Abstract
Severe asthma (SA) presents in about 3%–5% of adult asthmatics and is responsible for over 60% of asthma-related medical expenses, posing a heavy socioeconomic burden. However, to date, a precise definition of or clear diagnostic criteria for SA have not been established, and therefore, it has been challenging for clinicians to diagnose and treat this disease. Currently, novel biologics targeting several molecules, such as immunoglobulin E, interleukin (IL)5, and IL4/IL13, have emerged, and many new drugs are under development. These have brought a paradigm shift in understanding the mechanism of SA and have also provided new treatment options. However, we need to agree on a precise definition of and its diagnostic criteria for SA. Additionally, it is necessary to explain the diagnostic criteria and to summarize current standard and additional treatment options. This review is an experts' opinion on SA from the Korean Academy of Asthma, Allergy, and Clinical Immunology, the Working Group on Severe Asthma, and aims to provide a definition of and diagnostic criteria for SA, and propose future direction for SA diagnosis and management in Korea.
Keywords: Severe asthma; biologics; diagnosis; eosinophil; expert opinion; IgE; treatment, IL5; IL4
INTRODUCTION
Asthma is a highly prevalent airway disease. Severe asthma (SA) is a life-threatening condition that affects only a minor percentage of the patient population, but which accounts for more than 60% of the total asthma-related medical expenses, imposing a heavy socioeconomic burden.1 The appropriate management of SA remains challenging. Insights into the unmet needs of treating SA have facilitated understanding of the disease pathophysiology, resulting in development of novel biological treatments and a paradigm shift in the management of SA.
Most clinicians currently adopt the latest SA definition proposed by the European Respiratory Society (ERS)/American Thoracic Society (ATS) in 2014: asthma that requires high-dose inhaled corticosteroids (ICS) plus a second controller and/or systemic corticosteroids (SCS) to prevent it from becoming uncontrolled, or asthma that remains uncontrolled despite such therapy.2 Recently, the ERS/ATS published new guidelines for managing SA, including the use of novel drugs.3 Nevertheless, clinicians remain unsure when and for whom biologic treatment should be considered. Additionally, it is not clear which biologics should be tried as a first-line option. Therefore, a practical guideline for SA is required that considers the distinct regional circumstances and newly available biologics. The members of the Korean Working Group for Severe Asthma from the Korean Academy of Allergy and Clinical Immunology (KAAACI) have formulated such a guide, thoroughly reviewing the definition, epidemiology, diagnosis, phenotyping, and general controller medications of SA, and providing practical guidance for the management of SA, addressing the role of emerging therapies, defining potential responders.
METHODS
Target users
The target users of this document are specialists in allergy and respiratory medicine managing adult asthmatic patients. General internists and primary care physicians may also benefit from this document as they need to decide when it is appropriate to refer their patients to specialists.
Development methods
The Working Group for Severe Asthma of the KAAACI undertook this review of difficult-to-treat and SA. The members of this Working Group are specialists in allergy, pulmonology, and immunology. Key topics were selected by the representative members of the Working Group, and all participating members were assigned a topic based on their expertise or interest. Literature reviews included comprehensive domestic and international electronic database searches. Data from relevant studies were summarized and the document was constructed through a series of face-to-face meetings, and the completed document was reviewed by 2 asthma experts outside the Working Group. This expert opinion paper comprises 4 parts: 1) definition, prevalence, and burden of SA; 2) diagnosis of SA; 3) treatment of SA; and 4) specific situations.
DEFINITION, PREVALENCE, AND BURDEN OF SA
Evolution in the understanding of asthma severity
The severity of asthma has been evaluated differently across studies based on various factors, such as lung function, clinical symptoms, or medication for decades. In 1995, the Global Initiative for Asthma (GINA) guidelines suggested a classification of asthma severity based on its clinical features, including the degree of symptoms and exacerbations, frequency of nighttime symptoms, consumption of a reliever, limitation of physical activities, and lung function. However, clinicians became aware of asthma subgroups whose disease was not easily controlled with conventional treatment, thus giving rise to the current concept of SA. Several nomenclatures and definitions were used to describe this problematic population (Table 1). Since previous guidelines did not take into account the treatment response when assessing asthma severity, an ATS-sponsored workshop presented the diagnostic criteria of “refractory asthma” in 2000.4 It was a combination of medication requirements to achieve controlled status, and had other minor characteristics in terms of symptoms, exacerbation history, and airflow limitation.4 In 2009, the World Health Organization meeting suggested 3 subgroups of SA5: 1) untreated SA; 2) difficult-to-treat SA; and 3) treatment-resistant SA. The last group was defined as uncontrolled asthma despite use of the highest level of treatment and controlled only by maintaining the highest level of treatment.5 This definition was also similar to an international consensus from the Innovative Medicine Initiative and ERS/ATS.2,6 Accordingly, the essential components defining SA include the level of symptom control, future risk of adverse outcomes, and medication required to control symptoms. The control status of symptoms has been determined by either impact on daily life or the exacerbation history. Risk factors for poor asthma outcomes consist of fixed airflow limitation and side-effects of medications, in addition to the exacerbation itself. Finally, the required medication should be maximally optimized therapy after treating modifiable risk factors and comorbidities. In 2019, GINA reported guidelines regarding the term “difficult-to-treat asthma and SA.”7 In the guidelines, “uncontrolled asthma,” “difficult-to-treat asthma,” and “SA” are defined separately, emphasizing the need for several management steps before making a final diagnosis of SA.7
Table 1. Published definitions of severe asthma.
| Guidelines | Definition | Required medications | Additional details | |||
|---|---|---|---|---|---|---|
| ATS (2000) | • Refractory asthma requires 1 or both major criteria and 2 minor criteria | • Major criteria | • Minor criteria; at least 2 of following categories | |||
| 1) Continuous or near-continuous (≥ 50% of the year) OCS | 1) Requirement for daily treatment with additional controller | |||||
| 2) High-dose ICS | 2) Requirement for short-acting inhaled β2-agonist | |||||
| 3) Persistent airway obstruction | ||||||
| 4) 1 or more urgent care visits for asthma per year | ||||||
| 5) 3 or more oral steroid “bursts” per year | ||||||
| 6) Prompt deterioration with ≤ 25% reduction in oral or ICS dose | ||||||
| 7) Near-fatal asthma event in the past | ||||||
| WHO (2009) | • Treatment-resistant severe asthma | • High-dose ICS or a high-dose ICS–LABA combination | • Level of control assessed based on following categories: | |||
| 1) “Control” is not achieved despite highest level treatment | • Frequent or chronic use of SCS | 1) Daytime symptoms | ||||
| 2) “Control” maintained only with highest level treatment | 2) Limitations on activities | |||||
| 3) Nocturnal symptoms/awakenings | ||||||
| 4) Need for short-acting inhaled β2-agonist | ||||||
| 5) Lung function | ||||||
| 6) Exacerbations | ||||||
| IMI (2011) | • Refractory asthma despite high-intensity treatment | • High-dose ICS with or without SCS | • Uncontrolled and/or frequent (≥ 2/year) exacerbations | |||
| ERS/ATS (2014) | • Asthma that requires high-intensity medication to prevent it becoming “uncontrolled” or that remains “uncontrolled” despite therapy | • Medications for GINA steps 4–5 asthma for the previous year or | • Uncontrolled asthma; at least 1 of the following: | |||
| • Controlled asthma that worsens on tapering of these high doses of ICS or SCS (or additional biologics) | • SCS for ≥ 50% of the previous year | 1) Poor symptom control | ||||
| 2) Frequent severe exacerbations | ||||||
| 3) Serious exacerbations | ||||||
| 4) Airflow limitation | ||||||
| GINA (2019) | • Uncontrolled asthma despite adherence to maximal optimized therapy and treatment contributory factors | • Medications for GINA steps 4–5 asthma | • Uncontrolled asthma; at least 1 of the following categories: | |||
| • Asthma worsens when high-dose treatment is decreased | 1) Poor symptom control | |||||
| 2) Frequent or serious exacerbations | ||||||
| • Good adherence and inhaler technique | ||||||
ATS, American Thoracic Society; WHO, World Health Organization; IMI, Innovative Medicine Initiative; ERS, European Respiratory Society; GINA, Global Initiative for Asthma; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; OCS, oral corticosteroids; SCS, systemic corticosteroids.
Prevalence of SA
Given the historical changes in the definitions of SA, its prevalence varies depending on the definitions applied, defined populations, and methodologies used. In population-based studies, the Asthma Insights and Reality surveys showed that severe persistent asthma in adults ranged from 12.5% in the Asia-Pacific region to 20.5% in Western Europe, using the GINA severity classification and symptom severity index.8,9 In hospital-based studies, severe persistent asthma prevalence was reported as 3.9%-7.8% based on clinicians' judgment and the GINA severity classification.10,11 Thus, heterogeneous SA definitions may have affected the estimation of its true prevalence.
