Abstract
Antihistamines with cationic amphiphilic drug (CAD) characteristics induce cancer-specific cell death in experimental studies. Epidemiologic evidence is, however, limited. In a Danish nationwide cohort of ovarian cancer patients diagnosed during 2000–2015 (n = 5075), we evaluated the association between filled antihistamine prescriptions and cancer mortality. We used Cox regression models to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for ovarian cancer mortality. In an in vitro cell viability assay, we evaluated cell death in three ovarian cancer cell lines after treatment with clinically relevant doses of eight antihistamines. In our cohort study, CAD antihistamine use (≥1 prescription; n = 133) was associated with a hazard ratio of 0.63 (95% CI = 0.40 to 0.99) compared to use of non-CAD antihistamines (n = 304), and we found a tendency toward a dose-response association. In our cell viability assay, we found consistent and dose-dependent cytotoxicity for all CAD but not non-CAD antihistamines. In this nationwide cohort study, use of antihistamines with CAD characteristics is associated with a prognostic benefit in ovarian cancer patients.
Despite advances in ovarian cancer treatment, survival rates remain low, and the identification of strategies to improve outcomes has high clinical priority (1). Antihistamines are used for relief of allergic symptoms (2). Repurposing these drugs for cancer therapy has gained considerable attention, following laboratory studies reporting antineoplastic effects (3–5). Recently, we found that use of antihistamines with cationic amphiphilic drug (CAD) characteristics was associated with reduced mortality among patients with advanced stage cancer (6). This prompted us to evaluate the potential of prescribed CAD antihistamines to improve prognosis in a large nationwide cohort of ovarian cancer patients, linking data from several nationwide Danish registries, including the cancer and the prescription registry. Additionally, we evaluated cytotoxicity of commonly used antihistamines on ovarian cancer cell lines.
We identified all women in Denmark aged 30–84 years with an incident diagnosis of epithelial ovarian cancer during 2000–2015. Antihistamine use was defined as one or more filled prescriptions within 6 months before cancer diagnosis and start of follow-up. Follow-up started 1 year (1-year baseline) or 3 years (3-year baseline) after the cancer diagnosis, and ended at time of death, emigration, or end of the study (December 31, 2016), whichever came first. We used Cox proportional hazard regression models to estimate multivariable-adjusted hazard ratios (HRs) and two-sided 95% confidence intervals (CIs) for the association between filled antihistamine prescriptions and ovarian cancer mortality. Other-cause mortality was evaluated as a secondary outcome to estimate the effect of competing events. The proportional hazards assumption was tested using scaled Schoenfeld residuals. We tested the robustness of our findings by repeating the analyses using inverse probability of treatment weighting with propensity scores (7). Results were considered statistically significant if the 95% confidence intervals of the hazard ratio did not cross 1.00. Data sources and methods are described in more detail in the Supplementary Materials (Supplementary Methods, Supplementary Table 1, Supplementary Figure 1, available online).
In our cohort of 5075 ovarian cancer patients (Supplementary Tables 2–3, available online), use of CAD antihistamines compared to use of non-CAD antihistamines was associated with reduced ovarian cancer mortality in analyses with baseline at 1 year (HR = 0.74, 95% CI = 0.51 to 1.06) or 3 years (HR = 0.63, 95% CI: 0.40 to 0.99) (Table 1). We found some evidence of a more pronounced effect with increasing cumulative amount (Table 1). Compared to nonuse of any antihistamine, a tendency toward reduced mortality was found for CAD antihistamines, but not for non-CAD antihistamines (Table 1). We found no strong evidence for effect modification by chemotherapy (Supplementary Table 4, available online). Results were not meaningfully different in sensitivity analyses, restricting to serous ovarian cancer patients (Supplementary Table 5, available online), including clemastine among CAD antihistamines (Supplementary Table 6, available online), or using propensity score–weighted Cox models (Supplementary Table 7 available online). We also found no indication of a meaningful influence of competing events (Supplementary Table 8 and Supplementary Figure 2, available online).
