Abstract
Background
Patients with cancer may be at risk of high opioid use due to physical and psychosocial factors, although little data exist to inform providers and policymakers. Our aim is to examine overdoses from opioids leading to emergency department (ED) visits among patients with cancer in the United States.
Methods
The Healthcare Cost and Utilization Project Nationwide Emergency Department Sample was queried for all adult cancer-related patient visits with a primary diagnosis of opioid overdose between 2006 and 2015. Temporal trends and baseline differences between patients with and without opioid-related ED visits were evaluated. Multivariable logistic regression analysis was used to identify risk factors associated with opioid overdose. All statistical tests were two-sided.
Results
Between 2006 and 2015, there were a weighted total of 35 339 opioid-related ED visits among patients with cancer. During this time frame, the incidence of opioid-related ED visits for overdose increased twofold (P < .001). On multivariable regression (P < .001), comorbid diagnoses of chronic pain (odds ratio [OR] 4.51, 95% confidence interval [CI] = 4.13 to 4.93), substance use disorder (OR = 3.54, 95% CI = 3.28 to 3.82), and mood disorder (OR = 3.40, 95% CI = 3.16 to 3.65) were strongly associated with an opioid-related visit. Patients with head and neck cancer (OR = 2.04, 95% CI = 1.82 to 2.28) and multiple myeloma (OR = 1.73, 95% CI = 1.32 to 2.26) were also at risk for overdose.
Conclusions
Over the study period, the incidence of opioid-related ED visits in patients with cancer increased approximately twofold. Comorbid diagnoses and primary disease site may predict risk for opioid overdose.
Prescription opioids are commonly used in the management of pain in patients with cancer (1). Such patients may present with pain due to the disease process (2) or adverse effects from treatment (3). Patients with cancer are also vulnerable to emotional or psychological distress that might complement or exacerbate their physical pain, increasing their likelihood of nonmedical opioid use (4,5). Furthermore, cancer survivors continue to have higher opioid prescribing rates long after their disease has been treated (6–11). This may be in part due to chronic pain syndromes, which have been described in long-term cancer survivors (12).
Although guidelines exist for the treatment of cancer-related pain in adult patients (13,14), the optimal management of such symptoms can be challenging. Patients with advanced cancer can be on high doses of narcotics with various times of onset along with other sedating medications. Confusion may abound as to how to properly take these medications, leading to unintended aberrant opioid use. Patients engaging either unintentionally or actively in high-risk opioid use may be susceptible to both nonfatal and fatal overdoses. Such overdoses may lead to emergency department (ED) visits, and severe cases may result in respiratory intubation or even death (15,16). ED visits for opioid overdose have been studied extensively among the general population (17–20), although little is known about such visits among patients with cancer. One cohort study found that patients with cancer who used opiates after surgery were more likely to visit the ED compared with noncancer opiate users (21). Another study characterized temporal trends in and factors associated with opioid-related hospitalizations among patients with cancer (22). Yet not all overdoses result in hospitalizations, so lower acuity patients who were discharged from the ED would not have been captured.
To date, no study to our knowledge has examined opioid-related ED visits among patients with cancer on a national level. A national analysis of ED visits and hospitalizations from opioid overdoses in patients with cancer would help illuminate the current state of high-risk opioid use in this population, thereby enabling providers and policymakers to devote greater attention and resources to these patients. This study describes recent trends in opioid-related ED visits among patients with cancer and identifies clinical risk factors associated with opioid overdose in the cancer population.
Methods
Study Sample and Covariates
This study used the Nationwide Emergency Department Sample (NEDS) published by the Healthcare Cost and Utilization Project (HCUP) of the Agency for Healthcare Research and Quality. The NEDS is the largest all-payer ED database in the United States, yielding approximately 25 to 35 million ED visits each year across more than 950 hospitals in 34 states. It represents a 20% stratified sample of US hospital-based EDs. Each ED visit is given a discharge weight so that a national estimate may be obtained. These weights are assigned by HCUP during the sampling process based on ratios of total ED visits to ED visits sampled in the NEDS. All diagnoses reported in the NEDS were based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) system until September 30, 2015, after which the Tenth Revision (ICD-10-CM) was used. This study was granted an institutional review board exemption by the Yale Human Investigations Committee. Informed consent was also waived because the study was retrospective and data were deidentified.
