Abstract
Background
The natural history of human papillomavirus (HPV)-induced cervical cancer (CC) is not directly observable, yet the age of HPV acquisition and duration of preclinical disease (dwell time) influences the effectiveness of alternative preventive policies. We performed a Cancer Intervention and Surveillance Modeling Network (CISNET) comparative modeling analysis to characterize the age of acquisition of cancer-causing HPV infections and implied dwell times for distinct phases of cervical carcinogenesis.
Methods
Using four CISNET-cervical models with varying underlying structures but fit to common US epidemiological data, we estimated the age of acquisition of causal HPV infections and dwell times associated with three phases of cancer development: HPV, high-grade precancer, and cancer sojourn time. We stratified these estimates by HPV genotype under both natural history and CC screening scenarios, because screening prevents cancer development that affects the mix of detected cancers.
Results
The median time from HPV acquisition to cancer detection ranged from 17.5 to 26.0 years across the four models. Three models projected that 50% of unscreened women acquired their causal HPV infection between ages 19 and 23 years, whereas one model projected these infections occurred later (age 34 years). In the context of imperfect compliance with US screening guidelines, the median age of causal infection was 4.4–15.9 years later compared with model projections in the absence of screening.
Conclusions
These validated CISNET-CC models, which reflect some uncertainty in the development of CC, elucidate important drivers of HPV vaccination and CC screening policies and emphasize the value of comparative modeling when evaluating public health policies.
Despite intensive cervical cancer (CC) control efforts in the United States, an estimated 13 240 women developed CC and more than 4170 died from CC in 2018 (1). Ongoing evaluations of alternative screening modalities [eg, primary human papillomavirus [HPV]-based screening (2)] and expanded use of HPV vaccination (eg, up to age 45 years as recently evaluated by the US Advisory Committee on Immunization Practices) examine new opportunities to further reduce the burden of CC; however, the optimal combination and use of these interventions has not reached consensus in the United States (2).
Understanding the natural history pathway of HPV to CC can provide guidance in HPV vaccination and screening policies (3); however, much of this pathway is unobservable. For example, the age at which women acquire their “causal” HPV infection—the HPV infection that develops into invasive CC—is an important determinant of vaccine impact at different ages. Currently available HPV vaccines, which are prophylactic, would have diminished population-level impact if administered to women after the peak age of causal infections (4). Similarly, the length of time between HPV acquisition and cancer development is critical in determining the optimal frequency and target ages of preventive screening interventions.
Clinical studies can neither differentiate causal infections nor follow a cohort for the decades required to observe the progression to invasive cancer; they do, however, provide indirect information on these processes. This information can inform mathematical simulation models, which are increasingly being used to capture the complex natural history process and project the health benefits and economic consequences of alternative CC prevention approaches. Although numerous CC models have been developed worldwide (5), direct comparisons between model structures and projections are limited, particularly comparisons that have standardized the epidemiological setting and common inputs. Because model-based analyses are increasingly being used for US policy guidance (6) and model complexity increases, a more comprehensive understanding of model structures and assumptions is imperative to improve model transparency and provide decision makers with confidence in model-based results (7). Model comparisons can also generate insights on unobservable epidemiological phases of the carcinogenic process.
The CC working group of the Cancer Intervention and Surveillance Modeling Network (CISNET-cervical) comprises four independently developed microsimulation models of HPV infection and cervical carcinogenesis and one model of HPV-HIV coinfection. These models can be used to conduct standardized, comparative analyses to enhance model transparency and help guide public health research and priorities (https://cisnet.cancer.gov/). We leveraged multiple models to gain insights on the natural history of CC and the impact of screening interventions using four independent CC models. In this analysis, we compared the underlying natural history of CC by estimating the dwell times for preclinical phases of cervical disease and characterizing the age of acquisition of causal HPV infections among women who develop clinical CC. Second, we describe how CC screening may, by selection, influence the timing of these key events.
Methods
Analytic Overview
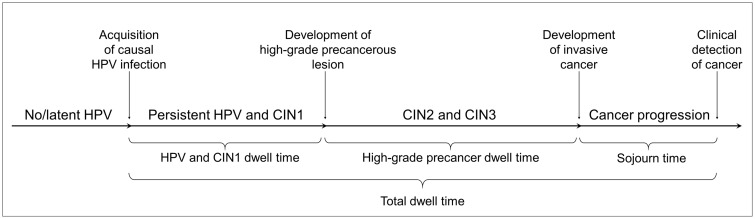
Four CISNET-cervical microsimulation models (Harvard, Microsimulation Screening Analysis [MISCAN]-Cervix [Erasmus Medical Center], Policy1-Cervix [Cancer Council NSW], and University of Minnesota-HPV Cancer [UMN-HPV CA]) were used to project outcomes for a hypothetical cohort of individual women. All models applied common inputs from the US population and were fit to match common, standardized observed data in the United States but varied in their underlying structure and assumptions of the carcinogenic process (Table 1; Supplementary Table 1, available online). For women diagnosed with CC in the absence of screening or vaccination, the models estimated the age of acquisition of the causal HPV infection and dwell times associated with phases of cancer development, including “HPV/CIN1 dwell time,” defined as the time from acquisition of an HPV infection to development of a high-grade precancer (cervical intraepithelial neoplasia grade 2 [CIN2] and/or grade 3 [CIN3]); “high-grade precancer dwell time,” defined as the time from development of a high-grade precancer (CIN2 and/or CIN3) to asymptomatic cancer development; cancer “sojourn time,” defined as the time from asymptomatic cancer development to clinical detection; and “total dwell time,” defined as the time from HPV acquisition to cancer detection (Figure 1). Conditioned on developing cancer, we stratified these estimates by high-risk HPV, HPV16, and non-HPV16 genotypes. For each model, we calculated the median age of causal HPV infection and dwell times but also reported the interquartile ranges (IQR) and means.
