Abstract
We are currently facing the COVID-19 pandemic which is the consequence of severe acute respiratory syndrome coronavirus (SARS-CoV-2). Since no specific vaccines or drugs have been developed till date for the treatment of SARS-CoV-2 infection, early diagnosis is essential to further combat this pandemic. In this context, the reliable, rapid, and low-cost technique for SARS-CoV-2 diagnosis is the foremost priority. At present reverse transcription polymerase chain reaction (RT-PCR) is the reference technique presently being used for the detection of SARS-CoV-2 infection. However, in a number of cases, false results have been noticed in COVID-19 diagnosis. To develop advanced techniques, researchers are continuously working and in the series of constant efforts, nanomaterials-enabled biosensing approaches can be a hope to offer novel techniques that may perhaps meet the current demand of fast and early diagnosis of COVID-19 cases. This paper provides an overview of the COVID-19 pandemic and nanomaterials-enabled biosensing approaches that have been recently reported for the diagnosis of SARS-CoV-2. Though limited studies on the development of nanomaterials enabled biosensing techniques for the diagnosis of SARS-CoV-2 have been reported, this review summarizes nanomaterials mediated improved biosensing strategies and the possible mechanisms that may be responsible for the diagnosis of the COVID-19 disease. It is reviewed that nanomaterials e.g. gold nanostructures, lanthanide-doped polysterene nanoparticles (NPs), graphene and iron oxide NPs can be potentially used to develop advanced techniques offered by colorimetric, amperometric, impedimetric, fluorescence, and optomagnetic based biosensing of SARS-CoV-2. Finally, critical issues that are likely to accelerate the development of nanomaterials-enabled biosensing for SARS-CoV-2 infection have been discussed in detail. This review may serve as a guide for the development of advanced techniques for nanomaterials enabled biosensing to fulfill the present demand of low-cost, rapid and early diagnosis of COVID-19 infection.
Keywords: Coronavirus, SARS-CoV-2, COVID-19, Nanomaterials, Biosensors
Graphical abstract
No specific vaccine or drug has been developed till date for the treatment of SARS-CoV-2 infection, early diagnosis is very essential to manage COVID-19 pandemic. At present, the reliable, rapid, and low-cost technique for SARS-CoV-2 diagnosis is the foremost priority. In this context, nanomaterials enabled based biosensors can be a hope to offer novel techniques that may perhaps meet the current demand for early and rapid diagnosis of SARS-CoV-2 infections.
1. Introduction
The whole world is barely breathing under the shadow of the novel coronavirus (COVID-19). In December 2019, un-precedented pneumonia due to unknown cause was detected in the city Wuhan of China (Udugama et al., 2020; Morales-Narváez and Dincer, 2020). Subsequently, on 11 February 2020, the world health organization (WHO) identified it as the new coronavirus disease-19 (COVID-19) and it was declared as pandemic on 13 March 2020 [https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen]. The public health professionals have discovered that this new pathogen possesses about 80% similarity to the genome of the severe acute respiratory syndrome (SARS-CoV) and therefore, given the name as SARS-CoV-2 (Zhou et al., 2020; Gorbalenya et al., 2020; Lu et al., 2020). The SARS-CoV-2 has not only aroused severe health issues around the world but has led to an un-precedented socio-economic burden. SARS-CoV-2 is capable of spreading among humans to humans from the infected individuals, bearing no or mild symptoms (Bai et al., 2020; Rothe et al., 2020). In the beginning, the diagnosis of patients suffering from SARS-CoV-2 infection was done through the computed tomography (CT) of the chest, and was compared to the healthy lungs, varying opacities in the CT images were recorded in patients, leading to initial diagnosis of pneumonia (Li and Xia, 2020; Bernheim et al., 2020). Subsequently, in early January 2020 after the publication of the genetic code of coronavirus, the polymerase chain reaction (PCR) based technique was employed for the diagnosis of COVID-19 (Chu et al., 2020).
As per the WHO guidelines, confirmation of the COVID-19 infection should be done by detecting unique ribonucleic acid (RNA) sequences of SARS-CoV-2. Further, detection of virus RNA is based on the nucleic acid amplification tests through real-time reverse-transcription polymerase chain reaction (rRT-PCR), targeting N, E, S and RdRp genes [https://apps.who.int/iris/bitstream/handle/10665/331501/WHO-COVID-19-laboratory-2020.5-eng.pdf?sequence=1&isAllowed=y]. The characteristics of RT-PCR techniques (e.g. detection ability, detection limit, sensitivity, detection time etc.) towards the specific targets (RdRP gene, ORF1ab, N, S and Egene) of SARS-CoV-2 have been found to vary as per the manufacturer and compressively reviewed in recent studies [Wang et al., 2020b; Mahapatra and Chandra, 2020; LeBlanc et al., 2020; van Kasteren et al., 2020; Carter et al., 2020]. In a study by van Kasteren et al., diagnostic ability of seven different commercial RT-PCR kits for SARS-CoV-2 infection were evaluated [van Kasteren et al., 2020]. In all the assays, ≥96% efficiency of PCR was noticed whereas the estimated limit of detection (LOD95) [copy/ml] exhibited 6-fold variation. Similarly, Wang et al., observed a substantial difference in LOD of 6 commercial RT-PCR kits where the poorest LOD is estimated to be false negative result of SARS-CoV-2 diagnosis [Wang et al., 2020b]. Though the PCR-based technique relies on the RNA and is highly sensitive, there are several issues associated therein. These issues can be briefed as follows (i) complex and time-consuming, (ii) handling and transportation of samples by highly skilled staff (iii) high cost, (iii) risk to elicit false-negative and false-positive diagnosis etc. (Shen et al., 2020; Tahamtan and Ardebili, 2020). Further, the alternative techniques based on anti-bodies (serological testing) and CRISPR have been employed for the diagnosis of the SARS-CoV-2 infection (Broughton et al., 2020).
To impede the spreading of SARS-CoV-2 among the humans and thereby reducing the mortality rate, early and fast diagnosis is the demand of the hour. Moreover, to overcome numerous issues associated with PCR-based techniques, researchers are working hard to develop the advanced and low-cost techniques to offer rapid and reliable detection of SARS-CoV-2. In this context, biosensors based on nanomaterials have been predicted to fulfill the demand of the desired fast and low-cost diagnosis techniques. Owing to interesting physicochemical properties at nano-scale, nanomaterials enabled biosensing is advantageous in several aspects e.g. (i) easy to use (ii) requirement of a very low amount of the sample analytes (iii) fast and sensitive (iv) low-cost, and easy disposability. Further, nanomaterials-enabled biosensings are being developed and have shown their potential for the diagnosis of different types of virus infectious diseases including HIV/AIDS (Farzin et al., 2020), hepatitis B virus (Negahdari et al., 2019), herpes virus (Narang et al., 2018), dengue fever (Palomar et al., 2018) and influenza virus infections, etc. (Siuzdak et al., 2019; Lee et al., 2019).
In this review we focus on the role of nanomaterials-enabled biosensing techniques of different types of virus that have been considered as responsible for the onset of respiratory infections including MERS-CoV, SARS-CoV, and SARS-CoV-2. We summarize in particular the studies reported on the development of nanomaterials-enabled biosensing for the detection of SARS-CoV-2 that have been recently performed. Different types of nanomaterials such as gold based nanostructures, lanthanide-doped polysterene NPs, graphene and iron oxide NPs that have been used for the development of novel techniques for the diagnosis of SARS-CoV-2 with the help of colorimetric, fluorescence, amperometric, impedimetric and optomagnetic mediated improved biosensing have been reviewed. The emphasis has been made on the different methods for biosensing using a variety of nanomaterials and the associated mechanisms for the detection of SARS-CoV-2 infection. Moreover, future prospects to implement nanomaterials-enabled biosensing techniques for the SARS-CoV-2 infection have been discussed in detail.
