Abstract
The outbreak of coronavirus disease (COVID-19) has caused a significant public health challenge worldwide. A lack of effective methods for screening potential patients, rapidly diagnosing suspected cases, and accurately monitoring of the epidemic in real time to prevent the rapid spread of COVID-19 raises significant difficulties in mitigating the epidemic in many countries. As effective point-of-care diagnosis tools, simple, low-cost and rapid sensors have the potential to greatly accelerate the screening and diagnosis of suspected patients to improve their treatment and care. In particular, there is evidence that multiple pathogens have been detected in sewage, including SARS-CoV-2, providing significant opportunities for the development of advanced sensors for wastewater-based epidemiology that provide an early warning of the pandemic within the population. Sensors could be used to screen potential carriers, provide real-time monitoring and control of the epidemic, and even support targeted drug screening and delivery within the integration of emerging mobile health (mHealth) technology. In this communication, we discuss the feasibility of an integrated point-of-care biosensor system with mobile health for wastewater-based epidemiology (iBMW) for early warning of COVID-19, screening and diagnosis of potential infectors, and improving health care and public health. The iBMW will provide an effective approach to prevent, evaluate and intervene in a fast, affordable and reliable way, thus enabling real-time guidance for the government in providing effective intervention and evaluating the effectiveness of intervention.
Keywords: Point-of-use sensors, Wastewater-based epidemiology, Mobile health, Pandemic, Early warning
Highlights
-
•
Point-of-care biosensors for rapid and reliable COVID-19 diagnosis.
-
•
Community sewage sensors for tracing and early warning of COVID-19 within population.
-
•
Point-of-use sensor-based mHealth for health care and epidemic management and intervention.
-
•
Microfluidics for effective drug delivery for potential therapy of COVID-19.
1. Introduction
The COVID-19 pandemic caused by a s severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly throughout more than 200 countries and has led to a worldwide disaster. Some dilemmas are associated with COVID-19 care and management from the initial outbreak to the present situation, some of which have been resolved and some of which have not (more detailed description is seen in Table S1). A good understanding of these dilemmas and putting forward solutions will help us face COVID-19 and novel infectious disease epidemics in the future. It is critical to adopt strict and accurate public health measures for COVID-19 care to address these difficulties and risks in the processes of prevention, diagnosis, intervention, and even therapy (Dowell et al., 2016).
Point-of-care (POC) biosensors may achieve the intended goal, enabling the convenient acquisition of both pathogen information and host-response information in almost any location in a short time, which has the potential to facilitate prevention and rapid diagnosis and intervention for COVID-19 when combined with other useful technologies. We discuss the feasibility of an integrated POC biosensor system with mobile health for wastewater-based epidemiology (iBMW) for early warning of COVID-19, screening and diagnosis of potential infectors, improving patient health care and monitoring public health.
-
2.
iBMW for the prevention, surveillance, monitoring and intervention of the COVID-19 pandemic
1.1. Point-of-use biosensors for COVID-19 diagnosis
The first crucial step is the rapid and accurate diagnosis of COVID-19 to screen potential patients, confirm suspected cases, provide timely health care/treatment, monitor and manage the epidemic (Udugama et al., 2020). Biosensors offer great potential to meet diagnostic requirements (Part S1) and can be used to detect COVID-19-related indicators (Russell et al., 2020). Compared with traditional detection methods, biosensors can provide a fast response time and ultra-low limit of detection, enabling the potential for miniaturization and portable analysis but requiring minimal sample processing (Veber and Weidermann, 2018).
Biosensors are analytical devices with biological elements as receptors (e.g., antibodies, aptamer, peptides, protein, etc.) that can generate physicochemical signals (optical, electrochemical, etc.) in the presence of targets for biorecognition. Biosensors provide the possibility for rapid and real-time diagnostic monitoring of COVID-19 to solve the current dilemma, as they have become a powerful tool for detecting diseases (Mao et al., 2020a; Yang et al., 2017). In addition, this method can be used to determine both pathogens and blood glucose, blood pressure, platelets and other indicators related to the symptoms of COVID-19 (Yang et al., 2015b; Yang et al., 2016; Yang et al., 2015a). Currently, there are many mature portable sensor devices that perform real-time diagnosis and detection; these include thermometers to measure body temperature and blood glucose meters to measure blood glucose and other conventional indexes. There are significant opportunities for biosensors to contribute to the rapid diagnosis and screening of infectious disease, in particular together with nanotechnology with an ultrasensitive detection of a range of disease markers (Bhalla et al., 2020). However, the main difficulty is the need to quickly and effectively detect specific indicators of infectious diseases, such as pathogens. The results of these indicators will aid in the diagnosis of suspected and potential cases.
