Abstract
Background
The incidence of pain and inflammation in West Africa and in fact globally, continues to increase at an alarming rate. This research was conducted to investigate the analgesic and anti-inflammatory activities of leaf extracts of Chasmanthera dependens and Chenopodium ambrosioides; formulate and evaluate polyherbal gels from their combination in a bid to providing topical therapeutic solutions to pain and inflammation.
Methods
Pre-formulation studies (phytochemical analysis, in vitro analgesic and anti-inflammatory activities) were conducted on the methanol leaf extracts of Chasmanthera dependens and Chenopodium ambrosioides. Individual and polyherbal gels were prepared using polymer carbopol 940 (1%) at combination ratios of 0:100, 25:75, 50:50, 75:25 and 100:0 Chasmanthera:Chenopodium. These herbal gels were evaluated for physical parameters, pH, viscosity, extrudability and spreadability. Analgesic and anti-inflammatory activities of herbal gels were evaluated by their inhibitory activities (percentage inhibition) against COX-2, TNF-α, IL-10, PGE-2 and compared with commercial diclofenac gel.
Results
The phytochemicals of the two extracts detected gave varied contents of major classes of secondary metabolites. The pre formulation inhibitory studies of the two extracts exhibited dose dependent inhibitory activities against COX-2, TNF-α, IL-10, PGE-2. The physical appearance, homogeneity, and consistency of the herbal formulations were good. The herbal gels were spreadable with good extrudability. The pH of the herbal gels ranged from 4.5 ± 0.4 to 5.2 ± 0.4. The viscosity of the herbal gels ranged between 4.3 ± 0.2 and 4.7 ± 0.4 Pas. The herbal gels exhibited significant differences in inhibitory activities against COX-2, TNF-α, IL-10, PGE-2 when compared with control commercial diclofenac gel.
Conclusion
The outcomes, including the inhibition of mediators COX-2, TNF-α, IL-10, PGE-2, confirm the use of the plant extracts under study, the individual and polyherbal gels formulated for the potential topical therapeutic treatment of pain and inflammation.
Keywords: Pain, Inflammation, Polyherbal, Carbopol gel, Chasmanthera dependens, Chenopodium ambrosioides, Biological sciences, Plant biology, Pharmaceutical science, Health sciences, Pharmacology, Alternative medicine
Pain; Inflammation; Polyherbal; Carbopol Gel; Chasmanthera Dependens; Chenopodium Ambrosioides; Biological Sciences; Plant Biology; Pharmaceutical Science; Health Sciences; Pharmacology; Alternative Medicine.
1. Introduction
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage. Inflammation is the tissue's immunologic response to injury, characterized by mobilization of white blood cells and antibodies, swelling, and fluid accumulation according to the International Association for the Study of Pain (Merskey and Bogduk, 1994). Various cytokines interleukin (IL)-1, IL-6, IL-8, IL-10, tumor necrosis factor-alpha (TNF-α), and mediators such as prostaglandins, reactive oxygen species and nitric oxide are released which mediate the inflammatory process and tissue damage (Shinwan et al., 2019). In addition, prostaglandin E2 (PGE2), a major pain enhancing inflammatory mediator can be induced by cyclooxygenase 2 (COX-2) in the process of inflammation. NIH, 2013, cited pain as the most common reason for access to healthcare systems (NIH, 2013). It has been identified as a major cause of disability and a major influence of healthcare costs. Approximately 76.2 million, one in four Americans, have suffered from pain that lasts longer than 24 h and millions more suffer from acute pain.
The human skin is a barrier between the human body and the external environment. It is a protective organ with three layers: epidermis, dermis, and hypodermis. Lipids of the cornified layer are important in selective absorption of compounds from the skin surface. Drug absorption occurs through the epidermis and through the skin appendages. The primary route is through the epidermis (Boer et al., 2016).
Topical analgesics are pain medications applied to the skin. They work in different ways for different conditions but are commonly used to treat musculoskeletal and some types of neuropathic pain (Kraychete et al., 2016). Oral analgesic and anti-inflammatory drugs are usually prescribed for the treatment of acute and chronic pain and inflammation. These agents often have adverse systemic effects which are sometimes severe. Topical analgesics and anti-inflammatory drugs provide the same relief with reduced adverse systemic effects (Brayfield, 2018).
Herbal medicines are natural remedies derived from plants. However, they elicit concerted pharmacological intervention of several compounds that interact with multiple targets instead of a single compound that interacts with a single target (Zhang et al., 2012). The medical systems in developing countries involve both traditional herbal systems and orthodox medicine. Due to the poor economy, the people resort to the traditional herbal system for primary health care. Up to 80% of the population use traditional medicine due to cost especially in African and Asian countries (Ekor, 2014). Another report gave justification for low- and middle-income countries where Physicians are insufficient to meet the health care needs of the country. Traditional Medical Practitioners (TMPs) are considered an important resource for population health (Oyebode et al., 2016). The World Bank data on African development indicators 2018 showed that the statistics of Physicians per 1000 people in Nigeria was 0.381 (The World Bank Group, 2020). The resurgence of interest in herbal medicines is backed by several reasons which include the increasingly expensive and unavailability of orthodox drugs to average income earners (Linus, 2016).
