Abstract
African American women have the highest rate of preterm birth (PTB; <37 completed weeks’ gestation) of any racial and ethnic group in the United States (14.1%). Depressive symptoms (DS) have been linked to PTB risk of African American women. We hypothesized that maternal lipidomic profiles are related to prenatal DS and gestational age at birth among African American women. Women were enrolled at 9–25 weeks’ gestation, completed questionnaires, and provided plasma samples. Lipidomic profiles were determined by “shotgun” Orbitrap high-resolution/accurate mass spectrometry. Data were analyzed using SIMCA P+ software. There was a clear separation in the orthogonal projections to latent structures discriminant analysis score plot between women with Center for Epidemiologic Studies Depression Scale (CES-D) scores ≥23 and women with CES-D scores ≤22. Similarly, a clear separation was observed in the model between PTB and full-term birth. Corresponding S-plot, loading plot, and variable importance in projection plot/list were used to identify the lipids responsible for the groupings. Higher levels of specific triglyceride (TG) species and lower levels of specific phosphatidylcholines (PCs) PC(37:1), PC(41:6), and PC(39:3) were associated with PTB. PC PC(37:1) levels were also lower among women with CES-D scores ≥23, pointing toward a possible connection between DS and PTB. Although overweight pregnant women showed higher levels of TGs, the PTB model showed specific TGs unique to PTB. Lipidomic profiles in pregnant African American women are related to DS, and our data suggest a role for specific TGs and PCs in PTB.
Keywords: African American women, pregnancy, preterm birth, depressive symptoms, lipidomics, biomarkers, health disparities
African American women are 1.5 times more likely to have a preterm birth (PTB, <37 completed weeks’ gestation) than non-Hispanic Caucasian women (Hamilton et al., 2019; Martin et al., 2018). Pregnant African American women are also more likely to experience depressive symptoms (DS) compared with pregnant non-Hispanic Caucasian women (Culhane & Elo, 2005; Riis et al., 2016). DS have been associated with a higher risk of PTB among African American women (Nutor et al., 2018; Orr et al., 2002). Even though DS may not be at the root of all PTBs, the high rate of DS among pregnant African American women (Giurgescu et al., 2011) coupled with the link between DS and PTB suggests the need for further investigation. While numerous studies have focused on the biologic mechanisms mediating the effect of stress on PTB (Hoffman et al., 2016; Wadhwa et al., 2011), less work has been done to understand the pathways between DS and PTB.
Research has shown infections and inflammation to be causally related to PTB (Brocklehurst, 1999; Romero et al., 2007; Romero et al., 1994). Chronic stress is related to increased inflammation via dysregulation of the hypothalamic-pituitary-adrenal axis, which can alter cortisol levels, leading to systemic inflammation and increased risk of PTB (Corwin et al., 2015; Giurgescu, 2009; Nergiz Avcıoğlu et al., 2015; Wadhwa et al., 2011). Researchers have reported that chronic dyslipidemia is associated with obesity and inflammation, which can lead to altered lipid metabolism (Papoutsidakis et al., 2014; Smith et al., 2018). In one study, the association between dyslipidemia and PTB was similar for women across body mass index (BMI) categories, and African American women had a higher frequency of dyslipidemia (Smith et al., 2018). However, research relating lipids to DS has been limited thus far to lipids assessed by standard laboratory approaches. In two studies, pregnant women with DS had lower levels of polyunsaturated fatty acids (PUFAs) compared to those without DS (Rees et al., 2009; Vaz et al., 2014). As these studies were not conducted in the United States, they do not provide information with regard to how DS relate to PTB among African American women in the United States.
In a recent review of atherogenic lipids in nine cohorts and five case–control studies using standard laboratory assays, women with higher triglyceride (TG) and/or higher homocysteine levels had a higher risk of PTB (Moayeri et al., 2017). A 2018 retrospective cohort study also reported that higher TG levels in early pregnancy were related to PTB (Lin et al., 2018). Two recent studies went beyond standard assays but took a targeted approach to the lipidome. In a 2014 report, pregnant women with blood levels of nonesterified fatty acids in the highest tertile were more likely to have PTB compared with those with blood levels in the lowest tertile (Catov et al., 2014). In a 2016 publication targeting PUFA species, higher arachidonic acid (AA) level and a lower docosahexaenoic acid:AA ratio were both associated with lower gestational age at birth (Christian et al., 2016). A few published studies have reported on the lipidome in pregnancy using untargeted methods (Heazell et al., 2012). The earliest study reported changes in levels of 98 metabolites for women with poor pregnancy outcomes (total N = 40: n = 11 PTBs, n = 29 term births of small-for-gestational-age infants) compared with those of matched healthy controls (n = 40; Heazell et al., 2012). Differences were primarily in glycerolipids, free fatty acids, sterol lipids, sphingolipids, and metabolites related to progesterone and vitamin D metabolism. Specifically, serum levels of sphingolipids were increased for women with poor pregnancy outcomes compared with matched healthy controls. The later study found that acetylcarnitine, phosphatidylcholines (PCs), and sphingomyelins declined across gestation in healthy pregnancies, while several trichloroacetic acid intermediates and ketone body β-hydroxybutyrate increased (Lindsay et al., 2015).
