Abstract
The objective of the investigation was to identify biologically active polyphenols and to determine the antioxidant and antimicrobial capacity of Teucrium trifidum extracted with different organic solvents (acetone, ethanol and methanol) and distilled water. The results of the study revealed varying levels of polyphenols in the different solvent extracts. Condensed tannin, flavonoid and total phenolic content ranged from (77.339 ± 1.068) to (99.395 ± 1.490) mg CE/g; (3.398 ± 0.2410) to (53.253 ± 0.638) mg QE/g; (14.1087 ± 0.0915) to (21.7977 ± 0.0279) mg GAE/g, respectively. The extracts demonstrated high antioxidant activity in 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), nitric oxide (NO) and total antioxidant capacity (TAC) assays which were comparable to rutin and butylated hydroxytoluene (BHT) and increased with increasing concentrations of polyphenols extract (P < 0.05). The agar dilution assay of acetone, ethanol and methanol extracts revealed an appreciable broad-spectrum activity against tested pathogenic bacteria. The findings of this study provide evidence that T. trifidum can be used as a natural source of antioxidant and antimicrobial components.
Keywords: Food safety, Food science, Food technology, Nutrition, Antioxidant activity, Antimicrobial activity, Medicinal plants, Polyphenolic contents, Preservatives, Radical scavenging activity
Food safety, Food science, Food technology, Nutrition, Antioxidant activity, Antimicrobial activity, Medicinal plants, Polyphenolic contents, Preservatives, Radical scavenging activity.
1. Introduction
One of the main actions taken by the meat industry to extend the shelf-life and quality of meat and meat products is through the use of meat preservatives (Lorenzo et al., 2018). These meat preservatives prevent oxidation, enzymae reactions and microbial spoilage, which impair the nutritional value and quality of meat and meat products (Falowo et al., 2014). Synthetic antioxidants and antimicrobials have been and continue to be used in the meat industry as preservative agents to reduce economic losses and protect consumer health. However, there has been a serious concern about the use of synthetic preservatives in the food production chain due to the adverse effects on consumers (Lobo et al., 2010). Specifically, the incorporation of synthetic preservatives in meat and meat products has been reported to cause toxicity and to promote the development of cancer in consumers (Lobo et al., 2010). There has been some success in the use of synthetic antimicrobials to prevent microbial spoilage of meat and meat products (Gutierrez et al., 2009). However, certain spoilage microbes such as Listeria monocytogenes are resistant to these conventional synthetic antimicrobials (Gutierrez et al., 2009; Tajkarimi et al., 2010). Importantly, the use of such synthetic antimicrobials in the meat production chain has resulted in the emergency of bacterial-resistant strains that render the management and treatment of livestock, poultry and human diseases difficult (Chellat et al., 2016), thus creating a serious need for novel antibacterial agents.
As a result, the ability of synthetic preservative agents to cause secondary undesirable effects has encouraged consumers and the meat industry to call for natural preservatives (Karre et al., 2013). In addition, consumers are now insisting on high-quality meat and meat products with a long shelf life and no synthetic preservatives (Kumar et al., 2015). The meat industry is therefore currently responding to this call for natural preservatives to meet consumer demands (Karre et al., 2013; Kumar et al., 2015). In recent years, due to their ability as non-toxic preservatives in meat and meat products, considerable attention has been paid to natural preservatives, in particular, antioxidants and antimicrobials of plant origin (Kumar et al., 2015). Plants are an invaluable source of phytochemicals, among them alkaloids, phenolic acids, terpenes, terpenoids, flavonoids, tannins and essential oils (Aziz and Karboune, 2016) that have bioactive effects, including antioxidant and antimicrobial (Falowo et al., 2014; Şahin et al., 2017).
Several researchers have demonstrated the efficacy of various natural antioxidants and antimicrobial agents with the potential to reduce lipid oxidation, spoilage and microbial load in meat and meat products (Falowo et al., 2017; Ergezer et al., 2018). In addition, plant extracts have demonstrated activity against food-borne and pathogenic bacteria, including Bacillus cereus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella Typhimurium and Staphylococcus aureus (Mostafa et al., 2018). For that reason, the inclusion of natural antioxidants and antimicrobial agents in meat and meat products may increase the shelf-life and enhance the nutritional quality of meat and meat products.
Teucrium trifidum Retz. is one of the many plants with phytcohemicals exhibiting these beneficial biological activities. The shrub is widely distributed in South Africa. It belongs to the Lamiaceae family and is used as an ethnomedicine in the management of bacterial-induced diseases as well as an ethnoveterinary medicine in gastrointestinal parasites (Maphosa and Masika, 2010); gall sickness (Masika and Afolayan, 2003); digestive and respiratory ailments (Ruiters et al., 2016). Research has also shown that T. trifidum essential oil has antibacterial activity against Staphylococcus aureus (Ruiters et al., 2016). Despite the well documented antioxidant and antibacterial activity of T. trifidum, its polyphenol content, and its potential in vitro antioxidant and antibacterial activity have not been determined. This study therefore sought to determine the polyphenol content, antioxidant and antimicrobial activity of T. trifidum extracts.