Drug formulations of herbals can be with the use of one drug extract or the use of two or more drug extracts. The latter is referred to as polyherbal formulations. Single plants may not attain the desirable therapeutic effects due to the inadequacy of its active phytochemical constituents. However, polyherbals in combined ratios of multiple herbs, may give an enhanced therapeutic effect and decrease the toxicity (Karole et al., 2019). Therefore, adopting lower doses of more than one herb may exhibit improved safety (Benzie and Wachtel-Galor, 2011).
Anti-inflammatory agents derived from natural sources are chosen over orthodox anti-inflammatory agents due to lower risks of side effects (Juni et al., 2005). Several plants like Chasmanthera dependens (Menispermaceae) and Chenopodium ambrosioides (Amaranthaceae/Chenopodiaceae) have therapeutic properties. These plants are used locally to treat variety of ailments including pain and inflammation. Earlier reports confirmed their anti-inflammatory activities individually (Onabanjo et al., 1991; TrivellatoGrassi et al., 2013; Calado et al., 2015). These reports supported the safety and efficacy claims of Nigerian traditional bone setters who use C. dependens and C. ambrosioides in pain management and fracture healing. C. ambrosioides leaves have been used traditionally in West Africa as flavorings in soup, pounded leaves are applied to sores, or to swellings on the body and to areas of pain (Okhale et al., 2012). The aromatic smell of C. ambrosioides is inhaled for headache. Due to increasing number of people that suffer pain yearly, traditional medical practitioners make use of herbal formulations that are mostly not standardized.
Earlier reports had shown that the extracts and essential oil of C. ambrosioides and its fractions showed cytotoxic potential (Nowak et al., 2016; Degenhardt et al., 2016). Another report on the toxicity of aqueous extract of Chenopodium ambrosioides leaves, showed that no animals from either acute or sub chronic trial exhibited any signs of toxicity; however, slight hepatotoxic lesions in the rats were produced (da Silva et al., 2014). An earlier report opined that for Chasmanthera dependens, dose corresponding to 120 g wet weight kg−1 did not produce acute toxicity or lethality in mice, which suggested its safety when taken orally (Onabanjo et al., 1991).
The availability and safety of the herbal extracts in the traditional management of pain and inflammation backed up by previous scientific studies necessitated the formulation of topical single and polyherbal gels as investigated in this research. As there was no earlier attempt to further develop them into topical formulations. The study attempts to formulate herbal gels for the topical management of pain and inflammation using methanol leaf extracts of Chasmanthera dependens and Chenopodium ambrosioides.
2. Materials and methods
2.1. Materials
Methanol (Guandong Chemical Reagent, China), propylene glycol (BDH Chemicals Ltd., Poole England), triethanolamine (BDH Chemicals Ltd., Poole England), methyl paraben (BDH Chemicals Ltd., Poole England) and carbopol 940 (Guangdong Chemical Reagent, China), All chemical reagents for phytochemical screening and evaluation were of analytical grade. Anti-inflammatory assay reagents were obtained from Cayman Chemical, Ann Arbor, MI, USA.
2.2. Collection and treatment of plant materials
Leaves of Chasmanthera dependens were collected from a location in Ilorin, Kwara State, Nigeria. Leaves were identified at the Department of Pharmacognosy and Drug Development, Faculty of Pharmaceutical Sciences, University of Ilorin. Authentication was done at the herbarium of the Department of Plant Biology, University of Ilorin. A voucher number UILH/001/1042 was deposited for future references. Leaves were air dried at room temperature for 5 days and further dried in an oven at 45 °C for seven days. The leaves were then milled into powder and kept for extraction. This same sequence was repeated for the leaves of Chenopodium ambrosioides; a voucher number UILH/002/136 was deposited.
2.3. Preparation of crude extracts from plants
The powdered leaves of Chasmanthera dependens (750 g) was extracted in a Soxhlet apparatus using methanol as solvent. The extract was concentrated in a rotary evaporator (Buchi Switzerland). The dried extract was stored in small-capped plastic air-tight containers at 4 °C until required for analysis.
The powdered leaves of Chenopodium ambrosioides (750 g) was placed in a conical flask and methanol of up to four times of the proportion of powdered leaves was added. It was allowed to stand for a few hours and decanted. More solvent was added, and the process was repeated twice. The extract was concentrated in a rotary evaporator. The dried extract was stored in small-capped plastic air-tight containers at 4 °C until required for analysis.
The percentage yield was calculated for the two plant materials.
2.4. Pre-formulation screening of extracts
2.4.1. Physical characterization of extracts
The extracts of Chasmanthera dependens and Chenopodium ambrosioides were examined for physical characteristics such as their colour, texture, and odour.
2.4.2. Determination of pH of extracts
The pH values of extracts of Chasmanthera dependens and Chenopodium ambrosioides were measured by calibrated digital pH meter (Hanna, UK). The measurements were carried out in triplicates.
2.4.3. Phytochemical analyses of extracts
Phytochemical constituents were determined by subjecting the extracts to qualitative analysis using standard procedures (Harborne, 1998; Edeoga et al., 2005; Sofowora, 2006). Quantitative estimation of the phytoconstituents of phenols and tannins were determined by spectrophotometric method (Krishnaiah et al., 2009), while quantification of saponins, alkaloids, flavonoids and glycosides were estimated using procedure as reported by Harborne (1998).
2.4.4. In vitro anti-inflammatory and analgesic activity
A 0.5 g of Chasmanthera dependens was weighed in an analytical balance and was tested on TNF-α, Cox 2, IL-10 and PGE-2 using enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions. Diclofenac was used as a positive control.