The NEDS was queried from 2006 to 2015 for adult patients age 18 years and older. Patients with a diagnosis of cancer were identified using ED visits in which a cancer diagnosis was coded using Clinical Classifications Software (CCS) codes 11 to 45 as described previously (23). Patients with nonmelanoma skin cancer and carcinoma in situ were excluded from analysis given the low likelihood for cancer-related pain requiring opioids. Opioid-related ED visits were identified by selecting those with a primary diagnosis of any opioid overdose, which we categorized into prescription opioids (ICD-9-CM: 965.00, 965.02, 965.09; ICD-10-CM: T40.0, T40.2, T40.3, T40.4, T40.6) or heroin (ICD-9-CM: 965.01; ICD-10-CM: T40.1) as described previously (24). ICD-10-CM codes to identify opioid overdoses were provided by HCUP (25) and have been used in other published analyses (26,27). A flow diagram describing the patient selection criteria is depicted in Figure 1.
Figure 1.
Flow diagram demonstrating inclusion and exclusion criteria in the study.
ED visits and inpatient stays were characterized by demographic factors (age, sex, survey year, urban or rural designation), socioeconomic factors (insurance type, median household income by ZIP code), hospital characteristics (teaching status, trauma designation, region), comorbid risk factors for opioid overdose (bone metastases, substance use disorder, chronic pain, mood disorder), Elixhauser comorbidity index, primary cancer type, and inpatient outcomes (respiratory intubation, admission, death, length of stay, total charges). Urban or rural designation was based on the National Center for Health Statistics six-level classification scheme for US counties. Metropolitan counties with a population of at least 50 000 were defined as urban, consistent with the grouping used in an HCUP brief (28). The Elixhauser Comorbidity Index is a validated method to describe comorbidity burden in large administrative datasets (29). Index scores characterizing in-hospital mortality were created based on the code provided by HCUP. Of note, index scores were not created for ED visits with ICD-10-CM diagnoses, because a validated tool has yet to be created by HCUP for this new medical classification system. However, ICD-10-CM data only comprised a small (3%) proportion of the cohort.
Comorbid risk factors were obtained from secondary diagnoses for each ED visit. Patients with bone metastases were identified using codes previously validated in other administrative datasets (ICD-9-CM: 198.5; ICD-10-CM: C79.51, C79.52) (30,31). Substance use disorder was an aggregated category derived from CCS codes for alcohol (CCS: 660) and drug use disorder (CCS: 661) and ICD codes for tobacco use disorder or nicotine dependence (ICD-9-CM: 305.1; ICD-10-CM: F17, Z72.0). Because ICD-9-CM diagnosis codes for pain were created on October 1, 2006, analysis of comorbid chronic pain in this study began on January 1, 2007. This category included all ICD codes for chronic pain (ICD-9-CM: 338.2, 338.3, 338.4; ICD-10-CM: G89.2, G89.3, G89.4), because any etiology of chronic pain could potentially be related to a patient’s cancer diagnosis. Mood disorder used a single CCS code (CCS: 657), which primarily identifies patients with a history of depression or bipolar disorder. A full list of ICD and CCS codes used to define the study variables is detailed in Supplementary Table 1 (available online).
Statistical Analysis
Temporal trends and incidence rates of opioid-related ED visits, as well as comorbid risk factors for opioid overdose, among patients with cancer were analyzed. Similar trends were also evaluated among nonopioid-related cancer visits. Annual incidence was estimated by dividing the weighted number of ED visits by the number of adult cancer survivors for that given year, obtained from the National Health Interview Survey. For the purpose of this study, a cancer survivor was defined as any patient who had ever been diagnosed with cancer, in accordance with the definition from the National Cancer Institute (32). The Cochran-Armitage test for trend was used to assess for changes in the incidence of ED visits over time. Unadjusted univariate analysis was carried out through baseline comparison between patients with cancer presenting with or without an opioid-related ED visit using the Pearson’s χ2 test for categorical variables and an analysis of variance for continuous variables. Multivariable logistic regression was used to evaluate the association between comorbid risk factors and opioid overdose for both the entire cohort as well as for patients with metastatic disease (CCS: 42). Because temporality of cancer diagnosis cannot be determined from ICD coding, we used metastatic disease as a surrogate for patients with active cancer, given the low cure rates for most histologies. Further multivariable models were created for each of the top 10 most common primary cancers to determine the association between primary cancers and opioid-related ED visits. Hypothesis testing was two-sided, and P less than .05 was used to indicate statistical significance for all comparisons. A Bonferroni correction was applied to the multivariable models to adjust for multiple comparisons with a statistical significance threshold of P less than .004. Weighted frequencies were incorporated in all analyses to produce national estimates. Data analysis was carried out using STATA v16.0 (StataCorp LP, College Station, TX).