Table 1.
Key attributes of the four microsimulation models of the CISNET-cervical working group: Harvard, MISCAN-Cervix, Policy1-Cervix, and UMN-HPV CA
| Key attribute | Microsimulation model |
|||
|---|---|---|---|---|
| Harvard | MISCAN-Cervix | Policy1-Cervix | UMN-HPV CA | |
| Model structure | ||||
| Dynamic (interactive) or static (noninteractive)* | Static | Static | Static | Static |
| Mode of analysis, simulating life histories | Individual based | Individual based | Individual based | Individual based |
| Cycle length | Monthly | Continuous time | 6- or 12-monthly | Annual |
| HPV infection | ||||
| HPV types included | HPV16, HPV18, HPV31, HPV33, HPV45, HPV52, HPV58, pooled other high-risk HPV, pooled other low-risk HPV | HPV16; HPV18; pooled 31, 33, 45, 52, 58; and pooled high-risk genotypes | HPV16; HPV18; pooled 31, 33, 45, 52, 58; and pooled high-risk genotypes | HPV16; HPV18; pooled 31, 33, 45, 52, 58; and pooled other high-risk types |
| Natural immunity | Reduced probability of future type-specific infection | Reduced probability of future infection linked from dynamic model | Reduced probability of future type-specific infection from dynamic model | Reduced probability of future type-specific infection |
| Cervical carcinogenesis | ||||
| Health states included | Healthy, HPV, CIN2, CIN3, cancer (stage specific) | Healthy, HPV, CIN1, CIN2, CIN3, cancer (stage specific) | Healthy, HPV, CIN1, CIN2, CIN3, cancer (stage specific) | Healthy, HPV, CIN1, CIN2, CIN3, cancer (stage specific) |
| Progression and regression transitions | Age-specific, HPV and lesion persistence | Age- and type-specific | Age- and type-specific | Age- and type-specific |
| Model calibration | ||||
| Calibrated parameters | HPV incidence, HPV and CIN progression; HPV natural immunity; cancer symptom detection; progression of undetected asymptomatic cancer by stage | HPV and CIN progression and regression; duration of CIN3; cancer stages; cancer symptom detection; cytology test characteristics | HPV and CIN progression and regression rates; undetected asymptomatic cancer by stage | HPV incidence; CIN progression and regression rates; HPV natural immunity, cancer symptom detection |
All modeling groups have a companion dynamic transmission model. CIN = cervical intraepithelial neoplasia; CISNET = Cancer Intervention and Surveillance Modeling Network; HPV = human papillomavirus; MISCAN = Microsimulation Screening Analysis; UMN-HPV CA = University of Minnesota-HPV Cancer.
Figure 1.
General schematic of the natural history pathway of cervical cancer for distinct preclinical dwell times. For models that allow regression from a high-grade precancer to human papillomavirus (HPV) infection and progression back to high-grade precancer before progressing to invasive cancer, we assumed the time spent in each health state, regardless of sequential progression, contributed to the respective dwell times. The analysis stratified HPV-16, HPV-18, and other non-HPV16 or -HPV18 types. The Harvard model does not include an explicit CIN1 health state because it is interpreted as a microscopic manifestation of acute HPV infection and is therefore incorporated into the HPV-infected state. The Harvard model allows for nonsequential progression from HPV to cervical intraepithelial neoplasia grades 2 (CIN2) or 3 (CIN3). The natural history state of CIN at any grade is not the same as what is observed in the real world because of screening detection limitations.
Because dwell times and the age of causal infections are likely altered in the presence and intensity of screening (eg, because of screening’s preferential detection of precancers with longer dwell time during screening ages [ie, ages 21–65 years]) and to facilitate comparability with existing studies, we also projected these estimates in the context of imperfect compliance to US screening guidelines and full compliance to US screening guidelines. Screening scenarios involved triennial cytology-based screening with reflex HPV testing for women with atypical cells of undetermined significance and direct referral to diagnostic colposcopy for women with low-grade cytology results or worse.
Microsimulation Models
Each of the four CISNET-cervical models simulates the underlying natural history of HPV-induced CC (Figure 1) but differs structurally with respect to the type and number of health states, HPV genotype categorizations, cycle length, and data sources used to parameterize the baseline model before model fitting to the US setting (Table 1).
Brief model descriptions summary of key differences are available in the Supplementary Methods (available online), and standardized profiles of each model’s structure and underlying model parameters and assumptions, with additional references, are available at http://cisnet.cancer.gov/profiles/.
