Abstract
Periodontal disease is a chronic multifactorial infectious and inflammatory disease associated with several chronic systemic diseases, such as diabetes, cardiovascular diseases (CVD), chronic obstructive pulmonary disease, hypertension, Alzheimer’s disease and so on. These same systemic diseases have been associated with severe COVID-19 infections. Several recent studies have suggested hypotheses for the potential association between periodontal disease and severe COVID-19. Periodontal disease is also one of the most prevalent diseases globally. All this supports the importance of good oral health, also in the COVID-19 era. Thus, new strategies and approaches to identify patients at risk of periodontal disease could be beneficial to enhance secondary prevention, especially if targeted to COVID-19 risk groups. Diagnostic biomarkers for periodontal disease have been researched extensively. Potential biomarkers in oral fluid with currently available rapid non-invasive point-of-care technology, such as aMMP-8, could help to extend screening and identification of patients at risk for periodontal disease also to situations and places where professional dental expertise and equipment are limited or unavailable. i.e., nursing and care homes, and rural and distant places. The oral fluid point-of-care technologies could also be useful in the hands of medical professionals (diabetes, CVD, etc.) to identify patients at risk for undiagnosed periodontal disease and to refer them to a dentist for examination and evaluation. Finally, if there is a causality between periodontal disease and severe COVID-19 infections, these point-of-care oral fluid biomarker technologies could possibly also help in the assessment of the risk of deterioration and complications.
Keywords: Periodontitis, Prevention, Point-of-care testing, Teledentistry, aMMP-8, COVID-19
Introduction
Oral diseases, including periodontitis (gum disease), are one of the most prevalent diseases globally [1], [2]. They also represent a significant public health problem and have major health and economic burdens for individuals, communities and society [2]. In 2017, there were almost 800 million people globally with severe periodontitis [1]. Periodontitis is a chronic multifactorial infectious and inflammatory disease that not only leads to the destruction of tooth supporting tissues but can also have adverse systemic effects [3]. Mounting evidence in the literature suggests that periodontitis as a chronic low-burden inflammation is associated with several chronic systemic diseases, including diabetes, cardiovascular diseases (CVD), chronic obstructive pulmonary disease (COPD), hypertension, and cancer [4], [5], [6], [7], [8], [9], [10]. These same systemic diseases, as well as old age, have been identified as risk factors for severe COVID-19 infections [11], [12].
Recently, several studies have suggested hypotheses for the potential link between periodontitis and COVID-19 complications [13], [14], [15], [16], [17], [18], [19], [20], [21]. For example, bacterial superinfections have been common in severe COVID-19 cases, and the bacteria seem to be associated with the oral cavity [13], [14], [15]. Periodontitis may elevate the risk of invasion by bacterial pathogens [3], and potentially also viral pathogens such as SARS-CoV-2 [4], [20], [21], as periodontitis leads, even in mild and moderate forms, to the ulceration of the gingival epithelium. This exposed, ulcerated and inflamed surface area can be of substantial size, comparable with the surface area of the palm of a hand (8–20 cm2 vs. 78 cm2, respectively) [22]. Furthermore, periodontitis also increases the systemic inflammatory burden, as the inflamed periodontal tissues release host-derived proinflammatory cytokines and tissue destruction mediators to the circulatory system [3]. This can activate acute-phase response in the liver and amplify systemic inflammation [3]. The cytokine storm in severe COVID-19 infections has many components common with the cytokine expression profile of periodontitis suggesting a possible link between periodontitis and COVID-19 infection and complications, as well [16].
The aforementioned studies support the importance of good oral health also during the current COVID-19 pandemic. The spread of SARS-CoV-2 has been rapid. Already over 30 million people have been infected and more than 900,000 people have died of the COVID-19 disease globally. Senior citizens and individuals with underlying health conditions have been at higher risk of severe COVID-19 infections [23], [24], [25]. Moreover, the prevalence of underlying health conditions and periodontitis are higher among older individuals [1], [25]. This underscores the role of preventive oral health strategies with improvements in risk assessment and targeted disease prevention in reducing the disease burden of the COVID-19 pandemic.