Biosensors have great potential for rapidly diagnosing infectious disease and for the determination of infection transmission. For example, Trinh et al. constructed a foldable loop-mediated isothermal amplification (LAMP)-based biosensor integrating nucleic acid purification, amplification, and a detection method as a visual assay for multiple foodborne pathogens (Trinh and Lee, 2019). Although the genetic material of SARS-CoV-2 RNA is different from that of DNA-based pathogens, the sensor also has the capability to rapidly diagnose COVID-19 through the detection of SARS-CoV-2 RNA (Bhalla et al., 2020) (Pokhrel et al., 2020). For example, Lee et al. designed an easy-to-operate and highly sensitive analytical method for diagnosing the Zika virus through reverse transcription (RT)-LAMP and a lateral flow test that can produce results within 35 min (Lee et al., 2016). For the detection of another dangerous RNA-based virus pathogen, Ebola, Ahrberg et al. designed a palm-sized biosensor for rapid Ebola detection within 37 min (Ahrberg et al., 2016). These studies demonstrated that the biosensor has a clear potential for the rapid detection of SARS-CoV-2 and COVID-19 biomarkers.
An integrated biosensor has the potential to rapidly diagnose pathogens and efficiently monitor infection transmission through self-tests performed outside the hospital. Device integration can integrate all the steps of the biosensor into a small portable device, which is conducive to complex real-time diagnosis (Kozel and Burnham-Marusich, 2017). Recent advances in microfluidic technology (including paper microfluidic device) and nanotechnology have brought us closer than ever to the realization of simple yet highly sensitive and specific biosensors that can be used in complicated environments without a central laboratory (Zhuang et al., 2020). Highly sensitive and specific biosensors enable disease detection and diagnosis (Table. S2). Fig. 1 shows the real-time diagnostic biosensor concept for COVID-19 monitoring, which contains most of the elements needed to measure all the above targets related to COVID-19 diagnosis. We also provide two typical examples of fabrication and principles of related biosensors (nano-biosensor in Fig. S1 and integrated biosensor in Fig. S2).
Fig. 1.
The concept for an integrated POC biosensor for SARS-CoV-2 identification and COVID-19 biomarker quantification. A negative test is finished by adding the same channel and reaction cavity as the sample test process (seen in Fig. S3).
For example, we have recently developed a low-cost and rapid paper-based analytical tool that can integrate all the processes, including enrichment, extraction, purification, amplification, and visual detection for nucleic acid testing (Yang et al., 2018). The whole process can be completed with the simple folding-unfolding of the paper device without a power or pump supply, which can be used in limited resource areas. In addition, during the COVID-19 pandemic, areas with local infectious disease epidemics, such as Africa and the malaria epidemic (Reboud et al., 2019), have a high risk of simultaneous outbreaks together with COVID-19. The biosensor also has the ability to rapidly and accurately simultaneously identify and screen for COVID-19 and other local infectious diseases, serving as an early warning system in resource-poor areas. For example, a paper-origami device has been successfully employed to simultaneously diagnose two bovine bacteria and herpes virus-1 in rural India (Yang et al., 2018).
There are also reports of the simultaneous detection of human viruses. Acute febrile diseases, such as chikungunya and dengue, are related to the high burden of diseases in the tropics and have many non-specific symptoms. In the absence of available laboratory diagnostic tools, it is difficult to diagnose by routine examination alone. Wang et al., (2019) demonstrated a rapid analytical method based on colour-mixing encoding and a readout strategy with the capability of performing selective and sensitive multiplexed analyses of dengue and chikungunya in clinical samples within 30 min. Their multiplex assay could concurrently detect four targets using a low sample volume in a resource-limited setting. These studies demonstrate that paper-based biosensors with the ability to perform fast, precise and high-quality diagnostics enable multiplex, sensitive, and selective analysis of infectious diseases and pathogens.
