Abstract
Neonates and prematures are among the most transfused categories of patients. Adverse reactions due to transfusions, such as transfusion-transmitted infections, can affect the rest of their lives. In this systematic review, we revised the literature concerning transfusion-transmitted infection in neonates. We reported case-reports and case-series previously published and we integrated these data with our experience at local neonatal intensive care unit. Moreover, we illustrated strategies for mitigating transfusion-transmitted infections, including donor selection and testing, pathogen inactivation technologies and combined approaches, as for Cytomegalovirus infection, integrating leukoreduction and identification of seronegative donors.
Abbreviations: CMV, cytomegalovirus; GVHD, graft versus host disease; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IUFT, intrauterine fetal transfusion; PB, peripheral blood
Keywords: Neonatal transfusion, Cytomegalovirus, Hepatitis, Bacterial infections
1. Introduction
Neonatal transfusion medicine is a modern discipline presenting many areas of debate. For many aspects, including the indications to transfuse definite blood components (i.e. red blood cell - RBC, platelet, or plasma units), the precise triggers for transfusion, or the strategies to prevent adverse consequences of blood products, there is a great variability across neonatal transfusion guidelines and recommendations from different countries. Noteworthy, neonates, particularly those born before their due date, are among the most transfused categories of patients, and getting a transfusion-transmitted infection can affect the rest of their lives. A wide spectrum of transfusion-transmitted infections are reported in the neonatal setting. Most of them reflected those observed in adult people receiving transfusions, and consisted of transmission of hepatitis B virus (HBV), hepatitis C virus (HCV), or human immunodeficiency virus (HIV). Nevertheless, for distinct types of pathogens, such as cytomegalovirus (CMV), neonates represent by far the most frequently affected population.
In the first part of this review, we summarize the literature reports on transfusion-transmitted infections in neonates (i.e. infant in their first 28 days of life). In the second part, we describe the data gathered on this issue at our neonatology. Finally, we discuss which specific approaches to prevent transfusion-transmitted infections are currently recommended in the neonatal setting, with particular regard to the CMV infection.
2. Literature review
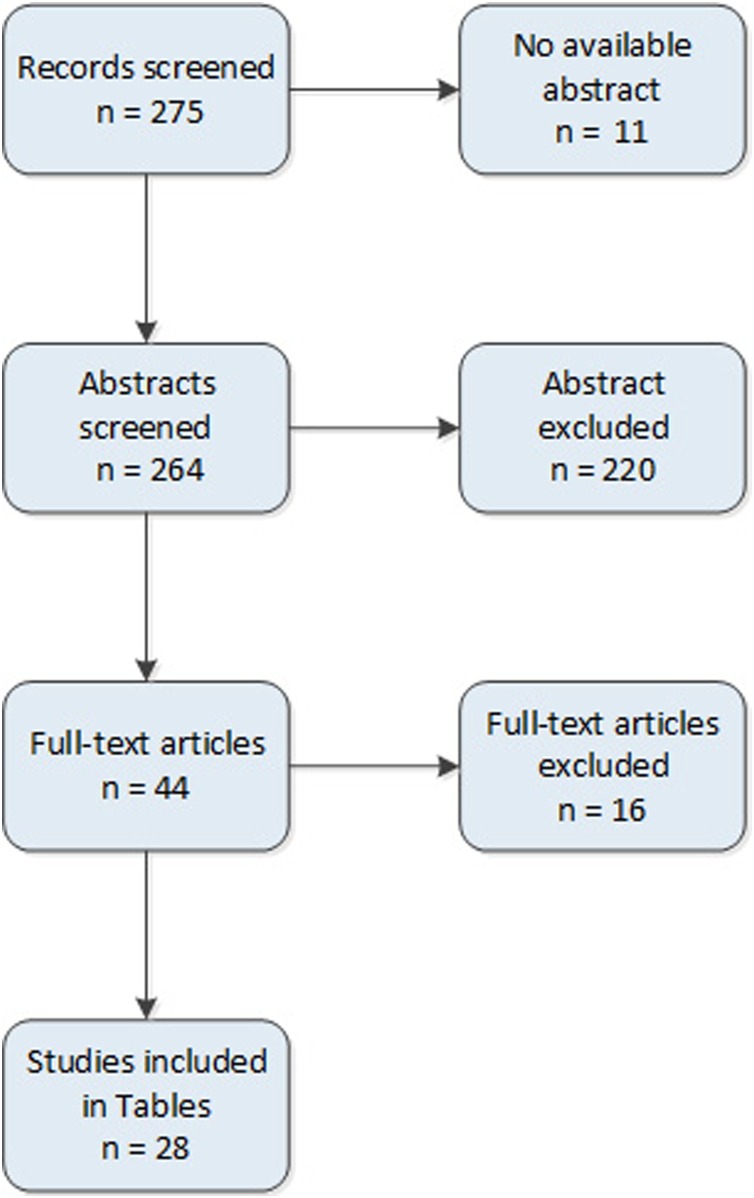
We performed a systematic search on the databases PubMed using the following queries: "Blood Transfusion"[Mesh] AND "Infant, Newborn "[Mesh] AND "Transfusion Reaction"[Mesh]. We excluded papers reporting non-infectious transfusion reactions, papers reporting data in paediatric population not allowing to extrapolate data on neonates, communications at congresses, duplicated studies and papers not in English. M.B, C.G.V. L.T and N.O. independently controlled all references, including observational studies, case report, case series and reviews. Discrepancies were discussed and resolved together. In total, 275 references were identified on May 2020. In the end, 44 papers were included and discussed in the review (Fig. 1 ). In Table 1, Table 2 we summarized case-reports and case-series of TTI from 1976 to 2018.
Fig. 1.
Study flow diagram.
Table 1.
