Abstract
Aims
Choosing the optimal palliative lung radiotherapy regimen is challenging. Guidance from The Royal College of Radiologists recommends treatment stratification based on performance status, but evidence suggests that higher radiotherapy doses may be associated with survival benefits. The aim of this study was to investigate the effects of fractionation regimen and additional factors on the survival of palliative lung cancer radiotherapy patients.
Materials and methods
A retrospective univariable (n = 925) and multivariable (n = 422) survival analysis of the prognostic significance of baseline patient characteristics and treatment prescription was carried out on patients with non-small cell and small cell lung cancer treated with palliative lung radiotherapy. The covariates investigated included: gender, age, performance status, histology, comorbidities, stage, tumour location, tumour side, smoking status, pack year history, primary radiotherapy technique and fractionation scheme. The overall mortality rate at 30 and 90 days of treatment was calculated.
Results
Univariable analysis revealed that performance status (P < 0.001), fractionation scheme (P < 0.001), comorbidities (P = 0.02), small cell histology (P = 0.02), ‘lifelong never’ smoking status (P = 0.01) and gender (P = 0.06) were associated with survival. Upon multivariable analysis, only better performance status (P = 0.01) and increased dose/fractionation regimens of up to 30 Gy/10 fractions (P < 0.001) were associated with increased survival. Eighty-five (9.2%) and 316 patients (34%) died within 30 and 90 days of treatment, respectively.
Conclusion
In this retrospective single-centre analysis of palliative lung radiotherapy, increased total dose (up to and including 30 Gy/10 fractions) was associated with better survival regardless of performance status.
Key words: Lung cancer, outcomes research, palliative care, radiotherapy
Highlights
-
•
Larger doses of palliative lung radiotherapy are associated with increased survival.
-
•
Performance status is independently linked to survival.
-
•
Palliative lung radiotherapy dose is independently linked to survival.
Introduction
Lung cancer is the malignancy with the highest incidence worldwide and the leading cause of cancer death [1]. In the UK, lung cancer accounts for 13% of new cancer cases and 22% of cancer deaths. Eighty-five per cent of lung cancer cases are non-small cell lung cancer (NSCLC), with most of the remainder being small cell lung cancer (SCLC) (13%). One-year survival in England and Wales ranges from 82% for patients with stage I NSCLC to 16% for patients with stage IV NSCLC [2]. At presentation, 57% of patients are not candidates for curative therapy due to tumour volume, presence of metastases, patient fitness and/or comorbidities [3]. An increasing number of patients are receiving immunotherapy (sometimes in combination with chemotherapy) or tyrosine kinase inhibitors, which have been shown to improve survival [4,5]. Across hospitals in England in the 2017–2018 financial year, 58% of all radiotherapy courses for lung cancer had palliative intent [6].
The primary treatment of patients with advanced lung cancer is systemic therapy (including chemotherapy, immunotherapy and targeted agents). However, palliative radiotherapy still has a role for those who are unresponsive to systemic therapy, those who relapse and those who have contraindications to, or are not fit for, systemic therapy [7]. Palliative radiotherapy is also often used to manage local symptoms [8,9]. These symptoms are often linked to local tumour effects, such as haemoptysis, chest pain, dyspnoea, cough, dysphagia and superior vena cava compression [10]. Palliative radiotherapy is intended to alleviate the aforementioned symptoms and improve quality of life. In a 2008 systematic review of palliative radiotherapy for lung cancer, improvement in total symptom score was reported in 65.4–77.1% of patients depending on the dose of radiotherapy administered [11].
The dose-fractionation schedule is selected when palliative radiotherapy is recommended to a patient. A balance between successful palliation of the symptoms, fitness of the patient, toxicity and convenience is sought in collaboration with the patient [10]. Toxicities of palliative radiotherapy may include: fatigue, dysphagia, odynophagia, dyspnoea, cough, skin erythema and, rarely, radiation myelopathy [10].
The choice of radiotherapy dose and fractionation scheme in the palliative setting is challenging because there is conflicting evidence regarding the optimal fractionation scheme in order to achieve palliation of symptoms and possibly improve survival. A 2015 meta-analysis found that when the patients were stratified by performance status no significant difference was found in 1-year overall survival [10]. More recently, two studies reported that higher fractionation schemes were associated with increased survival [12,13]. Fractionation schemes utilised varied from 10 Gy/one fraction up to doses more typically associated with the curative intent setting, such as 60 Gy/30 fractions [10]. Current Royal College of Radiologists (RCR) and American Society for Radiation Oncology (ASTRO) guidance suggest the use of palliative regimens with doses up to 39 Gy/13 fractions and 42 Gy/14 fractions, respectively, for patients with NSCLC [14,15]. Longer fractionation schemes can inconvenience patients with multiple hospital visits towards the end of their lives and also have healthcare resource implications.
The time taken for palliative radiotherapy to reach effect has been shown to occur at 5–7 weeks, with palliation occurring 2 weeks earlier in the 16 Gy/two fraction arm compared with the 30 Gy/three fraction arm [16]. Peak palliation occurs at 8–9 weeks. Frank et al. [17] defined radiotherapy as futile if the patient dies less than 30 days after treatment, as the patient has not yet benefitted fully from the treatment but has still been exposed to the risks of radiotherapy-related acute toxicity [17]. This issue has been debated at RCR forums and a consensus agreed that there should be a target of under 20% of patients that die within 30 days of palliative radiotherapy [18]. Patients with an acute presentation of symptoms, such as superior vena cava obstruction, often also have a short life expectancy and are incorporated into this figure.
There are predictive factors that have been investigated to guide the treatment decisions and give prognostic information in the context of palliative radiotherapy. From the literature, the following factors have been found to be significantly correlated with survival during multivariable analysis: T and N status, extrathoracic disease status, lactate dehydrogenase levels, completion of planned treatment, leukocyte count and C-reactive protein levels [10,12,13,17,19,20]. These factors have not been consistently examined through the literature and when included they are not always reproducibly significant and as such they are not incorporated in commonly used guidelines [14,15,21].
The aim of our study was to retrospectively analyse predictive factors for survival in palliative radiotherapy in lung cancer.