In resource-limited areas, health care services may be overwhelmed; therefore, it is important to develop testing kits with the capability for self-detection. Biosensors provide an important opportunity for family and community monitoring and have the potential to alleviate the current dilemma. At the same time, the use of biosensors to quantify host immune biomarkers in patients will aid in determining the severity of patients' symptoms, detecting the state of the host’s immune system and identifying organ disorders, and this information can be applied to strategically allocate resources to optimize health care by adapting the classification process, the requirement for admission or intensive care, and the administration of various treatments. The real-time diagnostic biosensor can easily obtain timely information on pathogens and host responses in almost any location, and its turnaround time is very fast. It can accurately identify potential patients, prompting corresponding interventions. This has a profound impact on understanding disease characteristics and transmission processes and guiding clinical treatment and health care, especially for infectious diseases (Bains, 2014; Peacock and Weinstock, 2014).
1.2. Community sewage sensors for tracing and early warning of COVID-19
Recently, it has been reported that the genetic material of SARS-CoV-2 has been detected in sewage treatment plants in several countries, including France (Wurtzer et al., 2020), the Netherlands (Medema et al., 2020), Australia (Ahmed et al., 2020), Italy (La Rosa et al., 2020), the USA (Nemudryi et al., 2020; Wu et al., 2020), Spain (Randazzo et al., 2020), Turkey (Kocamemi et al., 2020), India (Arora et al., 2020), China (Chen et al., 2020) and other countries (Haramoto et al., 2020; Mallapaty, 2020). Hence, the analysis of SARS-CoV-2 in raw sewage can trace the source of COVID-19 and determine whether there are potential virus carriers in certain areas (Mao et al., 2020b). Furthermore, wastewater analysis can also be used as a more effective approach to evaluate the extent of the COVID-19 spread in the population rather than testing each individual, more importantly, the cost and rapidness are much improved (Bhalla et al., 2020). Because the genetic material of viral RNA from people who have mild or no symptoms will also enter the wastewater treatment plant, wastewater analysis methods can test all people in the community. Murakami et al. further performed wastewater analysis to overcome representativeness and stigma issues related to COVID-19 (Murakami et al., 2020). Therefore, Bivins et al. further call for a global collaborative to maximize contributions of wastewater analysis in the fight against COVID-19 (Bivins et al., 2020).
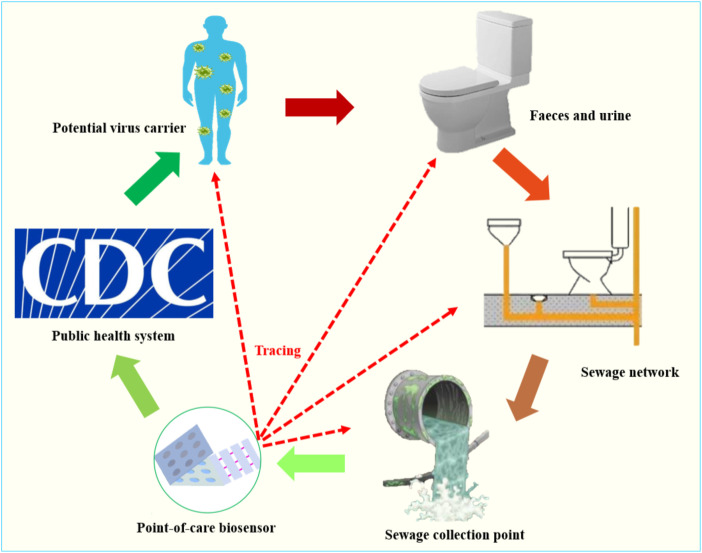
However, the current state-of-the-art analytical method for sewage involves sample enrichment, and the detection still heavily depends on RT-qRCR, which needs a well-equipped laboratory and well-trained personnel. Recently, much attention has been paid to sewage biosensors for monitoring public health (Yang et al., 2015b). These sensors play a key role in the rapid analysis of pathogens and other disease biomarkers in wastewater due to their excellent merits, such as rapid response, cost effectiveness, and small sample requirements (Yang et al., 2017). Therefore, community sewage biosensors can be used to collect timely information about COVID-19 for the whole community and report results to health institutions, facilitating early prevention measures and effects (Fig. 2 ). If SARS-CoV-2 can be detected in the local community at an early stage through a community sewage biosensor, an effective intervention can be implemented in a real-time fashion, and restrictions on SARS-CoV-2 transmission will minimize the spread of the disease and the threat to public health. Potential patients will also benefit from the community sewage sensor tracing of SARS-CoV-2 sources, which will provide information for accurate and timely treatment.
