Abstract
Recent research suggests that people can learn to link the control process of task switching to predictive cues so that switch costs are attenuated following informative precues of switch likelihood. However, the precise conditions that shape such contextual cuing of control are not well understood. Farooqui and Manly (2015) raised the possibility that cued task switching is more effective when cues of control demand are presented subliminally. In the current study, we aimed to replicate and extend these findings by more systematically manipulating whether cues of control demand are consciously perceived or are presented subliminally and whether participants have explicit prior knowledge of the cue meaning or acquire cue knowledge through experience. The direct replication was unsuccessful: We found no evidence for effective subliminal cuing but observed some evidence for participants reducing switch costs with explicit, supraliminal cues. Thus, cognitive control may be guided most effectively by explicitly understood and consciously perceived precues.
Keywords: cognitive control, consciousness, attention, memory, action control, open data, open materials, preregistered
Adaptive behavior is characterized by our ability to create, maintain, and flexibly update rules by which we categorize and respond to stimuli, otherwise known as task sets. The process of switching task sets is cognitively demanding and incurs switch costs, that is, slowed reaction times and higher error rates when compared with repeating the same task set (Monsell, 2003). Recent research suggests that people learn to link the cognitive control process of task switching to predictive cues or task statistics; as a result, switch costs are attenuated under conditions of high compared with low switch likelihood (Chiu & Egner, 2017; Crump & Logan, 2010; Dreisbach & Haider, 2006; Leboe, Wong, Crump, & Stobbe, 2008). However, the precise conditions that shape such contextual control learning are presently not well understood. A recent study by Farooqui and Manly (2015) raised the possibility that cued task switching is more effective when cues of control demand are presented subliminally, which would have major implications for theories of control. In the current study, we thus aimed to replicate and extend these findings by more systematically manipulating two major possible determinants of control learning: whether cues of control demand are consciously perceived or are presented subliminally (cue visibility) and whether participants have explicit prior knowledge of the cue meaning or acquire preparatory knowledge through experience (cue knowledge).
The role of conscious awareness in linking cues to control demand is unclear in part because typical experimental paradigms in this domain tend to confound conscious perception and knowledge with respect to awareness of cue presentation and predictive value. For instance, Farooqui and Manly (2015) subliminally cued the likelihood of task switching and concluded that subliminal cuing was more powerful than supraliminal cuing because subliminal cues caused a larger reduction in switch costs. These striking results, however, may include an important limitation: When the subliminal cues were made supraliminal (Experiments 9 and 10), participants were told to ignore those cues, an instruction explicitly telling participants that the cues were not useful. Thus, the lack of learning effects observed for supraliminal cuing may stem from a difference in prior understanding of cue value than of cue visibility per se. In fact, biased instructions alone can elicit cuing effects (cf. Entel, Tzelgov, & Bereby-Meyer, 2014), and task-irrelevant cues are not always sufficient to activate context-appropriate learned control settings (Gottschalk & Fischer, 2017).
Teasing apart the effects of conscious perception and knowledge on the cuing of control has important implications for current theories of action control and memory. Traditionally, the application of control has been closely tied to conscious intentions (Posner & Snyder, 1975). By contrast, more recent models of action control suggest that the role of consciousness in cognitive control, such as the performance benefits of strategies, is overstated (Hommel, 2013). This perspective is consistent with theories grounding cognitive control in associative learning (Abrahamse, Braem, Notebaert, & Verguts, 2016; Egner, 2014), which assume that control states can be implicitly learned. Most studies of contextual control cuing (e.g., Crump, Vaquero, & Milliken, 2008), however, are indifferent to how cues and contexts become associated with each other, ignoring potential effects of consciousness on control learning. If cue awareness and preparatory knowledge have independent effects on control learning, current theories of action control will need to better account for how consciousness, whether through intentional control or particular strategies, impacts our decisions.
Here, we manipulated conscious cue perception and predictive cue knowledge independently to better understand how people optimally adapt cognitive control settings across environments. Replicating the approach of Farooqui and Manly (2015), we used a standard task-switching paradigm in which task-repeat and task-switch trials were preceded by either predictive or nonpredictive cues. Crucially, across four experiments, we manipulated both whether these cues were subliminal or supraliminal and whether participants had been explicitly told the information provided by the cues or had to implicitly learn that the cues predict demand; our experiments formed a 2 (cue visibility: subliminal vs. supraliminal) × 2 (cue knowledge: explicit vs. nonexplicit) factorial design. This study thus linked streams of research on action control (Hommel, 2013), control and associative learning (Abrahamse et al., 2016; Bugg & Crump, 2012), and conscious processing (Kunde, Reuss, & Kiesel, 2012; van Gaal, de Lange, & Cohen, 2012).
We predicted that participants would use the contextual cues to modulate control; as a result, switch costs would be lower following predictive cues than nonpredictive cues. Because we manipulated cue visibility and preparatory knowledge separately, we could (a) systematically assess the necessary preconditions for observing cue benefits (tested within each experiment) and (b) compare the strength of such effects across conditions (treating experiment as a between-subjects factor). If the conclusions reached by Farooqui and Manly (2015) were warranted, we should observe smaller switch costs under subliminal cuing (Experiments 1 and 2) than supraliminal cuing (Experiments 3 and 4). By contrast, traditional views on control would predict the smallest switch costs under the supraliminal and explicit-knowledge (Experiment 4) conditions, and current theories of action control would predict the smallest switch costs under the nonexplicit-knowledge condition (Experiments 1 and 3). Finally, to the extent that task-switch/repeat operations are subject to the same constraints as motor actions, we predicted that switch costs would be reduced when participants developed explicit knowledge of the cues so that preemptive control operations can be prepared and triggered by the cues (cf. action triggers; Kunde, Kiesel, & Hoffmann, 2003). This would result in smaller switch costs in Experiments 2 through 4 than in Experiment 1.
Experiment 1
We attempted to directly replicate Experiment 2 from Farooqui and Manly (2015), in which subliminal cues preceded task-switch and task-repeat trials in a standard task-switching paradigm.
Method
Sample size
To determine the appropriate sample size per experiment, we used sequential Bayes factor (BF) analysis (Schönbrodt & Wagenmakers, 2018). We used an uninformative prior and reran the analysis each time that we recruited 5 additional participants until a BF of 10 or 1/10 was found in favor of the alternative or null hypothesis, respectively, for the interaction between cue validity and trial type, in line with recommendations for a previously observed effect (Schönbrodt & Wagenmakers, 2018). We recruited a minimum viable participant sample size of 20 (cf. Farooqui & Manly, 2015) and a maximum sample size of double our minimum (40) for every experiment. The effect that we sought to replicate (Farooqui & Manly, 2015; Experiment 2) was estimated to have a BF of 30 (Rouder, Speckman, Sun, Morey, & Iverson, 2009; http://pcl.missouri.edu/bf-one-sample), which suggested that these minimum and maximum sample sizes were appropriate.
