Abstract
Purpose
Recent studies have revealed the pathogenic role of interleukin (IL)-22 in atopic dermatitis and asthma. However, little is known about the role of IL-22 in the pathophysiology of chronic rhinosinusitis with nasal polyps. We aimed to investigate the expression of IL-22 and its pathogenic function in type 2 immune reactions of nasal polyps (NP).
Methods
Protein levels of inflammatory mediators were determined by multiplex immunoassay, and principal component analysis (PCA) was performed. Immunofluorescence analysis and mast cell culture were used to determine the cellular sources of IL-22. Normal human bronchial epithelial (NHBE) cells were stimulated using IL-22 in combination with diverse cytokines, and thymic stromal lymphopoietin (TSLP) was measured.
Results
IL-22 expression was not up-regulated in NP compared with control tissues, but IL-22-high NP revealed distinct features characterized by type 2 inflammatory cytokines such as chemokine (C-C motif) ligand (CCL)-11, CCL-24, and IL-5 on the PCA. Additionally, IL-22 positively correlated with type 2 immune mediators and the disease severity in NP. For the localization of the cellular sources of IL-22 in eosinophilic NP, it was expressed in cells mostly composed of eosinophil peroxidase-positive cells and partially of tryptase-positive cells. The human mast cell line, LAD2 cells, released IL-22 mediated by immunoglobulin E. Moreover, IL-22 receptor subunit alpha-1 (IL-22Ra1) expression was significantly increased in NP. IL-22Ra1 was up-regulated with poly(I:C) stimulation in NHBE cells. Furthermore, TSLP production was enhanced when stimulated with a combination of IL-13, poly(I:C), and IL-22. Treatment with anti-IL-22Ra1 also inhibited IL-22-induced enhancement of TSLP production.
Conclusion
IL-22 was associated with type 2 inflammatory reactions in NP. The IL-22/IL-22Ra1 axis was enhanced and might be involved in type 2 inflammatory reactions via TSLP production in NP.
Keywords: Interleukin-22, biomarkers, thymic stromal lymphopoietin, sinusitis, eosinophils, mast cells, nasal polyps, immunoassay, fluorescent antibody technique
INTRODUCTION
Chronic rhinosinusitis (CRS) is characterized by chronic inflammation of the paranasal sinus mucosa that persists for at least 12 weeks.1 Currently, the phenotype of CRS is classified into 2 groups: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP), depending on the presence or absence of nasal polyps (NP) during endoscopic examination.1 Endotypes can be classified according to the immunological patterns within the same phenotype of CRS.2,3,4 To date, several studies have demonstrated that a number of inflammatory markers and various immune cells have been engaged in the pathogenesis of CRS.5,6,7,8,9,10,11 However, different phenotypes of CRS show a very similar endotype, or its different endotypes are often presented in a single clinical phenotype. Thus, such disease heterogeneity in patients with CRS remains poorly understood.
Interleukin (IL)-22 is a member of the IL-10 family of cytokines and its signaling pathway plays crucial roles in regulating host defense, tissue homeostasis, and inflammation at mucosa barrier surface.12 Until now, signaling of IL-22 through its receptor (IL-22R) is known to promote antimicrobial immunity, inflammation, and tissue repair.12 In addition, IL-22 is also produced by various inflammatory cells, including Th1, Th2, Th17, Th22 cells, natural killer cells, and type 3 innate lymphoid cells,13,14 whereas IL-22R is expressed on epithelial cells rather than immune cells.15 However, several studies demonstrated that IL-22 plays a key role in the pathogenicity of allergic diseases.16,17,18 Recent studies showed that IL-22/IL-22R signaling regulates the pathogenesis of CRSwNP via alteration in MUC1 expression.19 Therefore, in this study, we aimed to investigate the expression level of IL-22 and its pathogenic function in patients with CRSwNP.
MATERIALS AND METHODS
Patients and tissue samples
Sinonasal and NP tissues were obtained from patients with CRS during routine functional endoscopic sinus surgery. All participants provided written informed consent prior to the study, which was approved by the Internal Review Board of Seoul National University Hospital, Boramae Medical Center (No. 30-2017-78). CRS diagnosis was made based on history taking, physical examination, nasal endoscopic exam, and computed tomography (CT) findings of the sinuses according to the 2012 European position paper on rhinosinusitis and NP guidelines.1 Exclusion criteria are as follows: (1) age younger than 18 years; (2) history of receiving treatment with antibiotics, systemic or topical corticosteroids, or other immune-modulating drugs during 4 weeks prior to surgery; and (3) having been diagnosed with unilateral rhinosinusitis, antrochoanal polyp, allergic fungal rhinosinusitis, cystic fibrosis, or immotile ciliary disease. Control tissues were obtained from patients without any sinonasal diseases during other types of rhinologic surgeries such as skull base and lacrimal duct surgeries. Uncinate process mucosal tissues were obtained, each from control subjects and patients with CRSsNP or CRSwNP; NP tissues in patients with CRSwNP were obtained for evaluation. As previously described,20,21,22 each tissue was divided into 3 parts: one was fixed in 10% formaldehyde and embedded in paraffin for histological analysis, another was immediately frozen and stored at −80ºC for subsequent isolation of mRNA, and the third was submerged in 1 mL phosphate-buffered saline supplemented with 0.05% Tween-20 (Sigma-Aldrich, St. Louis, MO, USA) and 1% PIC (Sigma-Aldrich) per 0.1 g of tissue.23 Then, the third samples were homogenized with a mechanical homogenizer at 1,000 rpm on ice for 5 minutes. After