Results
Characteristics of Study Sample
A weighted total of 43 891 464 ED visits occurred for adult patients with cancer between 2006 and 2015, of which 35 339 (0.08%) were opioid related. The overall incidence of opioid-related ED visits during the study period was 23.9 per 100 000 cancer survivors. In 2015, there were 5324 opioid overdoses among patients with cancer, increased from 2078 in 2006. The majority of opioid-related visits involved prescription opioids (94.3%), whereas heroin comprised a minority (5.7%) of overdoses. A comparison of baseline characteristics of opioid-related and nonopioid-related ED visits is detailed in Supplementary Table 2 (available online). The five most common primary cancers among opioid-related ED visits were breast (16.4%), lung (15.6%), prostate (7.2%), head and neck (6.9%), and colon (6.4%) (Supplementary Table 3, available online).
Temporal Trends in Opioid-Related ED Visits
During the study period, the incidence of opioid-related ED visits per 100 000 cancer survivors increased by twofold from 15.7 in 2006 to 32.3 in 2015 (Ptrend, <.001) (Figure 2). Meanwhile, the incidence of nonopioid-related ED visits in survivors increased by 1.3-fold from 25 946 in 2006 to 33 121 in 2015 (Ptrend, <.001) (Supplementary Figure 1, available online). The incidence of comorbid risk factors among patients with cancer both with and without opioid overdoses is described in Figure 3 and Supplementary Figure 2 (available online), respectively. Among patients with opioid overdoses, ED visits with comorbid chronic pain increased the most over time (threefold), followed by substance use disorder (2.9-fold) and mood disorder (2.8-fold), whereas those with bone metastases increased the least (1.3-fold) (Ptrend, <.001). Comorbid risk factors for nonopioid cancer visits demonstrated similar trends, with the largest increase seen in visits with secondary diagnoses of chronic pain (4.3-fold) and the smallest in patients with bone metastases (1.2-fold) (Ptrend, <.001).
Figure 2.
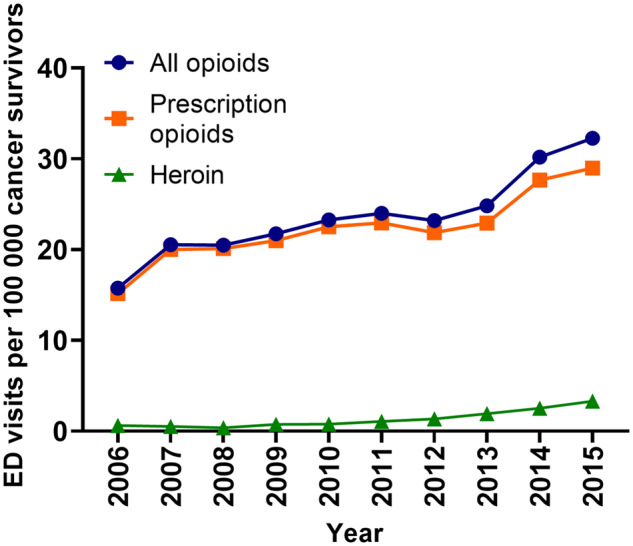
Temporal trends in opioid-related emergency department visits among patients with cancer by opioid type, 2006–2015. Two-sided Ptrend less than .001 for prescription opioid and heroin overdoses.
Figure 3.
