (0/1/2) | 1/17/2 | 3/31/7 | 0.699 | |
| Underlying disease | ||||
| Diabetes | 3 (15.0) | 9 (22.0) | 0.521 | |
| Hypertension | 3 (15.0) | 15 (36.6) | 0.083 | |
| Dyslipidemia | 3 (15.0) | 5 (12.2) | 0.761 | |
| Cardiovascular disease | 0 (0) | 3 (7.3) | 0.215 | |
| Chronic liver disease | 0 (0) | 1 (2.4) | 0.481 | |
| Respiratory disease | 0 (0) | 1 (2.4) | 0.481 | |
| Other cancer | 2 (10.0) | 4 (9.8) | 0.976 | |
Values are presented as mean ± standard deviation or number (%).
RDD, rapid drug desensitization; ECOG PS, Eastern Cooperative Oncology Group performance status.
Outcomes of RDD
The results of 108 desensitization cycles among 21 patients were analyzed. In the initial HSR evaluation, 9 (42.9%) patients of grade 1, 9 (42.9%) patients of grade 2 and 3 (14.3%) patients of grade 3 were observed. Out of 108 RDDs, 104 cycles were completed with a success rate of 96.3% (Supplementary Table S1). Among 108 RDDs cycles, no BTRs occurred in 74 cycles (68.5%) while grade 1 BTRs occurred in 26 cycles (24.1%). Six patients suffered from moderate to severe BTRs out of 8 RDDs, 1 of which was a severe reaction (Fig. 2A). Four patients had to change chemotherapy regimen to one that did not contain carboplatin due to BTR. In grade 2 or grade 3 BTRs, 6/8 were first or second cycles of RDD (Fig. 2B).
Fig. 2. Number and severity of BTRs during RDD. (A) Total RDD. (B) According to cycles of RDD.
BTR, breakthrough reaction; RDD, rapid drug desensitization.
ADRs
Primary outcome
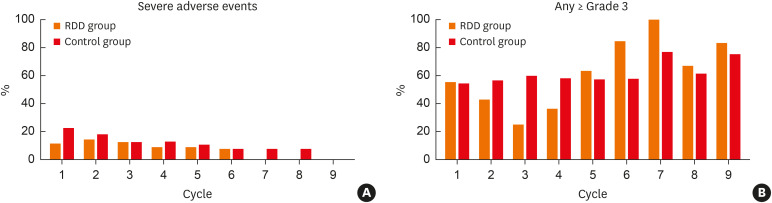
There were 8 SAEs (7.7%) in 104 RDD procedures and 34 SAEs (12.5%) in 272 standard procedures. The most frequent SAE was infection or infestations that were observed in 6 (5.8%) in the RDD group and 11 (4.1%) in the control group. Nausea and nutritional disorder were the second most common SAE, with 2 (1.9%) in the RDD group and 8 (3.0%) in the control group. Other kinds of SAE, such as GI and general disorders, occurred only in the control group. One drug-related death (pneumonia with neutropenic fever) was observed in the RDD group (Table 2). The SAEs were analyzed comparing the same chemotherapy cycle of the 2 groups. There was no significant increase in SAEs in the RDD group from the first to the ninth chemotherapy cycles (Fig. 3A and Supplementary Table S2). No significant increase in SAEs in the RDD group was observed even after correction for age, carboplatin dose, comorbid chemotherapy, and previous total chemotherapy cycle number by logistic regression analysis (Table 3).