2. Materials and methodology
2.1. Plant collection
The T. trifidum shrubs were collected from the natural veld in the village of Mbizana, Eastern Cape, South Africa. The plant was authenticated at the Albany Museum Herbarium in Grahamstown, South Africa (voucher specimen number IMAZ08/2018). The collected shrubs were rinsed with distilled water and air-dried for 7 days. Dry shrubs were ground into powder with a blender (Waring Products Division, Torrington, USA).
2.2. Plant extracts preparation
Four samples of 100 g of T. trifidum powder were soaked in 1 L of acetone, distilled water, ethanol and methanol. The resulting mixtures were shaken on an orbital shaker (Stuart Scientific Orbital shaker, UK) for 48 h at room temperature. The crude extracts were then filtered through a pressure filter using the Buchner funnel and Whatman No. 1 filter paper. The acetone, ethanol and methanol extracts were then condensed in a rotary evaporator (Laborator 4000-efficient, Heidolph, Germany), while the aqueous extract was freeze-dried (Vir Tis benchtop K, Vir Tis Co, Gardiner, NY). The extracts were stored at 4 °C in airtight glass bottles.
2.3. Determination of polyphenols
2.3.1. Condensed tannin content
The condensed tannin content was determined following the procedure by Hanen et al. (2009). Briefly, the reaction mixture contained 0.5 mL (1 mg/mL) of the extract plus 3 mL of vanillin-methanol (4 % w/v) and 1.5 mL of hydrochloric acid. The standard catechin (0.02–1 mg/mL) reaction mixture was also prepared in the same way. The reaction mixtures were then vortexed and left to stand at room temperature for 15 min. The absorbance of the reaction mixtures was then measured at 500 nm using a UV-3000 PC spectrophotometer (J.P. Selecta, Spain). Condensed tannin content was determined by the calibration curve equation: y = 0.8462x + 0.244, R2 = 0.998 and expressed as milligram of catechin equivalent (mg CE/g) using the equation:
| C = c × V/m |
Where C = total condensed tannin constituent in mg as CE/g extract, c = catechin concentration in mg/mL derived from the calibration curve, V = volume of the extract in the reaction solution in mL, and m = weight of the extract used in the assay in g.
2.3.2. Flavonoid content
The determination of the flavonoid content was done using the aluminium chloride colorimetric method of Abifarin et al. (2019) with some modifications. Briefly, 0.5 mL (1 mg/mL) of the different solvent extracts at varying concentrations (0.2–1 mg/mL) were added to the respective test tubes. Subsequently, 2 mL of distilled water and 0.15 mL of 5 % sodium nitrite were added to each of the test tubes and the mixture was left standing for 6 min. The quercetin standard reaction mixtures were similarly prepared. Then 0.15 mL of 10 % AlCl3 was pipetted into the reaction mixture which was then left standing for another 5 min. Then 1 mL of 1 M sodium hydroxide was added, followed by the addition of distilled water to make up a 5 mL solution. Absorbance was read at a wavelength of 420 nm. Flavonoid content was determined from the quercetin calibration curve equation: y = 1.1968x + 0.1572, R2 = 0.9856 and presented as milligram of quercetin equivalent (mg QE/g) using the equation: C = c × V/m.
2.3.3. Total phenolic content
The total phenolic content of acetone, water, ethanol and methanol extracts of T. trifidum were determined using the Folin–Ciocalteu reagent (Unuofin et al., 2018). Briefly, 0.5 mL of the plant extracts (1 mg/mL) and standard gallic acid (0.02–0.1 mg/mL) were pipetted into test tubes. Subsequently, 2.5 mL of 10 % (v/v) Folin–Ciocalteu reagent was added and the mixture was vortexed. The vortexed solution was left standing at room temperature for 5 min, followed by an addition of 2 mL of 7.5% (w/v) anhydrous sodium carbonate. The mixture was then incubated for 30 min in a water bath at 40 °C for colour development. The absorbance was measured at 765 nm. The phenolic content was then calculated using the gallic acid calibration curve (y = 27.398x + 0.1099, R2 = 0.984) and presented as milligram of gallic acid equivalent (mg GAE/g) using the equation: C = c × V/m.
2.4. Antioxidant assay
2.4.1. ABTS radical scavenging activity
The ABTS scavenging activity of the plant extracts was determined as described by Unuofin et al. (2018). The reaction mixture was made by adding equal volumes of 7 mM ABTS and 2.45 mM potassium persulfate. The mixture was left standing at room temperature in the dark for 12 h to release ABTS radicals. The resulting green-coloured solution was then diluted by adding 3 mL of the ABTS solution to 150 mL of methanol to get an absorbance of 0.700 ± 0.005 at 734 nm. Once the required absorbance was obtained, 1 mL of the resulting solution was combined with 1 mL of the plant extracts/or standards at varying concentrations (0.005–0.08 mg/mL). Approximately 7 min later, the decrease in absorbance was measured at 734 nm. The percentage inhibition of ABTS radicals by the extracts or standards was determined using the following equation: ABTS Scavenging activity (%) = [(Absorbance of control – Absorbance of the sample)/(Absorbance of control)] ×100.