2. An overview of SARS-CoV-2 and its consequences onCOVID-19 pandemic
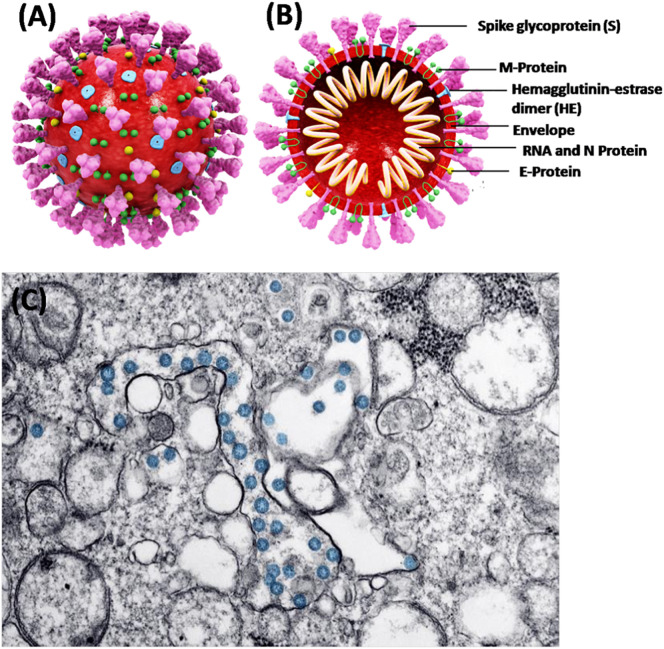
SARS-CoV-2, a β-coronavirus has been identified as a single positive-strand RNA genome (Grifoni et al., 2020). It consists of four different structural proteins namely spike (S), envelope (E), matrix (M), and nucleocapsid (N) [Fig. 1 ]. Through the phylogenetic analysis of SARS-CoV-2, the genomic similarties about 80% and 50% have been noticed with severe acute respiratory syndrome virus (SARS-CoV) and middle east respiratory syndrome virus (MERS-CoV), respectively (Zhang and Holmes, 2020). More importantly, SARS-CoV-2 expresses the genomic similarity of about 96% with the bat coronavirus RaTG13, and hence it is a matter of intense debate that SARS-CoV-2 may perhaps have originated from the bat (Zhang and Holmes, 2020). Many studies have explored that SARS-CoV-2 exploits the angiotensin converting enzyme-II (ACE2), as a receptor for the cellular entry. Further, ACE2 has been recognized for high affinity of the spike protein (S) of SARS-CoV-2 (Alifano et al., 2020).
Fig. 1.
Shows (A) 3D structure of SARS-CoV-2, (B) cross sectional representation of the viral structure with its proteins [modified background, adapted from: https://www.scientificanimations.com/coronavirus-symptoms-and-prevention-explained-through-medical-animation/] (https://en.wikipedia.org/wiki/Coronavirus, CC BY-SA 4.0) (C) Transmission electron microscope image of SARS-CoV-2. The virus is colorized in blue (adapted from the US Centers for Disease Control). [Adapted from: details on COVID-19; Public Health Image Library (PHIL), Centers for Disease Control and Prevention. https://phil.cdc.gov/Details.aspx?pid=23354 (accessed 2020/03/27).] (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Respiratory droplets and fomites are the possible medium and are responsible to transmit COVID-19 diseases. As per the clinical studies conducted on COVID-19, it has been estimated that SARS-CoV-2 can have the incubation period of up to 24 days. Whereas, a high basic reproductive number has been determined to be ~3.77 (Guan et al., 2020; Yang et al., 2020). In most of the clinical cases, this virus has been detected in the upper respiratory tract, suggesting nasopharynx as the main site of replication. Nevertheless, the gastrointestinal symptoms have been noticed in a number of patients suffering from COVID-19 (Gu et al., 2020). Moreover, even when the nasopharyngeal sample was found to be negative, the RNA trace of SARS-CoV-2 could be detected in rectal swabs (Wang et al., 2020a; Xiao et al., 2020; Lamers et al., 2020). These observations clearly suggest that transmission of SARS-CoV-2 through fecal-oral is also possible. It has been reported that usually, lungs are the most affected organs by SARS-CoV-2, but other organs like brain may also be severely infected. Gandhi et al., have reported that through the olfactory bulb, the central nervous system (CNS) is most likely to be infected by SARS-CoV-2 (Gandhi et al., 2020). Consequently, SARS-CoV-2 can easily target the thalamus and brain stem, leading to infection in the respiratory center of the brain. Thus, it is possible that SARS-CoV-2 can infect the respiratory center of the brain and is therefore, responsible for the respiratory breakdown in patients suffering from COVID-19. Besides this, the spectrum of this disease is partly recognized in patients. And, both the symptomatic and asymptomatic cases have been noticed. In asymptomatic case, no specific symptoms have been identified. Some of the symptoms may include respiratory issues, pneumonia, fever, cough, and dyspnea (Cascella et al., 2020). On the other hand, an asymptomatic patient is capable of transmitting this disease. However, technically one can say that the asymptomatic individuals are more dangerous since they can spread the disease unknowingly. Therefore, the development of a low-cost and rapid diagnosis technique is imperative and is urgently needed to identify the infected patients to further prevent and control this pandemic. In this context, nanomaterials enabled biosensing approaches can be imperative and are expected to fulfill the current demand for early, rapid and low-cost point- of- care diagnostic techniques for COVID-19 detection. As discussed in the next section chemical properties of nanomaterials can be a potential to design ultra-sensitive biosensing techniques for respiratory viral infections. In the meantime, to avoid the infection through SARS-CoV-2, the only options that an individual should follow are the precautionary measures [Fig. 2 ].
Fig. 2.
Schematic shows that one should take precautions to avoid infection through SARS-CoV-2 [credit: https://pixabay.com/; coronavirus 3D image adopted from https://www.scientificanimations.com/coronavirus-symptoms-and-prevention-explained-through-medical-animation/] (https://en.wikipedia.org/wiki/Coronavirus, CC BY-SA 4.0).
3. Nanomaterials-enabled biosensing of respiratory viral infection (MERS-CoV, SARS-CoV, SARS-CoV-2)
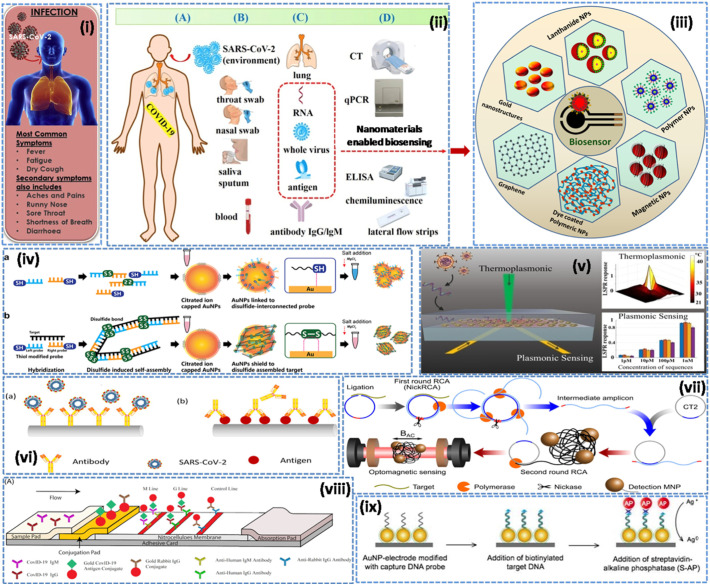
Recent advancements in the field of nanobiotechnology have given birth to advanced techniques for diagnosis and therapeutic applications (Augustine et al., 2017; Palmieri and Papi, 2020; Malhotra and Ali, 2018; Ravina et al., 2020; Cesewski and Johnson, 2020). In particular, properties of nanomaterials including high surface-to-volume ratio, quantum size effects, high adsorption and reactive capacity as compared to their bulk form are imperative to design biosensing techniques. Moreover, the size and shape of nanomaterials can be easily tailored, and, therefore, surface modification/immobilization with numerous biological species via covalent or non-covalent bonding are possible to enhance the biosensing characteristics in terms of low-detection limit (increased up to several order of magnitudes), high sensitivity, selectivity and rapid response towards the sample analytes (Maduraiveeran et al., 2018). Nevertheless, one of the biggest challenges in designing of biosensors (e.g. DNA/RNA) is to capture signal of very low magnitude which takes place between the biological species (bio-receptors and analytes). To overcome this issue, nanomaterials can be used as labels to achieve the significant enhancement of signal, high enough to be easily detectable. Labeling can be done using metal nanoparticles (Au NPs/Ag NPs) and quantum dots (Cd/Pb) by attaching on the targeted DNA/bio-recognizing probe [Kokkinos, 2019; Saylan et al., 2019]. The synergic effect due to nano-labeling results in significant amplification of the electrochemical signal and, therefore, it is possible to develop ultrasensitive and highly selective labeled-biosensing techniques [Saylan et al., 2019]. In this context, biosensors for the diagnosis of viral infected diseases are being developed (Saylan et al., 2019). Biosensors can be utilized to determine the virus-mediated infections that have been categorized into four major groups including (i) nucleic acid (ii) anti-body (iii) aptamer and (iv) antigen-dependent (Ozer et al., 2020). Thus, taking the advantages of the numerous physicochemical properties of nano-dimensions specifically electrical, optical, magnetic, and optomagnetic, attempts have been made to develop nano-enabled biosensing techniques for detection of virus, responsible for respiratory infections (MERS-CoV, SARS-CoV and SARS-CoV-2) [Fig. 3 ].
