Abstract
Background
The American Joint Committee on Cancer (AJCC) maintains that the eighth edition of its Staging Manual (AJCC8) has improved accuracy compared with the seventh (AJCC7). However, there are concerns that implementation may disrupt analysis of active clinical trials for stage III patients. We used an independent cohort of melanoma patients to test the extent to which AJCC8 has improved prognostic accuracy compared with AJCC7.
Methods
We analyzed a cohort of 1315 prospectively enrolled patients. We assessed primary tumor and nodal classification of stage I–III patients using AJCC7 and AJCC8 to assign disease stages at diagnosis. We compared recurrence-free (RFS) and overall survival (OS) using Kaplan-Meier curves and log-rank tests. We then compared concordance indices of discriminatory prognostic ability and area under the curve of 5-year survival to predict RFS and OS. All statistical tests were two-sided.
Results
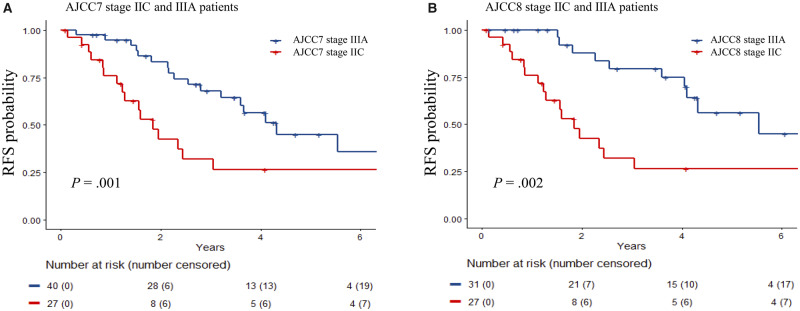
Stage IIC patients continued to have worse outcomes than stage IIIA patients, with a 5-year RFS of 26.5% (95% confidence interval [CI] = 12.8% to 55.1%) vs 56.0% (95% CI = 37.0% to 84.7%) by AJCC8 (P = .002). For stage I, removing mitotic index as a T classification factor decreased its prognostic value, although not statistically significantly (RFS concordance index [C-index] = 0.63, 95% CI = 0.56 to 0.69; to 0.56, 95% CI = 0.49 to 0.63, P = .07; OS C-index = 0.48, 95% CI = 0.38 to 0.58; to 0.48, 95% CI = 0.41 to 0.56, P = .90). For stage II, prognostication remained constant (RFS C-index = 0.65, 95% CI = 0.57 to 0.72; OS C-index = 0.61, 95% CI = 0.50 to 0.72), and for stage III, AJCC8 yielded statistically significantly enhanced prognostication for RFS (C-index = 0.65, 95% CI = 0.60 to 0.70; to 0.70, 95% CI = 0.66 to 0.75, P = .01).
Conclusions
Compared with AJCC7, we demonstrate that AJCC8 enables more accurate prognosis for patients with stage III melanoma. Restaging a large cohort of patients can enhance the analysis of active clinical trials.
The American Joint Committee for Cancer (AJCC) Staging Manual is the gold standard for prognostication of more than 50 types of cancer, including melanoma. The first edition was published in 1977 and revolutionized the standardization of cancer staging using the TNM System (1). This system follows the natural progression of oncogenesis in assessing the primary tumor thickness (T), regional lymph node involvement (N), and distant metastasis (M) and combines each classification to assign an overall disease stage. This stage is then used to provide prognostic information to the patient, assist the clinician in devising an appropriate treatment plan, and standardize baseline inclusion criteria for multi-site clinical trials.
The AJCC Staging Manual is continuously evaluated and periodically revised to incorporate newly discovered biomarkers, reflect advances in the understanding of the biological mechanisms of action in tumorigenesis, and consider improvements in treatment outcomes. In melanoma, the first six revisions focused on delineating T classification thickness cutoffs; clarifying N classification to include satellite and in-transit metastases and regional, but not distant, lymph node involvement; and identifying negative prognostic features, such as ulceration and high mitotic rate of the primary tumor (2–7). In 2018, the eighth edition of the AJCC Staging Manual proposed improved prognostication through a series of changes to the determinants of melanoma substaging, although overall staging was not revised. Such changes include removing mitotic index as a factor to assess T classification, increasing the influence of primary tumor thickness and ulceration status on substaging high-risk stage III patients, and adding the IIID substage (8).