To carry out the anti-inflammatory assay, 20, 40, 60 and 80 mg/mL of the Chasmanthera dependens and Chenopodium ambrosioides extracts were prepared using absolute methanol as the solvent. The positive control solution was prepared by dissolving 50 mg of diclofenac in 10 mL, methanol. The in-vitro anti-inflammatory activities of the extracts and diclofenac were assayed by measuring its inhibition of the enzyme-substrate reactions involving the chemokines; TNF-α, Cox-2, IL-10 and PGE-2.
Enzymes immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI, USA) was used to study the inhibitory activity of Chasmanthera dependens and Chenopodium ambrosioides extracts against Human Recombinant COX-2 according to the manufacturer's instructions. Reaction buffer solution (960 μL) containing 0.1 M Tris-HCl, 5 mM EDTA, and 2 mM phenol; COX-2 enzymes (10 μL) in the presence of heme (10 μL) with 10 μL of the prepared extract solutions was added to the wells. The solutions were incubated for 10 min at 37 °C. Then, 10 μL of arachidonic acid (AA) solution was added, and then the COX--2 reaction was stopped by adding 50 μL of 1 M HCl. The plate was washed to remove any unbound reagents and Ellman's reagent was added to the well. The distinct yellow colour obtained as a result of the enzymatic reaction was determined spectrophotometrically using a microtiter plate reader at a wavelength of 412 nm. The percentage inhibition (% inhibition) was obtained by comparison with the absorbance obtained from the blank/negative control. The procedure was repeated using TNF-α, IL-10 and PGE-2 ELISA kits respectively and the % inhibition of COX-2, TNF-α, IL-10 and PGE-2 by each prepared extract was calculated.
2.5. Preparation of herbal gel formulation
Methyl paraben (0.2 g) was weighed and added to heated water and stirred until it dissolved. The extract was weighed and dissolved in 15 mL of propylene glycol. The extract mix was poured into the dissolved methyl paraben. 1 g of carbopol was weighed, sieved, dispersed into the mixture, made up to 100 mL, and stirred continuously for 30 min using a stirrer. Triethanolamine was added to adjust the pH and stirred slowly until a clear and transparent gel was obtained. For each of the five formulations, Chasmanthera dependens and Chenopodium ambrosioides were combined at a ratio of 100:0, 75:25, 50:50, 25:75, 0:100, based on the composition presented in Table 1. The method was done according to Aslani et al. (2013) with slight variations to pH 6 using triethanolamine and stirred slowly until a clear and transparent gel was obtained.
Table 1.
Composition of herbal gel formulations.
| Ingredient (g) | F1 0:100 |
F2 25:75 |
F3 50:50 |
F4 75:25 |
F5 100:0 |
|---|---|---|---|---|---|
| Methyl paraben | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
|
C. dependens C. ambrosioides |
- 2.0 |
0.5 1.5 |
1.0 1 .0 |
1.5 0.5 |
2.0 - |
| Propylene glycol | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Triethanolamine | qs | qs | qs | qs | qs |
| Carbopol | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Water qs to | 100.0 | 100.0 | 100.0 | 100.0 | 100 .0 |
2.6. Post formulation studies
2.6.1. Physical evaluations
Organoleptic parameters such as odour, colour and texture were evaluated for the gels. Other physical evaluations such as ease of application, ease of removal on skin were conducted.
2.6.2. Determination of pH
The pH values of the herbal gels were measured by calibrated digital pH meter (Hanna, UK). The measurements were carried out in triplicates.
2.6.3. Spreadability
The spreadability of gel formulations was determined by measuring the spreading diameter of 1 g of gel between two horizontal plates (20 × 20 cm) as reported by Meera et al. (2010) with slight modifications. The horizontal plates weighed 125 g each, 1 g of the formulated gel was weighed and kept carefully on the horizontal glass slide. The second glass slide was placed on top of the gel and the time taken for the gel to spread in 1 min was recorded. A measuring rule was used to measure the diameter of the gel and the readings were taken from the three sides of the circle. The mean of the diameters was recorded. This test was repeated for all the formulations in triplicates.
2.6.4. Extrudability
A 10 g of the gel formulations were placed in collapsible tubes and the tubes compressed by adding 1 kg weight and the area of the extruded gel was measured. This was carried out in triplicates for all formulations.
2.6.5. Determination of viscosity
Herbal gel samples were placed at room temperature for 30 min. Viscosities were determined using NDJ-5S Digital Display Viscometer (Rinch, China). Number 4 spindle was attached then viscosity was determined at 25 °C and 60 rpm. The tests were done in triplicates and recorded.
2.6.6. Assay of in vitro analgesic and anti-inflammatory activity of formulated gels
Enzyme-Linked Immunosorbent Assay was used to measure the inhibitory activities of the herbal gels and commercial diclofenac gel on in vitro reactions involving IL-10, COX-2, PGE-2 and TNF-α.
IL-10 Human Elisa Kit, COX-2 EIA kit (Cayman Chemical, Ann Arbor, MI, USA), PGE-2, and TNF-α EIA kit were used to measure the %Inhibition of IL-10, COX-2, PGE-2 and TNF-α respectively by the herbal gels and diclofenac gel control following the manufacturers’ instructions. A target-specific antibody had been pre-coated in the wells of the supplied microplate to which the target chemokine, the prepared sample and reaction buffer were added. The solutions were incubated at 37 °C for 10 min after which the reaction was stopped by adding HCl, the plates were washed, the reagent to precipitate the colorimetrically assayed reaction was added and absorbance was taken spectrophotometrically at 412 nm, using the negative control in which the solvent (DMSO) was used as blank sample. The %Inhibition was calculated and recorded.