Table 1.
Association between antihistamine use and ovarian cancer–specific mortality: use of CAD antihistamines compared with non-CAD antihistamine use as an active comparator (upper panel), and compared with nonuse of any antihistamines (lower panel)
| Analysis | Antihistamine use | No. | Events | HR (95% CI)* | HR (95% CI)† |
|---|---|---|---|---|---|
| 1-y baseline | Non-CAD antihistamine‡ | 346 | 168 | 1.00 (Referent) | 1.00 (Referent) |
| CAD antihistamine§ | 138 | 58 | 0.77 (0.57 to 1.05) | 0.74 (0.51 to 1.06) | |
| Cumulative amount (/100 DDD)‖ | 0.92 (0.76 to 1.12) | ||||
| 3-y baseline | Non-CAD antihistamine‡ | 304 | 111 | 1.00 (Referent) | 1.00 (Referent) |
| CAD antihistamine§ | 133 | 37 | 0.74 (0.51 to 1.09) | 0.63 (0.40 to 0.99) | |
| Cumulative amount (/100 DDD)‖ | 0.95 (0.85 to 1.05) | ||||
| 1-y baseline | Nonuse | 4591 | 2213 | 1.00 (Referent) | 1.00 (Referent) |
| Antihistamine (any)¶ | 484 | 226 | 1.06 (0.92 to 1.22) | 0.97 (0.84 to 1.12) | |
| CAD antihistamine§ | 138 | 58 | 0.86 (0.66 to 1.11) | 0.80 (0.61 to 1.04) | |
| Non-CAD antihistamine‡ | 346 | 168 | 1.15 (0.99 to 1.35) | 1.05 (0.89 to 1.23) | |
| 3-y baseline | Nonuse | 2524 | 863 | 1.00 (Referent) | 1.00 (Referent) |
| Antihistamine (any)¶ | 437 | 148 | 1.05 (0.88 to 1.25) | 0.90 (0.75 to 1.08) | |
| CAD antihistamine§ | 133 | 37 | 0.81 (0.58 to 1.12) | 0.71 (0.50 to 0.99) | |
| Non-CAD antihistamine‡ | 304 | 111 | 1.16 (0.96 to 1.42) | 1.00 (0.81 to 1.23) |
Adjusted for age at diagnosis and year of diagnosis. CAD = cationic amphiphilic drug; CI = confidence interval; DDD = defined daily dose; HR = hazard ratio.
Adjusted for age at diagnosis, year of diagnosis, clinical stage, tumor histology, chemotherapy, comorbid conditions, use of other prescription drugs, and socioeconomic factors, including highest achieved education, income, and marital status (see Supplementary Methods E, Supplementary Table 1, available online).
One or more filled prescriptions for non-CAD antihistamines and no prescriptions for CAD antihistamines within 6 months before diagnosis and start of follow-up (baseline).
One or more filled prescriptions for CAD antihistamines (including ebastine, loratatadine, desloratadine, astemizole, terfenadine, and cyproheptadine) within 6 months before diagnosis and start of follow-up (baseline).
Association according to cumulative amount by including separate linear terms for CAD and non-CAD antihistamine use in the model. Presented as the change in the hazard ratio per increment of 100 DDDs, that is, comparing CAD and non-CAD antihistamine users with the same cumulative amount. The reference group, therefore, is not the entire group of non-CAD antihistamine users.
One or more filled prescriptions for any antihistamine within 6 months before diagnosis and start of follow-up (baseline).