Materials and Methods
Cohort Selection
Patients treated for lung tumours with palliative radiotherapy between 1 January 2013 and 8 May 2018 were identified from the UK Computer-Aided Theragnostics (ukCAT) database. The ukCAT database contains the anonymised electronic patient records from a single large cancer centre and was established to model clinical outcomes. Consent is on an opt-out basis (REC reference 17/NW/0060). For this study, consent for patient data access was granted by the ukCAT database management committee (reference: 2017–008). Data from this study were part of a clinical audit (reference: SE18/2221). All research was carried out in accordance with the Declaration of Helsinki. Further details are given in the Supplementary Material.
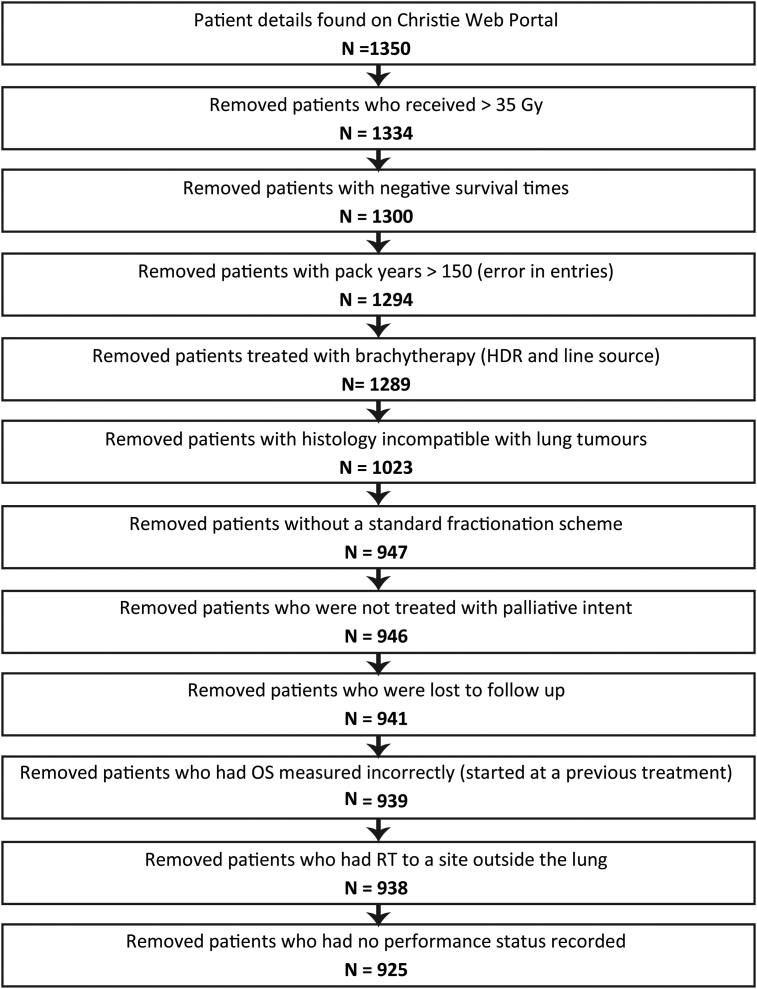
Detailed patient and tumour characteristics are collected prospectively at the time of the first appointment in our institution. All patients included within the analysis had confirmed histology consistent with lung cancer (see Figure 1). The data specification included the following items: treatment intent and lung or mediastinal cancer. Local guidance recommends the following fractionation schemes: 30 Gy/10 fractions, 20 Gy/five fractions or 10 Gy/one fraction. Therefore, 30 Gy/10 fractions was the highest fractionation scheme included in this study.
Fig 1.
A flowchart to show the selection of the dataset utilised.
Patients were staged with the IASLC seventh edition for TNM staging [22]. The comorbidity score was an overall score calculated with the Adult Comorbidity Evaluation 27 tool [23].
Primary Technique for Radiotherapy Delivery
The primary techniques for radiotherapy delivery were grouped into four larger groups that were deemed to be sufficiently similar. Parallel pair, two field or tangent pair (n = 890); single field (n = 28); three or more fields (n = 5); and all the intensity-modulated radiotherapy techniques were grouped together (n = 2). Brachytherapy was excluded.
Statistical Analysis
A combined NSCLC and SCLC patient cohort (n = 925) and NSCLC-only patient cohort (n = 664) were analysed.
Overall survival was measured from the date of the first fraction. Patients who had not died by 8 May 2018 were considered to be right-censored and were excluded from the analysis. The percentages of patients who died within 30 and 90 days of receiving radiotherapy were calculated.
A univariable and multivariable survival analysis was conducted using the Cox proportional hazards model. The multivariable model was built using a complete case (no missing variable data) analysis (n = 422). P-values and hazard ratios with 95% confidence intervals were reported. Survival curves were plotted using the Kaplan–Meier method and differences between survival curves were assessed using the Log-rank test. A SCLC patient cohort was not analysed separately, as the number of complete cases was deemed to be insufficient (n = 95).
Due to there being a low number of patients with performance status 0 and 4, performance status was grouped as follows: good (0–1), mid (2) and poor (3–4), for the survival analysis. Due to there being a low number of patients treated with 8 Gy/one fraction, they were grouped with the patients treated with 10 Gy/one fraction for the survival analysis. The software used for statistical analysis was R® Version 3.5.1.
Results
Patient Characteristics
In total, 925 patients with NSCLC and SCLC remained in the cohort for analysis after filtering the originally extracted patient data. Figure 1 shows how the initial patient data downloaded from the electronic patient records system was refined in order to provide a more complete and comparable dataset. Any outlying data were checked manually. There were 816 events within the cohort; 109 patients were censored. The median overall survival was 129 days (95% confidence interval 120–138).
Table 1 summarises the main patient, tumour and treatment characteristics. The gender distribution of the patients was 55:45 male to female. The most common performance status was 2 (35%). In total, 545 of 925 (76%) patients had stage IV disease; 261 of 925 (28%) patients were treated for SCLC and 664 of 925 (72%) patients were treated for NSCLC. Of the patients with NSCLC, most (97%) had either squamous cell carcinoma or adenocarcinoma. As expected, the patients with a high performance status had a high comorbidity score.
Table 1.
