Abstract
Films and edible coatings based on biopolymers have been developed as a packaging, which can be obtained from biodegradable materials and have properties similar to common plastics. These edible materials have many applications in the food industry, preventing mass transfer between the product and the surrounding environment. The objective of this study was to develop and evaluate the physicochemical and mechanical properties of edible films based on cassava starch (CS), whey protein (WP), and beeswax (BW). Response surface methodology has been used and the experiments were carried out based on face-centred composite design. On the other hand, three CS-based controls were formulated to evaluate the effect of the inclusion of WP and BW. The optimization of multiple responses established the optimal formulation: CS (3.17 %), WP (1.30 %), BW (0.50 %), presenting the following response variables: tensile stress (1.92 MPa), elongation (40.4 %), Young's modulus (42.1 MPa), water vapor permeability 1.79 × 10−11 (g mm/s cm2 Pa), swelling capacity (300.3 %), thickness (0.128 mm), moisture content (6.74 %), and colour: lightness (89.9), chromaticity a∗ (-1.8), chromaticity b∗ (7.7), saturation (9.9), tone (101.1°), and yellowness index (17.7). The selection and evaluation of this optimal formulation are essential because it is the material that shows the best possible mechanical and physicochemical properties using the studied components. The results, especially its good mechanical properties and low permeability to water vapour, would allow its application as a coating for fruits, vegetables, among others, effectively delaying its weight loss due to dehydration.
Keywords: Food science, Food technology, Materials science, Manihot esculenta, Biodegradable films, Optimal formulation, Physical properties
Food science, Food technology, Materials science, Manihot esculenta, Biodegradable films, Optimal formulation, Physical properties
1. Introduction
In recent decades, the worldwide consumption of plastic has been increasing, causing serious environmental problems due to its inability to biodegrade [1, 2, 3, 4, 5]. For this reason, the use of renewable resources is being explored, which can reduce the problems of waste disposal, and the development of films with biodegradable materials is a promising alternative that offers low cost, degradability, edibility, and properties similar to common plastics [6, 7, 8, 9].
Edible films and coatings play an important role in the quality, safety, transportation, storage, and display of a wide range of fresh and processed foods [1, 10]. They can minimize the principal degradation by preventing moisture losses and decreasing adverse chemical reaction rates, thus helping to prevent spoilage and microbial contamination of food [5, 11]. In addition, edible coatings can incorporate food additives, such as flavours, antimicrobials, antioxidants, among others, and this allows for expansion of its applications [12, 13]. Among the most common biopolymers for film formation are polysaccharides (starch, pectin, chitosan), proteins (gelatine, casein, gluten, whey protein, soy protein), and lipids (waxes), which are used alone or in blends [14, 15, 16, 17].
Numerous studies have focused on the development of starch-based films because it is one of the most abundant biopolymers in nature, being a low-cost material (lower cost than polyethylene), widely available, biodegradable, edible, tasteless, colourless, and easy to use in technological processes [2, 18, 19]. One of the main sources of starch is cassava (Manihot esculenta), and it is the crop with the highest production in Latin America, especially in Colombia, a country that has increased its demand due to its high level of industrialization [12].
The plastic biopolymers of cassava starch (CS) have shown excellent properties in film formation for obtaining flexible and extensible materials of homogeneous and smooth surfaces [12, 20], becoming packaging or coating alternatives in the food industry to extend the shelf life of the products [12, 21]. However, these films are relatively brittle materials that do not favour mechanical properties and have a high sensitivity to humidity, restricting their use especially in humid environments [17, 22]. On the other hand, whey protein concentrated (WP), generally used in formulations for sports and infant foods, is also used as an edible coating since it exhibits good film-forming capacity with good mechanical properties and forms a good gas barrier to respiration at low relative humidity [1, 23, 24, 25], as well as to volatile compounds (aromas, essential oils, among others) [26, 27].
The films based on CS and WP have a low barrier to water vapor, due to their hydrophilic nature [1, 26, 27]. Therefore, waxes (esters of long-chain fatty acids with high molecular weight alcohols) have been incorporated in various formulations to generate a barrier to water vapor, due to their hydrophobic nature [28]. In this context, beeswax (BW) has been widely studied in the formulation of edible films, firstly because of its viscoelastic behaviour [29, 30, 31] and also because it generates materials with greater resistance to water vapor due to its composition of long chain fatty acids. This decreases the transfer of gases and water vapor and, in turn, increases the hydrophobic capacity of some formulation materials such as proteins [32, 33, 34]. It has been shown that composite edible films present better characteristics to be used in food preservation: films based on WP, pectin, carrageenan, or konjac flour increased the tensile strength (Ts), Young's modulus (Ym), and the elongation (ε) of the materials obtained [35]. Films based on hydroxypropyl methyl cellulose, WP, and sunflower oil increased the water vapor barrier and decreased tensile properties [36]. On the other hand, films based on proteins and starch blends have generated a material of greater elasticity and lower permeability to water vapor with respect to films obtained with net starch [2].
The use of combined materials allows for improving the film properties and extend their application. The results of this study are essential for the region since it has been worked with autochthonous raw materials and an attempt is made to obtain a suitable coating for the preservation of food products such as fruits and vegetables. Subsequent studies will apply these coatings to assess shelf life of other food products. Therefore, the present research aims to develop and evaluate the physicochemical and mechanical properties of edible films based on CS, WP, and BW in order to form multipurpose materials for application in fruits.
2. Materials and methods
2.1. Materials
CS (Codipsa, Paraguay.), WP (WP80, Saputo, Canada) and BW (Guinama, Valencia, Spain) were used as a polymer matrix, glycerol (G) (Timur Oleochemical Malaysia, Sdn.Bhd) was applied as a plasticizer, and stearic acid (SA) (Timur Oleochemical Malaysia, Sdn.Bhd) was utilized as a surfactant.