3. Results
3.1. Pre-formulation studies
3.1.1. Crude extraction from plants
The methanolic extraction of Chasmanthera dependens exhibited 10% yield while Chenopodium ambrosioides was 18%.
3.1.2. Physical characterization of extracts
The organoleptic properties of Chasmanthera dependens and Chenopodium ambrosioides leaves extracts such as colour, odour and texture are presented in Table 2.
Table 2.
Organoleptic properties of extracts.
| Methanolic extract | Colour | Odour | Texture | pH |
|---|---|---|---|---|
| C. dependens | Light green | Slight | Smooth | 5.1 ± 0.1 |
| C. ambrosioides | Dark green | Pungent | Smooth | 4.9 ± 0.1 |
3.1.3. pH of extracts
The pH of the Chasmanthera dependens and Chenopodium ambrosioides extracts are presented in Table 2.
3.1.4. Phytochemical analyses of extracts
3.1.4.1. Qualitative analysis
Phytochemical constituents in methanol extracts of Chasmanthera dependens and Chenopodium ambrosioides leaves are presented in Table 3. Methanol Extract of C. dependens leaves contained major classes of secondary metabolites which included saponins, phenolics, steroids, flavonoids, coumarins, terpenoids and alkaloids. While C. ambrosioides contained saponins, tannins, phenolics, steroids, flavonoids, anthocyanins terpenoids and alkaloids.
Table 3.
Qualitative phytochemical constituents of methanol extracts of C. dependens and C. ambrosioides leaves.
| Phytochemicals | Chasmanthera dependens | Chenopodium ambrosioides |
|---|---|---|
| Saponins | + | + |
| Tannins | - | + |
| Phenolics | + | + |
| Phlobatannin | - | - |
| Steroids | + | + |
| Flavonoids | + | + |
| Coumarins | + | - |
| Anthocyanins | - | + |
| Amino acid | - | - |
| Terpenoids | + | + |
| Glycosides | - | - |
| Triterpenes | - | - |
| Alkaloids | + | + |
Note: + = present; - = absent.
3.1.4.2. Quantitative analysis
The methanol extract of C. dependens contained 166.46 mg/g flavonoids as its highest content and 0.71 mg/g saponins as its lowest content while C. ambrosioides's highest content is 29.87 mg/g of alkaloids and its least content is 0.71% of saponins as presented in Table 4.
Table 4.
Quantitative estimation of methanol extracts of C. dependens and C. ambrosioidesLeaves.
| Phytochemicals (mg/g) | Chasmanthera dependens | Chenopodium ambrosioides |
|---|---|---|
| Saponins | 0.71 ± 0.04 | 0.71 ± 0.02 |
| Tannins | 0.00 ± 0.00 | 3.52 ± 0.20 |
| Phenolics | 20.62 ± 0.40 | 15.23 ± 0.50 |
| Steroids | 37.10 ± 0.60 | 27.27 ± 0.20 |
| Flavonoids | 26.46 ± 0.20 | 25.32 ± 0.70 |
| Coumarins | 32.15 ± 0.40 | 0.00 ± 0.00 |
| Anthocyanins | 0.00 ± 0.00 | 0.37 ± 0.01 |
| Terpenoids | 33.52 ± 0.80 | 16.42 ± 0.30 |
| Alkaloids | 31.18 ± 0.60 | 29.87 ± 0.40 |
Data presented as Mean ± SEM (n = 3).
3.1.5. In vitro anti-inflammatory activity of extracts
The percentage inhibition of the methanol extracts of Chasmanthera dependens and Chenopodium ambrosioides on COX-2, IL-10, TNF-α, and PGE-2 were presented in Table 5. The plants extracts caused a dose dependent percentage inhibition of COX-2, IL-10, TNF-α, PGE-2 levels.
Table 5.
Percentage inhibition on COX-2, IL-10, TNF-α, PGE-2.
| Enzyme/Plant | % inhibition 20 mg/mL | % inhibition 40 mg/mL | % inhibition 60 mg/mL | % inhibition 80 mg/mL | % inhibition Diclofenac (5 mg/mL) |
|---|---|---|---|---|---|
| COX-2/C. dependens | 32.03 ± 0.86 | 44.83 ± 1.05 | 57.73 ± 1.70 | 70.40 ± 2.37 | 85.50 ± 3.86 |
| COX-2/C. ambrosioides | 39.43 ± 1.45 | 48.76 ± 1.76 | 58.10 ± 2.66 | 72.10 ± 1.81 | 85.50 ± 3.86 |
| IL-10/C. dependens | 9.21 ± 0.29 | 10.61 ± 0.46 | 11.10 ± 0.38 | 12.30 ± 0.42 | 14.83 ± 0.77 |
| IL-10/C. ambrosioides | 5.06 ± 0.57 | 7.06 ± 0.62 | 9.49 ± 0.13 | 10.45 ± 0.08 | 14.83 ± 0.77 |
| TNF-α/C. dependens | 37.74 ± 0.98 | 44.07 ± 2.25 | 62.71 ± 1.83 | 75.14 ± 1.65 | 52.33 ± 2.32 |
| TNF-α/C. ambrosioides | 31.41 ± 1.75 | 47.36 ± 1.05 | 38.10 ± 1.36 | 56.50 ± 2.78 | 52.33 ± 2.32 |
| PGE-2/C. dependens | 37.28 ± 1.22 | 40.91 ± 1.44 | 49.00 ± 1.71 | 57.32 ± 2.52 | 61.73 ± 2.32 |
| PGE-2/C. ambrosioides | 36.45 ± 1.84 | 42.66 ± 1.65 | 44.66 ± 0.92 | 46.42 ± 1.47 | 61.73 ± 2.32 |
Data presented as Mean ± SEM (n = 3).
