Chemical modifications of viral RNA are an integral part of the viral life cycle and are present in most classes of viruses. To date, more than 170 RNA modifications have been discovered in all types of cellular RNA. Only a few, however, have been found in viral RNA, and the function of most of these has yet to be elucidated. Those few we have discovered and whose functions we understand have a varied effect on each virus. They facilitate RNA export from the nucleus, aid in viral protein synthesis, recruit host enzymes, and even interact with the host immune machinery.
KEYWORDS: RNA modification, RNA modification detection, RNA virus, retroviruses, viral RNA
ABSTRACT
Chemical modifications of viral RNA are an integral part of the viral life cycle and are present in most classes of viruses. To date, more than 170 RNA modifications have been discovered in all types of cellular RNA. Only a few, however, have been found in viral RNA, and the function of most of these has yet to be elucidated. Those few we have discovered and whose functions we understand have a varied effect on each virus. They facilitate RNA export from the nucleus, aid in viral protein synthesis, recruit host enzymes, and even interact with the host immune machinery. The most common methods for their study are mass spectrometry and antibody assays linked to next-generation sequencing. However, given that the actual amount of modified RNA can be very small, it is important to pair meticulous scientific methodology with the appropriate detection methods and to interpret the results with a grain of salt. Once discovered, RNA modifications enhance our understanding of viruses and present a potential target in combating them. This review provides a summary of the currently known chemical modifications of viral RNA, the effects they have on viral machinery, and the methods used to detect them.
INTRODUCTION
Viruses are a phylogenetically diverse group of obligate intracellular parasites and it is estimated there are approximately 1031 of them in the world today (1). The Baltimore system divides viruses into seven (originally six) main categories based on their genome form: (i) double-stranded DNA (dsDNA); (ii) single-stranded DNA (ssDNA); (iii) double-stranded RNA (dsRNA); (iv) positive single-stranded RNA (+ssRNA); (v) negative single-stranded RNA (−ssRNA); (vi) positive single-stranded RNA retroviruses (ssRNA-RT); and (vii) double-stranded DNA retroviruses (dsDNA-RT) (2, 3). In successful completion of their life cycles, however, these viruses have several things in common. One of them is the creation of viral RNA, whether it be genomic RNA, mRNA, or only an intermediate RNA. As viruses do not have their own translational machinery, they must hijack a host cell apparatus in order to replicate, and they have developed various strategies for this purpose (4–6). Viruses also need to evade host immunity, facilitate RNA export from the nucleus, and improve their RNA stability, translational efficiency, packaging, etc. while avoiding cellular processing of viral RNA. One of the ways they achieve all this is through the use of RNA modifications (7, 8).
Chemical modifications of RNA have been known for over 50 years (9, 10). They affect a wide range of processes, from RNA stability to translational efficiency (11, 12). To date, more than 170 RNA modifications have been identified (13), and our understanding of their functions has improved greatly and been the topic of numerous reviews (14–17). Most of the known chemical modifications are present in rRNA and tRNA. Moreover, the discovery that modifications of mRNA are dynamic and reversible (18) led to the establishment of the new field of epitranscriptomics. Unfortunately, the minuscule amount of regulatory RNA and mRNA, in which these modifications potentially affect the function of the entire RNA molecule, represents a limitation that is difficult to overcome even with the techniques available today (19). The actual number of modifications per mRNA molecule is also rather small. The most abundant modification in mRNA is N6-methyladenosine (m6A), yet there is only about 1 m6A per 1,000 nucleotides (20). Because viral RNA may be more abundant than a given type of cellular mRNA, the difficulties of searching for new RNA modifications in low-abundance RNA species and of understanding their role might be overcome by employing viruses as model systems. Moreover, their simple intrinsic organization may help us understand the role of RNA modifications in virus-derived mRNAs.
The presence of RNA modifications in viral genomic RNA and viral mRNA has a diverse impact on viral machinery. Modifications on the Watson-Crick face, such as the methylation at position 1 of adenosine (m1A) or inosine, change the pairing properties, such that the original base is then read differently by a reverse transcriptase, an RNA-dependent RNA polymerase, or the translational apparatus. The presence of large RNA modifications, e.g., 2-methylthio-N6-threonylcarbamoyladenosine in tRNA, can even stop these processes (21, 22). Several reviews have been written on viral epitranscriptomics, but they tend to focus only on N6-methyladenosine (23–35). This review presents a summary of the current findings on viral RNA modifications in general and their effect on the viral life cycle, along with the detection methods used for their discovery. We discuss the effects that chemical modifications in viral genomic RNA and mRNA have on viral infection and attempt to summarize the majority of known methods developed for the detection and identification of RNA modifications, together with the pitfalls that accompany some of the methods.
Given the recent outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), it is clear that viruses pose a major threat and there is still much to discover about their life cycles and molecular mechanisms. Understanding viral RNA modifications and learning to exploit them may lead to the creation of attenuated vaccines or specifically targeted drugs that could give us an edge in dealing with the next viral pandemic when it strikes.
VIRAL RNA MODIFICATIONS
N6-methyladenosine.
One of the most abundant chemical modifications of eukaryotic mRNA is N6-methyladenosine (m6A) (36), which has been shown to be present across virtually all domains of life (37–40). The N6-methylation of adenosine is a very dynamic modification. It is added to the RNA by a methyltransferase complex comprising two catalytic subunits (METTL3 and METTL14), a novel protein (KIAA1429), a splicing factor (Wilms’ tumor associated protein [WTAP]), and two other as-yet-unidentified subunits (41) The modification can then be removed by two demethylases—the fat mass and obesity-associated protein (FTO) and AlkBH5 (42)—in a process that regulates RNA metabolism, stability, localization, and protein interactions, as well as transport and splicing (43–45). The methylation is preferentially located at translational start sites, stop codons, and the 3′ UTR (20). It has also been identified as a key component in cancer development and metastasis, as lower levels of m6A RNA keep the cell in a pluripotent state, and higher levels drive cellular differentiation (46).
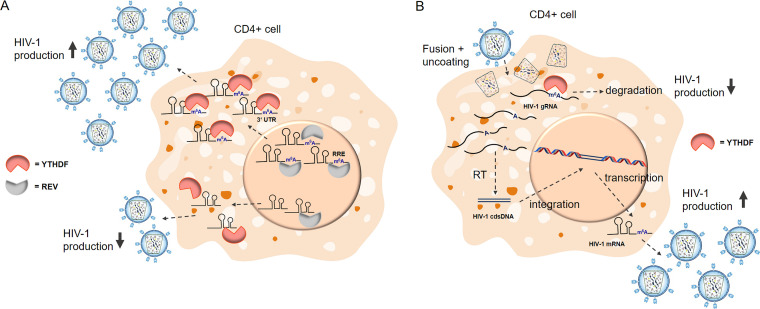
N6-methyladenosine is recognized by cytoplasmic readers, the YTHDF proteins (YTHDF1 to YTHDF3). In general, these proteins bind to the modified RNA through their C-terminal YTH domain. YTHDF1 facilitates the translation of modified mRNA and YTHDF2 localizes it to RNA decay sites, while YTHDF3 has a synergistic effect on both (43, 47, 48). In the case of HIV, several laboratories have published results showing different effects of m6A on the viral life cycle, suggesting that the role of m6A is very complex. The highly conserved YTH carboxy-terminal domain directly binds the m6A in the 3′ untranslated region of the HIV mRNA. The overexpression of these proteins greatly enhances viral expression, and their knockdown significantly reduces it (49). The mechanism remains unclear, but YTHDF2 can localize cellular mRNA to RNA decay sites and thus enhance the efficiency of viral mRNA translation (Fig. 1A) (44). Another study, however, showed that YTHDF proteins bound to m6A-modified HIV-1 RNA and inhibited genomic RNA and early HIV transcripts, but facilitated viral gene expression in m6A-modified late viral transcripts (50). Yet another study showed that m6A decreases viral protein expression and viral release (Fig. 1B). On the other hand, based on experiments demonstrating a significant decrease in viral replication upon METTL3 and METTL14 inhibition, along with an increase in viral replication upon AlkBH5 depletion, it seems that m6A plays an important role in the regulation of the HIV life cycle (25). This effect may be caused by the HIV-1 Rev protein preferentially binding to a Rev response element (RRE) containing the m6A modification and promoting nuclear export of the viral mRNA to the cytosol (Fig. 1A) (51). The effect of m6A in the RRE is still under debate, as some studies have shown that m6A has a minimal impact on the structure and stability of the RRE. Though the impact of methylation seems marginal, recent small molecule microarray screens have revealed that the change is sufficient for selective recognition by Rev (49, 51–54). The differences in results from studies of m6A in HIV can be attributed to several factors, such as the cell type used, the phase of the viral life cycle, the method used to detect m6A, etc. These discrepancies are thoroughly discussed in a review focusing specifically on m6A (25). It is also important to note that the viral infection can change the abundance of m6A in cellular RNA (51). For example, the binding of the CD4 receptor to the HIV-1 envelope glycoprotein GP120 increased the amount of cellular m6A by several fold, though the mechanism remains unclear (55).
FIG 1.
Various mechanisms by which m6A in viral RNA influences production of HIV-1. (A) In the nucleus, m6A present in the RRE of HIV-1 enhances viral mRNA export (51). In the cytoplasm, m6A in the 3′ UTR recruits YTHDF proteins and increases HIV-1 mRNA abundance while improving viral protein translation (49). (B) The m6A in HIV-1 genomic RNA leads to RNA degradation, a decrease in reverse transcription, and an overall decrease in infectivity. The m6A in the HIV-1 mRNA transcripts, however, results in an increase in viral gene expression (52).