2.2. Preparation and formation of edible films
First, a solution of CS and WP in distilled water was formed and homogenized at 13000 rpm (UltraTurrax IKA, T25) for 3 min. Then, glycerol was added in a 1:2 ratio to CS, and homogenization was continued for 3 more minutes. Subsequently, the dispersion was heated to 70 °C, and BW and SA were added in a ratio of 1:5 with respect to BW, and then, heating was continued until 85 °C for 30 min with constant agitation. The emulsion was cooled to 35 °C and homogenized cold at 21000 rpm for 1 min. Finally, they were poured into 9 cm diameter plastic petri dishes, dried at 27 °C in a drying oven for 48 h, and the films were demoulded and stored at 20 °C and 40 % RH until their respective measurements. For the CS-based films, the same procedure was followed above, without the addition of WP and BW, and additionally, the formed films were dried at 40 °C for 40 h.
2.3. Experimental design
15 emulsions were prepared using the response surface methodology with a face-centred composite design (α = 1), considering the following independent variables: CS (3.0–3.5 %), WP (0.5–1.5 %), and BW (0–0.5 %); and the following dependent variables: thickness (Th), moisture content (Xw), swelling capacity (Sc), water vapor permeability (WVP), tensile strength (Ts), elongation at break point (ε), young's modulus (Ym), and the colour parameters: lightness (L∗), chromaticity a∗, chromaticity b∗, saturation (Cab∗), tone (Hab∗), and yellowness index (Yi). The ranges of the main components were defined according to the literature: Chimma et al., 2015 (5% CS), Sapper et al., 2019 (2% CS), Basiak et al., 2017 (5% WPI90), García et al., 2020 and Castro et al., 2019 (10% WPC80), Zhang et al., 2018 (0.5–1% BW), Galus et al., 2019 (50% G, concerning the biopolymer) and from preliminary tests. In addition, three CS-based controls were formulated to assess the effect of the inclusion of WP and BW: control 1 (3% CS, 1.5% G and H20); control 2 (3.25% CS, 1.625% G and H20) and control 3 (3.5% CS, 1.750% G and H20).
2.4. Characterization of the films
The Xw was determined by the AOAC method 950.46 with some modifications proposed by Fama et al. (2012) [37]. The final Th of the films was determined from the arithmetic mean between measurements at the ends and centre [35], using a Starrentl micrometre No 426.2. The Sc was determined using areas of 2 × 2 cm films, then the pieces were immersed in distilled water at 25 °C for 10 min, and finally, the excess surface water was removed with filter paper to record the final weight [2]. The WVP was determined according to the ASTM E 96-80 standard method adapted to edible films and coatings proposed by Basiak et al. (2017) [1]with some modification, using a desiccator previously conditioned in a controlled environment with a supersaturated solution of MgCl2 hexahydrate (relative humidity: 32.8 %) at 25 °C. On the other hand, the weights were recorded at 1 h intervals for 6 h. The water vapor transfers rate (WVTR) was determined from Eq. (1), where m is the linear regression constant of the weight loss values versus time for a constant period and A is the film area (m2). The WVP was determined from Eq. (2), where Th is the thickness of the film (mm) and ΔP is the pressure gradient in Pa.
| (1) |
| (2) |
The mechanical properties associated with the tension of the films were evaluated according to the methodology described in ASTM D882-12 with modifications. The films were cut into 25 mm × 50 mm strips and attached to a TA. XT2 texturometer (Stable Micro System) with the self-tightening roller grips accessory (speed: 1 mm/s). The force vs. distance curve was recorded, the Ts being determined from the relationship between the breaking force (RF) and the cross-sectional area of the film (A∗) (Equation 3). In addition, the ε (%) was recorded according to Eq. (4) [36], where Df and Di are the length of elongation to rupture and the initial length (mm) of the film, respectively. On the other hand, the Ym was determined from the stress strain (Ts)- unit strain (ε) graph [38] (equation 5).
| (3) |
| (4) |
| (5) |
The colour parameters were determined from the CIE-L∗a∗b∗ coordinates, using an X-Rite spectrophotometer, model SP64, illuminant D65, and observer of 10°. From the reflection spectra, L∗, a∗, b∗, Cab∗, Hab∗, and Yi [36] were determined.
The statistical analysis was performed with the software Statgraphics Centurion XVI. II and, the ANOVA was performed with a confidence level of 95 % (p ≤ 0.05). All tests were performed in triplicate.
3. Results and discussion
Table 1 presents the mean values plus the standard deviation of Th, Xw, Sc, and colour of edible films based on CS, WP, and BW; and Table 2 presents the results of the ANOVA of each dependent variable as a function of the p-value, identifying the significant differences with respect to the independent variables and their linear and quadratic interactions. It is observed that most of the dependent variables (Xw, Sc, WVP, Ts, E, Ym, L ∗, a ∗, b ∗, Cab∗, Hab∗ and Yi) present statistically significant differences concerning the independent variables and/or their linear and/or quadratic interactions, denoting values of p < 0.05. Which validate the probability that these differences are sufficiently unlikely to be due to random.
Table 1.
Mean values plus standard deviation of Th, Xw, Sc, WVP, Ts, ε, Ym, and color of the films based on CS, WP, and BW.