Table 2. Severe adverse events according to rapid drug desensitization.
| Severe adverse events | RDD group (n = 104) | Control group (n = 271) |
|---|---|---|
| Death | 1 (1.0) | 0 (0.0) |
| Cardiac disorders | 0 (0.0) | 1 (0.4) |
| Gastrointestinal disorders (except nausea) | 0 (0.0) | 8 (3.0) |
| Infection and infestations | 6 (5.8) | 11 (4.1) |
| Injury, poisoning and procedural complications | 0 (0.0) | 1 (0.4) |
| Nausea and nutrition disorders | 2 (1.9) | 8 (3.0) |
| General disorders | 0 (0.0) | 3 (1.1) |
| Respiratory, thoracic and mediastinal disorders | 0 (0.0) | 1 (0.4) |
| Skin and subcutaneous tissue disorders | 0 (0.0) | 1 (0.4) |
| Total | 8 (7.7) | 34 (12.5) |
RDD, rapid drug desensitization.
Fig. 3. Severe adverse drug reactions according to the cycle of palliative chemotherapy containing carboplatin. (A) Severe adverse events. (B) Over grade 3 of any adverse drug reactions.
RDD, rapid drug desensitization.
Table 3. Risk assessment of comparison of severe adverse events in the RDD group by multivariate analysis.
| Chemotherapy cycles | Severe adverse events | ||
|---|---|---|---|
| Odds ratio | 95% CI | P value | |
| 1st | 0.714 | 0.062–8.218 | 0.787 |
| 2nd | 0.632 | 0.030–13.278 | 0.768 |
| 3rd | 0.507 | 0.032–8.124 | 0.507 |
| 4th | 0.587 | 0.027–12.691 | 0.734 |
| 5th | 1.573 | 0.070–35.485 | 0.776 |
| 6th | 1.777 | 0.097–32.589 | 0.699 |
RDD, rapid drug desensitization; CI, confidence interval.
Secondary outcome
Over grade 3 of any ADRs were observed in 70 (67.3%) of the RDD group and in 160 (59.0%) of the control group. Over grade 3 of any ADRs were analyzed comparing the same chemotherapy cycle of the 2 groups. There was no statistically significant difference between the 2 groups from the first cycle to the ninth cycle (Fig 3B and Supplementary Table S2). When analyzed through multiple logistic regression analysis, the risk of over grade 3 of ADRs was significantly lower in RDD group in the 3rd cycle, and there was no statistical difference in the other cycles (Supplementary Table S3). Over grade 3 of neutropenia was significantly higher in the RDD group on the 6th cycle (84.6% vs. 30.8%, P = 0.002) (Supplementary Fig. S2D and Supplementary Table S2). This continued even after adjustment by logistic regression (odds ratio, 10.249; 95% confidence interval [CI], 1.669–62.922; P = 0.012; Supplementary Table S3). Other cytopenia indicators (like anemia and thrombocytopenia) did not show a statistically significant difference in the RDD group. Over grade 3 of the ADRs by other organ system were not increased by the RDD group (Table 4 and Supplementary Fig. S2).
Table 4. Adverse drug reactions according to organ class.
| Characteristics | RDD group (n = 104) | Control group (n = 271) | |
|---|---|---|---|
| Any ≥ Grade 3 | 70 (67.3) | 160 (59.0) | |
| Gastrointestinal disorder (except nausea) | |||
| ≥ Grade 2 | 61 (58.7) | 160 (59.0) | |
| ≥ Grade 3 | 0 (0.0) | 8 (3.0) | |
| Infection and infestation | |||
| ≥ Grade 2 | 15 (13.9) | 26 (9.6) | |
| ≥ Grade 3 | 6 (5.6) | 11 (4.1) | |
| Nausea and nutrition disorders | |||
| ≥ Grade 2 | 46 (44.2) | 136 (50.2) | |
| ≥ Grade 3 | 2 (1.9) | 8 (3.0) | |
| Nervous system disorder | |||
| ≥ Grade 2 | 33 (30.6) | 98 (36.2) | |
| ≥ Grade 3 | 0 (0.0) | 0 (0.0) | |
| Hepatotoxicity | |||
| ≥ Grade 2 | 5 (4.6) | 8 (3.0) | |
| ≥ Grade 3 | 1 (1.0) | 2 (0.7) | |
| Nephrotoxicity | |||
| ≥ Grade 2 | 1 (1.0) | 5 (1.8) | |
| ≥ Grade 3 | 1 (1.0) | 0 (0.0) | |
| Neutropenia | |||
| ≥ Grade 2 | 84 (77.8) | 184 (67.9) | |
| ≥ Grade 3 | 62 (57.4) | 107 (39.5) | |
| Anemia | |||
| ≥ Grade 2 | 82 (75.9) | 202 (74.5) | |
| ≥ Grade 3 | 25 (23.1) | 73 (26.9) | |
| Thrombocytopenia | |||
| ≥ Grade 2 | 45 (41.7) | 89 (32.8) | |
| ≥ Grade 3 | 22 (20.4) | 66 (24.4) | |
RDD, rapid drug desensitization.