Fig. 2.
The community sewage biosensor for tracing COVID-19 sources and implementing effective interventions.
1.3. Biosensor-based mHealth for health care and epidemic management for effective intervention
Mobile health (mHealth)-based biosensors have great potential to overcome the limitations of a shortage of medical resources and alleviate this dilemma to facilitate effective intervention. mHealth is the application of mobile devices, components, and related technologies in the field of health care. mHealth provides an ideal framework for real-time and effective health care and epidemic management, which is achieved through targeted behavioural changes (through interactive applications), care roadmaps (based in the community or the clinic), and monitoring by connected diagnosis. mHealth technology improves the speed, efficiency, and appropriateness of the comprehensive public health and clinical response to the epidemic mainly through two mechanisms: increased access to health care outside the clinical environment (such as self-testing) and reporting of the diagnostic results to medical professionals and public health departments in (nearly) real-time to identify an epidemic outbreak in an area, thus ensuring efficient clinical and public health responses. Patients report their self-test results to the hospital and public health management department through the mobile health system; then, the hospital puts forward diagnosis and treatment suggestions depending on the patient’s actual situation (Fig. 3 ).
Fig. 3.
POC biosensor-based mHealth system. The stages, stakeholders, and possible outcomes of deploying an effective mHealth intervention that uses a connected biosensor.
The implementation of these mechanisms involves the fast transmission and storage of data as well as the connection of all related parties, all of which require a sufficient hardware foundation and technical support. The rapid development of portable communication technology, such as 5G, and digital computing, such as the concepts of “big data” and “blockchain”, improves the speed and efficiency of data processing and exchange. Additionally, the widespread use of smartphones and the networks needed to support them reduce data collection and transmission costs. This powerful handheld computer with sensors/biosensors and wireless connections provides scientists and medical systems with the possibility of capturing and processing data (Perkel, 2017). Regardless of whether a setting has abundant or limited resources, the application scope and capability of smartphones and their related technologies are increasing. Currently, these smartphones offer low-cost sensing and processing capabilities comparable to expensive “high-end” devices. In addition, the mHealth system can also promote efficiency by improving the automation of inventory and supply chain management systems, reducing the workload and errors related to paper reports, and preventing materials from running out (Namisango et al., 2016).
mHealth has been proven to be useful for internet-based clinical and public health responses to some infectious diseases. In a study from the United Kingdom, a comprehensive online prevention, diagnosis, and health care plan was presented (Estcourt et al., 2017). Chlamydia-infected patients were managed by online clinical consultations; the patients received a link to access their clinical records and even obtain antibiotics from pharmacies. The system also integrated partner notifications, health promotion and automated monitoring data collection to help prevent the spread of this potentially asymptomatic sexually transmitted infectious disease.
A biosensor can be a simple (a self-test), fast (real-time or nearly real-time) diagnosis method or detection technology to determine the relevant diagnostic target of an infectious disease (Bissonnette and Bergeron, 2017) and automatically transmit the diagnosis results to the mHealth system, which will greatly speed up the patient's access to treatment and consultation. COVID-19 detection and the application of the online diagnostic and symptom reporting application combined with a standardized epidemiological and clinical data collection have a substantial opportunity to improve the speed and efficiency of monitoring and management of the epidemic (Fallah et al., 2017). The rapid results acquired by POC biosensors can be monitored through geospatial maps utilizing geographical markers (Chunara et al., 2012) or by social network and internet search analyses, which provide an integration tool that can be applied to epidemic control (Lampos et al., 2015; Yom-Tov et al., 2015). COVID-19 can be detected and treated rapidly with mobile surveillance, and the real-time monitoring of epidemic areas can be intensified (Hayward et al., 2014). Moreover, the public health departments can monitor the epidemic with the real-time mHealth system and take appropriate measures, such as regional isolation and the allocation of strategic materials. All participants in the mobile system, including potential patients, medical staff and public health departments, can quickly understand the mobile health system, which can facilitate the diagnosis and treatment of potential patients. Medical staff can better guide patients' health care, and public health departments can better monitor the epidemic and implement interventions such as the timely isolation of confirmed patients, protection of healthy people, and allocation of public resources. Mobile systems, in combination with internet-connected diagnostic biosensors, provide new methods for the diagnosis, tracking, and control of infectious diseases while improving the efficiency of the health system (Fig. 3).