While a body of evidence is growing that supports the association of lipids with DS and/or PTB, most studies have relied on examining the alteration of a few targeted lipid classes rather than conducting global untargeted analyses. The lipidome encompasses several hundreds or thousands of lipid species that are important as structural and signaling molecules, far beyond the usual clinical chemistry measures of cholesterol and TG (Lydic & Goo, 2018). In the present study, we hypothesized that lipidomic profiles relate to DS and PTB in African American women. Using an untargeted, comprehensive, global lipidome profiling method, we determined the molecular compositions of several hundred lipid analytes in plasma from our sample of 31 pregnant African American women and explored associations between lipidome profiles and both DS and risk of PTB among these participants.
Method
Design and Sample
We enrolled self-identified African American/Black women from a prenatal clinic at a medical center in the Midwest in the present prospective cohort study between February and November 2015. We included women in the study if they self-identified as Black/African American, were ≥18 years of age, had a singleton pregnancy, and were at 9–25 weeks’ gestation. Women were excluded if they had multiple gestations (Dove-Medows et al., 2019). We limited the sample to African American/Black women, the group at highest risk of PTB (Martin et al., 2018).
Variables and Instruments
Maternal characteristics
We collected maternal sociodemographic characteristics by self-report and medical and obstetrical history from the prenatal medical records.
DS
To assess DS, we administered the Center for Epidemiologic Studies Depression Scale (CES-D), which measures DS within the past 7 days (Radloff, 1977). Scores range from 0 to 60, with higher scores indicating greater number and/or severity of DS. Scores ≥23 correlate with a diagnosis of major depression disorder (Orr et al., 2002). The Cronbach’s α for this instrument was .80 in our sample.
Lipidomic profiles
We used “shotgun” Orbitrap high-resolution/accurate mass spectrometry (MS) to measure plasma glycerolipids, phospholipids, sphingolipids, sterols, fatty acids, and signaling lipids (we searched >30,000 lipids with putative identification of 450–500 species per sample). Profiles were determined from plasma lipid extracts by nano-electrospray (nESI) direct infusion high-resolution/accurate MS utilizing an LTQ-Orbitrap Velos MS (Thermo Scientific, Waltham, MA) with the Fourier transform analyzer operating at 100,000 resolving power. An Advion NanoMate Triversa (Advion Biosciences, Ithaca, NY) served as the nESI source and high-throughput autosampler. Data were acquired for 2 min for each MS analysis and in both positive and negative ionization modes to enable detection of a broad range of lipid analytes. Peak finding and quantification for global lipidomics were performed with Lipid Mass Spectrum Analysis (LIMSA) Version 1.0 software (Haimi et al., 2006). Limits of detection range from 0.01 nM to 10 nM across all lipid classes. Screening for interfering isobaric lipid species was also conducted using LIMSA. Lipid species in the Results, below, are shown as a combination of lipid class and fatty acid total carbons: total double bonds.
PTB
We obtained gestational age at birth from medical records, defining PTB as less than 37 completed weeks’ gestation (Bick, 2012; Quinn et al., 2016).
Procedures
The institutional review boards at the university and clinical site approved the study, and we obtained written informed consent from participants. At enrollment (9–25 weeks’ gestation), women completed questionnaires in the clinic or at home. Venous blood was drawn with antecubital venipuncture (within 30 s of venipuncture), and 10 ml of blood was collected in one ethylenediaminetetraacetic acid tube. Samples were centrifuged for 15 min within 1 hr of collection. Aliquoted plasma samples were transported on ice to the laboratory for storage at −80 °C until analysis.
Data Management and Statistical Analysis
We used descriptive statistics to describe maternal characteristics, DS, and PTB using SPSS Version 25 (IBM, Armonk, NY). To perform multivariate analysis for lipidomic profiles, we used SIMCA P+ Version 15 (Sartorius Stedim Data Analytics AB, Sweden). Data were scaled based on unit variance. We produced a score plot using principal component analysis (PCA) to identify outliers. Specifically, we drew a tolerance ellipse based on Hotelling’s T2 (a multivariate generalization of the student’s t-distribution). Observations outside and away from the ellipse represent strong outliers. We removed three major outliers before further analysis. We also tested for outliers by inspecting the normal probability plot of residuals, considering points outside −4 and +4 standard deviations to be outliers. We identified and removed one additional outlier using this process. Missing data included CES-D, education, or BMI for five women and household income for six. In addition, two women had missing birth data. We removed women with missing data for variables within a model from the corresponding models only. SIMCA multivariate analysis software has the capability to handle missing data effectively, meaning there was no need to remove these observations completely from the data set.