CMV infection in neonates undergoing blood transfusions. Legend. BW, birth weight; CMV, Cytomegalovirus; ET, exchange transfusion; RBC, red blood cell; VLBWI, very low birth weight infant.
| Reference | Population | Patients (n)/blood components | CMV status of the mothers |
Results | Comments |
|---|---|---|---|---|---|
| Nankervis [1] | Newborns | 24/ET | All mothers were tested but not data were reported | 21 % (5/24) were infected with CMV at6−12 weeks after birth: - One of 5 received blood from seronegative donor - 4 received blood from seropositive donors No clinical disease was detected in this population. |
Transplacental antibodies were not protective. |
| Spector et al. [2] | Prematures | Total enrolled 93: 93/blood transfusion 14/ET |
NA | 13/93 with cytomegaloviruria All received blood from a mean of 10.45 different donors (only 5 underwent ET) 80/93 without cytomegaloviruria All received blood from a mean of 5.10 different donors (only 9 underwent ET) |
No clinical data were reported. The number of transfusions was significant higher in positive patients (p < 0.002). Human milk was used in only two patients: one of these was not frozen |
| Benson et al. [3] | Infants | Retrospectively 42: 32/blood 16/ET untested blood |
NA | 8 babies became infected: - 4 after blood transfusions not showing clinical signs of infection; - 4 after ET: 3 showed no clinical signs attributable to CMV, 1 died. |
Blood donor CMV status has an impact on the probability to be infected from CMV |
| Prospectively 22: 14/blood 2/ET Seronegative donors |
NA | 16 babies who received only CMV seronegative blood remained uninfected. | |||
| Kumar et al. [4] | Newborns | 29/ET Group 1: patients receiving blood from seropositive blood donors (n = 15) |
Mothers were screened but data are not reported | Four babies had CMV from urine and throat after 6−12 weeks after transfusion. Three of these had transplacental antibody to CMV | Blood from seropositive or unknown donors has a higher risk to transmit CMV. In case newborns with transplacental antibody, these seem not be protective. |
| Group 2: patients receiving blood from seronegative blood donors (n = 14) | Mothers were screened but data are not reported | One infant became infected. She received blood from two donors: one seronegative, the other with unknown serological status. | |||
| Yeager et al. [5] | Prematures | Total enrolled 355: 353/RBC 134 seropositive donors 221 seronegative donors |
164 seronegative mothers | Ten of the 74 infants who were exposed to CMV seropositive donors become infected. Four of the 10 infected infants had fatal infection or died from chronic lung disease. One infants had serious complications. All of these 5 had <28 weeks and <1200 g of weight |
In prematures, there is a higher risk to be infected from CMV when receiving blood from seropositive donors. Maternal antibodies do not prevent CMV transmission. None of the babies received fresh milk from his/her mother. |
| 191 seropositive mothers | Nine of the 60 infants who were exposed to CMV seropositive donors become infected. Twenty-three of 131 infants who were exposed to CMV seronegative donors become infected None had fatal infection or serious complications. |
||||
| Adler et al. [6] | Newborns | 178/RBC | Mothers were screened but data are not reported | Eight newborns acquired CMV infection during their hospital stay. Seven of these have a seronegative mother. Six infants who acquired CMV infection developed morbidity associated with this infection, three of them died. |
Significant correlation between the number of different blood donors from whom an infant received blood and CMV infection (p < 0.0001) No infant was fed nonmaternal breast milk. |
| Preiksaitis et al. [7] | Phase A: all infant |
114 RBC (no ultrafiltrated, washed or irradiated) platelets |
Seronegative mothers | Only one of 126 seronegative infants (114 from phase A and 12 from phase B) developed CMV infection. Only one of 16 seropositive infants developed CMV infection. No mortality and very little morbidity were attibuited to transfusion acquired CMV infection. |
Infants rarely received more than two transfusion from any one donor. All infants received only maternal or sterilized donated breast milk. |
| Phase B: infant with BW < 1,250g |
28 RBC (no ultrafiltrated, washed or irradiated) platelets |
Seronegative (12) and seropositive (16) mothers | |||
| Lamberson et al. [8] | Infants | Phase 1: 549 /RBC CMV serological assay were performed retrospectively |
NA | Eight infants acquired CMV: - Phase 1: 7 were transfused with blood from one or more ELISA-positive donors and from one or more IgM-positive donors, - Phase 2: 1 received negative blood for CMV IgM but positive at Elisa test performed later. Two of the eight seronegative infants developed characteristic symptoms. One of these infants died of respiratory distress. |
Most infected neonates received multiple transfusions, however an infants received only a small volume of blood from a donor. Screening donors for CMV IgM was effective to reducing transfusion associated-CMV. |
| Phase 2: 439 /RBC, CMV IgM was performed on the day of donation: only CMV IgM negative donors were used. |
NA | ||||
| Bhumbra et al. [9] | Newborns with BW < 2,000g | Total enrolled 137: 137/RBC 13/fresh frozen plasma Not specified/granulocyte At beginning, all donors were seronegative but 16 (0.7 %) seroconverted during the study. |
All mothers were seronegative | Two infants developed CMV infection and viruria after each received CMV seropositive blood (these units were mistakenly identified as CMV seronegative due to technical errors or poor sensitivity of the test kit). One infant acquired CMV infection through granulocyte transfusions. Neither infant had any symptoms attributable to CMV infection. |
The use of seronegative red blood cell is highly successful in the prevention of primary CMV infection. |
| Galea et al. [10] | Newborns with BW < 1,500g | 83/RBC and fresh frozen plasma 70 exposed to seropositive blood components |
NA | Seven of 83 (8.4 %) infants became infected with CMV in all transfused population. No clinical data were reported. |
Incidence of CMV infection was only present (7/70, 10 %) in newborns receiving blood from seropositive donors. |
| de Cates et al. [11] | Prematures ≤32 weeks | Part 1: 53 | Mothers were screened but data are not reported for all babies | 10/53 (19 %) infants acquired CMV infection (three of them had seronegative mothers and seven had seropositive mothers). One patient died at two months of age |
Preterms babies should be transfused with seronegative CMV blood regardless the serological status of the mother. |
| Part 2: 75 | 26 had seropositive mothers, 49 had seronegative mothers. |
6/72 (8.3 %) infants acquired CMV infection (three of them had seronegative mothers and three had seropositive mothers) No clinical data were reported, |
|||
| Kim et al. [12] |
Newborns with BW < 1500g | 80/filtered, irradiated RBC and platelets | NA | 2/80 (2.5 %) acquired CMV infection None received breast milk |
The use of filtered and irradiated do not differ from the use of no-filtered, no-irradiated RBC and platelets (p > 0.05) in hyperendemic area. |
| 20/no-filtered, no-irradiated RBC and platelets All donors were screened for CMV |
2/20 (10 %) acquired CMV infection. One received frozen breast milk. |
Table 2.