In addition, microfluidics sensing technology for effective drug screening and delivery holds the potential for therapy of SARS-CoV-2. Microfluidic chips afford considerable advantages in drug release, such as precise and multi-dosing release, targeted precise release, sustainable control of delivery, and small side effects, etc., which are important assets for drug delivery systems (see Part S3). Microfluidic technology has been gradually applied to the preparation of drug carriers, direct drug delivery systems, drug preparation and fixation. Inexpensive and easily manufactured materials are rich substrates that naturally integrate multiple functions, which include filtration, storage, transport, valves, multiplexing, and concentration. Microfluidics has great potential to be used in the research of COVID-19 therapies to avoid ineffectiveness and health risks.
2. Conclusions and perspectives
Effective prevention, monitoring, and interventions are important for slowing the spread of the disease and reducing the prevalence of COVID-19. We have proposed to use iBMW to provide an ideal framework to manage pandemics, from the perspectives of prevention, detection and intervention. The innovative miniaturization and portability of community sewage biosensors provide the possibility to trace potential sources in the field, and iBMW can directly identify pathogens and provide required biomarker data in a short period of time through self-testing. The real-time data collected and transmitted by the iBMW not only provide timely health care and treatment for patients but also allow for the timely implementation of epidemic control measures. COVID-19 can be accurately controlled by public health prevention measures according to the epidemic situation in different regions. Considering this timely information regarding the SARS-CoV-2 infection status and host reactions, the mHealth system can be used to monitor and control the epidemic. Hence, the use of iBMW could reduce the time from the onset of infection to the appropriate therapeutics. In addition, the fast growth of microfluidic sensing technology has provided new opportunities for effective drug screening and drug release in vitro tests, which will be beneficial for the development of effective therapeutic drugs and vaccines without a safety risk.
Although it still remains some challenges of biosensors for COVID-19 remain (listed in Table S3), the application of biosensors provides a systematic approach for alleviating the current dilemma associated with the prevention, monitoring and intervention of COVID-19. In particular, biosensors can trace potential virus carriers early, rapidly confirm suspected cases with self-tests, increase the chance of patients obtaining precise medical treatment, decrease the risk of medical staff infection, monitor and manage the pandemic in real time, support therapeutic drug screening and drug delivery systems, and finally control the epidemic through timely response.
Author Contribution
Kang Mao: Conceptualization, Writing - original draft, Writing - review & editing. Hua Zhang: Writing - review & editing, Project administration. Zhugen Yang: Conceptualization, Writing - original draft, Writing - review & editing, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is funded by the Royal Academy of Engineering Frontier Follow-up Grant (FF\1920\1\36), the China Postdoctoral Science Foundation (2020M673302), STS of CAS (KFJ-STS-QYZD-185), China and the UK NERC Fellowship grant (NE/R013349/2).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bios.2020.112617.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrberg C.D., Manz A., Neužil P. Anal. Chem. 2016;88(9):4803–4807. doi: 10.1021/acs.analchem.6b00278. [DOI] [PubMed] [Google Scholar]