We used orthogonal projections to latent structures (OPLS) to assess the correlation between the CES-D scores and lipidomic profiles. Higher BMI was a potential confounder, as obesity is related to both dyslipidemia (Klop et al., 2013) and PTB (Cnattingius et al., 2013). Research has also shown obesity to be related to higher levels of TGs and free fatty acids, decreased levels of high-density lipoprotein (HDL) cholesterol, and a slight increase in levels of low-density lipoproteins (LDL) cholesterol (Klop et al., 2013). We conducted OPLS to assess whether BMI was correlated with the lipidomic profiles for sensitivity analyses relating to BMI. We developed multivariate models for gestational age at testing, CES-D scores, education level, and gestational age at birth and compared these models for potential overlap and covariation by making shared and unique structures (SUS) plots. SUS plots of these models showed no clear overlap, shared structures, or covariation.
We performed OPLS discriminant analysis (OPLS-DA) to identify differences in lipidome profiles between women with and without PTB based on changes related to higher CES-D scores. We calculated variable importance in projection (VIP) and S-plots for CES-D scores and PTB models. A higher predictive VIP score indicates the variable’s greater importance to the model. The S-plot was used to identify biomarkers with lower variability, lower false discovery rates, and higher significance. The S-plot represents the predictive component loading of the OPLS-DA model for the model interpretation and it combines modeled covariance and correlation in the scatter plot. The p1 (x-axis) represents the variable magnitude and p(corr)1 (y-axis) represents the reliability of each variable in X (Wiklund et al., 2008). Ideal biomarkers have higher magnitude and reliability, so lipids on the lower left and upper right are the most reliable biomarkers.
Results
Sample Characteristics
We enrolled 38 women and we had questionnaire and lipidomics data for 35 (Table 1). We excluded four women from analysis based on PCA and a normal probability plot of residuals. Of the remaining 31 women, 8 had CES-D scores ≥23 and 9 women had a PTB. The sample PTB rate was much higher than rates for African American/Black women in Detroit and Michigan, likely due to the variability resulting from a small sample.
Table 1.
Maternal Characteristics.
| Variable | n | Valid % |
|---|---|---|
| Preterm birth | ||
| Yes | 9 | 31 |
| No | 20 | 69 |
| Living with baby’s father | ||
| Yes | 14 | 54 |
| No | 12 | 46 |
| Household income | ||
| <$20,000 | 13 | 52 |
| ≥$20,000 | 12 | 48 |
| Education level | ||
| ≤High school | 14 | 54 |
| >High school | 12 | 46 |
| Prepregnancy BMI | ||
| <25 kg/m2 | 6 | 23 |
| ≥25 kg/m2 | 20 | 76 |
| CES-D score | ||
| >23 | 18 | 69 |
| ≥23 | 8 | 31 |
Note. N = 31. BMI = body mass index; CES-D = Center for Epidemiologic Studies Depression Scale.
Lipidomic Profiles and DS
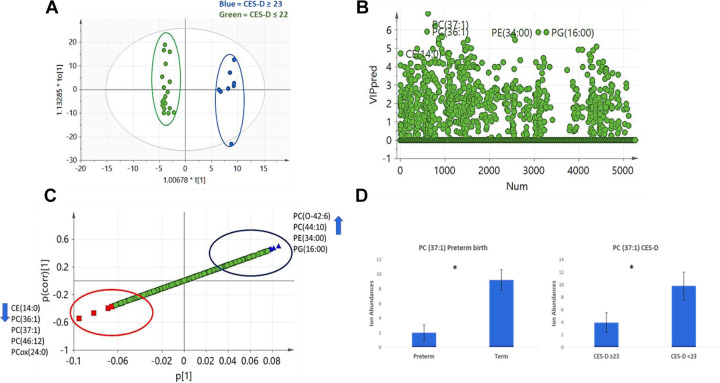
The OPLS-DA model compared lipidomic profiles of women with CES-D scores ≥23 versus those with scores ≤22. The score plot showed very good separation between the two groups, indicating differences in lipidomic profiles (Figure 1A). We calculated VIP scores (Figure 1B) and created loading and S-plots (Figure 1C). We also conducted peak identification of lipid biomarkers identified by multivariate analysis in LIMSA software. Women with CES-D scores ≥23 had higher levels of the O-alkenyl linked PC species PC(O-42:6) and the diacyl PC species PC(44:10), as well as the phosphatidylethanolamine (PE) species PE(34:00) and phosphatidylglycerol (PG) species PG(16:00). Conversely, plasma from the same women contained lower levels of the cholesteryl ester (CE) species CE(14:0), PC species PC(36:1), PC(37:1), PC(46:12), and oxidized PC PCox(24:0) compared with women with CES-D scores ≤22.