Calibration and Validation
The CISNET-cervical working group identified US calibration targets from epidemiological studies, including age- and genotype-specific HPV prevalence (8); HPV genotype distribution in CIN1, CIN2, and CIN3 (9); and age-specific HPV genotype distribution in invasive CC from seven US population-based cancer registries (10). To standardize each model to the US-specific HPV and cervical disease burden, selected parameters from each model (see Supplementary Table 1, available online) were adjusted to fit these standardized common data sources (ie, calibration targets). In general, all four models showed good fit to the standardized common calibration targets (Supplementary Figures 1–3, available online). In a validation exercise, model outputs were generally consistent with age-specific cancer incidence from the Connecticut Tumor Registry (11) before widespread cytology-based screening (1950–1969) as well as the cumulative proportion of detected cancers by age (Supplementary Figures 4 and 5, available online).
Results
Dwell Times
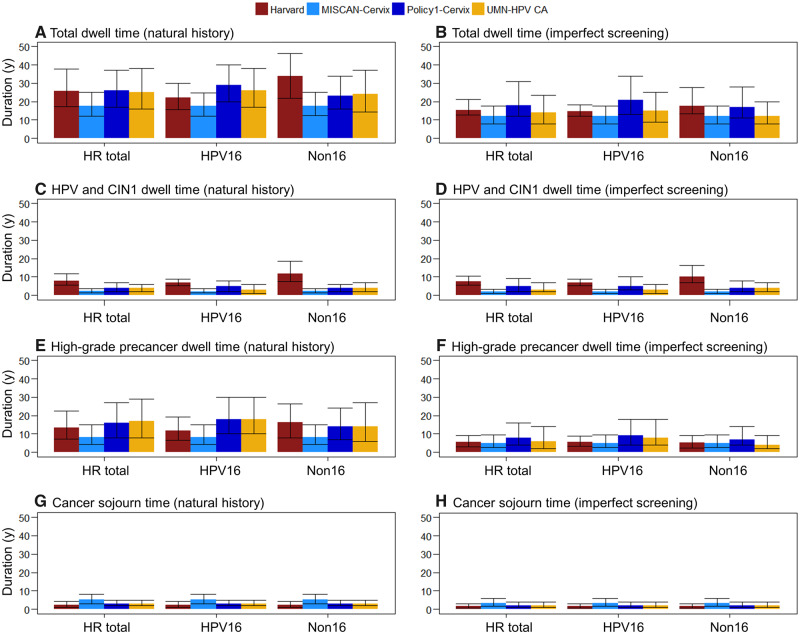
In the absence of primary (ie, HPV vaccination) or secondary (ie, screening) prevention, there were important similarities and differences among the natural history models with respect to the duration of preclinical disease and their variation by HPV genotype (Figure 2, left panels). The total median dwell time from acquisition of a high-risk HPV infection to cancer detection was shortest for MISCAN-Cervix (17.5 years, IQR = 12.2–25.0 years), followed by UMN-HPV CA (25.0 years, IQR = 16.0–38.0 years), Harvard (25.7 years, IQR = 16.7–39.6 years), and Policy1-Cervix (26.0 years, IQR = 17.0–37.0 years) (Figure 2A;Supplementary Table 3, available online). When we stratified by HPV genotype, the Harvard model projected a shorter median total dwell time for HPV16-related cancers compared with non-HPV16-related cancers (ie, 22.0 vs 33.7 years), whereas Policy1-Cervix and UMN-HPV CA projected total median dwell times that were 2–6 years longer for HPV16-related cancers compared with non-HPV16-related cancers. MISCAN-Cervix did not project differences in median dwell times for HPV16-related vs non-HPV16-related cancers. For all models, the mean dwell times were generally longer than the median dwell times (19.8–28.8 years), but the overall trends across models and HPV genotypes remained consistent (Supplementary Table 3, available online).
Figure 2.
Median dwell times for women who developed cervical cancer for preclinical phases of cancer development for four Cancer Intervention and Surveillance Modeling Network (CISNET) modeling groups. Estimates are provided under natural history assumptions (left panels) and in the context of imperfect compliance to US screening guidelines (right panels); stratified by any high-risk (HR) human papillomavirus (HPV) infection, HPV16 infections, and non-HPV16 (Non16) infections for the Harvard (red), Microsimulation Screening Analysis (MISCAN)-Cervix (light blue), Policy1-Cervix (dark blue), and University of Minnesota-HPV Cancer (UMN-HPV CA) (yellow) simulation models. Error bars reflect the interquartile ranges across the individual-level simulations in each model. For the imperfect compliance scenario, we assumed 70% compliance with primary testing and 90% compliance with follow-up management as recommended, including diagnostic colposcopy or biopsy and treatment to remove high-grade lesions. The Harvard model does not explicitly capture cervical intraepithelial neoplasia grade 1 (CIN1) because it is interpreted as a microscopic manifestation of acute HPV infection and is therefore incorporated into the HPV-infected state.