Baseline patient characteristics of this large cancer centre's cohort
| Covariate | Number of patients (% proportion of patients with data present) |
|---|---|
| Gender | |
| Male | 510 (55%) |
| Female | 415 (45%) |
| Age | |
| Range | 36–93 |
| Interquartile range | 14 |
| Mean | 69 |
| ECOG performance status | |
| 0 | 65 (7%) |
| 1 | 315 (34%) |
| 2 | 325 (35%) |
| 3 | 213 (23%) |
| 4 | 7 (1%) |
| Good (0+1) | 380 (41%) |
| Mid (2) | 325 (35%) |
| Poor (3+4) | 220 (24%) |
| Histology | |
| Small cell lung cancer | 261 (28%) |
| Non-small cell lung cancer | 664 (72%) |
| Non-small cell lung cancer subgroups | |
| Adenocarcinoma | 323 (35%) |
| Adenosquamous cell carcinoma | 7 (1%) |
| Large cell carcinoma | 14 (1%) |
| Squamous cell carcinoma | 320 (35%) |
| Comorbidities | |
| 0 | 221 (28%) |
| 1 | 257 (33%) |
| 2 | 202 (26%) |
| 3 | 96 (12%) |
| N/A | 149 |
| TNM stage | |
| 2a | 9 (1%) |
| 2b | 11 (2%) |
| 3a | 61 (8%) |
| 3b | 95 (13%) |
| 4 | 545 (76%) |
| N/A | 204 |
| Tumour location | |
| Lung, upper lobe | 533 (65%) |
| Lung, middle lobe | 60 (7%) |
| Lung, lower lobe | 230 (28%) |
| Lung, not otherwise specified (N/A) | 102 |
| Tumour side | |
| Left | 301 (40%) |
| Right | 462 (60%) |
| N/A | 162 |
| Smoking | |
| Lifelong never | 27 (3%) |
| Light former | 7 (1%) |
| Ex-smoker | 457 (60%) |
| Current smoker | 272 (36%) |
| N/A | 159 |
| Pack years | |
| Range | 0–150 |
| Interquartile range | 20 |
| Mean | 42 |
| N/A | 234 |
| Fractionation scheme | |
| 8 Gy/1 fraction | 10 (1%) |
| 10 Gy/1 fraction | 97 (10%) |
| 20 Gy/5 fractions | 267 (29%) |
| 30 Gy/10 fractions | 551 (60%) |
| 8 Gy + 10 Gy/1 fraction | 107 (12%) |
| Primary radiotherapy technique | |
| Intensity-modulated radiotherapy | 2 (0%) |
| Parallel pair and two field | 890 (96%) |
| Single field | 28 (3%) |
| Three+ field | 5 (1%) |
The most frequently used fractionation scheme was 30 Gy/10 fractions, with 551/925 (60%) patients being prescribed this regimen.
Death Within 30 and 90 Days of Treatment
Eighty-five patients (9%) in the combined NSCLC and SCLC cohort died within 30 days of treatment. Three hundred and sixteen patients (34%) of the combined NSCLC and SCLC cohort died within 90 days of treatment. Seventy-two patients (11%) with NSCLC died within 30 days of treatment. Two hundred and forty-five patients (37%) with NSCLC died within 90 days of treatment.
Univariable Survival Analysis
The univariable analysis, see Table 2, highlighted six covariates: performance status, fractionation scheme, comorbidities, small cell histology, gender and ‘lifelong never’ smoking status that were associated with patient survival.
Table 2.
Univariable and multivariable survival analysis
| Univariable survival analysis | Multivariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| NSCLC + SCLC cohort | NSCLC + SCLC cohort | NSCLC-only cohort | |||||
| N = 422, E = 367 | N = 327, E = 284 | ||||||
| Covariat | N (E) | Hazard ratio (95% confidence interval) | P-value | Hazard ratio (95% confidence interval) | P-value | Hazard ratio (95% confidence interval) | P-value |
| Sex | |||||||
| Female (reference) | 925 (816) | 1 | 1 | 1 | |||
| versus male | 1.14 (0.99–1.31) | 0.06 | 1.03 (0.83–1.27) | 0.82 | 0.95 (0.74–1.22) | 0.67 | |
| Age | 925 (816) | 1.00 (1.00–1.01) | 0.35 | 1.00 (0.99–1.01) | 0.85 | 0.99 (0.98–1.01) | 0.48 |
| Performance status | |||||||
| Good (0–1) versus mid (2) versus poor (3–4) | 925 (816) | 1.32 (1.21–1.45) | <0.001 | 1.22 (1.05–1.42) | 0.01 | 1.25 (1.05–1.49) | 0.01 |
| Histology | |||||||
| Adenocarcinoma (reference) | 925 (816) | 1 | 1 | 1 | |||
| versus adenosquamous | 1.25 (0.59–2.65) | 0.56 | 1.51 (0.55–4.17) | 0.42 | 1.54 (0.56–4.26) | 0.41 | |
| versus large cell | 0.68 (0.36–1.28) | 0.24 | 0.91 (0.42–2.01) | 0.82 | 0.84 (0.38–1.87) | 0.67 | |
| versus squamous cell | 1.00 (0.85–1.18) | 0.98 | 1.06 (0.83–1.36) | 0.62 | 1.08 (0.84–1.39) | 0.56 | |
| versus small cell | 0.80 (0.68–0.96) | 0.02 | 0.76 (0.56–1.01) | 0.06 | NA | NA | |
| Comorbidities | |||||||
| 0 versus 1 versus 2 versus 3 | 776 (673) | 1.09 (1.02–1.18) | 0.02 | 1.06 (0.94–1.18) | 0.34 | 1.08 (0.94–1.23) | 0.27 |
| Combined TNM stage | |||||||
| IV (reference) | 721 (642) | 1 | 1 | 1 | |||
| versus III | 0.89 (0.74–1.08) | 0.23 | 0.98 (0.75–1.28) | 0.87 | 1.05 (0.76–1.43) | 0.78 | |
| versus I+II | 1.03 (0.63–1.67) | 0.92 | 0.74 (0.38–1.44) | 0.38 | 0.70 (0.33–1.49) | 0.36 | |
| Tumour location | |||||||
| Lower lobe (reference) | 823 (720) | 1 | 1 | 1 | |||
| versus middle lobe | 0.87 (0.64–1.17) | 0.35 | 1.83 (0.79–4.23) | 0.16 | 1.37 (0.49–3.79) | 0.55 | |
| versus upper lobe | 0.93 (0.80–1.11) | 0.46 | 0.90 (0.71–1.14) | 0.36 | 0.87 (0.67–1.14) | 0.32 | |
| Tumour side | |||||||
| Left (reference) | 763 (669) | 1 | 1 | 1 | |||
| versus right | 0.99 (0.85–1.15) | 0.88 | 1.04 (0.83–1.29) | 0.75 | 0.98 (0.76–1.25) | 0.85 | |
| Smoking status | |||||||
| Current smoker (reference) | 766 (663) | 1 | 1 | 1 | |||
| versus light former | 0.88 (0.36–2.14) | 0.78 | 0.42 (0.13–1.39) | 0.16 | 0.43 (0.13–1.43) | 0.17 | |
| versus ex-smoker | 0.92 (0.78–1.08) | 0.31 | 0.95 (0.75–1.21) | 0.69 | 0.98 (0.74–1.29) | 0.88 | |
| versus lifelong never | 0.57 (0.37–0.89) | 0.01 | NA∗ | NA∗ | NA∗ | NA∗ | |
| Pack years | 691 (598) | 1.00 (1.00–1.01) | 0.4 | 1.00 (0.99–1.00) | 0.59 | 1.00 (0.99–1.00) | 0.30 |
| Fractionation scheme | |||||||
| 30 Gy/10F versus 20 Gy/5F versus 8 + 10 Gy/1F | 925 (816) | 1.73 (1.56–1.91) | <0.001 | 1.48 (1.23–1.77) | <0.001 | 1.54 (1.25–1.89) | <0.001 |
| Primary radiotherapy technique | |||||||
| IMRT (reference) | 925 (816) | 1 | 1 | ||||
| versus parallel pair and two field | 2.03 (0.29–14.41) | 0.48 | 2.03 (0.28–14.86) | 0.49 | 2.30 (0.31–17.05) | 0.42 | |
| versus single field | 2.13 (0.29–15.74) | 0.46 | 2.03 (0.25–16.50) | 0.51 | 2.23 (0.27–18.30) | 0.46 | |
| versus 3+ field | 1.64 (0.19–14.06) | 0.65 | 1.54 (0.16–15.15) | 0.71 | 1.83 (0.18–18.15) | 0.61 | |
IMRT, intensity-modulated radiotherapy; N, number of patients; E, number of events.