2.4.2. DPPH radical scavenging assay
The procedure described by Abifarin et al. (2019) was followed to determine the free radical scavenging activity of extracts on DPPH radical. A mixture of 0.135 mM DPPH radical in methanol was made and 1 mL of this mixture was combined with 1 mL (0.005–0.08 mg/mL) of each extract. The reaction mixture was allowed to stand The procedure described by Abifarin et al. (2019) was followed to determine the free radical scavenging activity of extracts on DPPH radical. A mixture of 0.135 mM DPPH radical in methanol was made and 1 mL of this mixture was combined with 1 mL (0.005–0.08 mg/mL) of each extract. The reaction mixture was allowed to stand at room temperature in the dark for 30 min. Absorbance was then measured at 517 nm. The reduction of the absorbance was recorded against the control. Rutin and BHT were used as standards (0.005–0.08 mg/mL). in the dark for 30 min. Thereafter, absorbance was measured at 517 nm. The reduction in absorbance was recorded against the control. Rutin and BHT were used as standards (0.005–0.08 mg/mL). The scavenging ability of the extracts was determined using the equation:
| DPPH scavenging activity (%) = [(Absorbance of control – Absorbance of the sample)/(Absorbance of control)] × 100 |
2.4.3. Nitric oxide scavenging activity
The nitric oxide assay was done as described by Ohikhena et al. (2018). Briefly, 0.5 mL of each extract and standard at varying concentrations (0.025–0.400 mg/mL) was combined with 2 mL of 10 mM sodium nitroprusside made in 0.5 mM phosphate buffer saline (pH 7.4). The resulting solution was then left standing in a water bath at 25 °C for 2.5 h. Thereafter, 0.5 mL of Griess mixture and 1 mL of naphthalene diamine dichloride [0.1% w/v]) were added. The Griess mixture consisted of 1 mL sulfanilic acid reagent that is 0.33 % made in 20 % glacial acetic acid. The mixture was left standing in a water bath at room temperature for another 30 min. The absorbance was then read at 540 nm. A solution containing water instead of the extract/standard was used as a control. The number of nitric oxide radicals inhibited by the extracts was extrapolated from the following equation:
| (%) inhibition of NO = [(Absorbance of control – Absorbance of the sample)/(Absorbance of control)] × 100 |
2.4.4. Total antioxidant capacity (phosphomolybdenum assay)
The TAC of the plant extracts was determined using the phosphomolybdenum assay as described by Ohikhena et al. (2018). Briefly, 0.3 mL of the extracts and standards (0.025–0.4 mg/mL) were pipetted into test tubes and combined with 3 mL of the reaction mixture (0.6 M sulphuric acid, 4 mM ammonium molybdate and 28 mM sodium phosphate). The test tubes were then capped and incubated at 95 °C in a water bath for 95 min. Then the solution mixture was left to cool and the absorbance was read at 695 nm. As a control, a mixture consisting of distilled water was used instead of the extract/standard. The percentage of inhibition was determined using the following equation:
| % Inhibition = [(Absorbance of sample − Absorbance of control)/(Absorbance of the sample)] × 100 |
2.5. Antibacterial activity of the plant extracts
2.5.1. Bacterial strains
The rationale for the selection of microorganisms was the use of bacteria responsible for food poisoning and pathological effects. The bacteria were acquired from the Microbiology Unit of the Botany Department of the University of Fort Hare, South Africa. Strains from the American Type Culture Collection (ATCC) were used. Four Gram-positive bacteria: Staphylococcus aureus (ATCC 18824), Streptococcus pyogenes (ATCC 19615), Bacillus subtilis (ATCC 6633), Bacillus cereus (ATCC 10702) and four Gram-negative bacteria: Pseudomonas aeruginosa (ATCC 19582), Klebsiella pneumonia (ATCC 4352), Vibrio cholera (ATCC 14033), Salmonella Typhimurium (ATCC 13311) were used to determine the antibacterial activity of T. trifidum extracts.
2.5.2. Bacterial inoculum preparation
The procedure of suspension of the colony by the European Committee for Antimicrobial Susceptibility Testing (EUCAST, 2003) was adopted in the preparation of the inoculum. All the test bacteria were cultured on nutrient agar slants, recovered in sterile Muller-Hinton broth (MHB) and incubated at 37 °C for 24 h. The previous cultures were mixed 1:100 v/v with fresh sterile MHB and grown on Mueller-Hinton agar (MHA) overnight at 37 °C. Similar colonies from the culture were dispersed in 0.85 % sterile saline, diluted with saline, and balanced with 0.5 McFarland standards to obtain a suspension density equivalent to 1 x 106 CFU/mL. The suspensions were then confirmed by spectrophotometric measurement at a wavelength of 600 nm. Finally, the cell suspensions were adjusted 1:100 by taking 0.1 mL of the bacterial suspension to 9.9 mL of sterile broth to give an estimated inoculum of 1 × 104 CFU/mL. Inocula suspensions were used for inoculation in less than 15 min.
