Abstract
The incidence of melanoma in the United States has been increasing over the past several decades. Prognosis largely depends on disease stage, with 5-year melanoma-specific survival ranging from as high as 99% in patients with stage I disease to less than 10% for some patients with stage IV (distant metastatic) disease. Fortunately, in the last 5–10 years, there have been remarkable treatment advances for patients with high-risk resectable melanoma, including approval of targeted and immune checkpoint blockade therapies. In addition, results of recent clinical trials have confirmed the importance of sentinel lymph node biopsy and continue to refine the approach to regional lymph node basin management. Lastly, the melanoma staging system was revised in the eighth edition AJCC Cancer Staging Manual, which was implemented on January 1, 2018. Here we discuss these changes and the clinicopathological features that confer high risk for locoregional and distant disease relapse and poor survival. Implications regarding the management of melanoma in the metastatic and adjuvant settings are discussed, as are future directions for neoadjuvant therapies.
Melanoma incidence in the United States has risen over the past several decades (1). Most patients with stages I–II cutaneous melanoma have a favorable, albeit heterogeneous prognosis; those with stages III–IV melanoma have a historically poorer prognosis. In the past 5–10 years, the clinical landscape for patients with stages III–IV melanoma has markedly improved with the introduction of more effective systemic therapies, including molecularly targeted agents and immune checkpoint blockade (ICB) (2–11) in the adjuvant and metastatic arenas, resulting in notable improvements in survival. Surgical management also continues to evolve, with confirmation of the importance of sentinel lymph node (SLN) biopsy (SLNB), refinement of our approach to completion lymph node dissection (CLND) (12–14), and the development of neoadjuvant treatment strategies. Thus, prognostic features affecting recurrence risk and outcomes across the continuum of stages II–IV melanoma must be considered during clinical decision-making with respect to nodal staging, adjuvant therapy, and neoadjuvant therapy protocols. Here, we discuss the evolving landscape of high-risk melanoma, including staging, clinical features, and contemporary and future directions in the multidisciplinary management of patients with stages II–IV resectable melanoma.
Melanoma Staging
AJCC Eighth Edition Melanoma Staging System
The American Joint Committee on Cancer (AJCC) Melanoma Expert Panel (MEP) revised the melanoma staging system, published in the eighth edition (8e) AJCC Cancer Staging Manual (15) in 2017 (Figure 1). Revisions (Table 1) were based on analyses from the International Melanoma Database and Discovery Platform, containing prospective data for over than 46 000 patients with stages I–III melanoma diagnosed between 1998 and 2014 (16). Additional input was obtained from the legacy AJCC seventh edition (7e) stage IV analysis, supplemented by published clinical trial data (16).
Figure 1.
AJCC 8th edition pathological prognostic groups (TNM) for stage I to III cutaneous melanoma*†. NX = Regional nodes not assessed (eg, SLN biopsy not performed, regional nodes previously removed for another reason); SLN = sentinel lymph node; T0 = no evidence of primary tumor (eg, unknown primary or completely regressed melanoma); Tis = melanoma in situ; TX = thickness cannot be assessed. Exception: pathological N category is not required for T1 melanomas, use cN. *Pathological stage is IV for any T, any N, and M1 disease. †Adapted and used with permission of the American Joint Committee on Cancer (AJCC), Chicago, IL. The original and primary source for this information is the AJCC Cancer Staging Manual, Eighth Edition (2017), published by Springer International Publishing (Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin AB, Edge SB, Greene, FL, et al. eds. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017:563–585).
Table 1.
Summary of major changes in the AJCC 8e Melanoma Staging System*
| Change | Summary of change |
|---|---|
| Definition of primary tumor (T) |
|
| Definition of regional lymph node (N) |
|
| Definition of distant metastasis (M) |
|
| AJCC prognostic stage groups |
|
Adapted (with permission of the American Joint Committee on Cancer [AJCC], Chicago, IL] from: the AJCC Cancer Staging Manual, Eighth Edition (2017) published by Springer International Publishing (Gershenwald JE, et al. Melanoma of the skin. In: Amin MB, Edge SB, Greene FL, et al, ed. AJCC Cancer Staging Manual. 8th ed. New York: Springer International Publishing; 2017:563–585). CNS = central nervous system; 8e = AJCC Cancer Staging Manual 8th edition; LDH = lactate dehydrogenase.
Primary Melanoma Clinicopathological Features
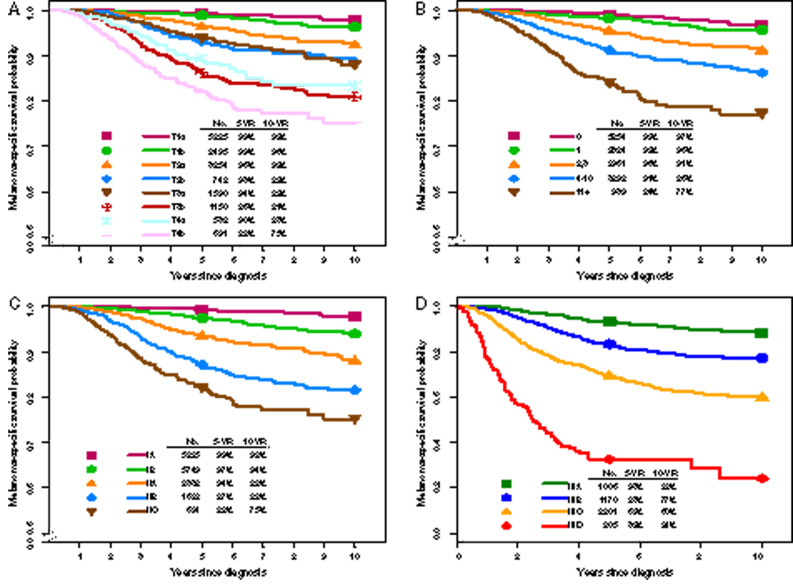
Breslow (tumor) thickness has been validated in multiple studies, including recent AJCC analyses (Figure 2A), and continues to represent a foundational component of melanoma staging (16–22). Previous studies have suggested that survival among T1 category patients is related to tumor thickness, with a possible clinically important “breakpoint” of 0.7–0.8 mm (18,19,21). In the 8e multivariable melanoma-specific survival (MSS) analysis of 7565 T1N0 patients (17), 0.8-mm tumor thickness threshold, mitotic rate (MR, dichotomized as <1 vs ≥1 mitosis/mm2), and ulceration were evaluated. Based on these analyses, T1a is defined as nonulcerated and less than 0.8 mm, and T1b as 0.8–1.0 mm (regardless of ulceration status) or ulcerated if less than 0.8 mm (15,17).
Figure 2.
Melanoma-specific survival (MSS) according to T subcategory and mitotic rate (MR) for patients with stage I and II melanoma and according to stage I–III subgroups from the eighth edition International Melanoma Database.* MSS according to (A) T subcategory and (B) MR for patients with stage I and II melanoma, and according to (C) stage I–II subgroup and (D) stage III subgroup. *Adapted and used with permission from Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition Cancer Staging Manual. CA Cancer J Clin. 2017; 67:472–492. 2017 John Wiley & Sons, Inc.
Primary tumor ulceration is an adverse prognostic factor for node-negative patients and patients with stage III disease (16,23–30). In the 8e, ulceration is designated as absent or present in each T category; patients with ulcerated primary melanomas have outcomes similar to those without ulceration in the next highest T category (Figure 2A) (15–17,31).
Numerous studies have shown a negative association between MR and survival (19,32–39). Based on these studies, MR was incorporated as a dichotomous variable and T1 subcategory criterion in the 7e. Although MR was not a statistically significant factor (as a dichotomous variable) for T1 MSS in the 8e analyses and was removed as a T1 criterion, increasing MR as a continuous variable was associated with decreasing MSS among patients with clinically node-negative primary melanoma (Figure 2B) (17). MR continues to have important prognostic value regardless of thickness and is associated with increased risk of SLN metastasis (15,17,37–41). The AJCC MEP and National Comprehensive Cancer Network (NCCN) guidelines recommend that MR be recorded for all primary melanomas (17, 42).
Other features of primary melanoma not included in the 8e that should be recorded include Clark level (18,20,43–48), presence and density of tumor-infiltrating lymphocytes (49–56), and lymphovascular invasion (57–61).