Microbial TTI in neonates undergoing blood transfusions. Legend. BW, birth weight; ET, exchange transfusion; RBC, red blood cell.
| Reference | Population | Patients (n)/ Product transfused |
Patogen | Outcome |
|---|---|---|---|---|
| Seeberg et al. [17] | Infant | 1/ET with blood from four different donors | HAV | A female baby was the source of the outbreak in a pediatric surgical ward. TTI is associated with a period of viremia and viremia of 25 days before the onset of jaundice. One blood donor was responsible for HAV transmission and he was identified one month after donation. |
| Ammann et al. [18] | Infant | 1/6 ET, 5 platelets and 7 partial ET from eighteen donors in the first 2 weeks of life | HIV | Eighteen months after birth HIV infection was diagnosed. The child died at 2 two years of age because of P.carinii pneumonia. One of the platelet donors was identified as a patient with AIDS who had died 17 months after blood donation. |
| Noble et al. [19] | Neonates | 2/RBC and fresh frozen plasma | HAV | One male donor donated blood and this was transfused to 11 neonates (pedi-pack units). Later, he developed symptomatic HAV. Two neonates had HAV following blood transfusion from this donor. |
| Azimi et al. [20] | Prematures | 2/RBC from 26 donors | HAV | Two patient were infected by blood transfusion from a IgM anti-HAV positive donor. The donor was a male who developed clinical symptoms some days after donation. Only after HAV diagnosis, he revealed a sexual intercourse with a male not tested. |
| O’Riordan et al. [21] | Infant | 20/RBC, platelets | HCV | 11/20 (55 %) presented HCV serological testing positive and only 5 had positive molecular testing (genotype 1b). All of the reported children are clinically asymptomatic. Six donors were identified: 1 viremic mother who performed direct donation to her child; 5 donors who were infected following anti-D administration (4) or not specified other source (1). |
| Lee et al. [22] | Infant | 3/Plasma unit was divided into three aliquots assigned to three different patients | HAV | Identification of index case was performed following outbreaks among personnel in a NICU. The index case was one of the three children receiving one aliquot of plasma. The other two neonates died within some days from transfusion. The donor was a female with a negative medical history, but her boyfriend was diagnosed with HAV shortly after her donation. |
| Vareil et al. [23] | Newborn | 1/whole blood | P. falciparum | The patient received a 60-mL whole blood transfusion in Senegal. The chronology of events and exposure to blood are highly suggestive of transfusion-transmitted malaria. The donor not could be traced. |
| Herwaldt et al. [24] | Prematures and full-term infant | 13/RBC or platelets | Babesia microti | From 1997–2009, 13 transfusion–associated Babesia cases occurred in the United States. Most cases were associated with RBC transfusion while 2 to whole blood-derived platelets. |
| Simonsen et al. [25] | Prematures (≤32 week of gestational age) |
7/RBC | Babesia microti | Transfusion from 2 infected units of blood resulted in 7 cases of neonatal transfusion-associated babesiosis. The extremely low birth weight neonates were the most severely affected. Double-volume exchange blood transfusion with prolunged multidrug treatment was required for 2 most severe cases. |
| Martini et al. [26] | Newborn | 1/platelets | S.epidermidis | Female newborn underwent platelets transfusion. Six hours after, she developed fever (39.8 °C). She died of septic shock the same days. |
| Niederhauser et al. [27] | Newborns | 3/RBC | HBV | Three received aliquots from the same unit: 2 had HBV infection and the third had no HBV markers. Blood donor was negative for HBsAg and not tested for anti-HBc or HBV DNA. |
| Waldenström et al. [28] | Newborn (9-day old age) |
1/RBC | HCV | A 9-day old neonate received two RBC units from two different donors during surgery. HCV infection in one donor was identified 29 days after transfusion in plasma sent to industries for pharmaceutical products. |
| Van Schalkmyk et al. [29] | Neonates | 48/blood | Candida krusei | A large outbreak of Candida krusei candidemia in a neonatal unit occuring during 4 months. The source of this outbreak could not be estabilished, however blood transfusion were identified as a risk factor in Candidemia positive infants. |
| Glanternik et al. [30] | Newborn | 4/RBC | Babesia microti | Four infants were transfused with the same packed RBC from a donor unknowingly infected with Babesia microti. Two of the infants developed high-grade of parasitemia. |
In Table 1, we reported CMV infection in neonates undergoing blood transfusions in a wide range of years with different transfusion practices [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]]. Transfusions and viral shedding in the milk of seropositive mothers are the two main routes implicated in transmitting post-natal CMV infection. Several papers showed that blood components from CMV-seropositive donors are potentially the major cause of CMV infections in neonates. Testing CMV status of the donor is the most effective strategy in preventing transfusion-associated CMV infection [[3], [4], [5],[8], [9], [10], [11]]. Moreover, exposing neonates to a low number of donors may be also preventive [2,6,8]. Only one paper reported different data but, in this study, infants rarely received more than two transfusions [7]. The role of passive maternal antibody transmission seems to be not protective in preventing CMV infection [1,4]. Some authors proposed blood components treatments to prevent CMV transmission. In the 80’, we reviewed experiences dealing with RBC freezing in a glycerol medium [13,14] and washing to remove leukocyte [15,16]. These methods may be considered obsolete. In fact, frozen RBCs need additional technologies, they are expensive and not based on a cost-effectiveness analysis. Washing to remove leukocyte may be easily overcome with last-generation leukoreduction filters.
In Table 2, we reported TTI due to different etiological agents [[17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]]. For hepatitis A, post-transfusion transmission was confirmed in all cases and index patients were also responsible to spread HAV through fecal-oral via causing nosocomial infection in other patients, their parents and personnel [17,19,20,22]. For Hepatitis B and C, asymptomatic or untested donors (i.e. NAT testing not yet available) were responsible for transmission [21,27,28]. Only one TTI HIV infection was reported and it happened at the beginning of the 80’s [18]. Some microbes are mainly transmitted by some blood components such as RBC (malaria parasites, Babesia) or platelet concentrates (bacteria) [[23], [24], [25], [26],29,[18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]].