For the first time, the AJCC used a completely new cohort to revise the staging manual that did not include legacy data from the previous edition. This new cohort included only patients with tumor thickness greater than 1.0 mm who received a sentinel lymph node biopsy (SLNB), as indicated by National Comprehensive Cancer Network guidelines. This is important because the seventh edition guidelines were created using stage II patients, some of whom with occult nodal disease would have been detected by SLNB if they had undergone the procedure. Thus, the new edition of the staging manual was based on the most up-to-date treatment paradigms for melanoma.
Given that this revised staging manual was introduced amid accrual to and analysis of clinical trials for novel immunotherapies and targeted therapies, there is debate that implementing these revisions may compromise the stability of outcome analysis (9). Critics maintain that it would be premature to revise the staging criteria without the discovery of a new biomarker that would change prognostic prediction, and they assert that the revised system be implemented for stages I, II, and IV only. Despite these criticisms, the AJCC reported that the revised edition has clinically significantly improved prognostic estimates (10). To assess the validity of this improvement in the newest edition and examine the feasibility and potential value in restaging a large number of patients, we used an independent cohort of melanoma patients to compare the prognostic accuracy of the AJCC Staging Manual Eighth Edition (AJCC8) with that of the AJCC Staging Manual Seventh Edition (AJCC7).
Methods
Patient Selection
We examined primary melanoma patients from the NYU Langone Health Interdisciplinary Melanoma Cooperative Group (IMCG) prospective cohort database. The eligibility criteria for this cohort include patients who have a diagnosis of primary cutaneous melanoma and enrolled within 2 months of initial diagnosis or 6 months within first recurrence or metastasis. Upon enrollment, trained supervised data managers capture clinical and pathological information from patients’ electronic Epic medical charts in 335 clinical and pathological data points, which are then stored in the Research Electronic Data Capture database. After enrollment, patients are prospectively followed up at regularly scheduled intervals. Follow-up information is recorded every 3 months for metastatic patients, every 6 months for primary invasive patients, and every 12 months for primary melanoma in situ patients (noninvasive melanoma with <1% chance of metastasis).
In this study, we aimed to incorporate the impact of contemporary immunotherapy in improving overall survival (OS) for patients with metastatic disease while maximizing the interval for clinical follow-up. To that end, we included patients who were accrued to the IMCG database between January 1, 2010 and December 31, 2016. We excluded patients who had multiple primary melanomas, an initial diagnosis of stage IV disease, melanoma in situ, no SLNB performed when indicated, or incomplete staging information from our analysis. Patients were then pathologically staged using both AJCC7 and AJCC8 guidelines.
Ethics Approval and Consent to Participate
The patient data used in this manuscript come from patients who were prospectively enrolled in the institutional review board-approved New York University IMCG database, which has been in existence since 2002 (institutional review board #10362). Biospecimens, clinicopathological data, and follow‐up information are collected using developed protocols and standard operating procedure. Upon consent, each patient is assigned a unique identification number. Demographic background, personal and family history of disease, pathological diagnoses, radiological imaging reports, sentinel lymph node mapping, disease staging, treatment, and continuing clinical follow‐up information, all compiled from thorough review of medical records and clinician interview, are incorporated into the database. Neither the name nor identifying information for an individual patient is used in program publications.
Primary Tumor Classification (T)
As per the AJCC7 guidelines, T classification was determined based on Breslow thickness (rounded to 0.01 mm), ulceration status, and mitotic rate (classified as a dichotomous variable ≥1 or <1). According to AJCC8 guidelines, T classification was determined using Breslow thickness (rounded to 0.1 mm) and ulceration. For analysis of AJCC7 guidelines, primary tumors less than 1.01 mm thick, without ulceration, and with a mitotic index less than 1/mm2 were classified as T1a, and those with a thickness less than 1.01 mm and ulceration or mitotic index 1/mm2 or greater were classified as T1b. For AJCC8, primary tumors with thickness less than 0.8 mm without ulceration were classified as T1a, and primary tumors with thickness less than 0.8 mm with ulceration or thickness from 0.8 to 1.0 mm were classified as T1b.
Regional Lymph Node Classification (N)
N classification was assessed according to the number of melanoma-positive nodes from SLNB plus complete lymph node dissection if performed, macroscopic vs microscopic nodal disease (AJCC8: clinically occult vs detected), and presence of satellite or microsatellitosis and in-transit disease. The presence of matted nodes was used for nodal classification for both AJCC7 and AJCC8, but the presence of extracapsular invasion was only used for N classification of AJCC7. T and N classifications were combined to assign an overall disease stage using AJCC7 and AJCC8 guidelines.