homogenization, the floating materials were centrifuged at 3,000 rpm for 10 minutes at 4ºC, and the supernatants were separated and stored at −80ºC for further analysis of cytokines and other inflammatory mediators. The atopic status of the study subjects was evaluated using the ImmunoCAP® assay (ThermoFisher Scientific, Waltham, MA, USA) to detect specific Immunoglobulin E (IgE) antibodies against 6 mixtures of common aeroallergens (house dust mites, molds, trees, weeds, grass pollen, and animal danders). Subjects were considered atopic if the allergen-specific IgE level was greater than 0.35 KU/L to more than 1 allergen.24 An asthmatic patient was defined as one who experienced chronic airway symptoms (dyspnea, cough, wheezing, and/or sputum) and reversible airflow limitation and had forced expiratory volume in 1 second increased by ≥ 12% or 200 mL after using a bronchodilator or a methacholine provocation test result of PC20 ≤ 16 mg/mL. Disease severity was evaluated by CT images using the Lund-Mackay scoring system. Patient characteristics are presented in Supplementary Table S1.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA was extracted from tissue samples using the TRI reagent (Invitrogen, Carlsbad, CA, USA). The 1 µg of total RNA was reversely transcribed into cDNA using the cDNA Synthesis Kit (amfiRivert Platinum cDNA Synthesis Master Mix; GenDEPOT, Katy, TX, USA). The qRT-PCR was performed. Analysis of IL-22 receptor subunit alpha-1 (IL-22Ra1; Hs00222035_m1), IL-22Ra2 (Hs00364814_m1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Hs02758991_g1) was performed using pre-developed assay reagent kits of primers and probes from TaqMan assays (Life Technologies Korea, Seoul, Korea). Pre-developed assay reagent kits containing primers and probes were purchased from Applied Biosystems (Foster City, CA, USA). The expression of GAPDH was used as an internal control for normalization. Cycling conditions are as follows: 95°C for 5 minutes, followed by 60 cycles at 95°C for 15 seconds, 60°C for 20 seconds, and 72°C for 20 seconds. To analyze the data, Sequence Detection Software version 1.9.1 (Applied Biosystems) was utilized. Relative gene expression was calculated using the comparative 2−ΔΔCT method.
Measurement of inflammatory mediators in tissue homogenates
As previously described,25,26 the protein concentrations of tissue extracts were determined using the Pierce 660nm Protein Assay Kit (ThermoFisher Scientific) and samples were thawed at room temperature and vortexed for thorough mixing. Tissue homogenates were then assayed for periostin proteins by using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA). Multiple cytokine analysis kits (IL-1ɑ, IL-1β, IL-5, IL-6, IL-10, IL-13, IL-17A, IL-22, IL-23, IL-33, chemokine [C-C motif] ligand [CCL]-11, CCL-24, chemokine [C-X-C motif] ligand [CXCL]-1, CXCL-2, CXCL-8, interferon (IFN)-γ, myeloperoxidase, transforming growth factor-β, S100A8, glycoprotein130, and B cell activating factor) were obtained from R&D Systems (Cat. No. LMSAHM), and data were collected using Luminex 100 (Luminex, Austin, TX, USA). Data analysis was performed using the MasterPlex QT version 2.0 (MiraiBio, Alameda, CA, USA). The levels of total IgE, Staphylococcal enterotoxins (SE)-specific IgE (SEA, SEB, and SEC), and eosinophil cationic protein (ECP) in nasal tissue homogenates were measured using the ImmunoCAP® assay (ThermoFisher Scientific). All assay procedures mentioned were run in duplicate according to the manufacturer's protocol. All the protein levels in the tissue homogenate were normalized to the concentration of total protein.
Immunofluorescence analysis
To verify the cellular source of IL-22 in NP tissues, immunofluorescence analysis was conducted using primary antibodies directed against anti-IL-22 (1:100; Abcam, Cambridge, MA, USA), anti-mast-cell tryptase (1:500; Abcam), anti-eosinophil peroxidase (EPX) (1:200; Abcam), and anti-human neutrophil elastase (HNE) (1:500; Abcam). After 24 hours of incubation of primary antibodies at 4°C, the secondary antibody Alexa Fluor 488-conjugated goat anti-mouse IgG (1:1,000; Abcam) or Cy3-conjugated goat anti-rabbit (1:500; Abcam) was incubated for 1 hour at room temperature. Then, the nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole) (1:1,000; Sigma-Aldrich) for 2 minutes. The tissues were mounted with Fluoroshield Mounting Medium (ab104135; Abcam). Fluorescent images were obtained using the CELENA® S Digital Fluorescence Imaging System (Logos Biosystems, Annandale, VA, USA). The proportion of IL-22-positive cells on each of the tryptase, EPX, and HNE-positive cells was calculated and analyzed in 3 randomly selected fields, and non-specific signals were excluded.
Human mast cell culture and measurement of cytokine production
LAD2 mast cells were cultured in serum-free media (StemPro-34 SFM; ThermoFisher Scientific) supplemented with 2 mM L-glutamine, 100 U/mL penicillin, 50 µg/mL streptomycin, and 100 ng/mL stem cell factor (SCF). Cell suspensions were cultured at a density of 105 cells/mL, and were maintained at 37°C and 5% CO2. Sensitized cells were re-suspended at 5 × 104 cells/mL with biotin-conjugated IgE protein (0.5 μg/mL, ABIN457505; Antibodies-online, Aachen, Germany) in fresh media (without SCF) overnight and pre-treated with recombinant human IL-4 (10 ng/mL, 200-04; PeproTech, Philadelphia, PA, USA), recombinant human IL-13 (10 ng/mL, 200-13; PeproTech), or SEB (5 ng/mL; List Biologic Laboratories, Campbell, CA, USA) for 1 hour. Cells were then treated with 0.5 μg/mL streptavidin (S4762; Sigma-Aldrich) for 4, 8, and 24 hours at 37°C. Secreted IL-22 was measured in cell supernatants using the ELISA kit according to the manufacturer's instructions (R&D Systems). The detection limit for the assay was 31.3 pg/mL.