There were no lifelong never smokers when performing a complete case analysis.
Univariable Subset Survival Analysis
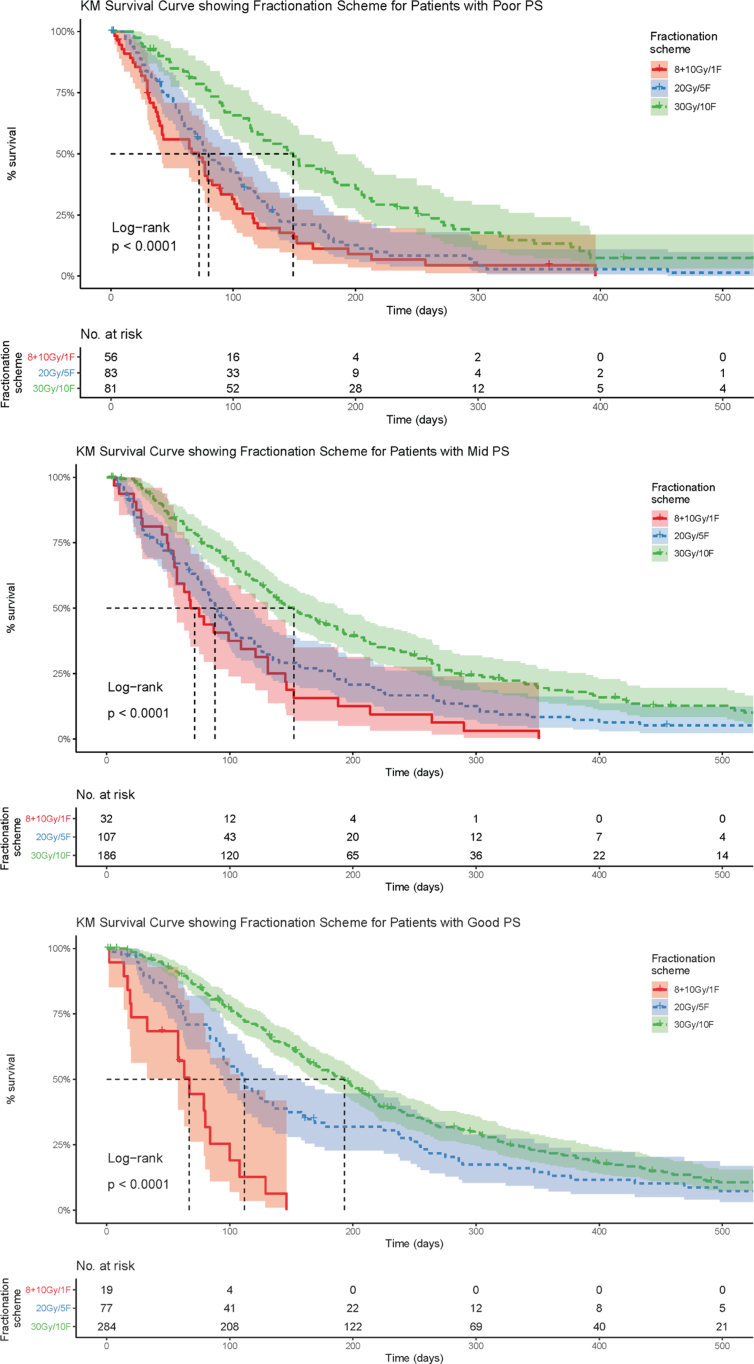
When the patients were subdivided into good, mid and poor performance status the fractionation scheme was still found to be a predictor of patient survival. The 30 Gy/10 fractions scheme showed a clear survival advantage in each performance status subset (see Figure 2). This correlation of increased survival with increased fractionation persisted when SCLC patients were removed from the dataset for patients with good, mid and poor performance status (see Table 2, Table 3).
Fig 2.
Univariable subset analysis Kaplan–Meier survival curves examining varying fractionation schemes and the correlating overall survival when all patients were divided by performance status strata in the combined non-small cell lung cancer/small cell lung cancer patient cohort.
Table 3.