Statistical Analysis
Descriptive statistics are presented as means with SDs for continuous variables and as frequencies with proportions for categorical variables. Recurrence-free survival (RFS) and OS were calculated as time from the time of initial diagnosis to the time of first recurrence or death, respectively. Time of the last follow-up was used for censored patients. To estimate the RFS and OS in each staging system, Kaplan-Meier curves were generated and compared using the log-rank test. Five-year RFS and OS percentage probabilities and their 95% confidence intervals (CIs) were also estimated for each substage in each staging system. We then estimated prognostic discrimination ability of staging as a single predictor for each disease stage for AJCC7 and AJCC8. The concordance index (C-index), which is similar to the area under the curve for binary outcomes, was used to indicate discriminatory ability to predict RFS and OS, respectively. A value of 0.5 indicates that the model has no discriminatory ability and a value of 1.0 indicates that the model has perfect discrimination ability. For comparison of C-indices from AJCC7 and AJCC8 on the same set of patients, a nonparametric test was used to compare the two correlated C-indices (11). In addition, we estimated prognostic discrimination ability of staging by identifying the area under the receiver operating characteristic curve values for 5-year RFS and OS, respectively. For this purpose, we defined cases as patients with events and time to event of less than 5 years and control patients as event-free patients with follow-up of 5 or more years. All data were analyzed using R version 3.5.1, with packages “survival” and “compare C.” A two-tailed P value, generated from the log-rank test and nonparametric test, of less than .05 was used to establish statistical significance. All tests were two-sided.
Results
We enrolled 1958 patients in the IMCG database between January 1, 2010 and December 31, 2016. We excluded 643 patients from the analysis due to having multiple primaries (N = 64), an initial diagnosis of stage IV (N = 43), melanoma in situ (N = 308), SLNB not performed when indicated (N = 113), or incomplete staging information (N = 115). Table 1 shows the baseline characteristics of the study cohort of 1315 patients in our analysis (741 male; 574 female). At the time of diagnosis, the mean age was 58.6 years and the mean primary thickness was 1.5 mm. The overall stage, sex, and age distribution in our cohort was representative of melanoma in the general population (12). A total 105 (8.0%) patients were accrued at the time of first recurrence.
Table 1.
Baseline characteristics of 1315 melanoma patients accrued to the IMCG database from January 1, 2010 to December 31, 2016, representative of melanoma in the general population*
| Characteristic | No. (%) |
|---|---|
| No. (%) | 1315 (100.0) |
| Age (SD), y | 58.6 (16.6) |
| Sex | |
| Male | 741 (56.3) |
| Female | 574 (43.7) |
| Race | |
| White | 1281 (97.4) |
| African American | 17 (1.3) |
| Other | 17 (1.3) |
| Stage at initial diagnosis | |
| Stage I | 980 (74.5) |
| Stage II | 179 (13.6) |
| Stage III | 156 (11.9) |
| Ulceration | |
| Absent | 1070 (81.4) |
| Present | 210 (16.0) |
| Unclassified | 35 (2.7) |
| Histologic type | |
| Superficial spreading melanoma | 430 (32.7) |
| Nodular melanoma | 158 (12.0) |
| Other | 189 (14.4) |
| Unclassified | 538 (40.9) |
| Primary tumor thickness in mm, mean (SD) | 1.5 (2.3) |
| Recurrence status | |
| Not recurred | 1078 (82.0) |
| Recurred | 237 (18.0) |
| Alive status | |
| Alive | 1190 (90.5) |
| Dead | 125 (9.5) |
| Months of follow-up from initial diagnosis, median (range) | 34.7 (0–372) |
IMCG = Interdisciplinary Melanoma Cooperative Group.