Human bronchial epithelial cell culture
Normal human bronchial epithelial (NHBE) cells were purchased from Lonza (CC-2540; Basel, Switzerland) and cultured in BEGMTM bronchial epithelial growth medium (CC-3170; Lonza) at 37°C in a humidified environment containing 5% CO2. NHBE cells were plated on 12-well culture plates coated with 6-10 µg/cm2 collagen (C8919; Sigma-Aldrich) and were grown to 80% confluence. Before treatment, NHBE cells were maintained in BEGM in the absence of hydrocortisone for at least 2 days. For IL-22Ra1 expression after stimulation of major cytokines and poly(I:C), NHBE cells were treated with recombinant human IL-4 (100 ng/mL; R&D Systems), IL-13 (100 ng/mL; R&D Systems), IL-17A (100 ng/mL; R&D Systems), IFN-γ (100 ng/mL; R&D Systems), and poly(I:C) (5 μg/mL; InvivoGen, San Diego, CA, USA). Supernatants were removed after 24 hours of stimulation and cells were used for RNA extraction. For TSLP measurement, NHBE cells were stimulated with recombinant human IL-4 (100 ng/mL; R&D Systems), IL-13 (100 ng/mL; R&D Systems), IL-22 (1 or 10 or 100 ng/mL; R&D Systems) for 72 hours. Additionally, in each experiment, NHBE cells were stimulated with poly(I:C) (5 μg/mL; InvivoGen) 1 hour after treatment with IL-4, IL-13, and/or IL-22. Anti- IL-22Ra1 (2.5 µg/mL; R&D Systems) was added to confirm the reversibility of IL-22-induced thymic stromal lymphopoietin (TSLP) production. Cell culture supernatants were collected and used for measuring TSLP with the ELISA (R&D Systems).
Statistical analysis
Statistical analyses were performed using GraphPad Prism software 7.0 (GraphPad Software Inc., La Jolla, CA, USA). Data were analyzed with Mann-Whitney U and Kruskal-Wallis tests with Dunn's multiple comparison test. Correlations were tested by Spearman's rank correlation coefficients. The Pearson correlation test was also used to determine variable relationships. If the data were not normally distributed, the Spearman correlation coefficient was utilized. The significance level was set at an α value of 0.05. Factor analysis based on principal component analysis (PCA) was used to describe the patterns of inflammatory mediators in varying IL-22 concentrations (interquartile range).
RESULTS
Expression of IL-22 in CRS
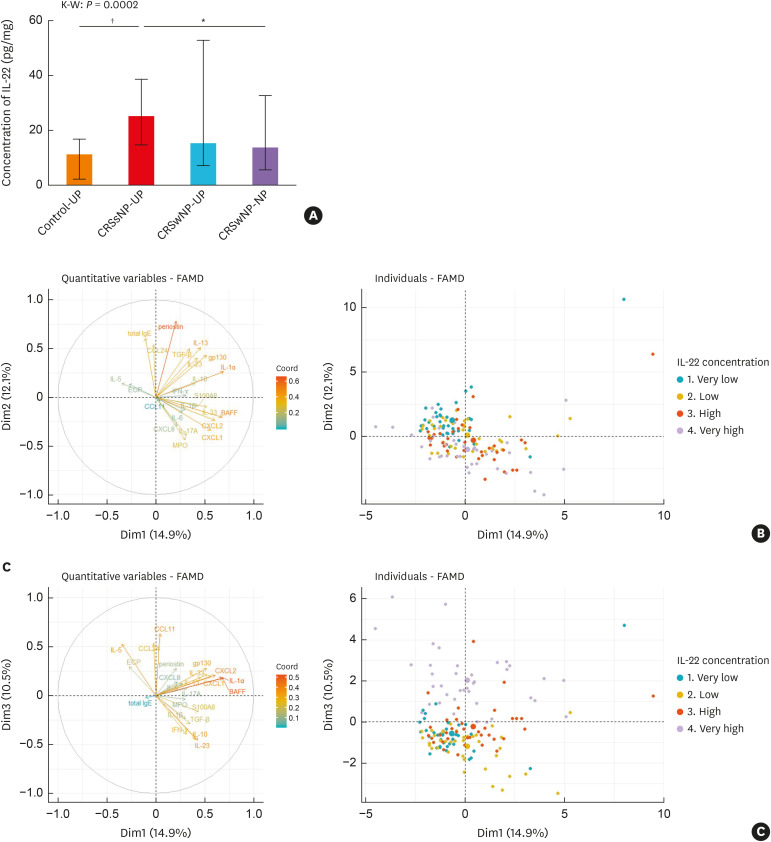
ELISA assays were performed to examine the expression of IL-22 at protein levels. The expression level of IL-22 was significantly increased in CRSsNP compared to control and CRSwNP, whereas there was no significant difference in IL-22 expression between control and CRSwNP (Fig. 1A). Next, to determine the influence of IL-22 expressions on the pathogenesis of NP development, we performed exploratory factor analysis using PCA according to the concentration of IL-22 expression.
Fig. 1. (A) IL-22 protein levels in the sinonasal tissues of each CRS subtype. (B, C) Factor analysis based on principal component analysis according to the concentration of IL-22 in CRSwNP patients.