3. Five-year experience at our neonatology
Records relative to patients admitted at our neonatology department from January 2014 to December 2019 were revised. At our hospital, blood donor testing does not include CMV serology at donation. Fresh RBC concentrates (<5 days) destined to neonates are always filtered pre-storage. Plasma units consisted of pharmaceutical grade plasma (Kedrion S.p.A Barga, Italy) since 2013. Platelet transfusions consisted of single units (until January 2016), apheresis units, or pool platelets (from February 2016 onward). Pathogen inactivation started on February 2016, and was performed through Intercept™ Blood System technology (Cerus Europe BV, Amersfoort, Netherlands). RBC and PLT units were subjected to γ-irradiation before to be administered. All neonates were fed with fresh milk from the own mothers, or, if maternal milk was not available, with preterm formula milk. Milk was collected only from mothers with negative IgM serology for toxoplasmosis, rubella virus, CMV, Herpes simplex virus, and varicella-zoster virus.
In total 4268 units (2,626 RBC, 1,192 PLT and 450 PLT units) were distributed to 729 patients. Apart from CMV, no further transfusion-transmitted infections were recorded among our patients. Considering only neonates who had never received maternal milk, CMV TTI occurred in 10 patients (0.9 %). All patients were preterm infants: the median gestational age was 27.6 weeks (range 24.0–30.0) and median birth weight 775 g (range 580–1,550). In all CMV-TTI, the diagnosis was made through the DNA virus detection in pharyngeal swabs and urine (10 patients), and bloodstream (5 patients). Each patient received a median number of 5.5 RBC units (range 1–19). Four patients were also given PLT (2 patients) or plasma (2 patients) transfusions. One RhD positive neonate born from a mother with anti-RhD iso-immunization, received from the 19th week of gestation 6 intra-uterine RBC units, and after the birth, additional 5 units. Conceivably, in this case, CVM transmission had occurred in the pre-natal period. In fact, this neonate displayed since her birth a hypo-regenerative anemia unresponsive to erythropoietin, with persistently low reticulocyte count and bilirubin levels, high serum levels of ferritin and soluble transferrin receptor. The CMV infection required treatment in 9 out of 10 cases; 2 patient died for severe bronchopulmonary dysplasia. Three additional cases of CMV infection were observed: neonates were fed with maternal milk and in all cases was documented the CMV presence in the milk.
4. Prevention of transfusion-transmitted infections
Transfusion of blood components is known to carry infectious risks to the recipients. Several actions can be put in place to prevent infections such as careful donor selection and deferral, leukoreduction, implementation of testing and pathogen inactivation.
Donor selection and consequent deferral for high-risk donors represents the first-line option in preventing infections. For infections such Hepatitis B, C, HIV and syphilis candidate donors undergo a careful evaluation of sexual behavior, high-risk activities (piercing, tattoo, etc.) or medical interventions (surgery, endoscopy, etc.). Travelling information may add useful information to reduce the risk of transmission of infection in donors from endemic area (i.e. malaria, Chagas’ disease) or in case of seasonal outbreaks (i.e. Dengue, Chikungunya, West Nile Virus, etc.). Moreover, donors are asked about signs of recent infections such as gastrointestinal symptoms (which is a risk factor for Yersinia species contamination), recent fever and/or respiratory symptoms (for influenza virus, Coronavirus, etc.) or antibiotic treatment [31]. In addition, at the time of collection, health professionals must carefully inspect donor skin to avoid venipuncture in scared skin and, most important, perform diversion of the initial blood (from 10 to 30 mL) after venipuncture, in a satellite pouch, to avoid bacterial contamination, especially Gram-positive organisms [32].
Leukoreduction is the reduction of white blood cell (WBC) concentration in blood components, RBCs, platelet concentrates and plasma obtained from the fractionation of whole blood or apheresis. There are many methods of LR but, currently, this process may be performed using selective last-generation LR filters, which allow obtaining less than one million residual WBCs in an RBC or PC unit. Over the past thirty years, it has been demonstrated that LR can reduce some adverse reactions due to blood component transfusion such as febrile non-hemolytic transfusion reactions, immunization against HLA and HPA antigens, which may cause refractoriness to platelet transfusion, and transmission of CMV [33,34]. Some early studies demonstrated that both policies, namely the use of CMV seronegative blood components and LR, may be able to determine a significant reduction of CMV infection in high-risk patients. However, controversies concerning “the gold standard” practice (only LR, only CMV-donor status, and LR plus CMV-donor status) are still ongoing [[35], [36], [37]].
Nowadays, infections still represent a great risk but the epidemiology of TTI is changed over the years. The introduction of NAT testing (Nucleic Acid Amplification) for HIV, HBV and HCV had significantly lowered the residual risk of 1:1 million to 1:10 million, while risk for bacterial contamination is still high (1:2,000–1:5,000) in platelet concentrates, with a high rate of fatal sepsis (10 %) [38]. Underestimation of these phenomena may be also due to clusters of patients receiving platelet concentrates. Adult and pediatric patients with onco-hematological diseases as well as prematures who easily present sepsis, comorbidities and immune system deficits during the course of their disease often received several platelet transfusions from different donors [38].
In the last years, an important strategy to minimize the risk of TTI is pathogen reduction technologies. These are effective on several well-known viruses as well emerging viruses, bacteria and protozoa. Different technologies have been developed for acellular (plasma) and cellular blood components (platelet concentrates). Acellular blood components may be inactivated with solvent-detergent (SD) for plasma pool or with methylene blue (MB) for single plasma units. SD plasma is generally performed by pharmaceutical industries using tri(n-butyl) phosphate and octonynol for 1–1.5 h at +30 °C. The main limitation of this method is the lack of efficiency on envelope-free viruses such as Parvovirus B19, Hepatitis A and E. This limit is overcame with additional testing on donors. MB plasma is not indicated for pediatric patients since adverse events were reported, as well as interference with phototherapy treatment [39,40]. SD or MB method cannot be used for cellular blood components because may be responsible for cellular damages with a consequent effect on transfusion efficacy and risks of adverse events.
For cellular blood components different technologies have been developed mainly based on UV light and addition of photosensitive compounds. At the moment three technologies are available: INTERCEPT™ (Cerus Corporation, USA), Mirasol® (Terumo BCT, USA) and THERAFLEX® (Macopharma, France) [38,41,42]. INTERCEPT™ is a CE-marked and FDA-approved device for plasma and platelets concentrates using amatosalen, which intercalates between DNA bases; after UVA exposure, there is an inhibition of transcription and cellular reproduction. Mirasol® is a CE-marked device using riboflavin and UVB light, while THERAFLEX® has different technologies for plasma, where MB is used, and only UVC light for platelets, which induces the formation of pyrimidine dimers and prevents DNA replication. In addition to well-known viruses (HIV, HBV, HCV and HTLV), these technologies may be used for preventing transmission of emerging infections affecting world population in the last decades. Pathogen-inactivation technologies have found a wide application in preventing transmission of Chikungunya Virus during outbreaks in La Reunion, West Nile Virus, Zika virus and Coronavirus (SARS) in addition to NAT testing, when available [[43], [44], [45], [46]].