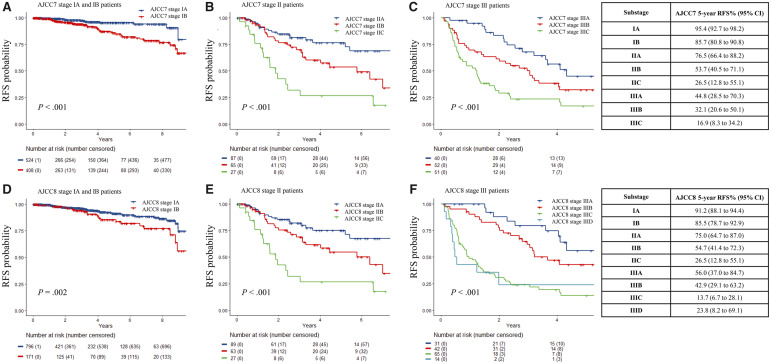
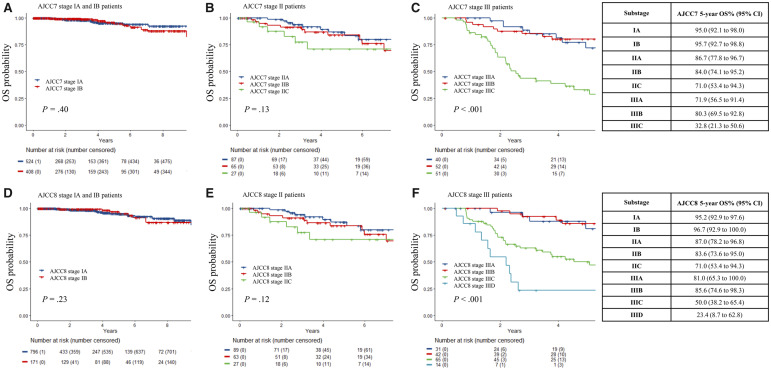
Figures 1 and 2 illustrate the RFS and OS, respectively, for each stage using both editions of the staging system. Stage IIC patients in both editions exhibited statistically significant worse RFS than stage IIIA patients, with a 5-year RFS of 26.5% (95% confidence interval [CI] = 12.8% to 55.1%) vs 56.0% (95% CI = 37.0% to 84.7%) by AJCC8 (Figure 3, P = .002). There was a lower 5-year OS for IIID than for IIIC (23.4%, 95% CI = 8.7% to 62.8% vs 50.0%, 95% CI = 38.2% to 65.4%), but this was not statistically significant due to the small number of patients with more than 5 years of follow-up. The area under the receiver operating characteristic curve values of AJCC7 and AJCC8 substaging for 5-year RFS and OS showed very similar results and patterns for every stage (Table 2). The rate of recurrence in our study is higher than the general melanoma population due to the hospital-based nature of our cohort.
Figure 1.
Recurrence-free survival (RFS) probability of stage I–III melanoma patients using American Joint Committee on Cancer Staging Manual Seventh Edition (AJCC7) and Eighth Edition (AJCC8). A) Stage IA and IB melanoma patients using AJCC7. B) Stage IIA, IIB, and IIC melanoma patients using AJCC7. C) Stage IIIA, IIIB, and IIIC melanoma patients using AJCC7. D) Stage IA and IB melanoma patients using AJCC8. E) Stage IIA, IIB, and IIC melanoma patients using AJCC8. F) Stage IIIA, IIIB, IIIC, and IIID melanoma patients using AJCC8. The Kaplan-Meier curves were generated for each substage with data from patients enrolled in the Interdisciplinary Melanoma Cooperative Group database at NYU Langone Health. The curves were compared, and P values were calculated using a two-sided log-rank test. The 5-year RFS probabilities with corresponding 95% confidence intervals for each substage are reported for both staging systems in the tables on the right.
Figure 2.
Overall survival (OS) probability of stage I–III melanoma patients using American Joint Committee on Cancer Staging Manual Seventh Edition (AJCC7) and Eighth Edition (AJCC8). A) Stage IA and IB melanoma patients using AJCC7. B) Stage IIA, IIB, and IIC melanoma patients using AJCC7. C) Stage IIIA, IIIB, and IIIC melanoma patients using AJCC7. D) Stage IA and IB melanoma patients using AJCC8. E) Stage IIA, IIB, and IIC melanoma patients using AJCC8. F) Stage IIIA, IIIB, IIIC, and IIID melanoma patients using AJCC8. The Kaplan-Meier curves were generated for each substage with data from patients enrolled in the Interdisciplinary Melanoma Cooperative Group database at NYU Langone Health. The curves were compared, and P values were calculated using a two-sided log-rank test. The 5-year OS probabilities with corresponding 95% confidence intervals for each substage are reported for both staging systems in the tables on the right.
Figure 3.
Recurrence-free survival (RFS) probability of stage IIC and IIIA melanoma patients using American Joint Committee on Cancer Staging Manual Seventh Edition (AJCC7) and Eighth Edition (AJCC8). A) Stage IIC and IIIA melanoma patients using AJCC7. B) Stage IIC and IIIA melanoma patients using AJCC8. The Kaplan-Meier curves were generated for each substage with data from patients enrolled in the Interdisciplinary Melanoma Cooperative Group database at NYU Langone Health. The curves were compared, and P values were calculated using a two-sided log-rank test.