Hypothesis
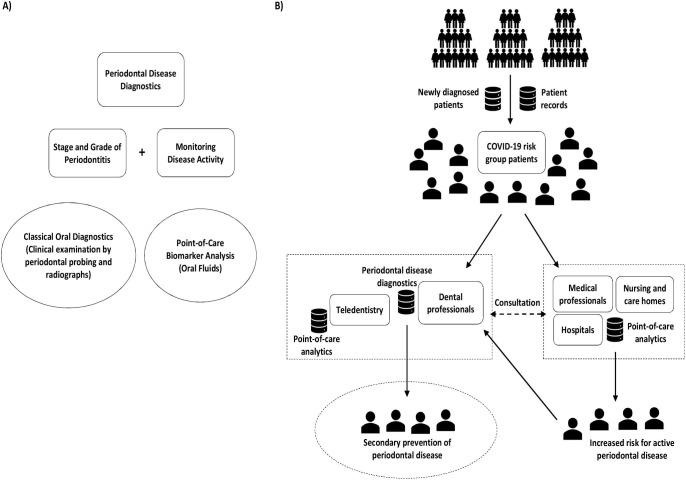
This study proposes to evaluate if non-invasive oral fluid point-of-care tests based on biomarkers, such as active matrix metalloproteinase-8 (aMMP-8), could be beneficial for interdisciplinary screening and identification of individuals with severe periodontitis and active periodontal breakdown during COVID-19 pandemic to refer them to targeted periodontal disease prevention (Fig. 1 ). Periodontitis could be associated with severe COVID-19 infections. Thus, the proposed preventive oral health strategy with point-of-care testing could mitigate the risk of adverse systemic effects of periodontitis and possibly also the risk of severe COVID-19 infections, especially among older patients and those with underlying health conditions. Moreover, it could also increase awareness of periodontal disease among individuals and encourage them to seek oral care before periodontal disease has progressed to severe stage.
Fig. 1.
A) Periodontal disease diagnostics and B) related preventive oral health strategy.
Evaluation of hypothesis: Preventive oral health strategy and point-of-care diagnostics for better oral health
Point-of-care oral fluid testing in periodontitis diagnostics
Typically, patients suffering from periodontitis are mostly not aware of their disease especially in the early phase because the oral symptoms are often painless and subclinical. As periodontitis advances and symptoms become more apparent that is usually the time when patients start to seek care. However, the loss of tooth supporting periodontal tissues is usually irreversible in periodontitis. As periodontitis becomes more generalized, the local inflammation might markedly extend its effect into the systemic circulation. Therefore, early and accurate diagnosis of periodontitis is crucial in controlling periodontitis and preventing further attachment loss and disease progression.
Periodontitis is diagnosed based on clinical and radiographic examination [26], [27]. The examination includes clinical measurements such as clinical attachment level and bleeding on probing by using a periodontal probe. These classical oral diagnostic methods provide information about the extent and severity of periodontitis and the degree of past attachment loss. However, accurately identifying patients with periodontitis, especially in its initial/early stage, requires lots of training and experience [27]. Furthermore, it is still unclear whether the clinical measurements alone are accurate enough to monitor disease activity, progression and treatment effects for all patients [27], [28], [29]. This is where accurate biomarkers come in.
Point-of-care oral fluid biomarker research has actively sought for suitable biomarker(s) and methods to improve the accuracy of oral health and periodontal disease diagnostics [27], [28], [29]. This is very important because of the aforementioned limitations related to the classical oral diagnostic methods. Oral fluids (e.g., mouth rinse, saliva, gingival crevicular fluid [GCF], peri-implant sulcular fluid [PISF]) contain several locally and systemically derived biomarkers associated with periodontitis, which makes them a potential diagnostic medium for periodontitis [30], [31]. Unlike periodontal probing and associated bacteremia [32], collection of oral fluids can be done in an atraumatic, non-invasive and sterile way that reduces the risk of bacteremia to the barest minimum [28], [29], [31]. Many potential biomarkers for periodontal disease have been studied and identified including, but not limiting to, macrophage inflammatory protein-1α (MIP-1α), neutrophil elastase, interleukin-1β (IL-1β), myeloperoxidase and neutrophil collagenase, also known as matrix metalloproteinase-8 (MMP-8) and gelatinase B, also known as matrix metalloproteinase-9 (MMP-9) [33], [34], [35]. Most potential biomarkers, in saliva, include MMP-8, MMP-9, IL-1β, MIP-1α and, in GCF, MMP-8 and neutrophil elastase [33], [34], [35]. Recently, aMMP-8 was proposed as a diagnostic biomarker for the updated periodontitis classification system [27] and presented a lower risk of false positives compared to traditionally used clinical measure bleeding on probing [36].