| Sample | CS (%) | WP (%) | BW (%) | Th (mm) | Xw (%) | Sc (%) | WVP (g mm/s cm2 Pa) | Ts (MPa) | ε (%) | Ym (MPa) | L∗ | a∗ | b∗ | Cab∗ | Hab∗ | Yi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.00 | 0.50 | 0.00 | 0.13 ± 0.01 | 6.4 ± 0.6 | 415.2 ± 12.6 | 7.2 ± 1.2 × 10−11 | 0.730 ± 0.135 | 87.8 ± 7.9 | 7.7 ± 0.5 | 88.7 ± 0.1 | -1.4 ± 0.0 | 3.0 ± 0.1 | 3.3 ± 0.1 | 115.4 ± 0.2 | 4.8 ± 0.3 |
| 2 | 3.00 | 1.50 | 0.50 | 0.12 ± 0.01 | 6.2 ± 0.4 | 278.3 ± 13.7 | 1.9 ± 0.1 × 10−11 | 1.070 ± 0.162 | 37.4 ± 2.8 | 18.2 ± 2.5 | 89.5 ± 0.1 | -1.9 ± 0.0 | 9.1 ± 0.3 | 9.3 ± 0.3 | 102.0 ± 0.1 | 16.0 ± 0.5 |
| 3 | 3.50 | 0.50 | 0.50 | 0.12 ± 0.01 | 7.3 ± 0.8 | 302.6 ± 12.8 | 2.2 ± 0.5 × 10−11 | 0.717 ± 0.042 | 65.7 ± 12.8 | 8.3 ± 0.5 | 89.7 ± 1.3 | -1.4 ± 0.1 | 2.5 ± 0.7 | 2.9 ± 0.6 | 121.0 ± 5.4 | 3.8 ± 1.3 |
| 4 | 3.25 | 1.50 | 0.25 | 0.13 ± 0.01 | 5.8 ± 0.5 | 285.7 ± 10.7 | 3.7 ± 1.3 × 10−11 | 1.13 ± 0.071 | 64.3 ± 9.5 | 19.6 ± 2.0 | 89.1 ± 0.2 | -1.8 ± 0.0 | 9.4 ± 1.0 | 9.5 ± 1.0 | 101.1 ± 1.2 | 16.7 ± 1.9 |
| 5 | 3.00 | 1.00 | 0.25 | 0.13 ± 0.01 | 6.6 ± 0.4 | 284.5 ± 9.1 | 4.5 ± 3.4 × 10−11 | 0.848 ± 0.077 | 78.9 ± 15.1 | 11.4 ± 2.7 | 89.1 ± 0.8 | -1.72 ± 0.1 | 5.5 ± 0.6 | 5.8 ± 0.5 | 107.4 ± 2.6 | 9.5 ± 1.1 |
| 6 | 3.25 | 1.00 | 0.25 | 0.13 ± 0.01 | 6.3 ± 0.8 | 290.5 ± 9.7 | 3.7 ± 0.7 × 10−11 | 1.889 ± 0.361 | 42.3 ± 3.8 | 37.2 ± 2.3 | 89.3 ± 0.3 | -1.7 ± 0.0 | 7.1 ± 0.4 | 7.3 ± 0.4 | 103.5 ± 0.5 | 12.5 ± 0.7 |
| 7 | 3.25 | 1.00 | 0.25 | 0.13 ± 0.01 | 6.2 ± 1.0 | 292.7 ± 10.9 | 4.2 ± 0.1 × 10−11 | 1.527 ± 0.315 | 42.9 ± 9.6 | 40.3 ± 3.9 | 89.1 ± 0.4 | -1.7 ± 0.0 | 7.7 ± 0.8 | 7.9 ± 0.8 | 102.4 ± 1.1 | 13.7 ± 1.5 |
| 8 | 3.25 | 0.50 | 0.25 | 0.13 ± 0.01 | 7.8 ± 6.7 | 308.2 ± 12.1 | 3.7 ± 0.1 × 10−11 | 1.817 ± 0.421 | 51.7 ± 13.1 | 47.1 ± 5.2 | 90.5 ± 0.5 | -1.5 ± 0.0 | 3.6 ± 0.2 | 3.9 ± 0.2 | 113.2 ± 1.2 | 5.8 ± 0.4 |
| 9 | 3.25 | 1.00 | 0.50 | 0.13 ± 0.01 | 7.7 ± 0.4 | 308.7 ± 11.8 | 2.0 ± 0.4 × 10−11 | 2.192 ± 0.156 | 49.0 ± 12.3 | 51.5 ± 7.6 | 90.3 ± 0.7 | -1.6 ± 0.0 | 5.1 ± 0.2 | 8.0 ± 0.1 | 105.4 ± 1.0 | 14.4 ± 2.1 |
| 10 | 3.25 | 1.00 | 0.25 | 0.13 ± 0.01 | 6.3 ± 0.5 | 294.9 ± 10.2 | 3.5 ± 0.1 × 10−11 | 2.546 ± 0.528 | 43.3 ± 11.3 | 52.4 ± 7.4 | 89.3 ± 0.2 | -1.7 ± 0.0 | 6.2 ± 0.3 | 6.5 ± 0.3 | 105.0 ± 0.8 | 10.9 ± 0.7 |
| 11 | 3.25 | 1.00 | 0.25 | 0.13 ± 0.01 | 7.5 ± 0.6 | 293.4 ± 12.7 | 3.9 ± 0.4 × 10−11 | 1.856 ± 0.361 | 46.0 ± 6.9 | 49.9 ± 8.2 | 89.3 ± 0.3 | -1.8 ± 0.0 | 7.4 ± 0.6 | 7.6 ± 0.6 | 103.7 ± 1.2 | 13.1 ± 1.2 |
| 12 | 3.25 | 1.00 | 0.25 | 0.13 ± 0.01 | 6.8 ± 0.6 | 292.1 ± 12.5 | 3.9 ± 0.2 × 10−11 | 1.986 ± 0.282 | 55.2 ± 11,0 | 44.4 ± 2.5 | 89.6 ± 0.3 | -1.8 ± 0.0 | 6.7 ± 0.4 | 6.9 ± 0.4 | 104.9 ± 0.8 | 11.6 ± 0.9 |
| 13 | 3.50 | 1.00 | 0.25 | 0.13 ± 0.01 | 7.1 ± 0.7 | 337.7 ± 11.5 | 4.6 ± 0.1 × 10−11 | 2.065 ± 0.214 | 41.1 ± 5.0 | 53.8 ± 7.9 | 89.6 ± 0.1 | -1.6 ± 0.1 | 5.4 ± 0.7 | 5.7 ± 0.7 | 106.8 ± 1.2 | 9.4 ± 1.4 |
| 14 | 3.50 | 1.50 | 0.00 | 0.13 ± 0.01 | 6.3 ± 0.3 | 381.5 ± 12.4 | 5.7 ± 0.6 × 10−11 | 2.211 ± 0.584 | 44.9 ± 2.6 | 49.0 ± 6.2 | 89.0 ± 0.3 | -1.7 ± 0.1 | 6.6 ± 0.5 | 6.8 ± 0.5 | 104.4 ± 0.9 | 11.5 ± 1.0 |
| 15 | 3.25 | 1.00 | 0.00 | 0.13 ± 0.01 | 8.1 ± 1.0 | 396.5 ± 14.0 | 7.4 ± 0.5 × 10−11 | 2.547 ± 0.08 | 50.8 ± 13.6 | 68.9 ± 8.9 | 88.7 ± 0.2 | -1.6 ± 0.0 | 5.6 ± 0.6 | 5.8 ± 0.6 | 106.3 ± 1.4 | 9.8 ± 1.1 |