- Arora S., Nag A., Sethi J., Rajvanshi J., Saxena S., Shrivastava S.K., Gupta A.B. 2020. MedRxiv. [DOI] [PubMed] [Google Scholar]
- Bains R.K. Genome Biol. 2014;15(11):529. doi: 10.1186/s13059-014-0529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla N., Pan Y., Yang Z., Payam A.F. ACS Nano. 2020 doi: 10.1021/acsnano.0c04421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette L., Bergeron M.G. Expert Rev. Mol. Diagn. 2017;17(5):471–494. doi: 10.1080/14737159.2017.1310619. [DOI] [PubMed] [Google Scholar]
- Bivins A., North D., Ahmad A., Ahmed W., Alm E., Been F., Bhattacharya P., Bijlsma L., Boehm A.B., Brown J., Buttiglieri G., Calabro V., Carducci A., Castiglioni S., Cetecioglu Gurol Z., Chakraborty S., Costa F., Curcio S., de los Reyes F.L., Delgado Vela J., Farkas K., Fernandez-Casi X., Gerba C., Gerrity D., Girones R., Gonzalez R., Haramoto E., Harris A., Holden P.A., Islam M.T., Jones D.L., Kasprzyk-Hordern B., Kitajima M., Kotlarz N., Kumar M., Kuroda K., La Rosa G., Malpei F., Mautus M., McLellan S.L., Medema G., Meschke J.S., Mueller J., Newton R.J., Nilsson D., Noble R.T., van Nuijs A., Peccia J., Perkins T.A., Pickering A.J., Rose J., Sanchez G., Smith A., Stadler L., Stauber C., Thomas K., van der Voorn T., Wigginton K., Zhu K., Bibby K. Environ. Sci. Technol. 2020;54(13):7754–7757. doi: 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L., Yang Y., Liu B., Wang W., Wei C. J. Med. Virol. 2020;92(7):833–840. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- Chunara R., Freifeld C.C., Brownstein J.S. Parasitology. 2012;139(14):1843–1851. doi: 10.1017/S0031182012000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell S.F., Blazes D., Desmond-Hellmann S. Nature. 2016;540(7632):189–191. [Google Scholar]
- Estcourt C.S., Gibbs J., Sutcliffe L.J., Gkatzidou V., Tickle L., Hone K., Aicken C., Lowndes C.M., Harding-Esch E.M., Eaton S., Oakeshott P., Szczepura A., Ashcroft R.E., Copas A., Nettleship A., Sadiq S.T., Sonnenberg P. Lancet Public Health. 2017;2(4):e182–e190. doi: 10.1016/S2468-2667(17)30034-8. [DOI] [PubMed] [Google Scholar]
- Fallah M.P., Skrip L.A., Raftery P., Kullie M., Borbor W., Laney A.S., Blackley D.J., Christie A., Dokubo E.K., Lo T.Q., Coulter S., Baller A., Vonhm B.T., Bemah P., Lomax S., Yeiah A., Wapoe-Sackie Y., Mann J., Clement P., Davies-Wayne G., Hamblion E., Wolfe C., Williams D., Gasasira A., Kateh F., Nyenswah T.G., Galvani A.P. PLoS Med. 2017;14(1) doi: 10.1371/journal.pmed.1002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. 2020. MedRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward A.C., Fragaszy E.B., Bermingham A., Wang L., Copas A., Edmunds W.J., Ferguson N., Goonetilleke N., Harvey G., Kovar J., Lim M.S.C., McMichael A., Millett E.R.C., Nguyen-Van-Tam J.S., Nazareth I., Pebody R., Tabassum F., Watson J.M., Wurie F.B., Johnson A.M., Zambon M. Lancet Respir.Med. 2014;2(6):445–454. doi: 10.1016/S2213-2600(14)70034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocamemi B.A., Kurt H., Hacioglu S., Yarali C., Saatci A.M., Pakdemirli B. 2020. MedRxiv. [Google Scholar]
- Kozel T.R., Burnham-Marusich A.R. J. Clin. Microbiol. 2017;55(8):2313–2320. doi: 10.1128/JCM.00476-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Ferraro G.B., Veneri C., Bonadonna L., Lucentini L., Suffredini E. Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampos V., Miller A.C., Crossan S., Stefansen C. Sci. Rep. 2015;5(1):12760. doi: 10.1038/srep12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Shin Y., Chung S., Hwang K.S., Yoon D.S., Lee J.H. Anal. Chem. 2016;88(24):12272–12278. doi: 10.1021/acs.analchem.6b03460. [DOI] [PubMed] [Google Scholar]
- Mallapaty S. Nature. 2020;580(7802):176–177. doi: 10.1038/d41586-020-00973-x. [DOI] [PubMed] [Google Scholar]