Figure 1.
Lipidomic profiles and depressive symptoms (DS). (A) Orthogonal projections to latent structures discriminant analysis (OPLS-DA) score plot showed clear separation between women with Center for Epidemiologic Studies Depression Scale (CES-D) scores ≥23, which have been correlated with DS, and the women with CES-D scores ≤22. Blue = CES-D ≥ 23, green = CES-D ≤22. (B) DS model predicted variable importance in projection (VIP) scatter plot showing lipids with high VIP scores. (C) S-plot of lipids identified to be related to DS. Blue triangles indicate lipid species higher with CES-D score ≥23 and red squares indicate lipid species lower with CES-D ≥ 23. (D) PC(37:1) levels in preterm birth (PTB) and DS models. Values represent mean, and error bars shows standard error of the mean (*p value < .05, Student’s t test).
Lipidomic Profiles and PTB
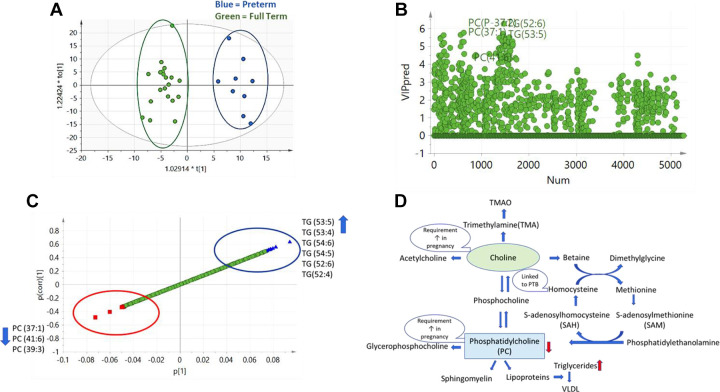
The OPLS-DA score plot showed a clear separation between the PTB and term birth groups, indicating differences in lipidomic profiles between the two groups (Figure 2A). Further investigation of lipids using VIP scores (Figure 2B) and S-plot (Figure 2C) led to the identification of a panel of lipids related to PTB. After peak identification and screening for potential isobaric lipids, we found that women with PTB had higher plasma levels of some TG species including TG(53:5), TG(53:4), TG(54:5), TG(54:6), TG(52:6), and TG(52:4) and lower levels of PC species including PC(37:1), PC(41:6), and PC(39:3) compared with women with term birth.
Figure 2.
Lipidomic profiles and preterm birth (PTB). (A) Orthogonal projections to latent structures discriminant analysis (OPLS-DA) score plot showing separation of PTB group from the full-term birth group. Blue = preterm, green = full term. (B) PTB model predicted variable importance in projection (VIP) scatter plot showing lipids with high VIP scores. (C) S-plot of lipid biomarkers of PTB. Blue triangles indicate lipid species higher with PTB and red squares indicate lipid species lower with PTB. (D) Choline and phosphatidylcholine (PC) metabolism. Specific PCs were found to be in lower levels with PTB and some TG species were found to be higher with PTB.
We observed differences in levels of some phospholipids including PC in the women with CES-D scores ≥23 compared to ≤22. PC species PC(37:1) levels were lower with CES-D scores ≥23 and women with PTB (Figure 1D). These results suggest that PC(37:1) could be a potential pathway between DS and PTB.
Higher TG levels have been associated with overweight/obesity status (Feingold & Grunfeld, 2000). Thus, we explored the relationship between TG levels and overweight/obesity. We created an OPLS-DA model with two groups: overweight/obese (prepregnancy BMI ≥ 25 kg/m2) and nonoverweight/nonobese (prepregnancy BMI < 25 kg/m2). The VIP plots and S-plot indicated that overweight/obese women had higher levels of certain lipid species including specific TGs such as TG(49:1), TG(49:2), TG(55:6), TG(64:7), and TG(66:9) compared with nonoverweight/nonobese women. These TGs were different from the TGs identified related to PTB.
Discussion
PC Association With PTB and DS
We found phospholipids, PC, and glycerophosphocholines to be related to DS in the plasma lipidomic profiles of our sample. Furthermore, we found that women with PTB had lower levels of three specific PC species, PC(37:1), PC(41:6), and PC(39:3), compared with women with term birth. PC plays an important role in fetal development, serving as choline donors, and is used for production of acetylcholine. Choline and PC requirements increase during pregnancy for normal development (Yan et al., 2013). The lower levels of PC in the plasma of women with PTB could indicate lower biosynthesis of PC or lower intake of choline in diet and/or metabolism by microbiota. Detailed investigation of PC biosynthesis and diet intake is needed to understand choline and PC metabolism in PTB.