| Control 1 | 3.00 | 0.00 | 0.00 | 0.08 ± 0.01 | 6.5 ± 0.6 | 435.8 ± 11.1 | 1.1 ± 1.1 × 10−11 | 0.579 ± 0.013 | 97.0 ± 19.9 | 3.5 ± 0.6 | 89.0 ± 0.3 | -1.3 ± 0.1 | 0.1 ± 0.2 | 1.3 ± 0.1 | 174.0 ± 7.9 | -0.7 ± 0.3 |
| Control 2 | 3.25 | 0.00 | 0.00 | 0.08 ± 0.01 | 7.1 ± 0.6 | 445.0 ± 12.0 | 9.9 ± 0.1 × 10−11 | 0.658 ± 0.106 | 76.6 ± 15.6 | 5.0 ± 0.9 | 90.2 ± 1.4 | -1.2 ± 0.1 | -0.0 ± 0.1 | 1.2 ± 0.1 | 180.2 ± 5.1 | -1.0 ± 0.3 |
| Control 3 | 3.50 | 0.00 | 0.00 | 0.08 ± 0.01 | 7.6 ± 0.6 | 479.9 ± 11.4 | 9.8 ± 0.5 × 10−11 | 1.031 ± 0.167 | 83.4 ± 20.1 | 7.8 ± 0.8 | 89.1 ± 0.2 | -1.3 ± 0.0 | -0.1 ± 0.0 | 1.3 ± 0.0 | 185.4 ± 2.0 | -1.3 ± 0.1 |
Table 2.
ANOVA results for films based on CS, WP, and BW.
| Source | p-value |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Th | Xw | Sc | WVP | Ts | ε | Ym | L∗ | a∗ | b∗ | Cab∗ | Hab∗ | Yi | |
| A: Cassava Starch | 0.8974 | 0.4424 | 0.0000 | 0.8898 | 0.0002 | 0.0001 | 0.0000 | 0.1999 | 0.0484 | 0.8772 | 0.8147 | 0.6840 | 0.8880 |
| B: Whey Protein | 0.4706 | 0.0037 | 0.0365 | 0.9629 | 0.0221 | 0.1471 | 0.0000 | 0.0020 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| C: Beeswax | 0.3294 | 0.4702 | 0.0000 | 0.0000 | 0.2239 | 0.8390 | 0.0040 | 0.0005 | 0.7715 | 0.4354 | 0.0001 | 0.5384 | 0.0001 |
| AA | 0.4954 | 0.1356 | 0.1038 | 0.3132 | 0.0001 | 0.0383 | 0.0000 | 0.2307 | 0.4479 | 0.0028 | 0.0000 | 0.0001 | 0.0000 |
| AB | 0.1769 | 0.2591 | 0.1211 | 0.3203 | 0.5302 | 0.2203 | 0.7398 | 0.0998 | 0.0111 | 0.0510 | 0.0703 | 0.1690 | 0.0343 |
| AC | 0.8522 | 0.1056 | 0.6102 | 0.3885 | 0.0001 | 0.0001 | 0.0000 | 0.0090 | 0.1609 | 0.3406 | 0.2554 | 0.1118 | 0.2942 |
| BB | 0.8891 | 0.1386 | 0.1501 | 0.1809 | 0.0001 | 0.1154 | 0.0000 | 0.3313 | 0.2438 | 0.5872 | 0.1767 | 0.0002 | 0.1004 |
| BC | 0.9354 | 0.9410 | 0.0001 | 0.4886 | 0.0718 | 0.0062 | 0.0005 | 0.6104 | 0.7222 | 0.0600 | 0.0364 | 0.0132 | 0.0535 |
| CC | 0.1782 | 0.0260 | 0.0000 | 0.1869 | 0.0105 | 0.5843 | 0.0003 | 0.6137 | 0.0025 | 0.0012 | 0.8120 | 0.0377 | 0.8380 |
The Th of the composite films did not present significant differences with respect to the study factors, as their mean values fluctuated between 0.12 and 0.13 mm. On the other hand, the films formulated only with CS (control 1, 2, 3) presented lower Th (≈0.08 mm). This difference could be attributed to several phenomena. On the one hand, the interaction between the polymeric components used in the formulation of the emulsion, which after forming presents changes in the segment-segment and segment-chain interactions, mainly favouring the interaction between the hydrophilic groups of the polymeric segments, but it also decreases the segment-chain interaction. In addition, it could be due to the effect that the drying has on the formation of the final structure of the films; increased emulsion viscosity; or because of a nonhomogeneous distribution of the solids per cm2 of drying surface. A similar increase in the thickness was reported for edible films based on wheat starch/whey protein [2], where the Th of films made with wheat starch or with milk protein was lower than the composite film, and edible films based on sago starch/guar gum with essential oils (carvacrol and citral), where Th of films with essential oil was upper than control film [3]. These authors affirm that the kinetics of drying and the formulations in solution directly influence the Th.