3.2. Post-formulation studies
3.2.1. Physical evaluation
The descriptions of the various formulated gels with regards to their organoleptic descriptions and other physical features are presented in Table 6.
Table 6.
Physical characterization of herbal gel.
| Gels | Appearance | Colour | Odour | Texture | Ease of Application | Ease of Removal | Feel on Skin |
|---|---|---|---|---|---|---|---|
| F1 | Less viscous, | Deep green | Leafy smell | Very smooth | Easy to apply | Easy to remove | Smooth, minty feel on skin. |
| F2 | Thick, glossy | Dark green | Unpleasant | Smooth | Easy to apply | Easily removed | Spreads on skin, smooth, minty feel. |
| F3 | Thick, smooth | Dark green | Slight astringent | Smooth | Very easy | Easily removed | Spreads on skin, smooth. |
| F4 | Thick, glossy | Dark green | Herb like | Smooth | Easy to apply | Easily removed | Spreads on skin, smooth, minty feel. |
| F5 | Thick, smooth | Dark green | Slight | Smooth | Easy to apply | Very easy | Smooth, spreads on skin, minty feel. |
3.2.2. Determination of pH
The pH of the formulated herbal gels are presented in Table 7.
Table 7.
pH, Spreadability, Extrudability and Viscosity of Herbal Gels.
| Formulations | pH | Spreadability (mm) | Extrudability (g/cm2) | Viscosity (Pas) |
|---|---|---|---|---|
| F1 | 5.2 ± 0.4 | 16.0 ± 0.5 | 1086.95 | 4.7 ± 0.4 |
| F2 | 4.7 ± 0.2 | 18.0 ± 0.4 | 952.38 | 4.4 ± 0.2 |
| F3 | 4.8 ± 0.5 | 17.0 ± 0.6 | 952.38 | 4.5 ± 0.3 |
| F4 | 4.5 ± 0.4 | 18.0 ± 0.3 | 917.43 | 4.4 ± 0.1 |
| F5 | 4.9 ± 0.5 | 19.0 ± 0.3 | 934.58 | 4.3 ± 0.2 |
Data presented as Mean ± SEM (n = 3).
3.2.3. Spreadability
The spreadability of the formulated herbal gels are presented in Table 7.
3.2.4. Extrudability
The tube extrudability of the herbal gels are presented in Table 7.
3.2.5. Determination of viscosity
The viscosity of the formulated herbal gels are presented in Table 7.
3.2.6. Assay of anti-inflammatory activity of formulated gels
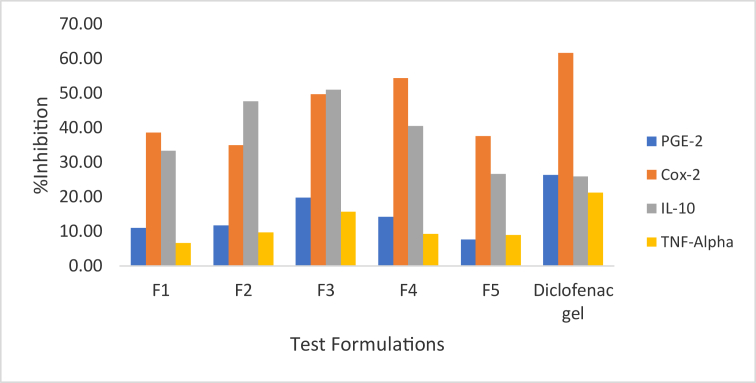
The percentage inhibition of the formulations on COX-2, IL-10, TNF-α, and PGE-2 were recorded and presented in Table 8 and Figure 1.
Table 8.
Analgesic and anti-inflammatory activity of gels.
| Mediators | % Inhibition F1 (0:100) | F2 (25:75) | F3 (50:50) | F4 (75:25) | F5 (100:0) | Diclofenac Gel |
|---|---|---|---|---|---|---|
| COX-2 | 38.58 ± 3.84b | 34.86 ± 2.72b | 49.70 ± 2.14b | 54.36 ± 3.96b | 37.49 ± 1.76b | 61.67 ± 4.72a |
| IL-10 | 6.60 ± 0.56b | 9.72 ± 0.83b | 15.70 ± 0.55b | 9.22 ± 0.47b | 8.88 ± 1.41b | 21.25 ± 2.12a |
| TNFα | 33.32 ± 3.71b | 47.62 ± 2.16b | 51.05 ± 3.18b | 40.43 ± 3.54b | 26.66 ± 1.03a | 25.89 ± 1.36a |
| PGE | 10.94 ± 1.60b | 11.70 ± 1.05b | 19.79 ± 0.73b | 14.15 ± 3.36b | 7.65 ± 0.51b | 26.28 ± 1.36a |
Formulation ratios in the order of Chasmanthera dependens:Chenopodium ambrosioides. Data presented as Mean ± SEM (n = 3). Values carrying superscripts different from the control gel for each parameter are significantly different (p < 0.05).