Our data also point toward PC as a possible link between DS and PTB in pregnant Black women. One of the PC species, PC(37:1), was lower for both outcomes: women with CES-D scores ≥23 and women with PTB (Figure 1D). Choline metabolism and PC have been reported to be related to anxiety and depression in nonpregnant adults (Muller et al., 2015; Riley & Renshaw, 2018) and in one study of pregnant women (van Lee et al., 2017). In a 2017 report from China, researchers investigated urinary metabolomic profiles and identified a panel of markers associated with postpartum (not prenatal) depression and identified a panel of five metabolites, including dimethylamine, as a biomarker of postpartum depression (Lin et al., 2017). In a metabolomics study in Finland, Toffol et al. (2017) reported that prenatal anxiety was linked to homocysteine, a metabolite related to choline and PC metabolism (Toffol et al., 2017). In a study investigating metabolites in plasma collected intrapartum, diacyl PC, PCaaC38:6 was independently inversely associated with gestational age at birth (Li et al., 2016).
Lipoproteins and PTB
We found concentrations for a set of TGs to be higher and some species of PCs to be lower in prenatal plasma from African American women with PTB compared to those with term birth (Figure 2C). Our finding is consistent with other reports of high TG levels being associated with an elevated risk of PTB (Lin et al., 2018; Moayeri et al., 2017). Lower PC could be related to higher TG due to poor assembly of lipoproteins, but these findings need further investigation. Phospholipids and nonesterified cholesterol are major components of the surface of plasma lipoproteins (Lydic & Goo, 2018). PCs are very important for lipoprotein assembly and insufficient biosynthesis will result in reduced levels of lipoproteins (very low-density lipoprotein and HDL) in plasma (Cole et al., 2012). In a recent review, second-trimester levels of both TGs and homocysteine were also associated with PTB (Moayeri et al., 2017).
When we explored whether maternal obesity confounded our results, we found a novel set of TGs that were related to PTB but not to overweight/obesity status. Specifically, TG(53:5), TG(53:4), TG(54:5), TG(54:6), TG(52:6), and TG(52:4) were positively associated with PTB. Lin et al. (2018) also reported that the higher TG levels they found were related to PTB in both overweight and normal-weight women, suggesting that the association is independent of BMI.
Odd Chain Fatty Acids (OC-FA) Associated With DS and PTB: A Novel Finding
We observed changes in levels of odd chain lipids with both DS and PTB. Until recently, OC-FA were thought to be of no, or very little, biological significance (Jenkins et al., 2015). But there has been a growing interest in investigating OC-FA and their roles in metabolism and biosynthesis (Forouhi et al., 2014; Jenkins et al., 2017). OC-FA are mainly consumed in dietary milk products but may also be biosynthesized in the body by α-oxidation (Jenkins et al., 2015). Researchers have reported concentrations of C15:0 and C17:0 to be inversely related to the incidence of Type II diabetes and heart disease among other conditions (Jenkins et al., 2015).
Future Directions
A strength of our study was its focus on African American women, a group that is highly vulnerable to DS during pregnancy and is at the highest risk of PTB in the United States. While our sample was small, it did include several women with PTB and high levels of DS. Future research is needed to examine the associations among DS, lipidomic profiles, and PTB in a larger sample of Black women. A second strength was our application of an innovative untargeted comprehensive omics approach to investigate the lipid profiles. We measured lipidomic profiles at a single time during pregnancy. Examining repeated measures of lipidomic profiles across pregnancy and their relationship with PTB should be the next step. Validation by targeted tandem mass spectrometry lipid analysis and confirmation of the structure of the lipid species should also be undertaken. Finally, the association of PTB and DS with choline metabolism suggests that studies using a global metabolomics approach that includes polar and nonpolar choline metabolites might be fruitful.
Nurses should continue to assess for DS, provide resources such as prenatal support groups, and make needed referrals to mental health care professionals. More evidence is needed before recommending regular monitoring of prenatal lipids. We found that DS were related to alterations in lipidomic profiles for pregnant African American women, and alterations in certain lipids species were related to PTB. Future research needs to investigate these lipid species with a larger sample size as well as data collected at multiple time points across pregnancy.
Conclusions
We identified a set of lipid biomarkers for PTB among African American women. We also found differences in lipidomic profiles between those with higher and lower levels of DS. Our data suggest roles of CEs, phospholipids, and glycerophospholipids in DS and roles of TG and PC in PTB.
Footnotes
Author Contributions: Nadia Saadat contributed to conception, design, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Todd A. Lydic contributed to acquisition and analysis, critically revised the manuscript, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Dawn P. Misra contributed to conception, design, and interpretation; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Rhonda Dailey contributed to acquisition, critically revised the manuscript, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Deborah S. Walker contributed to interpretation, critically revised the manuscript, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Carmen Giurgescu contributed to conception, design, and interpretation; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health, the National Institute on Minority Health and Health Disparities (NIH/NIMHD) grant no. R01MD01157502 (PI Giurgescu), NIH/NIMHD grant no. R15MD011465-01 (PI Walker), and the National Institute of Environmental Health Sciences (NIEHS) grant no. P30 ES 020957 (PI Runge-Morris; pilot dual PIs: Giurgescu and Stemmer).