Efficacy
Primary outcome
We compared the efficacy of 17 carboplatin-desensitized patients and 41 control patients. The median TTP for the total regimen was 17.14 months (95% CI, 12.28–22.00) in the RDD group and 14.27 months (95% CI, 10.95–17.59) in the control group. When limited to the 2nd line regimen, the median TTP was 19.94 months in the RDD group, and 17.23 months in the control group. There was no significant difference in TTP between the 2 groups (Fig. 4). TTP for total regimen after correction for age, time to relapse, previous chemotherapy history and ECOG PS, hazard ratio of desensitization was 0.733 (95% CI, 0.320–1.680; P = 0.463). When comparing only 2nd line regimen, hazard ratio of desensitization was 1.945 (95% CI, 0.476–7.951; P = 0.355) (Table 5).
Fig. 4. Curves for time to progression of the RDD group and the control group, defined as the time from 1st cycle of regimen to cancer progression. (A) Total regimen. (B) Second line regimen.
RDD, rapid drug desensitization.
Table 5. Multivariate cox regression analysis of TTP and OS.
| Characteristics | TTP | OS | |||||
|---|---|---|---|---|---|---|---|
| Total | 2nd line regimen | ||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Rapid desensitization of carboplatin | 0.733 (0.320–1.680) | 0.463 | 1.945 (0.476–7.951) | 0.355 | 1.310 (0.432–3.974) | 0.634 | |
| Age (yr) | 1.010 (0.960–1.063) | 0.700 | 1.042 (0.970–1.121) | 0.262 | 0.942 (0.871–1.019) | 0.138 | |
| Time to relapse (mon) | 0.970 (0.943–0.999) | 0.042 | 0.885 (0.809–0.967) | 0.007 | 0.952 (0.909–0.998) | 0.040 | |
| Previous chemotherapy cycle (number) | 1.086 (1.024–1.151) | 0.006 | 0.983 (0.882–1.096) | 0.759 | 0.988 (0.913–1.069) | 0.767 | |
| ECOG PS | |||||||
| 1 | 1.302 (0.399–4.250) | 0.661 | 2.832 (0.385–20.841) | 0.669 | 1.776 (0.208–15.163) | 0.600 | |
| 2 | 1.840 (0.437–7.744) | 0.405 | 8.674 (0.843–89.314) | 0.101 | 3.734 (0.307–45.415) | 0.301 | |
| Concurrent chemotherapeutic drug (gemcitabine) | 2.018 (0.696–5.849) | 0.196 | 3.356 (0.783–14.391) | 0.103 | 0.652 (0.194–2.191) | 0.489 | |
TTP, time to progression; OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status.
Secondary outcome
The 12-month survival rate was not significantly different between the RDD and control groups (14/15, 93.3% vs. 27/31, 87.1%). The 24-month survival rate was also 66.7% (8/12) for RDD group and 68.2% (15/22) for the control group, there was no significant difference between the 2 groups. The estimated median survival time was 47.84 months (95% CI, 33.23–63.45) for the RDD group and 41.22 months (95% CI, 33.86–48.59) for the control group. There was no significant difference in OS between the 2 groups (Log-rank P = 0.944) (Fig. 5). OS after correction for age, time to relapse, previous chemotherapy history and ECOG PS, hazard ratio of desensitization was 1.310 (95% CI, 0.432–3.974; P = 0.634) (Table 5).