Univariable subset survival analysis results examining varying fractionation schemes and the correlating overall survival when all patients were divided by performance status strata in both the combined non-small cell lung cancer/small cell lung cancer (NSCLC/SCLC) patient cohort and the NSCLC patient-only cohort
| Fractionation scheme 8 + 10 Gy/1 fraction | Fractionation scheme 20 Gy/5 fractions | Fractionation scheme 30 Gy/10 fractions | |
|---|---|---|---|
| Good performance status (0–1) | Median overall survival 67 days | Median overall survival 112 days | Median overall survival 193 days |
| Combined NSCLC and SCLC cohorts | (n = 19, 95% confidence interval 58–108) | (n = 77, 95% confidence interval 95–158) | (n = 284, 95% confidence interval 170–213) |
| Mid performance status (2) | Median overall survival 71.5 days | Median overall survival 88 days | Median overall survival 152 days |
| Combined NSCLC and SCLC cohorts | (n = 32, 95% confidence interval 57–131) | (n = 107, 95% confidence interval 78–109) | (n = 186, 95% confidence interval 131–187) |
| Poor performance status (3–4) | Median overall survival 72 days | Median overall survival 80 days | Median overall survival 149 days |
| Combined NSCLC and SCLC cohorts | (n = 56, 95% confidence interval 40–90) | (n = 83, 95% confidence interval 66–107) | (n = 81, 95% confidence interval 115–185) |
| Good performance status (0–1) | Median overall survival 67 days | Median overall survival 106 days | Median overall survival 185 days |
| NSCLC cohort only | (n = 17, 95% confidence interval 58–129) | (n = 57, 95% confidence interval 92–137) | (n = 189, 95% confidence interval 162–207) |
| Mid performance status (2) | Median overall survival 67 days | Median overall survival 84 days | Median overall survival 139 days |
| NSCLC cohort only | (n = 29, 95% confidence interval 55–131) | (n = 88, 95% confidence interval 67–102) | (n = 130, 95% confidence interval 118–187) |
| Poor performance status (3–4) | Median overall survival 64 days | Median overall survival 75 days | Median overall survival 139 days |
| NSCLC cohort only | (n = 42, 95% confidence interval 38–103) | (n = 58, 95% confidence interval 56–108) | (n = 54, 95% confidence interval 100–185) |
Multivariable Survival Analysis
The multivariable analysis highlighted that only fractionation scheme and performance status were predictors of patient survival (see Table 2).
The lack of interaction between fractionation scheme and performance status via Kaplan–Meier survival plots discussed above was further assessed in a multivariable analysis including only these two variables (n = 925). No interaction was found (interaction hazard ratio = 0.97, confidence interval = 0.85–1.10, P = 0.60) and both performance status and fractionation scheme retained independent effects on overall survival (performance status hazard ratio = 1.20; confidence interval = 1.07–1.35; P = 0.002 and fractionation scheme hazard ratio = 1.70; confidence interval = 1.42–2.02; P < 0.001).
Discussion
In this single-centre retrospective analysis of palliative lung radiotherapy, performance status and fractionation scheme were the only covariates shown to have a significant correlation with patient survival on multivariable analysis. Performance status was correlated with overall survival in a predictable manner: those with a good performance status out-survived those with a mid performance status, who out-survived those with a poor performance status.
In this cohort, when examining both NSCLC and SCLC together, every increase in fractionation regimen through all performance status strata resulted in an increased median overall survival. The difference in median overall survival between receiving 10 Gy/one fraction and 30 Gy/10 fractions in patients with a good, mid and poor performance status was 126, 80.5 and 77 days, respectively. The cohort of NSCLC patients was also examined in isolation to ensure that there was not a confounding effect from the SCLC patients. The results were similar, with every increase in fractionation regimen through all performance status strata resulting in an increased median overall survival. It should be noted that to date all published prospective studies comparing different palliative thoracic radiotherapy fractionation schemes have been carried out in the NSCLC setting.
Performance Status
The finding that performance status is significantly correlated with survival is in concordance with other survival analyses [12,15,17]. There has been less clarity as to the optimal fractionation scheme in order to increase survival in palliative lung radiotherapy.
Radiotherapy Fractionation and Overall Survival
Janssen et al. [13] reported in a retrospective analysis of 125 patients that increasing equivalent dose in 2 Gy fractions (EQD2) led to significantly better survival outcomes. These patients had stage III and IV lung cancer, including both NSCLC and SCLC. EQD2 of 31–40, 41–46 and 47–52 Gy led to 6-month overall survival of 30, 38 and 57%, respectively, and 1-year overall survival of 11, 26 and 36%, respectively [13]. On multivariable analysis, EQD2 was significant, although the confidence intervals were wide (n = 125, relative risk = 1.43, confidence interval = 1.06–1.94, P = 0.018) [13]. The doses of radiotherapy were higher compared with those used in this study. It should be noted that 29% of the patients could not complete their full fractionation course due to acute toxicity [13].
Nieder et al. [12] found that lower dose/fractionation regimens (17 Gy/two fractions and 20–24 Gy/five to six fractions) were significantly associated with lower overall survival on multivariable analysis when compared with regimens with an EQD2 of 45Gy. Unlike our study, when Nieder et al. [12] carried out a subset analysis and excluded those with performance status 3–4 this survival advantage was no longer significant.
More recently, Nieder et al. [19] compared the following palliative fractionation regimens of 17 Gy/two fractions versus 30 Gy/10 fractions versus regimens with an EQD2 of 34–50 Gy for those aged 80 years and over. They found a median overall survival difference of 2.4, 2.6 and 11.8 months, respectively, with significant differences in survival for doses ≤30 Gy and doses >30 Gy. This could be an analysis of a subset of the patients of those included in the previously mentioned study, although this is not explicitly mentioned (the cohort is selected from the same hospital and the time period) [12,19].
In the 2015 Cochrane analysis, a meta-analysis incorporating 14 trials, the authors were unable to obtain enough original individual patient data in order to conduct a time-to-event analysis [16,[24], [25], [26]]. Therefore, the authors were only able to perform a meta-analysis of 1-year overall survival. This meta-analysis of 1-year overall survival for all patients regardless of performance status showed that receiving more fractions and a higher dose was favourable for survival, depending on which model was used (fixed effects versus random effects model) [10]. Due to large heterogeneity in the data for good performance status patients, the authors did not present these data in a summary statistic [10]. Although the data for poor performance status patients showed low heterogeneity and no 1-year overall survival advantage in using a more fractionated regimen, the evidence was rated as moderate [10]. In addition, Frank et al. [17] investigated 159 patients with NSCLC and compared 30 Gy/10 fractions, 25 Gy/five fractions, 15 Gy/three fractions and 10 Gy/one fraction, finding no statistically significant correlation between overall survival and radiotherapy regimen.