Fig. 3.
Nanomaterials-enabled biosensing approaches for respiratory viral infections (i) SARS-CoV-2 infection; revealing symptoms [modified from Mahapatra and Chandra, 2020] (ii) current diagnostic methods and portable biosensors for SARS-CoV-2 infection [modified from Cui and Zhou, 2020] (iii) different types of nanomaterials based biosensing for respiratory viral infections (iv) colorimetric detection of DNA based on disulfide induced self-assembly for MERS-CoV [Kim et al., 2019]. (v) dual-functional plasmonic photo-thermal biosensors for SARS-CoV-2 using AuNIs [Qiu et al., 2020]. (vi) optical fiber enabled biosensing [Nag et al., 2020], (vii) real-time optomagnetic detection of SARS-CoV-2 following homogeneous circle-to-circle amplification [Tian et al., 2020b], (viii) SARS-CoV-2 IgM-IgG combined antibody test [Li et al., 2020b, https://creativecommons.org/licenses/by/4.0/]. (ix) enzymatic electrochemical detection of SARS [Draz and Shafiee, 2018, Creative Commons Attribution (CC BY-NC) license, and also credit to report by Martínez-Paredes et al., 2009].
Layqah et al., reported an electrochemical immunosensor prepared by using AuNPs modified carbon electrode and the recombinant spike protein S1 as the biomarker for the detection of MERS-CoV [Layqah and Eissa, 2019]. This immunosensor has been found to be capable of simultaneous detection of different coronavirus (CoVs) including MERS-CoV and human corona virus (HCoVs). This biosensor exhibits liner response as 0.001 to 100 ng.mL−1 and 0.01 to 10,000 ng.mL−1 for the detection of MERS-CoV and HCoV, respectively. And the detection limit was determined to be 1.0 and 0.4 pg.mL−1 for MERS-CoV and HCoV, respectively. This assay can be performed within 20 min and successfully employed to detect MERS-CoV and HCoVs protein in spiked nasal samples. Using AuNPs a label-free colorimetric biosensing approach has been reported for the detection of MERS-CoV [Kim et al., 2019]. This colorimetric assay is based on the detection of DNA that employs disulfide induced self-assembly and the MgCl2 salt-induced aggregation and prevention of AuNPs in the absence and presence of the target, respectively. The MgCl2 induced aggregation of AuNPs leads to change in the optical properties and thereby detects the presence of MERS-CoV. The LOD of the colorimetric assay was calculated to be 1 pmol/μL (6 × 1011 copies/μL), and the detection of MERS-CoV could be done within 10 min.
Ishikawa et al., reported a label-free In2O3 nanowires based FET biosensor that employed antibody mimic proteins (Fibronectin, Fn) to detect N protein, a biomarker of SARS [Ishikawa et al., 2009]. This FET biosensor worked on the impedimetric effect and was capable of detecting the N protein concentration at subnanomolar having a response time ~10 min. Martinez-Paredes et al., designed a genosensor using AuNPs modified screen-printed carbon electrodes for the detection of SARS virus [Martínez-Paredes et al., 2009]. This genosensor exhibited a linear response in the range of 2.5 to 50 pmol/L having LOD 2.5 pmol/L and sensitivity as 1.76 mA/pmol/L towards the biotinylated target. A technique based on the localized surface plasmon coupled fluorescence (LSPCF) fiber-optic enabled biosensing of the recombinant N-protein (SARS-CoV-N) using AuNPs has been reported [Huang et al., 2009]. This LSPCF fiber-optic enabled biosensor exhibited LOD of 1 pg/mL in the human serum with a linear response, 0.1 pg/mL to 1 ng/mL. Moreover, compared to the enzyme-linked immunosorbent assay (ELISA), the LSPCF fiber-optic biosensor exhibited 104-fold enhanced LOD and thus may be helpful for the early diagnosis of SARS infection. And it is anticipated that using such fiber optics based nanoenabled biosensing can detect the viral load as small as 106 particles/mL within 15 min [Murugan et al., 2020; Nag et al., 2020]. These studies suggest that nanomaterials-enabled biosensing can be potential approach for the early and rapid detection of the virus originated respiratory infections.
4. Biosensors for COVID-19 diagnosis
4.1. Gold nanostructures enabled-biosensing
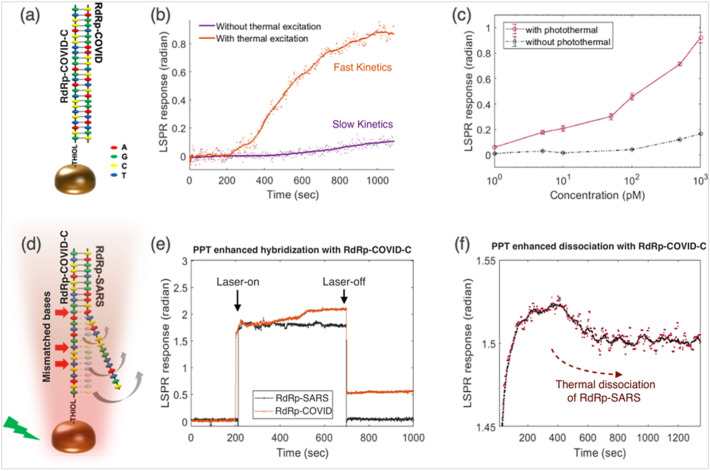
Owing to exceptional physicochemical properties, gold-based nanostructures have been widely employed for biomedical applications. Particularly, gold nanostructures have been exploited as the signal transducers in terms of optical signal amplifier, current amplifier, and resonance light scattering to fabricate biosensors for virus detection (Draz and Shafiee, 2018). A unique dual-functional biosensing platform based on plasmonic effect for the sensing of SARS-CoV-2 has been developed by Qiu et al. (Qiu et al., 2020). The developed biosensor exploits the combination of plasmonic photothermal (PPT) and the localized surface plasmon resonance (LSPR) effect. This biosensor was designed using two-dimensional gold nano-islands (AuNIs) [2D-AuNIs] chip following the self-assembly of thermally de-wetted gold (Au) nanofilm on the BK7 glass surface, wherein the Au-film was firstly prepared by magnetron-sputtering. Further, 2D-AuNIs were functionalized with the complementary DNA receptors, leading to sensitive detection of SARS-CoV-2 facilitated by nucleic acid hybridization. To enhance the sensing properties laser beam was allowed to fall at two different angles and hence it was possible to excite the plasmonic resonances of PPT and LSPR at two different wavelengths. In addition, the in situ PPT enhancement was recorded to significantly improve the hybridization kinetics and thus specific detection of nucleic acid, resulting in accurate discrimination between the different gene sequences [Fig. 4 ]. Thus, the developed biosensor was capable of real-time as well as label-free detection of desired virus sequences. The detection limit for the biosensor was calculated to be 0.22 pM [entire RNA strands ~2.26 × 104copies] (Qiu et al., 2020).
Fig. 4.