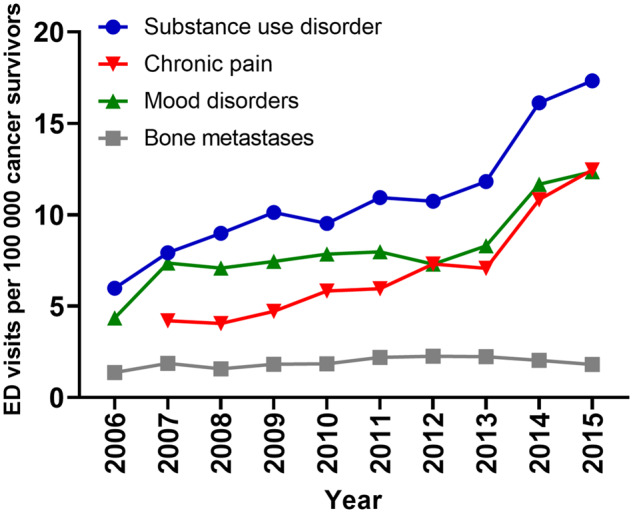
Temporal trends in opioid-related emergency department visits among patients with cancer by comorbid risk factor, 2006–2015. Two-sided Ptrend less than .001 for all risk factors. Temporal trends for chronic pain were analyzed beginning in 2007 to account for new diagnosis codes created on October 1, 2006.
On multivariable logistic regression, comorbidities statistically significantly associated with opioid-related ED visits among patients with cancer (P < .001 for all; reference: not having that comorbidity) included having bone metastases (odds ratio [OR] = 1.52, 95% confidence interval [CI] = 1.34 to 1.72), substance use disorder (OR = 3.54, 95% CI = 3.28 to 3.82), mood disorder (OR = 3.40, 95% CI = 3.16 to 3.65), and chronic pain (OR = 4.51, 95% CI = 4.13 to 4.93) (Table 1). Similarly, among patients with metastatic cancer (P < .001 for all), chronic pain (OR = 2.84, 95% CI = 2.33 to 3.46) and substance use disorder (OR = 2.72, 95% CI = 2.27 to 3.25) most strongly predicted for opioid overdose (Supplementary Table 4, available online). Primary cancers associated with the greatest odds for an opioid-related visit (P < .001 for all; reference: not having that cancer) included head and neck cancer (OR = 2.04, 95% CI = 1.82 to 2.28) and multiple myeloma (OR = 1.73, 95% CI = 1.32 to 2.26) (Figure 4). Among sociodemographic and hospital-related factors (P < .004 for all), younger age, higher Elixhauser comorbidity index, Medicare or Medicaid insurance (vs private), and West location (vs Northeast) were associated with a higher likelihood for opioid-related visits (Supplementary Table 5, available online).
Table 1.
Multivariable analysis for comorbidities associated with opioid-related ED visits among patients with cancer, 2006–2015
| Variable* | OR (95% CI) | P † |
|---|---|---|
| Bone metastases | 1.52 (1.34 to 1.72) | <.001 |
| Substance use disorder | 3.54 (3.28 to 3.82) | <.001 |
| Mood disorder | 3.40 (3.16 to 3.65) | <.001 |
| Chronic pain | 4.51 (4.13 to 4.93) | <.001 |
Model adjusted for sociodemographic factors, including age, sex, Elixhauser comorbidity index, household income, survey year, insurance type, and urban or rural designation; hospital-related factors, including geographic region, teaching status, and trauma designation; and primary cancer type. Reference categories are not having that comorbidity. CI = confidence interval; ED = emergency department; OR = odds ratio.
All P values were two-sided and derived from a multivariable logistic regression analysis.
Figure 4.
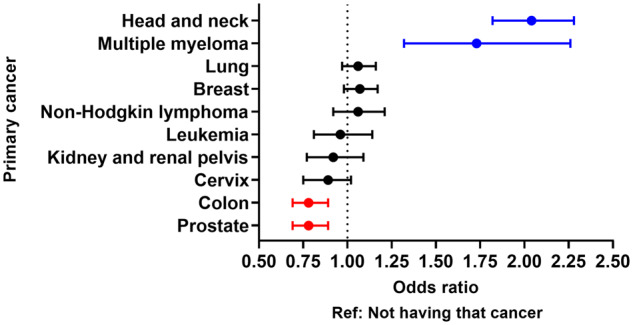
Forest plot depicting the association between primary cancers and opioid-related emergency department visits as assessed by multivariable models, adjusting for sociodemographic and hospital-related characteristics. Reference is not having that cancer. Two-sided P less than .001 for head and neck, multiple myeloma, colon, and prostate.