Matrix metalloproteinase-8 (MMP-8)
Matrix metalloproteinase-8 (MMP-8, neutrophil collagenase or collagenase-2) is a member of matrix metalloproteinases (MMPs) family of genetically distinct but structurally related endopeptidases. MMPs have catalytic properties responsible for tissue remodeling and degradation of structural components of the extracellular matrix (ECM) [37]. MMP-8 degrades particularly interstitial collagens (types I–III), which are important structural components of the extracellular matrix [37], [38], [39]. The enzyme also degrades non-matrix bioactive proteins such as pro- and anti-inflammatory cytokines, chemokines, insulin-receptor, fibrinogen, angiotensin, bradykinin and several others [37], [38], [39]. MMP-8 was first found/identified in neutrophils, but MMP-8 expression has been observed in other cells such as chondrocytes, human rheumatoid synovial fibroblasts, smooth muscle cells and activated macrophages [37]. Furthermore, various inflammatory cytokines, for example, IL-1β, tumor necrosis factor-α CD40 ligand (CD40L), can induce and upregulate the expression of MMP-8 [37]. PMN-derived MMP-8 is stored in latent pro-MMP-8 form and is activated to aMMP-8 when released at inflammatory sites. The activity of MMP-8 in the tissue is regulated by a complex network of enzymatic activators and inhibitors and degranulating stimuli [37], [38]. The imbalance between MMPs and tissue inhibitors of matrix metalloproteinases (TIMPs) is believed to initiate the degradation of the ECM, basement membrane, and alveolar bone, and eventually periodontal disease [37], [38]. Moreover, periodontopathogenic bacteria T. denticola and P. gingivalis can increase the activation of MMP-8 with their proteases, as well [37], [38]. MMP-8 seems to have a role in several chronic inflammatory diseases such as diabetes, rheumatoid arthritis, dental peri-implantitis, and particularly periodontal disease [37], [38], [39].
Active MMP-8 (aMMP-8) mouthrinse point-of-care test
According to several studies, the excessive elevation and activation of MMP-8 (aMMP-8) is linked to the progression of periodontitis, which is also reflected in oral fluids [40], [41], [42]. However, this is not the case regarding total or latent MMP-8 [40], [41], [42], [43], [44]. Monitoring aMMP-8 levels in oral fluids can be utilized in the assessment of the risk of increased collagenolytic activity and disease progression [36], [37], [38]. Increased levels of aMMP-8 indicate patients at risk of periodontal disease and requiring periodontal examination and evaluation of treatment needs [36], [37], [38]. Low levels of aMMP-8 represent a low risk of periodontal disease activity, which can be interpreted as a sign of controlled or eliminated active periodontal disease in the periodontal treatment and maintenance phase [36], [37], [38], [45].
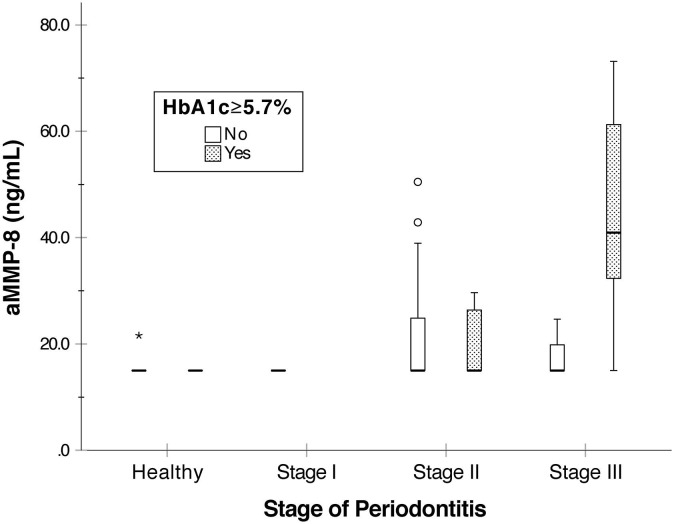
The screening procedure with the aMMP-8 mouthrinse point-of-care test is simple, low-cost and requires no professional dental equipment and expertise. Patients simply rinse with the provided sterile water, which is then quantitatively analyzed by using a reader-equipped lateral flow immunoassay within five minutes [45], [46]. Previous studies have demonstrated successful results for aMMP-8 mouthrinse point-of-care testing in identifying patients with periodontitis [36], [45], [46], [47], [48], [49], [50], [51], [52]. A recent pilot study also demonstrated promising results of the possibility to screen and identify patients at risk of prediabetes by an aMMP-8 mouthrinse point-of-care test [53]. Severe periodontal disease status and problems in HbA1c levels were reflected in elevated aMMP-8 levels (Fig. 2 ) [53].
Fig. 2.
aMMP-8 levels measured from 69 Greek patients by aMMP-8 point-of-care mouth rinse test (PerioSafe® with the ORALyzer®), test cut-off 20 ng/ml. Patients classified by their stage of periodontitis and HbA1c levels. Calculated from the data in Grigoriadis et al. (2019) [53].