PPT enhancement in LSPR biosensing. (a) Schematic illustration of the hybridization of two complementary strands. (b) Real-time hybridization of RdRp-COVID and its cDNA sequence (RdRp-COVID-C) with or without the thermoplasmonic enhancement. (c) PPT enhancement on RdRp-COVID sequence detection at different concentrations. The error bars refer to the standard deviations of LSPR responses after reaching the steady conditions following the buffer flushing. (d) Schematic illustration of inhibited hybridization of two partially matched sequences. The red arrows indicated the mismatch bases of RdRp-SARS and functionalized cDNA of RdRp-COVID. (e) Discrimination of two similar sequences with PPT heat. The laser was applied at 200 s and switched off at 700 s. (f) RdRp-SARS sequence dissociation from the immobilized RdRp-COVID-C sequence. The original phase responses (red dots) and the corresponding smoothed means (black curve) are shown. [Reproduced with permission from: Qiu et al., 2020]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
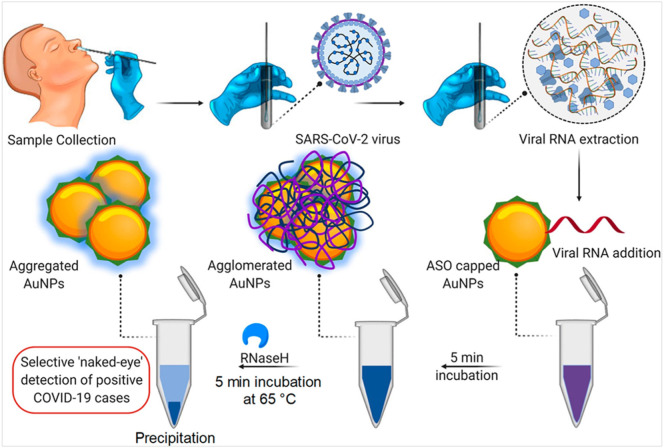
The colorimetric assay can be a simple and reliable approach for the naked-eye detection of viral infectious diseases, requiring no sophisticated technique. Using AuNPs capped with thiol-modified antisense oligonucleotides (ASOs) [AuNPs-ASOs], a colorimetric biosensing approach has been reported for the detection of SARS-CoV-2 (Moitra et al., 2020). The AuNPs-ASOs enabled biosensing was specific for the nucleocapsid phosphor protein (N-gene) from the RNA sample (oropharyngeal swab) and, therefore, can be utilized to detect the SARS-CoV-2 infection within 10 min. The colorimetric detection involved a phenomenon wherein AuNPs-ASOs nanostructures were selectively agglomerated in the presence of the targeted RNA sequence of SARS-CoV-2, leading to a red-shift in the UV-absorbance spectrum owing to the SPR effect. Further, it was found that the addition of RNaseH was responsible for cleaving the RNA strand from the RNA–DNA hybrid. And it was thus possible to visually detect precipitation from the solution due to additional agglomeration of AuNPs. Fig. 5 shows a schematic representation of the mechanism for the selective naked-eye detection of SARS-CoV-2. Moreover, the selectivity of the developed colorimetric assay was investigated towards the MERS-CoV viral RNA and the detection limit of 0.18 ng/μL for SARS-CoV-2 RNA were determined along with the dynamic range of 0.2–3 ng/μL (Moitra et al., 2020). In another study, a similar colorimetric dependent biosensing has been reported to detect RdRp gene of SARS-CoV-2 [Kumar et al., 2020]. This assay exhibited LOD of 0.5 ng for the SARS-CoV-2 RNA and was capable to detect SARS-CoV-2 infection in the human nasopharyngeal sample within 30 min. Moreover, the specificity of this colorimetric assay was investigated using a cervical DNA sample [obtained from Human papillomavirus infected women] exhibited selective detection of SARS-CoV-2.
Fig. 5.
Schematic representation for the Selective Naked-Eye Detection of SARS-CoV-2 RNA Mediated by the Suitably Designed ASO-Capped AuNPs [reproduced with permission from: Moitra et al., 2020].
A lateral flow immunoassay (LFIA) prepared by using AuNPs which simultaneously detected immunoglobulin M (IgM) and IgG antibodies of SARS-CoV-2 was reported (Li et al., 2020b). In this assay, the testing strip was prepared using the NC membrane, where anti-human-IgM, anti-human-IgG, and anti-rabbit-IgG (control) were immobilized at three different test lines. Thereafter, conjugation pad was sprayed using the mixture of AuNPs-COVID-19 recombinant antigen conjugate and AuNPs rabbit-IgG. This LIFA could detect the SARS-CoV-2 infection within 15 min using the human blood sample. Besides, owing to the capability of simultaneous detection of IgM and IgG, it is possible to diagnose COVID-19 cases in patients at different infection stages. Further, using the blood sample of the COVID19 patients, it was noticed that the LIFA exhibited good clinical detection sensitivity (88.66%) and specificity (90.63%), and therefore may be helpful to rapid detection of COVID-19 infections (Li et al., 2020b).
Mahari et al., reported Au-NPs based electrochemical biosensor to detect COVID-19 (spikeS1 protein) antigen (Mahari et al., 2020). This biosensor was prepared using fluorine-doped tin oxide (FTO) based substrate wherein Au-NPs were used as signal amplifier owing to very high electrical conductivity. To prepare the biosensing platform, firstly Au-NPs having size ~29 nm were drop cast, and subsequently, the monoclonal antibodies of COVID-19 [COVID-19Ab] were immobilized to fabricate FTO/AuNPs/COVID-19Ab immunosensor. The biosensing performance of the FTO/AuNPs/COVID-19Ab was also compared with COVID-19Ab immobilized screen-printed carbon electrode (SPCE). Further, electrochemical performances of both types of immunosensors were determined towards the specific COVID-19 antigen (spike protein) [COVID-19Ag]. The specific interaction between COVID-19 antibody(Ab) and COVID-19Ag led to the change in electrical conductivity and therefore increase in current was measured for varying concentrations of COVID-19Ag [1 f. to 1 μM]. The detection limit of FTO/AuNPs/COVID-19Ab immunosensor was determined to be 10 f. for COVID-19Ag detection. The interference study was also performed in the presence of different viral antigen (Ag) including HIV, JEV, or AIV and the observed results revealed that FTO/AuNPs/COVID-19Ab immunosensor had good selectivity towards the COVID-19Ag (spike protein). In addition, the repeatability test revealed that the FTO/AuNPs/COVID-19Ab immunosensor could be used up to 3 times, and could be utilized to detect COVID-19Ag concentration up to 120 fM. This immunosensor was found to be stable up to 21 days. Apart from this, the performance of the screen-printed carbon electrode (SPEC) based biosensor [SPCE/COVID-19Ab] was investigated using COVID-19Ag (spike protein) in spiked saliva sample. The SPCE/COVID-19Ab electrode-based sensing device exhibited limit of detection as 90 f. and the rapid response time of ~1 min. Thus, it could be used for the rapid diagnosis of COVID-19 patient (Mahari et al., 2020). Similarly, Xiang et al. in their study evaluated the biosensing efficiency of colloidal gold-immuno-chromatographic (GICA) and enzyme-linked immunoassay (ELISA) kit for the detection SARS-CoV-2 infection (Xiang et al., 2020). It was noticed that the GICA kit that relied on the combined detection of GICA-IgM and GICA-IgG (IgM and IgG antibodies) was 82.4% sensitive. On the other hand, ELISA kit showed a sensitivity of 87.3%. Moreover, both the GICA and ELISA kits were 100% specific in case of normal patients and no significant difference between these two kits could be noticed. This study suggests the feasibility of colloidal gold NPs to prepare immuno-chromatographic kit that may assist to relieve huge pressure of the clinical diagnosis of COVID-19 cases through rRT-PCR based technique.
4.2. Lanthanide-doped polysterene NPs enabled biosensing
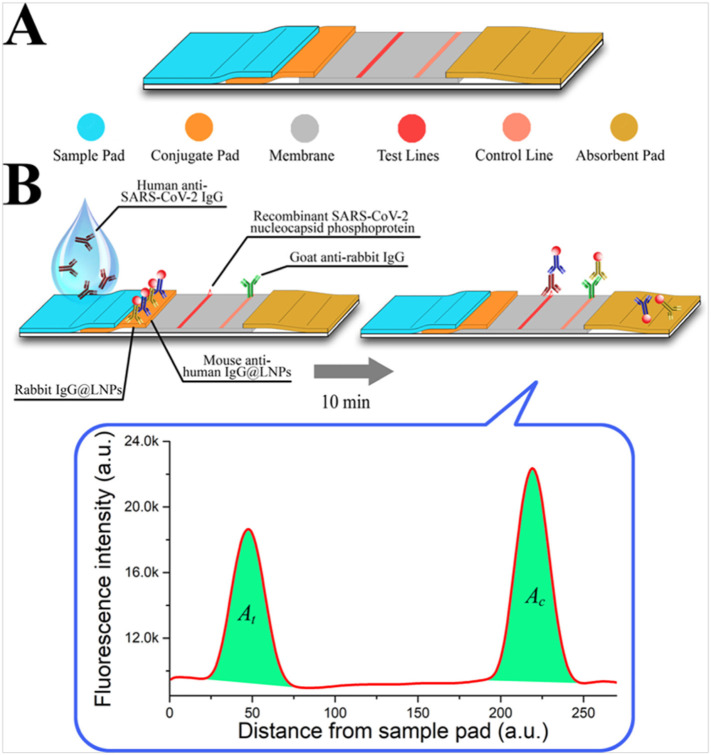
Lanthanides possess unique electronic configuration, enable lanthanide-doped NPs numerous interesting optical characteristics including long luminescence life-times, large and sharp emission bands. Specially, owing to their long luminescence life-times, lanthanide-doped NPs have been widely used for highly sensitive biosensing applications [Ma et al., 2019]. Using lanthanide-doped NPs, LFIA based biosensor has been reported as the point of care diagnostic for infectious agents [Banerjee and Jaiswal, 2018]. In a study, LFIA based biosensing for the diagnosis of SARS-CoV-2 has been developed by Chen el al. (Chen et al., 2020). The developed LFIA relies on the principle to detect anti-SARS-CoV-2 IgG in human serum sample. This biosensing platform was fabricated using lanthanide-doped polystyrene nanoparticles (LNPs), prepared by mini-emulsion polymerization method. Further, surface modifications of LNPs that acted as the fluorescent probe was done using mouse anti-human IgG antibody (MH-IgG) and rabbit IgG (R-IgG) following EDC/NHS chemical reaction. A nitrocellulose membrane was used as the template to immobilize a recombinant nucleocapsid phospho-protein of SARS-CoV-2, responsible to confine the specific IgG. Fig. 6 illustrates a schematic diagram, showing sequential steps for the fabrication process of LFIA. The fabricated LIFA could detect anti-SARV-CoV-2 IgG in human serum approximately in 10 min. Further, to validate the clinical application of LIFA, these authors compared results of the anti-SARS-CoV-2 IgG detection through the RT-PCR technique. It was noticed that the results through LIFA were the same as obtained through RT-PCR technique except for one sample, which showed the contrary result. Thus, the authors affirmed that though the developed LFIA did not yield accurate quantitative results due to non-availability of any anti-SARS-CoV-2 IgG standard, it can be of high interest for rapid diagnosis of the suspicious COVID-19 cases (Chen et al., 2020).