Discussion
This study provides a national analysis of opioid overdoses among patients with cancer resulting in ED visits and inpatient hospitalizations. During the study time frame, the incidence of opioid-related ED visits in patients with cancer doubled and increased at a faster rate compared with nonopioid-related visits in patients with cancer. ED visits with comorbid chronic pain, substance use disorder, and mood disorder increased the most during the study period and were most strongly associated with opioid overdoses. Diagnoses of head and neck cancer and multiple myeloma predicted for opioid-related ED visits.
These findings provide important insight into recent temporal trends in opioid overdoses in patients with cancer. To date, much media attention and scrutiny has been focused on opioid overdoses across the general population (33), although little is known about high opioid use among patients with cancer. Multiple studies suggest the risk of nonmedical opioid use among patients with cancer may be higher than previously thought, with estimated rates of 18–20% (5,34,35), and the rate of fatal overdoses is about 0.5–0.6 per 100 000 (36). Although our reported incidence of opioid-related ED visits in patients with cancer was small (23.9 visits per 100 000 cancer survivors), the absolute number of overdoses per year was just over 5000 in 2015, with a rate that is increasing and outpacing the rise in nonopioid-related cancer visits. This increase may be partially explained by a concurrent rise in comorbid risk factors for high opioid use, including chronic pain, mood disorders, and substance use disorder. Chronic pain was the strongest risk factor for opioid overdose in our analysis, and multiple reports show that nearly 30% of cancer survivors may be living with chronic pain (37,38), particularly those who undergo multimodality treatment (9). Mood disorders such as depression may exist in 10–20% of cancer survivors (39), and fear of recurrence may be reported in up to 80% of survivors. Patients with cancer and an underlying anxiety disorder may also receive a greater number of opioid prescriptions (40). A history of substance use disorder has been shown to be a strong risk factor for prolonged opioid use or misuse in patients with cancer (22,41,42). Notably, comorbid chronic pain or substance use disorder were most strongly associated with opioid overdose in patients with metastatic cancer, highlighting the need for appropriate pain management and addiction counseling for patients with active cancer. Together, these findings indicate a growing need for providers to address issues related to cancer survivorship and ensure that patients maintain a high quality of life both during and after treatment.
This study also revealed that diagnoses of head and neck cancer and multiple myeloma independently predicted for opioid overdose among the top 10 most common cancers in this cohort. These findings suggest that disease-specific factors might explain high-risk opioid use in patients with these cancers. Examples of such factors include locally advanced cancer stage, receipt of prior systemic therapy or radiotherapy, or severity of pain. In head and neck cancer, multiple reports have demonstrated a higher T-stage (42,43) and induction chemotherapy predict for long-term opioid use (41). Meanwhile, patients with multiple myeloma are known to develop osteolytic metastases, which are not only more painful than osteoblastic lesions (44) but also are more prone to fractures. Given the high incidence of bone metastases (90%) and fractures (80%) in patients with multiple myeloma (45,46), those with advanced disease may experience a high degree of pain, which could translate to increased opioid use. Notably, these factors could not be accounted for in this analysis, because they are either not captured or undercoded in the NEDS. Therefore, further exploration into disease-specific factors might help elucidate the impact of a patient’s primary cancer on the risk for opioid overdose.
Finally, it is important to place the findings of this study in the context of the opioid epidemic and ongoing legislative efforts to curb opioid overdoses. The time frame of this investigation coincides with a period of widespread national adoption of controlled-substance and dispensing laws regulating opioid prescribing. One report found no association between enactment of these laws and reductions in potentially hazardous use or overdose of opioids in the disabled Medicare population (47). These findings dovetail with the results from our study showing a steady increase in opioid overdose rates among patients with cancer over time. Therefore, we believe that clinicians should be aware of the risk of overdose among patients with cancer who are prescribed opioids. A thorough understanding of this issue as well as comorbidities that may increase the risk of this outcome will likely help encourage oncologists to more closely monitor the appropriateness of opioid use among their patients. Heightened awareness of this problem may also serve to increase patient referrals to specialized multidisciplinary palliative care and pain management services. Furthermore, given the concerns about national legislation restricting opioid access to patients with cancer-related pain (48), perhaps strategies to reduce overdoses in the oncologic population, such as targeted outpatient monitoring and improved patient education, should occur in the clinic. Going forward, it will be imperative to devise solutions that both protect patient access to pain medications as well as reduce potentially hazardous opioid use among patients with cancer.