The average values of the Xw of the films were between 5.83 to 8.13 %, showing a tendency to increase when CS levels were high and both BW and WP low, which was consistent with their water affinity. The interaction between the polymers (CS and WP) with BW may be the cause of the decrease of Xw of the films since the protein has a hydrophobic part and the BW is extremely hydrophobic, which limits the interaction between water and hydrophilic groups of the polymers. This parameter is directly related to the useful life of the films and is affected by the moisture content contributed by the components present in each formulation, by the relative humidity of the surrounding environment, and by the hydrophilic/lipophilic balance of the emulsifier. Similar results were reported for sago starch/guar gum edible films where Xw decreased from 22.1% to 16.1% with the addition of essential oils [3]; Amin et al. (2019) [32] reported higher moisture content ranges in films based chitosan (30%) than both Chitosan/Aloe vera (13–25%) and Chitosan/Aloe vera/BW (11–21%), and results were according for edible films based on pectin/alginate/W with results between 6.6 and 13% [11]. Furthermore, they concluded that the addition of G also influences the Xw, increasing its value in films that contained less, and this attributed to the hydrophilic nature of the plasticizer.
3.1. Swelling capacity and water vapor permeability
The behaviour of the Sc and WVP as a function of the CS and BW concentrations is illustrated in Figure 1. The Sc showed a tendency to decrease (430 % → 260 %) when it increases the contents of BW and WP in the formulation. This decrease could be attributed mainly to the hydrophobic character of BW, which interacts with the polymer matrix and decreases the Sc of the films [31], this behaviour was reported by Dhumal et al. (2019) [3] for edible films based on sago starch and guar gum with essential oil, where Sc reduction was attributed to extreme lipophilic nature of carvacrol and citral. It is also observed that the Sc of the composite films was lower than controls 1, 2, and 3, where the latter fluctuated between 435.8 ± 11.1 and 479.9 ± 11.4%. The results showed an inverse behaviour of the Sc to those obtained by Basiak et al. (2015) [2] for edible films composed of wheat starch and whey protein isolate, where the Sc increased with the increase of WP and helped to retain more water in the films. It could be to the interaction between hydrogen bonds, hydrophobic chains with ionic bonds which were generated on composite films [27]. In this way, strong intermolecular interaction among hydroxyl and amino groups of WP and amylose and amylopectin chains, improving the permeability of films [22, 27].
Figure 1.
Response surface graphs of the Sc and WVP of the films based on CS, WP, and BW: a) Swelling index films based on CS and WP; b) Swelling index films based on BW and WP; and c) Water vapor permeability of films based on CS and BW.
With respect to WVP, it is observed that this decreases with the increase of the BW content in the formulation. This effect is attributed to the hydrophobic character of the BW that increases the tortuous passage of the water vapor molecules through the film, decreasing its diffusivity coefficient. These results are in agreement with those reported by Ochoa et al. (2017) [33] in the evaluation of edible films formulated with corn starch, beeswax and natural antimicrobial. On the other hand, the lowest level of WVP is reached at a concentration of 0.5 % of WP when CS and BW concentrations are 3.0 % and 0.5 %, respectively. This low WVP condition is very important in the application of edible films in fruits [11]. The interactions that occur between the WP and CS generate denser matrices, capable of reducing the free volume in the film and limiting the diffusion of water vapor [17]. Similar results were observed by Basiak et al. (2017) [1], where starch-based films presented WVP around 7.87 ± 0.65 × 10−10 g m−1 s−1 Pa−1, and when protein increased WVP reduced 5.3–16.4%. On the other hand, the presence of BW in the films forms a barrier that decreases the diffusion of water vapor because of its hydrophobic character [29, 32]. However, CS-based films have a higher WVP because their interaction with G provides flexibility and modifies the polymer structure of the film by increasing water mobility, resulting in an increase in the water vapor pressure [8, 10]. Some authors have reported a decrease in WVP in films based on CS and soy protein with results among 4.3 ± 0.2 to 2.6 ± 0.1 g mm/m2 day kPa [10] and in films of gelatine and BW with results among 13.2 × 10−8 to 5 × 10−8 g∗mm∗h−1∗cm−2∗Pa−1 [31].
3.2. Mechanical properties
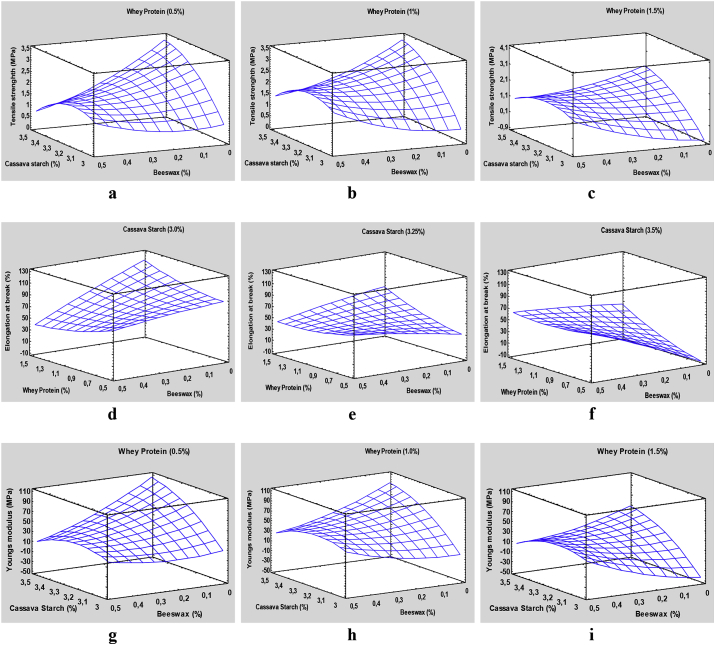
The behaviour of the mechanical properties is illustrated in Figure 2, TS at different concentrations of WP and as a function of CS and BW concentrations; ε at different concentrations of CS and depending on the concentrations of WP and BW; and Ym at different concentrations of WP and depending on the concentrations of CS and BW. The response surface plots showed similar Ts behaviour in the WP ranges evaluated, being higher at low WP concentrations (0.5 %–1.0 %). On the other hand, the films promote a higher TS in formulations with high concentration of CS (3.5 %), and low concentrations of BW result in lower values (0–0.1 %). Some authors have reported low values of Ts in films based on protein, polysaccharides, and glycerol. The materials had greater flexibility due to the protein-polysaccharides interaction and the plasticizing effect of glycerol, and this increases the mobility of polymer chains [3]. This behaviour also can be explained by a possible higher molecular affinity between WP-BW than with CS-BW. When the film contains high concentration of CS (3.5 %), the addition of high proportions of BW could interrupt the polymer network and weaken its structure. The opposite effect occurs at low levels of BW, a situation in which a reinforcing effect of the matrix is observed. Some authors indicate that in films based on CS, there is an decrease in Ts due to the use of waxes, and this is caused by both the formation of rigid polymer matrices [29] and whey protein because it is more easily added improving the mechanical strength [2].