As the definition of SA is refined, recent population-based asthma studies have demonstrated that the prevalence of SA depends on the definition used, from 3.6% (Severe Asthma Research Program), to 4.8% (ERS/ATS Taskforce), and 6.1% (GINA), among subjects with current asthma.12 The current definition of SA emphasizes maximal optimal treatment, including drug adherence and management of comorbidities. A few studies have shown the precise prevalence of SA using this approach. In data from Danish nationwide registers, 8.1% of the patients identified were classified as having SA; their symptoms could be controlled with high-dose ICS and second controllers, including omalizumab.13 Based on 65 Dutch pharmacy databases, 3.6% of all adults with asthma were controlled by high-dose ICS plus at least 2 consecutive 3-month prescriptions of oral corticosteroids (OCS).14
In Korea, the number of reliable population studies exactly meeting the SA definition is limited. Nonetheless, there are some indirect estimates. A study using nationwide claims data showed that approximately 11% of patients had SA, defined as those using high-dose ICS.15 A survey of Korean allergy or pulmonology specialists showed that about 13% of their patients seemed to have SA.16 A recent study using national health claims data reported that the SA prevalence in Korea had increased from 3.5% to 6.1% over a decade.17 Future investigation on the epidemiology of SA is needed with the establishment of a definition of SA.
Mortality, OCS use, and quality of life
From a claims database in France in 2012, the 3-year cumulative mortality rate was markedly higher in SA patients than in patients without asthma (7.1% vs. 4.5%).18 The asthma mortality data from Korea were initially reported as 0.21 per 100,000 population in 1995, and then decreased to 0.08 in 2006 and 0.04 in 2012.19 In contrast, a recent analysis of heath claims data and the Statistics Korea database showed that asthma mortality had increased over a decade, with the highest mortality due to SA.17
Although long-term OCS use in SA patients causes numerous side-effects, OCS is widely used as controller or exacerbation drugs for SA. The rate of long-term OCS use in SA patients varies across countries (21.4%–71.9%).20 In the Korean Severe Asthma Registry, 60.1% of patients at GINA step 5 took OCS continuously.21 A population-based matched cohort study from Korea revealed that long-term use of SCS was associated with an increased mortality risk in a dose-dependent manner in patients with asthma.22 Moreover, quantitative studies reported that exacerbation of asthma and long-term OCS-use negatively impacted professional life and made maintaining a normal life challenging.23
Healthcare use and costs
The estimated healthcare use and costs of treatment for SA are greater than those for non-SA. While fewer than 10% of all individuals with asthma have SA, their healthcare accounts for at least 60% of all asthma-related healthcare costs.24 According to a Korean claims database, the annual healthcare cost for SA patients was $1,635, 9.4 times higher than that for patients with mild asthma ($174), and 40.8% of the healthcare cost was due to asthma exacerbation.25 The overall asthma-related healthcare cost has increased over years, with the highest cost related to SA.17 Recurrent exacerbations and uncontrolled asthma are also related to higher asthma-related medical costs.26 Moreover, the cost of high-dose OCS users increased more than twice as much as that of low-dose OCS users. This might be directly related to OCS-induced morbidities and adverse events.
Pathogenesis of SA
Type 2 inflammation in asthma refers to asthmatics who show eosinophilic, and/or allergic airway inflammation. Identification of sputum eosinophil and/or high fractional exhaled nitric oxide (FeNO) is evidence of eosinophilic airway inflammation. Sensitization to common inhalant allergens in a skin-prick test or serum allergen-specific immunoglobulin E (IgE) tests implies allergic or atopic features of asthma. Recently, the focus on “type 2” rather than “Th2” asthma reflects the important contribution of innate lymphoid type 2 cells (ILC2) and other non-classic Th2 cells, in addition to the original Th2 cells, to the type 2 cytokine milieu. On the other hand, SA without type 2 features is currently defined as the “apparent” absence of type 2 cytokines and their downstream signatures. Detailed information on the mechanisms of type 2 SA and non-type 2 SA and other mechanisms, including corticosteroid (CS) resistance, airway remodeling, smoking, and air pollution, which also contribute to the evolution of SA, are provided in Supplement S1.
DIAGNOSIS OF SA
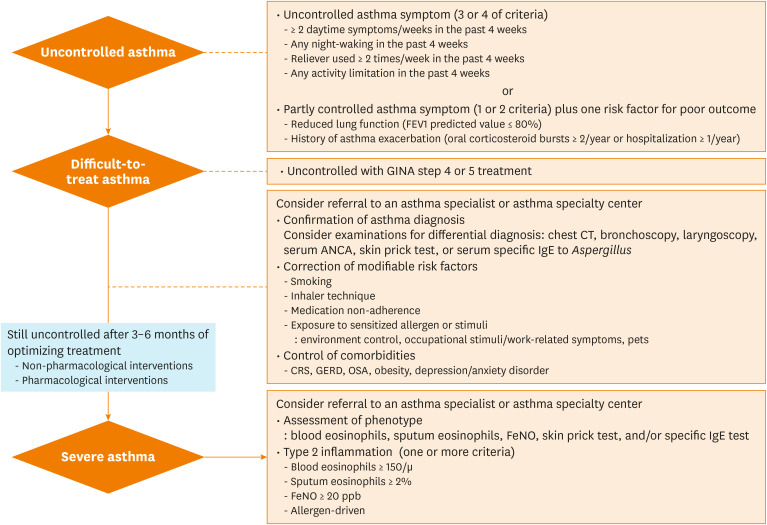
There are 3 steps in diagnosing SA (Fig. 1): 1) determining uncontrolled asthma; 2) defining and evaluating difficult-to-treat asthma (confirmation of an asthma diagnosis, correction of modifiable risk factors, and controlling comorbidities); 3) optimizing treatments in difficult-to-treat asthma. A diagnosis of SA is eventually made when asthma remains poorly controlled at 3-6 months after treatment optimization.
Fig. 1. Approach to uncontrolled, difficult-to-treat, and severe asthma.
ANCA, anti-neutrophil cytoplasmic antibodies; FEV1, forced expiratory volume in 1 second; GINA, Global Initiative for Asthma; ICS, inhaled corticosteroids; CRS, chronic rhinosinusitis; GERD, gastroesophageal reflux disease; OSA, obstructive sleep apnea; FeNO, fractional exhaled nitric oxide; CT, computed tomography; IgE, immunoglobulin E.
Determination of uncontrolled asthma
In GINA 2019, the asthma control status should be assessed in terms of asthma symptom control and risk factors for future poor outcomes, particularly exacerbation.7 Uncontrolled asthma symptoms are indicated when 3 or 4 of the following criteria have been fulfilled over the past 4 weeks: 1) daytime symptoms more than twice a week; 2) any night waking due to asthma in the past 4 weeks; 3) symptom relievers were used more than twice a week; and 4) any activity limitation. In addition to uncontrolled symptoms, although reduced lung function and a history of asthma exacerbation are important risk factors for poor outcomes,27 symptom perception showed a poor correlation with lung function.28 Therefore, lung function measurements can be considered as an objective tool when asthma control is assessed. In Korea, the National Health Insurance Service, the only public healthcare service provider, emphasizes the importance of lung function tests and annually evaluates the rate of lung function tests to assure good quality asthma management. Moreover, the lung function test is widely available in primary care clinics in Korea. Accordingly, we recommend that lung function should be considered to determine uncontrolled asthma. Therefore, uncontrolled asthma includes partly controlled symptoms (1 or 2 of above criteria) plus 1 of the following risk factors for poor outcomes: 1) reduced lung function (forced expiratory volume in 1 second [FEV1] predicted value ≤ 80%); 2) history of asthma exacerbations (serious exacerbation: OCS bursts ≥ 2/year or hospitalization ≥ 1/year).
Definition and evaluation of difficult-to-treat asthma
Difficult-to-treat asthma is defined when asthma is uncontrolled despite GINA step 4 or 5 treatment (Fig. 1). During the treatment optimization period, physicians should check the following: 1) confirming the asthma diagnosis; 2) correcting modifiable risk factors; and 3) controlling comorbidities. Physicians are also recommended to consider referral of patients with difficult-to-treat asthma to asthma specialists or asthma specialty centers. We have included a checklist for real practice use (Table 2).
Table 2. Checklist to distinguish ‘severe asthma’ from ‘difficult-to-treat asthma’.
| Checklist | |
|---|---|
| Is the patient a current smoker? Have you encouraged him/her to quit smoking? | |
| Do you check how well the patient uses the inhaler and educate them on how to use it properly (at each visit)? | |
| Do you understand the factors that keep patients not adherent to their medications? | |
| Are there any adverse events due to asthma medications? (e.g., oral candidiasis, cough, hoarseness, dry mouth, or palpitation) | |
| Has the patient informed of avoidance of the sensitized allergens or non-specific stimuli? | |
| Environment control (HDM, pollens, molds, fine dust, air pollution, cold air, or other seasonal factors) | |
| Occupational stimuli/work-related symptoms | |
| Pets (dogs, cats, birds) | |
| Drug adverse effects (e.g., cough, chest tightness, or dyspnea due to aspirin, ACEi, or β-blockers) | |
| Does the patient need to be encouraged to exercise or lose weight? | |
| Have you ever considered assessing and managing the comorbidities of the patient? | |
| Chronic rhinosinusitis (with or without nasal polyps) by imaging studies (X-ray or CT scan of the PNS) | |
| GERD by endoscopy or preemptive treatment with proton pump inhibitors | |
| Obstructive sleep apnea by polysomnography | |
| Obesity | |
| Psychological distress (anxiety and depression) | |
| Structural lung diseases (COPD or bronchiectasis) by imaging studies (chest CT scan) | |
HDM, house dust mites; ACEi, angiotensin-converting enzyme inhibitors; PNS, paranasal sinuses; GERD, gastroesophageal reflux disease; COPD, chronic obstructive pulmonary diseases; CT, computed tomography.