Table 2.
C-index and AUC table demonstrate that AJCC8 has a higher prognostic value than AJCC7 for stage III patients, while it remains the same for stage I and II patients
| Stage | Recurrence (%) | RFS C-index (95% CI) |
5-year RFS AUC (95% CI) |
Death No. (%) | OS C-index (95% CI) |
5-y OS AUC (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AJCC7 | AJCC8 | P * | AJCC7 | AJCC8 | AJCC7 | AJCC8 | P * | AJCC7 | AJCC8 | |||
| Stage I | 63 (6.8) | 0.63 (0.56 to 0.69) | 0.56 (0.49 to 0.63) | .07 | 0.63 (0.55 to 0.70) | 0.55 (0.47 to 0.62) | 38 (4.3) | 0.48 (0.38 to 0.58) | 0.48 (0.41 to 0.56) | .90 | 0.55 (0.43 to 0.67) | 0.54 (0.46 to 0.63) |
| Stage II | 60 (33.5) | 0.65 (0.57 to 0.72) | 0.65 (0.57 to 0.72) | .83 | 0.68 (0.58 to 0.78) | 0.68 (0.57 to 0.78) | 28 (15.6) | 0.61 (0.50 to 0.72) | 0.61 (0.50 to 0.72) | .17 | 0.60 (0.47 to 0.74) | 0.60 (0.47 to 0.74) |
| Stage III | 89 (62.2) | 0.65 (0.60 to 0.70) | 0.70 (0.66 to 0.75) | .01 | 0.63 (0.50 to 0.77) | 0.70 (0.57 to 0.83) | 52 (36.4) | 0.69 (0.63 to 0.76) | 0.72 (0.66 to 0.78) | .27 | 0.74 (0.65 to 0.84) | 0.76 (0.67 to 0.84) |
A two-sided nonparametric test was used to calculate P values. AJCC7 = American Joint Committee on Cancer Staging Manual Seventh Edition; AJCC8 = American Joint Committee on Cancer Staging Manual Eighth Edition; AUC = area under the curve; CI = confidence interval; C-index = concordance index; OS = overall survival; RFS = recurrence-free survival.
All patients remained in the same stage category when using AJCC7 or AJCC8. Details of the shifts in substages can be seen in Supplementary Table 1 (available online). For stage I patients, there was 27.8% discordance as 272 of 980 patients were assigned different substages when using AJCC7 vs AJCC8. The removal of mitotic index as a factor in determining T1 classification led to the restaging of 35 patients from stage I to IA using the AJCC8 guidelines. With the revised T1a and T1b criteria, including the new classification of T1bN0 as stage IA, 237 stage IB patients were downstaged into the stage IA substage in AJCC8. After restaging, the prognostic accuracy for stage I patients remained constant for OS in both editions (C-index = 0.48, 95% CI = 0.38 to 0.58; to 0.48, 95% CI = 0.41 to 0.56, P = .90) (Table 2). There was a trend for slightly less prognostic accuracy in AJCC8 for RFS, but this was not statistically significant (C-index = 0.63, 95% CI = 0.56 to 0.69; to 0.56, 95% CI = 0.49 to 0.63, P = .07).
Only 2 of 179 patients in stage II shifted substages (from IIB to IIA) when restaged according to AJCC8 due to the change in rounding primary tumor thickness to 0.1. Because only stage II patients who had SLNB performed were included in this analysis, the revised edition maintained the same prognostic accuracy as the previous edition for both OS and RFS (OS C-index = 0.61, 95% CI = 0.50 to 0.72; RFS C-index = 0.65, 95% CI = 0.57 to 0.72).
For stage III, 70 of 156 were assigned different substages in AJCC7 vs AJCC8, which represents 44.9% discordance. In AJCC8, nine patients were assigned a substage that were only classified as stage III in AJCC7: one from III to IIIA, four from III to IIIB, and four from III to IIIC. From AJCC7 to AJCC8, 53 patients were upstaged: 12 from IIIA to IIIB, three from IIIA to IIIC, 24 from IIIB to IIIC, and 14 from IIIC to IIID. Finally, after restaging, eight patients were downstaged after restaging from AJCC7 to AJCC8: five from IIIB to IIIA and three from IIIC to IIIB. For stage III patients overall, the prognostic accuracy of AJCC8 for RFS exhibited a statistically significant improvement from AJCC7 (C-index = 0.65, 95% CI = 0.60 to 0.70; to 0.70, 95% CI = 0.66 to 0.75, P = .01). Although there was a trend towards improved prognostic accuracy for OS in AJCC8, there was no statistically significant change in OS for stage III patients (C-index = 0.69, 95% CI = 0.63 to 0.76; to 0.72, 95% CI = 0.66 to 0.78, P = .27).