Figure 2.
Response surface graphs of Ts, ε, and Ym: a) Tensile strength of films based on CS and BW with 0.5% of WP; b) Tensile strength of films based on CS and BW with 1% of WP; c) Tensile strength of films based on CS and BW with 1.5% of WP; d) Elongation at break of films based on WP and BW with 3% of CS; e) Elongation at break of films based on WP and BW with 3.25% of CS; f) Elongation at break of films based on WP and BW with 3.5% of CS; g) Young's modulus of films based on CS and BW with 0.5% of WP; h) Young's modulus of films based on CS and BW with 1% of WP; and i) Young's modulus of films based on CS and BW with 1.5% of WP.
The average ε values of the edible films fluctuated between 41.1 - 97.0 %, which gives the films good tensile strength characteristics before their breaking. However, there is no definite trend with respect to the independent variables, as it is very evident that the response of ε depends mainly on the linear interactions between the CS-BW and WP-BW. The highest elongation at break point is highlighted (≈97 %) with formulations low in CS (3.0 %) and BW (0–0.15%) and high content in WP. On the other hand, the smaller ε is obtained with formulations with high contents of CS (3.5 %) and BW (0–0.15 %) and low content of WP. This situation shows that the effective action of BW as a plasticizer depends on the interactions between the independent variables, allowing for a greater or lesser molecular mobility and free volume. This variant behaviour has been reported by Amin et al. (2019) [32], where ε depended on the interactions between polymers and beeswax. On the other hand, Cecchini et al. (2017) [29] and Zhang et al. (2018) [31] concluded that the interactions between the materials play an important role on the elastic properties of the materials, considering the BW to be a component that decreases the film ε.
The average values of the Ym of the edible films fluctuated between 3.5 - 68.9 %, which enhances the elastic behaviour of the material. The Ym presented significant statistical differences with respect to the concentrations of CS, WP, and BW, as well as with the linear interactions between CS-BW and WP-BW. The highest elasticity of the material (Ym ≈ 100–105 MPa) was determined from the negative interaction between BW-CS: low content of BW (0–0.1 %) and high CS (3.5 %). This behaviour is similar to when the formulation presents WP levels <1.0 %; however, the less elastic films (Ym < 20 MPa) are obtained mainly at low contents of WP (0.5 %), CS (3.0 %), and over the whole range of BW. This situation could be attributable to the reduction of the amylose crystallinity in the preparation of the films, but on the other hand, the interactions of CS and WP decrease the availability of amylose and amylopectin, in turn, increase the modulus of elasticity [2]. In addition, according to Zhang et al. (2018) [31], the BW generates rigid and glassy materials that increase the modulus of elasticity of the obtained films. This variable behaviour has been reported by Chiumarelli and Hubinger (2014) [39], obtaining films with heterogeneous elastic modules dependent on the mixtures used, higher concentrations of carnauba wax materials resulted in higher modulus of elasticity, while low concentrations of CS decrease the modulus of elasticity in various investigations.
3.3. Colour parameters
The L∗, although it presented statistically significant differences (p < 0.05) with respect to the contents of WP and BW, presented ranges of variation were very low. Their average values fluctuated between 90.2 and 88.7, which is related to the degree of whiteness that the CS contributes. A similar situation occurs in the chromatic coordinates a∗ and b∗ with their mean values fluctuated between (-1.2 and -1.9) and (3.0 and 9.1), which denotes a location in the chromatic plane a∗b∗ in the gray area of the II quadrant. This situation configures the dry material in the CIE-L∗a∗b∗ colour space with a translucent appearance (the light passes but does not allow for the objects to be seen clearly), matte or not very bright, of a greenish-yellow colour, conferred mainly by the content of WP present [40]. With respect to the control samples, the b∗ chromaticity differences are highlighted, whose values were lower than all the treatments with an average value of approximately 0, given the zero contribution of the CS in yellow pigmentation.
Regarding the polar coordinates Cab∗ and Hab∗, the formulations of the films presented similarly low fluctuations: (9.5–3.9) and (101.1°–121.0°) respectively. They had significant differences with respect to the control samples: (1.20–1.3) and (174.0–185.5), respectively, which denotes a lower intensity of colour in the latter or that it is close to the achromatic stimulus (Cab∗≈0).
Finally, the Yi in the formulations showed fluctuations in the range 3.8–16.7, being values higher than those found in controls 1, 2, and 3 (-0.7 and -1.3). As this represents the colour change of the clear or white sample to yellow, it is suggested that there is a direct relationship with the WP/CS interaction, and additionally, this could be caused by a possible Maillard browning reaction during the drying process [41]. The Yi presented significant differences (p < 0.05) with respect to the WP and BW factors, increasing their value in the presence of these components in the matrix that form the edible films. With respect to the materials obtained from CS, this is mainly due to the non-enzymatic browning process that occurs during drying to obtain the films [2]. In addition, the addition of BW can absorb light to avoid transmission through the material and, therefore, generate a colour change [29]. In general, the colour does not represent a critical parameter in the definition of the colour of the films since the observed changes are not perceived by the human eye.
4. Conclusions
Films based on CS, WP, and BW were obtained through casting method with potential application in fruit and vegetable coating. The mechanical properties (Ts, ε, and Ym) did not show a well-defined behaviour, being affected by the proportion of the components and their interactions. In some formulations, the BW showed a plasticizing behaviour in the materials with importance given to contents of WP. There is also a reinforcement effect of the materials with a high content of CS, although it seems to interrupt the continuity of the matrix when it is in a high proportion, reflected in the values of Ts. In addition, it was observed that the BW decreased the affinity with water of the CS-based material to a greater extent than the WP content, and this affected both the Xw, WVP, and Sc of the films. The films obtained in this work have a potential application in the food industry, specifically in the conservation of fruits. This is because it was possible to reduce the WVP, which would promote the slowing down of some physiological processes of fruits and vegetables, as well as your weight loss by dehydration.