Pathogen-reduction technologies are promising in preventing bacterial infections where molecular and/or culture-based method may be ineffective. As previously described, bacterial contamination in blood components is the most frequent TTI and platelet concentrates are the principal blood components involved, mainly due to their storage conditions (20−24 °C). Gram-positive bacteria (i.e. Staphylococcus epidermidis, Staphylococcus aureus) are often involved and they normally are part of the skin flora. Gram-negative bacteria are rare and mainly due to asymptomatic bacteremia in blood donors, after red blood cell transfusions or use of blood cell-saver during surgeries [47]. Pathogen inactivation technologies are able to inactivate a wide range of Gram positive (from 3.6 to >6.9 log reduction) and negative bacteria (4.5 to >6.7 log reduction) without compromising platelet functions and reducing residual leukocytes in blood components (INTERCEPT™) as well as they are active on slow-growing bacteria (Mirasol ®) [42].
In terms of safety, some studies reported experiences in pediatric patients. Platelets concentrates treated with INTERCEPT™ system were successfully transfused in 46 neonates (less than 28 days) without any adverse events. Mirasol-platelet concentrates were transfused in 51 children showing no difference in the rate of adverse events when compared to patients no-receiving Mirasol-platelet concentrates. For THERAFLEX® the only concern is on the use of MB in neonates, which can determine, as already described, adverse effects [41].
Notwithstanding the efficacy of leukoreduction in removing residual white blood cells, viral transmission from blood components remains at risk for CMV and donations from CMV sero-positive donors may represent a risk for nonimmune patients.
5. What CMV-safe does mean?
CMV is a worldwide endemic herpesvirus and the prevalence of CMV seronegative individuals varies according age, country and ethnicity [48]. For example, a French study on general people reported a prevalence of 42 % for CMV-IgG antibodies among individuals aged 15–49, with a higher frequency in females than in males [49]. A German study carried out among blood donors showed a seroprevalence of 30 % for those aged 18–30, and of 80 % for those older than 65 years, with female donors having a 15 % higher risk at all ages [50]. Regarding ethnicity, a British study carried out among pregnant females showed a CMV seroprevalence of 49 % among White British women, of 89 % among South Asian UK born women and of 98 % among South Asian women born in South Asia [51].
During the primary infection, CMV replication occurs in several cells including myeloid cells, and is followed by an IgM response. Therefore, viral transmission can occur if the donor is in the window phase [52]. After the primary infection, CMV persists lifelong latent in leukocytes. Either the reactivation of a latent infection or the re-infection by a different strain can occur, accompanied by viral shedding in body fluids, including milk and blood. Indeed, CMV can be transmitted by blood donated in all these circumstances. Basically, CMV-seronegative patients undergoing hematopoietic stem cell transplant (HSCT) from seronegative donors and preterm neonates are the patient populations carrying the highest risk for transfusion-transmitted CMV infection. Blood components obtained from IgG/IgM seronegative donors could prevent CMV transmission in exchange-transfused neonates [53]. Indeed, seronegative blood products became the standard to prevent CMV transmission in patients at risk. Since CMV is mainly present in white blood cells, the modern filters for leukodepletion have significantly reduced this risk in cellular products, dropping from 10 to 59 % of fresh blood to 3 % or less in leukodepleted products [48]. In 1995, a study of Bowden et al. in HSCT patients, found that leukodepleted blood products reduced the day-21 risk for CMV infection/disease to a similar extent as using blood from CMV-seronegative donors [36]. At a secondary analysis carried out at post-transplant day 100, however, there were fewer CMV infection and disease in the seronegative arm [36]. At present, it is unknown if testing CMV DNA can further reduce the risk for CMV transmission of leukodepleted blood products. Noteworthy, among 2400 blood donor samples serially collected at 10-year intervals, CMV DNA was found in 4.3 % of samples from donors in their 60 s and in 1.0 % of samples from donors younger than 60 years, whereas none of the 562 seronegative samples was found DNA positive [54].
Maternal milk transmission seems to be the main responsible for post-natal CMV infection if CMV-seronegative and leukoreduced blood products are used. In a seminal study on 2061 CMV-seronegative and leukoreduced transfusions to 310 neonates, none of postnatal CMV infection was linked to transfusion, whereas all 28 infants with CMV infection were fed with maternal breast milk from CMV-seropositive mothers [55]. Although theoretically estimated below the threshold of 1 in 1 million [56], the residual CMV-transmission risk in leukodepleted products has never been evaluated in randomized or even large case-control neonate populations, but only in a pilot study including 20 neonates [57]. In 2005, a meta-analysis of controlled studies available at that time in HSCT patients, indicated that CMV-seronegative blood components are more efficacious than leukodepleted blood components in preventing transfusion-acquired CMV infection [58]. Similarly, our data gathered in neonates not receiving maternal milk suggest that leukoreduction alone might be not sufficient to defeat the risk for transfusion-transmitted CMV infection in fetuses or neonates. Notably, at variance with CMV infection transmitted by fresh maternal milk, which is mild and self-limiting in the majority of patients, 9 out of 10 patients in our series required antiviral therapy [59].