Stages I and II
8e pathological stage I and II subgroups are mostly unchanged from the 7e; pathological stage IA now includes T1bN0M0 (formerly stage IB), reflecting better survival of patients with T1b melanoma and pathologically negative SLNs (Table 1) (17). Because patients with clinically node-negative T2–T4 melanoma had to undergo SLNB for inclusion in 8e analyses (in contrast to the 7e), MSS was higher across pathological stages I–II groups compared with the 7e (as in the 7e such T2-T4 patients were included even if SLNB was not performed). Patients with stages IA–IIA melanoma have a favorable prognosis, with 5-year MSS of 94–99% (Figure 2C). Given the AJCC MEP’s intentional preservation of anatomic (TNM) stratification for staging purposes and revisions to the stage III groups, as in the 7e, there is prognostic overlap between stage II and stage III patients, with 8e stage IIB and IIC patients having similar or slightly lower 5-year MSS compared with 8e stage IIIA and IIIB patients (Figure 2, C and D) (17). Thus, from a clinical perspective, high-risk resectable melanoma should include stage IIB and IIC disease (Figure 1). Ongoing (eg, NCT03553836) or proposed adjuvant clinical trials for high-risk SLNB-negative patients highlight the interest in exploring adjuvant approaches to mitigate risk of relapse.
Clinicopathological Features of Regionally Metastatic Melanoma
In the 8e, the N category includes extent and number of tumor-involved regional lymph nodes (RLNs) (Figure 1) (15). “Clinically occult” nodal metastases describe patients without clinical evidence of RLN metastasis who have RLN metastasis identified by SLNB (termed “microscopic” in the 7e). “Clinically detected” nodal metastases describe patients with RLN metastasis detected by clinical or radiographic examination (termed “macroscopic” in the 7e) (15). Patients with clinically occult vs clinically detected regional disease generally have longer survival (29,45,62,63), although prognosis varies (Figure 2D) (15,16,29). The number of tumor involved RLNs is an important predictor of survival (Figure 2D) (44,64,65). Patients with clinically occult or clinically detected RLN metastases are subcategorized based on the number of tumor-involved nodes (15) (Figure 1).
Extranodal tumor extension (ENE) or extracapsular extension, defined as a nodal metastasis extending through the lymph node (LN) capsule into adjacent tissues, usually occurs with large clinically detected nodal metastases that demonstrate gross effacement of normal nodal architecture but are occasionally observed with smaller LN metastases. Although not included as an 8e N category criterion, it is recommended that ENE be recorded (15,17,66).
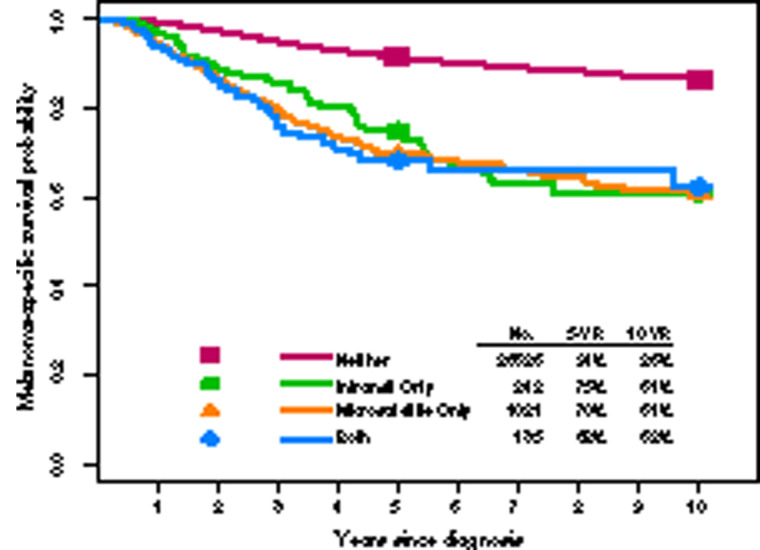
Presence of microsatellite, satellite, or in-transit metastases, thought to represent intralymphatic or angiotrophic metastases (44,67–74), constitute 8e N category nonnodal locoregional components (Figure 1). As there was no substantial difference in survival in 8e univariate analysis among these entities, they were grouped for staging and are designated N1c, N2c, or N3c depending on the number of involved RLNs (Figure 3) (17).
Figure 3.
Melanoma-specific survival according to presence of in-transit or satellite disease from the eighth edition International Melanoma Database.* *Adapted and used with permission from Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition Cancer Staging Manual. CA Cancer J Clin. 2017; 67:472–492. 2017 John Wiley & Sons, Inc.
Stage III
RLNs are the most common first metastatic site among patients with cutaneous melanoma. In the 8e, the N category includes patients with metastatic disease in RLNs and/or nonnodal locoregional sites (Figure 1). Patients with stage III melanoma have heterogeneous prognosis, with 5-year MSS varying from 32% to 93% depending on primary tumor characteristics, tumor burden within RLNs, number of RLNs involved, and presence of nonnodal locoregional metastases (17). Clinical management and design of future adjuvant therapy clinical trials should therefore reflect the wide variation in outcomes across stage III subgroups.
Although most patients with metastatic melanoma present with a known primary tumor, up to 10% of patients (with some higher estimates) with nodal metastases at diagnosis have no identifiable primary tumor and no history of primary melanoma (75–78). These patients demonstrate similar if not better prognosis than those with regional metastatic melanoma from a known primary site (76,77). Because the natural history of stage III melanoma from an unknown primary site is comparable with that of stage III melanoma with a known primary melanoma (1,79–84), patients with regional metastatic melanoma (LN involvement and/or pathologically confirmed skin or subcutaneous melanoma metastases) and no known primary site should be classified as stage III if distant metastases are not identified by appropriate evaluation and considered for surgery along with neoadjuvant and adjuvant treatment (15).
Clinicopathological Features of Melanoma Distant Metastasis
The 8e analysis yielded several changes from the 7e with respect to categorization based on site of distant metastasis (Table 1) (15,17). Patients with distant metastasis to the skin, subcutaneous tissue, muscle, or LNs are again categorized as M1a and have a more favorable prognosis than patients with another distant metastasis (16,83,85–88). Patients with lung metastasis are again categorized as M1b. Patients with central nervous system (CNS) disease have the worst prognosis (89–92) and have been frequently excluded from clinical trials, particularly if metastases are untreated and/or active (3,4,6–8,93–103). A new 8e subcategory (M1d) was added to stratify patients with CNS disease, given the importance of CNS metastasis in clinical decision-making and to facilitate clinical trial design, stratification, and analysis, as therapeutic options for these patients are actively explored (93,94,97,102,104,105). Patients with non-CNS visceral metastasis now constitute a refined M1c category.
Patients with distant metastasis and elevated serum lactate dehydrogenase (LDH) have worse survival compared with patients with similar sites of metastasis and normal LDH (15,16,84,106–113). Even with effective systemic therapies, elevated LDH level is negatively associated with response, progression-free survival, MSS, and overall survival (OS) (107–109,114–116). To better account for these associations, each 8e M subcategory now includes an LDH-related suffix (“[0]”, not elevated; “[1]”, elevated) to provide additional granularity for clinical decision-making and for clinical trial design, stratification, and analysis (15).
Stage IV
Treatment options have improved for patients with stage IV melanoma during the past 8 years. Because long-term survival data are not yet available for the most contemporary and still-evolving treatment approaches, the AJCC 8e MEP concluded that it was premature to embark on a broad-based analytic initiative. Rather, the 7e stage IV international database, which included patients who presented with or developed stage IV disease through 2008, supplemented by published clinical trial data, was used (3–8,16,96–103,117,118).
Surgical Approach to High-Risk Resectable Melanoma
Resection Margins
Prospective randomized controlled trials (RCTs) have investigated the optimal surgical resection margins for primary cutaneous melanoma and are incorporated into NCCN guidelines (42); they are sometimes modified to accommodate functional and/or anatomic considerations. There is ongoing interest in exploring a narrower surgical resection margin for patients with Breslow thickness greater than 2 mm or 1–2 mm with ulceration (pT2b-pT4b, AJCC 8e) in an ongoing phase III, multicenter, noninferiority-based RCT comparing 1-cm vs 2-cm margins (NCT03860883). Implications of narrower resection margins include decreased surgical morbidity and improved patient-reported outcomes; risks of decreased locoregional disease control and increased recurrence rate may be mitigated in this era of more effective systemic therapies.