Preventive oral health strategy
Due to the rapid spread of COVID-19, we need to think of new ways of strengthening the health care system. Oral health seems to be one part of the puzzles. Periodontitis is associated with several underlying health conditions that are also considered as risk factors for severe COVID-19 disease [4], [5], [6], [7], [8], [9], [10], [54]. Therefore, next to the typical recommendations of social distancing and respiratory, hand, and surface hygiene from the official health organizations, targeted prevention of periodontal disease among COVID-19 risk groups based on individual oral health risk seems important and becomes even more important as the epidemic may last longer than expected.
The preventive oral health strategy consists of screening and identification of COVID-19 risk patients with elevated risk profile (older age, underlying health conditions, and active periodontal disease) to offer them selective and proper dental care and/or regular anti-inflammatory treatment, e.g., antimicrobial mouthwash. Periodontal disease's activity and the risk of its progression could be screened in various situations and locations, such as in the dental, medical, or even at-home settings (Fig. 1). The identified risk patients could be then referred to a dentist for further evaluation and confirmation of their diagnosis. The goal should be to get their periodontal disease under control and to lower its adverse systemic effects in relation to various chronic systemic diseases such as diabetes. This could aid in reducing the severity of the COVID-19 infection and related complications among these patients.
Dentists have the best expertise and equipment for diagnosing periodontitis. Based on the examination, the stage and grade of periodontitis are defined to offer precise treatment for an individual patient [27]. However, there are most likely not enough dental professionals to make this screening of periodontitis patients feasible, even if targeted directly to the COVID-19 risk groups. Modern rapid point-of-care diagnostics technologies based on biomarkers in oral fluids, such as aMMP-8, could offer a solution to this problem [36], [45], [46], [47], [48], [49], [50], [51], [52]. The ability to screen and identify patients at risk of active periodontal disease. point-of-care and non-invasively. could extend the periodontal disease screening to places and situations where professional dental expertise and equipment are limited or not available (Fig. 1). COVID-19 has also inspired the use of novel approaches, namely teledentistry [55], [56], to provide medical and dental care remotely using information technology that reduces the need for direct person-to-person contacts with patients and also reduces infection transmission routes [56]. The first results from this new approach have been positive [57]. Yet, there is still much work needed to make teledentistry a routine among the dentists and patients [56]. Teledentistry with point-of-care testing could help to extend periodontal diagnostics and access to oral health care even to rural and distant places and also to developing countries, where dental care diagnostic resources can be insufficient [46], [58]. Performing a point-of-care test is simple, low-cost and possible even at home settings. A positive test result would indicate the risk of active periodontal disease and need to contact a dentist for oral care.
Point-of-care testing could be particularly effective among the institutionalized, nursing and care home senior citizens. Poor oral health and comorbidities are common among them [59], which makes them especially vulnerable against COVID-19 [60]. The evidence supports that the systemic inflammation connects periodontitis to several chronic diseases of aging [10]. Furthermore, hospitalized patients are more likely to suffer from nosocomial pneumonia if they have periodontitis [61]. Previous studies suggest that providing oral care has a positive effect especially on the incidence of aspiration pneumonia [62]. However, it should be noted that there are still barriers to accessing oral care services that need to be overcome among these elderly individuals, even if point-of-care testing could enhance their periodontal disease diagnostics [62], [63].
Medical professionals providing treatment to COVID-19 risk group patients could benefit from using point-of-care diagnostics for screening in case of undiagnosed active periodontal disease [36], [45], [46], [47], [48], [49], [50], [51], [52]. If the point-of-care test is positive indicating a risk of active periodontal disease, patients should be referred to a dentist for periodontal examination and evaluation. Systemic inflammation links periodontitis to several other chronic inflammatory diseases, such as diabetes, CVD and Alzheimer’s disease, and can alter their course [4], [5], [6], [7], [8], [9], [10], [64]. Previous studies also indicate the positive effect of periodontal therapy on systemic markers of inflammation [65]. For example, recent consensus reports recommend regular oral examination, monitoring and maintenance for patients with a diagnosis of CVD and diabetes [5], [6]. Finally, although not a COVID-19 risk group, evidence suggest that maternal periodontal disease is associated with pregnancy complications such as gestational diabetes with elevated aMMP-8 levels in oral fluids [66], [67]. In this regard, identifying patients at risk for periodontitis early enough by using an aMMP-8 point-of-care test could be beneficial also at the maternity outpatient clinics for referring the risk patients to targeted oral health promotion [66].