Participants
Fifty participants completed Experiment 1 for psychology course credits or compensation at a rate of $10 per hour. All reported normal or corrected-to-normal visual acuity. Our predetermined exclusion threshold was 60% accuracy for all four experiments, which resulted in the exclusion of 3 participants for Experiment 1. An additional 7 participants were excluded because their responses to the posttest questions (see below) indicated that they may have been aware of the subliminal cues, which was unexpected given the responses in Farooqui and Manly’s (2015) study. Consistent with the sample maximum we set, the final sample size was 40 (29 women, 10 men, 1 unspecified; age: M = 22.10 years, SD = 4.09).
Note that exclusions beyond the accuracy criterion were not planned in the preregistration. We therefore report and analyze the full sample for every experiment in the Supplemental Material available online.
Procedure
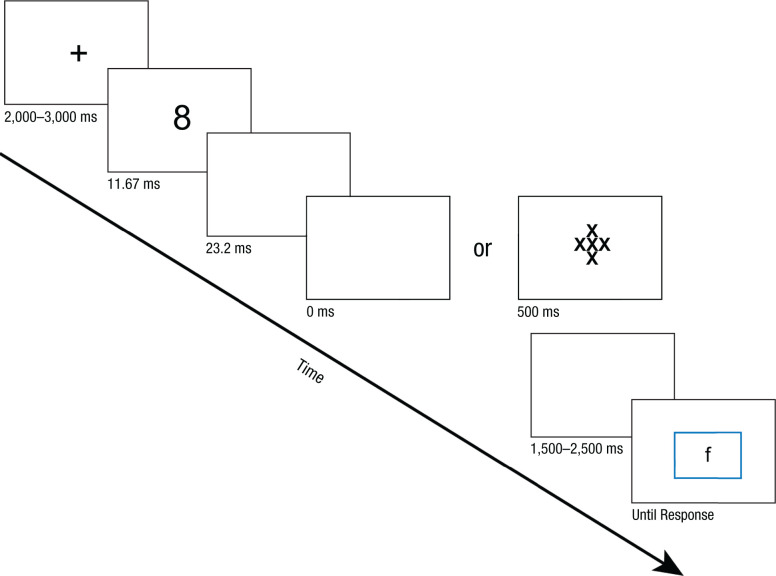
We followed the procedure outlined in Experiment 2 of Farooqui and Manly’s (2015) study. On each trial (Fig. 1), a number cue (“2,” “4,” “8”) was presented for 11.67 ms, followed by a blank screen for 23.2 ms. A mask of five Xs was shown for 500 ms to render the cue subliminal. Finally, after 1.5 s to 2.5 s, a letter stimulus appeared on screen surrounded by a color rectangle. Participants were instructed that when the rectangle was blue, they should press the Z key to categorize the letter as a vowel and the 3 key to categorize it as a consonant, and that when the rectangle was green, they should press the Z key if the letter is lowercase and the 3 key if it is uppercase. The stimulus was presented until a response was made. A low-frequency tone sounded if participants made an incorrect response. After receiving feedback, participants saw a fixation cross for 2 s to 3 s before the next trial started. Participants performed six practice trials for each letter categorization before the main experimental session.
Fig. 1.
Example trial sequence for the four experiments. On each trial, participants were briefly shown a cue that was either nonpredictive or predictive of the likelihood of a task-switch or task-repeat trial, followed by a blank screen. In Experiments 1 and 2, the cue presentation was followed by a visual mask, whereas in Experiments 3 and 4, the cues were rendered supraliminal by removing the mask. After cue presentation, there was a jittered delay before participants were shown a letter stimulus surrounded by a colored rectangle, which cued the category-appropriate judgment (consonant/vowel vs. uppercase/lowercase) that participants performed. Note that in Experiments 2 and 4, participants were explicitly informed of the predictive or nonpredictive relationships that each cue had with task-switch or task-repeat trials.
The key manipulation of interest involved the number cues. One number cue preceded half of all switch trials (predictive switch cue), one number cue preceded half of all repeat trials (predictive repeat cue), and one number cue preceded each trial type with equal frequency (nonpredictive cue). The global ratio of task-switch to task-repeat trials was 25:75 because Farooqui and Manly (2015) found less evidence that the subliminal cues modulated control at a 50:50 ratio. Tasks were presented in random order across trials, and the predictive identity of the cues was randomly determined across participants. Participants were not told about the cues or the cues’ predictive value.
After an initial run of 50 trials, response time-outs were tailored to individual participants to encourage faster responses and ensure high task difficulty. Participants were told that they had to respond both quickly and accurately and that accurate but slow responses were now counted as incorrect trials. The response threshold was set at the 60th percentile for correct responses on repeat trials. After another 100 trials, this threshold was revised again. Altogether, the experiment consisted of 250 trials, as did the study by Farooqui and Manly (2015), who found that the control cuing effect diminished over time.
After the main experiment, participants were tested about their knowledge of the cues with a posttest questionnaire and cue-perception task. Participants were first asked whether they were aware of anything beyond the visual mask being presented. Subsequent questions assessed whether they knew what was presented. All participants were also shown the three cues 10 times each, followed by the visual mask, and asked to guess what the cue identities were. They were encouraged to guess but also allowed to skip (by pressing enter) if they did not want to guess.
The task procedure was programmed with PsychoPy (Peirce et al., 2019) for a CRT monitor with a refresh rate of 85 Hz. All materials are available on the Open Science Framework (https://osf.io/7jfbp/). Because the predictive-cue identities were randomly presented and not fully counterbalanced, the six task versions were split among Experiment 1 participants as follows: 10, 6, 4, 8, 6, and 6 participants.
Data analysis
Median reaction time (RT) data were analyzed for trials with correct responses in which participants were not excessively fast (< 200 ms) or slow (+3 SDs) to respond. We also analyzed accuracy data and calculated and analyzed a global performance index—mean accuracy (%) divided by median RT (ms) times 100—consistent with the procedure of Farooqui and Manly (2015). This index indicated whether participants modulated their response time without a cost to accuracy.
We ran a repeated measures analysis of variance (ANOVA) with cue (predictive vs. nonpredictive) and trial type (task switch vs. task repeat) as within-subjects factors. We expected to find an interaction between cue and trial type showing that participants were quicker and more efficient to respond to task-switch trials following predictive cues than nonpredictive cues.
Quantifying evidence
We ran Bayesian repeated measures ANOVAs. This quantified the amount of evidence that conscious cue perception and knowledge impact predictive cue benefits, via inclusion BFs (https://github.com/mattansb/BFEffects), which address how likely statistical models with particular predictors are relative to models without those predictors. An inclusion BF of 5, for example, means that the model including that set of predictors is 5 times more likely than a model without them.