Figure 1.
Percentage inhibition of herbal formulations and diclofenac gel.
3.3. Statistical analysis
The mean of activities of each extract obtained as the average of their inhibition. The resulting average was used as the parameter for comparing the analgesic and anti-inflammatory activities of the gels against the control gel. On this basis, a null hypothesis (H) and alternate hypothesis (HA) was set and ANOVA was employed to compare the means.
H There is no significant difference between the means of the analgesic and anti-inflammatory activities of the gels F1, F2, F3, F4, F5 and the control diclofenac gel used.
HA = There is a significant difference between the means of the analgesic and anti-inflammatory activities of the herbal gels F1, F2, F3, F4, F5 and the control diclofenac gel used.
A p value of <0.05 as obtained implies that there is a significant statistical difference between the analgesic and anti-inflammatory activities of the herbal gels and the control. The alternate hypothesis is therefore taken, and the null rejected. In addition, the results obtained are statistically relevant.
4. Discussion
In this study, crude plant extracts were evaluated; as crude plant extracts often have greater potency rather than isolated constituents’ compounds (Karole et al., 2019). In traditional medicine, whole plants or mixtures of plants are used rather than isolated compounds (Karole et al., 2019). Polyherbals exhibit some benefits which are not accessible in single herbal formulations due to synergism.
Major secondary metabolites found in methanol leaves extracts of Chasmanthera dependens and Chenopodium ambrosioides within the levels of detection of methods used were presented in Table 3.
Quantitative analysis of the methanol extract of Chasmanthera dependens leaves showed that its highest phytochemical content was 37.1 mg/g steroids, while the least was 0.71 mg/g saponins. Methanol extract of Chenopodium ambrosioides leaves gave 29.87 mg/g alkaloids as its highest phytochemical content while 0.71 mg/g saponins was its least content as presented in Table 4. Presence of bioactive compounds which include secondary metabolites such as saponins, flavonoids, alkaloids, phenols, tannins and glycosides have been attributed to the biological activities of plants employed in the treatments of diseases. Earlier report shows that pain relief have been attributed to the bioactive constituent of C. ambrosioides, ascaridole (Okuyama et al., 1993).
Various nonsteroidal anti-inflammatory drugs can reduce pain and inflammation by blocking the metabolism of arachidonic acid by isoform of cyclooxygenase enzyme (COX-1 and/or COX-2), thereby reducing the production of prostaglandin. Unfortunately, there are many side effects associated with the administration of non-steroidal anti-inflammatory drugs. However, there are medicinal plants with low or no side effects (Oguntibeju, 2018). In recent times, plants have been used increasingly, as an alternative and/or complementary source in management of diseases, and the hunt for plant sources that relieve pain symptoms has become more dire.
According to (Onabanjo et al., 1991), methanol extract of Chenopodium ambrosioides has proven analgesic and anti-inflammatory properties, while same was reported for Chasmanthera dependens (Ibironke and Ajiboye, 2007). These attributes informed the selection of these plant species for the design of herbal formulations to be used in the management of pain and inflammation in this research. In confirming their activities, in vitro analgesic and anti-inflammatory screening of the methanol extracts was first carried out. The two plant extracts were tested for their inhibition against COX-2, PGE-2, TNF α and IL-10 and standard drug diclofenac was used as positive control. The plant extracts were tested at different concentrations of 20, 40, 60 and 80 mg/mL as presented in Table 5. The in vitro inhibitory activities of the two extracts showed a dose dependent increase in percentage inhibition of expression of COX-2 enzymes, production of PGE-2 mediators, TNF α and IL-10 inflammatory cytokines activities tested. At 80 mg/mL, Chasmanthera dependens extract showed highest inhibitory activities of 9.21, 57.32, 70.40 and 75.14% for IL-10, PGE-2, COX-2 and TNF α respectively. While Chenopodium ambrosioides extract at 80 mg/mL, showed highest inhibitory activities of 5.06, 46.42, 56.50 and 72.10% for IL-10, PGE-2, TNF α and COX-2 respectively. The two extracts activities were compared with 5 mg/mL of standard drug diclofenac which showed 14.83, 52.33, 61.73 and 85.50% inhibitory activities against IL-10, PGE-2, TNF α and COX-2 respectively.
Attributes such as long residence time on the skin, high viscosity, moisturizing effect on flaky skin due to their occlusive properties, more bio adhesiveness, less irritation, independent of water solubility of active ingredient, ease of application and better release has influenced the choice of gels over other topical semi-solid dosage forms (Loganathan et al., 2001).
Carbopol 940 was used as gelling agent in the formulation based on its biodegradable, bioadhesive, biocompatible and non-irritant properties. The percentage of polymer used was optimized in preliminary studies and 1% carbopol concentration was selected to prepare the herbal formulations. Carbopol gels (1%) were formulated by incorporating 2% concentration of Chasmanthera dependens and Chenopodium ambrosioides individually and in predetermined combination. This was to evaluate and observe the activities of the extracts when combined and when used individually in the formulation as potential anti-inflammatory agents.