Confirming asthma diagnosis
The diagnosis of asthma should be confirmed based on the patient's clinical history and diagnostic tests before diagnosing SA. Differential diagnoses should be considered according to patient age. For patients <40 years old, chronic upper airway cough syndrome, vocal cord dysfunction (VCD), hyperventilation, bronchiectasis, congenital heart diseases, or foreign body aspiration should be distinguished from asthma. For patients >40 years old, VCD, hyperventilation, chronic obstructive pulmonary disease (COPD), bronchiectasis, heart failure, medication-related cough (for example, angiotensin-converting enzyme inhibitors), parenchymal lung diseases, pulmonary embolism, and central airway obstruction should be considered as differential diagnoses of difficult-to-treat asthma. Furthermore, for some specific diseases, such as eosinophilic granulomatosis with polyangiitis (EGPA), allergic bronchopulmonary aspergillosis (ABPA), and chronic eosinophilic pneumonia, asthma is included in their diagnostic criteria. For differential diagnosis, clinicians should consider appropriate examinations, such as chest computed tomography, bronchoscopy, laryngoscopy, serum anti-neutrophil cytoplasmic antibodies, and serum specific IgE to Aspergillus.
Correcting modifiable risk factors
Modifiable risk factors should be corrected before diagnosing SA. Modifiable risk factors include smoking, incorrect inhaler technique, medication non-adherence, environmental exposure to sensitized allergens, or non-specific stimuli. Detailed information is provided in Supplement S2.
Controlling comorbidities
Comorbidities should be controlled before diagnosing SA. Comorbidities include chronic rhinosinusitis (CRS), gastroesophageal reflux disease, obstructive sleep apnea, obesity, and depression/anxiety disorder. These comorbidities can hamper diagnosis, reduce treatment response, increase acute deterioration, and result in patients receiving excessive treatment. Current guidelines emphasize the need for thorough check and correction of comorbidities before diagnosing patients as having SA. Detailed information is provided in Supplement S3.
Optimization of treatments in difficult-to-treat asthma
SA is diagnosed when asthma is still poorly controlled a 3-6 months after treatment optimization, including both non-pharmacological and pharmacological interventions.
Non-pharmacological interventions
Asthma education is necessary for SA management: lack of information results in poor adherence to treatment or under-perception of the asthma severity by patients, resulting in poorly controlled asthma. Therefore, to ensure better control, asthmatics should understand the nature of the disease, exacerbation signs and symptoms, treatment principles, the necessity for regular asthma medication use, avoidance of trigger factors, and self-management of asthma. Physicians should ensure that patients are aware that asthma is an inflammatory airway condition, which rationalizes regular long-term anti-inflammatory treatment. It is important to inform patients that several comorbidities and risk factors can worsen the disease.
Pharmacological interventions
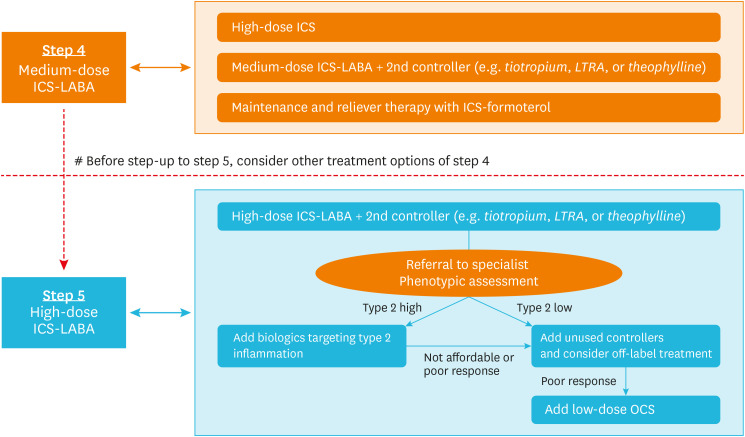
The combination of ICS and long-acting β2 agonists (LABA) is a mainstay of asthma treatment. In patients who do not achieve a well-controlled status with the use of a medium-dose ICS–LABA combination (GINA step 4), increasing the dose of ICS (a high-dose ICS–LABA combination) or adding a second controller, such as tiotropium or leukotriene receptor antagonists (LTRA), is recommended. Maintenance and reliever therapy with an ICS–formoterol combination can be considered before moving to GINA step 5 treatment (Fig. 2).
Fig. 2. Pharmacological treatment of severe asthma. Patients with uncontrolled asthma despite the use of a high-dose ICS–LABA combination and an add-on second controller (such as tiotropium, LTRA, or theophylline) should be referred to asthma specialists for phenotypic assessment and determination of the use of type 2 biologics. Those with type 2 high inflammation are eligible for type 2 biologics. Those with type 2 low inflammation need to be managed with unused second controller or an off-label treatment. Finally, OCS could be added as maintenance therapy for patients with severe asthma that is uncontrolled with the use of every medication available.
ICS, inhaled corticosteroids; LABA, long-acting β2 agonists; LTRA, leukotriene receptor antagonists; OCS, oral corticosteroids.
If asthma remains uncontrolled despite use of a high-dose ICS–LABA combination in GINA step 5 treatment, other controllers are needed for effective control (Fig. 2). If all of these attempts remain unsuccessful, physicians should consider referring patients to asthma specialists for phenotyping and add-on treatment, such as biologics. Biologics are helpful for SA with type 2 inflammation. Type 2 inflammation is defined when one of the following criteria is satisfied during high-dose ICS: 1) blood eosinophils ≥ 150/μL; 2) sputum eosinophils ≥ 2%; 3) FeNO ≥ 20 ppb; and 4) allergen-driven asthma.7 If a patient does not show type 2 inflammation (on at least 3 separate assessments), treatment options are very limited. Off-label and experimental options for these so-called non-type 2, patients are presented further in this paper. Finally, maintenance of low-dose OCS can be added to these medications, but adverse effects should be considered.
Phenotype and biomarkers in SA
Phenotype, endotype, and treatable traits
Phenotypes are collections of observable characteristics and can be organized according to their associations with specific triggers, patient characteristics, or clinical presentation features (Table 3). These phenotypes present as clusters in patients with SA.29 As part of the investigative work-up, clinicians endeavor to characterize each patient's asthma. SA is now recognized as a heterogeneous disease driven by multiple underlying pathobiological mechanisms (so-called endotypes), which can be described in terms of the cells, cytokines, and mediators involved. A deeper understanding of phenotype and endotype has proposed “precision medicine” for the management of patients with SA. However, endotypes are not fully understood as they are defined by different biological levels (genes, proteins, metabolites, cells, organs, and environment). Additionally, endotypes are not mutually exclusive. Consequently, it is not easy to implement their use in real practice. Recently, there has been a move away from the labeling of airway diseases and toward the identification of phenotypic “treatable traits,” linking them to therapeutic approaches.30 These traits can be treatable based on phenotypic recognition or deep understanding of the critical endotype recognition.31 A treatable trait must be clinically important, should be able to be effectively treated to be called a “treatable” trait, and needs a trait identification marker for objectively identifying the presence of the trait in preparation for targeted therapy (Table 4).30 As an example, a recent study examined 24 treatable traits of SA across 3 domains (pulmonary, extra-pulmonary, and behavioral/risk factors). This revealed 10 treatable traits, including depression and inhaler-device polypharmacy, that were significantly associated with an elevated risk of future asthma exacerbations.32 Among the treatable traits listed in Table 4, patients with SA expressed more pulmonary and extrapulmonary traits than those with non-SA. Allergic sensitization, upper airway disease, airflow limitation, eosinophilic inflammation, and frequent exacerbations were common in SA. Thus, one can say that a patient “has SA with a degree of fixed airflow limitation, likely due to smoking, nasal polyposis, absence of type 2 inflammation, and high expression of XX,” rather than “he has asthma,” if XX is a treatable trait. We acknowledge that the treatable trait approach is more efficient, effective, and safer than the traditional approach, and recommend wide use of this approach in clinical practice.