2.5.3. Agar dilution assay
The methods described by Wiegand et al. (2008) and the EUCAST (2003) were used in the antibacterial assay. The MHA was heated in an autoclave at 121 °C for 15 min, then left to cool to 50 °C in a water bath. One millilitre of the 2-fold serial dilutions was transferred to the liquefied agar (19 mL) and carefully whirled before pouring into sterile petri dishes and then left to harden. A 10 μl was taken from the bacterial inocula and then dotted on separate hardened agar to obtain the desired absolute inoculum of 1 × 104 CFU/mL. The concentrations of extracts used in the antibacterial assays ranged from 0.3125 mg/mL to 5 mg/mL. The extracts were compared against the antibacterial standard, erythromycin (0.001–0.016 mg/mL). The petri dishes with bacteria were incubated at 37 °C for 24 h. The minimum inhibitory concentrations (MICs) of the extracts were considered to be the lowest concentration without visible growth on the agar plates.
2.5.4. Preparation of the extracts
100 mg/mL stock mixture was made in a small quantity of dimethyl sulfoxide (DMSO) and topped up with MHB. A 2-fold serial dilution of the extracts (50, 25, 12.5, 6.25, 3.125, 1.5625 mg/mL) was prepared. Similarly, the standard antibiotic (erythromycin) was prepared.
2.6. Statistical analyses
The data were presented as mean ± standard deviation. MINITAB 17 statistical packages were used to analyze the data. One-way analysis of variance (ANOVA) was done to determine the variations in the antioxidant and antibacterial activity of the different T. trifidum extracts. The means were separated by the Fisher's Least Significant Difference (LSD). Significance was set at P < 0.05.
3. Results
3.1. Phytochemical content
Condensed tannin, flavonoid and total phenolic content in T. trifidum extracts are shown in Table 1. The polyphenol content of acetone, aqueous, ethanol and methanol extracts of T. trifidum were significantly different (P < 0.05). The aqueous extract had the most condensed tannin content (99.395 ± 1.490 mg CE/g) while the acetone extract had the lowest (77.339 ± 1.068 mg CE/g). The acetone extract had the highest flavonoid content (53.253 ± 0.638 mg QE/g), while the aqueous extract had the lowest content (3.398 ± 0.241 mg QE/g). Total phenolic content (21.798 ± 0.028 mg GAE/g) was highest in the acetone extract, while the aqueous extract had the lowest total phenolic content (14.109 ± 0.092 mg GAE/g).
Table 1.
Polyphenol content of various solvent extracts of T. trifidum.
| Solvent | Condensed tannin (mg CE/g) | Flavonoid (mg QE/g) | Total phenolic (mg GAE/g) |
|---|---|---|---|
| Acetone | 77.339d ± 1.068 | 53.253a ± 0.638 | 21.798a ± 0.028 |
| Aqueous | 99.395a ± 1.490 | 3.398d ± 0.241 | 14.109d ± 0.092 |
| Ethanol | 81.156c ± 0.648 | 45.733b ± 0.870 | 20.356b ± 0.046 |
| Methanol | 83.701b ± 0.245 | 21.084c ± 0.638 | 14.334c ± 0.087 |
Abbreviations: mg CE/g - milligram catechin equivalent per gram of extract; mg QE/g - milligram quercetin equivalent per gram of extract; mg GAE/g - milligram gallic acid equivalent per gram of extract.
Mean with a-d superscripts within a column indicates significant differences (P < 0.05).
3.2. ABTS radical scavenging activity
Figure 1 shows that the ABTS scavenging activity of the extracts and standards had an increasing trend as concentrations increased (P < 0.05). At the maximum concentration (0.08 mg/mL), the highest % mean inhibition was produced by rutin, BHT and ethanol extract (100 %) followed by methanol (97.52 %), acetone (96.71 %) and aqueous extract (69.36 %). The concentration required to scavenge 50 % of the radicals (IC50) was in the following order: rutin > BHT > ethanol extract > methanol extract > acetone extract > aqueous extract (Table 2).
Figure 1.
ABTS radical scavenging activity of T. trifidum extracts. A set of bars with different superscripts are significantly different (P < 0.05).
Table 2.
IC50 values of the various extracts of T. trifidum and standard drugs.a
| Extracts/Standard | ABTS |
DPPH |
Nitric Oxide |
TAC |
||||
|---|---|---|---|---|---|---|---|---|
| IC50 | R2 | IC50 | R2 | IC50 | R2 | IC50 | R2 | |
| Aqueous | 0,297 | 0,999 | 0,095 | 0,966 | 0,292 | 0,976 | 0,121 | 0,915 |
| Acetone | 0,086 | 0,942 | 0,018 | 0,951 | 0,290 | 0,988 | 0,044 | 0,977 |
| Ethanol | 0,067 | 0,942 | 0,012 | 0,917 | 0,150 | 0,995 | 0,035 | 0,836 |
| Methanol | 0,182 | 0,944 | 0,017 | 0,950 | 0,304 | 0,993 | 0,025 | 0,968 |
| BHT | 0,019 | 0,765 | 0,005 | 0,903 | 1,914 | 0,958 | 0,222 | 0,960 |
| Rutin | 0 | 0.500 | 0 | 0,675 | 0,100 | 0,875 | 0,020 | 0,948 |
Abbreviations: IC50 – the concentration (mg/mL) required to scavenge/inhibit 50% of the radical; R2 – coefficient of determination.