It is difficult to directly compare this study with others finding a positive correlation between increased fractionation and overall survival, as the fractionation schemes utilised in each study are variable with a large range in EQD2. The maximum palliative lung fractionation scheme used in this cohort was 30 Gy/10 fractions (EQD2 32.5), whereas Nieder et al. [12] and Janssen et al. [13] reported much higher doses used, with maximum radiotherapy doses of EQD2 45 and 47–52 Gy, respectively.
There were several potential biases in our study that need to be explored. This was a retrospective, single-centre study. The conclusions that can be drawn are therefore limited due to both known and unknown confounding factors. It is likely that the patients receiving a larger number of fractions are also the patients receiving systemic treatment. It would have been beneficial to include previous systemic therapies (chemotherapy, tyrosine kinase inhibitors and immunotherapies) and previous radiotherapy in our analysis, as these treatments could have a major impact on survival. It is likely that patients with SCLC would have only received higher fractionation schemes if they had shown a good response to systemic therapies, as recommended in RCR guidance (see Table 4). This could have been a confounding factor, as these patients showing a good response to systemic therapy would probably go on to have longer survival. Unfortunately, due to some patients receiving systemic treatment at multiple hospitals and a lack of integrated databases there was not sufficient access to information on these previous treatments. Improvements in electronic registration and database integration mean that in future analysis we expect to take the role of systemic treatments in palliative lung radiotherapy into account.
Table 4.
Palliative radiotherapy guidelines from The Royal College of Radiologists (RCR) and American Society for Radiation Oncology (ASTRO)
| RCR guidance [14] | |
|---|---|
| Good performance∗ NSCLC patients | 20 Gy/5 fractions over 1 week or |
| 30 Gy/10 fractions over 2 weeks or | |
| 36 Gy/12 fractions over 2.5 weeks or | |
| 39 Gy/13 fractions over 2.5 weeks | |
| Poor performance status∗ NSCLC patients | 17 Gy/2 fractions over 8 days or |
| 10 Gy/1 fraction | |
| Metastatic SCLC patients† |
30 Gy/10 fractions |
| ASTRO guidance [15] | |
| Performances status 0–2, stage III NSCLC and life expectancy of >3 months patients | 30 Gy/10 fractions to 42 Gy/14 fractions + concurrent chemotherapy |
| Good performance status∗ NSCLC patients | 30 Gy/10 fractions or more |
| Poor performance status∗ NSCLC patients | 20 Gy/5 fractions or 17 Gy/2 fractions or 10 Gy/1 fraction |
NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer.
Good and poor performance status undefined.
Patients who respond well to primary chemotherapy but who have persistent intrathoracic disease/symptoms.
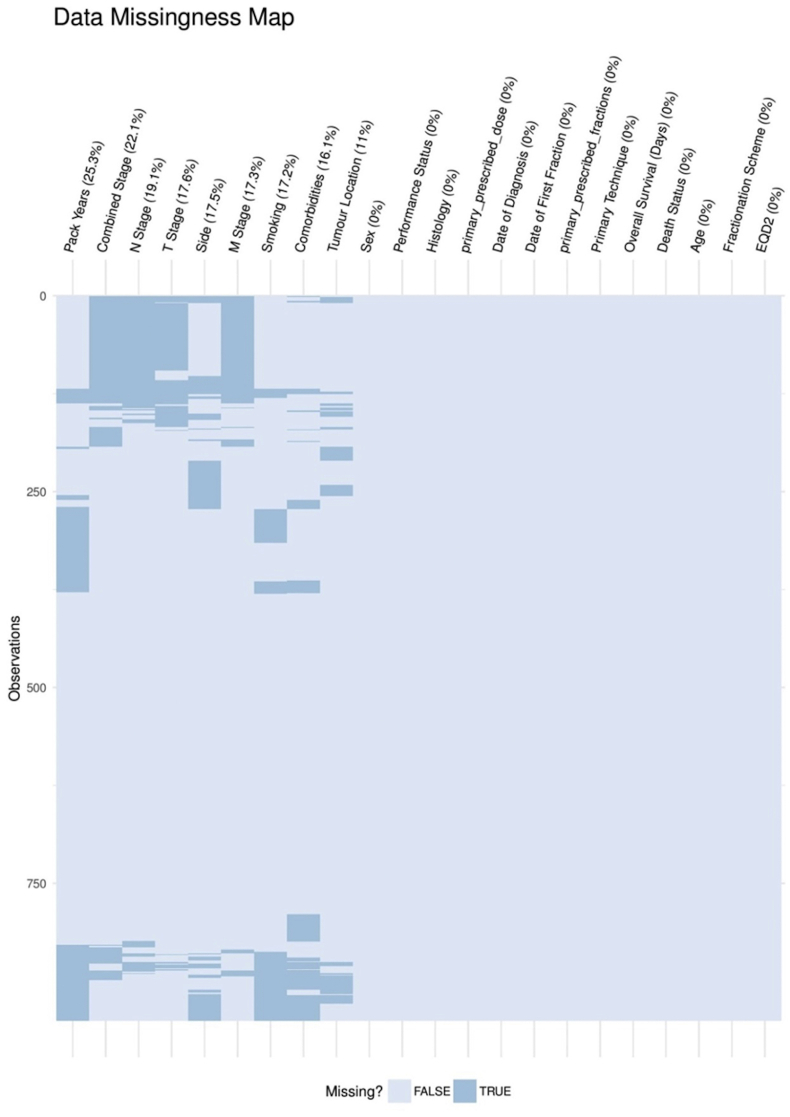
It would have also been interesting to see if mutation status (such as epidermal growth factor receptor and anaplastic lyphoma kinase rearrangement) was correlated to survival, but unfortunately these data were unavailable. There was a large proportion of missing data in several of the covariates examined (see Supplementary Material) and this reduced the power of the multivariable analysis. It would be informative to carry out regular audits on which data are not being fully recorded and why.
Treatment field size was another important prognostic indicator that would have been valuable to include, as tumour volume is known to be a factor associated with poorer survival, but this was also unavailable [27]. There was little variance in the radiotherapy technique, with the vast majority of patients treated with parallel pair/two field (96%). Therefore, a meaningful comparison of radiotherapy technique effect on overall survival did not take place. Granton et al. [28] have recently reported a decreased incidence of dysphagia following oesophageal-sparing intensity-modulated radiotherapy as opposed to parallel pair beams. If this technique becomes standard of care, it would be worthwhile investigating its effect on overall survival, toxicity and patient-reported symptoms.