Conclusion
In conclusion, currently available oral fluid point-of-care tests could be useful for interdisciplinary screening and identification of individuals with severe periodontitis and active periodontal breakdown. They could extend periodontal disease diagnostics to situations and places where professional oral health care and resources are limited or unavailable, such as distant and rural areas, or nursing homes and care homes. This could help to identify individuals at potential risk for undiagnosed periodontitis and be an important step towards helping patients initiate access to oral care for early intervention. After all, periodontitis is one of the most prevalent diseases globally, which underscores the need for new approaches to identify patients at risk to enhance secondary prevention for periodontitis. This study presented a rapid oral fluid point-of-care technology based on aMMP-8 that has had successful results in the periodontal disease diagnostics. There is enough data to warrant further research to determine the benefits of using the aMMP-8 point-of-care testing also outside the dentist’s office, for example, in the hands of medical professionals in relation to chronic systemic diseases, such as diabetes and CVD. Finally, if there is a causality between periodontal disease and severe COVID-19 infections, aMMP-8 oral fluid analytics could help to assess the risk of deterioration and complications. Therefore, further research on the association between aMMP-8 levels in oral fluids and severity of COVID-19 seems important.
Declaration of Competing Interest
Timo Sorsa is an inventor of US-patent 10488415B2, and a Japanese patent 2016-554676. Other authors report no conflicts of interest related to this study. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Acknowledgements
This research was funded by the University of Helsinki Research Foundation (grant number TYH2016251, TYH2017251, TYH2018229, TYH2019319, Y1014SL017, Y1014SL018, Y1014SULE1), Helsinki, Finland, and the Karolinska Institutet, Stockholm, Sweden.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110276.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.GBD 2017 Oral Disorders Collaborators, Bernabe E., Marcenes W., Global Regional, and national levels and trends in burden of oral conditions from 1990 to 2017: A systematic analysis for the global burden of disease 2017 study. J Dent Res. 2020;99(4):362–373. doi: 10.1177/0022034520908533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peres M.A., Macpherson L.M.D., Weyant R.J. Oral diseases: a global public health challenge [published correction appears in Lancet. 2019 Sep 21;394(10203):1010] Lancet. 2019;394(10194):249–260. doi: 10.1016/S0140-6736(19)31146-8. [DOI] [PubMed] [Google Scholar]
- 3.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slots S.J. Periodontitis: facts, fallacies and the future. Periodontol 2000. 2017;75(1):7–23. doi: 10.1111/prd.12221. [DOI] [PubMed] [Google Scholar]
- 5.Sanz M., Ceriello A., Buysschaert M. Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International diabetes Federation and the European Federation of Periodontology. Diabetes Res Clin Pract. 2018;137:231–241. doi: 10.1016/j.diabres.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Sanz M., Marco Del Castillo A., Jepsen S. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. 2020;47(3):268–288. doi: 10.1111/jcpe.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes-Filho I.S., Cruz S.S.D., Trindade S.C. Periodontitis and respiratory diseases: a systematic review with meta-analysis. Oral Dis. 2020;26(2):439–446. doi: 10.1111/odi.13228. [DOI] [PubMed] [Google Scholar]
- 8.Del Pinto R., Pietropaoli D., Munoz-Aguilera E. Periodontitis and hypertension: is the association causal? [published online ahead of print, 2020 Jun 4] High Blood Press Cardiovasc Prev. 2020 doi: 10.1007/s40292-020-00392-z. [DOI] [PubMed] [Google Scholar]
- 9.Heikkilä P., But A., Sorsa T., Haukka J. Periodontitis and cancer mortality: register-based cohort study of 68,273 adults in 10-year follow-up. Int J Cancer. 2018;142(11):2244–2253. doi: 10.1002/ijc.31254. [DOI] [PubMed] [Google Scholar]
- 10.Kornman K.S. Future of preventing and managing common chronic inflammatory diseases [published online ahead of print, 2020 Jun 24] J Periodontol. 2020 doi: 10.1002/JPER.20-0134. [DOI] [PubMed] [Google Scholar]