Fig. 6.
Design and fabrication of the developed LFIA assay. (A) Lateral flow test strip. (B) Assay. [Reproduced with permission from: Chen et al., 2020].
4.3. Graphene enabled-biosensing
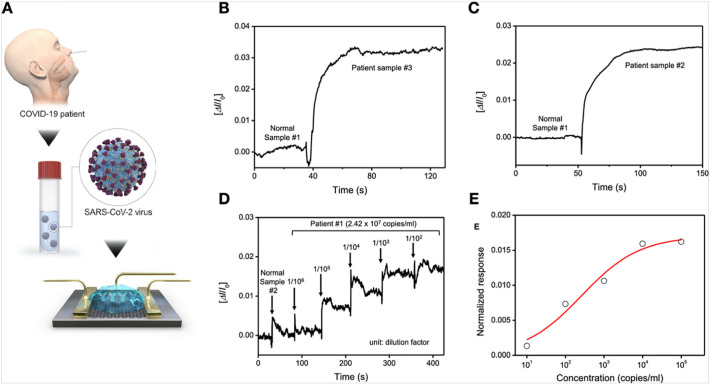
Graphene, 2D hexagonally arranged carbon based single atom thick layer has proven its worth towards the development of advanced biosensing platforms (Li et al., 2020a). This is mainly because of numerous exceptional properties such as high specific surface area, high electrical, and ionic mobility; thus, it has the potential to develop ultrasensitive biosensors (Peña-Bahamonde et al., 2018). By exploiting these properties of graphene, Seo et al. developed a field-effect transistor (FET) based biosensing platform to detect SARS-CoV-2 in human nasopharyngeal swabs (Seo et al., 2020). The FET-based biosensing platform was developed by transferring graphene onto the SiO2/Si substrate following the wet-transfer method. Further, this graphene based-FET device was possible to develop by using the photolithography technique. Subsequently, the graphene surface was coated with a specific antibody of SARS-CoV-2 spike protein to finally prepare the FET-based biosensing device. The clinical application of the FET-based biosensing device was investigated using different sample analytes e.g. antigen protein, cultured virus, and nasopharyngeal swab of COVID-19 patients. Detection of SARS-CoV-2 spike protein was measured at the concentration of 1 fg/mL in phosphate-buffered saline whereas 100 fg/mL in case of the universal transport medium. This graphene-based FET sensor exhibited LOD, 1.6 × 101 pfu/mL, and 2.42 × 102 copies/mL in case of SARS-CoV-2 spiked culture and clinical samples, respectively [Fig. 7 ]. It may be noted that there was no measurable interference that could be detected with MERS-CoV antigen, suggesting practical application for diagnosis of COVID-19 patients (Seo et al., 2020).
Fig. 7.
Detection of SARS-CoV-2 virus from clinical samples. (A) Schematic diagram for the COVID-19 FET sensor for detection of SARS-CoV-2 virus from COVID-19 patients. (B, C) Comparison of response signal between normal samples and patient ones. (D) Real-time response of COVID-19 FET towards SARS-CoV-2 clinical sample and (C) related dose-dependent response curve. [Reproduced with permission from: Seo et al., 2020].
4.4. Magnetic nanoparticles enabled biosensing
Magnetic nanoparticles (MNPs) have emerged as promising candidates to develop biosensors for the detection of the deadly respiratory viral infections [Islam and Ahsan, 2020; Barnett et al., 2020]. Using MNPs different biosensing techniques have been developed such as magnetic resonance (NMR/MRI) [Perez et al., 2003], fluorescence [Zheng et al., 2020], electrochemical [Khan et al., 2020], and rolling circle amplification [Tian et al., 2020a; Tian et al., 2020b]. Further, compared to PCR-based DNA diagnosis, rolling circle amplification (RCA) is a simple and powerful technique. In case of RCA, amplification takes place in the isothermal conditions, utilizing DNA or RNA polymerases and thereby produces ssDNA or RNA molecules. Further, a typical RCA-based cascade amplification known as circle to circle amplification (C2CA) are employed for the analysis of nucleic acid and single molecule detection [Tian et al., 2020a, Tian et al., 2020b]. Tian et al., reported a unique strategy for the quantification of nucleic acid with the help of integrated homogeneous and isothermal C2CA and optomagnetic analysis of iron oxide NPs (IONPs) assembly [Tian et al., 2020b]. In this approach, the end amplicons (ssDNA) resulting from the homogeneous circle-to-circle amplification (HC2CA) process hybridize with the detection probes which was grafted onto IONPs. This phenomenon leads to the assembly of IONPs. Since an individual IONPs and the assembly of IONPs exhibits the specific properties towards the scattering/absorption of light, it varies with the rotation of such types of magnetic objects under the influence of an external time varying magnetic field. By exploiting this effect, an optomagnetic biosensing approach was employed for the analysis of IONPs state in the HC2CA suspension, and thus it was possible to establish a real-time volumetric sensing system. Further, biosensing capability of this technique was investigated towards the detection of RdRp sequence (synthetic complementary DNA of SARS-CoV-2). This technique exhibited LOD 0.4 f. having total assay time ~100 min along with a dynamic detection range of 3 order of magnitude. This technique was found to be specific to differentiate SARS-CoV-2 and SARS-CoV viral sequences [Tian et al., 2020b]. Zhao et al., synthesized carboxyl polymer coated magnetic NPs (pcMNPs) and employed this nano-system for efficient RNA extraction to detect COVID-19 infection [Zhao et al., 2020]. The pcMNPs based nano-system exhibited the combined properties including virus lysis and RNA binding in a single step. Thus, resulting pcMNPs-RNA complex could be directly used for the subsequent RT-PCR reactions. Further, with the identification of ORFlab and N gene of the viral RNA, the pcMNPs enabled RT-PCR based biosensing was found to be 10-copy sensitive, and having a linearity of 10-105 copies of the SARS-CoV-2 pseudovirus particles. This study suggests that the pcMNPs-enabled RNA extraction approach can be a promising alternative to overcome issues in the rapid diagnosis of SARS-CoV-2 through RT-PCR based technique. Table 1 summarizes the characteristics of the nanomaterials-based biosensors, reported for SARS-CoV-2 detection.
Table 1.