Performance status has been shown in this study to be a prognostic indicator of overall survival and is used in multiple guidelines to determine treatment. Yet, performance status is not entirely objective, with the clinician's judgement playing a large role in determining the patient's score. Discrepancies between clinician- and patient-reported performance status have been documented and been shown to be associated with poorer survival [29]. As performance status determines treatment regimen and is sometimes an entry criteria to clinical trials, the treating clinician may be assigning performance status to fit the treatment rather than vice versa [30].
Previous studies have established that fractionation does not have a bearing on symptom control but can increase acute toxicity [10]. Unfortunately, in this cohort it was not possible to carry out an analysis on symptomatic improvement or toxicity due to its retrospective nature. The question remains: does a higher fractionation scheme not only lead to a longer overall survival, but also to a better quality of life?
RCR and ASTRO palliative lung radiotherapy fractionation guidance is summarised in Table 4. Neither ASTRO nor RCR guidance defines good, mid or poor performance status [14,15]. This makes it difficult to determine if clinicians are following the guidance explicitly. In the 2015 Cochrane review on palliative lung radiotherapy, good performance status was defined as a score of 0–1 and poor as 2–4 [10]. Other studies have classified a score of 0–1 as good, 2 as moderate and 3–4 as poor [31]. A number of patients in this study were prescribed an alternative dose of radiotherapy than that recommended by the RCR or ASTRO. According to RCR guidance, in this cohort, only 17 patients (2.6%) with a good performance status were undertreated (with 8 Gy/one fraction or 10 Gy/one fraction) and 110 patients (16.6%) with a poor performance status were over-treated (with 20 Gy/five fractions or 30 Gy/10 fractions). According to ASTRO guidance, 74 patients (11.1%) with a good performance status were undertreated as they were given <30 Gy/10 fractions and 54 patients (8.1%) with a poor performance status were over-treated as they were given >20 Gy/five fractions. Moderate performance status patients are not defined within either guidance.
Nine per cent of patients within this large centre's cohort died within 30 days of receiving palliative radiotherapy. This is consistent with other published data, including Spencer et al. [32,33], who examined the 30-day mortality of 3628 patients who received palliative lung radiotherapy, resulting in a 30-day mortality rate of 14%. It is also within the RCR forum suggested limit of 20% [18].
Future
Although this study has limitations, it adds to the justification for the need of a prospective multicentred randomised controlled trial to examine the effects of varying fractionation schemes and radiotherapy techniques on survival in today's era of modern systemic therapies. This future study should include both doctor- and patient-reported outcomes. The TOURIST (Thoracic Umbrella Radiotherapy Study in Stage IV) trial, a UK-based trial, is currently under development and is aiming to answer these questions (Woolf D, Lee C, Shah R, Ahmed M, Fraser I, Billingham L et al., unpublished data).
An area of unmet need are studies evaluating dose/fractionation regimens in the SCLC setting. To our knowledge there is only one prospective trial, currently recruiting, that is looking into a dose–effect relationship in patients with extensive stage SCLC. This trial compares 30 Gy/10 fractions versus 45 Gy/15 fractions in patients who have shown a response to standard of care chemotherapy (Clinicaltrials.gov identifier: NCT02675088).
The data coming from ongoing and future studies should be used to create decision tools to help patients with lung cancer considered for palliative treatment to balance survival gain and quality of life.
Conclusions
In this retrospective, single-centre analysis of palliative lung radiotherapy, although limited by a lack of data on systemic anticancer treatments, toxicity and quality of life, we found that increased fractionation regimens (up to and including 30 Gy/10 fractions) were associated with better survival regardless of performance status.
Conflict of interest
C. Faivre-Finn has declared research grants from AstraZeneca, MSD and Elekta and sits on the advisory boards of AstraZeneca and Pfizer. D. Woolf has declared travel grants, consultancy and speaker fees from AstraZeneca and travel grants from Roche. G. Price acknowledges the support of Cancer Research UK via funding to the Cancer Research Manchester Centre (C147/A18083) and (C147/A25254).
Acknowledgements
The key results presented in this paper have been published previously as part of a poster presentation at the 2019 British Thoracic Oncology Group and European Society for Radiotherapy and Oncology conferences. The authors are grateful for the assistance of Dr Kate Wicks in preparing the manuscript. This work was supported by Cancer Research UK via the funding to Cancer Research UK Manchester Centre: (C147/A18083) and (C147/A25254). Cancer Research UK did not have any input into the study design, data analysis or interpretation, the writing of the report, or the decision to submit the article for publication. C. Faivre-Finn was supported by the National Institute for Health Research: Manchester Biomedical Research Centre.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clon.2020.05.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Royal College of Physicians . 2018. National lung cancer audit annual report 2017. 1–29.https://www.rcplondon.ac.uk/projects/outputs/nlca-annual-report-2017 Available at: [Google Scholar]
- 3.Royal College of Physicians . 2019. National lung cancer audit annual report 2018. 1–22.https://www.rcplondon.ac.uk/projects/outputs/nlca-annual-report-2018 Available at: [Google Scholar]
- 4.Wao H., Mhaskar R., Kumar A., Miladinovic B., Djulbegovic B. Survival of patients with non-small cell lung cancer without treatment: a systematic review and meta-analysis. Syst Rev. 2013;2:10. doi: 10.1186/2046-4053-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moya-Horno I., Viteri S., Karachaliou N., Rosell R. Combination of immunotherapy with targeted therapies in advanced non-small cell lung cancer (NSCLC) Therapeut Adv Med Oncol. 2018;10:1–12. doi: 10.1177/1758834017745012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The National Cancer Registration and Analysis Service . 2019. The radiotherapy dataset.https://www.cancerdata.nhs.uk/radiotherapy Available at: [Google Scholar]