The limitations of this study are inherent to those of observational datasets and retrospective analyses. First, opioid-related visits were defined by the primary discharge diagnosis, and it is possible that this may fail to capture overdoses that were coded using a secondary diagnosis. However, including secondary diagnoses would potentially compromise the specificity of the cohort and include nonopioid-related visits. Second, because the NEDS provides only visit-level data, it is not possible to calculate the true incidence of opioid overdoses at the patient level. Third, treatment-level data are sparse, so it is not possible to accurately determine whether patients were undergoing active treatment for cancer. Although treatment history of radiotherapy or chemotherapy is available through International Classification of Diseases coding, this is underused in the NEDS and therefore was not examined in our study. Similarly, cancer staging data are limited except for secondary diagnoses of metastatic disease. Fourth, our definition of chronic pain may capture patients with long-term pain from etiologies unrelated to their cancer diagnosis. Finally, the NEDS does not code for race or ethnicity, a demographic variable that has a known association with opioid use. Yet given the overall paucity of data on opioid-related overdoses in patients with cancer, the NEDS is one of the few databases that can be used to study this topic effectively.
Opioid overdoses in patients with cancer are a major national issue. Although opioid-related ED visits comprised a small proportion of all cancer-related visits, the incidence increased twofold during the study period, totaling over 5000 visits in 2015 and outpacing the growth rate of nonopioid visits in patients with cancer. Visits with secondary diagnoses of chronic pain, substance use disorder, and mood disorder increased markedly over time and were strongly associated with opioid overdoses. A growing proportion of cancer survivors with comorbid conditions linked to high opioid use may in part explain the rising rate of opioid-related ED visits. As patients with cancer experience greater longevity, it will be vitally important for providers both to understand the realities of cancer survivorship and meet the needs of this burgeoning population.
Funding
The authors report no sources of funding for this study.
Notes
Vikram Jairam, MD, and Daniel X. Yang, MD, have no conflicts of interest to report. James B. Yu, MD, MHS, has received research funding from 21st Century Oncology and served as a consultant for Augmentix. Henry S. Park, MD, MPH, has received honoraria from RadOncQuestions LLC.
Supplementary Material
References
- 1. Swarm RA, Dans M.. NCCN frameworks for resource stratification of NCCN guidelines: adult cancer pain and palliative care. J Natl Compr Canc Netw. 2018;16(5S):628–631. [DOI] [PubMed] [Google Scholar]
- 2. Cipta AM, Pietras CJ, Weiss TE, et al. Cancer-related pain management in clinical oncology. J Community Support Oncol. 2015;13(10):347–355. [DOI] [PubMed] [Google Scholar]
- 3. Paice JA. Chronic treatment-related pain in cancer survivors. Pain. 2011;152(suppl 3):S84–S89. [DOI] [PubMed] [Google Scholar]
- 4. Turk DC, Monarch ES, Williams AD.. Cancer patients in pain: considerations for assessing the whole person. Hematol Oncol Clin N Am. 2002;16(3):511–525. [DOI] [PubMed] [Google Scholar]
- 5. Arthur J, Bruera E.. Balancing opioid analgesia with the risk of nonmedical opioid use in patients with cancer. Nat Rev Clin Oncol. 2019;16(4):213–226. [DOI] [PubMed] [Google Scholar]
- 6. Sutradhar R, Lokku A, Barbera L.. Cancer survivorship and opioid prescribing rates: a population-based matched cohort study among individuals with and without a history of cancer. Cancer. 2017;123(21):4286–4293. [DOI] [PubMed] [Google Scholar]