In comparison to HIV-1 infection, the role of m6A during a flaviviral infection is more unambiguous. Based on current studies, m6A has an inhibitory effect on flaviviruses such as the hepatitis C virus (HCV) and the Zika virus (ZIKV). An increase in the amount of m6A in the viral RNA hinders viral replication and, consistently with this effect, a lowering of m6A leads to an increase in viral production (56). In HCV, the E1 gene region showed an ability to bind YTHDF proteins. In infected cells, these proteins subsequently relocalize to the lipid droplets in which viral particle assembly takes place and where they inhibit the packaging of the virus. When YTHDF is overexpressed, it binds to the RNA and hinders viral production. When m6A is absent, viral production accelerates due to an increase in HCV core protein binding to the E1 site (57). The YTHDF proteins had a similar effect on the Zika virus. Their knockdown through small interfering RNA (siRNA) led to an increase in ZIKV replication, while their overexpression inhibited the virus, pointing to a conserved mechanism among flaviviruses (58). It is important to note that when a virus infects a cell, it also affects its immune response by modifying the amount of m6A present in cellular RNA (34).
m5C.
5-Methylcytosine was first discovered as a chemical modification of DNA more than 70 years ago (59). Its presence in RNA was detected in the late 1970s. Then, 3H labeling was used to confirm the presence of m5C in the mRNA of hamster cells infected with Sindbis virus (60). More specifically, the viral 26S mRNA coding for viral structural proteins is substantially modified by m5C. The 42S mRNA also possesses several m5C sites, but significantly fewer than 26S mRNA (61).
In viral RNA, m5C has been shown to affect the host innate immune response, binding to the pattern recognition receptor RIG-1 but failing to induce the necessary conformational change that would cause the antiviral signaling cascade (62). The ability of m5C to modify viral RNA properties has led some to believe that this modification could facilitate the transfer of viroidal RNA into cellular nuclei or chloroplasts. The presence of m5C in viroids, however, was ruled out through bisulfite sequencing (63).
The m5C modification has also recently been linked to enhanced retroviral gene expression. In general, the methyltransferase NSUN2 is responsible for the methylation of cytosine in tRNA and mRNA (64), and can also add m5C to retroviral transcripts and thus affect their life cycle (65). The genomic RNA of the murine leukemia virus contains as many as 40 m5C sites, and their removal inhibits viral replication. Specifically, downregulation of the m5C writer NSUN2 through RNA interference (RNAi) caused an overall decrease in Gag protein expression, proving that the presence of m5C positively regulates viral replication (66). It has recently been reported that m5C is also present in the genomic RNA of SARS-CoV-2 genomic RNA (67). Although the effect of m5C on the viral life cycle is clearly visible, more research is necessary to elucidate the mechanisms by which it acts.
Inosine.
Inosine (I) is an essential modification created through the deamination of adenosine in a process called RNA editing (68). This is done by specific deaminases termed ADAT for tRNA and ADAR for noncoding RNA and mRNA (69). Inosine has been detected in several types of viral RNA, including dsRNA viral transcripts of human herpesvirus 8, negative-sense ssRNA viruses such as human orthopneumovirus, and even virusoids like hepatitis delta virus (HDV) (70–72). The ADARs and the modification itself have been shown to affect the viral life cycle with several mechanisms, either directly by means of the interaction of the modification, or through the inhibition of an immune response against the virus (73, 74). There is a specific isoform of ADAR1, known as p150, which is generated through an interferon (IFN)-inducible alternative promoter, meaning p150 is part of a direct antiviral response (75). ADAR1 has also been shown to positively regulate viral replication by binding and inhibiting the protein kinase R (PKR), which acts as an inhibitor of translation by phosphorylating eukaryotic initiation factor 2 (eIF2α). Phosphorylation of eIF2α stops the cellular mRNA translation and thus prevents the viral mRNA from being translated as well (76). HIV-1 is another example of a virus that actively uses ADAR-1; in fact, ADAR-1 can bind to the HIV-1 p55 Gag protein and is readily incorporated into the virion, pointing to an even more important role of this enzyme and, potentially, inosine in the viral life cycle (77).
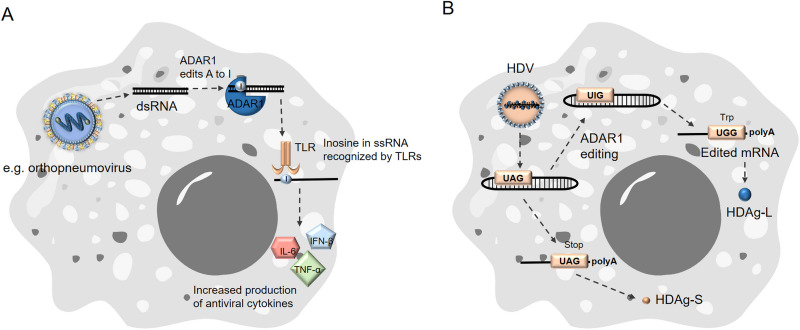
In the viral RNA of the human orthopneumovirus (also called the respiratory syncytial virus [RSV]), inosine acts as an innate immune recognition element. An in vitro-prepared RNA containing this modification also elicits an immune response. The ssRNA with this modification induces a stronger inflammatory cytokine response (Fig. 2A). It facilitates the release of interferon β (IFN-β), tumor necrosis factor α (TNF-α), and interleukin 6 (IL-6) through binding with a scavenger receptor class-A molecule. This receptor then activates the mitogen-activated protein kinase (MAPK) pathways through toll-like receptor 3 (TLR3). The authors have also demonstrated that inosine-RNA decreases replication of the respiratory syncytial virus (RSV) in epithelial cells in vitro (72). It has been suggested that the changes in RNA secondary structures associated with inosine are detected through TLR7 and TLR8. These changes also lead to an increase in TNF-α production, which would mean that inosine serves as a molecular pattern to be recognized in the phagocytosed RNA, as these receptors are mainly expressed by antigen-presenting dendritic cells (78).
FIG 2.
Effect of A to I editing on viral RNA. (A) Orthopneumoviridal negative-sense ssRNA is transcribed into dsRNA by an RNA-dependent RNA polymerase. The dsRNA is then edited by ADAR1 and recognized by Toll-like receptors, which triggers production of antiviral cytokines (e.g., IL-6, IFN-β, and TNF-α). (B) Editing of HDV circular ssRNA by ADAR1 leads to the production of two distinct mRNAs from the same ORF, the unedited HDAg-S and the edited HDAg-L.
Inosine has been shown to play a major role in the life cycle of hepatitis delta virus (HDV). HDV is a negative-strand RNA virusoid that exists in the form of a satellite associated with hepatitis B virus (HBV) (79). HDV produces its two proteins, called the small delta antigen (HDAg-S) and the large delta antigen (HDAg-L), from the same open reading frame (ORF) (80). HDV uses host ADARs, which edit an A to I in the HDAg-S amber codon in the antigenomic RNA. A UIG codon is thus transcribed as a UGG codon, and the resulting mRNA is translated as the HDAg-L (Fig. 2B) (81, 82). The general ability of inosine to change the ORF in the viral RNA enables the virus to compress more genetic information into the same sequence. The possibility that other viruses utilize the same feature should be entertained, along with the potential immunogenic effects of this modification.
2′-O-methylations.
2′-O-methylations (Nm) (the addition of a methyl group to the 2′-OH of a ribose) of RNA have been a subject of interest since the 1960s, when they were discovered and observed by means of radioactive labeling of RNA (83, 84). Based on their position within the RNA molecule, 2′-O-methylations can be divided into two categories. The first category includes methylation of eukaryotic mRNA at the first and second nucleotide behind the 5′ cap (85). These structures, called Cap1 and Cap2 based on the position of the methylated nucleoside (86), are responsible for efficiency of processing, translation, overall stability, and susceptibility to degradation of mRNA (87, 88). Some viruses, such as coronaviruses, flaviviruses, orthomyxoviruses, and picornaviruses, rely on such a cap-dependent mechanism of translation. They can either use the host cell capping apparatus, snatch the caps from host mRNA (e.g., influenza), or code for their own capping machinery (89, 90). While the aforementioned viruses produce 5′ capped RNA, the lack of 2′-O-methylation may still alert the cell to their presence (91).
In fact, the absence of 2′-O-methylation on the first nucleotide of the 5′ cap (Cap0) is strongly immunogenic. The cytoplasmic pattern recognition receptor Mda5 is activated through binding to the Cap0 RNA (92). The activated Mda5 interacts with the mitochondrial antiviral signaling proteins (MAVS) through its N-terminal caspase activation and recruitment domains (CARDs). Working in a multiprotein complex, the MAVS recruit the inhibitor of nuclear factor kappa-B kinase subunit epsilon (IKKε) and the serine/threonine-protein kinase 1 (TBK1). This leads to the phosphorylation and transport of interferon regulatory factors 3 and 7 (IRF3 and IRF7) into the nucleus, where they activate the transcription of type I interferon genes IFN-α and IFN-β (Fig. 2) (93–97).
Another type of molecule capable of recognizing the Cap0 structure belongs to the IFIT family (interferon-induced proteins with tetratricopeptide repeats) (98). These molecules serve not only as detectors but also as effectors capable of inhibiting the viral life cycle (99). In particular, IFIT1 competes with the eukaryotic initiation factor 4E (eIF4E), which is part of the eukaryotic initiation factor 4F (eIF4F) that binds to the 5′ cap of mRNA (100). The eIF4E has a higher affinity for the Cap1 and Cap2 structures than IFIT1. On the other hand, IFIT1 has a higher affinity for the Cap0 structure. Binding to the viral RNA, IFIT1 leads to the abortion of viral translation (101). It also inhibits the formation of the 43S-mRNA complex and blocks the recruitment of eIF3 to the ternary complex, etc. (102).
The second type of 2′-O-methylation is present in internal RNA sites. These methylations are added to the viral RNA by hijacking the cellular methyltransferase FTSJ3. Cells in which the FTSJ3 methyltransferase is knocked down produce HIV-1 RNA with fewer methylations, and the virus induces higher expression of IFN-α and IFN-β (103).