- 7.Stewart L.A. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence . 2019. Lung cancer: diagnosis and management.https://pathways.nice.org.uk/pathways/lung-cancer#path=view%3A/pathways/lung-cancer/supportive-and-palliative-care-for-lung-cancer.xml&content=view-node%3Anodes-palliative-radiotherapy Available at: [PubMed] [Google Scholar]
- 9.Rodrigues G., Videtic G.M., Sur R., Bezjak A., Bradey J., Hahn C.A. Palliative thoracic radiotherapy in lung cancer: an American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1:60–71. doi: 10.1016/j.prro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens R., Macbeth F., Toy E., Coles B., Lester J.F. Palliative radiotherapy regimens for patients with thoracic symptoms from non-small cell lung cancer. Cochrane Database Syst Rev. 2015;1:CD002143. doi: 10.1002/14651858.CD002143.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairchild A., Harris K., Barnes E., Wong R., Lutz S., Bezjak A. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26:4001–4011. doi: 10.1200/JCO.2007.15.3312. [DOI] [PubMed] [Google Scholar]
- 12.Nieder C., Tollali T., Yobuta R., Reigstad A., Flatoy L.R., Pawinski A. Palliative thoracic radiotherapy for lung cancer: what is the impact of total radiation dose on survival? J Clin Med Res. 2017;9:482–487. doi: 10.14740/jocmr2980w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssen S., Kaesmann L., Schild S.E., Rades D. Impact of the radiation dose and completion of palliative radiotherapy on survival in patients treated for locally advanced lung cancer. Anticancer Res. 2016;36:1825–1828. [PubMed] [Google Scholar]
- 14.The Royal College of Radiologists . Second Edition. 2019. Radiotherapy dose fractionation; pp. 49–57.https://www.rcr.ac.uk/system/files/publication/field_publication_files/bfco193_radiotherapy_dose_fractionation_third-edition-lung-cancer_0.pdf Available at: [Google Scholar]
- 15.Moeller B., Balagamwala E.H., Chen A., Creach K.M., Giaccone G., Koshy M. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8:245–250. doi: 10.1016/j.prro.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Kramer G.W., Wanders S.L., Noordijk E.M., Vonk E.J., van Houwelingen H.C., van den Hout W.B. Results of the Dutch national study of the palliative effect of irradiation using two different treatment schemes for non-small-cell lung cancer. J Clin Oncol. 2005;23:2962–2970. doi: 10.1200/JCO.2005.01.685. [DOI] [PubMed] [Google Scholar]
- 17.Frank M.S., Nørøxe D.S., Nygård L., Persson F.G. Fractionated palliative thoracic radiotherapy in non-small cell lung cancer – futile or worth-while? BMC Palliat Care. 2018;17:15. doi: 10.1186/s12904-017-0270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lees K. vols. 1–3. 2012. https://www.rcr.ac.uk/audit/audit-30-day-mortality-following-palliative-radiotherapy (Audit of 30 day mortality following palliative radiotherapy). Available at: [Google Scholar]
- 19.Nieder C., Yobuta R., Mannsaker B., Dalhaug A. How should palliative thoracic radiotherapy be fractionated for octogenarians with lung cancer? In Vivo. 2018;32:331–336. doi: 10.21873/invivo.11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lotayef M., Elkader Y.A., Amin A., Taher A., Ehab E.K., Abdelall M. A prospective study of the effect of different palliative radiotherapy fractionation schedules on tumor response and toxicity in advanced non-small cell lung cancer (NSCLC) patients. J Cancer Ther. 2016;7:924–938. [Google Scholar]
- 21.Planchard D., Popat S., Kerr K., Novello S., Smit E.F., Faivre-Finn C. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 22.Mirsadraee S. The 7th lung cancer TNM classification and staging system: Review of the changes and implications. World J Radiol. 2012;4:128–134. doi: 10.4329/wjr.v4.i4.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binder P.S., Peipert J.F., Kallogjeri D., Brooks R.A., Massad L.S., Mutch D.G. Adult Comorbidity Evaluation 27 score as a predictor of survival in endometrial cancer patients. Am J Obstet Gynecol. 2016;215:766. doi: 10.1016/j.ajog.2016.07.035. e1–766.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bezjak A., Dixon P., Brundage M., Tu D., Palmer M.J., Blood P. Randomized phase III trial of single versus fractionated thoracic radiation in the palliation of patients with lung cancer. Int J Radiat Oncol Biol Phys. 2002;54:719–728. doi: 10.1016/s0360-3016(02)02989-9. [DOI] [PubMed] [Google Scholar]
- 25.Reinfuss M., Glinski B., Kowalska T., Kulpa J., Zawila K., Reinfuss K. Radiotherapy in stage III, unresectable, asymptomatic non-small cell lung cancer. Final results of a prospective randomized study (240 patients) Cancer Radiothér. 1999;3:475–479. doi: 10.1016/s1278-3218(00)88254-2. [DOI] [PubMed] [Google Scholar]
- 26.Macbeth F.R., Bolger J.J., Hopwood P., Bleehen N.M., Cartmell J., Girling D.J. Randomized trial of palliative two-fraction versus more intensive 13-fraction radiotherapy for patients with inoperable non-small cell lung cancer and good performance status. Clin Oncol. 1996;8:167–175. doi: 10.1016/s0936-6555(96)80041-0. [DOI] [PubMed] [Google Scholar]
- 27.Plataniotis G., Theofanopoulou M.A., Sotiriadou K., Theodorou K., Mavroidis P., Kyrgias G. Palliative hypofractionated radiotherapy for non-small-cell lung cancer (NSCLC) patients previously treated by induction chemotherapy. J Thorac Dis. 2009;1:5–10. [PMC free article] [PubMed] [Google Scholar]
- 28.Granton P.V., Palma D.A., Louie A.V. Intentional avoidance of the esophagus using intensity modulated radiation therapy to reduce dysphagia after palliative thoracic radiation. Radiat Oncol. 2017;12:1–5. doi: 10.1186/s13014-017-0771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnadig I.D., Fromme E.K., Loprinzi C.L., Sloan J.A., Mori M., Li H. Patient-physician disagreement regarding performance status is associated with worse survivorship in patients with advanced cancer. Cancer. 2008;113:2205–2214. doi: 10.1002/cncr.23856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blagden S.P., Charman S.C., Sharples L.D., Magee L.R., Gilligan D. Performance status score: Do patients and their oncologists agree? Br J Cancer. 2003;89:1022–1027. doi: 10.1038/sj.bjc.6601231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prigerson H.G., Bao Y., Shah M.A., Paulk M.E., LeBlanc T.W., Schneider B.J. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778–784. doi: 10.1001/jamaoncol.2015.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park K.R., Lee C.G., Tseng Y.D., Liao J.J., Reddy S., Bruera E. Palliative radiation therapy in the last 30 days of life: a systematic review. Radiother Oncol. 2017;125:193–199. doi: 10.1016/j.radonc.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Spencer K., Morris E., Dugdale E., Newsham A., Sebag-Montefiore D., Turner R. 30 day mortality in adult palliative radiotherapy - a retrospective population based study of 14,972 treatment episodes. Radiother Oncol. 2015;115:264–271. doi: 10.1016/j.radonc.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.