IL, interleukin; CRS, chronic rhinosinusitis; CRSsNP, Chronic rhinosinusitis without nasal polyps; CRSwNP, Chronic rhinosinusitis with nasal polyps; UP, uncinate process mucosa; NP, nasal polyps; FAMD, factor analysis of mixed data; IgE, immunoglobulin E; CCL, chemokine (C-C motif) ligand; CXCL, chemokine (C-X-C motif) ligand; ECP, eosinophil cationic protein; MPO, myeloperoxidase; BAFF, B cell activating factor; IFN, interferon; TGF, transforming growth factor.
*P < 0.01, and †P < 0.001 using the Kruskal-Wallis test with Dunn's multiple comparison test.
The PCA retained 9 components, accounting for 68.6% of the overall variance in the data (Supplementary Table S2). On the PCA plot, the first and second components accounted for 14.9% and 12.1% of the variance in the dataset, respectively, but there were overlapping patterns of inflammatory mediators according to the IL-22 concentration (Fig. 1B). However, distinct differences were observed in the third component which accounted for 10.5% of the variance in the dataset (Fig. 1C). NP with very high IL-22 levels seemed distinguishable from the other groups by type 2 inflammatory markers such as CCL-11, CCL-24, and IL-5.
Correlations between IL-22 expression and inflammatory markers in CRS
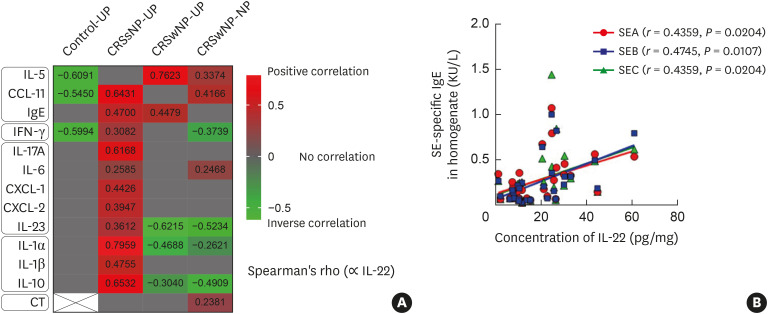
To elucidate the role of IL-22 in CRSwNP, we evaluated the correlation between IL-22 and other major inflammatory mediators (Fig. 2A). We found that there was a positive correlation between IL-22 expression and type 2 immune mediators, whereas IL-22 showed no or negative correlations with type 1 and 3 cytokines or proinflammatory mediators in CRSwNP patients. We also observed a significant correlation between SE-specific IgE and IL-22 in NP tissues, although there was no correlation between total IgE and IL-22 levels in NP tissues (Fig. 2B). Moreover, on subgroup analysis according to the IL-5 activity, 2 we found a higher positive association between IL-22 and IL-5 expression in CRSwNP patients who had high IL-5 (> 12.98 pg/mL) activity (r = 0.5904, P = 0.0061) than in those with low IL-5 (≤ 12.98 pg/mL) activity (r = 0.2700, P = 0.0354). Additionally, IL-22 expression in NP tissues was significantly associated with disease severity based on CT scores. In contrast, IL-22 expression positively correlated with various inflammatory mediators (type 1, 2, and 3 inflammatory cytokines and pro-inflammatory mediators) but showed no association with disease extent in CRSsNP patients.
Fig. 2. (A) Correlation between IL-22 and major inflammatory mediators in the sinonasal tissues of each CRS subtype. (B) Correlation between IL-22 and SE-specific IgE in homogenates of NPs. A heat map of correlation analysis between IL-22 and major cytokines according to CRS phenotypes and controls. The color bar presents Spearman's rho, red color bar represents positive correlation, and green color bar represents inverse correlations.
IL, interleukin; CRS, chronic rhinosinusitis; IgE, immunoglobulin E; CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; UP, uncinate process mucosa; NP, nasal polyps; CT, Lund-Mackay CT score; SE, Staphylococcal enterotoxin; SEA, Staphylococcal enterotoxin A; SEB, Staphylococcal enterotoxin B; SEC, Staphylococcal enterotoxin C.
The cellular source of IL-22 in NP
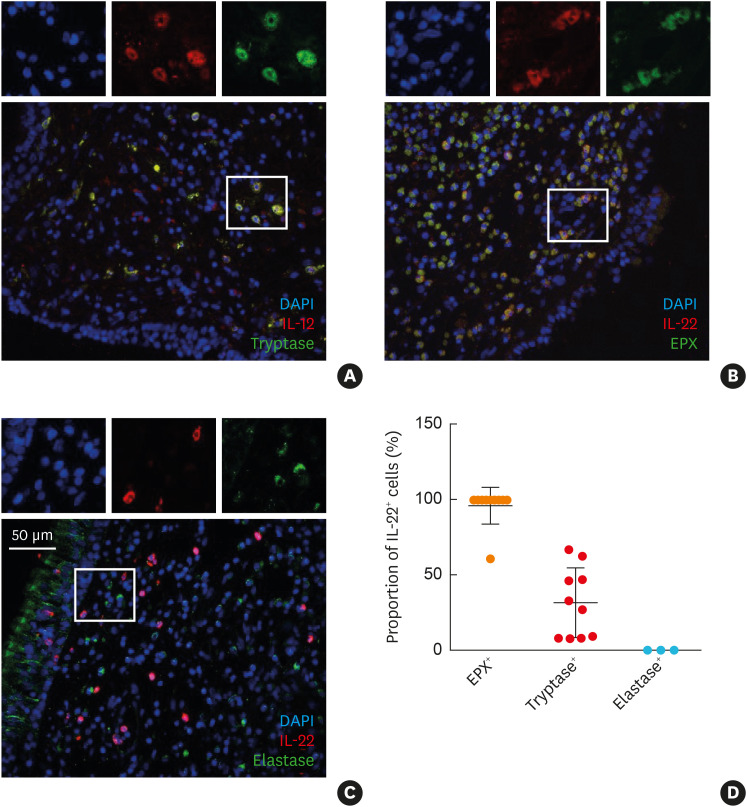
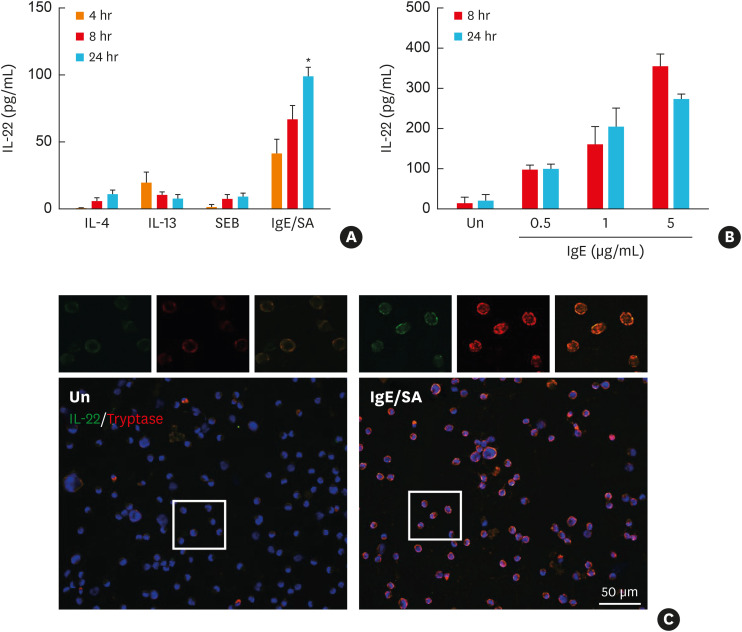