It has been suggested and tested both in vitro and in mouse models that some RNA viruses may be attenuated by creating mutants lacking the Nm modification. This is done by creating recombinant viruses with a specific defect in the S-adenosylmethionine (SAM) binding site of the methyltransferase responsible for 2′-O-methylation. An infection with this recombinant virus elicits strong humoral and cellular immune reactions (104, 105). One of the model viruses used for this type of vaccine research was the severe acute respiratory syndrome coronavirus (SARS-CoV). Mutations introduced into nonstructural protein 16 (nsp16) created an attenuated virus by preventing it from creating the 2′-O-methylation (106). It also conclusively proved that viral Nm is an integral RNA modification necessary for a successful viral life cycle, and that viruses may utilize host methylating machinery to hide from the immune system. Given the recent outbreak of the disease COVID-19 associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), it is definitely worthwhile to continue examining the modifications possessed by this virus and discover new ways of exploiting them for our benefit.
Pseudouridine.
Pseudouridine (Ψ), often called the fifth nucleotide, is created by the isomerization of uridine. It is present in all RNA and in very high quantities in noncoding RNA (107). While the detection and location of pseudouridine in viral RNA is still in its infancy, and pseudouridine has yet to be detected in viral RNA, it has recently been reported that the enzyme pseudouridine synthase PUS7L is integral to the life cycle of HCV (108). An in vitro-prepared part of the polyU/UC RNA domain of HCV has been shown to act as a pathogen-associated molecular pattern that activates the pattern recognition receptor RIG-1 and leads to IFN-β production (109). The complete replacement of uridine with pseudouridine in this transcript drastically decreased IFN-β production, even though the RNA motif still had a high affinity for the RIG-1 molecule. Specifically, the RNA binds to RIG-1 but fails to trigger the conformational change associated with the activation of the molecule, thus disrupting the IFN-β immune response at an early stage (62). As it may be an essential part of the viral life cycle and the evasion of the host immune response, further research into the effect of pseudouridine in viral RNAs is warranted.
Other RNA modifications.
Recently, a study based solely on liquid chromatography-mass spectrometry (LC-MS) analysis of viral RNA from ZIKV, dengue virus, HCV, poliovirus, and HIV-1 reported that the genomic RNA of these viruses contains, respectively, 32, 39, 42, 41, and 36 various chemical RNA modifications. Apart from numerous RNA modifications that were never before reported in mammalian systems, N1-methyladenosine (m1A) was also detected in all the tested viruses (110). Shortly thereafter, a study mapping m1A in RNA from the viral particle of HIV-1 showed that all the detected m1A comes from tRNA copacked in the viral particle, proving that HIV-1 genomic RNA does not contain m1A (22). This is in line with the finding that m1A is a typical tRNA modification and its presence in other types of RNA is somewhat rare (111).
Using ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analysis of the purified genomic viral RNA of two retroviruses, HIV-1 (65) and murine leukemia virus-MLV (66), it was recently shown that m1A, m1G, m5C, or m7G are present in viral RNA. These modifications, however, are present in tRNALys and tRNAPro, which serve as primers for the start of the reverse transcription of HIV-1 or MLV, respectively (13). It can be assumed that these tRNAs bind tightly to the genomic viral RNA. The sequencing libraries were prepared using the protocol for HIV-1 packageome analysis (112), which does not recover short RNAs (<50 nucleotides [nt]) or the highly structured tRNA. Even though the control sequencing analysis of the UPLC-MS/MS samples did not show any contamination from cellular RNA, the bound tRNAs could have been present and overlooked. It is important to note that, for example, 2-methylthio-N6-threonylcarbamoyladenosine (mS2t6A) causes a complete abortion of reverse transcription, and all the cDNA reads from tRNALys have an approximate length of only 36 nucleotides and thus are not included in the library (22). Therefore, it would be useful to map m1A or m1G in retroviral genomic RNA with a profiling technique to confirm the presence of these modifications. It is important to note that modifications such as m1A and m1G would affect the function of the viral RNA because they disrupt traditional Watson-Crick base pairing, unlike m6A or m5C. For example, they may weaken complementary pairing of the molecule and change its coding capacity.
Detection techniques.
Before a thorough study of the functions of a particular RNA modification, its existence and sequence position must be determined. Known viral RNA modifications and the methods of their detection are summarized in Table 1. Prior to the era of transcriptome sequencing (RNA-seq)-based techniques (113), mass spectrometry (MS) and radioactive labeling were commonly used to discover new RNA modifications. Even today, MS remains a very important tool capable of confirming the presence of almost all the chemical modifications (114). Nevertheless, it does not allow for the determination of the exact position of an RNA modification within the RNA sequence. The common procedure comprises the isolation of very pure target RNA material, followed by its digestion into the form of nucleosides or nucleotides. Analysis by means of MS usually requires a larger amount of starting material (isolated RNA) compared with RNA-seq-based methods. Although MS is a direct method and does not suffer from amplification bias created during library preparation, its main limitation lies in the purification of a particular RNA. Because rRNA represents about 85%, and tRNA about 12%, of cellular RNA (115), contamination of mRNA or viral RNA with these very abundant RNAs sometimes causes false positives when detecting RNA modifications.
TABLE 1.
Summary of all detected RNA modifications in viral genomic RNA or viral mRNA with the techniques used for their detection and other potentially useful detection techniques
| Modification | Type of virus by Baltimore classification and family: species | Type of viral RNA | Methods used for detection | Methods available for application |
|---|---|---|---|---|
N6-methyladenosine m6A 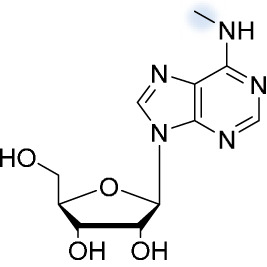
|
ss-RNA-RT, Retroviridae: | Analysis of unlabeled RNA: antibody based: (i) m6A-seq (20); (ii) MeRIP-seq (18); (iii) direct m6A seq (RT-KlenTaq DNA polymerase) (119); (iv) MAZTER-seq (120); (v) miCLIP (116); (vi) DART-seq (122) Metabolic labeling of cells: (i) metabolic propargyl labeling (130); (ii) PA-m6A-seq (antibody assisted) (129) Methods for the confirmation of m6A position: (i) SCARLET (121) | ||
| Avian sarcoma viruses | Genomic, mRNA | 32P-labeling (154–156), m6A-seq (52), PA-m6A-seq (49) | ||
| HIV-I | Genomic, mRNA | 32P-labeling (154–156), m6A-seq (52), PA-m6A-seq (49) | ||
| Murine leukemia virus (66) | Genomic | UPLC-MS/MS, PA-m6A-seq | ||
| (−)ss-RNA, Orthomyxoviridae: | ||||
| Influenza A (151) | Genomic, mRNA | PAR-CLIP with YTHDF (157) | ||
| Influenza A (151) | mRNA | PA-m6A-seq, radioactive labeling with [methyl-3H]methionine (158, 159) | ||
| ds-DNA, Polyomaviridae: | ||||
| Simian virus 40 (152) | mRNA | PAR-CLIP with YTHDF, PA-m6A-seq | ||
| (+)ss-RNA, Flaviviridae: | ||||
| Zika virus (58) | Genomic | UPLC-MS/MS, MeRIP-seq | ||
| HCV | Genomic | UPLC-MS/MS, MeRIP-seq | ||
| Dengue virus | Genomic | MeRIP-seq | ||
| Yellow fever virus | Genomic | MeRIP-seq | ||
| West Nile virus (57) | Genomic | MeRIP-seq | ||
| (+)ss-RNA, Picornaviridae: | ||||
| Enterovirus 71 (153) | mRNA | MeRIP-seq | ||
5-methylcytidine m5C 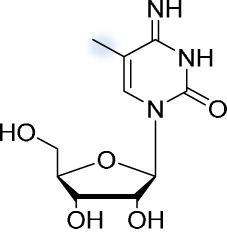
|
(+)ss-RNA, Togaviridae: | (i) Bisulfite sequencing (123–126); (ii) m5C-RIP (117); (iii) Aza-IP (131); (iv) miCLIP (132); (v) RBS-seq (128) | ||
| Sindbis virus | mRNA | Radioactive labeling (60, 61) | ||
| ss-RNA-RT, Retroviridae: | ||||
| HIV-I (65) | Genomic, mRNA | PA-m5C-seq | ||
| Murine leukemia virus (66) | Genomic | UPLC-MS, PA-m5C-seq | ||
| (+)ss-RNA, Betacoronaviridae: | ||||
| SARS-CoV-2 (67) | Genomic, mRNA | Nanopore sequencing | ||
Inosine I 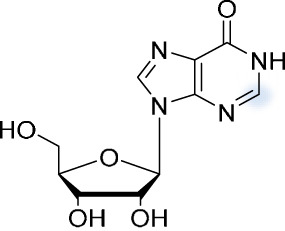
|
(−)ss-RNA, Paramyxoviridae: | (i) Sequencing comparison (133–135); (ii) ICE-seq (136) | ||
| Measles virus | mRNA | Sequence comparison (164, 165) | ||
| ss-RNA-RT, Retroviridae: | ||||
| HIV-I (74, 160) | mRNA | Sequence comparison | ||
| ds-DNA, Herpesviridae: | ||||
| Human herpesvirus 8 (71) | mRNA | Restriction enzyme cleavage of cDNA | ||
| (+)ss-RNA, Flaviviridae: | ||||
| Zika virus (58, 161, 162) | Genomic, mRNA | Sequence comparison | ||
| Subviral satellite, Ïncertae sedis: | ||||
| HDV (70, 163) | Genomic, mRNA | Sequence comparison | ||
2′-O-methylnucleoside Nm 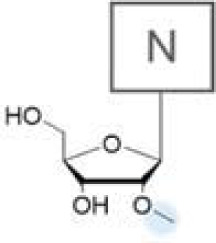
|
As part of cap: | (i) RiboMethSeq (139, 140); (ii) RibOxi-seq (141); (iii) Nm-seq (142, 143) | ||
| All viruses use cap-dependent translation (98) | ||||
| Internal location: | ||||
| (+)ss-RNA, Flaviviridae: | ||||
| Zika virus (U, C, G, and A) (58) | Genomic | UPLC/MS-MS | ||
| Dengue virus (166) | Genomic | UPLC/MS-MS, radioactive labeling | ||
| ss-RNA-RT, Retroviridae: | ||||
| HIV-1 (103) | Genomic, mRNA | RiboMeth-Seq | ||
In contrast, RNA-seq methods allow for the determination of the exact position of RNA modifications within the entire transcriptome. The main disadvantage is the necessity of developing a specific capture/profile technique for every RNA modification. Once such a method is available, captured RNA is reverse transcribed into cDNA (which does not contain any modifications) and then amplified. The majority of methods rely on selective antibodies against m6A, including m6A-seq (20); MeRIP-seq (18); miCLIP (116); and m5C (m5C-RIP) (117). The main issue with antibody-based methods is the lack of specificity and effectivity of the antibodies used. Nonspecific binding of the antibodies often introduces significant bias into the results, so a careful approach is thus required (118). To overcome this problem, alternative techniques combined with next-generation sequencing have been developed for m6A profiling, such as employment of RT-Klentaq DNA polymerase (119) or, more recently, MAZTER-seq (120). Other techniques for the detection of m6A, such as SCARLET, require prior knowledge of the position of m6A (121). Another antibody-independent method is called DART-seq (deamination adjacent to RNA modification targets). This uses the m6A-binding domain YTH fused to the cytidine deaminase APOBEC1. The C nucleotides next to the m6A are deaminated into U, which is subsequently recognized using RNA-seq (122). The development of selective chemical techniques for m6A profiling, which would overcome all the disadvantages of the previous methods, is limited by the similar chemical structure and similar reactivity of m6A to canonical adenosine.