Declarations
Author contribution statement
Misael Cortés-Rodríguez: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Camilo Villegas-Yépez: Performed the experiments; Wrote the paper.
Jesús H. Gil González, Pablo Emilio Rodríguez: Analyzed and interpreted the data.
Rodrigo Ortega-Toro: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Administrative Department of Science, Technology and Innovation from Colombia (COLCIENCIAS) (Project 52774, Contract RC 33–2016) and the CEIBA Foundation (Contract cc1085287640).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank to Michael James Stablein (University of Illinois, Urbana-Champaign) for the critical review of the manuscript.
References
- 1.Basiak E., Lenart A., Debeaufort F. Effects of carbohydrate/protein ratio on the microstructure and the barrier and sorption properties of wheat starch–whey protein blend edible films. J. Sci. Food Agric. 2017;97:858–867. doi: 10.1002/jsfa.7807. [DOI] [PubMed] [Google Scholar]
- 2.Basiak E., Galus S., Lenart A. Characterisation of composite edible films based on wheat starch and whey-protein isolate. Int. J. Food Sci. Technol. 2015;50:372–380. [Google Scholar]
- 3.Dhumal C.V., Ahmed J., Bandara N., Sarkar P. Improvement of antimicrobial activity of sago starch/guar gum bi-phasic edible films by incorporating carvacrol and citral. Food Packag. Shelf Life. 2019;21:1–9. [Google Scholar]
- 4.Pessanha Ferreira K.L., Farias Guimarães M., Carvalho Piler C.W., Godoy de Oliveira R.L. Starch films added of açaí pulp (Euterpe oleracea martius) Braz. Arch. Biol. Technol. 2018;61:1–14. [Google Scholar]
- 5.Yeddes W., Djebali K., Aidi Wannes W., Horchani-Naifer K., Hammami M., Younes I., Saidani Tounsi M. Gelatin-chitosan-pectin films incorporated with rosemary essential oil: optimized formulation using mixture design and response surface methodology. Int. J. Biol. Macromol. 2020;154:92–103. doi: 10.1016/j.ijbiomac.2020.03.092. [DOI] [PubMed] [Google Scholar]
- 6.García A., Pérez L.M., Piccirilli G.N., Verdini R.A. Evaluation of antioxidant, antibacterial and physicochemical properties of whey protein-based edible films incorporated with different soy sauces. LWT - Food Sci. Technol. Sci. Technol. 2020;117:108587. [Google Scholar]
- 7.Basiak E., Geyer M., Debeaufort F., Lenart A., Linke M. Relevance of interactions between starch-based coatings and plum fruit surfaces: a physical-chemical analysis. Int. J. Mol. Sci. 2019;20:1–17. doi: 10.3390/ijms20092220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sapper M., Talens P., Chiralt A. Improving functional properties of cassava starch-based films by incorporating xanthan, gellan, or pullulan gums. Int. J. Polym. Sci. 2019;2019:1–9. [Google Scholar]
- 9.Tongdeesoontorn W., Mauer L.J., Wongruong S., Sriburi P., Rachtanapun P. Physical and antioxidant properties of cassava starch-carboxymethyl cellulose incorporated with quercetin and TBHQ as active food packaging. Polymers (Basel) 2020;12:1–18. doi: 10.3390/polym12020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinma C.E., Ariahu C.C., Alakali J.S. Effect of temperature and relative humidity on the water vapour permeability and mechanical properties of cassava starch and soy protein concentrate based edible films. J. Food Sci. Technol. 2015;52:2380–2386. doi: 10.1007/s13197-013-1227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nallan Chakravartula S.S., Soccio M., Lotti N., Balestra F., Dalla Rosa M., Siracusa V. Characterization of composite edible films based on pectin/alginate/whey protein concentrate. Materials (Basel) 2019;12:1–19. doi: 10.3390/ma12152454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medina Jaramillo C., González Seligra P., Goyanes S., Bernal C., Famá L. Biofilms based on cassava starch containing extract of yerba mate as antioxidant and plasticizer. Starch - Stärke. 2015;67:780–789. [Google Scholar]
- 13.Md Nor S., Ding P. Trends and advances in edible biopolymer coating for tropical fruit: a review. Food Res. Int. 2020;134:109208. doi: 10.1016/j.foodres.2020.109208. [DOI] [PubMed] [Google Scholar]
- 14.Huntrakul K., Yoksan R., Sane A., Harnkarnsujarit N. Effects of pea protein on properties of cassava starch edible films produced by blown-film extrusion for oil packaging. Food Packag. Shelf Life. 2020;24:100480. [Google Scholar]
- 15.Mohamed S.A.A., El-Sakhawy M., El-Sakhawy M.A.-M. Polysaccharides, protein and lipid -based natural edible films in food packaging: a review. Carbohydr. Polym. 2020;238:1–14. doi: 10.1016/j.carbpol.2020.116178. [DOI] [PubMed] [Google Scholar]
- 16.Hassan B., Chatha S.A.S., Hussain A.I., Zia K.M., Akhtar N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: a review. Int. J. Biol. Macromol. 2018;109:1095–1107. doi: 10.1016/j.ijbiomac.2017.11.097. [DOI] [PubMed] [Google Scholar]
- 17.Santacruz S., Rivadeneira C., Castro M. Edible films based on starch and chitosan. Effect of starch source and concentration, plasticizer, surfactant’s hydrophobic tail and mechanical treatment. Food Hydrocolloids. 2015;49:89–94. [Google Scholar]
- 18.Pellá M.C.G., Silva O.A., Pellá M.G., Beneton A.G., Caetano J., Simões M.R., Dragunski D.C. Effect of gelatin and casein additions on starch edible biodegradable films for fruit surface coating. Food Chem. 2020;309:1–7. doi: 10.1016/j.foodchem.2019.125764. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt V., Porto L., Laurindo J., Menegalli F. Water vapor barrier and mechanical properties of starch films containing stearic acid. Ind. Crop. Prod. 2013;41:227–234. [Google Scholar]
