Abstract
As the Coronavirus disease 2019 (COVID-19) pandemic spread to the US, so too did descriptions of an associated coagulopathy and thrombotic complications. Hospitals created institutional protocols for inpatient management of COVID-19 coagulopathy and thrombosis in response to this developing data. We collected and analyzed protocols from 21 US academic medical centers developed between January and May 2020. We found greatest consensus on recommendations for heparin-based pharmacologic venous thromboembolism (VTE) prophylaxis in COVID-19 patients without contraindications. Protocols differed regarding incorporation of D-dimer tests, dosing of VTE prophylaxis, indications for post-discharge pharmacologic VTE prophylaxis, how to evaluate for VTE, and the use of empiric therapeutic anticoagulation. These findings support ongoing efforts to establish international, evidence-based guidelines.
Keywords: Coronavirus, Thrombosis, Venous thromboembolism (VTE), Pulmonary embolism (PE), Deep vein thrombosis (DVT), Coagulopathy, Anticoagulation
Highlights
-
•
COVID-19 protocols agreed on heparin-based venous thromboembolism prophylaxis.
-
•
Disagreement on thrombosis risk and diagnosis, D-dimer, empiric anticoagulation
-
•
Cumulative incidence of COVID-19 did not correlate with specific recommendations.
-
•
Framework for frontline providers and hospitals to evaluate practices and outcomes
1. Introduction
Descriptions of abnormal coagulation laboratory parameters and increased incidence of thrombotic complications emerged soon after the first case of COVID-19 was diagnosed in the US in January 2020 [1]. The subsequent months saw rapid accumulation of reports of coagulopathy and thrombosis but were often small or of variable quality [[2], [3], [4], [5], [6], [7], [8], [9]]. Faced with a need to respond rapidly to new evidence and no clear external guidelines, hospitals caring for COVID-19 patients were forced to react. To characterize their response, we collected and analyzed protocols from 21 US academic medical centers for inpatient management of COVID-19 coagulopathy and thrombosis that were developed in the 4 months following the arrival of COVID-19 in the US.
2. Methods
This study is part of the Hospital Medicine Reengineering Network (HOMERuN), a national network of investigators at 70 academic medical centers [10]. We invited HOMERuN collaborative participants to submit their local protocols for compilation via email and direct request during a HOMERuN webinar on COVID-19. Representatives from participating institutions submitted inpatient protocols for incorporation in the analysis between April 10, 2020 and May 5, 2020. For institutions that submitted additional updated protocols, we used the most recent version available at the time of the analysis. We collected additional information about the location, size of the hospital and date of protocol development. All institutions gave permission for inclusion of their protocols in this study.
We developed a potential set of domains and key questions based on review of existing international specialty society interim guidance and extant literature. One additional domain was added after it emerged through review of protocols. Two independent investigators reviewed each protocol submitted. Reviewers extracted and synthesized the content from each protocol. Disagreements were resolved via discussion until consensus was reached. We used descriptive statistics to quantify agreement and variation among protocols.
3. Results
A total of 21 academic medical centers submitted their protocols for analysis. Thirty-eight percent of centers (n = 8) were located in the Northeast, 33% (n = 7) were in the Midwest, 19% (n = 4) were in the South, and 10% (n = 2) were in the West. Thirty-eight percent (n = 8) were located in geographic regions with a cumulative incidence of COVID-19 above the national mean at the time of the analysis [11].
The mean number of hospital beds per institution was 996 (SD ± 626 beds). The mean date of development for the 13 protocols that supplied this information was April 17, 2020 (SD ± 7 days).
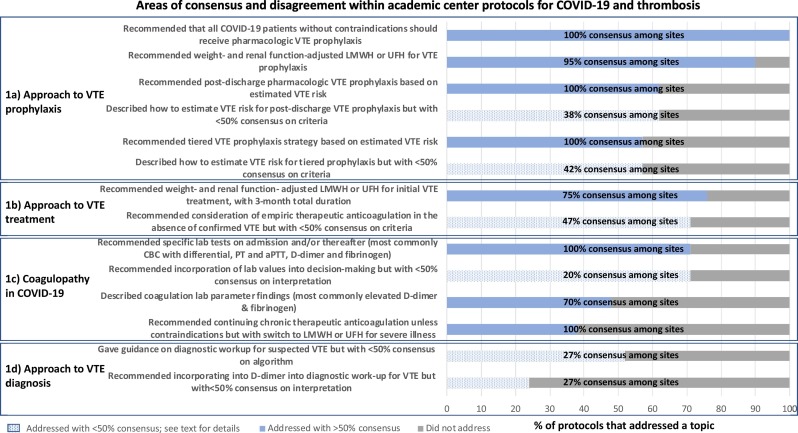
The final structure covered by institutional protocols consisted of 4 overarching themes on COVID-19-associated coagulopathy and thrombosis: 1) coagulopathy in COVID-19, 2) approach to VTE prophylaxis, 3) approach to VTE diagnosis, which was added after it emerged from protocol review, and 4) approach to VTE treatment. The areas with greatest representation from each theme are displayed in Fig. 1 . We reported the proportion of protocols that addressed or did not address a given question. Among the protocols that addressed a clinical question, we then reported the proportion of institutions that supported a specific practice (“consensus”). We provided additional information on topics that had less than 50% consensus below.