While there is an antibody-based approach for the detection of m5C, bisulfite sequencing is also frequently used. It relies on the selective chemical reaction of canonical cytidine and 5-methyl cytidine and is a functional alternative to the aforementioned antibody-based techniques (123–126). Relatively harsh reaction conditions, however, may destroy fragile RNA molecules, and other modifications (such as N4, 2′-O-dimethylcytidine) are sometimes mistaken for m5C. Moreover, the standard RNA bisulfite protocol has been shown to generate false-positive results when working with highly structured RNA (63). Nevertheless, the method has been used effectively to detect m5C in mouse embryonic and brain polyA RNA (127) and, recently, a modified bisulfite sequencing method called RBS-seq was introduced for simultaneous detection of Ψ, m1A, and m5C in a transcriptome-wide manner (128). To avoid the drawbacks of the aforesaid techniques, metabolic labeling methods were developed to detect both m6A (PA-m6A-seq [129] or metabolic propargyl labeling [130]) and m5C (Aza-IP [131] and miCLIP [132]). In general, the main disadvantage of metabolic labeling using propargyl, 5-azacytidine, or 4-thiouridine is that it introduces a major type of stress to the cell, such that the results do not represent the state of a healthy system.
Even though there are currently no antibody-based methods to detect inosine, a comparison of genomic sequences with the corresponding cDNA reveals its position within the RNA molecule. Inosine pairs with cytidine, and the cDNA thus contains a guanosine in its place (133). However, the method is prone to false positives, as it does not distinguish mapping errors, alignment errors, or single-nucleotide polymorphisms (134, 135). As an alternative, a chemical method called ICE-seq (RNA-seq based on the selective reaction of inosine with cyanoethyl) was developed in 2010 (136, 137).
There are no antibodies against 2′-O-methylation, and the modification is fairly unreactive. There are also two types of 2′-O-methylations distinguished by their position within the RNA molecule, where one is a part of the 5′ cap (85) and the other in internal RNA sites. The 2′-O-methylation within the cap structure can be detected mainly using UPLC-MS/MS (138). Identification of methylated internal sites is more complicated, and the developed techniques rely on a higher stability of the methylated position under basic conditions (RiboMethseq [139, 140]) or during oxidation (NaIO4) and β-elimination (RiboOxi-seq [141] and Nm-seq [142]). It was discovered, however, that in the case of less abundant RNA, Nm-seq is prone to mispriming and false positives (143).
In the future, all the problems caused by classical next-generation sequencing-based methods might be overcome with direct nanopore sequencing. This technique has the potential to detect RNA modifications directly without the need for reverse transcription and amplification. It has already been used in the sequencing of influenza virus RNA (144). In nanopore sequencing, the RNA moves through a pore and disrupts the electric current around it, causing so-called squiggles. Theoretically, every base and every modification disrupts the current differently and can thus be identified (145). Problems with alignment and current intensity changes, however, have prevented the creation of a successful detection algorithm. This issue can be circumvented by analyzing base-calling errors for some modifications, such as m6A, by comparing the target RNA with a nonmodified (or severely depleted) control RNA, or by employing artificial intelligence (AI) (146, 147).
Conclusion and outlook. While internal RNA modifications in mRNA or viral genomic RNA and mRNA do exist, they are not as diverse and abundant as many believed in 2012, when the field of epitranscriptomics was established. Nevertheless, the chemical modifications of viral RNA described above obviously play a role in the viral life cycle, in interactions with host innate immunity, and in the distinction between self and nonself RNA. Despite that several attempts have been made to exploit one of these modifications in order to create an attenuated vaccine in several viruses, to the best of our knowledge, no attempts to target the other modifications are currently being made. In comparison with internal modifications, new discoveries of modifications, such as the 5′ diphosphate termini in reoviruses, have shown that there is still much to be learned about the 5′ RNA moieties. The 5′ diphosphate RNA is recognized by RIG-I-like receptors in the cytoplasm and starts an antiviral cascade like the one described for the cap0 structure (107). Recently, a new mass spectrometry detection method called CapQuant has been used to identify several new types of RNA caps in purified dengue virions, including FAD (flavin adenine dinucleotide); UDP-Glc; UDP-GlcNAc; and m7Gpppm6A (108). We have recently described a new class of RNA caps in bacteria; dinucleoside polyphosphates were discovered using a combination of biochemical methods together with mass spectrometry (148). Given that some dsDNA viruses (e.g., poxviruses) encode their own NudiX enzymes that may process the 5′ dinucleoside polyphosphate RNA caps, the existence of such alternative caps in viral RNA cannot be ruled out (149, 150). The roles of most of these modifications and whether they are present in viral RNA remain to be determined. There is also a need for a careful and controlled approach when preparing RNA samples in order to generate reproducible and trustworthy data, or when applying a particular detection technique. Although new detection methods are constantly being developed based on ingenious new techniques, such as mutated enzymes that specifically interact with given modifications (120, 122), AI analysis of collected data, together with nanopore sequencing, seems the most promising. It is clear that viral RNA and the roles played by its modifications still hold a number of secrets. Thanks to the high abundance of viral RNA molecules in infected cells, they may well be a crucial model for understanding the role of similarly modified cellular RNA and further expanding our knowledge in the field of RNA modifications.
ACKNOWLEDGMENTS
We acknowledge funding from the Ministry of Education, Youth and Sports (Czech Republic), program ERC CZ (LL1603).
We gratefully thank Ondřej Nešuta, Roberto Benoni, Oldřich Hudeček, and Lucia Fehérová for a critical review of the manuscript.
Conceptualization, resources, writing, and visualization were done by J.F.P. and H.C. Supervision, project administration, and funding acquisition were done by H.C.
We declare no conflicts of interest.
Footnotes
Citation Potužník JF, Cahová H. 2020. It’s the little things (in viral RNA). mBio 11:e02131-20. https://doi.org/10.1128/mBio.02131-20.
Contributor Information
Vinayaka R. Prasad, Albert Einstein College of Medicine.
Danielle A. Garsin, University of Texas Health Science Center at Houston.