Fig. 5. Curves for overall survival of the RDD group and the control group, defined as the time from cancer recurrence to death.
RDD, rapid drug desensitization.
DISCUSSION
Our study demonstrated that RDD does not increase the frequency of severe ADRs compared with the standard administration method and has similar efficacy. Our study is meaningful in that it compared a relatively similar group of patients for the first time. Based on our findings, we suggest that RDD is not only an effective way to reduce the risk of HSRs, but also a way to administer drugs without affecting efficacy and other severe ADRs.
Common SAEs of chemotherapy include infection, cytopenia, nausea, GI motility symptoms, malnutrition and constitutional/dehydration symptoms.20 Platinum compounds are also known to have neurotoxicity, nephrotoxicity, and ototoxicity, but carboplatin is rarer than cisplatin.21,22,23 In our study, infection or infestations was the most common SAE. Malnutrition by nausea and anorexia, and GI symptoms including diarrhea and abdominal distension were also observed in some patients. As for neurotoxicity, more than 30% procedures were associated with neurotoxicity below grade 2 and grade 3 neurotoxicity was not observed. Only 6 procedures were accompanied by an increase in creatinine above grade 2, and there was no increase above grade 3 except in the fatal cases who progress multi-organ failure due to infection, so nephrotoxicity was tolerable.
GI disorder, nausea, anorexia, malnutrition and general disorder tended to be lower in the RDD group, especially as regards grade 3 reactions requiring hospitalization or emergency room visits. ADRs particularly common in platinum compounds, such as neurotoxicity and nephrotoxicity, also tended to be lower. However, the infection and infestations tended to be higher in the RDD group. Among all patients, there was one death related to ADR of chemotherapy, and this was the death caused by infection with neutropenia in the RDD group. In addition, neutropenia above grade 3 tended to increase in the RDD group as the order of chemotherapy cycle increased, especially after the sixth cycle, showing statistically significant differences. Cochrane review for paclitaxel presents the lower prevalence of neutropenia in short duration infusions than long duration infusions of paclitaxel.15 Our study showed similar results to the above study that the incidence of neutropenia was higher in the desensitized group, which required more than 4 hours of administration time than the control group that received carboplatin for 1 hour, which is the conventional administration time. Since, in our study, the number of patients with anemia and thrombocytopenia did not significantly change, further research is required to determine whether RDD causes bone marrow suppression but more careful observation of bone marrow suppression may be required in rapid desensitization.
Previous studies showed the relationship between RDD and OS. In relapsed ovarian cancer, there was no difference in OS between patients with RDD and the control patients.8 In a recent study, oxaliplatin administration in colon cancer showed that there was no difference in OS between RDD and control groups.24 Another study showed that patients who were desensitized to carboplatin hypersensitivity had longer OS than patients without hypersensitivity to carboplatin not undergoing desensitization.25 TTP was analyzed for the first time in this study. Although there was no statistical difference, the RDD group showed a longer TTP, despite the time to relapse and the number of previous chemotherapy cycles being higher. Through this, administration of carboplatin through RDD is not expected to decrease efficacy, and it is thought that it can be applied to other chemotherapeutic agents. In addition, 12-month survival, 24-month survival and OS were not statistically different, so it can be confirmed again that RDD does not affect OS.