When we stratified dwell times by preclinical phases, we found that the median dwell time of HPV and CIN1 for any high-risk HPV infections ranged from 2.2 years (MISCAN-Cervix) to 8.0 years (Harvard) (Figure 2C). For high-grade precancers, the median dwell times for any high-risk HPV infections were the shortest for MISCAN-Cervix (8.2 years) and longest for UMN-HPV CA (17.0 years) (Figure 2E), with a mean ranging from 11.0 to 20.0 years (Supplementary Table 3 available online). When we stratified by HPV genotype, the Harvard model projected a median high-grade precancer dwell time that was 4.4 years shorter for HPV16-related precancers compared with cancers related to non-HPV16 infections (ie, HPV16-related lesions progressed more quickly). Conversely, both Policy1-Cervix and UMN-HPV CA projected median dwell times that were 4 years longer for HPV16-related high-grade precancers compared with non-HPV16-related precancers. All four models projected comparable median cancer sojourn times, ranging from 2.3 to 5.3 years (Figure 2G).
In the context of cytology-based screening, total median dwell times were shorter in all models, and there was less variation across the four models (Figure 2, right panels), ranging from 12.0 years (MISCAN-Cervix) to 18.0 years (Policy1-Cervix) in the context of imperfect compliance to screening guidelines. Under assumptions of perfect compliance to guidelines, median dwell times were even shorter, ranging from 10.0 years (UMN-HPV CA) to 14.5 years (Harvard) (Supplementary Table 5, available online). Mean dwell time for all models was longer than median dwell times, reflecting a right-skewed distribution across all models (Supplementary Tables 4 and 5, available online).
Cumulative Incidence of Causal HPV Infections
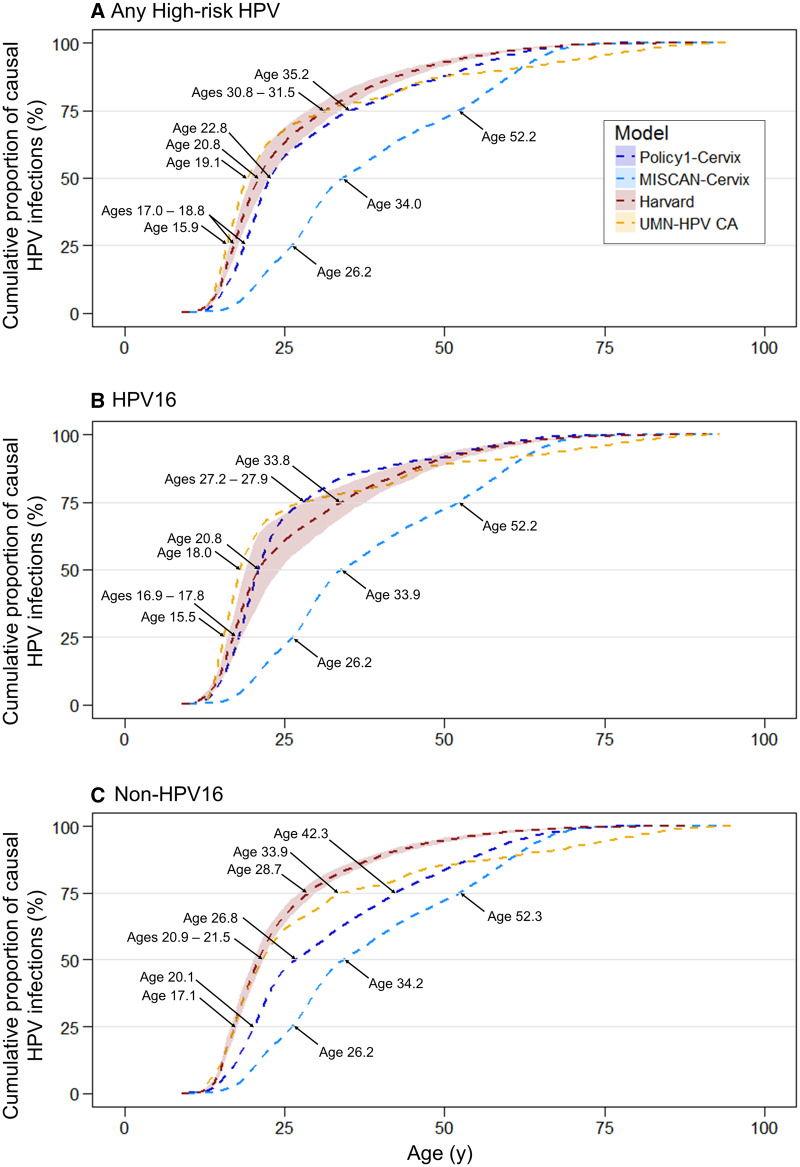
Three models projected that 50% of the women diagnosed with cancer acquired their causal high-risk HPV infection between ages 19 and 23 years (Harvard, Policy1-Cervix, and UMN-HPV CA), and one model projected that 50% of the women acquired their causal infection by age 34 years (MISCAN-Cervix) (Figure 3A). The same three models projected that 75% of causal HPV infections occurred before age 35 years, whereas MISCAN-Cervix projected that 75% of causal HPV infections occurred 17 years later (ie, by age 52 years). When we stratified by HPV genotype, all four models projected that causal HPV16 infections occurred between 0.1 and 6.0 years earlier compared with non-HPV16 infections (Figure 3, B and C).
Figure 3.