REFERENCES
- 1.Breitbart M, Rohwer F. 2005. Here a virus, there a virus, everywhere the same virus? Trends Microbiol 13:278–284. doi: 10.1016/j.tim.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Baltimore D. 1971. Expression of animal virus genomes. Bacteriol Rev 35:235–241. doi: 10.1128/MMBR.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King AMQ, Lefkowitz E, Adams MJ, Carstens EB. 2011. Virus taxonomy. Elsevier, Amsterdam, the Netherlands. [Google Scholar]
- 4.Walsh D, Mathews MB, Mohr I. 2013. Tinkering with translation: protein synthesis in virus-infected cells. Cold Spring Harb Perspect Biol 5:a012351. doi: 10.1101/cshperspect.a012351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bushell M, Sarnow P. 2002. Hijacking the translation apparatus by RNA viruses. J Cell Biol 158:395–399. doi: 10.1083/jcb.200205044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López-Lastra M, Ramdohr P, Letelier A, Vallejos M, Vera-Otarola J, Valiente-Echeverrkía F. 2010. Translation initiation of viral mRNAs. Rev Med Virol 20:177–195. doi: 10.1002/rmv.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson BL, White KA. 2015. Exploring the architecture of viral RNA genomes. Curr Opin Virol 12:66–74. doi: 10.1016/j.coviro.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Cross ST, Michalski D, Miller MR, Wilusz J. 2019. RNA regulatory processes in RNA virus biology. Wiley Interdiscip Rev RNA 10:e1536. doi: 10.1002/wrna.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limbach PA, Crain PF, McCloskey JA. 1994. Summary: the modified nucleosides of RNA. Nucleic Acids Res 22:2183–2196. doi: 10.1093/nar/22.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis FF, Allen FW. 1957. Ribonucleic acids from yeast which contain a fifth nucleotide. J Biol Chem 227:907–915. [PubMed] [Google Scholar]
- 11.Roundtree IA, Evans ME, Pan T, He C. 2017. Dynamic RNA modifications in gene expression regulation. Cell 169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baudin-Baillieu A, Fabret C, Liang XH, Piekna-Przybylska D, Fournier MJ, Rousset JP. 2009. Nucleotide modifications in three functionally important regions of the Saccharomyces cerevisiae ribosome affect translation accuracy. Nucleic Acids Res 37:7665–7677. doi: 10.1093/nar/gkp816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boccaletto P, MacHnicka MA, Purta E, Pitkowski P, Baginski B, Wirecki TK, De Crécy-Lagard V, Ross R, Limbach PA, Kotter A, Helm M, Bujnicki JM. 2018. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helm M, Alfonzo JD. 2014. Posttranscriptional RNA modifications: playing metabolic games in a cell’s chemical legoland. Chem Biol 21:174–185. doi: 10.1016/j.chembiol.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phizicky EM, Alfonzo JD. 2010. Do all modifications benefit all tRNAs? FEBS Lett 584:265–271. doi: 10.1016/j.febslet.2009.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saletore Y, Meyer K, Korlach J, Vilfan ID, Jaffrey S, Mason CE. 2012. The birth of the Epitranscriptome: deciphering the function of RNA modifications. Genome Biol 13:175. doi: 10.1186/gb-2012-13-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Mason CE. 2014. The pivotal regulatory landscape of RNA modifications. Annu Rev Genomics Hum Genet 15:127–150. doi: 10.1146/annurev-genom-090413-025405. [DOI] [PubMed] [Google Scholar]
- 18.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. 2012. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finka A, Sood V, Quadroni M, De Los Rios PDL, Goloubinoff P. 2015. Quantitative proteomics of heat-treated human cells show an across-the-board mild depletion of housekeeping proteins to massively accumulate few HSPs. Cell Stress Chaperones 20:605–620. doi: 10.1007/s12192-015-0583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, Sorek R, Rechavi G. 2012. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 21.Potapov V, Fu X, Dai N, Corrêa IR, Tanner NA, Ong JL. 2018. Base modifications affecting RNA polymerase and reverse transcriptase fidelity. Nucleic Acids Res 46:5753–5763. doi: 10.1093/nar/gky341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Šimonová A, Svojanovská B, Trylčová J, Hubálek M, Moravčík O, Zavřel M, Pávová M, Hodek J, Weber J, Cvačka J, Pačes J, Cahová H. 2019. LC/MS analysis and deep sequencing reveal the accurate RNA composition in the HIV-1 virion. Sci Rep 9:8697. doi: 10.1038/s41598-019-45079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batista PJ. 2017. The RNA modification N6-methyladenosine and its implications in human disease. Genomics Proteomics Bioinformatics 15:154–163. doi: 10.1016/j.gpb.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Netzband R, Pager CT. 2019. Epitranscriptomic marks: emerging modulators of RNA virus gene expression. Wiley Interdiscip Rev RNA 11:e1576. doi: 10.1002/wrna.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riquelme-Barrios S, Pereira-Montecinos C, Valiente-Echeverría F, Soto-Rifo R. 2018. Emerging roles of N6-methyladenosine on HIV-1 RNA metabolism and viral replication. Front Microbiol 9:576. doi: 10.3389/fmicb.2018.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gokhale NS, Horner SM. 2017. RNA modifications go viral. PLoS Pathog 13:e1006188. doi: 10.1371/journal.ppat.1006188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy EM, Courtney DG, Tsai K, Cullen BR. 2017. Viral epitranscriptomics. J Virol 91:e02263-16. doi: 10.1128/JVI.02263-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereira-Montecinos C, Valiente-Echeverría F, Soto-Rifo R. 2017. Epitranscriptomic regulation of viral replication. Biochim Biophys Acta Gene Regul Mech 1860:460–471. doi: 10.1016/j.bbagrm.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Wang H, Zhang W. 2019. Regulation of virus replication and T cell homeostasis by N 6 -methyladenosine. Virol Sin 34:22–29. doi: 10.1007/s12250-018-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manners O, Baquero-Perez B, Whitehouse A. 2019. m6A: widespread regulatory control in virus replication. Biochim Biophys Acta Gene Regul Mech 1862:370–381. doi: 10.1016/j.bbagrm.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan B, Gao SJ. 2018. RNA epitranscriptomics: regulation of infection of RNA and DNA viruses by N6-methyladenosine (m6A). Rev Med Virol 28:e1983. doi: 10.1002/rmv.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan B, Gao S-J. 2018. The RNA epitranscriptome of DNA viruses. J Virol 92:e00696-18. doi: 10.1128/JVI.00696-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dang W, Xie Y, Cao P, Xin S, Wang J, Li S, Li Y, Lu J. 2019. N6-methyladenosine and viral infection. Front Microbiol 10:417. doi: 10.3389/fmicb.2019.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams GD, Gokhale NS, Horner SM. 2019. Regulation of viral infection by the RNA modification N6-methyladenosine. Annu Rev Virol 6:235–253. doi: 10.1146/annurev-virology-092818-015559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu F, Cheng W, Zhao F, Tang M, Diao Y, Xu R. 2019. Association of N6-methyladenosine with viruses and related diseases. Virol J 16:133. doi: 10.1186/s12985-019-1236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao BS, Roundtree IA, He C. 2017. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol 18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nichols JL. 1979. N6-methyladenosine in maize poly(A)-containing RNA. Plant Sci Lett 15:357–361. doi: 10.1016/0304-4211(79)90141-X. [DOI] [Google Scholar]
- 38.Perry RP, Kelley DE. 1974. Existence of methylated messenger RNA in mouse L cells. Cell 1:37–42. doi: 10.1016/0092-8674(74)90153-6. [DOI] [Google Scholar]
- 39.Deng X, Chen K, Luo GZ, Weng X, Ji Q, Zhou T, He C. 2015. Widespread occurrence of N6-methyladenosine in bacterial mRNA. Nucleic Acids Res 43:6557–6567. doi: 10.1093/nar/gkv596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kowalak JA, Dalluge JJ, McCloskey JA, Stetter KO. 1994. The role of posttranscriptional modification in stabilization of transfer RNA from hyperthermophiles. Biochemistry 33:7869–7876. doi: 10.1021/bi00191a014. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, Mertins P, Ter-Ovanesyan D, Habib N, Cacchiarelli D, Sanjana NE, Freinkman E, Pacold ME, Satija R, Mikkelsen TS, Hacohen N, Zhang F, Carr SA, Lander ES, Regev A. 2014. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5’ sites. Cell Rep 8:284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Wei L-H, Wang Y, Xiao Y, Liu J, Zhang W, Yan N, Amu G, Tang X, Zhang L, Jia G. 2019. Structural insights into FTO’s catalytic mechanism for the demethylation of multiple RNA substrates. Proc Natl Acad Sci U S A 116:2919–2924. doi: 10.1073/pnas.1820574116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. 2015. N6-methyladenosine modulates messenger RNA translation efficiency. Cell 161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, Ren B, Pan T, He C. 2014. N 6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. 2015. N6 -methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kandimalla R, Gao F, Li Y, Huang H, Ke J, Deng X, Zhao L, Zhou S, Goel A, Wang X. 2019. RNAMethyPro: a biologically conserved signature of N6-methyladenosine regulators for predicting survival at pan-cancer level. NPJ Precis Oncol 3:13. doi: 10.1038/s41698-019-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C. 2017. YTHDF3 facilitates translation and decay of N 6-methyladenosine-modified RNA. Cell Res 27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, Ma J, Wu L. 2016. YTHDF2 destabilizes m 6 A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun 7:12626. doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kennedy EM, Bogerd HP, Kornepati AVR, Kang D, Ghoshal D, Marshall JB, Poling BC, Tsai K, Gokhale NS, Horner SM, Cullen BR. 2016. Posttranscriptional m6A editing of HIV-1 mRNAs enhances viral gene expression. Cell Host Microbe 22:830. doi: 10.1016/j.chom.