Carboplatin is one of the most important chemotherapeutic agents in ovarian cancer, and is mostly used as 1st line chemotherapy.26 Despite progress in the first-line treatment of ovarian cancer, the majority of patients relapse, so it is important to re-administer carboplatin for carboplatin sensitive ovarian cancers.27,28 However, one of the major problems with re-administration of carboplatin is the frequent occurrence of immediate HSR. Carboplatin immediate HSRs are known to increase as the cycle is repeated (6.5% for 6 cycles and 27% for 7 cycles, and 44% for 3rd line re-administration).29,30,31 When a carboplatin immediate HSR occurs in patients with carboplatin sensitive ovarian cancer, it has been well known from previous studies that administration of drugs through RDD significantly benefits OS compared with avoiding carboplatin. Therefore, a safe method for administering carboplatin was needed in these patients, and successful administration through RDD was reported.8,32 In our study, RDD had to be performed for a known HSR in approximately 1/3 of patients who used carboplatin for recurrent ovarian cancer patients who used carboplatin as the 1st line.
The success rate and number of BTR in the RDD group were similar to those results in previous carboplatin desensitization studies. Platinum compounds, such as carboplatin, were reported to have a higher BTR incidence than those of paclitaxel or monoclonal antibodies.8,33,34 Carboplatin infusion through RDD was reported to cause grade 2 or higher BTR in 5% to 10%.7,35,36,37 According to Sloane et al.,8 the BTR results of 1,069 desensitization therapies of carboplatin showed a similar rate to the BTR results of our study. It was also demonstrated in this study that the risk of BTR decreases as the number of desensitization therapies is repeated.10,38
In this study, we adopted a 1-bag desensitization protocol. RDD using 1-bag protocol has proven to be good in previous studies. In a previous study, 1-bag 12-step chemotherapy was successfully performed with platinum compounds,19 In another retrospective cohort study, success rate and BTR frequency were not statistically different between 3-bag system and 1-bag system for paclitaxel administration.10 In addition, when using the 1-bag system, it was possible to reduce the infusion time compared with the multi-bag protocol, thereby reducing the effort of medical staff.10 In this study, carboplatin 400 mg was administered for 254 minutes, showing a similar infusion time to previous 1-bag studies.
There are some limitations to this study. First, our study was conducted retrospectively in a single hospital with a resultant small sample size and reduced statistical power. ADRs may be sensitive to the patient's underlying condition. Prospective double-blind randomized trials would be ideal for ADR evaluation. Despite these limitations, however, our research was conducted consistently, so the desensitization protocol, treatment to ADRs, and cancer treatment protocol were able to follow up the patient under the most unified conditions, allowing a valid comparison between the 2 groups. In addition, due to the nature of RDD, it is not possible to conduct a randomized clinical trial. In order to further generalize as a conclusion of our research, there should be larger multicenter research in the future. Secondly, in our study, in patients with carboplatin immediate HSR, there was no comparison between the group that gave up carboplatin and the carboplatin RDD group. We attempted to re-administer all patients who developed carboplatin hypersensitivity during the study period, and only patients with moderate or higher BTR despite RDD tried other treatments than carboplatin. There were 4 patients who changed carboplatin, and the number of patients was too small to analyze separately. Thirdly, this study did not evaluate the mechanism of carboplatin hypersensitivity prior to desensitization. However, this study was aimed at analyzing the drug efficacy and toxicity according to infusion methods, so drug hypersensitivity mechanisms would not have had a significant influence. In addition, carboplatin is generally known to induce immunoglobulin E-mediated hypersensitivity as the carboplatin cycle repeats. The RDD group in our study showed almost one-third of patients who used carboplatin for recurrent ovarian cancer and had administered more than 10 times before RDD. This is reasonable considering the previous evidence of carboplatin hypersensitivity in more than 25% of patients after 6 cycles.39
In conclusion, RDD did not increase severe ADRs compared to the standard drug administration method, and it was confirmed that there was no difference in efficacy of drug. Based on the results of this study, it is suggested that rapid desensitization of carboplatin is similar to the standard infusion method in terms of efficacy and severe ADRs.
ACKNOWLEDGMENTS
This work was supported by Biomedical Research Institute grant, Kyungpook National University Hospital (2018).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Clinical characteristic of initial HSR and BTR
Analysis of univariate for each cycle for severe ADRs according to RDD
Risk assessment of comparison of ADRs over grade 3 and neutropenia over grade 3 in the RDD group by multivariate analysis
Flow chart of study patient selection.