We also ran equivalence tests using the two one-sided tests (TOST) procedure (Lakens, Scheel, & Isager, 2018) to provide support for the potential lack of a meaningful effect, which here refers to the interaction between cue and trial type. For example, although we may not find a statistically significant effect, we cannot then conclude that the observed effect is of little theoretical and practical relevance unless an equivalence test against meaningful equivalence bounds is also significant. In the case of a nonsignificant equivalence test, we would not be able to reject the null hypothesis that the observed effect is at least as large as the equivalence bounds, and we would instead assume that the design was underpowered to detect the two-way interaction. With respect to the latter possibility, we also ran exploratory Bayesian simulations that analyzed the sample size needed for a given design to yield a BF of 1/10 or 10 in favor of the null or alternate hypotheses, respectively, and we report the output of these analyses in Table S1 in the Supplemental Material to complement the equivalence tests.
Note that for both the sequential BF sample estimation and equivalence tests, we used Cohen’s dz as our effect-size metric (t/) because this measure provided the most conservative estimate (e.g., as opposed to converting partial eta squared or calculating standardized mean difference) and was reported by Farooqui and Manly (2015). Our equivalence bounds were based on the smallest Cohen’s dz (0.43) reported in Experiments 1 to 3 of Farooqui and Manly, showing that participants used subliminal cues to modulate control, which we tried to replicate. Because Farooqui and Manly reported only one effect size for RT, with analyses primarily focused on the performance index, we matched equivalence bounds across the RT and index.
Results
Main task
Participants were significantly slower to respond (M = 141 ms)—trial type: F(1, 39) = 106.91, p < .001, ηp2 = .73, inclusion: BF = 1.68 × 1019—and were less efficient (index: M = 2.25)—trial type: F(1, 39) = 93.71, p < .001, ηp2 = .71, inclusion BF = 1.30 × 1016—on switch trials than on repeat trials but showed no significant accuracy switch costs (M = 1.1%)—trial type: F(1, 39) = 1.33, p = .256, ηp2 = .03, inclusion BF = 0.30 (see Table 1). Contrary to our predictions and the results reported by Farooqui and Manly (2015), our findings showed that participants had smaller switch costs for RT—Cue Type × Trial Type: F(1, 39) = 6.16, p = .018, ηp2 = .14, inclusion BF = 1.54; cue type: F < 1.4—and the performance index—Cue Type × Trial Type: F(1, 39) = 4.62, p = .038, ηp2 = .11, inclusion BF = 2.38—following nonpredictive cues than predictive cues. This was driven primarily by performance on switch trials. Indeed, participants were also more accurate—cue type: F(1, 39) = 4.66, p = .037, ηp2 = .11, inclusion BF = 0.72; Cue Type × Trial Type, F < 0.1—and slightly more efficient—cue type: F(1, 39) = 3.86, p = .057, ηp2 = .09, inclusion BF = 1.09—following nonpredictive cues than following predictive cues. Altogether, these results suggest that participants were not using the predictive subliminal cues to reduce switch costs; rather, the predictive subliminal cues may have impaired switching.
Table 1.
Performance Data Across All Experiments
| Variable, cue type, and trial type | Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | Across experiments |
|---|---|---|---|---|---|
| RT (ms) | |||||
| Nonpredictive | |||||
| Switch | 760 [710, 810] | 807 [749, 864] | 829 [762, 896] | 811 [735, 887] | 879 [851, 907] |
| Repeat | 644 [617, 671] | 654 [615, 693] | 661 [616, 705] | 650 [611, 689] | 726 [707, 745] |
| Predictive | |||||
| Switch | 797 [747, 847] | 817 [756, 878] | 873 [797, 950] | 704 [640, 768] | 878 [849, 908] |
| Repeat | 631 [603, 658] | 648 [613, 684] | 660 [614, 707] | 601 [561, 641] | 712 [692, 733] |
| Accuracy (% correct) | |||||
| Nonpredictive | |||||
| Switch | 80.74 [78.43, 83.04] | 80.45 [77.03, 83.86] | 80.83 [78.19, 83.47] | 77.52 [73.57, 81.47] | 79.89 [78.31, 81.46] |
| Repeat | 81.84 [79.73, 83.95] | 81.94 [80.04, 83.83] | 79.45 [77.30, 81.60] | 76.38 [73.66, 79.10] | 79.90 [78.74, 81.06] |
| Predictive | |||||
| Switch | 79.25 [76.36, 82.13] | 80.93 [78.35, 83.50] | 80.42 [77.35, 83.50] | 82.38 [79.16, 85.61] | 80.75 [79.27, 82.22] |
| Repeat | 80.29 [78.33, 82.25] | 81.70 [79.14, 84.25] | 78.89 [76.39, 81.40] | 82.01 [79.04, 84.99] | 80.72 [79.46, 81.99] |
| Performance index: accuracy (%)/RT (ms) | |||||
| Nonpredictive | |||||
| Switch | 11.04 [10.34, 11.75] | 10.38 [9.67, 11.08] | 10.30 [9.54, 11.06] | 10.16 [9.36, 10.96] | 9.38 [9.10, 9.67] |
| Repeat | 12.87 [12.38, 13.36] | 12.89 [12.23, 13.56] | 12.47 [11.71, 13.24] | 12.00 [11.44, 12.57] | 11.25 [10.98, 11.52] |
| Predictive | |||||
| Switch | 10.26 [9.65, 10.86] | 10.36 [9.66, 11.07] | 9.83 [9.04, 10.62] | 12.58 [11.46, 13.70] | 9.58 [9.23, 9.93] |
| Repeat | 12.93 [12.38, 13.48] | 12.88 [12.24, 13.52] | 12.44 [11.64, 13.24] | 14.10 [13.25, 14.95] | 11.65 [11.33, 11.97] |
Note: Values in brackets are 95% confidence intervals. We report the mean of the medians for reaction time (RT) for the individual experiments and means generally for RT across experiments.
Equivalence tests for both RT (Cohen’s dz = 0.39) and the global performance index (Cohen’s dz = 0.34) were nonsignificant, t(39) = 0.24, p = .406; t(39) = −0.57, p = .286, respectively. Taken together with the significant null hypothesis tests, this suggests that the observed interaction is statistically different from, and not equivalent to, zero. See Table S1 for sample-size recommendations.
In sum, we did not replicate the main result from Farooqui and Manly’s (2015) study and instead found weak evidence that, on average, participants were less efficient at reducing their switch costs following subliminal predictive than nonpredictive cues. We speculate that because predictive cues were overall more rare than nonpredictive ones, the detection of these less common events may have delayed the switch process on predictive-cue trials.
Posttest
Thirty-four participants reported noticing “anything being briefly presented before the 5 Xs in each trial.” Of those 34, 25 participants also reported being aware of the identity of the stimulus, yet of the 40 total participants, only 3 participants correctly identified that the stimulus was a number, and not a string or letter, cue. The 7 participants who we excluded answered yes to the first and second questions and correctly identified the stimulus as a number cue, suggesting that they were more aware of the subliminal manipulation. Mean accuracy on the perception task was 2.4% and 5.0% for the nonpredictive and predictive cues, respectively, suggesting that participants largely could not perceive the cues.
Experiment 2
As with Experiment 1, task repetitions and switches were cued subliminally, but participants were also explicitly informed of each cue/trial-type association.