For localization of the cellular sources of IL-22 in eosinophilic NP, we performed double immunofluorescence staining of the NP with abundant eosinophils and mast cell infiltration (Fig. 3). We found that IL-22 was expressed mostly in EPX-positive cells, but not in HNE-positive cells. Moreover, a part of tryptase-positive cells also expressed IL-22. Thus, LAD2 cells, a human mast cell line, were cultured and stimulated with IgE, IL-4, IL-13, and SEB to confirm whether human mast cells can produce IL-22 and to identify upstream inducers. IL-22 was released from LAD2 in response to IgE stimulation in a dose-dependent manner, while IL-4, IL-13, and SEB were not (Fig. 4).
Fig. 3. Immunofluorescent staining for the cellular sources of IL-22 in eosinophilic NPs. (A) Tryptase and IL-22, (B) EPX and IL-22, (C) HNE and IL-22, (D) proportion of IL-22-positive cell in each inflammatory cell. Tryptase was used as a mast cell maker, EPX as an eosinophilic marker, and HNE for a neutrophil staining marker.
IL, interleukin; NP, nasal polyps; EPX, eosinophil peroxidase; HNE, human neutrophil elastase.
Fig. 4. IgE-mediated production and secretion of IL-22 from LAD2 cells. (A) IL-22 was measured by ELISA in the supernatants of LAD2-cell culture stimulated with IgE/SA, IL-4, IL-13, and SEB (n = 3 for each group). (B) IL-22 was measured by ELISA in the supernatants of LAD2-cell culture stimulated in variable concentrations (0.1, 1, or 5 μg/mL) of IgE with SA treatment (n = 3 for each group). (C) Representative double immunostaining (red: tryptase and green: IL-22). Box indicates representative findings (× 200). Scale bar = 50 μm.
IL, interleukin; ELISA, enzyme-linked immunosorbent assay; IgE, immunoglobulin E; SEB, Staphylococcal enterotoxin B; SA, streptavidin; Un, unstimulated control.
IL-22/IL-22Ra1 signaling pathway in NP
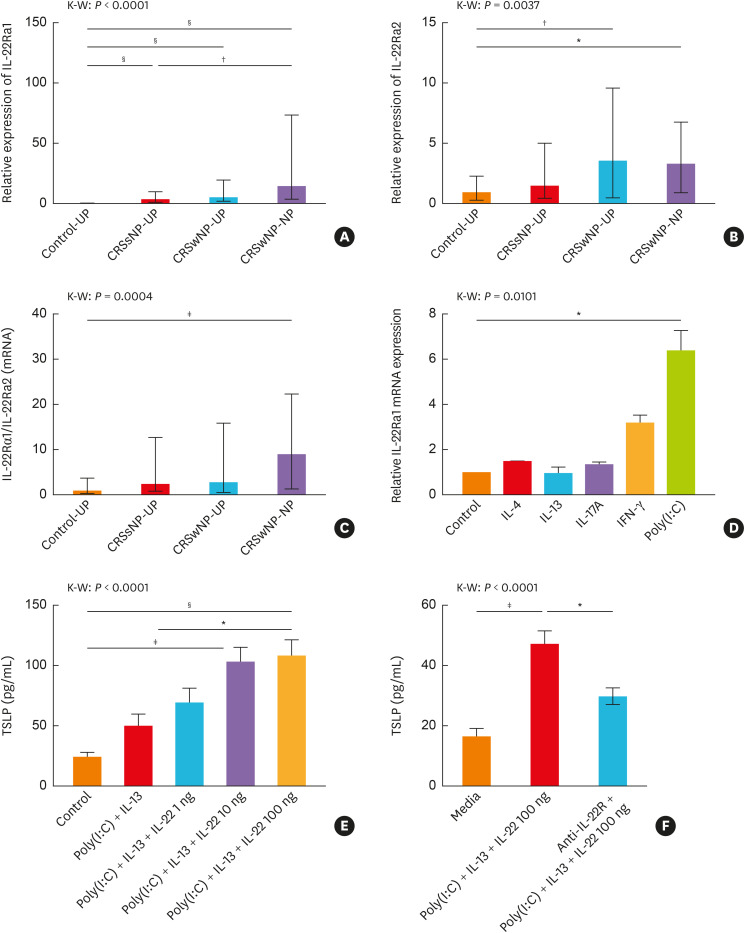
The IL-22 receptor complex consists of the receptor chains IL-22Ra1 and IL-10Ra2.13 Additionally, there is a soluble, secreted receptor of IL-22 (IL-22Ra2) which exists as a natural antagonist to IL-22.13 In this study, IL-22Ra1 was significantly higher in NP than in control and CRSsNP, whereas IL-22Ra2 was not upregulated in CRSwNP, compared with CRSsNP (Fig. 5A and B). The ratio of IL-22Ra1/IL-22Ra2 was the highest in NP (Fig. 5C). These findings imply that the IL-22/IL-22Ra1 axis is mainly activated in NP. Therefore, to confirm what conditions could promote IL-22Ra1 expression, NHBE cells were cultured and stimulated with IL-4, IL-13, IL-17A, IFN-γ (each 100 ng/mL), and poly(I:C). We observed that IL-22Ra1 expression on NHBE cells was up-regulated by poly(I:C) stimulation (Fig. 5D). Moreover, previous studies revealed that the expression of TSLP was induced by poly(I:C) in nasal epithelial cells of NP.27 Thus, we tested whether the IL-22/IL-22Ra1 axis could promote TSLP expression under poly(I:C) stimulation. NHBE cells stimulated with IL-4, IL-13, or IL-22 alone did not promote TSLP production (Supplementary Fig. S1A); however, when NHBE cells were stimulated with additional poly(I:C) in each experimental protocol, we were able to detect significantly up-regulated TSLP expression (Supplementary Fig. S1B). Furthermore, we found that the combination of IL-13 and poly(I:C) showed much enhanced production of TSLP on 100 ng/mL of IL-22. Treatment with anti-IL-22Ra1 also inhibited IL-22-induced enhancement of TSLP production (Fig. 5E and F).
Fig. 5. IL-22Ra1 expression in CRS and control tissues and in vitro experiment using NHBE cells. (A) IL-22Ra1 mRNA expression in controls and CRS subtypes. (B) IL-22Ra2 mRNA expression in controls and CRS subtypes. (C) The ratio of IL-22Ra1 to IL-22Ra2 mRNA expression in controls and CRS subtypes. (D) Relative mRNA expression of IL-22Ra1 in NHBE cells with stimulation of major cytokines and poly(I:C) (n = 3 for each group). (E) IL-22-induced TSLP production in NHBE cells after type 2 cytokines and poly(I:C) treatment (n = 6 for each group). (F) IL-22-induced TSLP production was reversed by anti-IL-22Ra1 (n = 7 for each group).