6. Conclusions
The numerous reports on transfusion-transmitted infections in newborn recipients in the past literature clearly show that blood donor testing has made over years blood neonatal transfusion progressively safer. The implementation of pathogen inactivation in plasma and platelet units further protects patients from non-screened infectious agents. Unfortunately, however, no similar methodologies are so far available for RBC concentrates. Our data clearly show that transfusion-transmitted CMV infection in premature neonates is still a concern in neonatal care units. In this context, an evidence-based clear equivalence of leukoreduced to seronegative-donor cellular products is still lacking, even for fetal and neonatal transfusions.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Nankervis G.A. Cytomegalovirus infections in the blood recipient. Yale J Biol Med. 1976;49(1):13–15. Mar. [PMC free article] [PubMed] [Google Scholar]
- 2.Spector S.A., Schmidt K., Ticknor W., Grossman M. Cytomegaloviruria in older infants in intensive care nurseries. J Pediatr. 1979;95(3):444–446. doi: 10.1016/s0022-3476(79)80532-6. Sep. [DOI] [PubMed] [Google Scholar]
- 3.Benson J.W., Bodden S.J., Tobin J.O. Cytomegalovirus and blood transfusion in neonates. Arch Dis Child. 1979;54(7):538–541. doi: 10.1136/adc.54.7.538. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A., Nankervis G.A., Cooper A.R., Gold E., Kumar M.L. Acquisition of cytomegalovirus infection in infants following exchange transfusion: a prospective study. Transfusion. 1980;20(3):327–331. doi: 10.1046/j.1537-2995.1980.20380214901.x. May–Jun. [DOI] [PubMed] [Google Scholar]
- 5.Yeager A.S., Grumet F.C., Hafleigh E.B., Arvin A.M., Bradley J.S., Prober C.G. Prevention of transfusion-acquired cytomegalovirus infections in newborn infants. J Pediatr. 1981;98(2):281–287. doi: 10.1016/s0022-3476(81)80662-2. Feb. [DOI] [PubMed] [Google Scholar]
- 6.Adler S.P., Chandrika T., Lawrence L., Baggett J. Cytomegalovirus infections in neonates acquired by blood transfusions. Pediatr Infect Dis. 1983;2(2):114–118. doi: 10.1097/00006454-198303000-00009. Mar–Apr. [DOI] [PubMed] [Google Scholar]
- 7.Preiksaitis J.K., Brown L., McKenzie M. Transfusion-acquired cytomegalovirus infection in neonates. A prospective study. Transfusion. 1988;28(3):205–209. doi: 10.1046/j.1537-2995.1988.28388219143.x. May–Jun. [DOI] [PubMed] [Google Scholar]
- 8.Lamberson H.V., Jr., McMillian J.A., Weiner L.B., et al. Prevention of transfusion-associated cytomegalovirus (CMV) infection in neonates by screening blood donors for IgM to CMV. J Infect Dis. 1988;157(4):820–823. doi: 10.1093/infdis/157.4.820. [DOI] [PubMed] [Google Scholar]
- 9.Bhumbra N.A., Lewandowski P., Lau P., Sererat M., Satish M., Nankervis G.A. Evaluation of a prescreening blood donor program for prevention of perinatal transfusion-acquired cytomegalovirus (CMV) infection. J Perinat Med. 1988;16(2):127–131. doi: 10.1515/jpme.1988.16.2.127. [DOI] [PubMed] [Google Scholar]
- 10.Galea G., Urbaniak S.J. The incidence and consequences of cytomegalovirus transmission via blood transfusion to low birth weight, premature infants in north east Scotland. Vox Sang. 1992;62(4):200–207. doi: 10.1111/j.1423-0410.1992.tb01199.x. [DOI] [PubMed] [Google Scholar]
- 11.de Cates C.R., Gray J., Roberton N.R., Walker J. Acquisition of cytomegalovirus infection by premature neonates. J Infect. 1994;28(1):25–30. doi: 10.1016/s0163-4453(94)94037-1. Jan. [DOI] [PubMed] [Google Scholar]
- 12.Kim A.R., Lee Y.K., Kim K.A., Chu Y.K., Baik B.Y., Kim E.S., et al. Transfusion-related cytomegalovirus infection among very low birth weight infants in an endemic area. J Korean Med Sci. 2006;21(1):5–10. doi: 10.3346/jkms.2006.21.1.5. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler S.P., Lawrence L.T., Baggett J., Biro V., Sharp D.E. Prevention of transfusion-associated cytomegalovirus infection in very low-birthweight infants using frozen blood and donors seronegative for cytomegalovirus. Transfusion. 1984;24(4):333–335. doi: 10.1046/j.1537-2995.1984.24484275576.x. Jul–Aug. [DOI] [PubMed] [Google Scholar]
- 14.Taylor B.J., Jacobs R.F., Baker R.L., Moses E.B., McSwain B.E., Shulman G. Frozen deglycerolyzed blood prevents transfusion-acquired cytomegalovirus infections in neonates. Pediatr Infect Dis. 1986;5(2):188–191. doi: 10.1097/00006454-198603000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Demmler G.J., Brady M.T., Bijou H., Speer M.E., Milam J.D., Hawkins E.P., et al. Posttransfusion cytomegalovirus infection in neonates: role of saline-washed red blood cells. J Pediatr. 1986;108(5 Pt 1):762–765. doi: 10.1016/s0022-3476(86)81062-9. May. [DOI] [PubMed] [Google Scholar]
- 16.Luban N.L., Williams A.E., MacDonald M.G., Mikesell G.T., Williams K.M., Sacher R.A. Low incidence of acquired cytomegalovirus infection in neonates transfused with washed red blood cells. Am J Dis Child. 1987;141(4):416–419. doi: 10.1001/archpedi.1987.04460040074018. Apr. [DOI] [PubMed] [Google Scholar]
- 17.Seeberg S., Brandberg A., Hermodsson S., Larsson P., Lundgren S. Hospital outbreak of hepatitis A secondary to blood exchange in a baby. Lancet. 1981;1(8230):1155–1156. doi: 10.1016/s0140-6736(81)92318-7. May 23. [DOI] [PubMed] [Google Scholar]
- 18.Ammann A.J., Cowan M.J., Wara D.W., Weintrub P., Dritz S., Goldman H., et al. Acquired immunodeficiency in an infant: possible transmission by means of blood products. Lancet. 1983;1(8331):956–958. doi: 10.1016/s0140-6736(83)92082-2. Apr 30. [DOI] [PubMed] [Google Scholar]
- 19.Noble R.C., Kane M.A., Reeves S.A., Roeckel I. Posttransfusion hepatitis A in a neonatal intensive care unit. JAMA. 1984;252(19):2711–2715. Nov 16. [PubMed] [Google Scholar]