- 7. Salz T, Lavery JA, Lipitz-Snyderman AN, et al. Trends in opioid use among older survivors of colorectal, lung, and breast cancers. J Clinc Oncol. 2019;37(12):1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clinc Oncol. 2014;32(16):1739–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Natalie M, Nessa C, Samuel E, et al. Chronic pain management in cancer survivors. J Natl Compr Canc Netw. 2010;8(9):1104–1110. [DOI] [PubMed] [Google Scholar]
- 10. Paice JA, Lacchetti C, Bruera E.. Management of chronic pain in survivors of adult cancers: ASCO clinical practice guideline summary. J Cinc Oncol. 2016;12(8):757–762. [DOI] [PubMed] [Google Scholar]
- 11. Lee J-J, Hu HM, Edelman AL, et al. New persistent opioid use among patients with cancer after curative-intent surgery. J Cinc Oncol. 2017;35(36):4042–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carmona-Bayonas A, Jimenez-Fonseca P, Castanon E, et al. Chronic opioid therapy in long-term cancer survivors. Clin Transl Oncol. 2017;19(2):236–250. [DOI] [PubMed] [Google Scholar]
- 13. Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(suppl 4):iv166–iv191. [DOI] [PubMed] [Google Scholar]
- 14. Dalal S, Bruera E.. Pain management for patients with advanced cancer in the opioid epidemic era. Am Soc Clin Oncol Educ Book. 2019;39:24–35. [DOI] [PubMed] [Google Scholar]
- 15. Hua A, Haight S, Hoffman RS, et al. Endotracheal intubation after acute drug overdoses: incidence, complications, and risk factors. J Emerg Med. 2017;52(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fox LM, Hoffman RS, Vlahov D, et al. Risk factors for severe respiratory depression from prescription opioid overdose. Addiction. 2018;113(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scheuermeyer FX, DeWitt C, Christenson J, et al. Safety of a brief emergency department observation protocol for patients with presumed fentanyl overdose. Ann Emerg Med. 2018;72(1):1–8.e1. [DOI] [PubMed] [Google Scholar]
- 18. Tadros A, Layman SM, Davis SM, et al. Emergency visits for prescription opioid poisonings. J Emerg Med. 2015;49(6):871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coben JH, Davis SM, Furbee PM, et al. Hospitalizations for poisoning by prescription opioids, sedatives, and tranquilizers. Am J Prev Med. 2010;38(5):517–524. [DOI] [PubMed] [Google Scholar]
- 20. Kay C, Bernstein J, Fergestrom N, et al. Opioid-related emergency department visits and hospitalizations among commercially insured individuals, 2009-2015. Clin J Pain. 2018;34(12):1121–1125. [DOI] [PubMed] [Google Scholar]
- 21. Kurteva S, Tamblyn R, Meguerditchian A.. EPR19-069: opioid use among cancer patients undergoing surgery and their associated risk of re-admissions and emergency department visits in the 1-year postsurgical period. J Natl Compr Canc Netw. 2019;17(3.5):EPR19-069. [Google Scholar]
- 22. Chua IS, Leiter RE, Brizzi KT, et al. US national trends in opioid-related hospitalizations among patients with cancer. JAMA Oncol. 2019;5(5):734–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rivera DR, Gallicchio L, Brown J, et al. Trends in adult cancer–related emergency department utilization: an analysis of data from the nationwide emergency department sample. JAMA Oncol. 2017;3(10):e172450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997 to 2012. JAMA Pediatr. 2016;170(12):1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weiss AM, Heslin KC. Opioid-related hospital stays among women in the United States, 2016: statistical brief #247. 2019. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs Rockville, MD: Agency for Healthcare Research and Quality (US); 2006; Table 1, ICD-10-CM diagnosis codes defining different opioid-related conditions. Available at: https://www.ncbi.nlm.nih.gov/books/NBK538344/table/sb247.tab1. Accessed November 26, 2019.