IL, interleukin; IL-22Ra, IL-22 receptor subunit alpha; CRS, chronic rhinosinusitis; CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; UP, uncinate process mucosa; NP, nasal polyps; TSLP, Thymic stromal lymphopoietin; NHBE, normal human bronchial epithelial.
*P < 0.05, †P < 0.01, ‡P < 0.001, and §P < 0.0001 using the Kruskal-Wallis test with Dunn's multiple comparison test.
DISCUSSION
IL-22 is essential not only for the host defense against extracellular pathogens, but also for tissue repair and wound healing. However, uncontrollably continued production of IL-22 can lead to certain diseases. To date, IL-22 has been known to have different functions, depending on the nature of the affected tissue and the local cytokine milieu. However, the role of IL-22 in the allergic airway diseases is still controversial (pro-inflammatory effects vs. anti-inflammatory properties).28 In this study, we showed that, in patients with CRSwNP, IL-22 expression was positively correlated with type 2 inflammatory cytokines. In addition to eosinophils, mast cells might be the potential source of IL-22 in NP tissues responding to subsequent IgE-mediated signals. The up-regulation of IL-22Ra1 was observed in NP tissues and was induced in NHBE cells by poly(I:C) treatment. Moreover, the enhancement of the IL-22/IL-22Ra1 axis promoted TSLP production in NHBE cells.
Previously, some studies have demonstrated that IL-22 can act a dual role in airway inflammation: anti-inflammatory or pro-inflammatory.18,29,30 Our study found that IL-22 expression and its correlation with other inflammatory mediators were different according to the CRS phenotype. Specifically, IL-22 expression was correlated with various inflammatory mediators (type 1, 2, and 3 cytokines and pro-inflammatory mediators) in CRSsNP, whereas there were positive correlations between IL-22 and type 2 cytokines in CRSwNP. Consistent with our findings, one cluster analysis study examined the up-regulation of IL-22 in 2 different phenotypes: the CRSsNP group and the IL-5-high CRS group which consist mostly of CRSwNP patients. They concluded that IL-22 might play different roles according to the cytokine milieu.2 Prior studies reported the association of IL-22 with disease severity, demonstrating that significantly higher level of IL-22 expression was detected in serum and sputum of patients with severe asthma than in those with moderate asthma.30,31,32 In accordance with these findings, we also observed that CRSwNP patients showed a significantly positive correlation between IL-22 expression and disease extent based on CT scores.
It is known that IL-22 is produced by immune cells, including helper T cell subsets and innate lymphocytes. Recently, one study described that IL-22 was expressed in CD4+ cells and ECP/EPX+ cells, not in CD68+ cells in the case of CRSwNP patients.19 Another study also demonstrated that skin mast cells are a predominant source of IL-22 in patients with psoriasis and atopic dermatitis.33 Interestingly, in this study, double immunofluorescence staining and LAD2 cell culture revealed that mast cells are another cellular source of IL-22 in NP tissues. Additionally, CRSwNP patients with high IL-5 activity showed a stronger correlation between IL-22 and IL-5 levels than those with low IL-5 activity. Taken together, our findings suggest that IL-22 may play a key role in type 2 airway inflammation in CRSwNP.