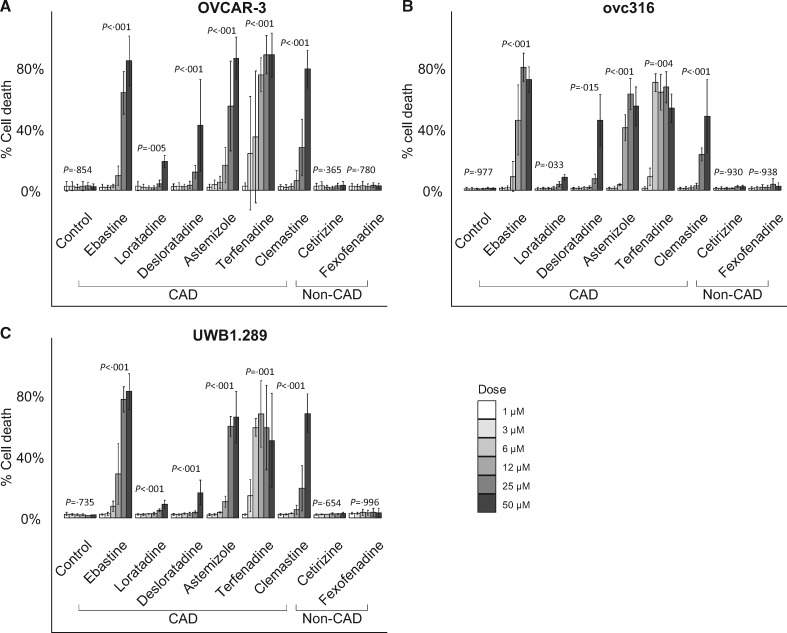
In our in vitro cell viability assay, we tested the effect of frequently used antihistamines on cell viability in three high-grade serous ovarian cancer cell lines (OVCAR-3, UWB1.289, and ovc316). All statistical tests were two-sided and a P value of less than .05 was considered statistically significant (Supplementary Methods, available online). We found a clear and consistent dose response for all CAD antihistamines (one-way analysis of variance, P < .03 for each drug), but not for non-CAD antihistamines (one-way analysis of variance, P > .37 for each drug) (Figure 1). Terfenadine was the most potent CAD antihistamine with 34.8%–70.4% cell death at low concentration (6 µM). In contrast, treatment with non-CAD antihistamines at the highest concentration (50 µM) resulted in 2.3%–3.3% cell death, not markedly different from control.
Figure 1.
In vitro assessment of cytotoxicity of cationic amphiphilic drug (CAD) and non-CAD antihistamines in ovarian cancer. Cell death (exclusion of propidium iodide) induced by treatment with antihistamines at different concentrations (1, 3, 6, 12, 25, and 50 µM) for 48 hours in three ovarian cancer cell lines, presented as means (SD) (n = 3): A) OVCAR-3, B) ovc316, and C) UWB1.289. P values derived from one-way analysis of variance after logit transformation, present comparison of group means by dose.
In our cohort study, use of CAD antihistamines was associated with a reduction of around 20%–35% in ovarian cancer mortality, whereas no association was found for non-CAD antihistamines. In our in vitro experiments, we confirmed the biological plausibility of these findings.
CADs are a diverse group of compounds, which because of their amphiphilic and weak basic properties, accumulate in acidic lysosomes where they can induce permeabilization of the lysosomal membrane, leading to cell death (8). Several CADs have shown cancer-specific cytotoxicity, in vivo and in vitro (8–12), with some evidence in ovarian cancer (8,13,14). The molecular basis for this specificity is that in cancer cells, as opposed to normal cells, lysosomes are more abundant, larger, and particularly susceptible to membrane permeabilization (8,15). CADs accumulate in acidic tumors, particularly in tumor lysosomes (up to 1000-fold) (16); therefore, the dose range used in our in vitro experiments may be relevant for the concentrations achieved after oral anthistamine use. CADs are also hypothesized to revert multidrug resistance in cancer cell lines (6,8,17,18), including ovarian cancer cells (19,20). Previously, we found more pronounced inverse associations between CAD antihistamine use and mortality among patients who had received chemotherapy compared to patients who did not (6). In the current study, a similar risk pattern did not emerge; however, the number of patients not receiving chemotherapy was low.