Values obtained from regression curve with 95% confidence level.
3.3. DPPH radical scavenging assay
The DPPH radical scavenging activities of the extracts and standards are shown in Figure 2. At the highest concentration (0.08 mg/mL), rutin demonstrated the highest DPPH scavenging activity (93.28 %), while acetone extract (92.67 %), ethanol extract (92.15 %), methanol extract (91.65 %), BHT (90.92 %) and the aqueous extract (39.64 %) followed in decreasing order (P < 0.05). The concentrations (mg/mL) required to scavenge 50 % of the radical (IC50) followed this trend: rutin > BHT > ethanol extract > methanol extract > acetone extract > aqueous extract. The least IC50 of the ethanol extract was indicative of its strong DPPH radical scavenging activity (Table 2).
Figure 2.
DPPH radical scavenging activity of T. trifidum extracts. A set of bars with different superscripts are significantly different (P < 0.05).
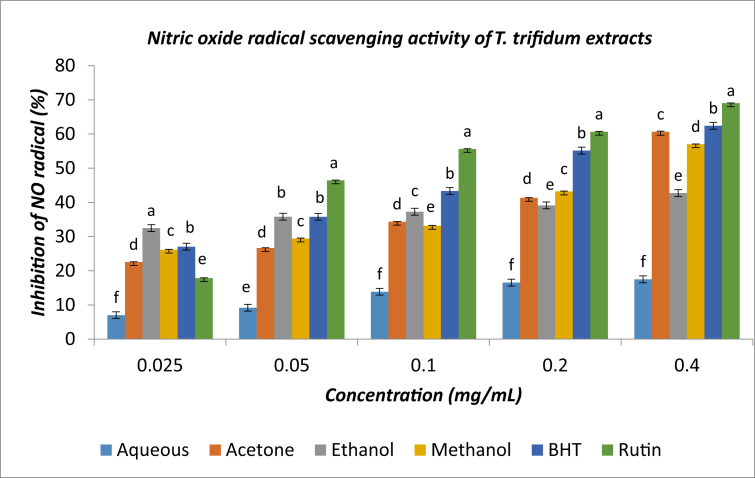
3.4. Nitric oxide scavenging activity
The percentage nitric oxide scavenging activity of the extracts and standards increased as concentrations increased (P < 0.05). At the highest concentration (0.40 mg/mL), the percentage of nitric oxide inhibitory activity followed this sequence: rutin > BHT > acetone extract > methanol extract > ethanol extract > aqueous extract (Figure 3). The IC50 values of the extracts/standards were in this sequence: rutin > BHT > acetone extract > ethanol extract > methanol extract > aqueous extract (Table 2).
Figure 3.
Nitric oxide radical scavenging activity of T. trifidum extracts. A set of bars with different superscripts are significantly different (P < 0.05).
3.5. Total antioxidant capacity
As shown in Figure 4, the extracts displayed a concentration-dependent total antioxidant capacity (P < 0.05). At the maximum concentration, the plant extracts showed high antioxidant activity (74.11–91.01 %) which was comparable to the standards (P < 0.05). On the basis of the IC50, rutin showed higher antioxidant activity compared to the rest. Antioxidant activity followed in this order: rutin > methanol extract > ethanol extract > acetone extract > aqueous extract > BHT.
Figure 4.
Total antioxidant capacity of T. trifidum extracts. A set of bars with different superscripts are significantly different (P < 0.05).
3.6. Antibacterial activity
Both Gram-positive and Gram-negative bacteria were susceptible to the T. trifidum extracts (Table 3). However, the aqueous extract exhibited the lowest antibacterial activity on both Gram-positive and Gram-negative bacteria with MIC values greater than 5 mg/mL. The standard erythromycin exhibited the highest antibacterial activity with MIC values within the range of 0.001–0.008 mg/mL. The acetone extract was active against all the bacterial isolates except B. cereus and S. aureus. All bacteria types were affected by methanol extracts except S. aureus.
Table 3.
Minimum inhibitory concentrations (MIC) of the various extracts of T. trifidum against selected pathogenic bacteria.
| Bacteria | MIC(mg/mL) |
||||
|---|---|---|---|---|---|
| Aqueous | Acetone | Ethanol | Methanol | Erythromycin | |
| Staphylococcus aureus (+) | >5 | >5 | >5 | >5 | 0.001 |
| Salmonella Typhimurium (-) | >5 | 5 | >5 | 5 | 0.002 |
| Vibrio cholera (-) | >5 | 5 | 5 | 5 | 0.008 |
| Klebsiella pneumonia (-) | >5 | 5 | 5 | 5 | 0.001 |
| Streptococcus pyogenes (+) | >5 | 5 | >5 | 5 | 0.004 |
| Bacillus subtilis (+) | >5 | 5 | 5 | 5 | 0.008 |
| Bacillus cereus (+) | >5 | >5 | >5 | 5 | 0.002 |
| Pseudomonas aeruginosa (-) | >5 | 5 | 5 | 5 | 0.002 |
4. Discussion
Phytochemicals present in plants exhibit health beneficial bioactivities including antioxidant (Lorenzo et al., 2018) and antimicrobial activities (Mahmud and Khan, 2018). Due to their antioxidant and antimicrobial function, plants can potentially be used as natural preservatives for meat and meat products. Phenolic compounds such as tannins, flavonoids and phenolic acids are the major antioxidant phytochemicals (Shahidi and Ambigaipalan, 2015). The redox properties of these phytochemicals are important for the absorption and neutralization of free radicals, degradation of peroxides, and also for the quenching of reactive oxygen species (Hossain et al., 2011).