The Role of SLNB and Lymphadenectomy
Although the most common site of melanoma metastasis is the RLN basin, most RLN metastases are clinically occult. Lymphatic mapping and SLNB to identify RLN metastases are the standard for RLN basin evaluation and staging for patients with cutaneous melanoma 1 mm or more in thickness (12,13,119–121). Because the risk of harboring occult RLN disease has been shown to be greater than or equal to 5% (and a minimum threshold for many clinicians to offer the procedure to otherwise healthy patients) for most patients with a primary tumor 0.8 mm or larger, for tumors 0.8–1.0 mm in thickness, NCCN guidelines state that SLNB may be discussed and considered, although there is no uniform consensus defining “high-risk features” in this prevalent patient group (40,42,122).
In 1999, Gershenwald et al. reported that SLN status was the most statistically significant prognostic factor for disease-free survival and MSS (121). These findings have been corroborated in subsequent literature (13,14,123–125), including the landmark Multicenter Selective Lymphadenectomy Trial-I (MSLT-I), which confirmed the prognostic significance of the SLN. Until recently, CLND was recommended for most patients with a positive SLNB (42). However, CLND carries risks, including wound infections and lymphedema. Furthermore, because only 10–20% of SLN-positive patients have tumor-involved non-SLNs at CLND, SLNB alone may be sufficient to confer the survival benefit seen in a subset of MSLT-I patients (14).
To address this question, two multicenter RCTs (DeCOG-SLT, MSLT-II) evaluated immediate CLND in patients with a positive SLN compared with nodal observation with or without ultrasound and showed that CLND did not provide clinically or statistically significant MSS benefits over nodal observation (14,126). Although prognostic information and regional control were improved with CLND, increased lymphedema was associated with CLND in both studies. Current NCCN guidelines recommend either nodal basin ultrasound surveillance or consideration of CLND for SLN-positive melanoma (42); however, the results of DeCOG-SLT and MSLT-II are clearly practice-changing and have begun to markedly reduce the fraction of SLN-positive patients undergoing CLND. Consideration of which patients, if any, benefit from CLND, as well as determining the “new” natural history of patients who do not undergo CLND in this era of more effective systemic therapy, are areas of ongoing clinical interest.
The evolving role of CLND coincides with an increase in more effective adjuvant therapy options. Importantly, it remains unclear the extent to which potential loss of prognostic information from CLND (ie, non-SLN tumor involvement as part of multivariable modeling and/or as a mechanism by which some patients may be upstaged to a higher stage III subgroup) affects decision-making regarding adjuvant therapy. Because the likelihood of patients with 8e stage IIIA melanoma having non-SLN tumor involvement on CLND is estimated to be quite low based on prior risk models, few would likely be upstaged (127,128). Prognostic models are currently being developed that may obviate the importance of non-SLN information for staging and decision-making purposes (129).
Adjuvant Therapy for High-Risk Resected Melanoma
The introduction of new targeted therapies and immune checkpoint inhibitors has markedly changed the adjuvant treatment landscape for patients with high-risk resected melanoma. Prior to this, adjuvant treatment was largely restricted to interferon therapy and limited by its poor tolerability and adverse events (AEs) that affect quality of life (130–132). Targeted combination regimens of BRAF and mitogen-activated protein kinase kinase (MEK) inhibitors, including dabrafenib plus trametinib, vemurafenib plus cobimetinib, and more recently, encorafenib plus cobimetinib, have improved outcomes for patients whose tumors test positive for the BRAF V600 driver mutation vs BRAF inhibitor monotherapy (4,5,11,99,101,109,117,133,134). Immune checkpoint inhibitors (anti-CTLA-4 and anti-PD1 antibodies) have also demonstrated favorable results, first for unresectable or metastatic melanoma and subsequently in the adjuvant setting (6,8,100,135,136).
Adjuvant Anti-CTLA-4 Therapy
Adjuvant ipilimumab, an anti-CTLA-4 therapy, was approved after demonstrating improved efficacy (recurrence-free survival [RFS]; distant metastasis-free survival; OS) compared with placebo in a phase III RCT in patients with high-risk resected melanoma (135,136) despite marked immune-related AEs and some deaths.
Adjuvant Anti-PD-1 Therapy
Following approval of adjuvant ipilimumab, nivolumab was compared with ipilimumab in the adjuvant setting for resected AJCC 7e stage IIIB, IIIC, or IV melanoma in the double-blind RCT CheckMate-238 (NCT02388906) (10). Patients in the nivolumab arm experienced statistically significantly longer RFS at 1 year (70.5% vs 60.8%, P < .001) and fewer grade 3 and 4 AEs (14.4% vs 45.9%). More patients completed 1 year of treatment (60.8% vs 26.9%) and fewer discontinued treatment (9.7% vs 42.6%) in the nivolumab vs ipilimumab arm. Based on these results, nivolumab received FDA approval for patients with LN involvement or metastatic disease who have undergone complete resection.
In KEYNOTE-054 (NCT02362594) (9), pembrolizumab was compared with placebo in patients with completely resected AJCC 7e stage III melanoma, with patients eligible for crossover to pembrolizumab upon disease recurrence. Eligible patients had either AJCC 7e stage IIIA melanoma (patients with stage IIIA melanoma had to have ≥1 LN metastasis >1 mm in greatest diameter) or IIIB or IIIC disease. Patients who received adjuvant pembrolizumab had a statistically significantly higher 1-year RFS (75.4% vs 61.0%, P < .001) with benefit independent of tumor PD-L1 status (9). In a recent post hoc analysis, AJCC 8e stage III subgroup had strong prognostic significance (with a caveat that longer follow-up is required to better assess treatment impact in the AJCC 8e stage IIIA cohort), as demonstrated by 1-year RFS rates with pembrolizumab vs placebo (IIIA, 92.7% vs 92.5%; IIIB, 79.0% vs 65.5%; IIIC, 73.6% vs 53.9%; IIID, 50.0% vs 33.3%), suggesting that treatment recommendations may need to be tailored to stage III subgroup (137).
Although most clinicians currently favor anti-PD-1–based approaches based on risk-benefit results to date, longer follow-up of RCTs evaluating adjuvant anti-PD-1 therapy will be needed to determine whether they improve MSS (138).
Adjuvant Targeted Therapies
For the approximately 40–50% of patients with BRAF V600-mutant melanoma, combined BRAF plus MEK inhibition with dabrafenib plus trametinib has resulted in improved survival in patients with this driver-mutation (3–5,109,115,139–141). In the COMBI-AD trial, patients with AJCC 7e stage IIIA (patients with stage IIIA melanoma had to have ≥1 LN metastasis >1 mm in greatest diameter), IIB, or IIIC BRAF V600-mutant melanoma who received adjuvant dabrafenib plus trametinib experienced statistically significantly higher 3-year RFS (58% vs 39%, P < .001) and 3-year OS (86% vs 77%, P = .0006) compared with placebo (11), with similar AEs to those reported in patients with BRAF V600-mutant metastatic melanoma (3–5,98,109,115). Most patients completed 1 year of scheduled treatment; however, 26% discontinued therapy due to AEs. Dabrafenib plus trametinib was approved by the FDA in April 2018 for the adjuvant treatment of BRAF V600E/K-mutant melanoma, and unlike CheckMate-238 and KEYNOTE-054, COMBI-AD included an early OS readout. Interestingly, in a recent exploratory analysis of extended study follow-up, RFS benefit was also observed across all AJCC 8e stage III subgroups (142), supporting that AJCC 8e and planned integrative risk models may help to inform ICB-related clinical decision-making going forward.
Adjuvant Radiation Therapy
In select circumstances, adjuvant radiation therapy (RT) can be considered for patients with high-risk resected melanoma. Multiple retrospective studies reported improved regional disease control in patients at high risk of relapse who undergo lymphadenectomy and receive adjuvant RT to nodal basins (143–146). Features associated with increased risk of regional failure include multiple positive LNs, 1 or more large node(s), ENE, and extranodal disease (147–151). A prospective, multicenter, phase III RCT (ANZMTG 01.02/TROG 02.01) in patients at high risk for LN field relapse after therapeutic lymphadenectomy demonstrated that adjuvant regional RT decreased the risk of local recurrence compared with nodal basin observation only, often with increased risk of lymphedema without improvement in OS (152,153). In view of these data, combined with exciting developments in the adjuvant systemic therapy arena, the role of adjuvant RT for high-risk resected melanoma remains limited but should be discussed with patients at high risk of nodal failure after lymphadenectomy in the context of a multidisciplinary team approach.