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu W, Tirumuru N, Gelais CS, Koneru Pc Liu C, Kvaratskhelia M, He C, Wu L. 2018. N 6 –Methyladenosine-binding proteins suppress HIV-1 infectivity and viral production. J Biol Chem 293:12992–13005. doi: 10.1074/jbc.RA118.004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lichinchi G, Gao S, Saletore Y, Gonzalez GM, Bansal V, Wang Y, Mason CE, Rana TM. 2016. Dynamics of the human and viral m(6)A RNA methylomes during HIV-1 infection of T cells. Nat Microbiol 1:16011. doi: 10.1038/nmicrobiol.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tirumuru N, Zhao BS, Lu W, Lu Z, He C, Wu L. 2016. N(6)-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. Elife 5:e15528. doi: 10.7554/eLife.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chu C-C, Liu B, Plangger R, Kreutz C, Al-Hashimi HM. 2019. m6A minimally impacts the structure, dynamics, and Rev ARM binding properties of HIV-1 RRE stem IIB. PLoS One 14:e0224850. doi: 10.1371/journal.pone.0224850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sherpa C, Grice SL. 2020. Structural fluidity of the human immunodeficiency virus Rev response element. Viruses 12:86. doi: 10.3390/v12010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tirumuru N, Wu L. 2019. HIV-1 envelope proteins up-regulate N 6 -methyladenosine levels of cellular RNA independently of viral replication. J Biol Chem 294:3249–3260. doi: 10.1074/jbc.RA118.005608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bradrick SS. 2017. Causes and consequences of flavivirus RNA methylation. Front Microbiol 8:2374. doi: 10.3389/fmicb.2017.02374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gokhale NS, McIntyre ABR, McFadden MJ, Roder AE, Kennedy EM, Gandara JA, Hopcraft SE, Quicke KM, Vazquez C, Willer J, Ilkayeva OR, Law BA, Holley CL, Garcia-Blanco MA, Evans MJ, Suthar MS, Bradrick SS, Mason CE, Horner SM. 2016. N6-methyladenosine in Flaviviridae viral RNA genomes regulates infection. Cell Host Microbe 20:654–665. doi: 10.1016/j.chom.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lichinchi G, Zhao BS, Wu Y, Lu Z, Qin Y, He C, Rana TM. 2016. Dynamics of human and viral RNA methylation during Zika virus infection. Cell Host Microbe 20:666–673. doi: 10.1016/j.chom.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hotchkiss RD. 1948. The quantitative separation of purines, pyrimidines, and nucleosides by paper chromatography. J Biol Chem 175:315–332. [PubMed] [Google Scholar]
- 60.Dubin DT, Stollar V. 1975. Methylation of sindbis virus “26S” messenger RNA. Biochem Biophys Res Commun 66:1373–1379. doi: 10.1016/0006-291x(75)90511-2. [DOI] [PubMed] [Google Scholar]
- 61.Dubin DT, Stollar V, Hsuchen CC, Timko K, Guild GM. 1977. Sindbis virus messenger RNA: the 5′-termini and methylated residues of 26 and 42 S RNA. Virology 77:457–470. doi: 10.1016/0042-6822(77)90471-8. [DOI] [PubMed] [Google Scholar]
- 62.Durbin AF, Wang C, Marcotrigiano J, Gehrke L. 2016. RNAs containing modified nucleotides fail to trigger RIG-I conformational changes for innate immune signaling. mBio 7:e00833-16. doi: 10.1128/mBio.00833-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Serio F, Torchetti EM, Daròs JA, Navarro B. 2019. Reassessment of viroid RNA cytosine methylation status at the single nucleotide level. Viruses 11:357. doi: 10.3390/v11040357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, Suter CM, Preiss T. 2012. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res 40:5023–5033. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Courtney D, Tsai K, Bogerd HP, Kennedy EM, Law BA, Emery A, Swanstrom R, Holley CL, Cullen BR. 2019. Epitranscriptomic addition of m5C to HIV-1 transcripts regulates viral gene expression. Cell Host Microbe 26:217–227.e6. doi: 10.1016/j.chom.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Courtney DG, Chalem A, Bogerd HP, Law BA, Kennedy EM, Holley CL, Cullen BR. 2019. Extensive epitranscriptomic methylation of A and C residues on murine leukemia virus transcripts enhances viral gene expression. mBio 10:e01209-19. doi: 10.1128/mBio.01209-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim D, Lee J-Y, Yang J-S, Kim JW, Kim VN, Chang H. 2020. The architecture of SARS-CoV-2 transcriptome. Cell 181:914–921. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zinshteyn B, Nishikura K. 2009. Adenosine-to-inosine RNA editing. Wiley Interdiscip Rev Syst Biol Med 1:202–209. doi: 10.1002/wsbm.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nishikura K. 2010. Functions and regulation of RNA editing by ADAR deaminases. Annu Rev Biochem 79:321–349. doi: 10.1146/annurev-biochem-060208-105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jayan GC, Casey JL. 2002. Increased RNA editing and inhibition of hepatitis delta virus replication by high-level expression of ADAR1 and ADAR2. J Virol 76:3819–3827. doi: 10.1128/jvi.76.8.3819-3827.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gandy SZ, Linnstaedt SD, Muralidhar S, Cashman KA, Rosenthal LJ, Casey JL. 2007. RNA editing of the human herpesvirus 8 kaposin transcript eliminates its transforming activity and is induced during lytic replication. J Virol 81:13544–13551. doi: 10.1128/JVI.01521-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liao JY, Thakur SA, Zalinger ZB, Gerrish KE, Imani F. 2011. Inosine-containing RNA is a novel innate immune recognition element and reduces RSV infection. PLoS One 6:e26463. doi: 10.1371/journal.pone.0026463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nie Y, Hammond GL, Yang J-H. 2007. Double-stranded RNA deaminase ADAR1 increases host susceptibility to virus infection. J Virol 81:917–923. doi: 10.1128/JVI.01527-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doria M, Neri F, Gallo A, Farace MG, Michienzi A. 2009. Editing of HIV-1 RNA by the double-stranded RNA deaminase ADAR1 stimulates viral infection. Nucleic Acids Res 37:5848–5858. doi: 10.1093/nar/gkp604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.George CX, Samuel CE. 1999. Human RNA-specific adenosine deaminase ADAR1 transcripts possess alternative exon 1 structures that initiate from different promoters, one constitutively active and the other interferon inducible. Proc Natl Acad Sci U S A 96:4621–4626. doi: 10.1073/pnas.96.8.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Toth AM, Li Z, Cattaneo R, Samuel CE. 2009. RNA-specific adenosine deaminase ADAR1 suppresses measles virus-induced apoptosis and activation of protein kinase PKR. J Biol Chem 284:29350–29356. doi: 10.1074/jbc.M109.045146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Orecchini E, Federico M, Doria M, Arenaccio C, Giuliani E, Ciafrè SA, Michienzi A. 2015. The ADAR1 editing enzyme is encapsidated into HIV-1 virions. Virology 485:475–480. doi: 10.1016/j.virol.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 78.Sarvestani ST, Tate MD, Moffat JM, Jacobi AM, Behlke MA, Miller AR, Beckham SA, McCoy CE, Chen W, Mintern JD, O’Keeffe M, John M, Williams BRG, Gantier MP. 2014. Inosine-mediated modulation of RNA sensing by Toll-like receptor 7 (TLR7) and TLR8. J Virol 88:799–810. doi: 10.1128/JVI.01571-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rizzetto M, Hoyer B, Canese MG, Shih JW, Purcell RH, Gerin JL. 1980. δ agent: association of δ antigen with hepatitis B surface antigen and RNA in serum of δ-infected chimpanzees. Proc Natl Acad Sci U S A 77:6124–6128. doi: 10.1073/pnas.77.10.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weiner AJ, Choo QL, Wang KS, Govindarajan S, Redeker AG, Gerin JL, Houghton M. 1988. A single antigenomic open reading frame of the hepatitis delta virus encodes the epitope(s) of both hepatitis delta antigen polypeptides p24 delta and p27 delta. J Virol 62:594–599. doi: 10.1128/JVI.62.2.594-599.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Polson AG, Bass BL, Casey JL. 1996. RNA editing of hepatitis delta virus antigenome by dsRNA-adenosine deaminase. Nature 381:346–346. doi: 10.1038/381346a0. [DOI] [PubMed] [Google Scholar]
- 82.Sato S, Wong SK, Lazinski DW. 2001. Hepatitis delta virus minimal substrates competent for editing by ADAR1 and ADAR2. J Virol 75:8547–8555. doi: 10.1128/jvi.75.18.8547-8555.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones JW, Robins RK. 1963. Purine nucleosides. III. Methylation studies of certain naturally occurring purine nucleosides. J Am Chem Soc 85:193–201. doi: 10.1021/ja00885a019. [DOI] [Google Scholar]
- 84.Hall RH. 1964. On the 2’-O-methylribonucleoside content of ribonucleic acids. Biochemistry 3:876–880. doi: 10.1021/bi00895a001. [DOI] [PubMed] [Google Scholar]