Discussion
In this study, we compared the prognostic accuracy of AJCC7 with that of AJCC8 by restaging a large cohort of melanoma patients with prospective follow-up. Our data support that AJCC8 is more informative and precise than AJCC7. Specifically, AJCC8 has a statistically significant improvement in the prognostic accuracy for RFS of stage III patients. On the other hand, as we continue to improve prognostic estimates for melanoma patients, more work needs to focus on better understanding the biological explanation for the worse prognosis of stage IIC disease compared with higher stage III disease.
The removal of mitotic index as a determinant of T classification did not affect prognostic accuracy. Mitotic rate is limited by the fact that it is an operator-dependent variable with poor inter-rater agreement. Published reports cited inter-rater agreement for mitotic rate as low as k = 0.345, which is markedly lower than other T classification parameters, such as thickness and ulceration (13,14).
Although AJCC8 reduced factors necessary for substaging stage I patients, the revised edition increased granularity for substaging stage III patients, with the addition of a fourth IIID substage and incorporation of tumor thickness. Importantly, the implemented changes resulted in a statistically significant improvement in prognostic accuracy for RFS from AJCC7 to AJCC8 in our cohort. Using thickness information to stratify stage III patients in AJCC8 identified a group of patients in the IIIA and IIIB substages with thin primary tumors and a low burden of nodal disease that had markedly improved outcome probabilities compared with patients with thicker primaries and comparable nodal disease. For example, the IIIA and IIIB substages in AJCC7 include patients with T3a, T3b, T4a, and T4b primary tumors, whereas AJCC8 guidelines dictate that all T3b, T4a, and T4b primary tumors are classified as IIIC or IIID in presence of nodal disease. Thus, better identification of more aggressive tumors in the IIIC and IIID substages can improve selection of patients to receive adjuvant therapy.
Critics have suggested that the oncologic community adopt the updated staging system for only stage I, II, and IV patients to allow more seamless analysis of active clinical trials for stage III patients (9). Here, we demonstrate the feasibility of restaging a large cohort of patients, such as one from a clinical trial. Our results suggest that using AJCC8 guidelines to restage patients at treatment initiation enables more accurate baseline prognosis, which potentially improves outcomes analyses.
Despite overall improvements in prognostication from AJCC7 to AJCC8, there are notable limitations that merit ongoing attention. First, the revised edition does not yield improved prognostic accuracy for patients with thin melanomas, which comprise nearly 70% of newly diagnosed cases and up to 28% of deaths caused by melanoma in absolute terms (15–20). Pathologist concordance in diagnosing these lesions remains low despite the revisions set forth in AJCC8, which highlights the complexity associated with thin melanoma behavior (21–24). There remains a need to identify biomarkers that can inform which thin tumors, in the absence of nodal disease, are more likely to behave aggressively. To that end, artificial intelligence has gained traction in the fields of dermatology and pathology because of its accuracy and reproducibility (25–29). We believe that the ongoing development of these and other novel technologies can help to standardize histopathologic evaluation and may contribute to improved staging. Given that mortality events associated with thin tumors are relatively uncommon, there is a parallel need for coordinated efforts across multiple institutions to assess larger cohorts of patient data and biospecimens.
In addition to the above, the AJCC8 remains limited as a staging tool given that increasing stage does not always correspond with poorer outcomes. As a group, patients classified as stage IIC continue to have worse RFS and OS than patients classified as stage IIIA. This draws broader attention to the limitations of the conventional TNM approach to staging, because nodal positivity itself does not necessarily portend worse outcomes. In fact, there is increasing evidence to suggest that lymphatic spread is not even prerequisite for hematological metastasis. Our earlier publication identified a subset of clinicopathologically distinct melanoma patients who have isolated brain metastases as the first visceral site of metastasis (30). Further improvement to patient stratification will depend on our ability to elucidate the mechanisms driving poor patient outcomes in node-negative disease. In the case of stage IIC melanoma, research should explore possible links between Breslow’s depth and worse survival, such as metastatic spread through direct extension into local vasculature.