Method
Participants
Fifty-nine participants completed Experiment 2 for psychology course credits or compensation at a rate of $10 per hour. All reported normal or corrected-to-normal visual acuity. We excluded 3 participants for poor accuracy and 16 participants for failing the manipulation checks (see below). The final sample size was 40 (25 women, 15 men; age: M = 22.6 years, SD = 3.86).
Procedure
The procedure for this experiment closely mirrored that of Experiment 1, except that participants were now told what the cues represented. Specifically, part of the instructions for Experiment 1 said, “On each trial, a pattern of five Xs will appear on-screen followed by a letter that you will categorize according to the color of its surrounding rectangle. The Xs are not relevant to the task that you are performing.” Experiment 2 added the following:
However, before the Xs are presented, number cues will flash on-screen. One of these cues (“2”) will precede trials in which the letter judgment (consonant/vowel vs. uppercase/lowercase) will switch from that of the previous trial. One of the cues (“4”) will precede trials in which the letter judgment will remain the same as in the previous trial. One of the cues (“8”) will precede trials in which the letter judgment will either switch or remain the same from the previous trial. You may use this information to aid your performance.
To ensure that participants paid attention to the instructions and encoded the cue/trial-type associations, we included a manipulation check: We periodically probed whether they remembered the instructed cue/trial-type associations over the course of the experiment. The first probe occurred right after participants finished the practice trials, the second probe after the RT threshold was reset the first time (Trial 50), the third probe after the RT threshold was reset the second time (Trial 150), and the fourth probe after participants answered the three posttest questions yet before the cue-perception task. The fifth probe was in a separate demographics form after participants performed the cue-perception task. Participants were forced to repeat Probes 1 through 3 until they pressed the correct key for all three associations. We excluded participants from the main analysis if they answered Probes 4 or 5 incorrectly, which indicated that they had not recalled the cue/trial-type associations (for the full data set, see the Supplemental Material). The six task versions were split among Experiment 2 participants as follows: 7, 9, 4, 8, 6, and 6 participants.
Results
Main task
As with Experiment 1, participants showed robust RT switch costs (M = 161 ms)—trial type: F(1, 39) = 127.99, p < .001, ηp2 = .77, inclusion BF = 1.18 × 1023—and performance-index switch costs (M = 2.51)—trial type: F(1, 39) = 140.63, p < .001, ηp2 = .78, inclusion BF = 1.30 × 1020—but had no significant accuracy switch costs (M = 1.1%)—trial type: F(1, 39) = 1.20, p = .279, ηp2 = .03, inclusion BF = 0.24 (see Table 1). No other effects were significant (Fs < 0.6).
Equivalence tests for both RT (Cohen’s dz = 0.12) and the performance index (Cohen’s dz = 0.002) were significant, t(39) = 1.97, p = .023; t(39) = 2.71, p = .005, respectively; taken with the nonsignificant null hypothesis test, the observed interaction is thus not statistically different from, and is equivalent to, zero. We can reject the null hypothesis that the true interaction effects are smaller than Cohen’s dz of −0.43 or larger than dz of 0.43, and the effect sizes should lie within these equivalence bounds most of the time.
In sum, even when participants were informed about the meaning of the subliminal control demand cues, we found little evidence that these cues aided task switching, similar to the results of Experiment 1.
Posttest
Twenty-two participants reported noticing something being presented before the mask, 13 participants reported being aware of the identity of the stimulus, and 28 participants correctly identified the stimulus as the number cue. Note that all participants were explicitly told and answered probes correctly about the cue/trial-type associations, so participants may have answered with respect to their perceptual cue awareness rather than their instructed cue knowledge; these posttest questions were the same as those used by Farooqui and Manly (2015) in their Experiment 1. Given that 12 more participants reported noticing something being presented before the mask in Experiment 1, when they were not notified about the presence of the subliminal cues, many of those responses may have reflected a bias toward answering “yes” on the posttest questions. Mean accuracy on the perception task was 15.3% and 17.9% for the nonpredictive and predictive cues, respectively (chance = 33%), suggesting that the subliminal presentation of cues across Experiments 1 and 2 was largely successful.
Experiment 3
In Experiment 3, the cues were no longer subliminal, but as in Experiment 1, participants were not informed about their relationship with task-switch and task-repeat trials.
Method
Participants
Fifty-two participants completed Experiment 3 for psychology course credits or compensation at a rate of $10 per hour. All reported normal or corrected-to-normal visual acuity. We excluded 4 participants for poor accuracy and 8 participants for gaining explicit knowledge of the cue/trial-type associations (see below). The final sample size was 40 (27 women, 13 men; age: M = 22.43 years, SD = 5.07).
Procedure
The procedure of this experiment closely mirrored that of Experiment 1, except that participants could now consciously perceive the cues. Following the procedure in Experiments 9 and 10 of Farooqui and Manly (2015), we removed the visual mask. Because the visual mask was no longer presented, the instructions reflected this change: “On each trial, a number cue will appear on-screen followed by a letter that you will categorize according to the color of its surrounding rectangle.”
Participants were no longer tested on whether they could visually perceive the cues but were tested with regard to their understanding of what each cue means (cf. Bejjani, Zhang, & Egner, 2018). They were asked whether they realized that there was a systematic relationship between the cues and control demand and were asked to match the cues with their respective trial types.
Because our experiment crucially depended on participants not obtaining explicit knowledge of the cue/trial-type associations, we excluded the 8 participants who matched more than one of the number cues (chance = 1/3) to its correct trial type in the posttest questionnaire on explicit cue knowledge. For the full data set, see the Supplemental Material. The six task versions were split among Experiment 3 participants as follows: 6, 7, 5, 9, 6, and 7 participants.
Results
Main task
As in Experiments 1 and 2, participants showed robust RT switch costs (M = 191 ms)—trial type: F(1, 39) = 96.72, p < .001, ηp2 = .71, inclusion BF = 2.88 × 1020—and performance-index switch costs (M = 2.39)—trial type: F(1, 39) = 87.20, p < .001, ηp2 = .69, inclusion BF = 9.74 × 1017—but no accuracy switch costs (M = −1.5%)—trial type: F(1, 39) = 2.66, p = .11, ηp2 = .06, inclusion BF = 0.47 (see Table 1). Participants were slightly slower to respond following the predictive than the nonpredictive cues—cue type: F(1, 39) = 3.60, p = .065, ηp2 = .08, inclusion BF = 0.47—which was driven by nominally larger RTs for switch trials following the predictive cue—Cue Type × Trial Type: F(1, 39) = 3.22, p = .081, ηp2 = .08, inclusion BF = 0.71. No other effects were significant—performance index: cue type: F(1, 39) = 2.11, p = .154, ηp2 = .05, inclusion BF = 0.30; Cue Type × Trial Type: F(1, 39) = 1.50, p = .229, ηp2 = .04, inclusion BF = 0.35; accuracy: Fs < 0.4.