Advances on the Horizon: Neoadjuvant Therapy for High-Risk Resectable Melanoma
Rationale
Interest in evaluating targeted therapies and immune checkpoint inhibitors in the neoadjuvant setting for locally and regionally advanced melanoma is growing (Supplementary Table 1 available online) (154). Neoadjuvant treatment may also facilitate surgical resection in patients with locally advanced disease who are at high risk for incomplete resection or positive resection margins, or in whom upfront surgery may not be feasible. Neoadjuvant approaches using chemotherapy and chemoradiation have been shown to improve survival and/or surgical outcomes in patients with multiple other solid malignancies (155–168). Neoadjuvant biochemotherapy in melanoma patients with locoregional metastases has also been explored (169).
Efficacy of systemic therapy can be evaluated preoperatively by monitoring tumor response and postoperatively by pathological evaluation of the resected tumor (170). Preclinical and early clinical studies suggest that neoadjuvant checkpoint blockade may facilitate resectability of high-risk or borderline resectable lesions and may improve recurrence and survival compared with adjuvant therapy (171–173).
Clinical Trials of Neoadjuvant Targeted Therapies and Immune Checkpoint Blockade in Resectable Stages III and IV Melanoma
Neoadjuvant trials include ongoing or actively recruiting early-phase studies of targeted combination therapy and ICB (Supplementary Table 1 available online). Interim analysis of the Combi-Neo trial (NCT02231775), in which patients with stage IIIB or IIIC or oligometastatic stage IV BRAF-mutant melanoma were randomly assigned to up-front surgery vs neoadjuvant combination dabrafenib plus trametinib, demonstrated a high pathological complete response rate (58%) along with statistically significantly improved event-free survival (median 19.7 vs 2.9 months, P < .0001) over surgery (174), and in the single-arm NeoCombi trial (NCT01972347) of 40 patients receiving neoadjuvant dabrafenib plus trametinib, 86% achieved RECIST response with a 49% pathological complete response rate (175).
Two studies of neoadjuvant ICB recently reported results. Blank et al. reported a randomized, phase Ib study to test the feasibility and compare the efficacy of neoadjuvant ipilimumab plus nivolumab with adjuvant therapy using the same regimen (NCT02437279) (176,177), and Amaria et al. reported a randomized, phase II study of neoadjuvant nivolumab vs ipilimumab plus nivolumab in 23 patients (NCT02519322) (178). Both studies found that neoadjuvant ipilimumab plus nivolumab combination therapy was associated with high response rates, albeit with clinically significant toxicity. Recent pooled analysis data from the International Neoadjuvant Melanoma Consortium also suggest that there may be durable prognostic significance associated with extent of pathological response to neoadjuvant therapy (179). A single-institution pilot study suggests that extent of surgery (eg, surgical removal of the “index” node) following neoadjuvant therapy represents an exciting new area of investigation (180).
The open-label, phase II OpACIN-neo (NCT02977052) trial randomly assigned patients to receive varying doses and sequences of ipilimumab and nivolumab followed by surgical resection (181) and identified a tolerable neoadjuvant dosing schedule (ipilimumab 1 mg/kg plus nivolumab 3 mg/kg) that might be suitable for broader clinical use. Other phase I and II studies will also provide insight into combining ICB with targeted therapies, oncolytic viral therapy, biochemotherapy with interferon, or other novel therapies (Supplementary Table 1 available online).
With increased interest in and use of neoadjuvant targeted and immune therapies, it is critical that clinical trial designs and correlative analyses across studies are aligned to facilitate comparison of results and optimal data organization for future regulatory review and to further strengthen translational research. Since 2016, the International Neoadjuvant Melanoma Consortium has met regularly to identify and address opportunities and challenges in establishing neoadjuvant systemic therapy among treatment options for high-risk, resectable melanoma and has recently published two white papers setting forth recommendations and guiding principles for neoadjuvant research, including pathological assessment of resection specimens (170,182).
Future Directions
Advances in our understanding of melanoma pathogenesis have led to the introduction of molecularly targeted therapies and immune checkpoint inhibitors that have improved the outlook and prognosis for melanoma patients. Simultaneously, the role and sequencing of surgery in the multimodal treatment of high-risk resectable and advanced and oligometastatic melanoma is evolving following the results of MSLT-II and DeCOG-SLT and development of effective systemic therapies. Neoadjuvant approaches have the potential to transform the treatment landscape in high-risk resectable stage III melanoma. Taken together, these advances offer exciting opportunities to further refine the development and validation of prognostic models, clinical tools, and future staging and risk stratification systems that incorporate data reflective of the contemporary era in which patients are routinely offered targeted- and immune-based therapies.
Currently, there is much enthusiasm surrounding the development of integrated risk models and clinical tools that enhance predictive and prognostic assessment and clinical decision-making. The ability to identify patients with higher risk than that predicted by conventional staging would assist clinicians in refining the use of adjuvant therapy or more comprehensive follow-up. Ongoing efforts to identify predictors of response and mechanisms of resistance to targeted therapies and immunotherapies, the evolving role of the microbiome, and other molecular and immunological signatures will help inform individualized prognostic and predictive models that can guide multidisciplinary care (17,129,183,184).
Despite these advances, many unmet needs remain. As fewer patients with tumor-involved SLNs undergo CLND, questions arise regarding the prognosis of and optimal systemic therapy for patients with stage III disease. The 8e staging system may have identified a favorable subset of patients with regional nodal disease (eg, stage IIIA and possibly IIIB) for whom adjuvant therapy may not be routinely recommended; development and validation of individualized integrated risk models are underway to further inform contemporary clinical decision-making. Whether results of CheckMate-238 and/or COMBI-AD are applicable for all patients with clinically occult nodal disease remains an area of ongoing interest. Going forward, we will continue to observe and better understand the natural history and prognosis of an increasingly prevalent group of patients with clinically occult RLN metastasis who do not undergo CLND. The role and impact of CLND at the time of regional failure in these patients remains an unanswered question. Lastly, further research is required in many melanoma patient subgroups, including those who cannot receive ICB, who relapse following first-line adjuvant therapy, have BRAF wild-type melanoma, have brain metastases, and/or have less prevalent melanoma subtypes such as uveal and mucosal melanoma, for whom effective treatment options remain limited and/or outcomes in advanced-stage disease remain poor.
Funding
This work was supported in part by The University of Texas MD Anderson Cancer Center Melanoma Moon Shots Program; the Robert and Lynne Grossman Family Foundation; and the Michael and Patricia Booker Melanoma Research Endowment. EZK was supported by National Institutes of Health grant T32 CA009599.
Notes
The funders had no role in the writing of this commentary or decision to submit it for publication.
Dr Gershenwald has served on advisory boards and/or as a consultant for Merck, Syndax, Novartis, Bristol-Myers Squibb, and Castle Biosciences. Dr Keung has no relevant disclosures.
Editorial support was provided by ArticulateScience LLC and funded by Novartis Pharmaceuticals Corporation. Neither Novartis Pharmaceuticals Corporation nor ArticulateScience LLC influenced the content of this manuscript, nor did the authors receive financial compensation.