Cumulative age of human papillomavirus (HPV) acquisition by HPV genotype for four Cancer Intervention and Surveillance Modeling Network modeling groups. Estimates are stratified by HPV type: A) any high-risk HPV infections, B) HPV16 infections, and C) non-HPV16 infections for the Harvard (red), Microsimulation Screening Analysis (MISCAN)-Cervix (light blue), Policy1-Cervix (dark blue), and University of Minnesota-HPV Cancer (UMN-HPV) CA (yellow) simulation models. Shaded area for the Harvard model represents the upper and lower bounds across the 50 good-fitting natural history parameter sets.
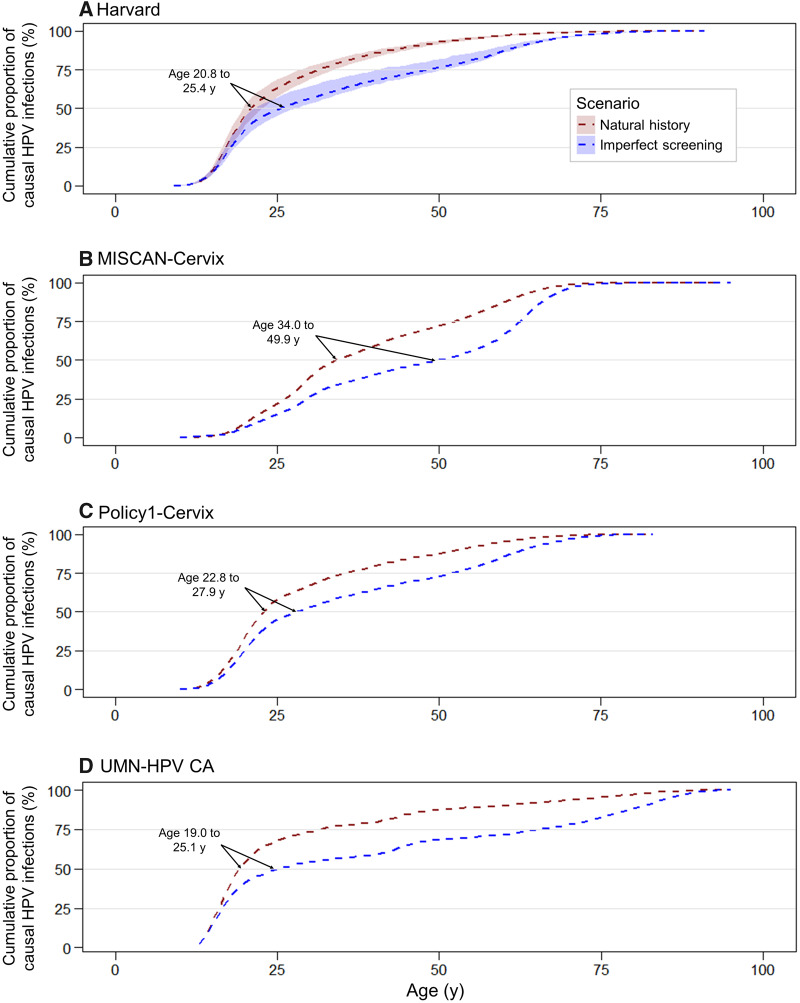
In the context of triennial cytology-based screening assuming imperfect compliance with US guidelines, the median age of the causal infection occurred between 4.4 years (Harvard) and 15.9 years (MISCAN-Cervix) later compared with the model projections in the absence of a screening program (Figure 4). Screening reduced the absolute risk of cancer between 60% and 80% in all models and delayed the median age of acquiring the causal high-risk infection associated with the remaining cancers to ages 25.1, 25.4, 27.9, and 49.9 years for the UMN-HPV CA, Harvard, Policy1-Cervix, and MISCAN-Cervix models, respectively. Under assumptions of perfect compliance with guidelines, the median age of causal infection was further delayed between 15 and 29 years compared with no screening (Supplementary Figure 8; Supplementary Table 2, available online).
Figure 4.
Cumulative age of human papillomavirus (HPV) acquisition in the context of imperfect compliance to US screening guidelines and natural history for four Cancer Intervention and Surveillance Modeling Network modeling groups. Projections of the casual age of infection under assumptions of imperfect screening (blue) are compared with natural history (red) for A) Harvard, B) Microsimulation Screening Analysis (MISCAN)-Cervix, C) Policy1-Cervix, and D) University of Minnesota-HPV Cancer (UMN-HPV CA). For the imperfect compliance scenario, we assumed 70% compliance with primary testing and 90% compliance with follow-up management as recommended, including diagnostic colposcopy or biopsy and treatment to remove high-grade lesions. Shaded area for the Harvard model represents the upper and lower bounds across the 50 good-fitting natural history parameter sets.
Discussion
Although all four of the CISNET-cervical natural history models are generally consistent with observed empirical data in the United States, this comparative analysis highlights important similarities and differences in the duration of preclinical disease that is targeted by screening, the inferred age of acquiring cancer-causing HPV infections targeted by vaccines, the role of HPV genotypes, and the potential impact of screening on these outcomes. To our knowledge, this is the first such comparative analysis. Due to ethical reasons and feasibility constraints of empirical studies (eg, observation period, treatment of high-grade lesions), we are unlikely to ever have direct observations of the acquisition age of the causal infection or dwell times among women who progress to CC.