It is known that IL-22Ra1 activates the signal transducer and activator transcription-3 signaling pathway, but IL-22Ra1 expression is restricted to cells within epithelial cells, hepatocytes, and acinar cells.15 A previous study described that nasal epithelial cells obtained from the ethmoid mucosae of CRSwNP are associated with significantly decreased expression of IL-22R1, implying impaired protective function.34 Some studies on the protective role of IL-22 in airway inflammation demonstrated that IL-22 inhibits the expression of lung epithelial cell-derived cytokines and attenuates the development of allergic airway inflammation.35,36 However, in this study, the up-regulated expression of IL-22Ra1 was observed in NP tissues. This implies that IL-22 produced by eosinophils and mast cells act on epithelium via IL-22Ra1. The enhanced IL-22/IL-22Ra1 axis may contribute to the development of nasal polypogenesis by initiating TSLP expression under IL-13 and poly(I:C). However, this is still unclear and should be further investigated when and how the role of IL-22 switches between protective and pathologic ones.
In conclusion, this study indicates that IL-22 may play a pathologic role in patients with CRSwNP. Eosinophils and mast cells may be the major cellular sources of IL-22 in eosinophilic NP tissues. RNA viral infection may mediate up-regulation of IL-22Ra1, activating IL-22/IL-22Ra1 signaling in airway epithelial cells. The enhanced IL-22/IL-22Ra1 axis promotes TSLP production under a type 2 microenvironment in airway epithelial cells, and such a pathway may contribute to the development of NP.
ACKNOWLEDGMENTS
This research was supported by a clinical research grant-in-aid from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2018R1D1A3B07040862 to Dong-Kyu Kim and NRF-2019R1A2C2087170 to Dae Woo Kim) and by a fund from the Seoul National University Hospital (05-2016-0020 to Dae Woo Kim). We are grateful to professor Arnold S. Kirshenbaum for providing the cell lines LAD2.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Patient characteristics
Coordinates of principal component analysis for the first 9 principal components
In vitro experiment using NHBE cells. (A) TSLP production in NHBE cells after type 2 cytokine and IL-22 treatment (n = 7 for each group). (B) TSLP production in NHBE cells after treatment of type 2 cytokine and IL-22 in combination with poly(I:C) (n = 7 for each group).
References
- 1.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 2.Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449–1456.e4. doi: 10.1016/j.jaci.2015.12.1324. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138:1344–1353. doi: 10.1016/j.jaci.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 4.Liao B, Liu JX, Li ZY, Zhen Z, Cao PP, Yao Y, et al. Multidimensional endotypes of chronic rhinosinusitis and their association with treatment outcomes. Allergy. 2018;73:1459–1469. doi: 10.1111/all.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryu G, Kim DK, Dhong HJ, Eun KM, Lee KE, Kong IG, et al. Immunological characteristics in refractory chronic rhinosinusitis with nasal polyps undergoing revision surgeries. Allergy Asthma Immunol Res. 2019;11:664–676. doi: 10.4168/aair.2019.11.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DK, Kim JY, Han YE, Kim JK, Lim HS, Eun KM, et al. Elastase-positive neutrophils are associated with refractoriness of chronic rhinosinusitis with nasal polyps in an Asian population. Allergy Asthma Immunol Res. 2020;12:42–55. doi: 10.4168/aair.2020.12.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim DK, Kang SI, Kong IG, Cho YH, Song SK, Hyun SJ, et al. Two-track medical treatment strategy according to the clinical scoring system for chronic rhinosinusitis. Allergy Asthma Immunol Res. 2018;10:490–502. doi: 10.4168/aair.2018.10.5.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DK, Kim DW. Does inflammatory endotype change in patients with chronic rhinosinusitis? Allergy Asthma Immunol Res. 2019;11:153–155. doi: 10.4168/aair.2019.11.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YS, Han D, Kim J, Kim DW, Kim YM, Mo JH, et al. In-depth, proteomic analysis of nasal secretions from patients with chronic rhinosinusitis and nasal polyps. Allergy Asthma Immunol Res. 2019;11:691–708. doi: 10.4168/aair.2019.11.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DW. Can neutrophils be a cellular biomarker in Asian chronic rhinosinusitis? Clin Exp Otorhinolaryngol. 2019;12:325–326. doi: 10.21053/ceo.2019.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Pan L, Liu Z. Neutrophils as a protagonist and target in chronic rhinosinusitis. Clin Exp Otorhinolaryngol. 2019;12:337–347. doi: 10.21053/ceo.2019.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 13.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 14.Eyerich K, Eyerich S. Th22 cells in allergic disease. Allergo J Int. 2015;24:1–7. doi: 10.1007/s40629-015-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Leyva-Castillo JM, Yoon J, Geha RS. IL-22 promotes allergic airway inflammation in epicutaneously sensitized mice. J Allergy Clin Immunol. 2019;143:619–630.e7. doi: 10.1016/j.jaci.2018.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang Y, Al-Alwan L, Risse PA, Roussel L, Rousseau S, Halayko AJ, et al. TH17 cytokines induce human airway smooth muscle cell migration. J Allergy Clin Immunol. 2011;127:1046–1053.e1-2. doi: 10.1016/j.jaci.2010.12.1117. [DOI] [PubMed] [Google Scholar]