- 85.Werner M, Purta E, Kaminska KH, Cymerman IA, Campbell DA, Mittra B, Zamudio JR, Sturm NR, Jaworski J, Bujnicki JM. 2011. 2′-O-ribose methylation of cap2 in human: function and evolution in a horizontally mobile family. Nucleic Acids Res 39:4756–4768. doi: 10.1093/nar/gkr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Byszewska M, Mietański M, Purta E, Bujnicki JM. 2014. RNA methyltransferases involved in 5′ cap biosynthesis. RNA Biol 11:1597–1607. doi: 10.1080/15476286.2015.1004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smietanski M, Werner M, Purta E, Kaminska KH, Stepinski J, Darzynkiewicz E, Nowotny M, Bujnicki JM. 2014. Structural analysis of human 2′-O-ribose methyltransferases involved in mRNA cap structure formation. Nat Commun 5:3004. doi: 10.1038/ncomms4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Picard-Jean F, Brand C, Tremblay-Létourneau M, Allaire A, Beaudoin MC, Boudreault S, Duval C, Rainville-Sirois J, Robert F, Pelletier J, Geiss BJ, Bisaillon M. 2018. 2’-O-methylation of the mRNA cap protects RNAs from decapping and degradation by DXO. PLoS One 13:e0193804. doi: 10.1371/journal.pone.0193804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koonin EV, Moss B. 2010. Viruses know more than one way to don a cap. Proc Natl Acad Sci U S A 107:3283–3284. doi: 10.1073/pnas.0915061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Decroly E, Ferron F, Lescar J, Canard B. 2012. Conventional and unconventional mechanisms for capping viral mRNA. Nat Rev Microbiol 10:51–65. doi: 10.1038/nrmicro2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bamming D, Horvath CM. 2009. Regulation of signal transduction by enzymatically inactive antiviral RNA helicase proteins MDA5, RIG-I, and LGP2. J Biol Chem 284:9700–9712. doi: 10.1074/jbc.M807365200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Züst R, Cervantes-Barragan L, Habjan M, Maier R, Neuman BW, Ziebuhr J, Szretter KJ, Baker SC, Barchet W, Diamond MS, Siddell SG, Ludewig B, Thiel V. 2011. Ribose 2’-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat Immunol 12:137–143. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reikine S, Nguyen JB, Modis Y. 2014. Pattern recognition and signaling mechanisms of RIG-I and MDA5. Front Immunol 5:342. doi: 10.3389/fimmu.2014.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fang R, Jiang Q, Zhou X, Wang C, Guan Y, Tao J, Xi J, Feng JM, Jiang Z. 2017. MAVS activates TBK1 and IKKε through TRAFs in NEMO dependent and independent manner. PLoS Pathog 13:e1006720. doi: 10.1371/journal.ppat.1006720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jacobs JL, Coyne CB. 2013. Mechanisms of MAVS regulation at the mitochondrial membrane. J Mol Biol 425:5009–5019. doi: 10.1016/j.jmb.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dias Junior AG, Sampaio NG, Rehwinkel J. 2019. A balancing act: MDA5 in antiviral immunity and autoinflammation. Trends Microbiol 27:75–85. doi: 10.1016/j.tim.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brisse M, Ly H. 2019. Comparative structure and function analysis of the RIG-I-like receptors: RIG-I and MDA5. Front Immunol 10:1586. doi: 10.3389/fimmu.2019.01586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hyde JL, Diamond MS. 2015. Innate immune restriction and antagonism of viral RNA lacking 2’-O methylation. Virology 479–480:66–74. doi: 10.1016/j.virol.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Diamond MS. 2014. IFIT1: a dual sensor and effector molecule that detects non-2’-O methylated viral RNA and inhibits its translation. Cytokine Growth Factor Rev 25:543–550. doi: 10.1016/j.cytogfr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abbas YM, Laudenbach BT, Martínez-Montero S, Cencic R, Habjan M, Pichlmair A, Damha MJ, Pelletier J, Nagar B. 2017. Structure of human IFIT1 with capped RNA reveals adaptable mRNA binding and mechanisms for sensing N1 and N2 ribose 2′-O methylations. Proc Natl Acad Sci U S A 114:E2106–E2115. doi: 10.1073/pnas.1612444114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kumar P, Sweeney TR, Skabkin MA, Skabkina OV, Hellen CUT, Pestova TV. 2014. Inhibition of translation by IFIT family members is determined by their ability to interact selectively with the 5’-terminal regions of cap0-, cap1- and 5’ppp- mRNAs. Nucleic Acids Res 42:3228–3245. doi: 10.1093/nar/gkt1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diamond MS, Farzan M. 2013. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat Rev Immunol 13:46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ringeard M, Marchand V, Decroly E, Motorin Y, Bennasser Y. 2019. FTSJ3 is an RNA 2′-O-methyltransferase recruited by HIV to avoid innate immune sensing. Nature 565:500–504. doi: 10.1038/s41586-018-0841-4. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Y, Wei Y, Zhang X, Cai H, Niewiesk S, Li J. 2014. Rational design of human metapneumovirus live attenuated vaccine candidates by inhibiting viral mRNA cap methyltransferase. J Virol 88:11411–11429. doi: 10.1128/JVI.00876-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li S-H, Dong H, Li X-F, Xie X, Zhao H, Deng Y-Q, Wang X-Y, Ye Q, Zhu S-Y, Wang H-J, Zhang B, Leng Q-B, Zuest R, Qin E-D, Qin C-F, Shi P-Y. 2013. Rational design of a flavivirus vaccine by abolishing viral RNA 2’-O methylation. J Virol 87:5812–5819. doi: 10.1128/JVI.02806-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Menachery VD, Yount BL, Josset L, Gralinski LE, Scobey T, Agnihothram S, Katze MG, Baric RS. 2014. Attenuation and restoration of severe acute respiratory syndrome coronavirus mutant lacking 2’-O-methyltransferase activity. J Virol 88:4251–4264. doi: 10.1128/JVI.03571-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schwartz S, Bernstein DA, Mumbach MR, Jovanovic M, Herbst RH, León-Ricardo BX, Engreitz JM, Guttman M, Satija R, Lander ES, Fink G, Regev A. 2014. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell 159:148–162. doi: 10.1016/j.cell.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marceau CD, Puschnik AS, Majzoub K, Ooi YS, Brewer SM, Fuchs G, Swaminathan K, Mata MA, Elias JE, Sarnow P, Carette JE. 2016. Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature 535:159–163. doi: 10.1038/nature18631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M. 2008. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454:523–527. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McIntyre W, Netzband R, Bonenfant G, Biegel JM, Miller C, Fuchs G, Henderson E, Arra M, Canki M, Fabris D, Pager CT. 2018. Positive-sense RNA viruses reveal the complexity and dynamics of the cellular and viral epitranscriptomes during infection. Nucleic Acids Res 46:5776–5791. doi: 10.1093/nar/gky029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Safra M, Sas-Chen A, Nir R, Winkler R, Nachshon A, Bar-Yaacov D, Erlacher M, Rossmanith W, Stern-Ginossar N, Schwartz S. 2017. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature 551:251–255. doi: 10.1038/nature24456. [DOI] [PubMed] [Google Scholar]
- 112.Eckwahl MJ, Arnion H, Kharytonchyk S, Zang T, Bieniasz PD, Telesnitsky A, Wolin SL. 2016. Analysis of the human immunodeficiency virus-1 RNA packageome. RNA 22:1228–1238. doi: 10.1261/rna.057299.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. 2008. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 114.Limbach PA, Paulines MJ. 2017. Going global: the new era of mapping modifications in RNA. Wiley Interdiscip Rev RNA 8. doi: 10.1002/wrna.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lodish H. 2013. Molecular cell biology. W.H. Freeman and Co., New York, NY, USA. [Google Scholar]
- 116.Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. 2015. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods 12:767–772. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Edelheit S, Schwartz S, Mumbach MR, Wurtzel O, Sorek R. 2013. Transcriptome-wide mapping of 5-methylcytidine RNA modifications in bacteria, archaea, and yeast reveals m5C within archaeal mRNAs. PLoS Genet 9:e1003602. doi: 10.1371/journal.pgen.1003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Slama K, Galliot A, Weichmann F, Hertler J, Feederle R, Meister G, Helm M. 2019. Determination of enrichment factors for modified RNA in MeRIP experiments. Methods 156:102–109. doi: 10.1016/j.ymeth.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 119.Aschenbrenner J, Werner S, Marchand V, Adam M, Motorin Y, Helm M, Marx A. 2018. Engineering of a DNA polymerase for direct m 6 A sequencing. Angew Chem Int Ed Engl 57:417–421. doi: 10.1002/anie.201710209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garcia-Campos MA, Edelheit S, Toth U, Safra M, Shachar R, Viukov S, Winkler R, Nir R, Lasman L, Brandis A, Hanna JH, Rossmanith W, Schwartz S. 2019. Deciphering the “m6A code” via antibody-independent quantitative profiling. Cell 178:731–747.e16. doi: 10.1016/j.cell.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 121.Liu N, Parisien M, Dai Q, Zheng G, He C, Pan T. 2013. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA 19:1848–1856. doi: 10.1261/rna.041178.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meyer KD. 2019. DART-seq: an antibody-free method for global m6A detection. Nat Methods 16:1275–1280. doi: 10.1038/s41592-019-0570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li Y, Tollefsbol TO. 2011. DNA methylation detection: bisulfite genomic sequencing analysis. Methods Mol Biol 791:11–21. doi: 10.1007/978-1-61779-316-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gu W, Hurto RL, Hopper AK, Grayhack EJ, Phizicky EM. 2005. Depletion of Saccharomyces cerevisiae tRNA(His) guanylyltransferase Thg1p leads to uncharged tRNAHis with additional m(5)C. Mol Cell Biol 25:8191–8201. doi: 10.1128/MCB.25.18.8191-8201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sabban EL, Bhanot OS. 1982. The effects of bisulfite-induced C to U transitions on aminoacylation of Escherichia coli glycine tRNA. J Biol Chem 257:4796–4805. [PubMed] [Google Scholar]
- 126.Schaefer M, Pollex T, Hanna K, Lyko F. 2009. RNA cytosine methylation analysis by bisulfite sequencing. Nucleic Acids Res 37:e12. doi: 10.1093/nar/gkn954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Amort T, Rieder D, Wille A, Khokhlova-Cubberley D, Riml C, Trixl L, Jia XY, Micura R, Lusser A. 2017. Distinct 5-methylcytosine profiles in poly(A) RNA from mouse embryonic stem cells and brain. Genome Biol 18:1. doi: 10.1186/s13059-016-1139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khoddami V, Yerra A, Mosbruger TL, Fleming AM, Burrows CJ, Cairns BR. 2019. Transcriptome-wide profiling of multiple RNA modifications simultaneously at single-base resolution. Proc Natl Acad Sci U S A 116:6784–6789. doi: 10.1073/pnas.1817334116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen K, Lu Z, Wang X, Fu Y, Luo GZ, Liu N, Han D, Dominissini D, Dai Q, Pan T, He C. 2015. High-resolution N6-methyladenosine (m6A) map using photo-crosslinking-assisted m6A sequencing. Angew Chem Int Ed Engl 54:1587–1590. doi: 10.1002/anie.201410647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hartstock K, Nilges BS, Ovcharenko A, Cornelissen NV, Püllen N, Lawrence-Dörner AM, Leidel SA, Rentmeister A. 2018. Enzymatic or in vivo installation of propargyl groups in combination with click chemistry for the enrichment and detection of methyltransferase target sites in RNA. Angew Chem Int Ed Engl 57:6342–6346. doi: 10.1002/anie.201800188. [DOI] [PubMed] [Google Scholar]
