Dupilumab, a fully humanized antibody, which inhibits IL-4 and IL-13 by blocking the IL-4 receptor α, is approved for the treatment of atopic dermatitis (AD) and some adverse effects were reported.1,2 Dupilumab facial redness (DFR) is a development of an eczematous facial rash after initiation of dupilumab and is an adverse event not described in the clinical trials. We herein report a case series of DFR to improve clinical knowledge of this possible new adverse event.
We reviewed 4 cases of DFR from November 2018 to September 2019 at the Department of Dermatology, CHA Bundang Medical Center. (Tables 1 and 2) Concomitant treatment during the dupilumab treatment included antihistamines, topical corticosteroids and topical calcineurin inhibitors (TCI). Patients did not receive any other systemic drugs.
Table 1. Patient characteristics (n = 4).
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Sex | F | F | M | F |
| Age (yr) | 43 | 22 | 37 | 18 |
| Asthma/allergic rhinitis/allergic conjunctivitis | +/+/+ | +/+/+ | +/+/+ | +/+/+ |
| Previous treatments | CsA, prednisone, antihistamine, TCS, TCI | CsA, prednisone, antihistamine, TCS, TCI | CsA, AZA, MTX, prednisone, antihistamine, TCS, TCI | Prednisone, antihistamine, TCS, TCI |
CsA. Cyclosporine A; TCS, topical corticosteroids; TCI, topical calcineurin inhibitors; AZA, azathioprine; MTX, methotrexate.
Table 2. Clinical characteristics (n = 4).
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Onset of DFR (treatment duration of dupilumab, wk) | 27 | 25 | 20 | 17 |
| Signs and symptom of facial redness (+/−) erythema/scale/itching/pain | +/+/−/− | +/+/−/− | +/+/−/− | +/−/−/− |
| Skin biopsy (+/−) | − | − | + | − |
| Patch test (+/−) | + | − | − | − |
| Concomitant treatment | Emollients, TCS, TCI, antihistamine | Emollients, TCS, TCI, prednisone, antihistamine | Emollients, TCS, TCI | Emollients, TCS, TCI, antihistamine |
| Prescriptions for DFR | Minocycline, TCI, brimonidine tartrate 0.33% topical gel | Minocycline, TCI, TCS | Minocycline, TCI, TCS, brimonidine tartrate 0.33% topical gel | Minocycline, TCI, TCS |
| Duration of treatment for DFR (wk) | 28 | 10 | 22 | 32 |
DFR, dupilumab facial redness; TCS, topical corticosteroids; TCI, topical calcineurin inhibitors.
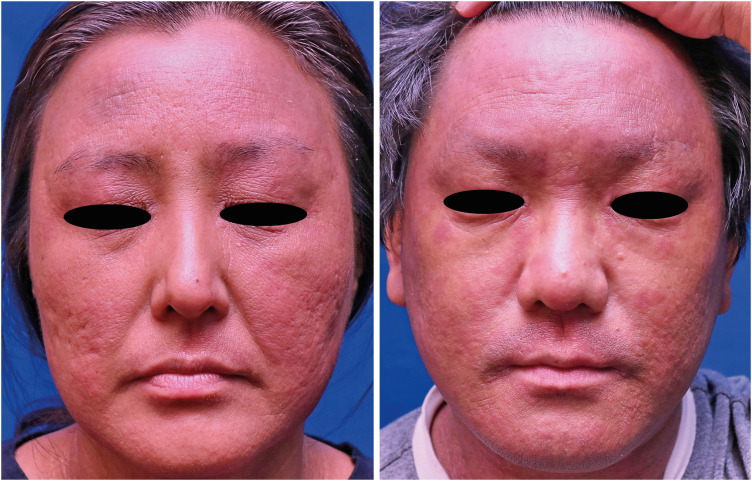
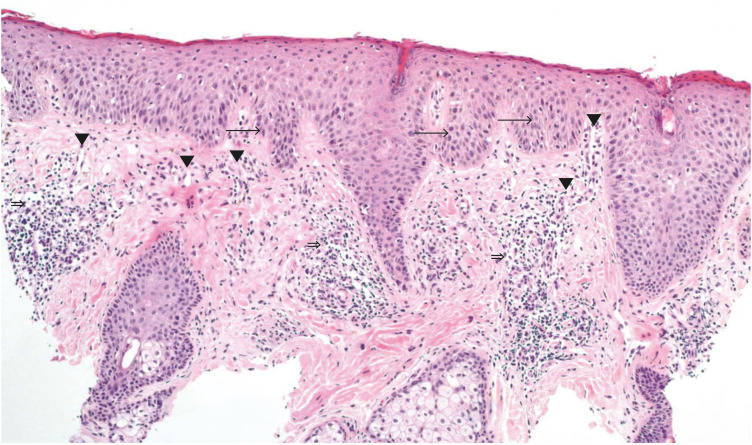
The mean onset of facial redness was 22.25 weeks after initiation of dupilumab treatment. Most patients presented erythematous and scaly patches on the whole face including the periocular area (Fig. 1). In all patients, there were no other side effects other than facial redness. In one patient, histopathology showed mild dermal edema and perifollicular chronic inflammation (Fig. 2); in another patient, the patch test was positive for nickel.