- 20.Azimi P.H., Roberto R.R., Guralnik J., Livermore T., Hoag S., Hagens S., et al. Transfusion-acquired hepatitis A in a premature infant with secondary nosocomial spread in an intensive care nursery. Am J Dis Child. 1986;140(1):23–27. doi: 10.1001/archpedi.1986.02140150025024. Jan. [DOI] [PubMed] [Google Scholar]
- 21.O’Riordan J.M., Conroy A., Nourse C., Yap P.L., McDonald G.S., Kaminski G., et al. Risk of hepatitis C infection in neonates transfused with blood from donors infected with hepatitis C. Transfus Med. 1998;8(4):303–308. doi: 10.1046/j.1365-3148.1998.00172.x. Dec. [DOI] [PubMed] [Google Scholar]
- 22.Lee K.K., Vargo L.R., Lê C.T., Fernando L. Transfusion-acquired hepatitis A outbreak from fresh frozen plasma in a neonatal intensive care unit. Pediatr Infect Dis J. 1992;11(2):122–123. doi: 10.1097/00006454-199202000-00012. Feb. [DOI] [PubMed] [Google Scholar]
- 23.Vareil M.O., Tandonnet O., Chemoul A., Bogreau H., Saint-Léger M., Micheau M., et al. Unusual transmission of Plasmodium falciparum, Bordeaux, France, 2009. Emerg Infect Dis. 2011;17(2):248–250. doi: 10.3201/eid1702.100595. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herwaldt B.L., Linden J.V., Bosserman E., Young C., Olkowska D., Wilson M. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med. 2011;155(8):509–519. doi: 10.7326/0003-4819-155-8-201110180-00362. Oct 18. [DOI] [PubMed] [Google Scholar]
- 25.Simonsen K.A., Harwell J.I., Lainwala S. Clinical presentation and treatment of transfusion-associated babesiosis in premature infants. Pediatrics. 2011;128(4):e1019–24. doi: 10.1542/peds.2010-0502. Oct. [DOI] [PubMed] [Google Scholar]
- 26.Martini R., Horner R., Rodrigues Mde A., Kempfer C.B., Tizotti M.K., Ratzlaff V. Bacteriological analysis of platelets and cases of septic reactions associated with transfusion of contaminated samples. Transfus Apher Sci. 2012;47(3):313–318. doi: 10.1016/j.transci.2012.06.011. Dec. [DOI] [PubMed] [Google Scholar]
- 27.Niederhauser C., Candotti D., Weingand T., Maier A., Tinguely C., Stolz M., et al. Reverse vertical transmission of hepatitis B virus (HBV) infection from a transfusion-infected newborn to her mother. J Hepatol. 2012;56(3):734–737. doi: 10.1016/j.jhep.2011.07.034. Mar. [DOI] [PubMed] [Google Scholar]
- 28.Waldenström J., Konar J., Ekermo B., Norder H., Lagging M. Neonatal transfusion-transmitted hepatitis C virus infection following a pre-seroconversion window-phase donation in Sweden. Scand J Infect Dis. 2013;45(10):796–799. doi: 10.3109/00365548.2013.797601. Oct. [DOI] [PubMed] [Google Scholar]
- 29.van Schalkwyk E., Iyaloo S., Naicker S.D., Maphanga T.G., Mpembe R.S., Zulu T.G., et al. Large outbreaks of fungal and bacterial bloodstream infections in a neonatal unit, South Africa, 2012–2016. Emerg Infect Dis. 2018;24(7):1204–1212. doi: 10.3201/eid2407.171087. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glanternik J.R., Baine I.L., Rychalsky M.R., Tormey C.A., Shapiro E.D., Baltimore R.S. A cluster of cases of Babesia microti among neonates traced to a single unit of donor blood. Pediatr Infect Dis J. 2018;37(3):269–271. doi: 10.1097/INF.0000000000001803. Mar. [DOI] [PubMed] [Google Scholar]
- 31.Grossman B.J., Kollins P., Lau P.M., Perreten J.L., Bowman R.J., Malcolm S., et al. Screening blood donors for gastrointestinal illness: a strategy to eliminate carriers of Yersinia enterocolitica. Transfusion. 1991;31(6):500–501. doi: 10.1046/j.1537-2995.1991.31691306245.x. Jul–Aug. [DOI] [PubMed] [Google Scholar]
- 32.de Korte D., Curvers J., de Kort W.L., Hoekstra T., van der Poel C.L., Beckers E.A., et al. Effects of skin disinfection method, deviation bag, and bacterial screening on clinical safety of platelet transfusions in the Netherlands. Transfusion. 2006;46(3):476–485. doi: 10.1111/j.1537-2995.2006.00746.x. Mar. [DOI] [PubMed] [Google Scholar]
- 33.Fergusson D., Khanna M.P., Tinmouth A., Hébert P.C. Transfusion of leukoreduced red blood cells may decrease postoperative infections: two meta-analyses of randomized controlled trials. Can J Anaesth. 2004;51:417–424. doi: 10.1007/BF03018302. [DOI] [PubMed] [Google Scholar]
- 34.Blumberg N., Zhao H., Wang H., Messing S., Heal J.M., Lyman G.H. The intention-to-treat principle in clinical trials and meta-analyses of leukoreduced blood transfusions in surgical patients. Transfusion. 2007;47(4):573–581. doi: 10.1111/j.1537-2995.2007.01158.x. Apr. [DOI] [PubMed] [Google Scholar]
- 35.Blajchman M.A., Goldman M., Freedman J.J., Sher G.D. Proceedings of a consensus conference: prevention of post-transfusion CMV in the era of universal leucoreduction. Transfus Med Rev. 2001;15:1–20. doi: 10.1053/tmrv.2001.19946. [DOI] [PubMed] [Google Scholar]
- 36.Bowden R.A., Slichter S.J., Sayers M., Weisdorf D., Cays M., Schoch G., et al. A comparison of filtered leukocyte-reduced and cytomegalovirus (CMV) seronegative blood products for the prevention of transfusion-associated CMV infection after marrow transplant. Blood. 1995;86(9):3598–3603. Nov 1. [PubMed] [Google Scholar]
- 37.Nichols W.G., Price T.H., Gooley T., Corey L., Boeckh M. Transfusion-transmitted cytomegalovirus infection after receipt of leukoreduced blood products. Blood. 2003;101(10):4195–4200. doi: 10.1182/blood-2002-10-3143. May 15. [DOI] [PubMed] [Google Scholar]
- 38.Schlenke P. Pathogen inactivation technologies for cellular blood components: an update. Transfus Med Hemother. 2014;41(4):309–325. doi: 10.1159/000365646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porat R., Gilbert S., Magilner D. Methylene blue-induced phototoxicity: an unrecognized complication. Pediatrics. 1996;97(5):717–721. [PubMed] [Google Scholar]