Consistent with the well-established understanding of cervical carcinogenesis (12), all four models projected that the natural history from acquisition of an HPV infection to clinical cancer generally required 18–26 years, although MISCAN-Cervix had a shorter median total dwell time compared with the other models. The differences in total dwell time were primarily driven by the length of time spent with HPV and CIN1, followed by length of time with a high-grade lesion. For the Harvard model, transition probabilities following acquisition of HPV are a function of duration of infection or lesion, not age, based on longitudinal data that suggests that viral persistence is a function of duration of the infection or lesion, irrespective of a woman’s age (13–15). This assumption contributed to a longer HPV and CIN1 dwell time among women who developed cancer compared with the other models, an outcome for which data are not available. In contrast, in two models (Policy1-Cervix and UMN-HPV CA), progression (regression) probabilities increase (decrease) with age, consistent with the literature (16–20); finally, in MISCAN-Cervix, progression potential but not dwell time is modified by age. Age may act at least in part as a proxy for duration; therefore, age-based transitions could potentially overestimate the oncogenic potential for infections acquired later in life, resulting in a shorter dwell time and an overestimation of the age of causal infection (and overestimation of the impact of vaccination at older ages). The magnitude and impact of these structural differences may become more apparent as we begin to evaluate HPV vaccination policies or as we consider more nuanced screening strategies. For example, in all four models, there would generally be at least two full screening rounds (assuming a 3-year screening interval) to detect a CIN2 or CIN3 lesion before cancer development. In contrast, when evaluating extended primary HPV-based screening intervals (eg, 5 years), MISCAN-Cervix, which had the shortest median high-grade precancer dwell time (8.2 years), would be expected to favor strategies with shorter intervals compared with other models.
There is considerable variability in the several efforts to estimate high-grade precancer dwell times in different settings. Vink et al. (21) projected a median dwell time of 23.5 years, which is longer than the median high-grade precancer dwell times projected by the CISNET-cervical models, under either assumption of natural history or screening. Several additional studies demonstrate similar variability, reporting mean high-grade dwell times ranging between 11.8 and 24.3 years (22–25). The mean natural history high-grade precancer dwell times reported across the CISNET-cervical models varied from 11 to 20 years; however, under assumptions of imperfect compliance to US cytology-based screening, the CISNET models projected mean high-grade precancer dwell times that were substantially shorter and more consistent, ranging between 7 and 12 years. However, there are complex interactions with the specifics of screening being conducted (eg, test, interval, age range, compliance) that make comparisons difficult between the aforementioned studies and our model projections.
We found that in the absence of screening, the projected median age of acquisition of the causal HPV infection was between ages 19 and 23 years for three models but was much later (age 34 years) for MISCAN-Cervix. The age of causal infection is a function of 1) the age at which women develop clinical cancer, and 2) the length of time required to develop clinical cancer (ie, total dwell time). Because the age distribution of the background cancer incidence in all models is similar, the differences and similarities in median age of causal infection stem from total dwell time. In particular, the later age of causal infection in MISCAN-Cervix is explained by shorter dwell time (described above), and the total median dwell time in the other models is similar. Under assumptions of imperfect compliance with screening, the relatively small number of remaining cancers compared with no screening occur among women: after age 65 years (screening end age), with shorter dwell times (cancer developed between screening intervals), with a false negative screen(s), or did not attend screening. Both the first and second reasons, by selection, are expected to result in a later age of infection. Indeed, all four models projected that the causal infection among the cancers occurring because of these underlying causes was delayed. These findings have important implications for prevention policies in the United States and internationally. For example, the proportion of vaccine-preventable cancers decreases with age, and therefore the added value of HPV vaccination policies that extend to mid-adult individuals diminishes [eg, HPV-FASTER (26) or US Advisory Committee on Immunization Practices (27)] in all of the models but is less pronounced in MISCAN-Cervix. Even though the median age of the causal infection is delayed with screening, vaccinating mid-adult women has been found to be either inefficient or associated with very high cost-effectiveness ratios in a recent comparative modeling analysis (27).