- 11.Yang J., Zheng Y., Gou X. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L., Mao Y., Chen G. Risk factors for 2019 novel coronavirus disease (COVID-19) patients progressing to critical illness: a systematic review and meta-analysis. Aging (Albany NY) 2020;12(12):12410–12421. doi: 10.18632/aging.103383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitones-Rubio V., Chávez-Cortez E.G., Hurtado-Camarena A., González-Rascón A., Serafín-Higuera N. Is periodontal disease a risk factor for severe COVID-19 illness? [published online ahead of print, 2020 Jun 19] Med Hypotheses. 2020;144:109969. doi: 10.1016/j.mehy.2020.109969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampson V., Kamona N., Sampson A. Could there be a link between oral hygiene and the severity of SARS-CoV-2 infections? Br Dent J. 2020;228(12):971–975. doi: 10.1038/s41415-020-1747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampson V. Oral hygiene risk factor. Br Dent J. 2020;228(8):569. doi: 10.1038/s41415-020-1545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahni V., Gupta S. COVID-19 & periodontitis: the cytokine connection [published online ahead of print, 2020 May 30] Med Hypotheses. 2020;144:109908. doi: 10.1016/j.mehy.2020.109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel J., Woolley J. Necrotizing periodontal disease: oral manifestation of COVID-19 [published online ahead of print, 2020 Jun 7] Oral Dis. 2020 doi: 10.1111/odi.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S., Sahni V. The intriguing commonality of NETosis between COVID-19 & periodontal disease [published online ahead of print, 2020 Jun 7] Med Hypotheses. 2020;144:109968. doi: 10.1016/j.mehy.2020.109968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madapusi Balaji T., Varadarajan S., Rao U.S.V. Oral cancer and periodontal disease increase the risk of COVID 19? A mechanism mediated through furin and cathepsin overexpression [published online ahead of print, 2020 Jun 1] Med Hypotheses. 2020;144:109936. doi: 10.1016/j.mehy.2020.109936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badran Z., Gaudin A., Struillou X., Amador G., Soueidan A. Periodontal pockets: a potential reservoir for SARS-CoV-2? [published online ahead of print, 2020 May 30] Med Hypotheses. 2020;143:109907. doi: 10.1016/j.mehy.2020.109907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kheur S., Kheur M., Gupta A.A., Raj A.T. Is the gingival sulcus a potential niche for SARS-Corona virus-2? [published online ahead of print, 2020 May 27] Med Hypotheses. 2020;143:109892. doi: 10.1016/j.mehy.2020.109892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loos B.G. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76(11 Suppl):2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J., Wang X., Jia X. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767–772. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stokes E.K., Zambrano L.D., Anderson K.N. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(24):759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark A., Jit M., Warren-Gash C. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study [published online ahead of print, 2020 Jun 15] Lancet Glob Health. 2020:30264–30273. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinane D.F., Stathopoulou P.G., Papapanou P.N. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 27.Tonetti M.S., Greenwell H., Kornman K.S. Staging and grading of periodontitis: framework and proposal of a new classification and case definition [published correction appears in J Periodontol. 2018 Dec;89(12):1475] J Periodontol. 2018;89(Suppl 1):S159–S172. doi: 10.1002/JPER.18-0006. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava N., Nayak P.A., Rana S. Point of care- a novel approach to periodontal diagnosis-a review. J Clin Diagn Res. 2017;11(8):ZE01–ZE06. doi: 10.7860/JCDR/2017/26626.10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He W., You M., Wan W., Xu F., Li F., Li A. Point-of-care periodontitis testing: biomarkers, current technologies, and perspectives. Trends Biotechnol. 2018;36(11):1127–1144. doi: 10.1016/j.tibtech.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Barros S.P., Williams R., Offenbacher S., Morelli T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontol 2000. 2016;70(1):53–64. doi: 10.1111/prd.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rathnayake N., Gieselmann D.R., Heikkinen A.M., Tervahartiala T., Sorsa T. Salivary Diagnostics-Point-of-Care diagnostics of MMP-8 in dentistry and medicine. Diagnostics (Basel) 2017;7(1):7. doi: 10.3390/diagnostics7010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsen I. Update on bacteraemia related to dental procedures. Transfus Apher Sci. 2008;39(2):173–178. doi: 10.1016/j.transci.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Arias-Bujanda N., Regueira-Iglesias A., Balsa-Castro C., Nibali L., Donos N., Tomás I. Accuracy of single molecular biomarkers in gingival crevicular fluid for the diagnosis of periodontitis: a systematic review and meta-analysis. J Clin Periodontol. 2019;46(12):1166–1182. doi: 10.1111/jcpe.13188. [DOI] [PubMed] [Google Scholar]