The herbal gels exhibited different shades of green depending on the combination mixing ratio. Herbal gels containing higher concentrations of Chenopodium ambrosioides had a characteristic pungent smell as expected while gels containing higher concentrations of Chasmanthera dependens had herb like smell. The herbal gels had a smooth texture and were generally easy to apply and wash off with water as shown in Table 6.
The pH of the herbal gels were measured and presented in Table 7. The pH or the degree of acidity and alkalinity affects grossly the stability of pharmaceutical preparations. The optimum pH value of skin on most of our face and body lies between 4.5 and 5.5. Therefore, the skin's natural pH is mildly acidic. The herbal gels formulated had slightly acidic pH ranging from 4.5 ± 0.4 to 5.2 ± 0.4 and were in the range of the pH of the skin.
The viscosity of the herbal gels ranged between 4.3 ± 0.2 and 4.7 ± 0.4 Pas as presented in Table 7. The viscosity measurements remained in this range since 1% carbopol was used as the base for all the formulations prepared.
Spreadability test results presented in Table 7 with values between 16 ± 0.5 and 19 ± 0.3 mm which showed that the gels were easily spreadable due the optimum values obtained. Extrudability test results presented in Table 7 showed the gels had good extrudability with values between 917.43 and 1086.95 g/cm2.
Comparing the anti-inflammatory activities of the herbal formulations with the extracts, the gels exhibited lower inhibitory activity as compared to the extracts. This could be due to poor release from the carbopol base. F1 formulation contained 2% Chenopodium ambrosioides only, while F5 contained 2% Chasmanthera dependens only and therefore presented as individual herbal gel formulations. Further, Formulations F2, F3 and F4 contained mixed ratios of the two extracts and therefore presented as polyherbal gels. Polyherbal formulation F3 which had equal proportions of the extracts (50:50) i.e. 1% of each extract combined, exhibited significant anti-inflammatory activities on COX-2, IL-10, TNF-α and PGE compared with the other four formulations as presented in Table 8. Therefore, enhanced inhibitory activities were achieved by these polyherbal formulations. Significant differences (p < 0.05) were observed between the means of the analgesic and anti-inflammatory of the herbal gels F1, F2, F3, F4, F5 and the control commercial diclofenac gel.
These results demonstrate the potentials of the gel formulations from Chasmanthera dependens and Chenopodium ambrosioides leaves extracts against mediators commonly associated with pain and inflammation. For long term use, it may be preferable to use herbal drugs as a safer alternative treatment for pain relief over nonsteroidal drugs.
5. Conclusion
This present study demonstrated the effectiveness of carbopol based herbal gels containing methanol extracts of Chasmanthera dependens and Chenopodium ambrosioides leaves in inhibiting COX-2 enzymes, PGE-2 mediators, IL-10 and TNF α inflammatory cytokines. Thus, the formulated individual and polyherbal gels incorporated with Chasmanthera dependens and Chenopodium ambrosioides extracts show great promises in the management of pain and inflammation.
Declarations
Author contribution statement
A.T. Kola-Mustapha: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
K.A. Yohanna: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Y.O. Ghazali: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
H.T. Ayotunde: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Thanks goes to Dr Emeka Asogwa of Central Research Laboratory Ilorin for the laboratory support.
References
- Aslani A., Ghannadi A., Najafi H. Design, formulation and evaluation of a mucoadhesive gel from Quercus brantii L. and Coriandrum sativum L. as periodontal drug delivery. Adv. Biomed. Res. 2013;2:21. doi: 10.4103/2277-9175.108007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie I.F.F., Wachtel-Galor S. second ed. CRC Press/Taylor & Francis; 2011. Herbal Medicine: Bimolecular and Clinical Aspects. [PubMed] [Google Scholar]
- Boer M., Duchnik E., Maleszka R., Marchlewicz M. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postepy Dermatologii/Alergologii. 2016;33(1):1–5. doi: 10.5114/pdia.2015.48037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayfield A., editor. Martindale: the Complete Drug Reference. 38th Edition. London Pharmaceutical Press; 2018. www.medicinescomplete.com/mc/martindale/current/ Available at: [Google Scholar]
- Calado G.P., Lopes A.J.O., Costa Junior L.M., Lima F.C.A., Silva L.A., Pereira W.S., do Amaral F.M.M., Garcia J.B.S., Cartágenes M.S., Nascimento F.R.F. Chenopodium ambrosioides L. reduces synovial inflammation and pain in experimental osteoarthritis. PloS One. 2015;10(11) doi: 10.1371/journal.pone.0141886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva M.G., Amorim R.N., Câmara C.C., Fontenele Neto J.D., Soto-Blanco B. Acute and sub-chronic toxicity of aqueous extracts of Chenopodium ambrosioides leaves in rats. J. Med. Food. 2014;17(9):979–984. doi: 10.1089/jmf.2013.0134. [DOI] [PubMed] [Google Scholar]