ORCID iDs: Nadia Saadat  https://orcid.org/0000-0001-8726-9245
https://orcid.org/0000-0001-8726-9245
Carmen Giurgescu  https://orcid.org/0000-0002-4577-3184
https://orcid.org/0000-0002-4577-3184
References
- Bick D. (2012). Born too soon: The global issue of preterm birth. Midwifery, 28(4), 341–342. 10.1016/j.midw.2012.06.010 [DOI] [PubMed] [Google Scholar]
- Brocklehurst P. (1999). Infection and preterm delivery. BMJ, 318(7183), 548–549. 10.1136/bmj.318.7183.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catov J. M., Bertolet M., Chen Y. F., Evans R. W., Hubel C. A. (2014). Nonesterified fatty acids and spontaneous preterm birth: A factor analysis for identification of risk patterns. American Journal of Epidemiology, 179(10), 1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian L. M., Blair L. M., Porter K., Lower M., Cole R. M., Belury M. A. (2016). Polyunsaturated fatty acid (PUFA) status in pregnant women: Associations with sleep quality, inflammation, and length of gestation. PLoS ONE, 11(2). 10.1371/journal.pone.0148752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnattingius S., Villamor E., Johansson S., Edstedt Bonamy A. K., Persson M., Wikström A. K., Granath F. (2013). Maternal obesity and risk of preterm delivery. JAMA, 309(22), 2362–2370. 10.1001/jama.2013.6295 [DOI] [PubMed] [Google Scholar]
- Cole L. K., Vance J. E., Vance D. E. (2012). Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochimica et Biophysica Acta (BBA)—Molecular and Cell Biology of Lipids, 1821(5), 754–761. 10.1016/j.bbalip.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Corwin E. J., Pajer K., Paul S., Lowe N., Weber M., McCarthy D. O. (2015). Bidirectional psychoneuroimmune interactions in the early postpartum period influence risk of postpartum depression. Brain, Behavior, and Immunity, 49, 86–93. 10.1016/j.bbi.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culhane J. F., Elo I. T. (2005). Neighborhood context and reproductive health. American Journal of Obstetrics and Gynecology, 192(Suppl. 5), S22–S29. [DOI] [PubMed] [Google Scholar]
- Dove-Medows E., Deriemacker A., Walker D., Dailey R., Misra D., Kavanaugh K., Giurgescu C. (2019). Pregnant African American women’s perspectives about neighborhood environment, racial discrimination, and psychological distress. MCN: The American Journal of Maternal/Child Nursing, 45(1), 1 10.1097/NMC.0000000000000589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold K. R., Grunfeld C. (2000, updated 2018). Obesity and dyslipidemia In Feingold K. R., Anawalt B., Boyce A., Chrousos G., Dungan K., Grossman A., Hershman J. M., Kaltsas G., Koch C., Kopp P., Korbonits M., McLachlan R., Morley J. E., New M., Perreault L., Purnell J., Rebar R., Singer F., Trence D. L., Vinik A., Wilson D. P. (Eds.), Endotext. MDText.com; https://www.ncbi.nlm.nih.gov/books/NBK305895/ [Google Scholar]
- Forouhi N. G., Koulman A., Sharp S. J., Imamura F., Kröger J., Schulze M. B., Crowe F. L., Huerta J. M., Guevara M., Beulens J. W., van Woudenbergh G. J., Wang L., Summerhill K., Griffin J. L., Feskens E. J., Amiano P., Boeing H., Clavel-Chapelon F., Dartois L.…Wareham N. J. (2014). Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident Type 2 diabetes: The EPIC-InterAct case–cohort study. Lancet Diabetes & Endocrinology, 2(10), 810–818. 10.1016/S2213-8587(14)70146-9 [DOI] [PMC free article] [PubMed]
- Giurgescu C. (2009). Are maternal cortisol levels related to preterm birth? Journal of Obstetrics, Gynecologic & Neonatal Nursing, 38(4), 377–390. 10.1111/j.1552-6909.2009.01034.x [DOI] [PubMed] [Google Scholar]
- Giurgescu C., McFarlin B. L., Lomax J., Craddock C., Albrecht A. (2011). Racial discrimination and the Black–White gap in adverse birth outcomes: A review. Journal of Midwifery & Women’s Health, 56(4), 362–370. 10.1111/j.1542-2011.2011.00034.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimi P., Uphoff A., Hermansson M., Somerharju P. (2006). Software tools for analysis of mass spectrometric lipidome data. Analytical Chemistry, 78(24), 8324–8331. 10.1021/ac061390w [DOI] [PubMed] [Google Scholar]
- Hamilton B. E., Martin J. A., Osterman M. J. K., Rossen L. M. (2019). Births: Provisional data for 2018. https://www.cdc.gov/nchs/data/vsrr/vsrr-007-508.pdf