Table 3. Asthma phenotypes.
| Category | Phenotypes |
|---|---|
| Triggers | Atopic/non-atopic, aspirin-exacerbated, infection, exercise, occupational, smoking |
| Patient characteristics | Early/late onset, sex, peri-menopausal onset, race, obesity |
| Clinical presentation | Aggravated by exercise, corticosteroid dependent/resistant, low lung function, less reversible airway obstruction, frequent exacerbation |
Table 4. Treatable traits in the European U-BIOPRED adult asthma cohorts and defining criteria.
| Category | Treatable trait | Defining criteria |
|---|---|---|
| Pulmonary | Fixed airway obstruction | Post-bronchodilator FEV1/FVC < 0.7 |
| Airway reversibility | Post-bronchodilator increases in FEV1 and/or FVC ≥ 12% and ≥ 200 mL | |
| Type 2 inflammation | Sputum eosinophil proportion ≥ 2% and/or blood eosinophils ≥ 450 cells per mL and/or FeNO > 50 ppb | |
| Neutrophilic inflammation | Sputum neutrophil proportion > 60% | |
| Cough | Asthma Quality of Life Questionnaire Question 12 score ≤ 4 and/or Sino-Nasal Outcomes Test Question score 4 ≥ 3 | |
| Exercise-induced respiratory symptoms | Routine physical activity and/or physical exercise as an asthma trigger | |
| Sputum | Mucus production > 30 mL/day | |
| Extrapulmonary | Rhinosinusitis | Medical history of “allergic/non-allergic rhinitis active and/or sinusitis” |
| Nasal polyps | Medical history of “nasal polyps” | |
| Obese | BMI ≥ 25 kg/m2* | |
| Underweight | BMI < 18.5 kg/m2* | |
| Obstructive sleep apnea | Epworth sleepiness scale score ≥ 11 | |
| Gastroesophageal reflux | Medical history of “gastroesophageal reflux” | |
| Vocal cord dysfunction | Medical history of “vocal cord dysfunction” | |
| Osteoporosis | Medical history of “osteoporosis” | |
| Cardiovascular disease | Medical history of “cardiac disease” | |
| Eczema | Medical history of “eczema” | |
| Atopy | Positive skin prick test and/or increased blood IgE | |
| Risk/behavioral | Smoking | Medical history of “current smoker” |
| Poor medication adherence | Medication Adherence Rating Scale mean score < 4.5 | |
| Depression | Center for Epidemiologic Studies Depression scale ≥ 16 | |
| Anxiety | Generalized Anxiety Disorder-7 ≥ 5 |
Modified from reference 31.
BMI, body mass index; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IgE, immunoglobulin E.
*Based on the Korean criteria for obesity.
Trait identification markers and biomarkers
Blood eosinophil count is a well-known biomarker of type 2 airway inflammation. Examples of biomarkers include everything from laboratory-measured results to questionnaire results (e.g., the Hospital Anxiety and Depression Scale, used to recognize psychological dysfunctions; Table 4). There is no universally accepted biomarker that is easy to use, applicable, and meaningful in clinical practice. The most extensively investigated biomarkers are those used to identify type 2 inflammation. They are particularly important in current practice as most of the newer biologics target type 2 inflammation. Table 5 summarizes the details of currently available biomarkers for type 2 inflammation, such as serum IgE, sputum eosinophil count, blood eosinophil count, and FeNO, and their advantages and disadvantages. Moreover, a recently updated guideline about SA management from ERS/ATS3 suggested a cutoff level of blood eosinophil count and FeNO for choosing to use biologics, such as anti-IgE and anti-interleukin (IL)-5. By combining these biomarkers, we can get a decision chart for phenotype- (or endotype-) based SA management. Biomarkers for non-type 2 asthma have not been available until now. The pathogenesis of non-type 2 asthma has been investigated and revealed little evidence regarding inflammatory molecules, such as neutrophils and non-Th2 cytokines (interferon-γ, IL8, IL18, and IL17).
Table 5. Biomarkers for identifying type 2 inflammation: advantages and disadvantages.
| Biomarker | Advantages | Disadvantages |
|---|---|---|
| Serum IgE | • Easy to measure | Does not predict omalizumab responsiveness |
| • Identifies candidates for omalizumab therapy | ||
| Sputum eosinophils | • ERS/ATS recommended to guide treatment, along with clinical criteria | Technically challenging to perform; some patients cannot provide adequate samples |
| • Adjusting therapy based on sputum eosinophils was validated for reducing exacerbation frequency in adults | ||
| Blood eosinophils | • Correlated with sputum eosinophilia | Optimal cutoff not established |
| • Easy to measure | ||
| • ≥ 300 cells/μL, ↑ risk of asthma attacks, asthma-related ED visits | ||
| • ≥ 400 cells/μL, ↑ rate of severe exacerbations | ||
| FeNO | • Correlated with sputum and blood eosinophils | Not recommended for guiding therapy in patients with severe asthma |
| • ≥ 50 ppb, ↑ risk of asthma attacks, asthma-related ED | ||
| • Adjusting therapy based on FeNO reduced the risk of asthma exacerbation | ||
| Serum periostin | • Markers of airway eosinophilia and IL13 activity | Not specific to asthma |
ED, emergency department; ERS/ATS, European Respiratory Society/American Thoracic Society; FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E; IL, interleukin.
TREATMENT OF SA
Below, we summarize medications that can be added as second controllers in patients in whom asthma is poorly controlled by a medium- to high-dose ICS–LABA combination in GINA steps 4 or 5. The most preferred second controllers are tiotropium, LTRA, and theophylline. Additionally, combining these second controllers with biologics should be considered in patients with type 2 inflammation.
Second controllers as add-on treatment to an ICS–LABA combination
Long-acting muscarinic antagonist (LAMA)
LAMA, including tiotropium, are the preferred additional bronchodilator in SA patients with a history of exacerbation, because tiotropium improves lung function and asthma symptoms, and reduces risk of acute exacerbation (AE), regardless of the baseline characteristics, including age, allergic status, and disease duration.33 Moreover, there is accumulating evidence of the effects and benefits of LAMA with add-on therapy in SA patients. Tiotropium was compared to salmeterol and a placebo in patients with uncontrolled asthma, despite medium to high doses of ICS. In 2 replicate, randomized, controlled trials involving patients with poorly controlled SA despite the use of standard combination therapy, addition of tiotropium significantly increased the time to first exacerbation and provided modest, sustained bronchodilation.34 Tiotropium also lowered the rate of AE and improved trough FEV1 compared to a placebo.34 Thus, addition of once-daily tiotropium, via a soft mist inhaler, to severe, uncontrolled asthma treatment, including a high-dose ICS–LABA combination, significantly reduces exacerbation, and improves lung function and symptom control.
Theophylline
Theophylline had been used as a controller before the ICS era. Beyond its weak bronchodilator effect, theophylline has anti-inflammatory, immunomodulatory, and bronchoprotective effects. Although theophylline is not routinely recommended for mild-to-moderate asthma in modern treatment, it could be considered as an add-on controller in patients with SA. Theophylline is less effective than LABA35 and did not demonstrate superiority over LTRA when added to ICS.36
Theophylline administration requires care with monitoring of serum concentrations. The clearance of theophylline is increased in younger patients and smokers, and decreased in patients with cor pulmonale, cardiac decompensation, hepatic impairment, and elderly patients. Concurrent medications that could change theophylline clearance should be checked.
LTRA
Leukotrienes are proinflammatory mediators in tissue, sputum, and blood that contribute to eosinophilic inflammation. LTRA provides an additional bronchodilator effect when added to ICS and/or LABA.37 The median number of asthma exacerbation incidents per patient decreased from 1.6 to 1.2 per year when montelukast was added to SA treatment in elderly patients.38 In contrast, in a study of 72 adults with SA receiving an ICS–LABA combination, some of whom were also on OCS, the addition of montelukast did not improve the clinical outcomes over 14 days.39 LTRA has advantages as an oral agent when used once or twice daily. Additionally, it has an excellent safety profile and a good therapeutic index.
Type 2 biologics therapy
There are currently 3 types of biologics for the treatment of SA patients with type 2 inflammation: anti-IgE, anti-IL5/anti-IL5 receptor (R), and anti-IL4Rα (Table 6). These medications are specific for the type 2 SA phenotype. The target population should be selected carefully based on inflammatory phenotypes. If used in appropriate patients, they may modify underlying pathogenic mechanisms and prevent disease progression.
Table 6. Currently approved indications of biologics for severe asthma in Korea.
| Biologics | Action | Dose | Interval (wk) | Route | Indication |
|---|---|---|---|---|---|
| Omalizumab (Xolair®) | Anti-IgE | 0.016 mg/kg per IU | 2 or 4 | SC | ≥ 6 years old; positive allergy testing (allergic asthma); IgE: 30–700 IU/mL |
| Mepolizumab (Nucala®) | Anti-IL5 | 100 mg | 4 | SC | ≥ 18 years old; AEC ≥ 150 cells/µL or ≥ 300 cells/µL at least once a year |
| Reslizumab (Cinqair®) | Anti-IL5 | 3 mg/kg | 4 | IV | ≥ 18 years old; AEC ≥ 400 cells/µL |
| Benralizumab (Fasenra®) | Anti-IL5R | 30 mg | 8* | SC | ≥ 18 years old; severe eosinophilic asthma |
| Dupilumab (Dupixent®) | Anti-IL4Rα | 200 mg† | 2 | SC | ≥ 12 years old; AEC ≥ 150 cells/µL or FeNO ≥ 25 ppb |
| 300 mg‡ | 2 | SC | With OCS-dependent or moderate-to-severe atopic dermatitis |
SC, subcutaneous; IV, intravenous; IgE, immunoglobulin E; IL, interleukin; AEC, absolute blood eosinophil count; FeNO, fractional exhaled nitric oxide; OCS, oral corticosteroids.
*Every 4 weeks for the first 3 doses, followed by every 8 weeks for maintenance; †For the first cycle, 400 mg (two 200 mg injections); ‡For the first cycle, 600 mg (two 300 mg injections).