Adverse drug reactions according to the cycle of palliative chemotherapy containing carboplatin.
References
- 1.Romano A, Torres MJ, Castells M, Sanz ML, Blanca M. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127:S67–73. doi: 10.1016/j.jaci.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 2.Lee Y, Chang HY, Kim SH, Yang MS, Koh YI, Kang HR, et al. A prospective observation of psychological distress in patients with anaphylaxis. Allergy Asthma Immunol Res. 2020;12:496–506. doi: 10.4168/aair.2020.12.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montañez MI, Mayorga C, Bogas G, Barrionuevo E, Fernandez-Santamaria R, Martin-Serrano A, et al. Epidemiology, mechanisms, and diagnosis of drug-induced anaphylaxis. Front Immunol. 2017;8:614. doi: 10.3389/fimmu.2017.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castells M, Sancho-Serra MC, Simarro M. Hypersensitivity to antineoplastic agents: mechanisms and treatment with rapid desensitization. Cancer Immunol Immunother. 2012;61:1575–1584. doi: 10.1007/s00262-012-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cernadas JR, Brockow K, Romano A, Aberer W, Torres MJ, Bircher A, et al. General considerations on rapid desensitization for drug hypersensitivity - a consensus statement. Allergy. 2010;65:1357–1366. doi: 10.1111/j.1398-9995.2010.02441.x. [DOI] [PubMed] [Google Scholar]
- 6.Madrigal-Burgaleta R, Berges-Gimeno MP, Angel-Pereira D, Ferreiro-Monteagudo R, Guillen-Ponce C, Pueyo C, et al. Hypersensitivity and desensitization to antineoplastic agents: outcomes of 189 procedures with a new short protocol and novel diagnostic tools assessment. Allergy. 2013;68:853–861. doi: 10.1111/all.12105. [DOI] [PubMed] [Google Scholar]
- 7.Castells MC, Tennant NM, Sloane DE, Hsu FI, Barrett NA, Hong DI, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008;122:574–580. doi: 10.1016/j.jaci.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 8.Sloane D, Govindarajulu U, Harrow-Mortelliti J, Barry W, Hsu FI, Hong D, et al. Safety, costs, and efficacy of rapid drug desensitizations to chemotherapy and monoclonal antibodies. J Allergy Clin Immunol Pract. 2016;4:497–504. doi: 10.1016/j.jaip.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Madrigal-Burgaleta R, Bernal-Rubio L, Berges-Gimeno MP, Carpio-Escalona LV, Gehlhaar P, Alvarez-Cuesta E. A large single-hospital experience using drug provocation testing and rapid drug desensitization in hypersensitivity to antineoplastic and biological agents. J Allergy Clin Immunol Pract. 2019;7:618–632. doi: 10.1016/j.jaip.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Moon M, Kim YC, Chung SJ, Oh J, Kang DY, et al. a one-bag rapid desensitization protocol for paclitaxel hypersensitivity: a noninferior alternative to a multi-bag rapid desensitization protocol. J Allergy Clin Immunol Pract. 2020;8:696–703. doi: 10.1016/j.jaip.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 11.O'Cearbhaill R, Zhou Q, Iasonos A, Hensley ML, Tew WP, Aghajanian C, et al. The prophylactic conversion to an extended infusion schedule and use of premedication to prevent hypersensitivity reactions in ovarian cancer patients during carboplatin retreatment. Gynecol Oncol. 2010;116:326–331. doi: 10.1016/j.ygyno.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pornwattanakrilert W, Suprasert P. Comparison of hypersensitivity reactions to carboplatin retreatment in gynecologic cancer patients between one and two hour infusions: a randomized trial study. Asian Pac J Cancer Prev. 2017;18:425–430. doi: 10.22034/APJCP.2017.18.