Supplementary Material
References
- 1. Tripp MK, Watson M, Balk SJ, Swetter SM, Gershenwald JE.. State of the science on prevention and screening to reduce melanoma incidence and mortality: the time is now. CA Cancer J Clin. 2016;66(6):460–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keung EZ, Ukponmwan EU, Cogdill AP, Wargo JA.. The rationale and emerging use of neoadjuvant immune checkpoint blockade for solid malignancies. Ann Surg Oncol. 2018;25(7):1814–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. [DOI] [PubMed] [Google Scholar]
- 4. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386(9992):444–451. [DOI] [PubMed] [Google Scholar]
- 5. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39. [DOI] [PubMed] [Google Scholar]
- 6. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 7. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. [DOI] [PubMed] [Google Scholar]
- 9. Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–1801. [DOI] [PubMed] [Google Scholar]
- 10. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835. [DOI] [PubMed] [Google Scholar]
- 11. Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–1823. [DOI] [PubMed] [Google Scholar]
- 12. Wong SL, Faries MB, Kennedy EB, et al. Sentinel lymph node biopsy and management of regional lymph nodes in melanoma: American Society of Clinical Oncology and Society of Surgical Oncology Clinical Practice Guideline update. J Clin Oncol. 2018;36(4):399–413. [DOI] [PubMed] [Google Scholar]
- 13. Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin In: Amin M, Edge SB, Greene FL, et al. eds. AJCC Cancer Staging Manual. 8th ed Basel, Switzerland: Springer; 2017:563–585. [Google Scholar]
- 16. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition Cancer Staging Manual. CA Cancer J Clin. 2017;67(6):472–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172(5):902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gimotty PA, Elder DE, Fraker DL, et al. Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J Clin Oncol. 2007;25(9):1129–1134. [DOI] [PubMed] [Google Scholar]
- 20. Balch CM, Murad TM, Soong SJ, Ingalls AL, Halpern NB, Maddox WA.. A multifactorial analysis of melanoma: prognostic histopathological features comparing Clark’s and Breslow’s staging methods. Ann Surg. 1978;188(6):732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Green AC, Baade P, Coory M, Aitken JF, Smithers M.. Population-based 20-year survival among people diagnosed with thin melanomas in Queensland, Australia. J Clin Oncol. 2012;30(13):1462–1467. [DOI] [PubMed] [Google Scholar]
- 22. Lo SN, Scolyer RA, Thompson JF.. Long-term survival of patients with thin (T1) cutaneous melanomas: a Breslow thickness cut point of 0.8 mm separates higher-risk and lower-risk tumors. Ann Surg Oncol. 2018;25(4):894–902. [DOI] [PubMed] [Google Scholar]
- 23. Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–3634. [DOI] [PubMed] [Google Scholar]
- 24. Balch CM, Wilkerson JA, Murad TM, Soong SJ, Ingalls AL, Maddox WA.. The prognostic significance of ulceration of cutaneous melanoma. Cancer. 1980;45(12):3012–3017. [DOI] [PubMed] [Google Scholar]
- 25. McGovern VJ, Shaw HM, Milton GW, McCarthy WH.. Ulceration and prognosis in cutaneous malignant melanoma. Histopathology. 1982;6(4):399–407. [DOI] [PubMed] [Google Scholar]
- 26. Rousseau DL, Ross MI, Johnson MM, et al. Revised American Joint Committee on Cancer staging criteria accurately predict sentinel lymph node positivity in clinically node-negative melanoma patients. Ann Surg Oncol. 2003;10(5):569–574. [DOI] [PubMed] [Google Scholar]
- 27. In’t Hout FE, Haydu LE, Murali R, Bonenkamp JJ, Thompson JF, Scolyer RA.. Prognostic importance of the extent of ulceration in patients with clinically localized cutaneous melanoma. Ann Surg. 2012;255(6):1165–1170. [DOI] [PubMed] [Google Scholar]
- 28. Balch CM, Soong SJ, Murad TM, Ingalls AL, Maddox WA.. A multifactorial analysis of melanoma: III. Prognostic factors in melanoma patients with lymph node metastases (stage II). Ann Surg. 1981;193(3):377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balch CM, Gershenwald JE, Soong SJ, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28(14):2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murali R, Haydu LE, Long GV, et al. Clinical and pathologic factors associated with distant metastasis and survival in patients with thin primary cutaneous melanoma. Ann Surg Oncol. 2012;19(6):1782–1789. [DOI] [PubMed] [Google Scholar]
- 31. Balch CM, Melanoma of the skin In: Greene F, Page D, Fleming I, et al. eds. AJCC Cancer Staging Manual. 6th ed New York: Springer Verlag; 2002. [Google Scholar]
- 32. Lasithiotakis KG, Leiter U, Eigentler T, et al. Improvement of overall survival of patients with cutaneous melanoma in Germany, 1976-2001: which factors contributed? Cancer. 2007;109(6):1174–1182. [DOI] [PubMed] [Google Scholar]
- 33. Zogakis TG, Essner R, Wang H, Foshag LJ, Morton DL.. Natural history of melanoma in 773 patients with tumor-negative sentinel lymph nodes. Ann Surg Oncol. 2007;14(5):1604–1611. [DOI] [PubMed] [Google Scholar]
- 34. Barnhill RL, Katzen J, Spatz A, Fine J, Berwick M.. The importance of mitotic rate as a prognostic factor for localized cutaneous melanoma. J Cutan Pathol. 2005;32(4):268–273. [DOI] [PubMed] [Google Scholar]
- 35. Busam KJ. The prognostic importance of tumor mitotic rate for patients with primary cutaneous melanoma. Ann Surg Oncol. 2004;11(4):360–361. [DOI] [PubMed] [Google Scholar]
- 36. Nagarajan P, Curry JL, Ning J, et al. Tumor thickness and mitotic rate robustly predict melanoma-specific survival in patients with primary vulvar melanoma: a retrospective review of 100 cases. Clin Cancer Res. 2017;23(8):2093–2104. [DOI] [PubMed] [Google Scholar]
- 37. Mandalà M, Galli F, Cattaneo L, et al. Mitotic rate correlates with sentinel lymph node status and outcome in cutaneous melanoma greater than 1 millimeter in thickness: a multi-institutional study of 1524 cases. J Am Acad Dermatol. 2017;76(2):264–273. [DOI] [PubMed] [Google Scholar]
- 38. Thompson JF, Soong SJ, Balch CM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol. 2011;29(16):2199–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Azzola MF, Shaw HM, Thompson JF, et al. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: an analysis of 3661 patients from a single center. Cancer. 2003;97(6):1488–1498. [DOI] [PubMed] [Google Scholar]
- 40. Andtbacka RHI, Gershenwald JE.. Role of sentinel lymph node biopsy in patients with thin melanoma. J Natl Compr Canc Netw. 2009;7(3):308–317. [DOI] [PubMed] [Google Scholar]
- 41. Wat H, Senthilselvan A, Salopek TG.. A retrospective, multicenter analysis of the predictive value of mitotic rate for sentinel lymph node (SLN) positivity in thin melanomas. J Am Acad Dermatol. 2016;74(1):94–101. [DOI] [PubMed] [Google Scholar]
- 42.National Comprehensive Cancer Network. Version 3.2019. Melanoma. https://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. Accessed January 12, 2019.