Anti-IgE antibody
1) Omalizumab
Omalizumab is a recombinant, humanized, monoclonal IgG antibody against IgE. It binds to free, circulating IgE rather than mast cell-bound IgE, decreasing the total body IgE fraction which down-regulates FcεRI on the mast cell surface. In the pivotal INNOVATE study,40 patients (age: 12-75 years) with inadequately controlled asthma despite a high-dose ICS–LABA combination, with reduced lung function and asthma exacerbations, randomly received omalizumab (compared to placebo) for 28 weeks. All patients had atopic asthma and a serum total IgE level of 30–700 IU/mL and FEV1 40%–80% of predicted. Total asthma exacerbation, SA exacerbation, and emergency department visit rates were markedly decreased.
Omalizumab was approved for use in Korea by the Ministry of Food and Drug Safety (MFDS) in 2007. Currently approved indications of omalizumab are detailed in Table 6. Dosing should not be adjusted based on total IgE levels during treatment or <1 year following interruption of therapy. A maximum amount of 150 mg should be administered at a single injection site to avoid local reactions. Therapy should not be initiated during AE of asthma, nor should it be self-administered or administered outside of a medical setting, due to the risk of anaphylaxis.
Previous studies have shown that omalizumab is effective in only about 60%–70% of SA patients. If it is going to be effective, it usually works within 4 months,41 although it is unclear how long patients should remain on omalizumab. Studies have shown that, after more than 2 years of treatment, the condition of about half of patients with asthma did not worsen even after stopping omalizumab. It is currently advised that, for patients with a good response, a trial withdrawal of the biologic should be considered after at least 12 months of treatment, and only if asthma remains well-controlled on medium-dose ICS therapy and there is no further exposure to a previous, well-documented allergic trigger, omalizumab can be discontinued.
Treatment with omalizumab was well-tolerated in several clinical trials. Through a post-marketing investigation, omalizumab could cause anaphylaxis with a probability of 1/1,000.42 A significant proportion of anaphylactic reactions was delayed in onset and exhibited protracted symptom progression; 61% of these reactions occurred within the first 2 hours after the first 3 injections; 14% occurred within 0.5 hours after the 4th or later injection.43 Therefore, it has been recommended that patients should be observed for 2 hours after the first 3 injections of omalizumab and for 30 minutes after subsequent injections.
In addition to omalizumab, other anti-IgE drugs (ligelizumab, quilizumab, XmAb7195, and MEDI4212) are currently undergoing clinical trials.44
Anti-IL5 and anti-IL5R antibody
1) Mepolizumab
Mepolizumab is a humanized monoclonal antibody that inhibits IL5 selectively and targets eosinophils specifically. In the DREAM study, mepolizumab reduced the frequency of AE in severe eosinophilic asthma (SEA), which was defined as 1 or more following criteria in the previous year: sputum eosinophils ≥ 3%, FeNO ≥ 50 ppb, blood eosinophils ≥ 300/μL, and prompt deterioration of asthma after 25% or less reduction in regular ICS or OCS.45 In the MENSA study, the AE rate reduced by 53% and the mean FEV1 improved by 98 mL with the administration of subcutaneous (SC) mepolizumab.46 In the SIRIUS study, mepolizumab led to a 50% reduction in OCS dosage and reduced the AE rate by 32% in SEA, and improved health-related quality of life in the MUSCA trial.47,48
Mepolizumab (100 mg, SC, monthly) was approved in Korea by the MFDS in 2016 (Table 6). However, there is no clear guideline on how long treatment should continue. A clinical response to mepolizumab is usually observed within 4 months; the safety profile is similar to a placebo in clinical trials and even after long-term exposure (up to 4.5 years), it can be continued indefinitely if a clinical response is achieved.49 To date, there is no definite evidence of the development of neutralizing anti-drug antibodies to IL5. Mepolizumab should not be used to treat AE.
2) Reslizumab
Reslizumab is a humanized, monoclonal antibody of IgG4 subtype against IL5, with a similar mechanism as mepolizumab. A phase 2, randomized, placebo-controlled, multicenter trial (NCT02559791) demonstrated that intravenous (IV) reslizumab (3.0 mg/kg) significantly decreased sputum eosinophils and improved asthma symptoms in patients with SA and sputum eosinophils ≥ 3%.50 In phase 3 trials, 2 duplicate international, double-blind, parallel-group, randomized, placebo-controlled trials, patients who had blood eosinophils ≥ 400 cells/μL or ≥ 1 AE in the previous year had a significant reduction in AE frequency after receiving reslizumab (NCT01287039 and NCT01285323).51 Additionally, reslizumab improved lung function, asthma control and symptoms, and quality of life.52 No study to date has demonstrated an OCS-sparing effect. Weight-adjusted IV reslizumab administration showed more effective attenuation in sputum/blood eosinophils, with an improvement of asthma control, than fixed-dose SC mepolizumab.53
Although common adverse reactions were similar to those of placebo, 3 anaphylaxis cases occurred among 1,611 patients during randomized controlled trials.50,51 Therefore, this drug has a black box warning from the US Food and Drug Administration.51 Although it shows favorable long-term safety and efficacy (up to 2 years), blood eosinophil levels appear to return to previous levels after stopping, by approximately 4 months after the last dose.54 Reslizumab (3 mg/kg, IV, monthly) was approved in Korea by MFDS in 2017 (Table 6).
3) Benralizumab
Benralizumab, a humanized monoclonal antibody against the alpha subunit of IL5R, on the immune cell surface, induces rapid and nearly complete depletion of eosinophils by natural killer cell-mediated cellular cytotoxic reactions. In the phase 3 SIROCCO study, benralizumab reduced AE, improved prebronchodilator FEV1, and improved asthma symptoms in patients who had blood eosinophils ≥ 300 cells/μL or ≥ 2 AE in the previous year55; this was replicated in Korean patients and other trials.56,57 In the ZONDA trial, benralizumab reduced OCS use by 75% from baseline and the annual AE rate by 70%.58 In a matching-adjusted indirect comparison, benralizumab and mepolizumab showed similar efficacy.59 The phase 3 BORA trial showed the safety and efficacy of benralizumab over 2 years. The therapeutic effect of benralizumab was maintained for up to 2 years. The most common AE was worsening asthma, with no helminth infection cases reported.60 The eosinophil depletion was maintained through the 2nd year, and blood eosinophil counts began to increase after treatment discontinuation.60 Benralizumab was approved by the MFDS in 2019 for the treatment of SEA in Korea, at 30 mg SC every 2 months (every 4 weeks for the first 3 doses) (Table 6).
Anti-IL4Rα antibody
1) Dupilumab
Dupilumab is a fully human monoclonal IgG4 antibody to the alpha subunit of IL4R, which is activated by IL4 and IL13. Dupilumab blocks IL4Rα to inhibit the signaling effects of IL4 and IL13, which play a crucial role in asthma and are produced mainly by CD4+ Th2 cells and ILC2s. In a phase 2a study, dupilumab treatment reduced the AE rate by 87%, as well as the concentration of FeNO and total IgE, in patients with persistent, moderate-to-SA (blood eosinophil counts of ≥ 300 cells/μL or sputum eosinophil ≥ 3%).61 In the phase 2b trial, dupilumab reduced the AE rate, by 70%, and FeNO, and improved FEV1, quality of life, and asthma control in patients with uncontrolled, persistent asthma, who were on a moderate- to high-dose ICS–LABA combination, irrespective of the baseline eosinophilic count.62 A phase 3 trial (the Liberty Asthma QUEST study; 52 weeks) confirmed the earlier findings, such as the improvement of FEV1, asthma control, and quality of life, and reduction of severe AE, irrespective of the baseline eosinophil count or any other biomarkers. However, it was observed that it was more effective for cases with a higher blood eosinophil count.63
Dupilumab was approved as maintenance therapy for patients ≥ 12 years old with SEA (suggested blood eosinophil counts ≥ 150 cells/μL or FeNO ≥ 25 ppb) or with OCS-dependent asthma by the US Food and Drug Administration in 2018. Dupilumab was recently approved by the MFDS as a treatment for patients with SEA in 2020 (for first cycle 400 or 600 mg, followed by 200 or 300 mg every 2 weeks) (Table 6). The most common adverse effects were injection-site reactions and upper respiratory infections.62 Eosinophilia was found in 1.2%; 2 cases suffered from worsening of hypereosinophilia and chronic eosinophilic pneumonia, including a fatality due to pneumonia.63
Future drugs in development
1) Anti-thymic stromal lymphopoietin (TSLP) antibody (Tezepelumab)
Tezepelumab is a humanized monoclonal antibody specific for the epithelial cell-derived cytokine, TSLP. A phase 2 trial showed that it could reduce the AE rate and improve FEV1 as compared to placebo, regardless of blood eosinophil counts.64
2) Chemoattractant receptor-homologous molecule expressed on Th2 lymphocytes (CRTH2) antagonist (prostaglandin D2 [PGD2] receptor 2 [DP2] antagonist)
PGD2, generated by hematopoietic prostaglandin D synthase, acts on G protein-coupled receptors, including the CRTH2 and DP1. Fevipiprant, a PGD2 antagonist, binds to DP2 (also known as CRTH2), which mediates the migration of Th2 cells, stimulates them to produce type 2 cytokines, such as IL4, IL5, and IL13, and affects the cytokine release from ILC2s.65 Fevipiprant reduces eosinophilic airway inflammation and is well tolerated in patients with persistent moderate-to-SA and sputum eosinophilia despite ICS therapy.