Equivalence tests for both RT (Cohen’s dz = 0.28) and the global performance index (Cohen’s dz = 0.19) were nonsignificant, t(39) = 0.93, p = .180; t(39) = −1.50, p = .071, respectively; taken with the nonsignificant null hypothesis tests, the observed interaction between cue and trial type was statistically not different from, yet not equivalent to, zero.
In sum, when cues of control demand were supraliminal but participants were not explicitly informed about their meaning, we found little evidence that these cues aided task switching, a result similar to that found in Experiments 1 and 2.
Posttest
Six people reported noticing a systematic variation in how often number cues preceded easy/repeat and hard/switch trials. When rating the frequency at which number cues preceded either easy or hard trials, participants did not rate the predictive cues as being more predictive than the nonpredictive cues, t(16.6) = −1.26, p = .225; all other ts < 0.71. Participants included in the main analysis were thus largely unaware of the cue/trial-type associations.
Experiment 4
In Experiment 4, the cues were supraliminal, as in Experiment 3, and participants were explicitly informed of the cue/trial-type associations, as in Experiment 2.
Method
Participants
Fifty-two participants completed Experiment 4 for psychology course credits or compensation at a rate of $10 per hour. All reported normal or corrected-to-normal visual acuity. We excluded 2 participants for poor accuracy and 10 participants for failing the manipulation check (see below). The final sample size was 40 (24 women, 16 men; age: M = 22.18 years, SD = 3.75).
Procedure
In this experiment, we used the procedure of Experiment 3 for supraliminal presentation of the cues and the posttest questionnaire, and we mimicked the instructions of Experiment 2 for conscious knowledge of what the cues mean. We included a manipulation check to ensure that participants kept the cue/trial-type associations in mind, but we included only four of the five probes from Experiment 2. Without a cue-perception task, participants thus went straight from the main task into the demographics form that included the final cue/trial-type probe. If they answered this question incorrectly, they were excluded from the main analysis. For the full data set, see the Supplemental Material. The six task versions were split among Experiment 4 participants as follows: 5, 5, 7, 8, 9, and 6 participants.
Results
Main task
As with Experiments 1 to 3, participants showed robust RT switch costs (M = 132 ms)—trial type: F(1, 39) = 57.02, p < .001, ηp2 = .59, inclusion BF = 1.57 × 1011—and performance-index switch costs (M = 1.68)—trial type: F(1, 39) = 28.28, p < .001, ηp2 = .42, inclusion BF = 8.68 × 104—but no significant accuracy switch costs (M = −0.8%)—trial type: F(1, 39) = 0.31, p = .584, ηp2 = .01, inclusion BF = 0.17 (see Table 1). Participants were quicker to respond, and more accurate and efficient when responding, following the predictive than the nonpredictive cues—RT cue type: F(1, 39) = 25.83, p < .001, ηp2 = .40, inclusion BF = 1.07 × 104; accuracy: F(1, 39) = 20.19, p < .001, ηp2 = .34, inclusion BF = 1,081.52—and performance index: F(1, 39) = 37.77, p < .001, ηp2 = .49, inclusion BF = 6.68 × 108. Whereas these main effects were additive for the performance index (Cue Type × Trial Type: F < 0.85) and the interaction nonsignificant for accuracy (Cue Type × Trial Type: F < 0.23), there was a significant Cue Type × Trial Type interaction for RT, F(1, 39) = 5.31, p = .027, ηp2 = .12, inclusion BF = 4.18, whereby participants showed reduced switch costs following the predictive cues compared with the nonpredictive cues (Ms = 106 ms vs. 118 ms, respectively).
The equivalence test for RT was nonsignificant, t(39) = −0.42, p = .340. This suggests that the RT interaction (Cohen’s dz = 0.36) was statistically different from, and not equivalent to, zero. However, the equivalence test for the performance index (Cohen’s dz = 0.14) was significant, t(39) = 1.80, p = .040; taken with the nonsignificant null hypothesis test, the observed interaction was not statistically different from, and was equivalent to, zero. We can reject the null hypothesis that the true interaction effect is smaller than Cohen’s dz of −0.43 or larger than dz of 0.43, and the effect size should lie within these equivalence bounds most of the time.
In sum, when participants could perceive the cues and were informed of their respective trial-type associations, we found some evidence of predictive cue benefits whereby participants successfully adjusted their switch readiness.
Cross-experiment analysis
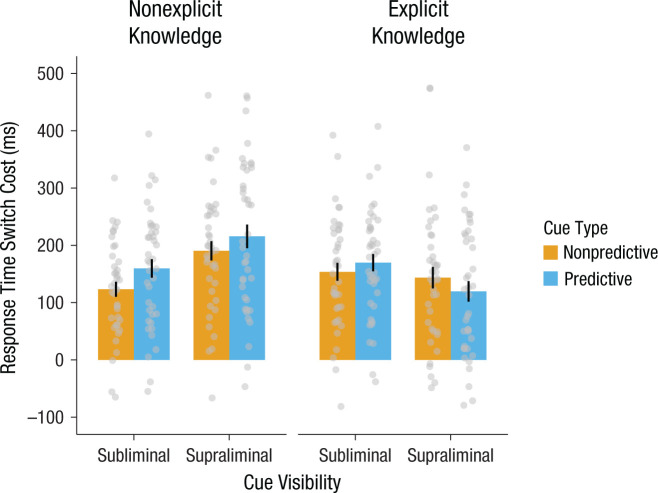
To quantify the respective and interactive effects of cue visibility and preparatory knowledge on cued task switching, we ran a repeated measures ANOVA including visibility (subliminal vs. supraliminal) and knowledge (nonexplicit vs. explicit) as between-subjects factors. Note that for within-experiment comparisons, median RT, instead of mean RT, was used to facilitate an optimal comparison with Farooqui and Manly’s (2015) study. However, because sequential BF analysis could have led to uneven sample sizes between experiments and because small sample sizes overestimate the true median (Miller, 1998), we used mean RT to compare effects between experiments.Because our experiments involved a 2 × 2 design with independent manipulations of conscious cue perception and knowledge, we combined the (trimmed) data across experiments and also ran a Bayesian repeated measures ANOVA that included cue visibility (subliminal vs. supraliminal) and preparatory cue knowledge (nonexplicit vs. explicit) as between-subjects factors, in addition to our within-subjects factors of cue (predictive vs. nonpredictive) and trial type (task switch vs. task repeat). As with each individual experiment, we report the inclusion BFs from this ANOVA. See Figure 2 for switch costs across the experiments and Table 1 for data across the experiments.