A potential limitation of our study is exposure misclassification because of over-the-counter use of antihistamines, which is around 40% of total antihistamine use in the general population in Denmark (21). In our study, however, the proportion of antihistamines obtained by prescription may expectedly be higher because of the increased medical surveillance of cancer patients. Nonetheless, such misclassification may have biased our estimates toward no observed association, particularly in analyses with nonuse as the reference group. We also had limited statistical power in analyses of CAD antihistamines, which prohibited an evaluation of histology-specific associations for nonserous ovarian cancer types and testing of drug-mortality associations for individual CAD antihistamines. Loratadine and its metabolite desloratadine constituted the vast majority (>80%) of CAD antihistamine use (Supplementary Table 9, available online). Finally, we cannot exclude residual confounding, which may be related to the indication for antihistamine use and selective prescribing. However, to the best of our knowledge, a clinician’s preference to prescribe a specific antihistamine is not related to its CAD characteristics. Thus, by using non-CAD antihistamines as an active comparator, we were able to minimize such biases (22), and the specificity of the inverse association to CAD antihistamines but not non-CAD antihistamines suggests that our results are not driven by confounding.
In conclusion, in a nationwide cohort study, we provide epidemiologic evidence suggesting that antihistamines with CAD characteristics at current doses may provide a prognostic benefit in ovarian cancer patients. The plausibility of this finding was confirmed in vitro in ovarian cancer cell lines. Further efforts are required to confirm our results in other study populations, and to elucidate the precise biological mechanism. Given that current antihistamines are well-tolerated (2), inexpensive, and already commonly used in cancer patients, CAD antihistamines may become promising candidates as adjuvants to standard ovarian cancer treatment and, therefore, merit further research.
Funding
This work was supported by the Independent Research Fund Denmark – Sapere Aude program (grant number 6110-00596B to FV); MERMAID III (to SKK); Danish National Research Foundation (grant number DNRF125 to MJ); European Research Council (grant number AdG 340751 to MJ); and Danish Cancer Society (grant number R167-A11061 to MJ).
Notes
Affiliations of authors: Unit of Virus, Lifestyle and Genes, Danish Cancer Society Research Centre, Copenhagen, Denmark (FV, SKK); Belgian Cancer Registry, Brussels, Belgium (FV); Unit of Statistics and Pharmacoepidemiology, Danish Cancer Society Research Centre, Copenhagen, Denmark (CD, SF); Cell Death & Metabolism, Centre for Autophagy, Recycling and Disease, Danish Cancer Society Research Center, Copenhagen, Denmark (MJ); Department of Cellular and Molecular Medicine, Faculty of Health Sciences, University of Copenhagen, Copenhagen, Denmark (MJ); Genome Integrity Unit, Danish Cancer Society Research Centre, Copenhagen, Denmark (RS); Clinical Pharmacology and Pharmacy, Department of Public Health, University of Southern Denmark, Odense, Denmark (AP, JH); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (SKK).
The funders had no influence on study design, collection, analysis, or interpretation of the data, nor on writing or submission of the manuscript. None of the authors have a conflict of interest to declare. We thank Tiina Naumanen Dietrich for technical assistance.
FV and CD contributed equally to this work.