- 34.Arias-Bujanda N., Regueira-Iglesias A., Balsa-Castro C., Nibali L., Donos N., Tomás I. Accuracy of single molecular biomarkers in saliva for the diagnosis of periodontitis: a systematic review and meta-analysis. J Clin Periodontol. 2020;47(1):2–18. doi: 10.1111/jcpe.13202. [DOI] [PubMed] [Google Scholar]
- 35.Kc S., Wang X.Z., Gallagher J.E. Diagnostic sensitivity and specificity of host-derived salivary biomarkers in periodontal disease amongst adults: systematic review. J Clin Periodontol. 2020;47(3):289–308. doi: 10.1111/jcpe.13218. [DOI] [PubMed] [Google Scholar]
- 36.Sorsa T., Alassiri S., Grigoriadis A. Active MMP-8 (aMMP-8) as a grading and staging biomarker in the periodontitis classification. Diagnostics (Basel) 2020;10(2):61. doi: 10.3390/diagnostics10020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorsa T., Tjäderhane L., Konttinen Y.T. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006;38(5):306–321. doi: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- 38.Sorsa T., Gursoy U.K., Nwhator S. Analysis of matrix metalloproteinases, especially MMP-8, in gingival creviclular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol 2000. 2016;70(1):142–163. doi: 10.1111/prd.12101. [DOI] [PubMed] [Google Scholar]
- 39.Lauhio A., Färkkilä E., Pietiläinen K.H. Association of MMP-8 with obesity, smoking and insulin resistance. Eur J Clin Invest. 2016;46(9):757–765. doi: 10.1111/eci.12649. [DOI] [PubMed] [Google Scholar]
- 40.Lee W., Aitken S., Sodek J., McCulloch C.A. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: role of active enzyme in human periodontitis. J Periodontal Res. 1995;30(1):23–33. doi: 10.1111/j.1600-0765.1995.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 41.Romanelli R., Mancini S., Laschinger C., Overall C.M., Sodek J., McCulloch C.A. Activation of neutrophil collagenase in periodontitis. Infect Immun. 1999;67(5):2319–2326. doi: 10.1128/iai.67.5.2319-2326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiili M., Cox S.W., Chen H.Y. Collagenase-2 (MMP-8) and collagenase-3 (MMP-13) in adult periodontitis: molecular forms and levels in gingival crevicular fluid and immunolocalisation in gingival tissue [published correction appears in J Clin Periodontol. 2004 Feb;31(2):149. Chen, HW [corrected to Chen, HY]] J Clin Periodontol. 2002;29(3):224–232. doi: 10.1034/j.1600-051x.2002.290308.x. [DOI] [PubMed] [Google Scholar]
- 43.Sorsa T., Hernández M., Leppilahti J., Munjal S., Netuschil L., Mäntylä P. Detection of gingival crevicular fluid MMP-8 levels with different laboratory and chair-side methods. Oral Dis. 2010;16(1):39–45. doi: 10.1111/j.1601-0825.2009.01603.x. [DOI] [PubMed] [Google Scholar]
- 44.Romero-Castro N.S., Vázquez-Villamar M., Muñoz-Valle J.F. Relationship between TNF-α, MMP-8, and MMP-9 levels in gingival crevicular fluid and the subgingival microbiota in periodontal disease. Odontology. 2020;108(1):25–33. doi: 10.1007/s10266-019-00435-5. [DOI] [PubMed] [Google Scholar]
- 45.Alassiri S., Parnanen P., Rathnayake N. The ability of quantitative, specific, and sensitive point-of-care/chair-side oral fluid immunotests for aMMP-8 to detect periodontal and peri-implant diseases. Dis Markers. 2018;2018:1306396. doi: 10.1155/2018/1306396. Published 2018 Aug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nwhator S.O., Ayanbadejo P.O., Umeizudike K.A. Clinical correlates of a lateral-flow immunoassay oral risk indicator. J Periodontol. 2014;85(1):188–194. doi: 10.1902/jop.2013.130116. [DOI] [PubMed] [Google Scholar]
- 47.Izadi Borujeni S., Mayer M., Eickholz P. Activated matrix metalloproteinase-8 in saliva as diagnostic test for periodontal disease? A case-control study. Med Microbiol Immunol. 2015;204(6):665–672. doi: 10.1007/s00430-015-0413-2. [DOI] [PubMed] [Google Scholar]
- 48.Heikkinen A.M., Nwhator S.O., Rathnayake N., Mäntylä P., Vatanen P., Sorsa T. Pilot study on oral health status as assessed by an active matrix metalloproteinase-8 chairside mouthrinse test in adolescents. J Periodontol. 2016;87(1):36–40. doi: 10.1902/jop.2015.150377. [DOI] [PubMed] [Google Scholar]
- 49.Johnson N., Ebersole J.L., Kryscio R.J. Rapid assessment of salivary MMP-8 and periodontal disease using lateral flow immunoassay. Oral Dis. 2016;22(7):681–687. doi: 10.1111/odi.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lorenz K., Keller T., Noack B., Freitag A., Netuschil L., Hoffmann T. Evaluation of a novel point-of-care test for active matrix metalloproteinase-8: agreement between qualitative and quantitative measurements and relation to periodontal inflammation. J Periodontal Res. 2017;52(2):277–284. doi: 10.1111/jre.12392. [DOI] [PubMed] [Google Scholar]