RT
Participants responded fastest when cues of control demand were supraliminal and explicitly instructed and slowest when these cues were supraliminal but without explicit instruction—Cue Knowledge × Visibility: F(1, 156) = 5.04, p = .026, ηp2 = .03, inclusion BF = 1,285.38. Having explicit as opposed to nonexplicit preparatory knowledge of the cue/trial-type associations sped responses following the predictive cues (mean difference = −46 ms) as opposed to the nonpredictive cues (mean difference = −7 ms)—Cue Knowledge × Cue Type: F(1, 156) = 12.48, p < .001, ηp2 = .07, inclusion BF = 430.28. Similarly, responses were faster for subliminal than for supraliminal presentation of the predictive cues (mean difference = −8 ms) as opposed to the nonpredictive cue (mean difference = −36 ms)—Cue Visibility × Cue Type: F(1, 156) = 6.53, p = .012, ηp2 = .04, inclusion BF = 126.31. Finally, these three factors interacted: Differences between RTs to predictive and nonpredictive cues showed that participants speeded their responses most when cues were either explicit and supraliminal (mean difference = −62 ms) or nonexplicit and subliminal (mean difference = 5 ms) and least when cues were either nonexplicit and supraliminal (mean difference = 20 ms) or explicit and subliminal (mean difference = 9 ms)—Cue Knowledge × Cue Visibility × Cue Type: F(1, 156) = 14.86, p < .001, ηp2 = .09, inclusion BF = 378.20.
As expected, all experiments showed RT switch costs—trial type: F(1, 156) = 595.70, p < .001, ηp2 = .79, inclusion BF = 7.23 × 1092. Switch costs were reduced when participants had explicit, instructed knowledge of the cue/trial-type associations (M = 147 ms) compared with when they had nonexplicit knowledge of these associations (M = 172 ms)—Cue Knowledge × Trial Type: F(1, 156) = 3.84, p = .052, ηp2 = .02, inclusion BF = 49.26. In particular, Figure 2 shows that switch costs were smallest when participants could perceive the cues and had explicit knowledge (M = 132 ms) and largest when participants could perceive the cues and had no explicit knowledge (M = 203 ms; subliminal, nonexplicit: M = 142 ms; subliminal, explicit: M = 162 ms)—Cue Knowledge × Cue Visibility × Trial Type: F(1, 156) = 12.28, p < .001, ηp2 = .07, inclusion BF = 175.78. No other results were significant—Cue Knowledge × Cue Type × Trial Type: F(1, 156) = 2.62, p = .108, ηp2 = .02, inclusion BF = 0.85; all other Fs < 1.60.
Fig. 2.
Switch costs across experiments. Mean reaction time switch cost is shown as a function of the within-subjects factor cue type (predictive vs. nonpredictive) as well as the between-subjects factors cue visibility (subliminal vs. supraliminal) and preparatory knowledge that participants had about cue/trial-type associations (nonexplicit vs. explicit). Error bars represent standard errors of the mean. Individual data from participants are shown as points.
In sum, these results suggest that participants used cues of control demand most to facilitate switching when they consciously perceived and had explicit knowledge of the cue associations. Moreover, switch costs were largest when cues were supraliminal and not explicitly instructed, with the widest distribution across individuals within this experiment, suggesting that individual differences in recognizing or using the cue associations may play a substantial role in exploiting cues to adjust control (Braver, 2012).
Accuracy
Participants were slightly more accurate following the predictive than the nonpredictive cues—cue type: F(1, 156) = 3.57, p = .061, ηp2 = .02, inclusion BF = 13.38—and particularly so when they had explicit (mean difference = 2.7%) rather than nonexplicit (mean difference = −1.0%) knowledge of the cue/trial-type associations—Cue Knowledge × Cue Type: F(1, 156) = 17.12, p < .001, ηp2 = .10, inclusion BF = 31.35—and when the cues were presented supraliminally (mean difference = 2.4%) rather than subliminally (mean difference = −0.7%)—Cue Visibility × Cue Type: F(1, 156) = 11.97, p < .001, ηp2 = .07, inclusion BF = 7.84. When cues of control demand were explicit and supraliminal, participants, on average, performed much better following the predictive than the nonpredictive cues (5.2%; subliminal, explicit: 0.1%; subliminal, nonexplicit: −1.5%; supraliminal, nonexplicit: −0.4%)—Cue Knowledge × Cue Visibility × Cue Type: F(1, 156) = 5.26, p = .023, ηp2 = .03, inclusion BF = 1.98. Finally, there were no significant accuracy switch costs except when we compared cues that were supraliminal (M = 1.1%) with those that were subliminal (M = −1.1%)—Cue Visibility × Trial Type: F(1, 156) = 4.23, p = .041, ηp2 = .03, inclusion BF = 0.16. No other effects were significant (all other Fs < 1.05).
Global performance index
Participants were most efficient at responding when the cues were supraliminal and explicit and least efficient when they were supraliminal and nonexplicit—Cue Knowledge × Cue Visibility: F(1, 156) = 4.51, p = .035, ηp2 = .03, inclusion BF = 7,677.38. Participants were also most efficient at responding following predictive than following nonpredictive cues—cue type: F(1, 156) = 8.03, p = .005, ηp2 = .05, inclusion BF = 1.74 × 1010—which was driven primarily by explicit knowledge (mean difference = 0.83) more than by nonexplicit knowledge (mean difference = −0.23)—Cue Knowledge × Cue Type: F(1, 156) = 24.97, p < .001, ηp2 = .14, inclusion BF = 8.57 × 107—and by supraliminal (mean difference = 0.74) than by subliminal (mean difference = −0.14) presentation of the cues—Cue Visibility × Cue Type: F(1, 156) = 17.68, p < .001, ηp2 = .10, inclusion BF = 1.44 × 106. Specifically, the difference between predictive and nonpredictive cues showed that participants responded quickest, without a cost to accuracy, when cues were supraliminal and explicit (mean difference = 1.72) and with the least efficiency when cues were supraliminal and nonexplicit (mean difference = −0.24; subliminal, explicit: −0.07; subliminal, nonexplicit: −0.22)—Cue Knowledge × Cue Visibility × Cue Type: F(1, 156) = 18.41, p < .001, ηp2 = .11, inclusion BF = 20,026.52.
As expected, we observed canonical switch costs—F(1, 156) = 361.04, p < .001, ηp2 = .70, inclusion BF = 4.16 × 1060. These switch costs were smallest in Experiment 4, when cues of control demand were supraliminal and explicit (M = 1.45), and largest in Experiment 2, when the cues were subliminal and explicit (M = 2.25; supraliminal, nonexplicit: M = 2.16; nonexplicit, subliminal: M = 2.00)—Cue Knowledge × Cue Visibility × Trial Type: F(1, 156) = 5.46, p = .021, ηp2 = .03, inclusion BF = 0.71. No other effects were significant—cue knowledge: F(1, 156) = 2.48, p = .117, ηp2 = .02, inclusion BF = 3.55 × 107; Cue Visibility × Trial Type: F(1, 156) = 2.35, p = .127, ηp2 = .01, inclusion BF = 0.34; all other Fs < 1.39.
Discussion
The conditions under which contextual information can be used to reduce switch costs are not well understood: Some research has investigated how people experientially learn to associate statistical regularities with the process of task switching, whereas other studies have examined how people use trial-by-trial predictive cues for performance benefits. This study independently manipulated the conscious perception and preparatory knowledge that participants had of predictive cues to determine their respective and interactive effects on cued task switching. We found inconclusive evidence for subliminal and not explicitly instructed adjustments in control and stronger evidence for benefits of explicit and supraliminal cues.