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson U, Seifert B, Schaffner A. Comparison of effects of amphotericin B deoxycholate infused over 4 or 24 hours: randomised controlled trial. BMJ. 2001;322:579–582. doi: 10.1136/bmj.322.7286.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hortobagyi GN, Frye D, Buzdar AU, Ewer MS, Fraschini G, Hug V, et al. Decreased cardiac toxicity of doxorubicin administered by continuous intravenous infusion in combination chemotherapy for metastatic breast carcinoma. Cancer. 1989;63:37–45. doi: 10.1002/1097-0142(19890101)63:1<37::aid-cncr2820630106>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Williams C, Bryant A. Short versus long duration infusions of paclitaxel for any advanced adenocarcinoma. Cochrane Database Syst Rev. 2011:CD003911. doi: 10.1002/14651858.CD003911.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams C, Collingwood M, Simera I, Grafton C. Short versus long duration infusions of paclitaxel for any adenocarcinoma. Cochrane Database Syst Rev. 2002:CD003911. doi: 10.1002/14651858.CD003911. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald JS. Toxicity of 5-fluorouracil. Oncology (Williston Park) 1999;13:33–34. [PubMed] [Google Scholar]
- 18.Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114:371–376. doi: 10.1016/j.jaci.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 19.Chung SJ, Kang SY, Kang RY, Kim YC, Lee KH, Kim TY, et al. A new non-dilution rapid desensitization protocol successfully applied to all-grade platinum hypersensitivity. Cancer Chemother Pharmacol. 2018;82:777–785. doi: 10.1007/s00280-018-3662-0. [DOI] [PubMed] [Google Scholar]
- 20.Hassett MJ, O'Malley AJ, Pakes JR, Newhouse JP, Earle CC. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98:1108–1117. doi: 10.1093/jnci/djj305. [DOI] [PubMed] [Google Scholar]
- 21.Rajeswaran A, Trojan A, Burnand B, Giannelli M. Efficacy and side effects of cisplatin- and carboplatin-based doublet chemotherapeutic regimens versus non-platinum-based doublet chemotherapeutic regimens as first line treatment of metastatic non-small cell lung carcinoma: a systematic review of randomized controlled trials. Lung Cancer. 2008;59:1–11. doi: 10.1016/j.lungcan.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Neijt JP, Engelholm SA, Tuxen MK, Sørensen PG, Hansen M, Sessa C, et al. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J Clin Oncol. 2000;18:3084–3092. doi: 10.1200/JCO.2000.18.17.3084. [DOI] [PubMed] [Google Scholar]
- 23.Steiner H, Höltl L, Wirtenberger W, Berger AP, Bartsch G, Hobisch A. Long-term experience with carboplatin monotherapy for clinical stage I seminoma: a retrospective single-center study. Urology. 2002;60:324–328. doi: 10.1016/s0090-4295(02)01708-9. [DOI] [PubMed] [Google Scholar]
- 24.Madrigal-Burgaleta R. Does rapid drug desensitization to chemotherapy affect survival outcomes? J Investig Allergol Clin Immunol. 2019 doi: 10.18176/jiaci.0425. [DOI] [PubMed] [Google Scholar]
- 25.Altwerger G, Florsheim EB, Menderes G, Black J, Schwab C, Gressel GM, et al. Impact of carboplatin hypersensitivity and desensitization on patients with recurrent ovarian cancer. J Cancer Res Clin Oncol. 2018;144:2449–2456. doi: 10.1007/s00432-018-2753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vergote I, Scambia G, O'Malley DM, Van Calster B, Park SY, Del Campo JM, et al. Trebananib or placebo plus carboplatin and paclitaxel as first-line treatment for advanced ovarian cancer (TRINOVA-3/ENGOT-ov2/GOG-3001): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20:862–876. doi: 10.1016/S1470-2045(19)30178-0. [DOI] [PubMed] [Google Scholar]