- Heazell A. E. P., Bernatavicius G., Warrander L., Brown M. C., Dunn W. B. (2012). A metabolomic approach identifies differences in maternal serum in third trimester pregnancies that end in poor perinatal outcome. Reproductive Sciences, 19(8), 863–875. [DOI] [PubMed] [Google Scholar]
- Hoffman M. C., Mazzoni S. E., Wagner B. D., Laudenslager M. L., Ross R. G. (2016). Measures of maternal stress and mood in relation to preterm birth. Obstetrics & Gynecology, 127(3), 545–552. 10.1097/AOG.0000000000001287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins B., West J. A., Koulman A. (2015). A review of odd-chain fatty acid metabolism and the role of pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0) in health and disease. Molecules, 20(2), 2425–2444. 10.3390/molecules20022425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins B. J., Seyssel K., Chiu S., Pan P. H., Lin S. Y., Stanley E., Ament Z., West J. A., Summerhill K., Griffin J. L., Vetter W., Autio K. J., Hiltunen K., Hazebrouck S., Stepankova R., Chen C. J., Alligier M., Laville M., Moore M.…Koulman A. (2017). Odd chain fatty acids; new insights of the relationship between the gut microbiota, dietary intake, biosynthesis and glucose intolerance. Scientific Reports, 7, 44845 10.1038/srep44845 [DOI] [PMC free article] [PubMed]
- Klop B., Elte J. W., Cabezas M. C. (2013). Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients, 5(4), 1218–1240. 10.3390/nu5041218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Lu Y. P., Reichetzeder C., Kalk P., Kleuser B., Adamski J., Hocher B. (2016). Maternal PCaaC38:6 is associated with preterm birth—A risk factor for early and late adverse outcome of the offspring. Kidney and Blood Pressure Research, 41(3), 250–257. 10.1159/000443428 [DOI] [PubMed] [Google Scholar]
- Lin L., Chen X. M., Liu R. H. (2017). Novel urinary metabolite signature for diagnosing postpartum depression. Neuropsychiatric Disease and Treatment, 13, 1263–1270. 10.2147/NDT.S135190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X. H., Wu D. D., Li C., Xu Y. J., Gao L., Lass G., Zhang J., Tian S., Ivanova D., Tang L., Chen L., Ding R., Liu X. M., Han M., Fan J. X., Li X. F, Sheng J. Z., O’Byrne K. T., Huang H. F. (2018). Maternal high triglyceride levels during early pregnancy and risk of preterm delivery: A retrospective cohort study. The Journal of Clinical Endocrinology and Metabolism, 104(4), 1249–1258. 10.1210/jc.2018-01372 [DOI] [PubMed] [Google Scholar]
- Lindsay K. L., Hellmuth C., Uhl O., Buss C., Wadhwa P. D., Koletzko B., Entringer S. (2015). Longitudinal metabolomic profiling of amino acids and lipids across healthy pregnancy. PLoS ONE, 10(12), e0145794 10.1371/journal.pone.0145794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydic T. A., Goo Y. H. (2018). Lipidomics unveils the complexity of the lipidome in metabolic diseases. Clinical and Translational Medicine, 7(1), 4 10.1186/s40169-018-0182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. A., Hamilton B. E., Osterman M. J. K., Driscoll A. K., Drake P. (2018). Births: Final data for 2017. National Vital Statistics Reports, 67(8), 1–50. [PubMed] [Google Scholar]
- Moayeri M., Heida K. Y., Franx A., Spiering W., de Laat M. W., Oudijk M. A. (2017). Maternal lipid profile and the relation with spontaneous preterm delivery: A systematic review. Archives of Gynecology and Obstetrics, 295(2), 313–323. 10.1007/s00404-016-4216-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C. P., Reichel M., Mühle C., Rhein C., Gulbins E., Kornhuber J. (2015). Brain membrane lipids in major depression and anxiety disorders. Biochimica et Biophysica Acta, 1851(8), 1052–1065. [DOI] [PubMed] [Google Scholar]
- Nergiz Avcıoğlu S., Demircan Sezer S., Küçük M., Zafer E., Yüksel H., Akcan B., Turgut O. (2015). Maternal serum concentrations of s-Endoglin and IL-6 in pregnancy complicated by preterm premature membrane rupture. Journal of Maternal-Fetal and Neonatal Medicine, 29(12), 1957–1962. 10.3109/14767058.2015.1070137 [DOI] [PubMed] [Google Scholar]
- Nutor J. J., Slaughter-Acey J. C., Giurgescu C., Misra D. P. (2018). Symptoms of depression and preterm birth among Black women. MCN: The American Journal of Maternal/Child Nursing, 43(5), 252–258. 10.1097/NMC.0000000000000464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr S. T., James S. A., Blackmore P. C. (2002). Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. American Journal of Epidemiology, 156(9), 797–802. [DOI] [PubMed] [Google Scholar]