Fig. 1.
The x-axis displays the proportion of protocols that addressed a clinical question. For protocols that addressed a clinical question, the proportion of protocols that supported a specific practice (“consensus”) is overlaid. Solid blue bars denote the proportion of protocols that addressed a clinical question with >50% consensus. Patterned blue bars denote the proportion of protocols that addressed a clinical question with <50% consensus. Grey bars denote the proportion of protocols that did not address a clinical question. Abbreviations: COVID-19 - Coronavirus disease 19, VTE - venous thromboembolism, LMWH - low-molecular-weight-heparin, UFH - unfractionated heparin, CBC - complete blood count, PT - prothrombin time, aPTT - activated partial thromboplastin time. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.1. Approach to VTE prophylaxis
Although there was near-universal agreement on the need for heparin-based VTE prophylaxis for COVID-19 inpatients without contraindications, recommended dosing strategies varied across institutions (Fig. 1a). Four protocols (19%) recommended using standard pharmacologic VTE prophylaxis dosing for all COVID-19 patients regardless of estimated VTE risk; conversely, two institutions (10%) recommended higher-than-standard prophylaxis dosing regardless of estimated VTE risk. The most common recommendation was a tiered prophylactic VTE dosing strategy, with standard dosing for patients considered at average VTE risk and higher-than-standard prophylaxis dosing for those at high estimated VTE risk (57%, n = 12). There was little consensus on dosing or patient selection for intensified dosing prophylaxis. For non-obese patients with normal renal function, the four intensified prophylaxis regimens recommended were: enoxaparin 30 mg subcutaneously every 12 h (19%, n = 4), enoxaparin 40 mg subcutaneously every 12 h (19%, n = 4), enoxaparin 1 mg/kg subcutaneously daily (10%, n = 2), or enoxaparin 0.5 mg/kg subcutaneously every 12 h (10%, n = 2). Criteria for selecting which patients warranted higher prophylaxis dosing also exhibited variation: nine protocols recommended selecting patients based on elevated D-dimer or fibrinogen with or without additional clinical criteria, while the remaining three protocols used clinical criteria alone to support prophylaxis intensification. The most common criteria for intensification referenced clinical status, including whether the patient was critically ill, required mechanical ventilation, clinically deteriorated, or had recurrent clotting of venous or arterial access or extracorporeal circuits, or baseline risk factors, including sickle cell disease, malignancy, prior VTE, or pregnancy. There was similar discord regarding which patients merit post-discharge pharmacologic VTE prophylaxis, as well as agent, dose and duration. The most common indications cited were if the patient had received intensified inpatient prophylaxis, had ongoing immobility or had an elevated D-dimer at discharge.
3.2. Approach to VTE treatment
For confirmed VTE, most protocols recommended initial therapeutic anticoagulation with low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH) adjusted for weight and renal function (Fig. 1b). The recommended duration was at least 3 months. In the absence of confirmed VTE, 15 protocols (71%) suggested empiric therapeutic anticoagulation for select populations, but there was no consensus on how to make this decision. Criteria ranged from consideration for all patients admitted to the intensive care unit or restricting to patients with elevated or rising D-dimer, clinical decompensation, or with high clinical suspicion for VTE but who are unable to undergo confirmatory imaging. Two protocols (10%) suggested consideration of tissue plasminogen activator for severely ill patients without confirmed VTE, one as part of a clinical trial and one as empiric treatment.
3.3. Coagulopathy in COVID-19
Although most protocols addressed incorporating laboratory values of coagulopathy into management decisions, there was wide variability in how to act upon these values (Fig. 1c). Seventy-one percent of protocols (n = 15) recommended specific laboratory testing on admission and periodically thereafter (range: daily to every 72 h). The most commonly recommended testing was a complete blood count with differential, prothrombin time and activated partial thromboplastin time, D-dimer and fibrinogen. Three protocols (14%) recommended against transfusion based on coagulation abnormalities unless a patient was bleeding or had other high-risk features. Three protocols (14%) recommended consideration of empiric therapeutic anticoagulation based on elevated D-dimer or fibrinogen alone. In contrast, four protocols (19%) specifically recommended against empiric therapeutic dosing of anticoagulation based on lab values in the absence of other clinical indications such as proven VTE. Nine protocols (43%) recommended escalation from standard to higher-dose prophylactic anticoagulation based on laboratory findings in combination with clinical variables.