- 43. Vollmer RT. Malignant melanoma. A multivariate analysis of prognostic factors. Pathol Annu. 1989;24(pt 1):383–407. [PubMed] [Google Scholar]
- 44. Buzaid AC, Ross MI, Balch CM, et al. Critical analysis of the current American Joint Committee on Cancer staging system for cutaneous melanoma and proposal of a new staging system. J Clin Oncol. 1997;15(3):1039–1051. [DOI] [PubMed] [Google Scholar]
- 45. Balch CM, Soong S, Ross MI, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Intergroup Melanoma Surgical Trial. Ann Surg Oncol. 2000;7(2):87–97. [DOI] [PubMed] [Google Scholar]
- 46. Prade M, Sancho-Garnier H, Cesarini JP, Cochran A.. Difficulties encountered in the application of Clark classification and the Breslow thickness measurement in cutaneous malignant melanoma. Int J Cancer. 1980;26(2):159–163. [DOI] [PubMed] [Google Scholar]
- 47. Breslow A. Problems in the measurement of tumor thickness and level of invasion in cutaneous melanoma. Hum Pathol. 1977;8(1):1–2. [DOI] [PubMed] [Google Scholar]
- 48. Clark WH, From L, Bernardino EA, Mihm MC.. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29(3):705–727. [PubMed] [Google Scholar]
- 49. Schatton T, Scolyer RA, Thompson JF, Mihm MC.. Tumor-infiltrating lymphocytes and their significance in melanoma prognosis. Methods Mol Biol. 2014;1102:287–324. [DOI] [PubMed] [Google Scholar]
- 50. Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS.. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007;25(7):869–875. [DOI] [PubMed] [Google Scholar]
- 51. Thomas NE, Busam KJ, From L, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol. 2013;31(33):4252–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eriksson H, Frohm-Nilsson M, Järås J, et al. Prognostic factors in localized invasive primary cutaneous malignant melanoma: results of a large population-based study. Br J Dermatol. 2015;172(1):175–186. [DOI] [PubMed] [Google Scholar]
- 53. Saldanha G, Flatman K, Teo KW, Bamford M.. A novel numerical scoring system for melanoma tumor-infiltrating lymphocytes has better prognostic value than standard scoring. Am J Surg Pathol. 2017;41(7):906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Duprat JP, Brechtbülh ER, Costa de Sá B, et al. Absence of tumor-infiltrating lymphocyte Is a reproducible predictive factor for sentinel lymph node metastasis: a multicenter database study by the Brazilian melanoma group. PLoS One. 2016;11(2):e0148160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30(21):2678–2683. [DOI] [PubMed] [Google Scholar]
- 56. Fortes C, Mastroeni S, Mannooranparampil TJ, et al. Tumor-infiltrating lymphocytes predict cutaneous melanoma survival. Melanoma Res. 2015;25(4):306–311. [DOI] [PubMed] [Google Scholar]
- 57. Kashani-Sabet M, Sagebiel RW, Ferreira CM, Nosrati M, Miller JR.. Vascular involvement in the prognosis of primary cutaneous melanoma. Arch Dermatol. 2001;137(9):1169–1173. [DOI] [PubMed] [Google Scholar]
- 58. Nagore E, Oliver V, Botella-Estrada R, Moreno-Picot S, Insa A, Fortea JM.. Prognostic factors in localized invasive cutaneous melanoma: high value of mitotic rate, vascular invasion and microscopic satellitosis. Melanoma Res. 2005;15(3):169–177. [DOI] [PubMed] [Google Scholar]
- 59. Pasquali S, Montesco MC, Ginanneschi C, et al. Lymphatic and blood vasculature in primary cutaneous melanomas of the scalp and neck. Head Neck. 2015;37(11):1596–1602. [DOI] [PubMed] [Google Scholar]
- 60. Storr SJ, Safuan S, Mitra A, et al. Objective assessment of blood and lymphatic vessel invasion and association with macrophage infiltration in cutaneous melanoma. Mod Pathol. 2012;25(4):493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Straume O, Akslen LA.. Independent prognostic importance of vascular invasion in nodular melanomas. Cancer. 1996;78(6):1211–1219. [DOI] [PubMed] [Google Scholar]
- 62. Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19(16):3635–3648. [DOI] [PubMed] [Google Scholar]
- 63. Cascinelli N, Belli F, Santinami M, et al. Sentinel lymph node biopsy in cutaneous melanoma: the WHO Melanoma Program experience. Ann Surg Oncol. 2000;7(6):469–474. [DOI] [PubMed] [Google Scholar]
- 64. Gershenwald JE, Colome MI, Lee JE, et al. Patterns of recurrence following a negative sentinel lymph node biopsy in 243 patients with stage I or II melanoma. J Clin Oncol. 1998;16(6):2253–2260. [DOI] [PubMed] [Google Scholar]
- 65. Gershenwald JE, Fischer D, Buzaid AC.. Clinical classification and staging. Clin Plast Surg. 2000;27(3):361–376. [PubMed] [Google Scholar]
- 66. Crookes TR, Scolyer RA, Lo S, Drummond M, Spillane AJ.. Extranodal spread is associated with recurrence and poor survival in stage III cutaneous melanoma patients. Ann Surg Oncol. 2017;24(5):1378–1385. [DOI] [PubMed] [Google Scholar]
- 67. Cascinelli N, Bufalino R, Marolda R, et al. Regional non-nodal metastases of cutaneous melanoma. Eur J Surg Oncol. 1986;12(2):175–180. [PubMed] [Google Scholar]
- 68. Day CL, Harrist TJ, Gorstein F, et al. Malignant melanoma: prognostic significance of “microscopic satellites” in the reticular dermis and subcutaneous fat. Ann Surg. 1981;194(1):108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Harrist TJ, Rigel DS, Day CL, et al. “Microscopic satellites” are more highly associated with regional lymph node metastases than is primary melanoma thickness. Cancer. 1984;53(10):2183–2187. [DOI] [PubMed] [Google Scholar]
- 70. León P, Daly JM, Synnestvedt M, Schultz DJ, Elder DE, Clark WH.. The prognostic implications of microscopic satellites in patients with clinical stage I melanoma. Arch Surg. 1991;126(12):1461–1468. [DOI] [PubMed] [Google Scholar]
- 71. Read RL, Haydu L, Saw RPM, et al. In-transit melanoma metastases: incidence, prognosis, and the role of lymphadenectomy. Ann Surg Oncol. 2015;22(2):475–481. [DOI] [PubMed] [Google Scholar]
- 72. Rao UNM, Ibrahim J, Flaherty LE, Richards J, Kirkwood JM.. Implications of microscopic satellites of the primary and extracapsular lymph node spread in patients with high-risk melanoma: Pathologic corollary of Eastern Cooperative Oncology Group Trial E1690. J Clin Oncol. 2002;20(8):2053–2057. [DOI] [PubMed] [Google Scholar]
- 73. Wilmott J, Haydu L, Bagot M, et al. Angiotropism is an independent predictor of microscopic satellites in primary cutaneous melanoma. Histopathology. 2012;61(5):889–898. [DOI] [PubMed] [Google Scholar]
- 74. Van Es SL, Colman M, Thompson JF, McCarthy SW, Scolyer RA.. Angiotropism is an independent predictor of local recurrence and in-transit metastasis in primary cutaneous melanoma. Am J Surg Pathol. 2008;32(9):1396–1403. [DOI] [PubMed] [Google Scholar]
- 75. Gershenwald J, Balch CM, Soong S, Al E.. Prognostic factors and natural history of melanoma In: Balch CM, Soong S, Sober AJ, et al. eds. Cutaneous Melanoma. 5th ed St Louis: Quality Medical Publishing; 2009:35–64. [Google Scholar]
- 76. Cormier JN, Xing Y, Feng L, et al. Metastatic melanoma to lymph nodes in patients with unknown primary sites. Cancer. 2006;106(9):2012–2020. [DOI] [PubMed] [Google Scholar]
- 77. Lee CC, Faries MB, Wanek LA, Morton DL.. Improved survival after lymphadenectomy for nodal metastasis from an unknown primary melanoma. J Clin Oncol. 2008;26(4):535–541. [DOI] [PubMed] [Google Scholar]
- 78. Rutkowski P, Nowecki ZI, Dziewirski W, et al. Melanoma without a detectable primary site with metastases to lymph nodes. Dermatol Surg. 2010;36(6):868–876. [DOI] [PubMed] [Google Scholar]
- 79. Weide B, Faller C, Elsässer M, et al. Melanoma patients with unknown primary site or nodal recurrence after initial diagnosis have a favourable survival compared to those with synchronous lymph node metastasis and primary tumour. PLoS One. 2013;8(6):e66953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Prens SP, van der Ploeg APT, van Akkooi ACJ, et al. Outcome after therapeutic lymph node dissection in patients with unknown primary melanoma site. Ann Surg Oncol. 2011;18(13):3586–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. de Waal AC, Aben KKH, van Rossum MM, Kiemeney L.. Melanoma of unknown primary origin: a population-based study in the Netherlands. Eur J Cancer. 2013;49(3):676–683. [DOI] [PubMed] [Google Scholar]
- 82. van der Ploeg APT, Haydu LE, Spillane AJ, et al. Melanoma patients with an unknown primary tumor site have a better outcome than those with a known primary following therapeutic lymph node dissection for macroscopic (clinically palpable) nodal disease. Ann Surg Oncol. 2014;21(9):3108–3116. [DOI] [PubMed] [Google Scholar]
- 83. Barth A, Wanek LA, Morton DL.. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181(3):193–201. [PubMed] [Google Scholar]