Characteristics of nanomaterials enabled biosensing for SARS-CoV-2 virus detection.
| Core nanomaterials | Biomarker | Principle | Sample analytes/type of sample | Characteristics/remarks | Ref. |
|---|---|---|---|---|---|
| Gold NPs | Thiol-c-DNA receptors/nucleic acid | Combined effect of plasmonic photothermal (PPT) and localized surface plasmon resonance (LSPR) mediated biosensing | ORF1ab-COVID, and E genes from SARS-CoV-2; viral sequences including RdRp-COVID | Low detection limit: 0.22 ± 0.08 pM [2.26 × 107 copies of the RdRp-COVID sequence whereas ~2.26 × 104 copies of the entire RNA strands from SARS-CoV-2]; detection range: 0.1 pM to 1 μM | [Qiu et al., 2020] |
| Gold NPs | Thiol-modified antisense oligonucleotides (ASOs) specific forN-gene (nucleocapsid phosphoprotein) of SARS-CoV-2 | Plasmonic effect based colorimetric biosensing | RNA [N-gene (nucleocapsid phosphoprotein)of SARS-CoV-2]; oropharyngeal swab | Detection limit: 0.18 ng/μL; detection range: 0.2–3 ng/μL; assay time was found to be 10 min | [Moitra et al., 2020] |
| Gold NPs | Oligo probe | Plasmonic effect based colorimetric biosensing | RNA [RdRp gene of SARS-CoV-2];human nasopharyngeal sample | Detection limit: 0.5 ng; total assay time was found to be ~30 min | [Kumar et al., 2020] |
| Gold NPs(colloid) | Recombinant antigen of SARS-CoV-2 and rabbit-IgG | Colorimetric dependent lateral flow immunoassay based biosensing | IgM and IgG antibodies; human blood sample | Assay time was found to be 15 min | [Li et al., 2020b] |
| Lanthanide-doped polysterene NPs | Protein [mouse anti-human IgG antibody] | Lateral flow immunoassay based on fluorescence biosensing | anti-SARS-CoV-2 IgG in positive sample, human serum sample | Assay time was determined to be 10 min; assay was reproducible | [Chen et al., 2020] |
| Graphene | Spike protein antibody | Field-effect transistor-based amperometric biosensing | SARS-CoV-2 antigen protein and MERS-CoV protein; nasopharyngeal swabs | FET based biosensing device could detect the SARSCoV-2 spike protein at concentrations of 1 fg/mL in phosphate-buffered saline and 100 fg/mL in clinical transport medium; limit of detection: 1.6 × 101 pfu/mL in culture medium whereas 2.42 × 102 copies/mL in clinical samples | [Seo et al., 2020] |
| Gold NPs | nCOVID-19 monoclonal antibody | Amperometric biosensing | COVID-19 spike antigen in saliva samples | Limit of detection: 10 fM; sensitive for the detection of COVID-19Ag, ranging from 1 f. to 1 μM; fabricated Au NPs based immunosensor could be used up to 3 times without major changes in peak current and could detect concentration up to 120 fM; stability up to 21 days | [Mahari et al., 2020] |
| Screen printed carbon electrode (SPCE) based sensing device | nCOVID-19 monoclonal antibody | Amperometric biosensing | COVID-19Ag spiked saliva sample | Detection of COVID-19Ag at 10 f. concentration in standard buffer sample; limit of detection was found to be 90fM in case of spiked saliva samples using SPCE mediated homemade biosensor device; detection of COVID-19Ag traces in patient saliva within 1 min; stability up to 28 days | [Mahari et al., 2020] |
| Iron oxide NPs | Biotinylated probe | Circle to circle amplification based optomagnetic biosensing | RdRp sequence (synthetic complementary DNA of SARS-CoV-2) | Limit of detection was found to be 0.4 fM; total assay time ~100 min; dynamic detection range having 3 order of magnitude | [Tian et al., 2020b] |
| Polymer nanoparticles coated with dye streptavidin (Crimson red) | Rabbit anti-fluorescein antibody, sheep anti-digoxigenin antibody, and biotinylated bovine serum albumin | Multiplex reverse transcription loop mediated isothermal amplification (mRT-LAMP) coupled with a NPs-based lateral flow biosensor (LFB) assay [mRT-LAMP-LFB] | ORF1ab and N genes of SARS-CoV-2; oropharynx swab | Limit of detection was found to be 12 copies (for each detection target) per reaction; total assay time ~60 min; analytical sensitivity 100%; specificity 100% | [Zhu et al., 2020] |
| Iron oxide NPs | Poly (amino ester) with carboxyl groups (PC)-coated MNPs (pcMNPs) | pcMNPs enabled improved RNA extraction and RT-PCR based biosensing | SARS-CoV-2 virus RNA | 10 copies sensitive; linear correlation 10 to 105 copies of SARS-CoV-2 pseudovirus particles | [Zhao et al., 2020] |
5. Conclusions and future prospects
The pandemic of COVID-19 has motivated researchers to make efforts towards the development of an advanced approach that must be highly efficient and should be capable of responding to the present demand of early diagnosis to manage this global issue. Since there is no explicit technique currently available for the treatment of COVID-19, therefore the management of this pandemic is only possible through detection, monitoring, and prevention of SARS-CoV-2 infection in time. Moreover, the asymptomatic cases of COVID-19 have made this issue more complex and, therefore, it is necessary to invent a rapid and low-cost technique for the early diagnosis of this suspicious infection at mass levels to discriminate against the infected and non-infected cases. In this context, since the nanomaterials-based biosensors have already shown their potential towards the diagnosis of numerous viral infections and hence may perhaps fulfill the current demand for early diagnosis of COVID-19 cases. And it appears that different types of nanostructures such as gold, lanthanide-doped polysterene NPs, graphene and IONPs could be successfully employed for the development of advanced biosensing techniques for the diagnosis of SARS-CoV-2. Besides, using these NPs, a number of methods including amperometric, impedimetric, colorimetric, magnetic and optomagnetic mediated biosensing have been established. These methods can be utilized to detect the SARS-CoV-2 within 10 min to 100 min and therefore may assist in the early and rapid diagnosis of COVID-19 cases. Though, limited studies on the development of nanomaterials-based biosensors for SARS-CoV-2 detection have been reported, these nanomaterials enabled biosensing techniques offer alternative approaches to PCR-based testing for COVID-19 infection. Moreover, under the outbreak of COVID-19, these nanomaterials-based biosensing can be imperative to deliver the requirement of easy, low-cost, rapid and real-time diagnosis technique. Thus, the nanomaterials-based biosensing is likely to reduce the stress on RT-PCR-based costly testing of COVID-19 cases. Further, by employing nanomaterials based biosensing devices as plug-and-play diagnostics approach are expected to be highly useful to fight against this pandemic.
It is predicted that the reported nanomaterials-enabled biosensors mainly rely on nucleic acid (RNA/DNA) and protein (antigen/antibody) mediated detection of SARS-CoV-2. Nevertheless, these techniques have not yet yielded 100% accuracy owing to the contamination of these highly sensitive bioreceptors. And even a single false result would be highly challenging and can spoil the efforts to overcome this pandemic. Thus, other alternatives including nanomaterials-mediated biosensing technique based on CRISPR can perhaps be explored to detect SARS-CoV-2 should be investigated (Lucia et al., 2020). In particular, nanomaterials -based biosensors that rely on the aerosol mediated diagnosis approach can be significant and are likely to offer numerous advantages e.g. fast response, sensitivity, as well as lack in sampler perturbation (Huffman et al., 2020). Besides, this, at lab-scale it is possible to achieve a low detection limit, good stability, and fast response time, mainly because of surface modification of sensing electrodes following several complex approaches. Nevertheless, this may differ in case of the real sample analysis and the parameters including LOD, stability, and response time may not be as much promising. The sensitivity and selectivity of the biosensing platform are highly dependent upon various factors like characteristics of the antigen, antibody, protein, types of nanomaterials, and other biomolecules. These factors actively participate and, therefore, can significantly influence the overall performance of the nanomaterials based biosensing devices. Therefore, a deep theoretical and experimental knowledge about these factors is highly demanding and is needed to be rigorously researched for the development of advanced nanomaterials enabled biosensing devices for SARS-CoV-2 detection. Moreover, technically it should be beneficial to develop universal nanomaterials-based biosensors that can be highly efficient to detect SARS-CoV-2 infection in different clinical samples e.g. urine, blood, saliva, and nasopharyngeal swabs. Finally, since only a few studies have been performed on the applications of nanomaterials-enabled biosensing for SARS-CoV-2, we are at very early stage of the nanomaterials-enabled biosensing technology. Therefore, efforts should be made to advance this approach to harvest the rich dividend in the near future to combat the battle against COVID-19 pandemic.
CRediT authorship contribution statement
Manish Srivastava: Conceptualization, Writing - original draft. Neha Srivastava: Writing - original draft. P.K. Mishra: Writing - review & editing. Bansi D. Malhotra: Conceptualization, Writing - review & editing.
Declaration of competing interest
Authors of the manuscript declare there is no conflict of interests.
Acknowledgments
Author M.S. acknowledges the Science and Engineering Research Board for SERB Research Scientist award and also to DST for DST INSPIRE Faculty award [IFA-13-MS-02]. Author N.S. thankfully acknowledges the Department of Chemical Engineering and Technology, IIT (BHU) Varanasi for providing Post-Doctoral Fellowship. Author N.S. and P.K.M. acknowledge the Department of Chemical Engineering and Technology, IIT (BHU) Varanasi for providing the experimental facilities. Author BDM thanks Science and Engineering Research Board for the award of a Distinguished Fellowship (SB/DF/011/2019). Authors of the manuscript also acknowledge https://pixabay.com/ for different vector images to design the graphical abstract.