The acetone extract of T. trifidum had the highest amounts of total phenolic content and flavonoids while the aqueous extract had the highest amount of condensed tannin content. Such results reveal similar patterns in phenolic and flavonoid content of Vernonia mespilifolia acetone and aqueous extracts, respectively (Unuofin et al., 2018). Polarity and solvent influence the amount of phenolic substances derived from plants. The variations in the extraction ability of solvents may therefore be the cause of the variations in the T. trifidum aqueous and acetone extracts.
Polyphenols are considered to have a beneficial impact on health, including antiulcerogenic (Issac et al., 2015), antiestrogenic (Basu et al., 2018), antidiabetic (Ohikhena et al., 2018), anticancer (Rodrigues et al., 2012) and anti-inflammatory (Wang et al., 2014). It can therefore be inferred that the presence of similar polyphenols in T. trifidum extracts justify its widespread use in ethnoveterinary medicine (Maphosa and Masika, 2010; Masika and Afolayan, 2003) and ethnomedicine (Van Wyk, 2011).
The study showed that synthetic antioxidants (BHT and rutin) had the greatest antioxidant effect on almost all the assays. The various extracts displayed significant free radical scavenging activity therefore T. trifidum can potentially be used as a natural source of antioxidants. As a result, the significant antioxidant activity demonstrated by T. trifidum extracts could be pointed to the presence of these phytochemicals in the extracts. For example, the ethanol extract had the highest ABTS scavenging potential compared to the other extracts. This may be due to the high phenolic content of the extract, which could have contributed to the electron transfer/hydrogen donation (Unuofin et al., 2018). The acetone extract showed superior scavenging ability for DPPH and nitric oxide radicals, while the methanol extract had higher total antioxidant capacity. Flavonoids and phenols are known to have antiradical and antioxidant activity (Heim et al., 2002). As a result, the acetone extract could have scavenged best due to its high phenolic content. In addition, the results indicate that T. trifidum extracts can potentially be used as natural sources of antioxidants due to their high free radical scavenging capabilities.
Owing to the use of conventional (synthetic) pharmacological agents such as antibiotics and growth promoters of livestock and poultry, there has been an increase in the problem of antibiotic resistance in consumers as well as livestock and poultry (Marshall and Levy, 2011; Ralte et al., 2019). The possible future threat of this antibiotic resistance has led to research into the use of natural alternatives that are considered to be safe. The antimicrobial capacity of plants is assumed to be the function of phytochemical compounds present in them. Which include alkaloids, tannins, saponins, flavonoids and phenolic compounds (Wintola and Afolayan, 2015). Although mechanisms for antimicrobial activity are not well understood, it is argued that these phytochemicals induce antimicrobial activity by specific mechanisms.
One of the postulated mechanisms is that the phytochemicals interact with and disrupt the phospholipid cell membranes, resulting in increased cell permeability, leading to the depletion of cell components (Omojate et al., 2014). Phenolic compounds inhibit hydrolytic enzymes thus disrupting both the energy production and the production of structural components (Pandey & Kumar, 2013). In bacterial cells, phytochemicals cause cell content coagulation and also inactivate deoxyribonucleic acid (DNA) which is manifested as growth retardation (Omojate et al., 2014).
The results of this study have shown that both gram-positive and gram-negative bacteria are susceptible to T. trifidum extracts. The observed antimicrobial effects of T. trifidum extracts are ascribed to the phytochemical-induced antibacterial activity as the extracts contained bioactive compounds known to have antibacterial activity. The presence of several phytochemicals with antibacterial activity in the extracts may have contributed to the synergistic destruction of the bacteria (Farasat et al., 2014). Although the organic solvent extracts showed efficacy, the aqueous extract was less effective mainly due to poor extraction of the key phytochemicals with antibacterial activity. Biswas, Rogers, McLaughlin, Daniels, and Yadav (2013) reported that gram-negative bacteria had resistance to the antimicrobial activity of plant-derived extracts. Nonetheless, in the current reaserch, the T. trifidum extracts showed activity against gram-negative and gram-positive bacteria. A related study, but using extracts from Bidens pilosa and Moringa oleifera (Falowo et al., 2016), also showed potency against gram-negative bacteria. It is noteworthy that like the T. trifidum extracts, the B. pilosa and M. oleifera extracts, also contained phytochemicals with antibacterial activity.