Fig. 1. Clinical pictures of dupilumab-induced facial redness.
Fig. 2. Histopathology of a left lower lid skin punch biopsy specimen shows irregular epidermal hyperplasia with bulbous elongated rete ridges (→), increased number of ectatic capillaries in the papillary dermis (▼) and a perivascular and perifollicular lymphocytic infiltration (⇒) (hematoxylin and eosin, × 100).
DFR was exacerbated by continued administration of dupilumab, but due to improvements in other skin lesions, patients did not want to stop dupilumab. Facial redness was considerably improved with minocycline, TCI and brimonidine tartrate 0.33% topical gel on the average within 23 weeks (range, 10 to 32 weeks) of treatment.
There are several case reports of DFR, but the cause of this new side effect is not clear. Many hypotheses have been proposed to explain the development of DFR, including 1) allergic contact dermatitis (ACD), 2) hypersensitivity/photosensitivity reactions to dupilumab, 3) Malassezia furfur associated seborrheic dermatitis-like reactions and 4) Demodex associated rosacea like dermatosis.3,4
Patch testing for ACD was performed in 1 patient, which showed positivity to nickel. However, avoidance of the allergens did not improve erythema. The distribution of the lesions was also suggestive of photosensitivity reactions. None of the patients were using any photosensitive drug or had any history of overexposure to ultraviolet light.5 It has been suggested that M. furfur, a normal skin flora, probably plays a role in the pathophysiology. However, in the mouse models, Malassezia causes massive infiltration of neutrophils and monocytes into the skin, but we could not find this in the skin biopsy specimens of our patients.6 Dupilumab inhibits T-helper cell 2 signaling, which may include immune responses against helminth infections. In theory, the treatment of dupilumab could promote Demodex proliferation in follicles and increase IL-17-mediated inflammation involved in the pathophysiology of rosacea.7 The clinical presentation in our patients was not typical for rosacea and none of them had a history of rosacea before. However, the histologic findings of 1 patient was considered to be rosacea.
In our experience, DFR is an underappreciated adverse event of dupilumab. We reported a case series of facial redness in 4 patients treated with dupilumab for AD to improve clinical knowledge of this new adverse event.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 2.Albader SS, Alharbi AA, Alenezi RF, Alsaif FM. Dupilumab side effect in a patient with atopic dermatitis: a case report study. Biologics. 2019;13:79–82. doi: 10.2147/BTT.S195512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Beer FS, Bakker DS, Haeck I, Ariens L, van der Schaft J, van Dijk MR, et al. Dupilumab facial redness: positive effect of itraconazole. JAAD Case Rep. 2019;5:888–891. doi: 10.1016/j.jdcr.2019.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Wijs LE, Nguyen NT, Kunkeler AC, Nijsten T, Damman J, Hijnen DJ. Clinical and histopathological characterization of paradoxical head and neck erythema in patients with atopic dermatitis treated with dupilumab: a case series. Br J Dermatol. 2019 doi: 10.1111/bjd.18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim WB, Shelley AJ, Novice K, Joo J, Lim HW, Glassman SJ. Drug-induced phototoxicity: a systematic review. J Am Acad Dermatol. 2018;79:1069–1075. doi: 10.1016/j.jaad.2018.06.061. [DOI] [PubMed] [Google Scholar]
- 6.Sparber F, De Gregorio C, Steckholzer S, Ferreira FM, Dolowschiak T, Ruchti F, et al. The skin commensal yeast Malassezia triggers a type 17 response that coordinates anti-fungal immunity and exacerbates skin inflammation. Cell Host Microbe. 2019;25:389–403.e6. doi: 10.1016/j.chom.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Thyssen JP. Could conjunctivitis in patients with atopic dermatitis treated with dupilumab be caused by colonization with demodex and increased interleukin-17 levels? Br J Dermatol. 2018;178:1220. doi: 10.1111/bjd.16330. [DOI] [PubMed] [Google Scholar]