- Papoutsidakis N., Deftereos S., Giannopoulos G., Panagopoulou V., Manolis A. S., Bouras G. (2014). Treating dyslipidemias: Is inflammation the missing link? Medicinal Chemistry, 10(7), 643–652. 10.2174/1573406410666140318101936 [DOI] [PubMed] [Google Scholar]
- Quinn J. A., Munoz F. M., Gonik B., Frau L., Cutland C., Mallett-Moore T., Kissou A, Wittke F., Das M., Nunes T., Pye S., Watson W., Ramos A. A., Cordero J. F., Huang W. T., Kochhar S., Buttery J., & Brighton Collaboration Preterm Birth Working Group. (2016). Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine, 34(49), 6047–6056. 10.1016/j.vaccine.2016.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. S. (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. [Google Scholar]
- Rees A. M., Austin M. P., Owen C., Parker G. (2009). Omega-3 deficiency associated with perinatal depression: Case control study. Psychiatry Research, 166(2–3), 254–259. [DOI] [PubMed] [Google Scholar]
- Riis V., Sammel M. D., Elovitz M. A. (2016). Racial differences in perceived stress and depression among pregnant women throughout pregnancy. American Journal of Obstetrics and Gynecology, 214(Suppl. 1), S98 10.1016/j.ajog.2015.10.186 [DOI] [Google Scholar]
- Riley C. A., Renshaw P. F. (2018). Brain choline in major depression: A review of the literature. Psychiatry Research: Neuroimaging, 271, 142–153. 10.1016/j.pscychresns.2017.11.009 [DOI] [PubMed] [Google Scholar]
- Romero R., Espinoza J., Goncalves L. F., Kusanovic J. P., Friel L., Hassan S. (2007). The role of inflammation and infection in preterm birth. Seminars in Reproductive Medicine, 25(1), 21–39. 10.1055/s-2006-956773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R., Mazor M., Munoz H., Gomez R., Galasso M., Sherer D. M. (1994). The preterm labor syndrome. Annals of the New York Academy of Sciences, 734, 414–429. 10.1111/j.1749-6632.1994.tb21771.x [DOI] [PubMed] [Google Scholar]
- Smith C. J., Baer R. J., Oltman S. P., Breheny P. J., Bao W., Robinson J. G., Dagle J. M., Liang L., Feuer S. K., Chambers C. D., Jelliffe-Pawlowski L. L., Ryckman K. K. (2018). Maternal dyslipidemia and risk for preterm birth. PLoS ONE, 13(12), e0209579 10.1371/journal.pone.0209579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffol E., Elomaa A. P., Glover V., Kivimäki P., Pasanen M., Keski-Nisula L., Huuskonen P., Voutilainen S., Velagapudi V., Lehto S. (2017). Symptoms of anxiety during pregnancy and metabolism: A pilot metabolomics study. European Psychiatry, 41, S169 10.1016/j.eurpsy.2017.01.2059 [DOI] [Google Scholar]
- van Lee L., Quah P. L., Saw S. M., Yap F. K. P., Godfrey K. M., Chong Y. S., Meaney M. J., Chen H., Chong M. F. (2017). Maternal choline status during pregnancy, but not that of betaine, is related to antenatal mental well-being: The growing up in Singapore toward healthy outcomes cohort. Depression and Anxiety, 34(10), 877–887. 10.1002/da.22637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz J. S., Kac G., Nardi A. E., Hibbeln J. R. (2014). Omega-6 fatty acids and greater likelihood of suicide risk and major depression in early pregnancy. Journal of Affective Disorders, 152–154(1), 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa P. D., Entringer S., Buss C., Lu M. C. (2011). The contribution of maternal stress to preterm birth: Issues and considerations. Clinics in Perinatology, 38(3), 351–384. 10.1016/j.clp.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund S., Johansson E., Sjöström L., Mellerowicz E. J., Edlund U., Shockcor J. P., Gottfries J., Moritz T., Trygg J. (2008). Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Analytical Chemistry, 80(1), 115–122. 10.1021/ac0713510 [DOI] [PubMed] [Google Scholar]
- Yan J., Jiang X., West A. A., Perry C. A., Malysheva O. V., Brenna J. T., Stabler S. P., Allen R. H., Gregory J. F.,III., Caudill M. A. (2013). Pregnancy alters choline dynamics: Results of a randomized trial using stable isotope methodology in pregnant and nonpregnant women. The American Journal of Clinical Nutrition, 98(6), 1459–1467. 10.3945/ajcn.113.066092 [DOI] [PMC free article] [PubMed] [Google Scholar]