References
- Alifano M., Alifano P., Forgez P., Iannelli A. Renin-angiotensin system at the heart of COVID-19 pandemic. Biochimie. 2020;174:30–33. doi: 10.1016/j.biochi.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine S., Singh J., Srivastava M., Sharma M., Das A., Malhotra B.D. Recent advances in carbon based nanosystems for cancer theranostics. [10.1039/C7BM00008A] Biomaterials Science. 2017;5(5):901–952. doi: 10.1039/c7bm00008a. [DOI] [PubMed] [Google Scholar]
- Bai Y., Yao L., Wei T., Tian F., Jin D.-Y., Chen L., Wang M. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R., Jaiswal A. Recent advances in nanoparticle-based lateral flow immunoassay as a point-of-care diagnostic tool for infectious agents and diseases. [10.1039/C8AN00307F] Analyst. 2018;143(9):1970–1996. doi: 10.1039/c8an00307f. [DOI] [PubMed] [Google Scholar]
- Barnett J.M., Monnier B.M., Tyler S., West D., Ballantine-Dykes H., Regan E.…Luxton R. Initial trail results of a magnetic biosensor for the rapid detection of Porcine Reproductive and Respiratory Virus (PRRSV) infection. Sensing and Bio-Sensing Research. 2020;27 doi: 10.1016/j.sbsr.2019.100315. [DOI] [Google Scholar]
- Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N.…Chung M. Chest CT Findings in Coronavirus disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020;295(3) doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J.…Chiu C.Y. CRISPR–Cas12-based detection of SARS-CoV-2. Nature Biotechnology. 2020 doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L.J., Garner L.V., Smoot J.W., Li Y., Zhou Q., Saveson C.J.…Liu C. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Central Science. 2020;6(5):591–605. doi: 10.1021/acscentsci.0c00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M, Rajnik M, Cuomo A, et al. Features, Evaluation, and Treatment of Coronavirus (COVID-19) [Updated 2020 Aug 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554776/. [PubMed]
- Cesewski E., Johnson B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020;159 doi: 10.1016/j.bios.2020.112214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z., Zhang, Z., Zhai, X., Li, Y., Lin, L., Zhao, H., … Lin, G. (2020). Rapid and sensitive detection of anti-SARS-CoV-2 IgG, using lanthanide-doped nanoparticles-based lateral flow immunoassay. Analytical Chemistry. doi: 10.1021/acs.analchem.0c00784. [DOI] [PubMed]
- Chu D.K.W., Pan Y., Cheng S.M.S., Hui K.P.Y., Krishnan P., Liu Y.…Poon L.L.M. Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clinical Chemistry. 2020;66(4):549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui F., Zhou H.S. Diagnostic methods and potential portable biosensors for coronavirus disease 2019. Biosens. Bioelectron. 2020;165 doi: 10.1016/j.bios.2020.112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draz M.S., Shafiee H. Applications of gold nanoparticles in virus detection. [review] Theranostics. 2018;8(7):1985–2017. doi: 10.7150/thno.23856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin L., Shamsipur M., Samandari L., Sheibani S. HIV biosensors for early diagnosis of infection: the intertwine of nanotechnology with sensing strategies. Talanta. 2020;206 doi: 10.1016/j.talanta.2019.120201. [DOI] [PubMed] [Google Scholar]
- Gandhi S., Srivastava A.K., Ray U., Tripathi P.P. Is the collapse of the respiratory center in the brain responsible for respiratory breakdown in COVID-19 patients? ACS Chem. Neurosci. 2020 doi: 10.1021/acschemneuro.0c00217. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A.…Coronaviridae Study Group of the International Committee on Taxonomy of, V The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni, A., Sidney, J., Zhang, Y., Scheuermann, R. H., Peters, B., & Sette, A. (2020). A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host & Microbe, 27(4), 671–680.e672. doi: 10.1016/j.chom.2020.03.002. [DOI] [PMC free article] [PubMed]
- Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020;158(6):1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W.-J., Ni, Z.-Y., Hu, Y., Liang, W.-H., Ou, C.-Q., He, J.-X., … Zhong, N.-S. (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine, 382(18), 1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed]
- Huang, J. C., Chang, Y.-F., Chen, K.-H., Su, L.-C., Lee, C.-W., Chen, C.-C., … Chou, C. (2009). Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in human serum using a localized surface plasmon coupled fluorescence fiber-optic biosensor. Biosensors and Bioelectronics, 25(2), 320–325. doi: 10.1016/j.bios.2009.07.012. [DOI] [PMC free article] [PubMed]
- Huffman J.A., Perring A.E., Savage N.J., Clot B., Crouzy B., Tummon F.…Pan Y. Real-time sensing of bioaerosols: Review and current perspectives. Aerosol Science and Technology. 2020;54(5):465–495. doi: 10.1080/02786826.2019.1664724. [DOI] [Google Scholar]
- Ishikawa F.N., Chang H.-K., Curreli M., Liao H.-I., Olson C.A., Chen P.-C.…Zhou C. Label-Free, Electrical Detection of the SARS Virus N-Protein with Nanowire Biosensors Utilizing Antibody Mimics as Capture Probes. ACS Nano. 2009;3(5):1219–1224. doi: 10.1021/nn900086c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Aminul, Md., Ahsan Ziaul., Md. Plausible approach for rapid detection of SARS-CoV-2 virus by magnetic nanoparticle based biosensors. Am. J. Nanosci. 2020;6(2):6–13. doi: 10.11648/j.ajn.20200602.11. http://www.sciencepublishinggroup.com/j/ajn. [DOI] [Google Scholar]
- Khan M.Z.H., Hasan M.R., Hossain S.I., Ahommed M.S., Daizy M. Ultrasensitive detection of pathogenic viruses with electrochemical biosensor: state of the art. Biosens. Bioelectron. 2020;166 doi: 10.1016/j.bios.2020.112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Park M., Hwang J., Kim J.H., Chung D.-R., Lee K.-s., Kang M. Development of label-free colorimetric assay for MERS-CoV using gold nanoparticles. ACS Sensors. 2019;4(5):1306–1312. doi: 10.1021/acssensors.9b00175. [DOI] [PubMed] [Google Scholar]
- Kokkinos C. Electrochemical DNA biosensors based on labeling with nanoparticles. Nanomaterials. 2019;9(10) doi: 10.3390/nano9101361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, V., Mishra, S., Sharma, R., Agarwal, J., Ghoshal, U., Khanna, T., … Tiwari, S. (2020). Development of RNA-based assay for rapid detection of SARS-CoV-2 in clinical samples. bioRxiv, 2020.2006.2030.172833. doi: 10.1101/2020.06.30.172833. [DOI] [PMC free article] [PubMed]
- Lamers, M. M., Beumer, J., van der Vaart, J., Knoops, K., Puschhof, J., Breugem, T. I., … Clevers, H. (2020). SARS-CoV-2 productively infects human gut enterocytes. Science, eabc1669. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed]
- Layqah L.A., Eissa S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchim. Acta. 2019;186(4):224. doi: 10.1007/s00604-019-3345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc J.J., Gubbay J.B., Li Y., Needle R., Arneson S.R., Marcino D.…Bastien N. Real-time PCR-based SARS-CoV-2 detection in Canadian laboratories. Journal of Clinical Virology. 2020;128 doi: 10.1016/j.jcv.2020.104433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Park S.Y., Jang H., Kim G.-H., Lee Y., Park C.…Min J. Fabrication of electrochemical biosensor consisted of multi-functional DNA structure/porous au nanoparticle for avian influenza virus (H5N1) in chicken serum. Materials Science and Engineering: C. 2019;99:511–519. doi: 10.1016/j.msec.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Li Y., Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. Am. J. Roentgenol. 2020:1–7. doi: 10.2214/ajr.20.22954. [DOI] [PubMed] [Google Scholar]
- Li S., Ma L., Zhou M., Li Y., Xia Y., Fan X.…Luo H. New opportunities for emerging 2D materials in bioelectronics and biosensors. Current Opinion in Biomedical Engineering. 2020;13:32–41. doi: 10.1016/j.cobme.2019.08.016. [DOI] [Google Scholar]
- Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Ye F. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020 doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H.…Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucia, C., Federico, P.-B., & Alejandra, G. C. (2020). An ultrasensitive, rapid, and portable coronavirus SARS-CoV-2 sequence detection method based on CRISPR-Cas12. bioRxiv, 2020.2002.2029.971127. doi: 10.1101/2020.02.29.971127. [DOI]
- Ma Q., Wang J., Li Z., Lv X., Liang L., Yuan Q. Recent progress in time-resolved biosensing and bioimaging based on lanthanide-doped nanoparticles. Small. 2019;15(32) doi: 10.1002/smll.201804969. [DOI] [PubMed] [Google Scholar]