- 27.Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–4707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 28.Pfisterer J, Vergote I, Du Bois A, Eisenhauer E, et al. AGO-OVAR; NCIC CTG. Combination therapy with gemcitabine and carboplatin in recurrent ovarian cancer. Int J Gynecol Cancer. 2005;15(Suppl 1):36–41. doi: 10.1111/j.1525-1438.2005.15355.x. [DOI] [PubMed] [Google Scholar]
- 29.Makrilia N, Syrigou E, Kaklamanos I, Manolopoulos L, Saif MW. Hypersensitivity reactions associated with platinum antineoplastic agents: a systematic review. Met Based Drugs. 2010;2010:207084. doi: 10.1155/2010/207084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markman M, Kennedy A, Webster K, Elson P, Peterson G, Kulp B, et al. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol. 1999;17:1141. doi: 10.1200/JCO.1999.17.4.1141. [DOI] [PubMed] [Google Scholar]
- 31.Hoekstra AV, Hurteau JA, Kirschner CV, Rodriguez GC. The combination of monthly carboplatin and weekly paclitaxel is highly active for the treatment of recurrent ovarian cancer. Gynecol Oncol. 2009;115:377–381. doi: 10.1016/j.ygyno.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 32.Hesterberg PE, Banerji A, Oren E, Penson RT, Krasner CN, Seiden MV, et al. Risk stratification for desensitization of patients with carboplatin hypersensitivity: clinical presentation and management. J Allergy Clin Immunol. 2009;123:1262–1267.e1. doi: 10.1016/j.jaci.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 33.Picard M, Pur L, Caiado J, Giavina-Bianchi P, Galvão VR, Berlin ST, et al. Risk stratification and skin testing to guide re-exposure in taxane-induced hypersensitivity reactions. J Allergy Clin Immunol. 2016;137:1154–1164.e12. doi: 10.1016/j.jaci.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 34.Kendirlinan R, Gümüşburun R, Çerçi P, Özbek E, Altıner S, Çelebi Sözener Z, et al. Rapid drug desensitization with chemotherapeutics (platins, taxanes, and others): a single-center retrospective study. Int Arch Allergy Immunol. 2019;179:114–122. doi: 10.1159/000496745. [DOI] [PubMed] [Google Scholar]
- 35.Castells Guitart MC. Rapid drug desensitization for hypersensitivity reactions to chemotherapy and monoclonal antibodies in the 21st century. J Investig Allergol Clin Immunol. 2014;24:72–79. [PubMed] [Google Scholar]
- 36.Kang Y, Kwon OY, Jung H, Kang M, An J, Lee JH, et al. Breakthrough reactions during rapid drug desensitization: clinical outcome and risk factors. Ann Allergy Asthma Immunol. 2019;123:48–56.e1. doi: 10.1016/j.anai.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Castells M. Drug hypersensitivity and anaphylaxis in cancer and chronic inflammatory diseases: the role of desensitizations. Front Immunol. 2017;8:1472. doi: 10.3389/fimmu.2017.01472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Malley DM, Vetter MH, Cohn DE, Khan A, Hays JL. Outpatient desensitization in selected patients with platinum hypersensitivity reactions. Gynecol Oncol. 2017;145:603–610. doi: 10.1016/j.ygyno.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Zanotti KM, Rybicki LA, Kennedy AW, Belinson JL, Webster KD, Kulp B, et al. Carboplatin skin testing: a skin-testing protocol for predicting hypersensitivity to carboplatin chemotherapy. J Clin Oncol. 2001;19:3126–3129. doi: 10.1200/JCO.2001.19.12.3126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical characteristic of initial HSR and BTR
Analysis of univariate for each cycle for severe ADRs according to RDD
Risk assessment of comparison of ADRs over grade 3 and neutropenia over grade 3 in the RDD group by multivariate analysis
Flow chart of study patient selection.
Adverse drug reactions according to the cycle of palliative chemotherapy containing carboplatin.