- 20.Orozco-Parra J., Mejía C.M., Villa C.C. Development of a bioactive synbiotic edible film based on cassava starch, inulin, and Lactobacillus casei. Food Hydrocolloids. 2020;104:105754. [Google Scholar]
- 21.Gutiérrez T.J., Morales N.J., Pérez E., Tapia M.S., Famá L. Physico-chemical properties of edible films derived from native and phosphated cush-cush yam and cassava starches. Food Packag. Shelf Life. 2015;3:1–8. [Google Scholar]
- 22.Silva O.A., Pellá M.G., Pellá M.G., Caetano J., Simões M.R., Bittencourt P.R.S., Dragunski D.C. Synthesis and characterization of a low solubility edible film based on native cassava starch. Int. J. Biol. Macromol. 2019;128:290–296. doi: 10.1016/j.ijbiomac.2019.01.132. [DOI] [PubMed] [Google Scholar]
- 23.Castro F.V.R., Andrade M.A., Silva A.S., Vaz M.F., Vilarinho F. The contribution of a whey protein film incorporated with green tea extract to minimize the lipid oxidation of salmon (Salmo salar L.) Foods. 2019;8:1–16. doi: 10.3390/foods8080327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galus S., Kadzińska J. Whey protein edible films modified with almond and walnut oils. Food Hydrocolloids. 2016;52:78–86. [Google Scholar]
- 25.Kurek M., Galus S., Debeaufort F. Surface, mechanical and barrier properties of bio-based composite films based on chitosan and whey protein. Food Packag. Shelf Life. 2014;1:56–67. [Google Scholar]
- 26.Tsai M.J., Weng Y.M. Novel edible composite films fabricated with whey protein isolate and zein: preparation and physicochemical property evaluation. LWT - Food Sci. Technol. 2019;101:567–574. [Google Scholar]
- 27.Zhang X., Zhao Y., Li Y., Zhu L., Fang Z., Shi Q. Physicochemical, mechanical and structural properties of composite edible films based on whey protein isolate/psyllium seed gum. Int. J. Biol. Macromol. 2020;153:892–901. doi: 10.1016/j.ijbiomac.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Syahida N., Fitry I., Zuriyati A., Hanani N. Effects of palm wax on the physical, mechanical and water barrier properties of fish gelatin films for food packaging application. Food Packag. Shelf Life. 2020;23:100437. [Google Scholar]
- 29.Cecchini J.P., Spotti M.J., Piagentini A.M., Milt V.G., Carrara C.R. Development of edible films obtained from submicron emulsions based on whey protein concentrate, oil/beeswax and brea gum. Food Sci. Technol. Int. 2017;23:371–381. doi: 10.1177/1082013217695170. [DOI] [PubMed] [Google Scholar]
- 30.Bahrami A., Rezaei Mokarram R., Sowti Khiabani M., Ghanbarzadeh B., Salehi R. Physico-mechanical and antimicrobial properties of tragacanth/hydroxypropyl methylcellulose/beeswax edible films reinforced with silver nanoparticles. Int. J. Biol. Macromol. 2019;129:1103–1112. doi: 10.1016/j.ijbiomac.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., Simpson B.K., Dumont M.J. Effect of beeswax and carnauba wax addition on properties of gelatin films: a comparative study. Food Biosci. 2018;26:88–95. [Google Scholar]
- 32.Amin U., Khan M.A., Akram M.E., Al-Tawaha A.R.M.S., Laishevtcev A., Shariati M.A. Characterization of compisote edible films from aloe vera gel, beeswax and chitosan, Potravin. Slovak J. Food Sci. 2019;13:854–862. [Google Scholar]
- 33.Ochoa T.A., García-Almendárez B.E., Reyes A.A., Pastrana D.M.R., López G.F.G., Belloso O.M., González C.R. Design and characterization of corn starch edible films including beeswax and natural antimicrobials. Food Bioprocess Technol. 2017;10:103–114. [Google Scholar]
- 34.Pashova S., Radev R., Dimitrov G. Physical properties of edible films with different composition. Qual. - Access to Success. 2019;20:152–156. [Google Scholar]
- 35.Coughlan K., Shaw N.B., Kerry J.F., Kerry J.P. Combined effects of proteins and polysaccharides on physical properties of whey protein concetrate-based edible films. Food Eng. Phys. Prop. 2004;69:271–276. [Google Scholar]
- 36.Rubilar J.F., Zúñiga R.N., Osorio F., Pedreschi F. Physical properties of emulsion-based hydroxypropyl methylcellulose/whey protein isolate (HPMC/WPI) edible films. Carbohydr. Polym. 2015;123:27–38. doi: 10.1016/j.carbpol.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Famá L., Gañan-Rojo P., Bernal C., Goyanes S. Biodegradable starch based nanocomposites with low water vapor permeability and high storage modulus. Carbohydr. Polym. 2012;87:1989–1993. [Google Scholar]
- 38.Navarro-Tarazaga M., Massa A., Pérez-Gago M. Effect of beeswax content on hydroxypropyl methylcellulose-based edible film properties and postharvest quality of coated plums (Cv. Angeleno) LWT - Food Sci. Technol. 2011;44:2328–2334. [Google Scholar]
- 39.Chiumarelli M., Hubinger M.D. Evaluation of edible films and coatings formulated with cassava starch, glycerol, carnauba wax and stearic acid. Food Hydrocolloids. 2014;38:20–27. [Google Scholar]
- 40.Galus S., Lenart A. Optical, mechanical, and moisture sorption properties of whey protein edible films. J. Food Process. Eng. 2019;42:1–10. [Google Scholar]
- 41.Huntrakul K., Harnkarnsujarit N. Effects of plasticizers on water sorption and aging stability of whey protein/carboxy methyl cellulose films. J. Food Eng. 2020;272:1–11. [Google Scholar]