- Mao K., Zhang H., Wang Z.L., Cao H.R., Zhang K.K., Li X.Q., Yang Z.G. Biosens. Bioelectron. 2020;148:111785. doi: 10.1016/j.bios.2019.111785. [DOI] [PubMed] [Google Scholar]
- Mao K., Zhang H., Yang Z. Environ. Sci. Technol. 2020;54(7):3733–3735. doi: 10.1021/acs.est.0c01174. [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. 2020. MedRxiv. [DOI] [PubMed] [Google Scholar]
- Murakami M., Hata A., Honda R., Watanabe T. Environ. Sci. Technol. 2020;54(9) doi: 10.1021/acs.est.0c02172. 5311-5311. [DOI] [PubMed] [Google Scholar]
- Namisango E., Ntege C., Luyirika E.B.K., Kiyange F., Allsop M.J. BMC Palliat. Care. 2016;15(1):20. doi: 10.1186/s12904-016-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Surya K., Wiegand T., Buyukyoruk M., Wilkinson R., Wiedenheft B. 2020. MedRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock S.J., Weinstock G.M. Genome Med. 2014;6(11):103. doi: 10.1186/s13073-014-0103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel J.M. Nature. 2017;545(7652):119–121. doi: 10.1038/545119a. [DOI] [PubMed] [Google Scholar]
- Pokhrel P., Hu C., Mao H. ACS Sens. 2020 doi: 10.1021/acssensors.0c01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboud J., Xu G., Garrett A., Adriko M., Yang Z., Tukahebwa E.M., Rowell C., Cooper J.M. PANS (Pest. Artic. News Summ.) 2019;116(11):4834–4842. doi: 10.1073/pnas.1812296116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S.M., Alba-Patiño A., Barón E., Borges M., Gonzalez-Freire M., de la Rica R. ACS Sens. 2020;5(6):1506–1513. doi: 10.1021/acssensors.0c00979. [DOI] [PubMed] [Google Scholar]
- Trinh T.N.D., Lee N.Y. Lab Chip. 2019;19(8):1397–1405. doi: 10.1039/c8lc01389f. [DOI] [PubMed] [Google Scholar]
- Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y.C., Chen H., Mubareka S., Gubbay J.B., Chan W.C.W. ACS Nano. 2020;14(4):3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- Veber M., Weidermann A. Sirius. Zeitschrift für Strategische Analysen. 2018;2(1):85–86. [Google Scholar]
- Wang J.G., Xu C.C., Wong Y.K., He Y.K., Adegnika A.A., Kremsner P.G., Agnandji S.T., Sall A.A., Liang Z., Qiu C., Liao F.L., Jiang T.L., Krishna S., Tu Y.Y. Lancet. 2020;395(10230):1094–1096. doi: 10.1016/S0140-6736(20)30561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Gu X., Lee W.L., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N. 2020. MedRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.-M., Moulin L. 2020. MedRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Castrignanò E., Estrela P., Frost C.G., Kasprzyk-Hordern B. Sci. Rep. 2016;6(1):21024. doi: 10.1038/srep21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., d’Auriac M.A., Goggins S., Kasprzyk-Hordern B., Thomas K.V., Frost C.G., Estrela P. Environ. Sci. Technol. 2015;49(9):5609–5617. doi: 10.1021/acs.est.5b00637. [DOI] [PubMed] [Google Scholar]
- Yang Z., Kasprzyk-Hordern B., Frost C.G., Estrela P., Thomas K.V. Environ. Sci. Technol. 2015;49(10):5845–5846. doi: 10.1021/acs.est.5b01434. [DOI] [PubMed] [Google Scholar]
- Yang Z., Xu G., Reboud J., Ali S.A., Kaur G., McGiven J., Boby N., Gupta P.K., Chaudhuri P., Cooper J.M. ACS Sens. 2018;3(2):403–409. doi: 10.1021/acssensors.7b00825. [DOI] [PubMed] [Google Scholar]
- Yang Z., Xu G., Reboud J., Kasprzyk-Hordern B., Cooper J.M. Anal. Chem. 2017;89(18):9941–9945. doi: 10.1021/acs.analchem.7b02257. [DOI] [PubMed] [Google Scholar]
- Yom-Tov E., Johansson-Cox I., Lampos V., Hayward A.C. Influenza other respir. Viruses. 2015;9(4):191–199. doi: 10.1111/irv.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J., Yin J., Lv S., Wang B., Mu Y. Biosens. Bioelectron. 2020;163:112291. doi: 10.1016/j.bios.2020.112291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.