- 84. Manola J, Atkins M, Ibrahim J, Kirkwood J.. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18(22):3782–3793. [DOI] [PubMed] [Google Scholar]
- 85. Warso MA, Boddie AW.. The natural history of melanoma, including the pattern of metastatic spread and the biological basis for metastases--staging of melanoma. Cancer Treat Res. 1993;65:141–160. [DOI] [PubMed] [Google Scholar]
- 86. Garrison M, Nathanson L.. Prognosis and staging in melanoma. Semin Oncol. 1996;23(6):725–733. [PubMed] [Google Scholar]
- 87. Brand CU, Ellwanger U, Stroebel W, et al. Prolonged survival of 2 years or longer for patients with disseminated melanoma. An analysis of related prognostic factors. Cancer. 1997;79(12):2345–2353. [PubMed] [Google Scholar]
- 88. Cochran AJ, Bhuta S, Paul E, Ribas A.. The shifting patterns of metastatic melanoma. Clin Lab Med. 2000;20(4):759–783. [PubMed] [Google Scholar]
- 89. Staudt M, Lasithiotakis K, Leiter U, et al. Determinants of survival in patients with brain metastases from cutaneous melanoma. Br J Cancer. 2010;102(8):1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117(8):1687–1696. [DOI] [PubMed] [Google Scholar]
- 91. Sperduto PW, Jiang W, Brown PD, et al. The prognostic value of BRAF, C-KIT, and NRAS mutations in melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys. 2017;98(5):1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Frinton E, Tong D, Tan J, et al. Metastatic melanoma: prognostic factors and survival in patients with brain metastases. J Neurooncol. 2017;135(3):507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–465. [DOI] [PubMed] [Google Scholar]
- 94. Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Unger JM, Flaherty LE, Liu PY, Albain KS, Sondak VK.. Gender and other survival predictors in patients with metastatic melanoma on Southwest Oncology Group trials. Cancer. 2001;91(6):1148–1155. [DOI] [PubMed] [Google Scholar]
- 96. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. [DOI] [PubMed] [Google Scholar]
- 100. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–1876. [DOI] [PubMed] [Google Scholar]
- 102. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. [DOI] [PubMed] [Google Scholar]
- 103. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. [DOI] [PubMed] [Google Scholar]
- 104. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Glitza Oliva IC, Schvartsman G, Tawbi H.. Advances in the systemic treatment of melanoma brain metastases. Ann Oncol. 2018;29(7):1509–1520. [DOI] [PubMed] [Google Scholar]
- 106. Sirott MN, Bajorin DF, Wong GY, et al. Prognostic factors in patients with metastatic malignant melanoma. A multivariate analysis. Cancer. 1993;72(10):3091–3098. [DOI] [PubMed] [Google Scholar]
- 107. Kelderman S, Heemskerk B, van Tinteren H, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother. 2014;63(5):449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Efficacy and safety in key patient subgroups of nivolumab (NIVO) alone or combined with ipilimumab (IPI) versus IPI alone in treatment-naive patients with advanced melanoma (MEL) (CheckMate 067). Eur J Cancer. 2015;51(Supp 3):S664–665. [Google Scholar]
- 109. Long GV, Weber JS, Infante JR, et al. Overall survival and durable responses in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. J Clin Oncol. 2016;34(8):871–878. [DOI] [PubMed] [Google Scholar]
- 110. Menzies AM, Wilmott JS, Drummond M, et al. Clinicopathologic features associated with efficacy and long-term survival in metastatic melanoma patients treated with BRAF or combined BRAF and MEK inhibitors. Cancer. 2015;121(21):3826–3835. [DOI] [PubMed] [Google Scholar]
- 111. Bedikian AY, Johnson MM, Warneke CL, et al. Prognostic factors that determine the long-term survival of patients with unresectable metastatic melanoma. Cancer Invest. 2008;26(6):624–633. [DOI] [PubMed] [Google Scholar]
- 112. Keilholz U, Martus P, Punt CJA, et al. Prognostic factors for survival and factors associated with long-term remission in patients with advanced melanoma receiving cytokine-based treatments: second analysis of a randomised EORTC Melanoma Group trial comparing interferon-alpha2a (IFNalpha) and interleukin 2 (IL-2) with or without cisplatin. Eur J Cancer. 2002;38(11):1501–1511. [DOI] [PubMed] [Google Scholar]
- 113. Balch CM, Soong SJ, Gershenwald JE, et al. Melanoma of the skin In: Edge S, Byrd D, Compton C, et al. eds. AJCC Cancer Staging Manual. 7th ed New York: Springer Verlag; 2009:325–344. [Google Scholar]
- 114. Weide B, Martens A, Hassel JC, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. 2016;22(22):5487–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17(12):1743–1754. [DOI] [PubMed] [Google Scholar]
- 116. Nosrati A, Tsai KK, Goldinger SM, et al. Evaluation of clinicopathological factors in PD-1 response: derivation and validation of a prediction scale for response to PD-1 monotherapy. Br J Cancer. 2017;116(9):1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. [DOI] [PubMed] [Google Scholar]
- 119. Morton DL. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392. [DOI] [PubMed] [Google Scholar]
- 120. Wong SL, Balch CM, Hurley P, et al. Sentinel lymph node biopsy for melanoma: American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guideline. J Clin Oncol. 2012;30(23):2912–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17(3):976–983. [DOI] [PubMed] [Google Scholar]
- 122. Gershenwald JE, Coit DG, Sondak VK, Thompson JF.. The challenge of defining guidelines for sentinel lymph node biopsy in patients with thin primary cutaneous melanomas. Ann Surg Oncol. 2012;19(11):3301–3303. [DOI] [PubMed] [Google Scholar]
- 123. van Akkooi ACJ, Nowecki ZI, Voit C, et al. Sentinel node tumor burden according to the Rotterdam criteria is the most important prognostic factor for survival in melanoma patients: a multicenter study in 388 patients with positive sentinel nodes. Ann Surg. 2008;248(6):949–955. [DOI] [PubMed] [Google Scholar]
- 124. Gutzmer R, Satzger I, Thoms KM, et al. Sentinel lymph node status is the most important prognostic factor for thick (> or = 4 mm) melanomas. J Dtsch Dermatol Ges. 2008;6(3):198–203. [DOI] [PubMed] [Google Scholar]
- 125. Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355(13):1307–1317. [DOI] [PubMed] [Google Scholar]
- 126. Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(6):757–767. [DOI] [PubMed] [Google Scholar]
- 127. Murali R, Desilva C, Thompson JF, Scolyer RA.. Non-sentinel node risk score (N-SNORE): a scoring system for accurately stratifying risk of non-sentinel node positivity in patients with cutaneous melanoma with positive sentinel lymph nodes. J Clin Oncol. 2010;28(29):4441–4449. [DOI] [PubMed] [Google Scholar]
- 128. Gershenwald JE, Andtbacka RHI, Prieto VG, et al. Microscopic tumor burden in sentinel lymph nodes predicts synchronous nonsentinel lymph node involvement in patients with melanoma. J Clin Oncol. 2008;26(26):4296–4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gershenwald JE, Scolyer RA.. Melanoma staging: American Joint Committee on Cancer (AJCC) 8th edition and beyond. Ann Surg Oncol. 2018;25(8):2105–2110. [DOI] [PubMed] [Google Scholar]
- 130. Bottomley A, Coens C, Suciu S, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma: a phase III randomized controlled trial of health-related quality of life and symptoms by the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2009;27(18):2916–2923. [DOI] [PubMed] [Google Scholar]
- 131. Brandberg Y, Aamdal S, Bastholt L, et al. Health-related quality of life in patients with high-risk melanoma randomised in the Nordic phase 3 trial with adjuvant intermediate-dose interferon alfa-2b. Eur J Cancer. 2012;48(13):2012–2019. [DOI] [PubMed] [Google Scholar]
- 132. Kilbridge KL, Cole BF, Kirkwood JM, et al. Quality-of-life-adjusted survival analysis of high-dose adjuvant interferon alpha-2b for high-risk melanoma patients using intergroup clinical trial data. J Clin Oncol. 2002;20(5):1311–1318. [DOI] [PubMed] [Google Scholar]
- 133. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(10):1315–1327. [DOI] [PubMed] [Google Scholar]
- 134. Liszkay G, Gogas H, Mandala M, et al. Update on overall survival in COLUMBUS: A randomized phase III trial of encorafenib (ENCO) plus binimetinib (BINI) versus vemurafenib (VEM) or ENCO in patients with BRAF V600–mutant melanoma. J Clin Oncol. 2019;37(suppl): 9512. [Google Scholar]
- 135. Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. [DOI] [PubMed] [Google Scholar]
- 137. Eggermont AMM, Blank CU, Mandala M, et al. Prognostic and predictive value of AJCC-8 staging in the phase III EORTC1325/KEYNOTE-054 trial of pembrolizumab vs placebo in resected high-risk stage III melanoma. Eur J Cancer. 2019;116(116):148–157. [DOI] [PubMed] [Google Scholar]