- 131.Khoddami V, Cairns BR. 2013. Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nat Biotechnol 31:458–464. doi: 10.1038/nbt.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hussain S, Sajini AA, Blanco S, Dietmann S, Lombard P, Sugimoto Y, Paramor M, Gleeson JG, Odom DT, Ule J, Frye M. 2013. NSUN2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep 4:255–261. doi: 10.1016/j.celrep.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Levanon EY, Eisenberg E, Yelin R, Nemzer S, Hallegger M, Shemesh R, Fligelman ZY, Shoshan A, Pollock SR, Sztybel D, Olshansky M, Rechavi G, Jantsch MF. 2004. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat Biotechnol 22:1001–1005. doi: 10.1038/nbt996. [DOI] [PubMed] [Google Scholar]
- 134.Kleinman CL, Majewski J. 2012. Comment on “Widespread RNA and DNA Sequence Differences in the Human Transcriptome. Science 335:1302. doi: 10.1126/science.1209658. [DOI] [PubMed] [Google Scholar]
- 135.Pickrell JK, Gilad Y, Pritchard JK. 2012. Comment on “Widespread RNA and DNA Sequence Differences in the Human Transcriptome. Science 335:1302. doi: 10.1126/science.1210484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sakurai M, Yano T, Kawabata H, Ueda H, Suzuki T. 2010. Inosine cyanoethylation identifies A-to-I RNA editing sites in the human transcriptome. Nat Chem Biol 6:733–740. doi: 10.1038/nchembio.434. [DOI] [PubMed] [Google Scholar]
- 137.Sakurai M, Ueda H, Yano T, Okada S, Terajima H, Mitsuyama T, Toyoda A, Fujiyama A, Kawabata H, Suzuki T. 2014. A biochemical landscape of A-to-I RNA editing in the human brain transcriptome. Genome Res 24:522–534. doi: 10.1101/gr.162537.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang J, Alvin Chew BL, Lai Y, Dong H, Xu L, Balamkundu S, Cai WM, Cui L, Liu CF, Fu XY, Lin Z, Shi PY, Lu TK, Luo D, Jaffrey SR, Dedon PC. 2019. Quantifying the RNA cap epitranscriptome reveals novel caps in cellular and viral RNA. Nucleic Acids Res 47:e130. doi: 10.1093/nar/gkz751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Birkedal U, Christensen-Dalsgaard M, Krogh N, Sabarinathan R, Gorodkin J, Nielsen H. 2015. Profiling of ribose methylations in RNA by high-throughput sequencing. Angew Chem Int Ed Engl 54:451–455. doi: 10.1002/anie.201408362. [DOI] [PubMed] [Google Scholar]
- 140.Marchand V, Blanloeil-Oillo F, Helm M, Motorin Y. 2016. Illumina-based RiboMethSeq approach for mapping of 2′-O-Me residues in RNA. Nucleic Acids Res 44:e135–e135. doi: 10.1093/nar/gkw547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhu Y, Pirnie SP, Carmichael GG. 2017. High-throughput and site-specific identification of 2′-O-methylation sites using ribose oxidation sequencing (RibOxi-seq). RNA 23:1303–1314. doi: 10.1261/rna.061549.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dai Q, Moshitch-Moshkovitz S, Han D, Kol N, Amariglio N, Rechavi G, Dominissini D, He C. 2017. Nm-seq maps 2′-O-methylation sites in human mRNA with base precision. Nat Methods 14:695–698. doi: 10.1038/nmeth.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dai Q, Moshitch-Moshkovitz S, Han D, Kol N, Amariglio N, Rechavi G, Dominissini D, He C. 2018. Correction: corrigendum: Nm-seq maps 2′-O-methylation sites in human mRNA with base precision. Nat Methods 15:226–227. doi: 10.1038/nmeth0318-226c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Keller MW, Rambo-Martin BL, Wilson MM, Ridenour CA, Shepard SS, Stark TJ, Neuhaus EB, Dugan VG, Wentworth DE, Barnes JR. 2018. Author correction: direct RNA sequencing of the coding complete influenza A virus genome. Sci Rep 8:15746. doi: 10.1038/s41598-018-34067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Garalde DR, Snell EA, Jachimowicz D, Sipos B, Lloyd JH, Bruce M, Pantic N, Admassu T, James P, Warland A, Jordan M, Ciccone J, Serra S, Keenan J, Martin S, McNeill L, Wallace EJ, Jayasinghe L, Wright C, Blasco J, Young S, Brocklebank D, Juul S, Clarke J, Heron AJ, Turner DJ. 2018. Highly parallel direct RN A sequencing on an array of nanopores. Nat Methods 15:201–206. doi: 10.1038/nmeth.4577. [DOI] [PubMed] [Google Scholar]
- 146.Liu H, Begik O, Lucas MC, Ramirez JM, Mason CE, Wiener D, Schwartz S, Mattick JS, Smith MA, Novoa EM. 2019. Accurate detection of m6A RNA modifications in native RNA sequences. Nat Commun 10:4079. doi: 10.1038/s41467-019-11713-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leger A, Amaral PP, Pandolfini L, Capitanchik C, Capraro F, Barbieri I, Migliori V, Luscombe NM, Enright AJ, Tzelepis K, Ule J, Fitzgerald T, Birney E, Leonardi T, Kouzarides T. 2019. RNA modifications detection by comparative Nanopore direct RNA sequencing. bioRxiv 843136. doi: 10.1101/843136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hudeček O, Benoni R, Reyes-Gutierrez PE, Culka M, Šanderová H, Hubálek M, Rulíšek L, Cvačka J, Krásný L, Cahová H. 2020. Dinucleoside polyphosphates act as 5′-RNA caps in bacteria. Nat Commun 11:1052. doi: 10.1038/s41467-020-14896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Parrish S, Hurchalla M, Liu SW, Moss B. 2009. The African swine fever virus g5R protein possesses mRNA decapping activity. Virology 393:177–182. doi: 10.1016/j.virol.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Parrish S, Resch W, Moss B. 2007. Vaccinia virus D10 protein has mRNA decapping activity, providing a mechanism for control of host and viral gene expression. Proc Natl Acad Sci U S A 104:2139–2144. doi: 10.1073/pnas.0611685104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Courtney DG, Kennedy EM, Dumm RE, Bogerd HP, Tsai K, Heaton NS, Cullen BR. 2017. Epitranscriptomic enhancement of influenza A virus gene expression and replication. Cell Host Microbe 22:377–386. doi: 10.1016/j.chom.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tsai K, Courtney DG, Cullen BR. 2018. Addition of m6A to SV40 late mRNAs enhances viral structural gene expression and replication. PLoS Pathog 14:e1006919. doi: 10.1371/journal.ppat.1006919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hao H, Hao S, Chen H, Chen Z, Zhang Y, Wang J, Wang H, Zhang B, Qiu J, Deng F, Guan W. 2019. N 6 -methyladenosine modification and METTL3 modulate enterovirus 71 replication. Nucleic Acids Res 47:362–374. doi: 10.1093/nar/gky1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Beemon K, Hunter T. 1977. In vitro translation yields a possible Rous sarcoma virus src gene product. Proc Natl Acad Sci U S A 74:3302–3306. doi: 10.1073/pnas.74.8.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kane SE, Beemon K. 1985. Precise localization of m6A in Rous sarcoma virus RNA reveals clustering of methylation sites: implications for RNA processing. Mol Cell Biol 5:2298–2306. doi: 10.1128/mcb.5.9.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stoltzfus CM, Dane RW. 1982. Accumulation of spliced avian retrovirus mRNA is inhibited in S-adenosylmethionine-depleted chicken embryo fibroblasts. J Virol 42:918–931. doi: 10.1128/JVI.42.3.918-931.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, Rothballer A, Ascano M, Jungkamp AC, Munschauer M, Ulrich A, Wardle GS, Dewell S, Zavolan M, Tuschl T. 2010. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141:129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Krug RM, Morgan MA, Shatkin AJ. 1976. Influenza viral mRNA contains internal N6-methyladenosine and 5’-terminal 7-methylguanosine in cap structures. J Virol 20:45–53. doi: 10.1128/JVI.20.1.45-53.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Narayan P, Ayers DF, Rottman FM, Maroney PA, Nilsen TW. 1987. Unequal distribution of N6-methyladenosine in influenza virus mRNAs. Mol Cell Biol 7:1572–1575. doi: 10.1128/mcb.7.4.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Phuphuakrat A, Kraiwong R, Boonarkart C, Lauhakirti D, Lee T-H, Auewarakul P. 2008. Double-stranded RNA adenosine deaminases enhance expression of human immunodeficiency virus type 1 proteins. J Virol 82:10864–10872. doi: 10.1128/JVI.00238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Khrustalev VV, Khrustaleva TA, Sharma N, Giri R. 2017. Mutational pressure in Zika virus: local ADAR-editing areas associated with pauses in translation and replication. Front Cell Infect Microbiol 7:44. doi: 10.3389/fcimb.2017.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Piontkivska H, Frederick M, Miyamoto MM, Wayne ML. 2017. RNA editing by the host ADAR system affects the molecular evolution of the Zika virus. Ecol Evol 7:4475–4485. doi: 10.1002/ece3.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Casey JL. 2006. RNA editing in hepatitis delta virus. Curr Top Microbiol Immunol 307:67–89. doi: 10.1007/3-540-29802-9_4. [DOI] [PubMed] [Google Scholar]
- 164.Cattaneo R, Schmid A, Eschle D, Baczko K, ter Meulen V, Billeter MA. 1988. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell 55:255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pfaller CK, Mastorakos GM, Matchett WE, Ma X, Samuel CE, Cattaneo R. 2015. Measles virus defective interfering RNAs are generated frequently and early in the absence of C protein and can be destabilized by adenosine deaminase acting on RNA-1-like hypermutations. J Virol 89:7735–7747. doi: 10.1128/JVI.01017-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dong H, Chang DC, Hua MHC, Lim SP, Chionh YH, Hia F, Lee YH, Kukkaro P, Lok S-M, Dedon PC, Shi P-Y. 2012. 2′-O methylation of internal adenosine by flavivirus NS5 methyltransferase. PLoS Pathog 8:e1002642. doi: 10.1371/journal.ppat.1002642. [DOI] [PMC free article] [PubMed] [Google Scholar]