5. Conclusion
The aqueous, acetone, ethanol and methanol T. trifidum extracts contained condensed tannins, flavonoids and phenolic compounds which showed significant free radical mopping capabilities. In addition, the extracts demonstrated antibacterial activity against gram-negative and gram-positive bacteria. The T. trifidum extracts can potentially be used as a natural source of antioxidant with antimicrobial components and can therefore be used as preservatives.
Declarations
Author contribution statement
Irene R. MAZHANGARA, Emrobowansan M. IDAMOKORO: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Eliton CHIVANDI: Conceived and designed the experiments; Wrote the paper.
Anthony J. AFOLAYAN: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the National Research Fund Collaborative Postgraduate Training Programme, South Africa (Grant Number 105289).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to acknowledge the Govan Mbeki Research and Development Centre, University of Fort Hare (GMRDC, UFH) for their support during this research.
References
- Abifarin T.O., Afolayan A.J., Otunola G.A. Phytochemical and antioxidant activities of Cucumis africanus L.f.: a wild vegetable of South Africa. j. Evid. Base Integr. Med. 2019;24 doi: 10.1177/2515690X19836391. 2515690X19836391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz M., Karboune S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: a review. Crit. Rev. Food Sci. Nutr. 2016;58(3):1–26. doi: 10.1080/10408398.2016.1194256. [DOI] [PubMed] [Google Scholar]
- Basu P., Dixon D., Varghese S., Maier C. Detection of estrogenic, antiestrogenic, and drug synergistic activities of seven commercially available fruits by in Vitro reporter assays. Pharmacogn. Res. 2018;10(2):137. [Google Scholar]
- Biswas B., Rogers K., McLaughlin F., Daniels D., Yadav A. Antimicrobial activities of leaf extracts of guava (Psidium guajava L.) on two gram-negative and gram-positive bacteria. Int. J. Microbiol. 2013;2013:1–7. doi: 10.1155/2013/746165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellat M.F., Raguž L., Riedl R. Targeting antibiotic resistance. Angew. Chem. Int. Ed. 2016;55(23):6600–6626. doi: 10.1002/anie.201506818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergezer H., Kaya H.İ., Şimşek Ö. Antioxidant and antimicrobial potential of artichoke (Cynara scolymus L.) extract in beef patties. Food Technology and economy, Engineering and physical properties Czech. J. Food Sci. 2018;36(2):154–162. [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003;9:1–7. Retrieved from. [Google Scholar]
- Falowo A.B., Muchenje V., Hugo A., Aiyegoro O.A., Fayemi P.O. Antioxidant activities of Moringa oleifera L. and Bidens pilosa L. leaf extracts and their effects on oxidative stability of ground raw beef during refrigeration storage. CyTA - J. Food. 2017;15(2):249–256. [Google Scholar]
- Falowo A.B., Muchenje V., Hugo C.J., Charimba G. In vitro antimicrobial activities of Bidens pilosa and Moringa oleifera leaf extracts and their effects on ground beef quality during cold storage. CyTA - J. Food. 2016;14(4):541–546. [Google Scholar]
- Falowo Andrew B., Fayemi P.O., Muchenje V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: a review. Food Res. Int. 2014;64:171–181. doi: 10.1016/j.foodres.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Farasat M., Khavari-Nejad R.-A., Nabavi S.M.B., Namjooyan F. Antioxidant activity, total phenolics and flavonoid contents of some edible green seaweeds from Northern Coasts of the Persian gulf. Iran. J. Pharm. Res. (IJPR) 2014;13(1):163–170. http://www.ncbi.nlm.nih.gov/pubmed/24734068 Retrieved from. [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J., Barry-Ryan C., Bourke P. Antimicrobial activity of plant essential oils using food model media: efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009;26(2):142–150. doi: 10.1016/j.fm.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Hanen F., Riadh K., Samia O., Sylvain G., Christian M., Chedly A. Interspecific variability of antioxidant activities and phenolic composition in Mesembryanthemum genus. Food Chem. Toxicol. 2009;47(9):2308–2313. doi: 10.1016/j.fct.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Heim K.E., Tagliaferro A.R., Bobilya D.J. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. JNB (J. Nutr. Biochem.) 2002;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Hossain M.A., Shah M.D., Gnanaraj C., Iqbal M. In vitro total phenolics, flavonoids contents and antioxidant activity of essential oil, various organic extracts from the leaves of tropical medicinal plant Tetrastigma from Sabah. Asian Pacific J. Trop. Med. 2011;4(9):717–721. doi: 10.1016/S1995-7645(11)60180-6. [DOI] [PubMed] [Google Scholar]
- Issac A., Gopakumar G., Kuttan R., Maliakel B., Krishnakumar I.M. Safety and anti-ulcerogenic activity of a novel polyphenol-rich extract of clove buds (Syzygium aromaticum L) Food and Function. 2015;6(3):842–852. doi: 10.1039/c4fo00711e. [DOI] [PubMed] [Google Scholar]