3.4. Approach to VTE diagnosis
Most protocols (n = 11, 52%) included some guidance on diagnosis of suspected VTE, but specific recommendations were sometimes divergent (Fig. 1d). One protocol (5%) recommended that all patients receive a baseline screening deep vein thrombosis ultrasound regardless of symptoms. With regard to incorporating D-dimer into diagnostic decision-making for VTE, three protocols (14%) recommended that elevated or rising D-dimer should prompt additional imaging to evaluate for VTE. Conversely, two protocols (10%) commented that D-dimer elevation alone without other clinical suspicion should not trigger diagnostic work-up for VTE and also suggested that D-dimer below the upper limit of normal could be used to exclude VTE. Five protocols (24%) made specific mention of relying on diagnostic modalities performed at the bedside in lieu of CT-angiogram to evaluate for pulmonary embolism, such as deep vein thrombosis point-of-care ultrasound or transthoracic echocardiogram, to minimize the risk of infectious spread and conserve resources.
4. Discussion
Our review of 21 US academic medical center protocols for COVID-19 coagulopathy and thrombosis developed during the early stage of the pandemic showed considerable variation in the approach to the prevention, diagnosis, and treatment of VTE in COVID-19. We found no correlation between centers that had a cumulative incidence of COVID-19 above the national mean with specific recommendations. The area of greatest concordance was use of heparin-based pharmacologic VTE prophylaxis. However there was substantial disagreement on which patients should be considered high-risk for VTE and the indications for escalated pharmacologic prophylaxis dosing or post-discharge pharmacologic prophylaxis. Additionally, there was little consensus on how elevated D-dimer values should affect VTE prophylaxis or treatment, the optimal diagnostic strategy for VTE, and indications for empiric therapeutic anticoagulation for VTE. The areas of consensus and disagreement identified in our study are reflected in the recommendations from recently released international professional society guidelines [[12], [13], [14]]. Aside from consistently recommending pharmacologic prophylaxis with LMWH or UFH, society guidelines emphasize the lack of definitive data and vary in their recommendations on issues of VTE prevention, diagnosis and management.
Our study should not be considered representative of the wider US practice given the self-selected nature of protocol submission. We also can make no assertions on which specific practices should be recommended. Until high-quality comparative effectiveness data are available, our findings serve as a framework for frontline providers and hospitals to evaluate their own practices and outcomes and support the urgent need for evidence in this area.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Dr. Fang reports that research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K24HL141354. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Auerbach reports that research reported in this publication was supported by the Gordon and Betty Moore Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Gordon and Betty Moore Foundation.
Contributor Information
the Hospital Medicine Reengineering Network (HOMERuN):
Valerie M. Vaughn, Andrew S. Dunn, Anne S. Linker, Daniel P. Hunt, Justin J. Choi, Daniel J. Brotman, Michael B. Streiff, Melissa L.P. Mattison, Matthew A. Pappas, S. Ryan Greysen, David F. Hemsey, Kwame Dapaah-Afriyie, Neera Ahuja, William J. Collins, Shoshana J. Herzig, Sanjay Bhandari, Eric R. Schumacher, Vijay S. Duggirala, Kevin J. O'Leary, Geraldine E. Menard, and Michael Y. Lin
References
- 1.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Middeldorp S., Coppens M., Haaps T.F. van. April 19, 2020. Incidence of Venous Thromboembolism in Hospitalized Patients with COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. April 9, 2020 doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. May 4, 2020 doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. April 10, 2020 doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poissy Julien, Goutay Julien, Caplan Morgan, et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 0(0). 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed]
- 8.Thomas W., Varley J., Johnston A. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb. Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auerbach A.D., Patel M.S., Metlay J. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad. Med. J. Assoc. Am. Med. Coll. 2014;89(3):415–420. doi: 10.1097/ACM.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDCMMWR Geographic differences in COVID-19 cases, deaths, and incidence — United States, February 12–April 7, 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69 doi: 10.15585/mmwr.mm6915e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. April 2020 doi: 10.1016/j.jacc.2020.04.031. S0735109720350087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thachil J., Tang N., Gando S. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes G.D., Burnett A., Allen A. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J. Thromb. Thrombolysis. 2020;50(1):72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]