Supplementary Material
References
- 1. Cortez AJ, Tudrej P, Kujawa KA, Lisowska KM.. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol. 2018;81(1):17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Church MK, Church DS.. Pharmacology of antihistamines. Indian J Dermatol. 2013;58(3):219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Faustino-Rocha AI, Ferreira R, Gama A, Oliveira PA, Ginja M.. Antihistamines as promising drugs in cancer therapy. Life Sci. 2017;172:27–41. [DOI] [PubMed] [Google Scholar]
- 4. Medina VA, Rivera ES.. Histamine receptors and cancer pharmacology. Br J Pharmacol. 2010;161(4):755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falus A, Pós Z, Darvas Z.. Histamine in normal and malignant cell proliferation In: Histamine in Inflammation. Boston, MA: Springer; 2010:109–123. [DOI] [PubMed] [Google Scholar]
- 6. Ellegaard AM, Dehlendorff C, Vind AC, et al. Repurposing cationic amphiphilic antihistamines for cancer treatment. EBioMedicine. 2016;9:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Austin PC, Stuart EA.. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petersen NH, Olsen OD, Groth-Pedersen L, et al. Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell. 2013;24(3):379–393. [DOI] [PubMed] [Google Scholar]
- 9. Ostenfeld MS, Hoyer-Hansen M, Bastholm L, et al. Anti-cancer agent siramesine is a lysosomotropic detergent that induces cytoprotective autophagosome accumulation. Autophagy. 2008;4(4):487–499. [DOI] [PubMed] [Google Scholar]
- 10. Sukhai MA, Prabha S, Hurren R, et al. Lysosomal disruption preferentially targets acute myeloid leukemia cells and progenitors. J Clin Invest. 2013;123(1):315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jahchan NS, Dudley JT, Mazur PK, et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discov. 2013;3(12):1364–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shchors K, Massaras A, Hanahan D.. Dual targeting of the autophagic regulatory circuitry in gliomas with repurposed drugs elicits cell-lethal autophagy and therapeutic benefit. Cancer Cell. 2015;28(4):456–471. [DOI] [PubMed] [Google Scholar]
- 13. Lee CS, Kim YJ, Jang ER, Kim W, Myung SC.. Fluoxetine induces apoptosis in ovarian carcinoma cell line OVCAR-3 through reactive oxygen species-dependent activation of nuclear factor-kappaB. Basic Clin Pharmacol Toxicol. 2009;106(6):446–453. [DOI] [PubMed] [Google Scholar]
- 14. Kornhuber J, Tripal P, Reichel M, et al. Functional inhibitors of acid sphingomyelinase (FIASMAs): a novel pharmacological group of drugs with broad clinical applications. Cell Physiol Biochem. 2010;26(1):9–20. [DOI] [PubMed] [Google Scholar]
- 15. Kallunki T, Olsen OD, Jaattela M.. Cancer-associated lysosomal changes: friends or foes? Oncogene. 2013;32(16):1995–2004. [DOI] [PubMed] [Google Scholar]
- 16. Trapp S, Rosania GR, Horobin RW, Kornhuber J.. Quantitative modeling of selective lysosomal targeting for drug design. Eur Biophys J. 2008;37(8):1317–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jaffrezou JP, Chen KG, Duran GE, et al. Inhibition of lysosomal acid sphingomyelinase by agents which reverse multidrug resistance. Biochim Biophys Acta. 1995;1266(1):1–8. [DOI] [PubMed] [Google Scholar]
- 18. An L, Li DD, Chu HX, et al. Terfenadine combined with epirubicin impedes the chemo-resistant human non-small cell lung cancer both in vitro and in vivo through EMT and notch reversal. Pharmacol Res. 2017;124:105–115. [DOI] [PubMed] [Google Scholar]
- 19. Drinberg V, Bitcover R, Rajchenbach W, Peer D.. Modulating cancer multidrug resistance by sertraline in combination with a nanomedicine. Cancer Lett. 2014;354(2):290–298. [DOI] [PubMed] [Google Scholar]
- 20. Engelmann BJ, Ryan JJ, Farrell NP.. Antidepressants and platinum drugs. Anticancer Res. 2014;34(1):509–516. [PubMed] [Google Scholar]
- 21. Schmidt M, Hallas J, Laursen M, Friis S.. Data resource profile: Danish online drug use statistics (MEDSTAT). Int J Epidemiol. 2016;45(5):1401–1402. [DOI] [PubMed] [Google Scholar]
- 22. Lund JL, Richardson DB, Sturmer T.. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.