3) Anti-IL33 antibody (Etokimab, ANB020)
IL33 plays an important role in type-2 innate immunity via the activation of allergic inflammation through its receptor ST2. Phase 2 trials, targeting the IL33 and IL33/ST2 axis are currently ongoing.
Selection of biologics for SA
Selection of the most appropriate drug among the currently approved biologics for patients with SA is challenging. No head-to-head comparisons between currently available biologics have been conducted. There are no useful biomarkers for predicting or monitoring treatment responses. Thus, factors such as the mechanism of action of certain drugs, blood/sputum eosinophil levels, serum IgE levels, FeNO levels, and atopic status of asthma patients, as well as the cost of the drugs, should be considered when selecting biologics. For patients with allergic asthma, anti-IgE antibody (omalizumab) is recommended in the first place among biologics. Blood eosinophil counts ≥ 260 cells/μL or FeNO ≥ 19.5 could be used as biomarkers predicting a good response to anti-IgE treatment.3 For eosinophilic asthmatic patients (suggested blood eosinophil counts ≥ 150 cells/μL) with a history of AE, anti-IL5/5R therapy may be considered as the first choice. IL4Ra could be considered as a treatment option for severe eosinophilic type 2 asthma or patients requiring maintenance OCS.
Assessment of treatment response to biologics
Evaluation of therapeutic response to biologics is important, and we recommend it after 3–4 months of use. Unfortunately, at present, there are no criteria and/or biomarkers of a good response. To determine response, we need consider AEs, symptom control, lung function, OCS sparing, and patient satisfaction collectively. Although the GINA guidelines on SA recommend extending trials to 6–12 months if the treatment response is difficult to assess after use of biologics for 3–4 months,7 evidence is scarce and further studies are required. If patients have shown a good response to biologics, the first step is to reduce OCS carefully, and then stop other add-on therapy by evaluating asthma control at an interval of 3–6 months. Inhaler use should be maintained at least at medium-dose ICS and patients need to be informed to avoid stopping the inhaler use even if symptoms improve. Evidence on when to discontinue biologics remains insufficient. On the other hand, if patients are non-responders, the following should be considered. First, treatable trait, including the type 2 high inflammation phenotype and comorbidity, should be reviewed. Then, clinicians can attempt to switch different type 2 targeted biologics, although the timing of the drug switch has not yet been established. Another option might be combination therapy with 2 or more biologics in patients who have severe eosinophilic and allergic asthma despite receiving add-on therapy with a biologic agent.
Off-label treatments and therapeutic procedures
Macrolides
Macrolides have been considered as a therapeutic option for asthma due to their anti-bacterial and immunomodulating actions. There have been several randomized trials using clarithromycin and azithromycin for refractory asthma patients. Clarithromycin therapy (500 mg, twice daily) for 8 weeks, in a study of 23 SA patients, reduced sputum IL8 levels, neutrophil numbers, and improved the quality of life scores as compared to placebo.66 The AZISAST study used azithromycin 250 mg once daily for 26 weeks in 55 SA patients, and the AMAZES study used azithromycin 500 mg, 3 times a week, for 48 weeks in 213 partly controlled asthma patients.67 They showed reduction in AE and reduction in severe AE in those with non-eosinophilic SA (blood eosinophilia ≤ 200/μL). Although a Cochrane review published in 2015 was inconclusive because of the low quality of evidence,68 macrolides could be an additional therapy in SA patients. However, increasing antibiotic resistance, QT prolongation, and hearing loss should be considered.
Roflumilast
Roflumilast is known to exert an anti-inflammatory effect, decreasing neutrophilic and eosinophilic inflammation. According to Meltzer and colleagues,69 roflumilast provided additional improvements in FEV1 when given with ICS. In a phase 2 trial, the combination of roflumilast with montelukast improved lung function and asthma control, as compared to montelukast alone, in moderate-to-SA.70
Imatinib
In a randomized trial, imatinib decreased airway hyper-responsiveness, mast cell counts, and tryptase release in patients with SA.71 A similar drug, masitinib (c-Kit/PDGFR tyrosine kinase inhibitor) improved disease control in severe CS-dependent asthmatics.72
Bronchial thermoplasty (BT)
Airway smooth muscles play multiple roles in asthma pathogenesis, including regulation of bronchial tone, immunomodulation, and extracellular matrix deposition. BT ablates airway smooth muscles by radiofrequency energy. BT resulted in significant improvements in FEV1, the Asthma Control Questionnaire score, and a reduction of severe AE in SA patients, which remained over 52 weeks to 5 years.73
SCS
CS is effective in targeting numerous elements of the type 2/eosinophilic inflammatory pathway. The key mechanism is depletion of circulating eosinophils caused by ICS-unresponsive recruitment signals of the airway mucosa and resulting in a reduced response to ICS. To date, OCS have been widely used as a maintenance treatment in SA. However, chronic OCS use has serious side-effects. Moreover, CS-dependent asthma is associated with an increased risk of mortality22; therefore, CS should be considered as the last controller.
SPECIFIC SITUATIONS
EGPA
EGPA is a rare systemic necrotizing vasculitis accompanying asthma, blood, and tissue eosinophilia. The exact prevalence of EGPA in Korea is not known particularly in the SA population. However, a retrospective analysis from a single center in Korea showed that the respiratory tract (bronchial asthma) was the most commonly involved organ, and its involvement was associated with a more favorable outcome.74 EGPA occurs in a minority of SA patients. However, EGPA should be considered when dealing with severe uncontrolled asthma as these patients require additional treatment, such as immunosuppressants and OCS. Mepolizumab in high doses (300 mg every 4 weeks) can be used to treat EGPA.75
Aspirin-exacerbated respiratory disease (AERD)
AERD is characterized by asthma, CRS with nasal polyps, and hypersensitivity to aspirin/nonsteroidal anti-inflammatory drugs (NSAID). A recent meta-analysis reported the prevalence of AERD as 7% in adult patients with asthma and 14% in patients with SA.76 The risk of uncontrolled asthma, SA, and AE is reported to be higher in patients with AERD than in those with aspirin-tolerant asthma. Identifying patients with AERD is important because of its high morbidity. Additionally, education on aspirin/NSAID avoidance and trialing of other potential therapeutic options are needed.
ABPA
ABPA is caused by hypersensitivity to Aspergillus fumigatus. It manifests as poorly controlled asthma, recurrent pulmonary infiltrates, and bronchiectasis. ABPA is also a risk factor for accelerated loss of lung function. Therefore, recognizing ABPA is important to improve patient symptoms and to delay the development or prevention of bronchiectasis. Sensitization to Aspergillus without other characteristics of ABPA in SA is termed SA with fungal sensitization; one-third to one-half of SA patients have fungal sensitization mostly to A. fumigatus. Patients with asthma who display fungal sensitization are more likely to have SA. ABPA treatment consists of anti-inflammatory therapy with CSs and anti-fungal treatment to reduce the fungal load in the airways. SCS is recommended over several months, with an initial dose of 0.5 mg/kg of prednisone and tapered over 6-8 weeks. Although SCS is highly efficacious in ABPA management, almost 50% of patients relapse when the dose is tapered, and 20%–45% become CS-dependent. Anti-fungal therapy with itraconazole (200 mg qd) is recommended as a second-line treatment.
The total IgE level in ABPA patients is usually high. Therefore, omalizumab, which acts against serum free IgE, has been used for ABPA treatment. Recent systematic reviews of case reports of omalizumab treatment in ABPA showed that it reduced FeNO, total IgE, AE, and SCS, but not lung function.77 Double-blind placebo-controlled trials are needed to establish the efficacy and safety of omalizumab treatment in ABPA.
Asthma–COPD overlap (ACO)
ACO is a condition in which the clinical features of asthma and COPD coexist in a patient.7 Despite considerable effort to define ACO, there are no definite diagnostic criteria for this disease.7 Accordingly, the prevalence varies widely, depending on the criteria applied, and the clinical outcomes are inconsistent. However, ACO patients are generally considered to be more symptomatic, experience more frequent exacerbations, and have higher mortality than patients with asthma. Unfortunately, there have been no large-scale treatment trials for ACO patients. Therefore, the treatment strategy for ACO patients is not well established. Currently, an ICS–LABA combination is generally recommended as an initial treatment for ACO. However, given that the patients with ACO have more symptoms and frequent exacerbations, a significant proportion of patients are thought to be uncontrolled with an ICS–LABA combination.
A post hoc analysis of the PROSPERO study showed that ACO patients receiving omalizumab over 48 weeks had improved treatment outcomes in terms of AE and ACT scores similar to those of patients without ACO.78 In the post hoc subgroup analyses, using data from the DREAM and the MENSA phase III studies on SA, mepolizumab was effective in preventing AE in asthmatic patients who had COPD features (≥40 years of age, fixed airflow limitation, FEV1 < 80%, and poor bronchodilator response) and smoking history (former smoker ≤ 10 years). In contrast, patients who were enrolled in a study demonstrating the effect of mepolizumab on eosinophilic COPD patients (100 mg in METREX, 100 or 300 mg in METREO) had some asthma features (elevated blood eosinophil count and use of high-dose ICS therapy).79 Therefore, mepolizumab may be recommended for improving the treatment outcomes of ACO patients in some cases. A post hoc analysis using the data from a phase 2b study on the clinical efficacy of dupilumab in patients with uncontrolled, persistent asthma revealed that about 12% of patients had COPD features, such as fixed airflow limitation with FEV1 < 80% and smoking history ≤ 10 years. The preliminary study results showed significant improvements in FEV1 and reduced frequent exacerbations with the use of dupilumab (300 mg every 2 weeks). This may also apply to patients with ACO.