- 51.Räisänen I.T., Heikkinen A.M., Siren E. Point-of-care/chairside aMMP-8 analytics of periodontal diseases' activity and episodic progression. Diagnostics (Basel) 2018;8(4):74. doi: 10.3390/diagnostics8040074. Published 2018 Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmalz G., Hübscher A.E., Angermann H. Associations of chairside salivary aMMP-8 findings with periodontal parameters, potentially periodontal pathogenic bacteria and selected blood parameters in systemically healthy adults. Diagn Microbiol Infect Dis. 2019;95(2):179–184. doi: 10.1016/j.diagmicrobio.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Grigoriadis A., Sorsa T., Räisänen I., Pärnänen P., Tervahartiala T., Sakellari D. Prediabetes/diabetes can be screened at the dental office by a low-cost and fast chair-side/point-of-care aMMP-8 immunotest. Diagnostics (Basel) 2019;9(4):151. doi: 10.3390/diagnostics9040151. Published 2019 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma P., Dietrich T., Ferro C.J., Cockwell P., Chapple I.L. Association between periodontitis and mortality in stages 3–5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol. 2016;43(2):104–113. doi: 10.1111/jcpe.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jampani N.D., Nutalapati R., Dontula B.S., Boyapati R. Applications of teledentistry: a literature review and update. J Int Soc Prev Community Dent. 2011;1(2):37–44. doi: 10.4103/2231-0762.97695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghai S. Teledentistry during COVID-19 pandemic [published online ahead of print, 2020 Jun 16] Diabetes Metab Syndr. 2020;14(5):933–935. doi: 10.1016/j.dsx.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giudice A., Barone S., Muraca D. Can teledentistry improve the monitoring of patients during the covid-19 dissemination? A descriptive pilot study. Int J Environ Res Public Health. 2020;17(10):3399. doi: 10.3390/ijerph17103399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leppilahti J.M., Harjunmaa U., Järnstedt J. Diagnosis of newly delivered mothers for periodontitis with a novel oral-rinse aMMP-8 point-of-care test in a rural malawian population. Diagnostics (Basel) 2018;8(3):67. doi: 10.3390/diagnostics8030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong F.M.F., Ng Y.T.Y., Leung W.K. Oral health and its associated factors among older institutionalized residents-a systematic review. Int J Environ Res Public Health. 2019;16(21):4132. doi: 10.3390/ijerph16214132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abrams H.R., Loomer L., Gandhi A., Grabowski D.C. Characteristics of U.S. Nursing Homes with COVID-19 Cases [published online ahead of print, 2020 Jun 2] J Am Geriatr Soc. 2020 doi: 10.1111/jgs.16661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jerônimo L.S., Abreu L.G., Cunha F.A., Esteves Lima R.P. Association between periodontitis and nosocomial pneumonia: a systematic review and meta-analysis of observational studies. Oral Health Prev Dent. 2020;18(1):11–17. doi: 10.3290/j.ohpd.a44114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barnes C.M. Dental hygiene intervention to prevent nosocomial pneumonias. J Evid Based Dent Pract. 2014;14(Suppl):103–114. doi: 10.1016/j.jebdp.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Mathews R., O'Malley C., Hall J.M., Macaden L., MacRury S. Diabetes care homes, and the influence of technology on practice and care delivery in care homes: systematic review and qualitative synthesis. JMIR Diabetes. 2019;4(2) doi: 10.2196/11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsushita K., Yamada-Furukawa M., Kurosawa M., Shikama Y. Periodontal disease and periodontal disease-related bacteria involved in the pathogenesis of Alzheimer's Disease. J Inflamm Res. 2020;13:275–283. doi: 10.2147/JIR.S255309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Montero E., López M., Vidal H. Impact of periodontal therapy on systemic markers of inflammation in patients with metabolic syndrome: a randomized clinical trial [published online ahead of print, 2020 Jul 1] Diabetes Obes Metab. 2020 doi: 10.1111/dom.14131. [DOI] [PubMed] [Google Scholar]
- 66.Boggess K.A. Choosing the left fork: steven Offenbacher and understanding maternal periodontal disease and adverse pregnancy outcomes [published online ahead of print, 2020 Jul 2] J Periodontol. 2020 doi: 10.1002/JPER.20-0090. [DOI] [PubMed] [Google Scholar]
- 67.Padilla A.C., Fuentes O.R., Rios M.H. Early pregnancy levels of gingival crevicular fluid matrix metalloproteinases -8 and -9 are associated with the severity of periodontitis and the development of gestational diabetes mellitus. J Periodontol. 2020 doi: 10.1002/JPER.19-0743. In press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.