We failed to replicate the results found by Farooqui and Manly (2015), which suggested that participants were more effective at modulating switch costs following subliminal cues than following supraliminal (and nonexplicit) predictive cues. With double the sample size, we found that participants did not display reduced switch costs following predictive cue than following nonpredictive, nonexplicit cues but were generally faster and less accurate (although more efficient) than participants in Farooqui and Manly’s study. It is not clear why we observed no predictive cue benefits for switching; however, Bayesian simulations based on the effect size reported by Farooqui and Manly (2015; Cohen’s dz = 0.84) suggest that to achieve 80% power (i.e., 80% of the BFs are greater than or equal to 1/10 or 10), we needed 25 participants, so our sample size should have been sufficient. One possibility is that the true effect size is neither as robust nor as large as previously reported. Notably, participants in the current study had generally smaller switch costs in accuracy and RT than those in Farooqui and Manly’s study. The reasons for this difference are unclear, but it raises the possibility that subliminal cuing effects may be observed only when participants experience switching as particularly difficult (and baseline switch costs are very large).
To the extent that one construes a mental task-switch/repeat operation as an “action,” we also did not find evidence of cued control on the basis of action triggers (Kunde et al., 2003): When participants had explicit knowledge of the subliminal cue associations, they could not preemptively recruit control to reduce their switch costs, and equivalence tests suggested that the observed RT and efficiency-index effects are likely too small to be of much practical or theoretical relevance. When participants had no explicit knowledge of the cue associations and the cues were presented supraliminally, participants also had the largest switch costs and showed little cue benefits. Most theories of associative learning and action control assume that experiential learning is key, with control recruitment occurring primarily on an implicit level (Abrahamse et al., 2016; Hommel, 2013), yet both manipulations of nonexplicit cue associations (Experiments 1 and 3) resulted in less efficient responding toward task-switch trials. Indeed, we observed the expected modulation of control only when participants had explicit knowledge of the specific supraliminal cue associations. This suggests that when participants were told to ignore the cues in the supraliminal experiments in Farooqui and Manly’s (2015) study, they may have inferred that the cues were task irrelevant. Together, with the lack of benefits from subliminal cues in the present study, the results suggest that subliminal cuing does not result in greater cue benefits.
A number of studies (e.g., Lau & Passingham, 2007) have shown that a masked cue can be used for control when associated with a specific task set, whereas the present study suggests that masked cues cannot be used for control if they are not associated with a specific task set but with switching per se. One factor that may impact either type of cue usage, and which we did not manipulate here, is the cue-to-stimulus interval. Future work will need to clarify the role of the cue-to-stimulus interval in learning task sets.
Although traditional views of control suggest that supraliminal, explicit knowledge is necessary to recruit control, and we observed reduced switch costs with supraliminal, explicit knowledge, we do not propose that conscious intentions are necessary for such modulation (cf. Brosowsky & Crump, 2016) or that control cannot be adjusted on the basis of subliminal processes (cf. van Gaal, Lamme, & Ridderinkhof, 2010) or implicit learning (cf. Blais, Harris, Guerrero, & Bunge, 2012). In particular, when participants did not have explicit knowledge of the cue/trial-type associations, we observed the greatest individual variance in switch costs. Therefore, one important distinction may not be conscious intentions, per se, but individual differences in statistical learning of patterns within the environment (e.g., spontaneous structure learning or working memory capacity) or, when participants recognize such patterns, individual differences in deeming the expected payoff worth the cost of recruiting control (e.g., reward responsiveness, Braver, 2012; expected value of control, Shenhav, Botvinick, & Cohen, 2013). One particularly interesting and promising line of future research thus involves delineating the robustness of the kind of trial-by-trial predictive cuing effects investigated in the present study and experiential, frequency-based control-learning effects (cf. Bugg & Smallwood, 2016). The present study adds to this enterprise by showing that in the case of trial-by-trial cuing of control, consciously perceiving and explicitly understanding the predictive value of contextual cues result in greater benefits than subliminal cuing.
Supplemental Material
Supplemental material, Beijani_OpenPracticesDisclosure_rev for Disentangling the Roles of Cue Visibility and Knowledge in Adjusting Cognitive Control: A Preregistered Direct Replication of the Farooqui and Manly (2015) Study by Christina Bejjani, Jack Dolgin, Ziwei Zhang and Tobias Egner in Psychological Science
Supplemental material, Bejjani_Supplemental_Material_rev for Disentangling the Roles of Cue Visibility and Knowledge in Adjusting Cognitive Control: A Preregistered Direct Replication of the Farooqui and Manly (2015) Study by Christina Bejjani, Jack Dolgin, Ziwei Zhang and Tobias Egner in Psychological Science
Footnotes
ORCID iDs: Christina Bejjani  https://orcid.org/0000-0002-3404-5771
https://orcid.org/0000-0002-3404-5771
Jack Dolgin  https://orcid.org/0000-0002-6451-4663
https://orcid.org/0000-0002-6451-4663
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797620904045
Transparency
Action Editor: Edward S. Awh
Editor: D. Stephen Lindsay
Author Contributions
C. Bejjani, Z. Zhang, and T. Egner developed the study concept. C. Bejjani and T. Egner designed the study. C. Bejjani and J. Dolgin coded the experiments. J. Dolgin and Z. Zhang collected the data. C. Bejjani analyzed the data under the supervision of T. Egner. All the authors contributed to the manuscript and approved the final manuscript for submission.
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This work was supported by National Institute of Mental Health Grant R01-MH087610.
Open Practices: All data, analysis code, and materials have been made publicly available via the Open Science Framework and can be accessed at osf.io/7jfbp. The design and analysis plans for Experiments 1 through 4 were preregistered at https://osf.io/vs47x. Minor changes (e.g., wording choices, addition of a manipulation check) were made to the preregistration during early drafts of this article. These changes are clarified at https://osf.io/43wp5/. The complete Open Practices Disclosure for this article can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797620904045. This article has received the badges for Open Data, Open Materials, and Preregistration. More information about the Open Practices badges can be found at http://www.psychologicalscience.org/publications/badges.