- 40.Sills M.R., Zinkham W.H. Methylene blue-induced Heinz body hemolytic anemia. Arch Pediatr Adolesc Med. 1994;148(3):306–310. doi: 10.1001/archpedi.1994.02170030076017. [DOI] [PubMed] [Google Scholar]
- 41.Jacquot C., Delaney M. Pathogen-inactivated blood products for pediatric patients: blood safety, patient safety, or both? Transfusion. 2018;58(9):2095–2101. doi: 10.1111/trf.14811. [DOI] [PubMed] [Google Scholar]
- 42.Levy J.H., Neal M.D., Herman J.H. Bacterial contamination of platelets for transfusion: strategies for prevention. Crit Care. 2018;22(1):271. doi: 10.1186/s13054-018-2212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rasonglès P., Angelini-Tibert M.F., Simon P., Currie C., Isola H., Kientz D., et al. Transfusion of platelet components prepared with photochemical pathogen inactivation treatment during a Chikungunya virus epidemic in Ile de La Réunion. Transfusion. 2009;49(6):1083–1091. doi: 10.1111/j.1537-2995.2009.02111.x. Jun. [DOI] [PubMed] [Google Scholar]
- 44.Mohr H., Knüver-Hopf J., Gravemann U., Redecker-Klein A., Müller T.H. West Nile virus in plasma is highly sensitive to methylene blue-light treatment. Transfusion. 2004;44(6):886–890. doi: 10.1111/j.1537-2995.2004.03424.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y., Ren K., Liao X., Luo G., Kumthip K., Leetrakool N., et al. Inactivation of Zika virus in plasma and derivatives by four different methods. J Med Virol. 2019;91(12):2059–2065. doi: 10.1002/jmv.25538. Dec. [DOI] [PubMed] [Google Scholar]
- 46.Eickmann M., Gravemann U., Handke W., Tolksdorf F., Reichenberg S., Müller T.H., et al. Inactivation of three emerging viruses - severe acute respiratory syndrome coronavirus, Crimean-Congo haemorrhagic fever virus and Nipah virus - in platelet concentrates by ultraviolet C light and in plasma by methylene blue plus visible light. Vox Sang. 2020;115(3):146–151. doi: 10.1111/vox.12888. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parker R.I. Transfusion in critically ill children: indications, risks, and challenges. Crit Care Med. 2014;42(3):675–690. doi: 10.1097/CCM.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 48.Ziemann M., Thiele T. Transfusion-transmitted CMV infection - current knowledge and future perspectives. Transfus Med. 2017;27(4):238–248. doi: 10.1111/tme.12437. [DOI] [PubMed] [Google Scholar]
- 49.Antona D., Lepoutre A., Fonteneau L., Baudon C., Halftermeyer-Zhou F., LE Strat Y., et al. Seroprevalence of cytomegalovirus infection in France in 2010. Epidemiol Infect. 2017;145(7):1471–1478. doi: 10.1017/S0950268817000103. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hecker M., Qiu D., Marquardt K., Bein G., Hackstein H. Continuous cytomegalovirus seroconversion in a large group of healthy blood donors. Vox Sang. 2004;86(1):41–44. doi: 10.1111/j.0042-9007.2004.00388.x. [DOI] [PubMed] [Google Scholar]
- 51.Pembrey L., Raynor P., Griffiths P., Chaytor S., Wright J., Hall A.J. Seroprevalence of cytomegalovirus, Epstein Barr virus and varicella zoster virus among pregnant women in Bradford: a cohort study. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0081881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziemann M., Heuft H.G., Frank K., Kraas S., Görg S., Hennig H. Window period donations during primary cytomegalovirus infection and risk of transfusion-transmitted infections. Transfusion. 2013;53(5):1088–1094. doi: 10.1111/trf.12074. [DOI] [PubMed] [Google Scholar]
- 53.Luthardt T., Siebert H., Lösel I., Quevedo M., Todt R. Cytomegalievirus-infektionen bei Kindern mit Blutaustauschtransfusion im Neugeborenenalter [Cytomegalo-virus infections in infants with blood exchange transfusions after birth] Klin Wochenschr. 1971;49(2):81–86. doi: 10.1007/BF01497304. [DOI] [PubMed] [Google Scholar]
- 54.Furui Y., Satake M., Hoshi Y., Uchida S., Suzuki K., Tadokoro K. Cytomegalovirus (CMV) seroprevalence in Japanese blood donors and high detection frequency of CMV DNA in elderly donors. Transfusion. 2013;53(10):2190–2197. doi: 10.1111/trf.12390. [DOI] [PubMed] [Google Scholar]
- 55.Josephson C.D., Caliendo A.M., Easley K.A., Knezevic A., Shenvi N., Hinkes M.T., et al. Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants: a prospective cohort study. JAMA Pediatr. 2014;168(11):1054–1062. doi: 10.1001/jamapediatrics.2014.1360. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seed C.R., Wong J., Polizzotto M.N., Faddy H., Keller A.J., Pink J. The residual risk of transfusion-transmitted cytomegalovirus infection associated with leucodepleted blood components. Vox Sang. 2015;109(1):11–17. doi: 10.1111/vox.12250. [DOI] [PubMed] [Google Scholar]
- 57.Delaney M., Mayock D., Knezevic A., Norby-Slycord C., Kleine E., Patel R., et al. Postnatal cytomegalovirus infection: a pilot comparative effectiveness study of transfusion safety using leukoreduced-only transfusion strategy. Transfusion. 2016;56(8):1945–1950. doi: 10.1111/trf.13605. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vamvakas E.C. Is white blood cell reduction equivalent to antibody screening in preventing transmission of cytomegalovirus by transfusion? A review of the literature and meta-analysis. Transfus Med Rev. 2005;19(3):181–199. doi: 10.1016/j.tmrv.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 59.Capretti M.G., Lanari M., Lazzarotto T., Gabrielli L., Pignatelli S., Corvaglia L., et al. Very low birth weight infants born to cytomegalovirus-seropositive mothers fed with their mother’s milk: a prospective study. J Pediatr. 2009;154(6):842–848. doi: 10.1016/j.jpeds.2008.12.046. Jun. [DOI] [PubMed] [Google Scholar]