- Degenhardt R.T., Farias Ingrid V., Grassi Liliane T., Franchi, Gilberto C., Nowill Alexandre E., Bittencourt Christiane M. da S., Wagner Theodoro M., Souza Marcia M. de, Cruz Alexandre Bella, Malheiros Angela. Characterization and evaluation of the cytotoxic potential of the essential oil of Chenopodium ambrosioides. Revista Brasileira de Farmacognosia. 2016;26(1):56–61. [Google Scholar]
- Edeoga H., Okwu D., Mbaebie B. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005;4:685–688. [Google Scholar]
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne J.B. third ed. Chapman and Hall; New York, NY: 1998. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- Ibironke G.F., Ajiboye K.I.A. Studies on the anti-inflammatory and analgesic activity of Chenopodium ambrosioides leaf extracts in rats. Int. J. Pharmacol. 2007;3:111–115. [Google Scholar]
- Juni P., Reischenbach S., Egger M. Cox-2 inhibitors, traditional NSAIDs and the heart. Br. Med. J. 2005;330(7504):1342–1343. doi: 10.1136/bmj.330.7504.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karole S., Shrivastava S., Thomas S., Soni B., Khan S., Dubey J., Dubey S.P., Khan N., Jain D.K. Polyherbal formulation concept for synergic action: a review. Journal of Drug Delivery and Therapeutics Online. 2019;9(1-s):453–466. http://jddtonline.info/index.php/jddt/article/view/2339 Available from: [Google Scholar]
- Kraychete D.C., Palladini M.C., Castro A.P.C.R. Topic drug therapy for neuropathic pain. Revista Dor. 2016;17(Suppl. 1):95–97. [Google Scholar]
- Krishnaiah K.D., Devi T., Bano A., Sarbatly R. Studies on phytochemical constituents of six Malaysian medicinal plants. J. Med. Plants Res. 2009;3:67–72. [Google Scholar]
- Linus C.S. Nigerian folklore medicinal plants with potential antifertility activity in males: a Scientific Appraisal. Res. J. Med. Plant. 2016;10:201–227. [Google Scholar]
- Loganathan V., Manimaran S., Jaswanth A., Sulaiman A., Shivaprasadha R.M.V., Senthil Kumar B., Rajasekaran A. The effects of polymers and permeation enhancers on releases of flurbiprofen from gel formulations. Indian J. Pharmaceut. Sci. 2001;63(3):200–204. [Google Scholar]
- Meera C.S., Ajinkya S.N., Sawant S.D. Transdermal drug delivery system with major emphasis on transdermal patches. J. Pharmaceut. Res. 2010;3(10):2537–2543. [Google Scholar]
- Merskey H., Bogduk N. IASP Task Force on Taxonomy. second ed. IASP Press; Seattle: 1994. Part III: pain terms, A current list with definitions and notes on usage. Classification of chronic pain; pp. 209–214. [Google Scholar]
- NIH . NIH Research Portfolio Online Reporting Tool (RePORT) 2013. factsheets: pain management.https://report.nih.gov//nihfactsheets/ViewFactSheet.aspx?csid=57 Available from: [Google Scholar]
- Nowak R., Szewczyk K., Gawlik-Dziki U., Rzymowska J., Komsta Ł. Antioxidative and cytotoxic potential of some Chenopodium L. species growing in Poland. Saudi J. Biol. Sci. 2016;23(1):15–23. doi: 10.1016/j.sjbs.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguntibeju O.O. Medicinal plants with analgesic and anti-inflammatory activities from selected regions of Africa. J. Inflamm. Res. 2018;11:307–331. doi: 10.2147/JIR.S167789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okhale S.E., Egharevba H.O., Ona E.C., Kunle O.F. Phytochemical and proximate analyses and thin layer chromatography fingerprinting of the aerial part of Chenopodium ambrosioides Linn. (Chenopodiaceae) J. Med. Plants Res. 2012;6(12):2289–2294. [Google Scholar]
- Okuyama E.K., Umeyama K., Saito Y., Yamazaki M., Satake M. Ascaridole as a pharmacologically active principle of Paico, a medicinal Peruvian plant. Chem. Pharm. Bull. 1993;41:1309–1311. doi: 10.1248/cpb.41.1309. [DOI] [PubMed] [Google Scholar]
- Onabanjo A.O., John T.A., Sokale A.A., Samuel O.T. Analgesic and antiinflammatory effects of Chasmanthera dependens. Int. J. Pharmacogn. 1991;29(1):24–28. [Google Scholar]
- Oyebode O., Kandala N.B., Chilton P.J., Lilford R.J. Use of traditional medicine in middle-income countries: a WHO-SAGE study. Health Pol. Plann. 2016;31(8):984–991. doi: 10.1093/heapol/czw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinwan K., Jan T.V., Borna R. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019;20(23):6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofowora A. Spectrum Books Limited; Ibadan: 2006. Medicinal Plants and Traditional Medicine in Africa; p. 285. [Google Scholar]
- The World Bank Group . 2020. World Health Organization's Global Health Workforce Statistics, OECD, Supplemented by Country Data.https://data.worldbank.org/indicator/SH.MED.PHYS.ZS?end=2018&locations=NG&start=1960&view=chart [Google Scholar]
- TrivellatoGrassi L., Malheiros A., Meyre-Silva C., da Silva Buss Z., Monguilhott E., Fröde T., Bortolini K., da Silva S., de Souza M. From popular use to pharmacological validation: a study of the anti-Inflammatory, anti-Nociceptive and healing effects of Chenopodium ambrosioides extract. J. Ethnopharmacol. 2013;145(1):127–138. doi: 10.1016/j.jep.2012.10.040. [DOI] [PubMed] [Google Scholar]
- Zhang D., Luo G., Ding X., Lu C. Preclinical experimental models of drug metabolism and disposition in drug discovery and development. Acta Pharm. Sin. B. 2012;2:549–561. [Google Scholar]