In the context of screening, HPV16 infections, which are responsible for only 20% of HPV infections but more than 50% of CCs, are considered the most carcinogenic HPV genotype because these infections are less likely to clear and more likely to progress to precancer and cancer (12). HPV16-related cancers contribute to a larger proportion of cancers that develop among younger women (before age 50 years) compared with cancers that occur after age 50 years (10,28). A study by Wheeler et al. (29) also supports an earlier age of occurrence for HPV16-positive cancers compared with non-HPV16 cancers, although type attribution studies for cancer can be subject to biases (30). Conditioned on developing cancer, the Harvard model projected shorter median HPV and CIN1, high-grade precancer, and total dwell times for cancers related to HPV16 infections compared with non-HPV16 infections, whereas the Policy1-Cervix and UMN-HPV CA models projected longer total dwell times for HPV16 infections. A study by Wentzensen et al. (31) that evaluated patients with Loop electrosurgical excision procedure confirmed CIN2 or CIN3 cases to estimate the time from sexual activity onset to development of CIN2 or CIN3 found that HPV16-related CIN2 and CIN3s grew more rapidly than lesions related to other HPV genotypes, with a progression time that was on average 30% shorter for HPV16-related CIN3s. However, these estimates were not isolated to precancers that ultimately progressed to cancer. In contrast, the statistical model by Vink et al. (21) compared dwell times between HPV16- and non-HPV16-related lesions and did not find differences in the median high-grade lesion dwell times by HPV genotype, though that study did find a larger proportion of HPV16 CIN2 or CIN3s that progressed within the first 10 years. However, higher probability to progress does not rule out equal median dwell time. Observable information on the genotype specificity is fraught with complex interactions between age effects of HPV16, the differential accuracy of screening in detecting HPV16-related lesions, and the impact of screening on cancer prevention. The impact of different genotype-specific model assumptions will be relatively more important for screening strategies that involve genotyping for HPV16 and HPV18 infections or for screening among women vaccinated against HPV16 and HPV18 infections. Analyses from the Harvard model would be expected to differentially favor more intensive screening strategies for HPV16 infections compared with the Policy-Cervix and UMN-HPV CA models.
The differences by HPV genotype may in part stem from two of the models assuming age-specific transition probabilities, whereas the Harvard model assumes transitions that are duration based. The age-specific transition models that capture non-HPV16 infections occurring at older ages compared with HPV16 infections will necessarily have faster transitions (and shorter dwell times) associated with non-HPV16 infections because they occur at older ages and face increasing probabilities of progression. In contrast, the Harvard model transitions are a function of time and assume HPV16 infections are more likely to persist, and therefore progress, irrespective of age.
There are several notable limitations to this study. First, we report results from only four models, but there may be alternative plausible model structures that could fit the observed empirical data. Our imperfect screening compliance scenario was not intended to mimic current practice, and direct comparison with empirical data requires more refined model assumptions; however, simulating screening provides projections for the impact of screening on the age of causal infection. Generalizing these findings to other populations may be limited in that they are based on US patterns of HPV acquisition, benign hysterectomy utilization, and background mortality, all of which can affect age patterns in CC.
Ongoing analyses performed by the CISNET-cervical working group involve reevaluation of CC strategies outlined by the US Preventive Services Task Force (2). Despite structural differences in the natural history of the models reported in this study, preliminary analyses evaluating US Preventive Services Task Force strategies indicate similar policy findings across the models (32), strengthening the validity of policy conclusions. Using multiple models will serve as an important approach to understanding the drivers of potential model disagreements in identifying optimal cancer control policies. In addition, using multiple, independently developed models for policy analyses is the most robust approach to undertake sensitivity analysis on underlying model structure and on the “deep” unobservable model parameters, in line with good modeling practice (7).
This comparative analysis highlights important similarities and differences, in part because of evidence gaps, among four validated CISNET CC natural history models. Our findings elucidate important drivers of HPV prevention and CC screening policies and emphasize the value of comparative modeling when evaluating optimal public health policies.
Funding
This work was funded by the National Institutes of Health's National Cancer Institute as part of the Cancer Intervention and Surveillance Modeling Network (CISNET), Grant Number U01CA199334. The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. The article’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Notes
Affiliations of authors: Harvard T.H. Chan School of Public Health, Boston, MA (EAB, CR, SS, JJK); University of Oslo, Oslo, Norway (EAB); Erasmus Medical Center, Rotterdam, the Netherlands (IMCMdK, SM, MvB); University of Minnesota, Minneapolis, MN (EG, SK, FA-E, VV); Drug Policy Program, Center for Research and Teaching in Economics (CIDE)-CONACyT, Aguascalientes, Mexico (FA-E); Cancer Research Division, Cancer Council NSW, Sydney, Australia (JK, KC, KTS, MAS); School of Public Health, University of Sydney, Sydney, Australia (KC, KTS, MAS).
This study was approved by the State of Connecticut Department of Public Health (DPH) Human Investigations Committee. Certain data used in this study were obtained from DPH. The authors assume full responsibility for analyses and interpretation of these data.
EAB reports grants from the Norwegian Cancer Society during the conduct of the study. KC is is co-principal investigator of an unrelated investigator-initiated trial of cytology and primary human papillomavirus screening in Australia (Compass; ACTRN12613001207707 and NCT02328872), which is conducted and funded by the VCS Foundation, a government-funded health promotion charity. In 2013, the VCS Foundation received equipment and a funding contribution for the Compass trial from Roche Molecular Systems and Ventana. However, neither KC nor her institution (Cancer Council NSW) receives direct funding from industry for this trial or any other project. MAS reports grants from the National Health and Medical Research Council (Australia) and Cancer Institute NSW. All other authors declare no conflicts of interest.
We acknowledge Aanjaneya Shukla for work on developing earlier versions of the University of Minnesota (UMN) analysis.
Supplementary Material
References
- 1.American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018. [Google Scholar]
- 2.US Preventive Services Task Force. Draft recommendation statement: cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Accessed July 10, 2018.