We acknowledge the potential impact of adjuvant therapy in improving outcomes for stage III patients that was not available for stage II patients during the study period (31–33). Analyses from these trials have demonstrated that RFS and OS are statistically significantly better for patients who received treatment vs placebo, which suggests that adjuvant therapy does have an impact. Our study is limited in its capacity to rigorously assess the role of adjuvant treatment in the gap in prognosis between high-risk stage II disease and low-risk stage III disease by the relatively small sample of stage III patients receiving adjuvant therapy (7.0%). Nevertheless, given that clinical trials for adjuvant treatment of stage IIC disease are underway (NCT01295827), we anticipate that the poor prognosis for this subset of patients will improve (34,35). Considering that the results from these trials will inevitably be compared with those for stage III disease, it is imperative that all analyses of active trials restage patients according to AJCC8. We intend to prospectively investigate this when adequate follow-up time from these trials is reached.
In conclusion, there is unavoidable complexity in updating staging systems amid major breakthroughs in the surgical, adjuvant, and neo-adjuvant management of oncologic disease. The guidelines put forth in the AJCC8 more accurately prognosticate for initial staging of stage III melanoma patients than the AJCC7, statistically significantly for RFS. We endorse its implementation to evaluate active clinical trial data with the highest accuracy despite written arguments in opposition. Moreover, as our treatment arsenal grows, our staging must evolve to capture the changes in outcome based on the most accurate survival estimates (36). Our study demonstrates that AJCC8 provides more reliable prognostic estimates for melanoma than AJCC7 and better reflects paradigm shifts in treatment since the time when the previous edition was published.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health through the NYU Cancer Institute Cancer Center Support Grant (P30CA016087) and NYU Melanoma SPORE (P50CA016087) to IO, JW, JZ, and DP.
Note
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. The authors have no conflict of interest to declare.
Supplementary Material
References
- 1. American Joint Committee for Cancer Staging and End Results Reporting. Manual for Staging of Cancer. Philadelphia, PA: Lippincott Raven Publishers; 1977:131–136. [Google Scholar]
- 2. American Joint Committee on Cancer. Manual for Staging of Cancer 2nd ed. Philadelphia, PA: Lippincott Raven Publishers; 1983:117–122. [Google Scholar]
- 3. American Joint Committee on Cancer. Manual for Staging of Cancer 3rd ed Philadelphia, PA: Lippincott Raven Publishers; 1988:139–144. [Google Scholar]
- 4. American Joint Committee on Cancer. Manual for Staging of Cancer 4th ed Philadelphia, PA: Lippincott Raven Publishers; 1992:143–148. [Google Scholar]
- 5. American Joint Committee on Cancer. Manual for Staging of Cancer 5th ed Philadelphia, PA: Lippincott Raven Publishers; 1997:163–170. [Google Scholar]
- 6. American Joint Committee on Cancer. Manual for Staging of Cancer 6th ed. New York. NY: Springer-Verlag; 2002:209–220. [Google Scholar]
- 7. American Joint Committee on Cancer. Manual for Staging of Cancer 7th ed. New York. NY: Springer International Publishing; 2009:325–344. [Google Scholar]
- 8. American Joint Committee on Cancer. Manual for Staging of Cancer 8th ed. New York. NY: Springer International Publishing; 2017:563–588. [Google Scholar]
- 9. Grob JJ, Schadendorf D, Lorigan P, et al. Eighth American Joint Committee on Cancer (AJCC) melanoma classification: let us reconsider stage III. Eur J Cancer. 2018;91:168–170. [DOI] [PubMed] [Google Scholar]
- 10. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin. 2017;67(6):472–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang L, Chen W, Petrick NA, et al. Comparing two correlated C indices with right-censored survival outcome: a one-shot nonparametric approach. Stat Med. 2015;34(4):685–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Howlader N, Noone AM, Krapcho M, et al. , eds. SEER Cancer Statistics Review, 1975-2016. Bethesda, MD: National Cancer Institute; 2019. [Google Scholar]
- 13. Garbe C, Eigentler TK, Bauer J, et al. Mitotic rate in primary melanoma: interobserver and intraobserver reliability, analyzed using H&E sections and immunohistochemistry. J Dtsch Dermatol Ges. 2016;14(9):910–915. [DOI] [PubMed] [Google Scholar]
- 14. Scolyer RA, Shaw HM, Thompson JF, et al. Interobserver reproducibility of histopathologic prognostic variables in primary cutaneous melanomas. Am J Surg Pathol. 2003;27(12):1571–1576. [DOI] [PubMed] [Google Scholar]