- 18.Hirose K, Ito T, Nakajima H. Roles of IL-22 in allergic airway inflammation in mice and humans. Int Immunol. 2018;30:413–418. doi: 10.1093/intimm/dxy010. [DOI] [PubMed] [Google Scholar]
- 19.Noyama Y, Okano M, Fujiwara T, Kariya S, Higaki T, Haruna T, et al. IL-22/IL-22R1 signaling regulates the pathophysiology of chronic rhinosinusitis with nasal polyps via alteration of MUC1 expression. Allergol Int. 2017;66:42–51. doi: 10.1016/j.alit.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Kim DK, Jin HR, Eun KM, Mo JH, Cho SH, Oh S, et al. The role of interleukin-33 in chronic rhinosinusitis. Thorax. 2017;72:635–645. doi: 10.1136/thoraxjnl-2016-208772. [DOI] [PubMed] [Google Scholar]
- 21.Kim DK, Jin HR, Eun KM, Mutusamy S, Cho SH, Oh S, et al. Non-eosinophilic nasal polyps shows increased epithelial proliferation and localized disease pattern in the early stage. PLoS One. 2015;10:e0139945. doi: 10.1371/journal.pone.0139945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DW, Kulka M, Jo A, Eun KM, Arizmendi N, Tancowny BP, et al. Cross-talk between human mast cells and epithelial cells by IgE-mediated periostin production in eosinophilic nasal polyps. J Allergy Clin Immunol. 2017;139:1692–1695.e6. doi: 10.1016/j.jaci.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin HW, Kim DK, Park MH, Eun KM, Lee M, So D, et al. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2015;135:1476–1485.e7. doi: 10.1016/j.jaci.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Vazquez C, Torregrosa-Bertet MJ, Carvajal-Urueña I, Cano-Garcinuño A, Fos-Escrivà E, García-Gallego A, et al. Accuracy of ImmunoCAP Rapid in the diagnosis of allergic sensitization in children between 1 and 14 years with recurrent wheezing: the IReNE Study. Pediatr Allergy Immunol. 2009;20:601–609. doi: 10.1111/j.1399-3038.2008.00827.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim DW, Eun KM, Roh EY, Shin S, Kim DK. Chronic rhinosinusitis without nasal polyps in Asian patients shows mixed inflammatory patterns and neutrophil-related disease severity. Mediators Inflamm. 2019;2019:7138643. doi: 10.1155/2019/7138643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DK, Eun KM, Kim MK, Cho D, Han SA, Han SY, et al. Comparison between signature cytokines of nasal tissues in subtypes of chronic rhinosinusitis. Allergy Asthma Immunol Res. 2019;11:201–211. doi: 10.4168/aair.2019.11.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golebski K, van Tongeren J, van Egmond D, de Groot EJ, Fokkens WJ, van Drunen CM. Specific induction of TSLP by the viral rna analogue poly(I:C) in primary epithelial cells derived from nasal polyps. PLoS One. 2016;11:e0152808. doi: 10.1371/journal.pone.0152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamasauskiene L, Sitkauskiene B. Role of Th22 and IL-22 in pathogenesis of allergic airway diseases: pro-inflammatory or anti-inflammatory effect? Pediatr Neonatol. 2018;59:339–344. doi: 10.1016/j.pedneo.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207:1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besnard AG, Sabat R, Dumoutier L, Renauld JC, Willart M, Lambrecht B, et al. Dual role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am J Respir Crit Care Med. 2011;183:1153–1163. doi: 10.1164/rccm.201008-1383OC. [DOI] [PubMed] [Google Scholar]
- 31.Farfariello V, Amantini C, Nabissi M, Morelli MB, Aperio C, Caprodossi S, et al. IL-22 mRNA in peripheral blood mononuclear cells from allergic rhinitic and asthmatic pediatric patients. Pediatr Allergy Immunol. 2011;22:419–423. doi: 10.1111/j.1399-3038.2010.01116.x. [DOI] [PubMed] [Google Scholar]
- 32.Sherkat R, Yazdani R, Ganjalikhani Hakemi M, Homayouni V, Farahani R, Hosseini M, et al. Innate lymphoid cells and cytokines of the novel subtypes of helper T cells in asthma. Asia Pac Allergy. 2014;4:212–221. doi: 10.5415/apallergy.2014.4.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mashiko S, Bouguermouh S, Rubio M, Baba N, Bissonnette R, Sarfati M. Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. J Allergy Clin Immunol. 2015;136:351–359.e1. doi: 10.1016/j.jaci.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 34.Ramanathan M, Jr, Spannhake EW, Lane AP. Chronic rhinosinusitis with nasal polyps is associated with decreased expression of mucosal interleukin 22 receptor. Laryngoscope. 2007;117:1839–1843. doi: 10.1097/MLG.0b013e31811edd4f. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Hirose K, Kawashima S, Niwa Y, Wakashin H, Iwata A, et al. IL-22 attenuates IL-25 production by lung epithelial cells and inhibits antigen-induced eosinophilic airway inflammation. J Allergy Clin Immunol. 2011;128:1067–1076.e1-6. doi: 10.1016/j.jaci.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Ito T, Hirose K, Saku A, Kono K, Takatori H, Tamachi T, et al. IL-22 induces Reg3γ and inhibits allergic inflammation in house dust mite-induced asthma models. J Exp Med. 2017;214:3037–3050. doi: 10.1084/jem.20162108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient characteristics
Coordinates of principal component analysis for the first 9 principal components
In vitro experiment using NHBE cells. (A) TSLP production in NHBE cells after type 2 cytokine and IL-22 treatment (n = 7 for each group). (B) TSLP production in NHBE cells after treatment of type 2 cytokine and IL-22 in combination with poly(I:C) (n = 7 for each group).