- 26. Haffajee RL, Lin LA, Bohnert ASB, et al. Characteristics of US counties with high opioid overdose mortality and low capacity to deliver medications for opioid use disorder. JAMA Netw Open. 2019;2(6):e196373–e196373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Follman S, Arora VM, Lyttle C, et al. Naloxone prescriptions among commercially insured individuals at high risk of opioid overdose. JAMA Netw Open. 2019;2(5):e193209–e193209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Owens PL, Mutter R, Emergency department visits related to eye injuries, 2008: statistical brief #112. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2006. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb112.pdf. Accessed November 25, 2019. [PubMed] [Google Scholar]
- 29. Moore BJ, White S, Washington R, et al. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698–705. [DOI] [PubMed] [Google Scholar]
- 30. Liede A, Hernandez RK, Roth M, et al. Validation of International Classification of Diseases coding for bone metastases in electronic health records using technology-enabled abstraction. Clin Epidemiol. 2015;7:441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jensen AØ, Nørgaard M, Yong M, et al. Validity of the recorded International Classification of Diseases, 10th edition diagnoses codes of bone metastases and skeletal-related events in breast and prostate cancer patients in the Danish National Registry of Patients. Clin Epidemiol. 2009;1:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Cancer Institute. NCI Dictionary of Cancer Terms. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/survivor. Accessed October 26, 2019.
- 33.U.S. Department of Health and Human Services. What is the US Opioid Epidemic?https://www.hhs.gov/opioids/about-the-epidemic/index.html. Last Updated September 4, 2019. Accessed November 25, 2019.
- 34. Arthur J, Reddy A.. Opioid prescribing in an opioid crisis: what basic skills should an oncologist have regarding opioid therapy? Curr Treat Options in Oncol. 2019;20(5):39. [DOI] [PubMed] [Google Scholar]
- 35. Kwon JH, Tanco K, Park JC, et al. Frequency, predictors, and medical record documentation of chemical coping among advanced cancer patients. Oncologist. 2015;20(6):692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chino FL, Kamal A, Chino JP.. Opioid-associated deaths in patients with cancer: a population study of the opioid epidemic over the past 10 years. J Clinc Oncol. 2018;36(suppl 30):230–230. [Google Scholar]
- 37. Jiang C, Wang H, Wang Q, et al. Prevalence of chronic pain and high-impact chronic pain in cancer survivors in the United States. JAMA Oncol. 2019;5(8):1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sanford NN, Sher DJ, Butler SS, et al. Prevalence of chronic pain among cancer survivors in the United States, 2010-2017. Cancer. 2019;125(23):4310–4318. [DOI] [PubMed] [Google Scholar]
- 39. Yi JC, Syrjala KL.. Anxiety and depression in cancer survivors. Med Clin N Am. 2017;101(6):1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Henry M, Alias A, Frenkiel S, et al. Anxiety disorders contribute to extent of opioid prescription in head and neck cancer: a longitudinal study. J Pain Symptom Manag. 2018;56(6):e63–e64. [Google Scholar]
- 41. Smith WH, Luskin I, Resende Salgado L, et al. Risk of prolonged opioid use among cancer patients undergoing curative intent radiation therapy for head and neck malignancies. Oral Oncol. 2019;92:1–5. [DOI] [PubMed] [Google Scholar]
- 42. McDermott JD, Eguchi M, Stokes WA, et al. Short- and long-term opioid use in patients with oral and oropharynx cancer. Otolaryngol Head Neck Surg. 2019;160(3):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Silver N, Dourado J, Hitchcock K, et al. Chronic opioid use in patients undergoing treatment for oropharyngeal cancer. Laryngoscope. 2019;129(9):2087–2093. [DOI] [PubMed] [Google Scholar]
- 44. Parkes A, Warneke CL, Clifton K, et al. Prognostic factors in patients with metastatic breast cancer with bone-only metastases. Oncologist. 2018;23(11):1282–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zamagni E, Cavo M, Fakhri B, et al. Bones in multiple myeloma: imaging and therapy. Am Soc Clin Oncol Educ Book. 2018;38:638–646. [DOI] [PubMed] [Google Scholar]
- 46. Melton IIL, Kyle RA, Achenbach SJ, et al. Fracture risk with multiple myeloma: a population-based study. J Bone Miner Res. 2004;20(3):487–493. [DOI] [PubMed] [Google Scholar]
- 47. Meara E, Horwitz JR, Powell W, et al. State legal restrictions and prescription-opioid use among disabled adults. N Engl J Med. 2016;375(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Page R, Blanchard E.. Opioids and cancer pain: patients’ needs and access challenges. J Oncol Pract. 2019;15(5):229–231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.