Future direction of SA diagnosis and management in Korea
The development of type 2 biologics has led to an era of precision medicine. It also requires the physician to select the patients who would benefit most from particular drugs. To this end, accurate diagnosis of SA, and phenotypes and treatable traits need to be performed systematically. If a primary care physician does not have the capability to diagnose and manage uncontrolled or difficult-to-treat asthma, or encounters suspected SA patients, referral to an asthma specialist should be made. A SA clinic needs to enhance its ability to diagnose SA and comorbidities via a cooperative, multidisciplinary approach. Additionally, standardized protocols for phenotyping and assessing SA are required. We hope that this opinion paper will facilitate identification of patients with SA for referral and setting up SA clinics in referral hospitals.
While type 2 biologics have been approved and are available in clinical practice, they are not reimbursed by the public health insurance in Korea as yet. Most patients with SA cannot afford the high cost of biologics, and even those with very good responses to biologics often refuse to maintain biologic treatment because of economic reasons. Thus, a stratified approach is needed to use these highly expensive medications wisely in the management of SA. Making a new diagnostic code for the diagnosis of SA and reimbursement of biologics could be an option. Moreover, funds should be focused on the care of SA patients.
CONCLUSION
Difficult-to-treat and SA is associated with significant morbidity and mortality rates. A comprehensive and systematic approach is needed to manage uncontrolled asthma and to define difficult-to-treat asthma and SA. Applying the concept of treatable traits is a practical tool for managing SA. Since biologics targeting type 2 inflammation are available in clinical practice, physicians should consider referring patients with SA to specialists appropriately and to assess phenotypes for starting biologics. Moreover, novel treatments are needed to manage SA that is unresponsive to currently available medications.
ACKNOWLEDGMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC19C0318).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Other mechanisms contributing to severe asthma
Modifiable risk factors
Comorbidities of severe asthma
References
- 1.Song WJ, Lee JH, Kang Y, Joung WJ, Chung KF. Future risks in patients with severe asthma. Allergy Asthma Immunol Res. 2019;11:763–778. doi: 10.4168/aair.2019.11.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 3.Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55:1900588. doi: 10.1183/13993003.00588-2019. [DOI] [PubMed] [Google Scholar]
- 4.Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet J, Mantzouranis E, Cruz AA, Aït-Khaled N, Baena-Cagnani CE, Bleecker ER, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126:926–938. doi: 10.1016/j.jaci.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Bel EH, Sousa A, Fleming L, Bush A, Chung KF, Versnel J, et al. Diagnosis and definition of severe refractory asthma: an international consensus statement from the Innovative Medicine Initiative (IMI) Thorax. 2011;66:910–917. doi: 10.1136/thx.2010.153643. [DOI] [PubMed] [Google Scholar]
- 7.Global Initiative for Asthma. Global strategy for asthma management and prevention (2019 updated) Fontana: Global Initiative for Asthma; 2019. [Google Scholar]
- 8.Lai CK, De Guia TS, Kim YY, Kuo SH, Mukhopadhyay A, Soriano JB, et al. Asthma control in the Asia-Pacific region: the Asthma Insights and Reality in Asia-Pacific Study. J Allergy Clin Immunol. 2003;111:263–268. doi: 10.1067/mai.2003.30. [DOI] [PubMed] [Google Scholar]
- 9.Rabe KF, Adachi M, Lai CK, Soriano JB, Vermeire PA, Weiss KB, et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol. 2004;114:40–47. doi: 10.1016/j.jaci.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 10.Antonicelli L, Bucca C, Neri M, De Benedetto F, Sabbatani P, Bonifazi F, et al. Asthma severity and medical resource utilisation. Eur Respir J. 2004;23:723–729. doi: 10.1183/09031936.04.00004904. [DOI] [PubMed] [Google Scholar]
- 11.Quirce S, Plaza V, Picado C, Vennera M, Casafont J. Prevalence of uncontrolled severe persistent asthma in pneumology and allergy hospital units in Spain. J Investig Allergol Clin Immunol. 2011;21:466–471. [PubMed] [Google Scholar]
- 12.Backman H, Jansson SA, Stridsman C, Eriksson B, Hedman L, Eklund BM, et al. Severe asthma-A population study perspective. Clin Exp Allergy. 2019;49:819–828. doi: 10.1111/cea.13378. [DOI] [PubMed] [Google Scholar]
- 13.von Bülow A, Kriegbaum M, Backer V, Porsbjerg C. The prevalence of severe asthma and low asthma control among Danish adults. J Allergy Clin Immunol Pract. 2014;2:759–767. doi: 10.1016/j.jaip.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135:896–902. doi: 10.1016/j.jaci.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 15.Park SY, Kim JH, Kim HJ, Seo B, Kwon OY, Chang HS, et al. High prevalence of asthma in elderly women: findings from a Korean National Health Database and Adult Asthma Cohort. Allergy Asthma Immunol Res. 2018;10:387–396. doi: 10.4168/aair.2018.10.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SH, Moon JY, Lee JH, Ban GY, Kim S, Kim MA, et al. Perceptions of severe asthma and asthma-COPD overlap syndrome among specialists: a questionnaire survey. Allergy Asthma Immunol Res. 2018;10:225–235. doi: 10.4168/aair.2018.10.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee E, Kim A, Ye YM, Choi SE, Park HS. Increasing prevalence and mortality of asthma with age in Korea, 2002–2015: a nationwide, population-based study. Allergy Asthma Immunol Res. 2020;12:467–484. doi: 10.4168/aair.2020.12.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourdin A, Fabry-Vendrand C, Ostinelli J, Ait-Yahia M, Darnal E, Bouee S, et al. The burden of severe asthma in France: a case-control study using a medical claims database. J Allergy Clin Immunol Pract. 2019;7:1477–1487. doi: 10.1016/j.jaip.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 19.Ebmeier S, Thayabaran D, Braithwaite I, Bénamara C, Weatherall M, Beasley R. Trends in international asthma mortality: analysis of data from the WHO Mortality Database from 46 countries (1993–2012) Lancet. 2017;390:935–945. doi: 10.1016/S0140-6736(17)31448-4. [DOI] [PubMed] [Google Scholar]
- 20.van Bragt JJ, Adcock IM, Bel EH, Braunstahl GJ, Ten Brinke A, Busby J, et al. Characteristics and treatment regimens across ERS SHARP severe asthma registries. Eur Respir J. 2020;55:1901163. doi: 10.1183/13993003.01163-2019. [DOI] [PubMed] [Google Scholar]
- 21.Kim MH, Kim SH, Park SY, Ban GY, Kim JH, Jung JW, et al. Characteristics of adult severe refractory asthma in korea analyzed from the severe asthma registry. Allergy Asthma Immunol Res. 2019;11:43–54. doi: 10.4168/aair.2019.11.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee H, Ryu J, Nam E, Chung SJ, Yeo Y, Park DW, et al. Increased mortality in patients with corticosteroid-dependent asthma: a nationwide population-based study. Eur Respir J. 2019;54:1900804. doi: 10.1183/13993003.00804-2019. [DOI] [PubMed] [Google Scholar]
- 23.Eassey D, Reddel HK, Ryan K, Smith L. The impact of severe asthma on patients' autonomy: a qualitative study. Health Expect. 2019;22:528–536. doi: 10.1111/hex.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadatsafavi M, Lynd L, Marra C, Carleton B, Tan WC, Sullivan S, et al. Direct health care costs associated with asthma in British Columbia. Can Respir J. 2010;17:74–80. doi: 10.1155/2010/361071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YJ, Kwon SH, Hong SH, Nam JH, Song HJ, Lee JS, et al. Health care utilization and direct costs in mild, moderate, and severe adult asthma: a descriptive study using the 2014 South Korean Health Insurance Database. Clin Ther. 2017;39:527–536. doi: 10.1016/j.clinthera.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Golam S, Myers J, Bly C, Smolen H, Xu X. Systematic literature review of the clinical, humanistic, and economic burden associated with asthma uncontrolled by GINA steps 4 or 5 treatment. Curr Med Res Opin. 2018;34:2075–2088. doi: 10.1080/03007995.2018.1505352. [DOI] [PubMed] [Google Scholar]
- 27.Bateman ED, Buhl R, O'Byrne PM, Humbert M, Reddel HK, Sears MR, et al. Development and validation of a novel risk score for asthma exacerbations: the risk score for exacerbations. J Allergy Clin Immunol. 2015;135:1457–1464.e4. doi: 10.1016/j.jaci.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Chetta A, Gerra G, Foresi A, Zaimovic A, Del Donno M, Chittolini B, et al. Personality profiles and breathlessness perception in outpatients with different gradings of asthma. Am J Respir Crit Care Med. 1998;157:116–122. doi: 10.1164/ajrccm.157.1.9702093. [DOI] [PubMed] [Google Scholar]
- 29.Fitzpatrick AM, Moore WC. Severe asthma phenotypes—how should they guide evaluation and treatment? J Allergy Clin Immunol Pract. 2017;5:901–908. doi: 10.1016/j.jaip.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simpson AJ, Hekking PP, Shaw DE, Fleming LJ, Roberts G, Riley JH, et al. Treatable traits in the European U-BIOPRED adult asthma cohorts. Allergy. 2019;74:406–411. doi: 10.1111/all.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47:410–419. doi: 10.1183/13993003.01359-2015. [DOI] [PubMed] [Google Scholar]