- Maduraiveeran G., Sasidharan M., Ganesan V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018;103:113–129. doi: 10.1016/j.bios.2017.12.031. [DOI] [PubMed] [Google Scholar]
- Mahapatra S., Chandra P. Clinically practiced and commercially viable nanobio engineered analytical methods for COVID-19 diagnosis. Biosens. Bioelectron. 2020;165 doi: 10.1016/j.bios.2020.112361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahari, S., Roberts, A., Shahdeo, D., & Gandhi, S. (2020). eCovSens-ultrasensitive novel in-house built printed circuit board based electrochemical device for rapid detection of nCovid-19 antigen, a spike protein domain 1 of SARS-CoV-2. bioRxiv, 2020.2004.2024.059204. doi: 10.1101/2020.04.24.059204. [DOI]
- Malhotra Bansi, Ali Md. Azahar. Elsevier; 2018. Book: “Nanomaterials for Biosensors”, Fundamentals and Applications in Micro and Nano Technologies. [DOI] [Google Scholar]
- Martínez-Paredes G., González-García M.B., Costa-García A. Genosensor for SARS virus detection based on gold nanostructured screen-printed carbon electrodes. Electroanalysis. 2009;21(3–5):379–385. doi: 10.1002/elan.200804399. [DOI] [Google Scholar]
- Moitra P., Alafeef M., Dighe K., Frieman M.B., Pan D. Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano. 2020 doi: 10.1021/acsnano.0c03822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Narváez E., Dincer C. The impact of biosensing in a pandemic outbreak: COVID-19. Biosens. Bioelectron. 2020;163 doi: 10.1016/j.bios.2020.112274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan D., Bhatia H., Sai V.V.R., Satija J. P-FAB: a fiber-optic biosensor device for rapid detection of COVID-19. Transactions of the Indian National Academy of Engineering. 2020;5(2):211–215. doi: 10.1007/s41403-020-00122-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag P., Sadani K., Mukherji S. Optical fiber sensors for rapid screening of COVID-19. Transactions of the Indian National Academy of Engineering. 2020;5(2):233–236. doi: 10.1007/s41403-020-00128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narang J., Singhal C., Mathur A., Sharma S., Singla V., Pundir C.S. Portable bioactive paper based genosensor incorporated with Zn-Ag nanoblooms for herpes detection at the point-of-care. Int. J. Biol. Macromol. 2018;107:2559–2565. doi: 10.1016/j.ijbiomac.2017.10.146. [DOI] [PubMed] [Google Scholar]
- Negahdari B., Darvishi M., Saeedi A.A. Gold nanoparticles and hepatitis B virus. Artificial Cells, Nanomedicine, and Biotechnology. 2019;47(1):469–474. doi: 10.1080/21691401.2018.1546185. [DOI] [PubMed] [Google Scholar]
- Ozer T., Geiss B.J., Henry C.S. Review-chemical and biological sensors for viral detection. J. Electrochem. Soc. 2020;167(3):037523. doi: 10.1149/2.0232003jes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri V., Papi M. Can graphene take part in the fight against COVID-19? Nano Today. 2020 doi: 10.1016/j.nantod.2020.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomar Q., Gondran C., Marks R., Cosnier S., Holzinger M. Impedimetric quantification of anti-dengue antibodies using functional carbon nanotube deposits validated with blood plasma assays. Electrochim. Acta. 2018;274:84–90. doi: 10.1016/j.electacta.2018.04.099. [DOI] [Google Scholar]
- Peña-Bahamonde J., Nguyen H.N., Fanourakis S.K., Rodrigues D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. Journal of Nanobiotechnology. 2018;16(1):75. doi: 10.1186/s12951-018-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez J.M., Simeone F.J., Saeki Y., Josephson L., Weissleder R. Viral-induced self-assembly of magnetic nanoparticles allows the detection of viral particles in biological media. J. Am. Chem. Soc. 2003;125(34):10192–10193. doi: 10.1021/ja036409g. [DOI] [PubMed] [Google Scholar]
- Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020 doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- Ravina, Dalal, A., Mohan, H., Prasad, M., & Pundir, C. S. (2020). Detection methods for influenza A H1N1 virus with special reference to biosensors: a review. Bioscience Reports, 40(BSR20193852). doi: 10.1042/bsr20193852. [DOI] [PMC free article] [PubMed]
- Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C.…Hoelscher M. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. New England Journal of Medicine. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylan Y., Erdem Ö., Ünal S., Denizli A. An alternative medical diagnosis method: biosensors for virus detection. Biosensors. 2019;9(2) doi: 10.3390/bios9020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, G., Lee, G., Kim, M. J., Baek, S.-H., Choi, M., Ku, K. B., … Kim, S. I. (2020). Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano, 14(4), 5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed]
- Shen M., Zhou Y., Ye J., Abdullah Al-maskri A.A., Kang Y., Zeng S., Cai S. Recent advances and perspectives of nucleic acid detection for coronavirus. Journal of Pharmaceutical Analysis. 2020;10(2):97–101. doi: 10.1016/j.jpha.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuzdak K., Niedziałkowski P., Sobaszek M., Łęga T., Sawczak M., Czaczyk E.…Bogdanowicz R. Biomolecular influenza virus detection based on the electrochemical impedance spectroscopy using the nanocrystalline boron-doped diamond electrodes with covalently bound antibodies. Sensors and Actuators B: Chemical. 2019;280:263–271. doi: 10.1016/j.snb.2018.10.005. [DOI] [Google Scholar]
- Tahamtan A., Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert. Rev. Mol. Diagn. 2020;20(5):453–454. doi: 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Fock J., Minero G.A.S., Hansen M.F. Nicking-assisted on-loop and off-loop enzymatic cascade amplification for optomagnetic detection of a highly conserved dengue virus sequence. Biosens. Bioelectron. 2020;160 doi: 10.1016/j.bios.2020.112219. [DOI] [PubMed] [Google Scholar]
- Tian B., Gao F., Fock J., Dufva M., Hansen M.F. Homogeneous circle-to-circle amplification for real-time optomagnetic detection of SARS-CoV-2 RdRp coding sequence. Biosens. Bioelectron. 2020;165 doi: 10.1016/j.bios.2020.112356. [DOI] [PubMed] [Google Scholar]
- Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y.C.…Chan W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano. 2020;14(4):3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- van Kasteren P.B., van der Veer B., van den Brink S., Wijsman L., de Jonge J., van den Brandt A.…Meijer A. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. Journal of Clinical Virology. 2020;128 doi: 10.1016/j.jcv.2020.104412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Yao H., Xu X., Zhang P., Zhang M., Shao J.…Wang H. Limits of Detection of 6 Approved RT–PCR Kits for the Novel SARS-Coronavirus-2 (SARS-CoV-2) Clinical Chemistry. 2020;66(7):977–979. doi: 10.1093/clinchem/hvaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, J., Yan, M., Li, H., Liu, T., Lin, C., Huang, S., & Shen, C. (2020). Evaluation of enzyme-linked immunoassay and colloidal gold-immunochromatographic assay kit for detection of novel coronavirus (SARS-Cov-2) causing an outbreak of pneumonia (COVID-19). medRxiv, 2020.2002.2027.20028787. doi: 10.1101/2020.02.27.20028787. [DOI]
- Xiao, F., Tang, M., Zheng, X., Liu, Y., Li, X., & Shan, H. (2020). Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology, 158(6), 1831–1833.e1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed]
- Yang, Y., Lu, Q., Liu, M., Wang, Y., Zhang, A., Jalali, N., … Fang, L. (2020). Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv, 2020.2002.2010.20021675. doi: 10.1101/2020.02.10.20021675. [DOI]
- Zhang Y.-Z., Holmes E.C. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020;181(2):223–227. doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z., Cui, H., Song, W., Ru, X., Zhou, W., & Yu, X. (2020). A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. bioRxiv, 2020.2002.2022.961268. doi: 10.1101/2020.02.22.961268. [DOI]
- Zheng X., Zhao L., Wen D., Wang X., Yang H., Feng W., Kong J. Ultrasensitive fluorescent detection of HTLV-II DNA based on magnetic nanoparticles and atom transfer radical polymerization signal amplification. Talanta. 2020;207 doi: 10.1016/j.talanta.2019.120290. [DOI] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W.…Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Wang X., Han L., Chen T., Wang L., Li H.…Wang Y. Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19. Biosensors and Bioelectronics. 2020;166 doi: 10.1016/j.bios.2020.112437. [DOI] [PMC free article] [PubMed] [Google Scholar]