- 3. Schiffman M, Castle PE.. The promise of global cervical-cancer prevention. N Engl J Med. 2005;353(20):2101–2104. [DOI] [PubMed] [Google Scholar]
- 4. Burger EA, Kim JJ, Sy S, Castle PE.. Age of acquiring causal human papillomavirus (HPV) infections: leveraging simulation models to explore the natural history of HPV-induced cervical cancer. Clin Infect Dis. 2017;65(6):893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mendes D, Bains I, Vanni T, Jit M.. Systematic review of model-based cervical screening evaluations. BMC Cancer. 2015;15(1):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim JJ, Burger EA, Regan C, Sy S.. Screening for cervical cancer in primary care: a decision analysis for the US Preventive Services Task Force. JAMA. 2018;320(7):706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB.. Model transparency and validation: a report of the ISPOR-SMDM modeling good research practices task force-7. Med Decis Making. 2012;32(5):733–743. [DOI] [PubMed] [Google Scholar]
- 8. Wheeler CM, Hunt WC, Cuzick J, et al. A population-based study of HPV genotype prevalence in the United States: baseline measures prior to mass HPV vaccination. Int J Cancer. 2013;132(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joste NE, Ronnett BM, Hunt WC, et al. Human papillomavirus genotype-specific prevalence across the continuum of cervical neoplasia and cancer. Am Soc Prev Oncol. 2015;24:230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst. 2015;107(6):djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connecticut Tumor Registry, Connecticut Department of Public Health. Cervical Cancer Incidence 1950–1969. https://portal.ct.gov/DPH/Tumor-Registry/CTR-Home. Accessed March 28, 2017.
- 12. Schiffman M, Wentzensen N.. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(4):553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herrero R, Hildesheim A, Rodriguez AC, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26(37):4795–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102(5):315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM.. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195(11):1582–1589. [DOI] [PubMed] [Google Scholar]
- 16. Hildesheim A, Schiffman MH, Gravitt PE, et al. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis. 1994;169(2):235–240. [DOI] [PubMed] [Google Scholar]
- 17. Kjaer S, Hogdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66(21):10630–10636. [DOI] [PubMed] [Google Scholar]
- 18. Molano M, Van den Brule A, Plummer M, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol. 2003;158(5):486–494. [DOI] [PubMed] [Google Scholar]
- 19. Canfell K, Barnabas R, Patnick J, Beral V.. The predicted effect of changes in cervical screening practice in the UK: results from a modelling study. Br J Cancer. 2004;91(3):530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bulkmans NW, Berkhof J, Bulk S, et al. High-risk HPV type-specific clearance rates in cervical screening. Br J Cancer. 2007;96(9):1419–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vink MA, Bogaards JA, van Kemenade FJ, de Melker HE, Meijer CJ, Berkhof J.. Clinical progression of high-grade cervical intraepithelial neoplasia: estimating the time to preclinical cervical cancer from doubly censored national registry data. Am J Epidemiol. 2013;178(7):1161–1169. [DOI] [PubMed] [Google Scholar]
- 22. Gustafsson L, Adami HO.. Natural history of cervical neoplasia: consistent results obtained by an identification technique. Br J Cancer. 1989;60(1):132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Oortmarssen GJ, Habbema JD.. Epidemiological evidence for age-dependent regression of pre-invasive cervical cancer. Br J Cancer. 1991;64(3):559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bos AB, van Ballegooijen M, van Oortmarssen GJ, van Marle ME, Habbema JD, Lynge E.. Non-progression of cervical intraepithelial neoplasia estimated from population-screening data. Br J Cancer. 1997;75(1):124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Insinga RP, Dasbach EJ, Elbasha EH.. Epidemiologic natural history and clinical management of human papillomavirus (HPV) disease: a critical and systematic review of the literature in the development of an HPV dynamic transmission model. BMC Infect Dis. 2009;9(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bosch FX, Robles C, Diaz M, et al. HPV-FASTER: broadening the scope for prevention of HPV-related cancer. Nat Rev Clin Oncol. 2016;13(2):119–132. [DOI] [PubMed] [Google Scholar]
- 27.Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR. 2019;68(32);698–702. [DOI] [PMC free article] [PubMed]
- 28. Hammer A, Rositch A, Qeadan F, Gravitt PE, Blaakaer J.. Age‐specific prevalence of HPV 16/18 genotypes in cervical cancer: a systematic review and meta‐analysis. Int J Cancer. 2016;138(12):2795–2803. [DOI] [PubMed] [Google Scholar]
- 29. Wheeler CM, Hunt WC, Joste NE, Key CR, Quint WGV, Castle PE.. Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. J Natl Cancer Inst. 2009;101(7):475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gravitt PE, van Doorn LJ, Quint W, et al. Human papillomavirus (HPV) genotyping using paired exfoliated cervicovaginal cells and paraffin-embedded tissues to highlight difficulties in attributing HPV types to specific lesions. J Clin Microbiol. 2007;45(10):3245–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wentzensen N, Walker J, Schiffman M, et al. Heterogeneity of high-grade cervical intraepithelial neoplasia related to HPV16: implications for natural history and management. Int J Cancer. 2013;132(1):148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim JJ, Canfell K, van Ballegooijen M, et al. Estimating the benefits and harms of primary HPV screening in the United States: a comparative modeling study. In: International Papillomavirus Conference; October 2–6, 2018; Sydney, Australia.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.