- 15. Monshi B, Vujic M, Kivaranovic D, et al. The burden of malignant melanoma—lessons to be learned from Austria. Eur J Cancer. 2016;56:45–53. [DOI] [PubMed] [Google Scholar]
- 16. Landow SM, Gjelsvik A, Weinstock MA.. Mortality burden and prognosis of thin melanomas overall and by subcategory of thickness, SEER registry data, 1992-2013. J Am Acad Dermatol. 2017;76(2):258–263. [DOI] [PubMed] [Google Scholar]
- 17. Svedman FC, Pillas D, Taylor A, Kaur M, Linder R, Hansson J.. Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe—a systematic review of the literature. Clin Epidemiol. 2016;8:109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Minini R, Rohrmann S, Braun R, Korol D, Dehler S.. Incidence trends and clinical-pathological characteristics of invasive cutaneous melanoma from 1980 to 2010 in the Canton of Zurich, Switzerland. Melanoma Res. 2017;27(2):145–151. [DOI] [PubMed] [Google Scholar]
- 19. Shaikh WR, Dusza SW, Weinstock MA, Oliveria SA, Geller AC, Halpern AC.. Melanoma thickness and survival trends in the United States, 1989 to 2009. J Natl Cancer Inst. 2016;108(1): djv294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whiteman DC, Baade PD, Olsen CM.. More people die from thin melanomas (1 mm) than from thick melanomas (>4 mm) in Queensland, Australia. J Inves Dermatol. 2015;135(4):1190–1193. [DOI] [PubMed] [Google Scholar]
- 21. Elmore JG, Elder DE, Barnhill RL, et al. Concordance and reproducibility of melanoma staging according to the 7th vs 8th edition of the AJCC Cancer Staging Manual. JAMA Netw Open. 2018;1(1):e180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verver D, Louwman WJ, Koljenović S, et al. Improved stratification of pT1 melanoma according to the 8th American Joint Committee on Cancer Staging Edition criteria: a Dutch population-based study. Eur J Cancer. 2018;92:100–107. [DOI] [PubMed] [Google Scholar]
- 23. Elmore JG, Barnhill RL, Elder DE, et al. The reproducibility and accuracy of pathologists’ diagnosis of invasive melanoma and melanocytic proliferations. BMJ. 2017;357:j2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taylor L, Hood K, Reisch L, et al. Influence of variability in assessment of Breslow thickness, mitotic rate and ulceration among US pathologists interpreting invasive melanoma, for the purpose of AJCC staging. J Cutan Pathol. 2018;45(8):588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prathamesh M, Robinson E, Pradhan J, et al. Deep learning based on standard H&E images of primary melanoma tumors identifies patients at risk for visceral recurrence and death. Clin Cancer Res. 2020; doi: 10.1158/1078-0432.CCR-19-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ehteshami Bejnordi B, Veta M, Johannes van Diest P, et al. ; and the CAMELYON16 Consortium. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ehteshami Bejnordi B, Mullooly M, Pfeiffer RM, et al. Using deep convolutional neural networks to identify and classify tumor-associated stroma in diagnostic breast biopsies. Mod Pathol. 2018;31(10):1502–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tellez D, Balkenhol M, Otte-Holler I, et al. Whole-slide mitosis detection in H&E breast histology using PHH3 as a reference to train distilled stain-invariant convolutional networks. IEEE Trans Med Imaging. 2018;37(9):2126–2136. [DOI] [PubMed] [Google Scholar]
- 29. Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma MW, Qian M, Lackaye DJ, et al. Challenging the current paradigm of melanoma progression: brain metastasis as isolated first visceral site. Neuro Oncol. 2012;14(7):849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835. [DOI] [PubMed] [Google Scholar]
- 32. Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–1823. [DOI] [PubMed] [Google Scholar]
- 33. Eggermont AMM, Chiarion-Sileni V, Grob J-J, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brancaccio G, Napolitano S, Troiani T, et al. Eighth American Joint Committee on Cancer (AJCC) melanoma classification: what about stage IIC? Br J Dermatol. 2018;179(6):1422–1423. [DOI] [PubMed] [Google Scholar]
- 35. Grob JJ, Garbe C, Ascierto P, Larkin J, Dummer R, Schadendorf D.. Adjuvant melanoma therapy with new drugs: should physicians continue to focus on metastatic disease or use it earlier in primary melanoma? Lancet Oncol. 2018;19(12):e720–e725. [DOI] [PubMed] [Google Scholar]
- 36. Haydu LE, Thompson JF, Scolyer RA, Gershenwald JE.. Embracing changes to the American Joint Committee on Cancer 8th Edition Melanoma Staging System. Eur J Cancer. 2019;112:9–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.