- 32.McDonald VM, Hiles SA, Godbout K, Harvey ES, Marks GB, Hew M, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Respirology. 2019;24:37–47. doi: 10.1111/resp.13389. [DOI] [PubMed] [Google Scholar]
- 33.Kerstjens HA, Moroni-Zentgraf P, Tashkin DP, Dahl R, Paggiaro P, Vandewalker M, et al. Tiotropium improves lung function, exacerbation rate, and asthma control, independent of baseline characteristics including age, degree of airway obstruction, and allergic status. Respir Med. 2016;117:198–206. doi: 10.1016/j.rmed.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367:1198–1207. doi: 10.1056/NEJMoa1208606. [DOI] [PubMed] [Google Scholar]
- 35.Davies B, Brooks G, Devoy M. The efficacy and safety of salmeterol compared to theophylline: meta-analysis of nine controlled studies. Respir Med. 1998;92:256–263. doi: 10.1016/s0954-6111(98)90105-6. [DOI] [PubMed] [Google Scholar]
- 36.American Lung Association Asthma Clinical Research Centers. Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. Am J Respir Crit Care Med. 2007;175:235–242. doi: 10.1164/rccm.200603-416OC. [DOI] [PubMed] [Google Scholar]
- 37.Virchow JC, Jr, Prasse A, Naya I, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am J Respir Crit Care Med. 2000;162:578–585. doi: 10.1164/ajrccm.162.2.9905041. [DOI] [PubMed] [Google Scholar]
- 38.Bozek A, Warkocka-Szoltysek B, Filipowska-Gronska A, Jarzab J. Montelukast as an add-on therapy to inhaled corticosteroids in the treatment of severe asthma in elderly patients. J Asthma. 2012;49:530–534. doi: 10.3109/02770903.2012.680638. [DOI] [PubMed] [Google Scholar]
- 39.Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double-blind placebo-controlled trial. Lancet. 2001;357:2007–2011. doi: 10.1016/S0140-6736(00)05113-8. [DOI] [PubMed] [Google Scholar]
- 40.Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–316. doi: 10.1111/j.1398-9995.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 41.Bousquet J, Siergiejko Z, Swiebocka E, Humbert M, Rabe KF, Smith N, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy. 2011;66:671–678. doi: 10.1111/j.1398-9995.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 42.Cox L, Lieberman P, Wallace D, Simons FE, Finegold I, Platts-Mills T, et al. American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma & Immunology Omalizumab-Associated Anaphylaxis Joint Task Force follow-up report. J Allergy Clin Immunol. 2011;128:210–212. doi: 10.1016/j.jaci.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Lieberman P, Nicklas RA, Randolph C, Oppenheimer J, Bernstein D, Bernstein J, et al. Anaphylaxis--a practice parameter update 2015. Ann Allergy Asthma Immunol. 2015;115:341–384. doi: 10.1016/j.anai.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Busse WW. Biological treatments for severe asthma: a major advance in asthma care. Allergol Int. 2019;68:158–166. doi: 10.1016/j.alit.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 46.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 47.Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 48.Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390–400. doi: 10.1016/S2213-2600(17)30125-X. [DOI] [PubMed] [Google Scholar]
- 49.Khatri S, Moore W, Gibson PG, Leigh R, Bourdin A, Maspero J, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2019;143:1742–1751.e7. doi: 10.1016/j.jaci.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 50.Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184:1125–1132. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- 51.Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–366. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 52.Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789–798. doi: 10.1016/j.chest.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 53.Mukherjee M, Aleman Paramo F, Kjarsgaard M, Salter B, Nair G, LaVigne N, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38–46. doi: 10.1164/rccm.201707-1323OC. [DOI] [PubMed] [Google Scholar]
- 54.Murphy K, Jacobs J, Bjermer L, Fahrenholz JM, Shalit Y, Garin M, et al. Long-term safety and efficacy of reslizumab in patients with eosinophilic asthma. J Allergy Clin Immunol Pract. 2017;5:1572–1581.e3. doi: 10.1016/j.jaip.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 55.Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 56.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 57.Park HS, Lee SH, Lee SY, Kim MK, Lee BJ, Werkström V, et al. Efficacy and safety of benralizumab for Korean patients with severe, uncontrolled eosinophilic asthma. Allergy Asthma Immunol Res. 2019;11:508–518. doi: 10.4168/aair.2019.11.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–2458. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 59.Bourdin A, Husereau D, Molinari N, Golam S, Siddiqui MK, Lindner L, et al. Matching-adjusted indirect comparison of benralizumab versus interleukin-5 inhibitors for the treatment of severe asthma: a systematic review. Eur Respir J. 2018;52:1801393. doi: 10.1183/13993003.01393-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Busse WW, Bleecker ER, FitzGerald JM, Ferguson GT, Barker P, Sproule S, et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir Med. 2019;7:46–59. doi: 10.1016/S2213-2600(18)30406-5. [DOI] [PubMed] [Google Scholar]
- 61.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 62.Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388:31–44. doi: 10.1016/S0140-6736(16)30307-5. [DOI] [PubMed] [Google Scholar]
- 63.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486–2496. doi: 10.1056/NEJMoa1804092. [DOI] [PubMed] [Google Scholar]
- 64.Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377:936–946. doi: 10.1056/NEJMoa1704064. [DOI] [PubMed] [Google Scholar]
- 65.Gonem S, Berair R, Singapuri A, Hartley R, Laurencin MF, Bacher G, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4:699–707. doi: 10.1016/S2213-2600(16)30179-5. [DOI] [PubMed] [Google Scholar]
- 66.Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 2008;177:148–155. doi: 10.1164/rccm.200707-1134OC. [DOI] [PubMed] [Google Scholar]
- 67.Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659–668. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- 68.Kew KM, Undela K, Kotortsi I, Ferrara G. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015:CD002997. doi: 10.1002/14651858.CD002997.pub4. [DOI] [PubMed] [Google Scholar]
- 69.Meltzer EO, Chervinsky P, Busse W, Ohta K, Bardin P, Bredenbröker D, et al. Roflumilast for asthma: Efficacy findings in placebo-controlled studies. Pulm Pharmacol Ther. 2015;35(Suppl):S20–7. doi: 10.1016/j.pupt.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 70.Bateman ED, Goehring UM, Richard F, Watz H. Roflumilast combined with montelukast versus montelukast alone as add-on treatment in patients with moderate-to-severe asthma. J Allergy Clin Immunol. 2016;138:142–149.e8. doi: 10.1016/j.jaci.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 71.Cahill KN, Katz HR, Cui J, Lai J, Kazani S, Crosby-Thompson A, et al. KIT inhibition by imatinib in patients with severe refractory asthma. N Engl J Med. 2017;376:1911–1920. doi: 10.1056/NEJMoa1613125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Humbert M, de Blay F, Garcia G, Prud'homme A, Leroyer C, Magnan A, et al. Masitinib, a c-kit/PDGF receptor tyrosine kinase inhibitor, improves disease control in severe corticosteroid-dependent asthmatics. Allergy. 2009;64:1194–1201. doi: 10.1111/j.1398-9995.2009.02122.x. [DOI] [PubMed] [Google Scholar]
- 73.Pavord ID, Cox G, Thomson NC, Rubin AS, Corris PA, Niven RM, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185–1191. doi: 10.1164/rccm.200704-571OC. [DOI] [PubMed] [Google Scholar]
- 74.Kim MY, Sohn KH, Song WJ, Park HW, Cho SH, Min KU, et al. Clinical features and prognostic factors of Churg-Strauss syndrome. Korean J Intern Med. 2014;29:85–95. doi: 10.3904/kjim.2014.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376:1921–1932. doi: 10.1056/NEJMoa1702079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: a meta-analysis of the literature. J Allergy Clin Immunol. 2015;135:676–681.e1. doi: 10.1016/j.jaci.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 77.Li JX, Fan LC, Li MH, Cao WJ, Xu JF. Beneficial effects of omalizumab therapy in allergic bronchopulmonary aspergillosis: a synthesis review of published literature. Respir Med. 2017;122:33–42. doi: 10.1016/j.rmed.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 78.Hanania NA, Chipps BE, Griffin NM, Yoo B, Iqbal A, Casale TB. Omalizumab effectiveness in asthma-COPD overlap: post hoc analysis of PROSPERO. J Allergy Clin Immunol. 2019;143:1629–1633.e2. doi: 10.1016/j.jaci.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 79.Pavord ID, Chanez P, Criner GJ, Kerstjens HA, Korn S, Lugogo N, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377:1613–1629. doi: 10.1056/NEJMoa1708208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Other mechanisms contributing to severe asthma
Modifiable risk factors
Comorbidities of severe asthma