References
- Abrahamse E., Braem S., Notebaert W., Verguts T. (2016). Grounding cognitive control in associative learning. Psychological Bulletin, 142, 693–728. [DOI] [PubMed] [Google Scholar]
- Bejjani C., Zhang Z., Egner T. (2018). Control by association: Transfer of implicitly primed attentional states across linked stimuli. Psychonomic Bulletin & Review, 25, 617–626. doi: 10.3758/s13423-018-1445-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais C., Harris M. B., Guerrero J. V., Bunge S. A. (2012). Rethinking the role of automaticity in cognitive control. Quarterly Journal of Experimental Psychology, 65, 268–276. doi: 10.1080/17470211003775234 [DOI] [PubMed] [Google Scholar]
- Braver T. S. (2012). The variable nature of cognitive control: A dual-mechanisms framework. Trends in Cognitive Sciences, 16, 106–113. doi: 10.1016/j.tics.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosowsky N. P., Crump M. J. C. (2016). Context-specific attentional sampling: Intentional control as a pre-requisite for contextual control. Consciousness and Cognition, 44(Suppl. C), 146–160. doi: 10.1016/j.concog.2016.07.001 [DOI] [PubMed] [Google Scholar]
- Bugg J. M., Crump M. J. (2012). In support of a distinction between voluntary and stimulus-driven control: A review of the literature on proportion congruent effects. Frontiers in Psychology, 3, Article 367. doi: 10.3389/fpsyg.2012.00367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg J. M., Smallwood A. (2016). The next trial will be conflicting! Effects of explicit congruency pre-cues on cognitive control. Psychological Research, 80, 16–33. doi: 10.1007/s00426-014-0638-5 [DOI] [PubMed] [Google Scholar]
- Chiu Y.-C., Egner T. (2017). Cueing cognitive flexibility: Item-specific learning of switch readiness. Journal of Experimental Psychology: Human Perception and Performance, 43, 1950–1960. doi: 10.1037/xhp0000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump M. J. C., Logan G. D. (2010). Contextual control over task-set retrieval. Attention, Perception, & Psychophysics, 72, 2047–2053. doi: 10.3758/BF03196681 [DOI] [PubMed] [Google Scholar]
- Crump M. J. C., Vaquero J. M. M., Milliken B. (2008). Context-specific learning and control: The roles of awareness, task relevance, and relative salience. Consciousness and Cognition, 17, 22–36. doi: 10.1016/j.concog.2007.01.004 [DOI] [PubMed] [Google Scholar]
- Dreisbach G., Haider H. (2006). Preparatory adjustment of cognitive control in the task switching paradigm. Psychonomic Bulletin & Review, 13, 334–338. doi: 10.3758/BF03193853 [DOI] [PubMed] [Google Scholar]
- Egner T. (2014). Creatures of habit (and control): A multi-level learning perspective on the modulation of congruency effects. Frontiers in Psychology, 5, Article 1247. doi: 10.3389/fpsyg.2014.01247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entel O., Tzelgov J., Bereby-Meyer Y. (2014). Proportion congruency effects: Instructions may be enough. Frontiers in Psychology, 5, Article 1108. doi: 10.3389/fpsyg.2014.01108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui A. A., Manly T. (2015). Anticipatory control through associative learning of subliminal relations: Invisible may be better than visible. Psychological Science, 26, 325–334. doi: 10.1177/0956797614564191 [DOI] [PubMed] [Google Scholar]
- Gottschalk C., Fischer R. (2017). Activation of context-specific attentional control sets by exogenous allocation of visual attention to the context? Psychological Research, 81, 378–391. doi: 10.1007/s00426-016-0746-5 [DOI] [PubMed] [Google Scholar]
- Hommel B. (2013). Dancing in the dark: No role for consciousness in action control. Frontiers in Psychology, 4, Article 380. doi: 10.3389/fpsyg.2013.00380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunde W., Kiesel A., Hoffmann J. (2003). Conscious control over the content of unconscious cognition. Cognition, 88, 223–242. doi: 10.1016/S0010-0277(03)00023-4 [DOI] [PubMed] [Google Scholar]
- Kunde W., Reuss H., Kiesel A. (2012). Consciousness and cognitive control. Advances in Cognitive Psychology, 8, 9–18. doi: 10.5709/acp-0097-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D., Scheel A. M., Isager P. M. (2018). Equivalence testing for psychological research: A tutorial. Advances in Methods and Practices in Psychological Science, 1, 259–269. doi: 10.1177/2515245918770963 [DOI] [Google Scholar]
- Lau H. C., Passingham R. E. (2007). Unconscious activation of the cognitive control system in the human prefrontal cortex. The Journal of Neuroscience, 27, 5805–5811. doi: 10.1523/JNEUROSCI.4335-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboe J. P., Wong J., Crump M., Stobbe K. (2008). Probe-specific proportion task repetition effects on switching costs. Perception & Psychophysics, 70, 935–945. doi: 10.3758/PP.70.6.935 [DOI] [PubMed] [Google Scholar]
- Miller J. (1998). Effects of stimulus–response probability on choice reaction time: Evidence from the lateralized readiness potential. Journal of Experimental Psychology: Human Perception and Performance, 24, 1521–1534. [DOI] [PubMed] [Google Scholar]
- Monsell S. (2003). Task switching. Trends in Cognitive Sciences, 7, 134–140. doi: 10.1016/S1364-6613(03)00028-7 [DOI] [PubMed] [Google Scholar]
- Peirce J., Gray J. R., Simpson S., MacAskill M., Höchenberger R., Sogo H., . . . Lindeløv J. K. (2019). PsychoPy2: Experiments in behavior made easy. Behavior Research Methods, 51, 195–203. doi: 10.3758/s13428-018-01193-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M. I., Snyder C. R. R. (1975). Attention and cognitive control. In Solso R. L. (Ed.), Information processing and cognition: The Loyola symposium (pp. 55–85). Mahwah, NJ: Erlbaum. [Google Scholar]
- Rouder J. N., Speckman P. L., Sun D., Morey R. D., Iverson G. (2009). Bayesian t tests for accepting and rejecting the null hypothesis. Psychonomic Bulletin & Review, 16, 225–237. doi: 10.3758/PBR.16.2.225 [DOI] [PubMed] [Google Scholar]
- Schönbrodt F. D., Wagenmakers E.-J. (2018). Bayes factor design analysis: Planning for compelling evidence. Psychonomic Bulletin & Review, 25, 128–142. doi: 10.3758/s13423-017-1230-y [DOI] [PubMed] [Google Scholar]
- Shenhav A., Botvinick M. M., Cohen J. D. (2013). The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron, 79, 217–240. doi: 10.1016/j.neuron.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaal S., de Lange F. P., Cohen M. X. (2012). The role of consciousness in cognitive control and decision making. Frontiers in Human Neuroscience, 6, Article 121. doi: 10.3389/fnhum.2012.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaal S., Lamme V. A. F., Ridderinkhof K. R. (2010). Unconsciously triggered conflict adaptation. PLOS ONE, 5(7), Article e11508. doi: 10.1371/journal.pone.0011508 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Beijani_OpenPracticesDisclosure_rev for Disentangling the Roles of Cue Visibility and Knowledge in Adjusting Cognitive Control: A Preregistered Direct Replication of the Farooqui and Manly (2015) Study by Christina Bejjani, Jack Dolgin, Ziwei Zhang and Tobias Egner in Psychological Science
Supplemental material, Bejjani_Supplemental_Material_rev for Disentangling the Roles of Cue Visibility and Knowledge in Adjusting Cognitive Control: A Preregistered Direct Replication of the Farooqui and Manly (2015) Study by Christina Bejjani, Jack Dolgin, Ziwei Zhang and Tobias Egner in Psychological Science