- 138. Owen CN, Larkin JMG, Shoushtari AN, et al. A multicenter analysis of melanoma recurrence following adjuvant anti-PD1 therapy. J Clin Oncol. 2019;37(suppl):9502. [Google Scholar]
- 139. Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161(7):1681–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29(10):1239–1246. [DOI] [PubMed] [Google Scholar]
- 141. Luke JJ, Flaherty KT, Ribas A, Long GV.. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14(8):463–482. [DOI] [PubMed] [Google Scholar]
- 142. Hauschild A, Dummer R, Schadendorf D, et al. Longer follow-up confirms relapse-free survival benefit with adjuvant dabrafenib plus trametinib in patients with resected BRAF V600-mutant stage III melanoma. J Clin Oncol. 2018;36(35):3441–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Creagan ET, Cupps RE, Ivins JC, et al. Adjuvant radiation therapy for regional nodal metastases from malignant melanoma: a randomized, prospective study. Cancer. 1978;42(5):2206–2210. [DOI] [PubMed] [Google Scholar]
- 144. Ballo MT, Ross MI, Cormier JN, et al. Combined-modality therapy for patients with regional nodal metastases from melanoma. Int J Radiat Oncol Biol Phys. 2006;64(1):106–113. [DOI] [PubMed] [Google Scholar]
- 145. Agrawal S, Kane JM, Guadagnolo BA, Kraybill WG, Ballo MT.. The benefits of adjuvant radiation therapy after therapeutic lymphadenectomy for clinically advanced, high-risk, lymph node-metastatic melanoma. Cancer. 2009;115(24):5836–5844. [DOI] [PubMed] [Google Scholar]
- 146. Guadagnolo BA, Zagars GK.. Adjuvant radiation therapy for high-risk nodal metastases from cutaneous melanoma. Lancet Oncol. 2009;10(4):409–416. [DOI] [PubMed] [Google Scholar]
- 147. Miller EJ, Daly JM, Synnestvedt M, Schultz D, Elder D, Guerry D.. Loco-regional nodal relapse in melanoma. Surg Oncol. 1992;1(5):333–340. [DOI] [PubMed] [Google Scholar]
- 148. Monsour PD, Sause WT, Avent JM, Noyes RD.. Local control following therapeutic nodal dissection for melanoma. J Surg Oncol. 1993;54(1):18–22. [DOI] [PubMed] [Google Scholar]
- 149. Lee RJ, Gibbs JF, Proulx GM, Kollmorgen DR, Jia C, Kraybill WG.. Nodal basin recurrence following lymph node dissection for melanoma: implications for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2000;46(2):467–474. [DOI] [PubMed] [Google Scholar]
- 150. Shen P, Wanek LA, Morton DL.. Is adjuvant radiotherapy necessary after positive lymph node dissection in head and neck melanomas? Ann Surg Oncol. 2000;7(8):554–559; discussion 560–1. [DOI] [PubMed] [Google Scholar]
- 151. Beadle BM, Guadagnolo BA, Ballo MT, et al. Radiation therapy field extent for adjuvant treatment of axillary metastases from malignant melanoma. Int J Radiat Oncol Biol Phys. 2009;73(5):1376–1382. [DOI] [PubMed] [Google Scholar]
- 152. Burmeister BH, Henderson MA, Ainslie J, et al. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol. 2012;13(6):589–597. [DOI] [PubMed] [Google Scholar]
- 153. Henderson MA, Burmeister BH, Ainslie J, et al. Adjuvant lymph-node field radiotherapy versus observation only in patients with melanoma at high risk of further lymph-node field relapse after lymphadenectomy (ANZMTG 01.02/TROG 02.01): 6-year follow-up of a phase 3, randomised controlled trial. Lancet Oncol. 2015;16(9):1049–1060. [DOI] [PubMed] [Google Scholar]
- 154. van Zeijl MCT, van den Eertwegh AJ, Haanen JB, Wouters M.. (Neo)adjuvant systemic therapy for melanoma. Eur J Surg Oncol. 2017;43(3):534–543. [DOI] [PubMed] [Google Scholar]
- 155. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–866. [DOI] [PubMed] [Google Scholar]
- 156. Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel. J Clin Oncol. 1997;15(7):2483–2493. [DOI] [PubMed] [Google Scholar]
- 157. Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785. [DOI] [PubMed] [Google Scholar]
- 158. Von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. [DOI] [PubMed] [Google Scholar]
- 159. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. [DOI] [PubMed] [Google Scholar]
- 160. Mauri D, Pavlidis N, Ioannidis J.. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–194. [DOI] [PubMed] [Google Scholar]
- 161. Salvador-Coloma C, Cohen E.. Multidisciplinary care of laryngeal cancer. J Oncol Pract. 2016;12(8):717–724. [DOI] [PubMed] [Google Scholar]
- 162. Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098. [DOI] [PubMed] [Google Scholar]
- 163. Franco P, Arcadipane F, Strignano P, et al. Pre-operative treatments for adenocarcinoma of the lower oesophagus and gastro-oesophageal junction: a review of the current evidence from randomized trials. Med Oncol. 2017;34(3):1–7. [DOI] [PubMed] [Google Scholar]
- 164. Anderegg MCJ, van der Sluis PC, Ruurda JP, et al. Preoperative chemoradiotherapy versus perioperative chemotherapy for patients with resectable esophageal or gastroesophageal junction adenocarcinoma. Ann Surg Oncol. 2017;24(8):2282–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Noordman BJ, van Klaveren D, van Berge Henegouwen MI, et al. Impact of surgical approach on long-term survival in esophageal adenocarcinoma patients with or without neoadjuvant chemoradiotherapy. Ann Surg. 2018;267(5):892–897. [DOI] [PubMed] [Google Scholar]
- 166. van Hagen P, Hulshof M, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. [DOI] [PubMed] [Google Scholar]
- 167. Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28(5):859–865. [DOI] [PubMed] [Google Scholar]
- 168. Cercek A, Goodman KA, Hajj C, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw. 2014;12(4):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Buzaid AC, Colome M, Bedikian A, et al. Phase II study of neoadjuvant concurrent biochemotherapy in melanoma patients with local-regional metastases. Melanoma Res. 1998;8(6):549–556. [DOI] [PubMed] [Google Scholar]
- 170. Tetzlaff MT, Messina JL, Stein JE, et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann Oncol. 2018;29(8):1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Melero I, Berraondo P, Rodríguez-Ruiz ME, Pérez-Gracia JL.. Making the most of cancer surgery with neoadjuvant immunotherapy. Cancer Discov. 2016;6(12):1312–1314. [DOI] [PubMed] [Google Scholar]
- 172. Davar D, Tarhini AA, Kirkwood JM.. Adjuvant immunotherapy of melanoma and development of new approaches using the neoadjuvant approach. Clin Dermatol. 2013;31(3):237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Liu J, Blake SJ, Yong MCR, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6(12):1382–1399. [DOI] [PubMed] [Google Scholar]
- 174. Amaria RN, Prieto PA, Tetzlaff MT, et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: a single-centre, open-label, randomised, phase 2 trial. Lancet Oncol. 2018;19(2):181–193. [DOI] [PubMed] [Google Scholar]
- 175. Long GV, Saw RPM, Lo S, et al. Neoadjuvant dabrafenib combined with trametinib for single-centre, phase 2 trial. Lancet Oncol. 2019;2045(19):1–11. [DOI] [PubMed] [Google Scholar]
- 176. Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655–1661. [DOI] [PubMed] [Google Scholar]
- 177. Blank CU, Versluis J, Reijers I, et al. 3-year relapse-free survival (RFS), overall survival (OS) and long-term toxicity of (neo) adjuvant ipilimumab (IPI)+ nivolumab (NIVO) in macroscopic stage III melanoma (OpACIN trial). Paper presented at 44th ESMO Congress, September 27–1 October 1, 2019; Barcelona, Spain.
- 178. Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Menzies AM, Rozeman EA, Amaria RN, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). J Clin Oncol. 2019;37(suppl):9503. [DOI] [PubMed] [Google Scholar]
- 180. Schermers B, Franke V, Rozeman EA, et al. Surgical removal of the index node marked using magnetic seed localization to assess response to neoadjuvant immunotherapy in patients with stage III melanoma. Br J Surg. 2019;106(5):519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Rozeman EA, Menzies AM, Van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20(7):948–960. [DOI] [PubMed] [Google Scholar]
- 182. Amaria RN, Menzies AM, Burton EM, et al. Neoadjuvant systemic therapy in melanoma: recommendations of the International Neoadjuvant Melanoma Consortium. Lancet Oncol. 2019;20(7):e378–e389. [DOI] [PubMed] [Google Scholar]
- 183. Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA.. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25(3):377–388. [DOI] [PubMed] [Google Scholar]
- 184. McQuade JL, Daniel CR, Helmink BA, Wargo JA.. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019;20(2):e77–e91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.