- Karre L., Lopez K., Getty K.J.K. Natural antioxidants in meat and poultry products. Meat Sci. 2013;94(2):220–227. doi: 10.1016/j.meatsci.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Kumar Y., Yadav D.N., Ahmad T., Narsaiah K. Recent trends in the use of natural antioxidants for meat and meat products. Compr. Rev. Food Sci. Food Saf. 2015;14(6):796–812. [Google Scholar]
- Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Phcog. Rev. 2010;4(8):118. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo J.M., Munekata P.E.S., Sant’Ana A.S., Carvalho R.B., Barba F.J., Toldrá F.…Trindade M.A. Main characteristics of peanut skin and its role for the preservation of meat products. Trends Food Sci. Technol. 2018;77:1–10. [Google Scholar]
- Mahmud J., Khan R.A. Characterization of natural antimicrobials in food system. Adv. Microbiol. 2018;8:894–916. [Google Scholar]
- Maphosa V., Masika P.J. Ethnoveterinary uses of medicinal plants: a survey of plants used in the ethnoveterinary control of gastro-intestinal parasites of goats in the Eastern Cape Province, South Africa. Pharmaceut. Biol. 2010;48(6):697–702. doi: 10.3109/13880200903260879. [DOI] [PubMed] [Google Scholar]
- Marshall B.M., Levy S.B. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 2011;24(4):718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masika P.J., Afolayan A.J. An ethnobotanical study of plants used for the treatment of livestock diseases in the eastern Cape Province. Pharmaceut. Biol. 2003;41(1):16–21. [Google Scholar]
- Mostafa A.A., Al-Askar A.A., Almaary K.S., Dawoud T.M., Sholkamy E.N., Bakri M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018;25(2):361–366. doi: 10.1016/j.sjbs.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohikhena F.U., Wintola O.A., Afolayan A.J. Quantitative phytochemical constituents and antioxidant activities of the mistletoe, Phragmanthera capitata (sprengel) Balle extracted with different solvents. Pharmacogn. Res. 2018;10(1):16–23. doi: 10.4103/pr.pr_65_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omojate G.C., Enwa F.O., Jewo A.O., Eze C.O. Vol. 2. 2014. Mechanisms of antimicrobial actions of phytochemicals against enteric pathogens-A review.http://www.jpcbs.info (Journal of Pharmaceutical, Chemical and Biological Sciences). Retrieved from. [Google Scholar]
- Pandey A.K., Kumar S. Perspective on plant products as antimicrobials agents: a review. Pharmacologia. 2013;4(7):469–480. [Google Scholar]
- Ralte R., Kumar V., Wani M.Y., Randhawa S.N.S., Malik A., Kaur N. Antimicrobial resistance profile of livestock and poultry of North-western Punjab. Int. J. Curr. Microbiol. Appl. Sci. 2019;8(10):1250–1259. [Google Scholar]
- Rodrigues S., Calhelha R.C., Barreira J.C.M., Dueñas M., Carvalho A.M., Abreu R.M.V.…Ferreira I.C.F.R. Crataegus monogyna buds and fruits phenolic extracts: growth inhibitory activity on human tumor cell lines and chemical characterization by HPLC–DAD–ESI/MS. Food Res. Int. 2012;49(1):516–523. [Google Scholar]
- Ruiters A.K., Tilney P.M., Van Vuuren S.F., Viljoen A.M., Kamatou G.P.P., Van Wyk B.-E. The anatomy, ethnobotany, antimicrobial activity and essential oil composition of southern African species of Teucrium (Lamiaceae) South Afr. J. Bot. 2016;102:175–185. [Google Scholar]
- Şahin S., Samli R., Tan A.S.B., Barba F.J., Chemat F., Cravotto G., Lorenzo J.M. Solvent-free microwave-assisted extraction of polyphenols from olive tree leaves: antioxidant and antimicrobial properties. Molecules. 2017;22(7) doi: 10.3390/molecules22071056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi F., Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects – a review. J. Funct. Foods. 2015;18:820–897. [Google Scholar]
- Tajkarimi M.M., Ibrahim S.A., Cliver D.O. Antimicrobial herb and spice compounds in food. Food Contr. 2010;21(9):1199–1218. [Google Scholar]
- Unuofin J.O., Otunola G., Afolayan A. Polyphenolic content, antioxidant and antimicrobial activities of Vernonia mespilifolia less. Used in folk medicine in the eastern Cape Province, South Africa. j. Evid. Base Integr. Med. 2018;23 doi: 10.1177/2515690X18773990. 2515690X1877399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wyk B.-E. The potential of South African plants in the development of new medicinal products. South Afr. J. Bot. 2011;77(4):812–829. [Google Scholar]
- Wang S., Moustaid-Moussa N., Chen L., Mo H., Shastri A., Su R.…Shen C.-L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014;25(1):1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand I., Hilpert K., Hancock R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- Wintola O.A., Afolayan A.J. The antibacterial, phytochemicals and antioxidants evaluation of the root extracts of Hydnora africanaThunb. used as antidysenteric in Eastern Cape Province, South Africa. BMC Compl. Alternative Med. 2015;15:307. doi: 10.1186/s12906-015-0835-9. [DOI] [PMC free article] [PubMed] [Google Scholar]